Abstract
Wild animals are natural reservoir hosts for a variety of pathogens that can be transmitted to other wildlife, livestock, other domestic animals, and humans. Wild deer (family Cervidae) in Europe, Asia, and North and South America have been reported to be infected with gastrointestinal and vector-borne parasites. In Australia, wild deer populations have expanded considerably in recent years, yet there is little information regarding which pathogens are present and whether these pathogens pose biosecurity threats to humans, wildlife, livestock, or other domestic animals. To address this knowledge gap, PCR-based screening for five parasitic genera was conducted in blood samples (n = 243) sourced from chital deer (Axis axis), fallow deer (Dama dama), rusa deer (Rusa timorensis) and sambar deer (Rusa unicolor) sampled in eastern Australia. These blood samples were tested for the presence of DNA from Plasmodium spp., Trypanosoma spp., Babesia spp., Theileria spp. and Sarcocystis spp. Further, the presence of antibodies against Babesia bovis was investigated in serum samples (n = 105) by immunofluorescence. In this study, neither parasite DNA nor antibodies were detected for any of the five genera investigated. These results indicate that wild deer are not currently host reservoirs for Plasmodium, Trypanosoma, Babesia, Theileria or Sarcocystis parasites in eastern Australia. We conclude that in eastern Australia, wild deer do not currently play a significant role in the transmission of these parasites. This survey represents the first large-scale molecular study of its type in Australian wild deer and provides important baseline information about the parasitic infection status of these animals. The expanding populations of wild deer throughout Australia warrant similar surveys in other parts of the country and surveillance efforts to continually assess the level of threat wild deer could pose to humans, wildlife, livestock and other domestic animals.
Keywords: Wildlife diseases, Parasites, Deer, PCR, Immunofluorescence, Australia
Graphical abstract
Highlights
-
•
Deer carry pathogens potentially transmissible to livestock.
-
•
243 whole blood samples and 105 serum samples were collected from wild deer in eastern Australia.
-
•
PCR and antibody screening for five parasitic genera was performed on blood or serum samples.
-
•
This survey represents the first large-scale molecular study of its type in Australian deer.
-
•
Wild deer populations in eastern Australia are unlikely to be currently acting as reservoirs for the parasites investigated.
1. Introduction
The frequency of emerging and re-emerging infectious disease outbreaks in wildlife has increased during recent decades (Woods et al., 2019), raising new questions about disease pathogenesis and epidemiology. The increasing role of wildlife in the emergence of livestock diseases (Siembieda et al., 2011) is due to multiple changes occurring within wildlife and livestock populations, including encroachment on natural habitats, climate change and alteration of population demographics (Miller et al., 2013). Most notably, alteration of wildlife population demographics caused by anthropogenic landscape modification and introduction of non-native species has created new interfaces between livestock and wildlife, exacerbating processes that favour pathogen transmission (Gortazar et al., 2015). Importantly, transmission of an infectious agent at the wildlife-livestock interface may occur directly through interspecies contact, or indirectly through shared space or vectors (Gortázar et al., 2006; Miller et al., 2013).
Although Australia is currently free from some of the world's most important livestock diseases such as foot-and-mouth disease and avian influenza H5N1 (Australian Government Department of Agriculture, 2020a), other endemic pathogens impact on local livestock industry. For instance, the economic losses produced by Neospora caninum in Australian cattle were estimated at AU$85 million and AU$25 million per annum for the dairy and the beef cattle industries, respectively (Reichel, 2000). Moreover, exotic diseases constitute a major threat to Australia's livestock industry and a severe outbreak would considerably impact Australia's production and access to export markets (Australian Government Department of Agriculture, 2020b).
