Abstract
Background
The aim of this systematic review was to identify the current endoscopic surveillance strategies in use across the world and to determine whether these were sufficient or if any recommendations for changes in the guidelines could be made. This review focused on the cystoscopic follow-up of non-muscle invasive bladder cancer (NMIBC) patients and muscle invasive bladder cancer (MIBC) patients who had undergone bladder sparing treatments.
Methods
A literature search was carried out on Medline and Embase using OVID gateway according to a pre-defined protocol. Systematic screening of the identified studies was carried out by two authors. Quality assessment was performed using the Joanna Briggs critical appraisal tools. Data was extracted on various aspects including the follow-up regime utilised, patients included, outcomes investigated and a summary of the results. The studies were compared in a narrative nature.
Results
A total of 2,604 studies were identified from the search strategy, of which 14 were deemed suitable for inclusion following the screening process. The studies identified were from nine countries and were mainly observational or qualitative. There was a huge variation in the follow-up regimes utilised within the studies with no clear consensus as to which regime was the most suitable. However, all studies utilised an initial cystoscopy at three months post-TURBT. No studies were identified which investigated the endoscopic follow-up strategies for MIBC patients who opted for bladder conservation with chemoradiation.
Conclusions
There is no universally accepted protocol for endoscopic follow-up of patients with NMIBC bladder cancer. Guidance on cystoscopic monitoring of bladder in patients who have undergone chemoradiation for MIBC is also lacking.
Keywords: Endoscopic, surveillance, follow-up, bladder cancer
Introduction
Bladder cancer is one of the most expensive cancers to treat. Together with the cost of treating the tumours, the follow-up for bladder cancer poses a huge burden on healthcare systems across the world. This is because, bladder cancer is a highly recurrent disease, and this risk varies as to which risk category a particular patient’s disease falls defined by the European Organisation for Research and Treatment of Cancer (EORTC) risk tables (1).
The majority (~75%) of patients diagnosed with bladder cancer have non-muscle invasive disease. These patients will largely undergo treatments that ensure bladder preservation, such as transurethral resection of the bladder tumours (TURBT) followed by adjuvant intravesical chemotherapy or BCG immunotherapy. However, they are at variable risk of disease recurrence and progression. Thus they need regular follow-up with periodic cystoscopy and imaging at various intervals. Although the mainstay of management for muscle invasive bladder cancer (MIBC) is radical surgery with or without prior neo-adjuvant chemotherapy, highly select group of patients may either be recommended for bladder preservation with chemoradiation whereas others may make a choice of this alternative as a preferred option. Bladder preservation strategies though attractive, necessitate regular surveillance because of a significant risk of recurrence requiring salvage treatment. A large case series reported that 27% of patients required a salvage cystectomy following tri-modal treatment either due to a lack of complete response or to recurrence (as observed in 13% of patients) (2).
Several endoscopic follow-up guidelines for NMIBC currently exist including that from the European Association of Urology (EAU) and American Urological Association (AUA) and National Institute for Health and Care Excellence (NICE) to name a few (summarised in Table 1) (3-5). The aims of this review are to (I) identify the current endoscopic follow-up regimes practiced in different urology departments around the world, (II) assess whether these regimes were adequate in detecting recurrences.
Table 1. Summary of risk-stratified cystoscopic follow-up guidelines following treatment for NMIBC.
| Guideline | Low risk NMIBC | Intermediate risk NMIBC | High risk NMIBC |
|---|---|---|---|
| EAU (3) | • Cystoscopy at 3 months • Subsequent cystoscopy 9 months later • Then annually for 5 years • Stop follow-up after 5 years if tumour-free |
• Patients should have an “in-between (individualised)” follow-up schedule | • Cystoscopy and urine cytology at 3 months • Subsequent follow-up every 3 months for 2 years • Then every 6 months until 5 years • Life-long annual cystoscopies thereafter |
| AUA (4) | • Cystoscopy at 3 months • Subsequent cystoscopy 6–9 months later • Annually until 5 years • Shared-decision making about surveillance thereafter |
• Cystoscopy at 3 months • Subsequent cystoscopy and cytology every 3–6 months for 2 years • Then 6–12 months until 5 years • Life-long annual cystoscopies thereafter |
• Cystoscopy at 3 months • Subsequent cystoscopy every 3–4 months for 2 years • Then every 6 months until 5 years • Life-long annual cystoscopies thereafter |
| NICE (5) | • Cystoscopy at 3 months • Subsequent cystoscopy at 12 months • Do not offer prolonged cystoscopic follow-up after 12 months |
• Cystoscopy at 3 months • Subsequent cystoscopy at 9 and 18 months • Annually thereafter • Consider discharging patients to primary care if disease-free after 5 years |
• Cystoscopy every 3 months for 2 years • Then every 6 months for another 2 years • Life-long annual cystoscopies thereafter |
| NCCN (6) | • Cystoscopy at 3 and 12 months • Then annually for 5 years • Shared-decision making about surveillance thereafter |
• Cystoscopy at 3 months • Subsequent cystoscopy at 6 and 12 months • Then every 6 months for 1 year • Then annually until years 5 • Shared-decision making about surveillance thereafter |
• Cystoscopy every 3 months for 2 years • Then every 6 months for 2 years • Then annually until 10 years • Shared-decision making about surveillance thereafter |
AUA, American Urological Association; EAU, European Association of Urology; NCCN, National Comprehensive Cancer Network; NICE, National Institute for Health and Care Excellence; NMIBC, non-muscle invasive bladder cancer; MIBC, muscle invasive bladder cancer.