Among Australian wildlife capable of carrying pathogens transmissible to livestock, deer are of substantial concern. Indeed, wild deer commonly feed on pasture and crops in agricultural landscapes, they exhibit a widespread distribution and high local population densities and are susceptible to many livestock diseases (Cripps et al., 2019). Since their introduction into Australia as game animals in the 19th century, deer have successfully adapted to the climate and environmental conditions. In addition to the initial intentional releases, there are records of numerous animals establishing wild populations after escaping from deer farms (Davis et al., 2016). Originally, eighteen deer species were released in Australia, and six have established viable wild populations: chital deer (Axis axis), fallow deer (Dama dama), rusa deer (Rusa timorensis), red deer (Cervus elaphus), hog deer (Axis porcinus) and sambar deer (Rusa unicolor). Most of these species continue to expand their distribution and increase their abundance occupying a wide variety of Australian habitats including rangeland, farmland, plantation forests, and montane forest (Davis et al., 2016; Forsyth et al., 2016).
Numerous pathogens have been detected in several deer species worldwide, including protozoan parasites with epidemiological relevance to domestic animals and livestock (Asada et al., 2018; Cripps et al., 2019; de Las Cuevas et al., 2019; Desquesnes et al., 2013; Duncan et al., 2000; Fisher et al., 2013; Gunter et al., 2018; Holman et al., 2011; Hornok et al., 2017; Martinsen et al., 2016; Remesar et al., 2019; Yabsley et al., 2005; Zanet et al., 2014). However, there is little information about the overall infection status of Australian wild deer populations. Reports are restricted to Fasciola hepatica in fallow deer from New South Wales (Jenkins et al., 2020), parasitic helminths, Leptospira and some endemic livestock viruses in red deer from Queensland (McKenzie et al., 1985) and rusa deer from New South Wales (Moriarty, 2004). To date, the prevalence of vector-borne parasitic genera commonly detected in deer overseas such as Trypanosoma, Babesia or Theileria has not been investigated in Australian wild deer populations. Further, the role of wild deer in the spread of pathogens to livestock in Australia remains to be explored (Cripps et al., 2019). Addressing this knowledge gap is critical to establish appropriate management strategies for wild deer in Australia and to minimise potential impacts on livestock health.
To this end, we aimed to investigate the pathogen diversity carried by wild deer in Australia, including the detection of viral (Huaman et al., 2020) and parasitic organisms (this study). Babesia (Bock et al., 2006), Theileria (Jenkins, 2018) and Sarcocystis (Savini et al., 1992, 1993) parasites are endemic in Australia and cause infections in livestock. Therefore, we hypothesised that deer may be carriers of these parasites. Further, vector-borne parasites of the genera Plasmodium and Trypanosoma have been identified in wild deer populations in Europe (Desquesnes et al., 2013), North America (Guggisberg et al., 2018), and South America (Asada et al., 2018), although their presence in Australian deer populations has not been investigated. Given the number of Plasmodium and Trypanosoma spp. described in native Australian wildlife (Supplementary Table 1), it is likely that suitable vectors are widely present in Australia. Therefore, we hypothesised that wild deer might also be carriers of Plasmodium and Trypanosoma parasites in Australia. Although the species of Babesia, Theileria, and Sarcocystis organisms previously found in deer and livestock are the same, none of the Plasmodium or Trypanosoma species detected in Australian native wildlife (e.g., macropods (Botero et al., 2013)) have been described either in deer or livestock. Therefore, to investigate the parasitic diversity of wild deer in Australia, the present study combined molecular and serological methods to examine blood samples from wild deer inhabiting eastern Australia and detect the presence of Babesia, Theileria, Sarcocystis, Plasmodium and Trypanosoma parasites.