This review focused on the cystoscopic follow-up of non-muscle invasive bladder cancer (NMIBC) patients and MIBC patients who had undergone bladder sparing treatments. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1363).
Methods
Search strategy and criteria
A search was carried out on Medline and Embase using OVID gateway using the search terms (Surveillance OR follow-up OR follow-up regimens OR follow up OR follow up regimens) AND (Endoscope OR endoscopy OR endoscopic OR cystoscopy OR cystoscope)) AND (exp bladder cancer) OR (Non-muscle invasive bladder cancer OR NMIBC) OR ((Muscle invasive bladder cancer OR MIBC) AND (Radiotherapy OR radiation))) (Appendix). Additional search criteria included: published from 2000, full text available and written in English. Studies were excluded if they were an editorial, conference abstract, review, commentary or letter. Studies were deemed suitable for inclusion if they described the outcomes of a certain endoscopic follow-up regime, made recommendations for changes to the current guidelines or compared the outcomes for two or more follow-up regimes. All studies included must have described the frequency of an investigated follow-up regime e.g., how often and how long for? Two independent reviewers assessed the studies for inclusion (B Russell and P Kotecha). Initially the titles were screened, followed by the abstracts and subsequently the full texts.
Data extraction
All extracted data were summarized into tables. The extracted data included: country of study, type of study, type of bladder cancer patients included, the follow-up regime investigated, a summary of results (including any recommendations for follow-up). The various follow-up regimes and outcomes were compared between the studies in a qualitative manner.
Statistical analysis
The data extracted from the studies did not allow for a quantitative synthesis to take place.
Quality assessment
A quality assessment was completed for all studies using one of the Joanna Briggs Institute critical appraisal tools (7). These tools are a series of checklists including information on study design, statistical analyses and reporting of results to determine whether a study should be included within a review. These tools are advantageous when several different study types are to be assessed as the relevant appraisal tool can be selected depending on the study type in question. The checklists utilised for this study were for: case-series, cohort studies and qualitative research studies. This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (8).
Results
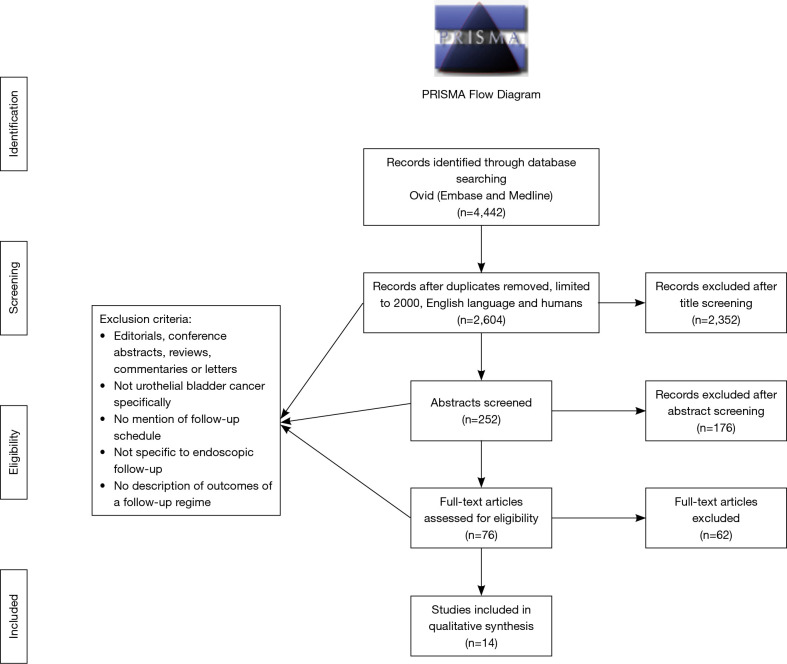
The search strategy identified 2,604 studies. After the title screening, 252 abstracts were screened for inclusion of which 76 full texts were subsequently screened. Consequently, 14 studies were deemed suitable for inclusion (Figure 1). The critical appraisal of the studies deemed all studies suitable for inclusion into the review (Appendix I). It is important to note however, that whilst only studies published from 2000 onwards were included, with the exception of the studies by Golabesk et al. (9) and Mariappan (10) which included data prior to 2000.
Figure 1.
PRISMA flow diagram for selection of studies.
Of the 14 studies included, four were case series, seven cohort and three qualitative research studies (Table 2). The studies were reported from across the globe with five from USA, three from UK, two from Spain and one from each of: Turkey, Italy, France and Europe (France, UK, Germany, Italy, Czech Republic). All studies investigated the follow-up schedules for NMIBC patients only. None of the studies identified included the follow-up for MIBC post-radiotherapy.
Table 2. Summary of studies.
| Study ID | Title | Authors | Year | Study type | Country of study | Type of BC patients |
|---|---|---|---|---|---|---|
| 1 | Is cystoscopy follow-up protocol safe for low-risk bladder cancer without muscle invasion? | Yucetas et al. (11) | 2020 | Retrospective cohort study (Case series) | Turkey | Low risk NMIBC |
| 2 | Extent of Risk-Aligned Surveillance for Cancer Recurrence Among Patients With Early-Stage Bladder Cancer | Schroeck et al. (12) | 2018 | Cohort study | USA | Patients with early stage bladder cancer (NMIBC) |
| 3 | Pathological Outcomes for Patients Who Failed To Remain Under Active Surveillance for Low-risk Non–muscle-invasive Bladder Cancer: Update and Results from the Bladder Cancer Italian Active Surveillance Project | Hurle et al. (13) | 2018 | Prospective observational cohort study | Italy | NMIBC |
| 4 | Discrepancy Between European Association of Urology Guidelines and Daily Practice in the Management of Non–muscle-invasive Bladder Cancer: Results of a European Survey | Hendricksen et al. (14) | 2019 | Qualitative study - Interviews | Europe (Germany, France, UK, Italy, Poland, Netherlands, Czech Republic, Austria and Belgium) | NMIBC |
| 5 | The impact of frequent cystoscopy on surgical care and cancer outcomes among patients with low-risk, non–muscle-invasive bladder cancer | Schroeck et al. (15) | 2019 | Retrospective cohort study | USA | Low risk NMIBC |
| 6 | Overuse of Cystoscopic Surveillance Among Patients With Low-risk Non–Muscle-invasive Bladder Cancer – A National Study of Patient, Provider, and Facility Factors | Han et al. (16) | 2019 | Retrospective cohort study | USA | Patients newly diagnosed with low-risk NMIBC |
| 7 | Multiple recurrences and risk of disease progression in patients with primary low-grade (TaG1) non-muscle-invasive bladder cancer and with low and intermediate EORTC-risk score. | Simon et al. (17) | 2019 | Retrospective cohort study – Single centre case series | France | Primary TaG1 bladder cancer |
| 8 | Long-term Bladder and Upper Urinary Tract Follow-up Recurrence and Progression Rates of G1-2 Non-muscle-invasive Urothelial Carcinoma of the Bladder | Golabesk et al. (9) | 2017 | Retrospective cohort study | Spain | NMIBC |
| 9 | Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer | Hernandez et al. (18) | 2016 | Cohort study | Spain | G1/G2 pTa-T1 |
| 10 | A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. | Mariappan and Smith (10) |
2005 | Prospective cohort study | Scotland, UK | G1pTa |
| 11 | Surveillance for bladder cancer: The management of 4.8 million people | Wright and Jones (19) | 2000 | Qualitative study - Questionnaires | UK | G1/G2 pTa-T1 |
| 12 | Comparison of surveillance strategies for low-risk bladder cancer patients | Zhang, Denton and Nielsen (20) | 2013 | Cohort Study | USA | NMIBC |
| 13 | Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy | Herr and Donat (21) | 2011 | Cohort study | USA | Recurrent low- grade papillary bladder tumours |
| 14 | Long-term surveillance of bladder tumours: Current practice in the United Kingdom and Ireland | Wazait et al. (22) | 2003 | Qualitative study - Questionnaires | UK and Ireland | NMIBC |
NMIBC, non-muscle invasive bladder cancer; TURBT, transurethral resection of the bladder tumour.
The studies varied somewhat in both the follow-up regimes and outcomes of interest (summarised in Table 3). For example, some looked at the number of recurrences, frequency of cystoscopies, time to recurrence or recurrence free survival. A similarity between all of the follow-up regimes included was the practice of initial cystoscopy at three months post-TURBT. Following this, some protocols included cystoscopies every 3–6 months until two years and then between 6–12 months thereafter until five years. Whilst, others only included cystoscopies every six months or even annually following the initial three-month post-TURBT cystoscopy. It is important to note however that the grade and stage of patients included within the studies did vary (Table 3). There were also some discrepancies between a recommendation to continue or cease endoscopic follow-up after a five-year period and as to which patients this would be recommended for. This was particularly evident in the survey of consultant urologists conducted by Wazait et al. (for which results are explain in more detail below) (22).
Table 3. Summary of follow-up regimes, outcomes and results.
| Study ID | Authors | Follow-up regimes investigated | Outcome(s) of interest | Summary of results |
|---|---|---|---|---|
| 1 | Yucetas et al. (11) |
3-month post-TURBT cystoscopy, then every 3 months for 2 years, then annually until the 5th year | Frequency of recurrences and pT stage progression | Most recurrences in the low-risk NMIBC patients occurred within the first 2 years. If the follow-up strategies described in the guidelines had been utilised, patients with relapses would have a delay of at least 6 months of diagnosis. The authors therefore recommend that cystoscopy should be performed every 3 months within the first two years |
| 2 | Schroeck et al. (12) |
Risk aligned cancer surveillance | Frequency of surveillance cystoscopy and length of follow-up for low-risk and high-risk NMIBC patients | The frequency of cystoscopic surveillance for patients with early-stage bladder cancer was comparable across both low and high risk groups Therefore, the risk-aligned strategies were not widely used |
| 3 | Hurle et al. (13) |
Cystoscopy every 3 months for the first year, and then every 6 months thereafter | Active surveillance failure | Active surveillance is a reasonable strategy in patients presenting with LG pTa/pT1a bladder tumours. The authors state the results from the study strengthen the role of active surveillance within this selected population with minimal risk of progression |
| 4 | Hendricksen et al. (14) | Urologists were given 10 options of follow-up schedules with varying frequencies and duration to choose from | Frequency and duration of follow-up | Patients with low-risk NMIBC are likely to be over-monitored and those with high-risk NMIBC under-monitored |
| 5 | Schroeck et al. (15) |
Recommended vs. frequent surveillance | Progression to muscle-invasive disease and bladder cancer death | Patients with low-risk NMIBC underwent too many cystoscopies. Subsequently, frequent cystoscopy was associated with twice as many transurethral resections and did not decrease the risk for bladder cancer progression or death, supporting current guidelines |
| 6 | Han et al. (16) |
Recommended cystoscopy regime (at 3, 12 and 24 months post-diagnosis) and overuse of surveillance (defined as undergoing 2 or more cystoscopies if followed for less than 1 year, 3 or more procedures if followed between 1 to less than 2 years, and 4 or more procedures if followed for 2 years after diagnosis) | Overuse of surveillance | The authors observed the overuse of cystoscopy surveillance in 75% of patients with low-risk NMIBC |
| 7 | Simon et al. (17) | (French Association of Urology guidelines) Cystoscopy at 3 months post-TURBT, then every 3 months for two years, every 6 months thereafter until five years, and then annually | Time to recurrence and/or progression (up to 10 years) | 17 of the 47 progression which occurred, did so after 5-year of follow-up. Therefore, the authors recommend that the endoscopic surveillance of patients with TaG1 should be continued beyond 5 years of follow-up but annually rather than 6 months |
| 8 | Golabesk et al. (9) |
Cystoscopy performed at 3 months after the therapy and, if no recurrence was found, alternate follow-up with cystoscopy or ultrasound and cytology was organized every 4 months for 2 years, and every 6 months thereafter | Frequency of recurrence and progression free survival | G1-2 urothelial bladder cancers recur and progress uncommonly in the long-term period. 37% of recurrences occurred within the first 5 years, whilst 4% occurred after 5 years. The authors suggest a re-examination of the follow-up schedule for patients G1-2 tumours who remain asymptomatic and disease-free for at least 5 years |
| 9 | Hernandez et al. (18) | Cystoscopy every 3 to 4 months for the first 2 years then every 6 months alternating between cystoscopy and ultrasound | End points were grade and pathological stage progression | The authors conclude that active surveillance in a high-selectivity group of patients (i.e., patients with Ta not T1 tumours) is feasible and oncologically safe in the long term |
| 10 | Mariappan and Smith (10) |
Initial cystoscopy 3 months after TURBT then again at 6–9 months and, if clear, annually thereafter | Frequency of recurrences | The authors state that patients with G1Ta disease who are free of recurrence for 5 years after presentation can be safely discharged |
| 11 | Wright and Jones (19) | N/A | Study aimed to get a consensus on what regime was used by consultant urologists | There were considerable variations among individuals in the type and timing of check cystoscopy |
| 12 | Zhang, Denton and Nielsen (20) | EAU and AUA guidelines plus 12 other strategies. All strategies begin surveillance 3 months post-TURBT and end after 5 years. They each differed in intervals between cystoscopies | Lifelong progression rate, total number of cystoscopies and QALYS associated with each surveillance strategy | The results suggest that both age and comorbidity significantly affected the optimal surveillance strategy. For example, younger patients should be screened more intensively than older patients, and patients with comorbidities should be screened less intensively |
| 13 | Herr and Donat (21) | Both white light and narrow-band imaging cystoscopy at 6-month intervals following prior recurrence | Frequency of recurrences and the recurrence-free survival | Narrow band imaging cystoscopy was associated with fewer patients having tumour recurrences, fewer numbers of recurrent tumours, and a longer recurrence-free survival time |
| 14 | Wazait et al. (22) | N/A | Study aimed to identify a consensus on the follow-up guidelines for NMIBC patients | The authors concluded that there was a lack of consensus regarding the long-term surveillance of bladder cancer in the UK and Ireland |
AS, active surveillance; AUA, American Urological Association; EAU, European Association of Urology; NMIBC, non-muscle invasive bladder cancer; QALYS, quality adjusted life years; TURBT, transurethral resection of the bladder tumour.
Three of the studies which investigated the frequency of cystoscopies were conducted by the same group of authors (12,15,16). The study by Han et al. investigated the frequency of cystoscopies to determine whether surveillance was being overused in a cohort of 1,135 NMIBC patients (16). They defined the recommended surveillance cystoscopy procedure at three months after diagnosis, then repeat at 12 and 24 months. They state that 75% of all patients considered surveillance too frequent totalling to an excess of 1,846 cystoscopies within two years when compared to the recommended surveillance strategy. The authors claimed that the recommended surveillance regime was adopted from the guidelines though they did not specify as to which guidelines they followed.
A similar study by Schroeck et al. (15) included 1,042 low risk NMIBC patients. The authors stated that the surveillance intervals were shorter among patients undergoing frequent surveillance as their recurrence rates was higher than those on the recommended surveillance strategy. Crucially, the authors conclude that a higher frequency of cystoscopies did not reduce the risk of progression or death in these patients thus supporting the use of the recommended surveillance regime.
Another study by these authors (12) included 1,278 low-risk and 2,115 high-risk NMIBC patients diagnosed between 2005–2011. Patients with low-risk cancer underwent a mean (SD) of 5.3 (3.4) cystoscopies during a median (IQR) follow-up of 2.6 (0.9–4.7) years. They stated that patients with high-risk cancer underwent a check cystoscopy at relatively shorter intervals due to a higher risk of recurrence. However, the mean number of cystoscopies was comparable to that of low-risk patients. For example, the adjusted frequency of surveillance cystoscopy ranged from 3.7 to 6.2 procedures over two years for low-risk patients and from 4.6 to 6.0 procedures over two years for high-risk patients. Therefore, the authors conclude that a risk-aligned cancer surveillance strategy was not utilised at a national level.
Three of the studies were qualitative studies whereby clinicians were given questionnaires to complete (14,19,22). Two of these were conducted within the UK whilst the other was a pan-European study. In the UK study by Wright and Jones, the majority of consultants surveyed said they would recommend a cystoscopy at 3–4 months post-TURBT for both pTa and pT1 G1/G2 tumours (19). Most of the consultants (44%) said they would then schedule the next cystoscopy at six months post-TURBT. A small minority reported that they would wait a year before performing the next cystoscopy (28% in pTa and 16% in pT1 G1/G2 tumours). Similarly, the study by Wazait et al. looked at pTa and pT1 tumours separately (22). In this study however, they found that consultant urologists tended to carry on the cystoscopy follow-up of pT1 patients for longer than pTa tumours. This was particularly evident for the G3 pT1 tumours. For example, 33% and 16% of the consultants surveyed said they would end the follow-up of pTaG1 and pTaG2 tumours after five years. In pT1, G3 tumours however, 70% said they would conduct lifelong follow-up. In the Europe-wide study by Hendricksen et al., a total of 498 physicians from nine European countries completed the questionnaires (14). The authors concluded that the patients with high-risk NMIBC appeared to be under-monitored, compared to patients with low- and moderate-risk NMIBC though they did not explicitly define what they meant by over or under monitored.
Numerous studies investigated the frequency of recurrences within their reported cohorts (9-11,17,21). Yucetas et al. for example retrospectively analysed 51 patients and found that recurrence occurred in 80% of the patients within the first two years and in 84% within five years (11). Yucetas and colleagues concluded that had their patients been followed up as per guidelines, the patients with recurrences would have had this diagnosis delayed by at least six months. They therefore recommend that within the first two years, cystoscopy should be performed every three months in order to detect recurrences without delay.
A similar study by Golabesk et al. included a larger cohort of 704 G1-G2 NMIBC patients from a single institution (9). In this cohort, recurrence occurred in 24% of patients after two years and in 40% of patients after five years. The authors concluded that in patients who have remained recurrence free for a minimum of five years, a less intensive and invasive follow-up regime could be considered including the elimination of upper urinary tract surveillance. Another study by Mariappan and Smith which included G1pTa patients only, found that recurrences occurred in 19% of patients at five years (10). This study benefitted from a long follow-up period of 20 years. However, as the follow-up was quite long, the study also included slightly older data compared to the other studies and hence slightly outdated follow-up regimes were in use during that study period.
Hurle and colleagues (13) investigated the active surveillance adherence in 167 low grade NMIBC patients. Of the 181 active surveillance events, 33.7% required initiation of treatment due to active surveillance failure. The follow-up regime utilised was cystoscopy every three months for the first year, and then every six months thereafter. The authors concluded that this regime was a reasonable strategy for low grade NMIBC patients.
The study by Zhang, Denton and Nielsen is somewhat different from the others, as in this study a mathematical modelling to estimate quality adjusted life years (QALYS) from various follow-up regimes (20). The authors investigated the follow-up regimes set out in the EAU and AUA guidelines as well as 12 other variations/dynamic strategies (D1-D12) each with increasing intervals until five years. QALYS were estimated for a male and a female aged 73 years as this was the average age at diagnosis for a male bladder cancer according to Surveillance Epidemiology and End Results (SEER). In both the male and female base cases, the EAU guideline resulted in higher mean QALYs compared with the AUA guideline which is more intensive. The highest QALYS were observed in the no surveillance group for the male case, however this was not found to be significant when compared to three other dynamic strategies. The authors found that the regime within the EAU guideline resulted in a higher expected lifelong progression rate but with around half the number of cystoscopies over the patient’s lifetime when compared to the AUA guideline.
T stage progression was another theme which was investigated by several of the studies with varying follow-up regimes. The study by Simon et al. included a cohort of 470 TaG1 patients (17). Of these, 10% progressed to a higher pathological T stage, either on early re-resection or after early recurrence on check cystoscopy. Almost half (47%) of the total cohort experienced a recurrence without disease progression. The authors concluded that surveillance should be continued after five years as 17 of the 47 patients who progressed, did so after five years. According to a cohort of 186, Ta and T1 patients in another study, around 14% experienced a stage progression with four of the T1G2 patients progressing to T2 (18). Subsequently, Hernandez et al. concluded that active surveillance beyond 5 years should only be recommended in a highly selected group of patients excluding patients with previous T1 disease.
Discussion
It is apparent from this systematic review of the studies from nine different countries, that the endoscopic follow-up regimes for NMIBC vary significantly across Europe and the United States. Furthermore, there is lack of studies on endoscopic follow-up of MIBC patients who have undergone bladder sparing treatments.
There are two main questions when considering a follow-up strategy: (I) how often should the cystoscopies be performed and (II) how long should this surveillance should continue? Deciding upon surveillance strategy also involves taking into account several aspects including risk level of the patient (i.e., low, intermediate and high), the quality of life for the patient, the cost effectiveness of the surveillance and likelihood of diagnosing a recurrence. The studies identified within this review touched upon most of these aspects in the context of NMIBC.
Both the EAU and AUA guidelines do not recommend the continuation of follow-up for low-risk NMIBC patients after five years if the patient has remained cancer free. However, a few of the studies identified within this review contest this recommendation. Simon et al. for example, observed many recurrences in low risk TaG1 patients even after five years of surveillance and hence proposed that cystoscopic surveillance should continue beyond this five-year (17). Another study (which was not included in our review as it was published prior to the year 2000), also supported the surveillance of TaG1 patients beyond five years stating that progression was not uncommon for these patients (23). Furthermore, almost half of the clinicians surveyed within the qualitative study by Wazait et al. said that they continue surveillance for these patients for 10 years with 18% saying this surveillance should be lifelong (22). On the contrary, the study by Mariappan and Smith recommended that patients with G1Ta disease who remain free of recurrence for five years after presentation can be safely discharged from further surveillance (10).
The EORTC risk tables were developed to help clinicians predict a patient’s risk of recurrence or progression (24). Both the AUA and EAU guidelines include risk stratified guidelines for NMIBC surveillance (25,26), with the higher risk patients undergoing a more intensive cystoscopy follow-up regime than their lower risk counterparts. However, the study by Schroeck et al. (12) suggested that these risk aligned guidelines are not always followed with comparable number of cystoscopies across both low and high risk groups. A previous systematic review recognised that the reality of implementing consistent, risk-aligned surveillance strategies within busy clinics is challenging (27).
A commonly investigated outcome was the number of recurrences detected by the numerous surveillance strategies. However, the number of recurrences that occurred at different time points varied greatly amongst the studies as did the surveillance strategies utilised. For example, the regime in the study by Yucetas et al. (11) constituted cystoscopy every three months for two years, then annually until five years. Conversely, in the study by Golabesk et al. (9), cystoscopies were performed every four months for two years then every six months until five years. Therefore, the largest difference between these regimes existed after the initial two years. Importantly, Yucetas et al. (11) state that had their patients followed the EAU and AUA recommended guidelines, many patients would have had their recurrence diagnosis delayed. They subsequently recommend that patients should undergo cystoscopy every three months within the initial two-year period. It is important to consider however, that the results from the two studies by Golabesk et al. and Yucetas et al. were based on very different population numbers (n=704 vs. n=51 respectively) (9,11). A further noteworthy point is that detected recurrences may have variable consequences depending upon their grade and stage at the time of diagnosis. Some may be serious in case of higher grade and higher stage but low grade and non-invasive recurrences may not have equivalent connotations.
Whilst it was hard to determine from the studies included within this review which follow-up regime is most suitable, the study by Zhang, Denton and Nielsen showed that a ‘one size fits all’ approach is not necessarily appropriate for follow-up regimes (20). Instead, the authors show that stratification by age should be taken into consideration. For example, the no active surveillance but watchful waiting strategy was deemed best for the older patients, whereas a moderately intense regime (cystoscopy at 3, 14, 33 and 60 months) was recommended for those in 50s. This study benefitted from not only taking into account the practicalities of surveillance i.e., effect on survival, but it also considered the quality of life of the patients through the calculation of QALYs.
The huge variation in surveillance strategies observed amongst the studies was also noted by van der Heijden and Witjes within the various follow-up policies (28). Despite the variation, almost all studies within the current review and the policies within the summary by van der Heijden and Witjes agreed that there should be an initial cystoscopy at three months after TURBT.
There was a paucity of identified studies investigating the endoscopic follow-up regime for MIBC patients who have undergone bladder preservation treatments. Currently, both the AUA and EAU guidelines provide recommendations for imaging rather than endoscopic strategies. For example, the AUA guidelines recommend CT or MRI imaging at 6–12 months intervals for 2–3 years then annually thereafter (29). Similarly, the EAU guidelines state that CT imaging should occur every 6 months for three years then annually thereafter (30). Therefore, one area for future research on this topic would be to identify whether there is a consensus on how is best to follow-up these MIBC patients through cystoscopies.
There are several reasons as to why clinicians may decide upon a cystoscopic follow-up regime. Some may choose to perform these cystoscopies more frequently than recommended due either to a deficient knowledge of the guidelines, poor pathological reporting with no comment on accurate grade and stage, absence of a multidisciplinary team (MDT) structure or lack of use of the recommended risk tables. There may also be financial incentives to perform cystoscopies more often within a private health care setting. Likewise, some clinicians may decide to carry out fewer cystoscopies on their patients due to a deficient knowledge of the guidelines, but also due to a lack of sub-specialisation or MDT structure within their hospital. Some countries have fragmented healthcare systems with poor access to health facilities which may hinder the frequency of cystoscopies and patient compliance can also play a major factor.
Whilst this review focused on the endoscopic follow-up of bladder cancer patients, it is important to note the emerging role of urinary biomarkers in the diagnosis and surveillance of bladder cancer. The advantage of urinary biomarkers is that they are a non-invasive technique. They are however, not as sensitive as the gold standard urine cytology and therefore may lead to false positives (31). Despite the non-invasive nature of urine biomarkers, a recent study in the UK found that patients require urine biomarkers to be as sensitive as cystoscopy before they would be willing to forgo cystoscopy in their surveillance regime (32). There are currently six urinary assays approved by the US Food and Drug Administration (FDA) but only for use alongside cytology (31).
A strength of the current review is the inclusion of studies from nine difference countries. This enabled the collation of information from several parts of the world. It is however a limitation that none of the studies included investigated the follow-up regime for MIBC patients who had undergone bladder sparing treatments specifically.
Conclusions
This review suggests, that whilst there are several guidelines with recommendations for follow-up regimes for NMIBC, there remains a lack of consensus among the practising clinicians on which is the best regime to follow. There did not appear to be any particular regime used throughout the studies which was deemed most suitable for universal acceptance or adoption.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mieke Van Hemelrijck and Netty Kinsella) for the series “Expectant Management in Genitourinary Malignancies (Prostate, Bladder, Kidney)” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1363
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1363). The series “Expectant Management in Genitourinary Malignancies (Prostate, Bladder, Kidney)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Kurth KH, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer 1995;31A:1840-6. 10.1016/0959-8049(95)00287-S [DOI] [PubMed] [Google Scholar]
- 2.Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term Outcomes After Bladder-preserving Tri-modality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur Urol 2017;71:952-60. 10.1016/j.eururo.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Compérat E, et al. Non-muscle-invasive Bladder Cancer Guidelines [Internet]. EAU Guidelines. 2021. Available online: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/
- 4.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Joint Guideline (2020). J Urol 2016;196:1021-9. 10.1016/j.juro.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health Care and Excellence. Bladder cancer: Diagnosis and Management [Internet]. NICE guideline. 2015. Available online: https://www.nice.org.uk/guidance/ng2/chapter/1-recommendations#follow-up-after-treatment-for-muscle-invasive-bladder-cancer
- 6.Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:329-54. 10.6004/jnccn.2020.0011 [DOI] [PubMed] [Google Scholar]
- 7.Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk [Internet]. Joanna Briggs Institute Reviewer’s Manual. 2017. Available online: https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 2009;6:e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golabesk T, Palou J, Rodriguez O, et al. Long-term Bladder and Upper Urinary Tract Follow-up Recurrence and Progression Rates of G1-2 Non-muscle-invasive Urothelial Carcinoma of the Bladder. Urology 2017;100:145-50. 10.1016/j.urology.2016.07.063 [DOI] [PubMed] [Google Scholar]
- 10.Mariappan P, Smith G. A surveillance schedule for G1Ta bladder cancer allowing efficient use of check cystoscopy and safe discharge at 5 years based on a 25-year prospective database. J Urol 2005;173:1108-11. 10.1097/01.ju.0000149163.08521.69 [DOI] [PubMed] [Google Scholar]
- 11.Yucetas U, Aglamis E, Ates HA, et al. Is cystoscopy follow-up protocol safe for low-risk bladder cancer without muscle invasion? Urol Ann 2020;12:25-30. 10.4103/UA.UA_143_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeck FR, Lynch KE, Chang JW, et al. Extent of Risk-Aligned Surveillance for Cancer Recurrence Among Patients With Early-Stage Bladder Cancer. JAMA Netw Open 2018;1:1-12. 10.1001/jamanetworkopen.2018.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurle R, Colombo P, Lazzeri M, et al. Pathological Outcomes for Patients Who Failed To Remain Under Active Surveillance for Low-risk Non-muscle-invasive Bladder Cancer: Update and Results from the Bladder Cancer Italian Active Surveillance Project. Eur Urol Oncol 2018;1:437-42. 10.1016/j.euo.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 14.Hendricksen K, Aziz A, Bes P, et al. Discrepancy Between European Association of Urology Guidelines and Daily Practice in the Management of Non-muscle-invasive Bladder Cancer: Results of a European Survey. Eur Urol Focus 2019;5:681-8. 10.1016/j.euf.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 15.Schroeck FR, Lynch KE, Li Z, et al. The impact of frequent cystoscopy on surgical care and cancer outcomes among patients with low-risk, non-muscle-invasive bladder cancer. Cancer 2019;125:3147-54. 10.1002/cncr.32185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han DS, Lynch KE, Chang JW, et al. Overuse of Cystoscopic Surveillance Among Patients With Low-risk Non-Muscle-invasive Bladder Cancer - A National Study of Patient, Provider, and Facility Factors. Urology 2019;131:112-9. 10.1016/j.urology.2019.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon M, Bosset PO, Rouanne M, et al. Multiple recurrences and risk of disease progression in patients with primary low-grade (TaG1) non-muscle-invasive bladder cancer and with low and intermediate EORTC-risk score. PLoS One 2019;14:e0211721. 10.1371/journal.pone.0211721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández V, Llorente C, de la Peña E, et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol 2016;34:165.e19-165.e23. 10.1016/j.urolonc.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 19.Wright MPJ, Jones DJ. Surveillance for bladder cancer: The management of 4.8 million people. BJU Int 2000;85:431-3. 10.1046/j.1464-410x.2000.00467.x [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Denton BT, Nielsen ME. Comparison of surveillance strategies for low-risk bladder cancer patients. Med Decis Making 2013;33:198-214. 10.1177/0272989X12465353 [DOI] [PubMed] [Google Scholar]
- 21.Herr HW, Donat SM. Reduced bladder tumour recurrence rate associated with narrow-band imaging surveillance cystoscopy. BJU Int 2011;107:396-8. 10.1111/j.1464-410X.2010.09547.x [DOI] [PubMed] [Google Scholar]
- 22.Wazait HD, Al-Bhueissi SZ, Patel HRH, et al. Long-term surveillance of bladder tumours: Current practice in the United Kingdom and Ireland. Eur Urol 2003;43:485-8. 10.1016/S0302-2838(03)00052-6 [DOI] [PubMed] [Google Scholar]
- 23.Leblanc B, Duclos AJ, Bénard F, et al. Long-term followup of initial Ta grade 1 transitional cell carcinoma of the bladder. J Urol 1999;162:1946-50. 10.1016/S0022-5347(05)68075-5 [DOI] [PubMed] [Google Scholar]
- 24.Sylvester RJ, van der Meijden A, Oosterlinck W, et al. Predicting Recurrence and Progression in Individual Patients With Stage Ta T1 Bladder Cancer Using EORTC Risk Tables: A Combined Analysis of 2596 Patients From Seven EORTC Trials. Eur Urol 2006;49:466-5. 10.1016/j.eururo.2005.12.031 [DOI] [PubMed] [Google Scholar]
- 25.Chang SS, Boorjian SA, Chou R, et al. Bladder Cancer: Non-Muscle Invasive Guideline - American Urological Association [Internet]. 2020 [cited 2020 Jul 7]. Available online: https://www.auanet.org/guidelines/bladder-cancer-non-muscle-invasive-guideline
- 26.Babjuk M, Burger M, Compérat E, et al. EAU Guidelines: Non-Muscle Invasive Bladder Cancer [Internet]. 2019. Available online: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/#1
- 27.Schroeck FR, Smith N, Shelton JB. Implementing risk-aligned bladder cancer surveillance care. Urol Oncol 2018;36:257-64. 10.1016/j.urolonc.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Heijden AG, Witjes JA. Recurrence, Progression, and Follow-Up in Non-Muscle-Invasive Bladder Cancer. Eur Urol Suppl 2009;8:556-62. 10.1016/j.eursup.2009.06.010 [DOI] [Google Scholar]
- 29.Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline (2017) - American Urological Association [Internet]. 2020 [cited 2020 Jul 8]. Available online: https://www.auanet.org/guidelines/bladder-cancer-non-metastatic-muscle-invasive-guideline
- 30.Witjes J., Bruins M, Cathomas R, et al. EAU Guidelines: Muscle-invasive and Metastatic Bladder Cancer [Internet]. 2019. Available online: https://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/#7
- 31.Ng K, Stenzl A, Sharma A, Vasdev N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Vol. 39, Urologic Oncology: Seminars and Original Investigations. Elsevier Inc.; 2021:41-51. [DOI] [PubMed] [Google Scholar]
- 32.van Osch FHM, Nekeman D, Aaronson NK, et al. Patients choose certainty over burden in bladder cancer surveillance. World J Urol 2019;37:2747-53. 10.1007/s00345-019-02728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as