2. Material and methods
2.1. Geographical location of sample collection
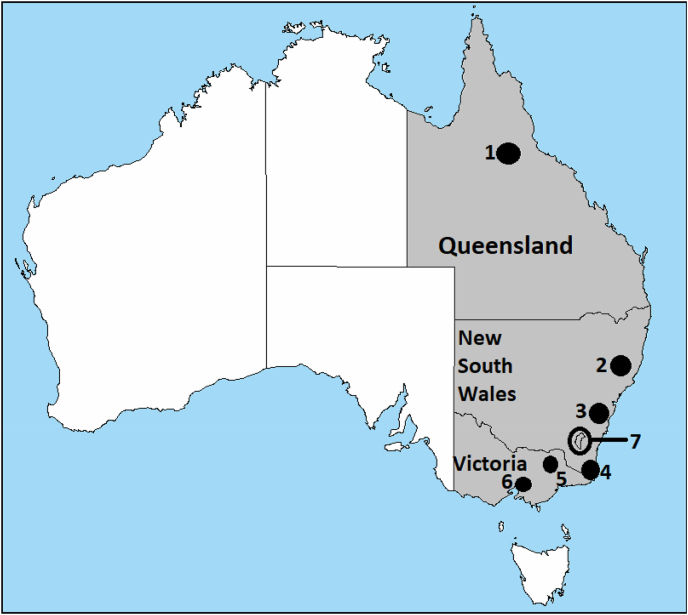
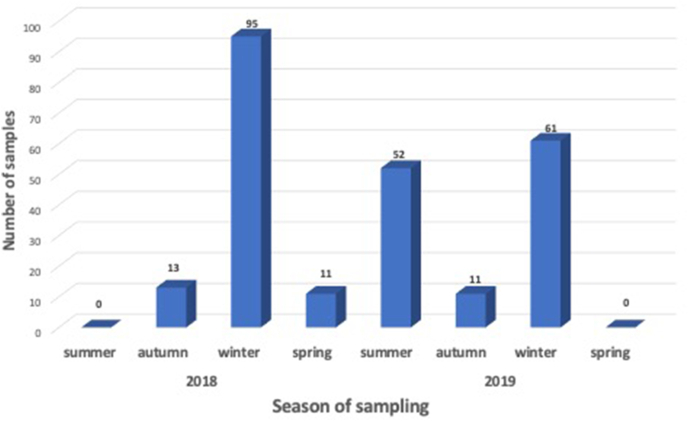
Between March 2018 and November 2019, blood samples from wild deer shot by professional shooters (i.e., culling operations), were collected from eight locations in eastern Australia, including New South Wales, Victoria, Queensland, and Australian Capital Territory (Fig. 1 and Table 1). Sample collection was possible through collaborations with state governments and local councils’ deer management programs. For safety and logistic reasons, field operations were mostly conducted in the colder winter months (Fig. 2). Indeed, sampling in summer is challenging due to the risk of bushfires, for welfare reasons (dependent young), reduced sampling efficiency (longer daylight hours reduces shooting time which occurs mostly at night), and problematic field samples storage due to elevated temperatures.
Fig. 1.
Location of eight deer sampling sites in eastern Australia. Queensland: north-east Queensland (1). New South Wales: Liverpool Plains (2), Wollongong (3), and Kiah (4). Victoria: Alpine National Park (5), Upper Yarra Flats and Yellingbo (6). ACT: Canberra (7).
Table 1.
Total number of deer blood samples analysed in this study. Geographical distribution, deer species and sample size are reported. * 105 additional serum samples were obtained from north-east Queensland.
| Australian state or territory | Sampling location | Deer species | Number of sampled animals |
|---|---|---|---|
| New South Wales (NSW) | Liverpool Plains | fallow (Dama dama) | 87 |
| Kiah | 18 | ||
| Wollongong | rusa (Rusa timorensis) | 63 | |
| Australian Capital Territory (ACT) | Canberra | fallow | 31 |
| Victoria (VIC) | Alpine National Park | sambar (Rusa unicolor) | 24 |
| Upper Yarra Flats | 8 | ||
| Yellingbo | 6 | ||
| fallow | 2 | ||
| Queensland (QLD) | north-east Queensland | chital (Axis axis) | 4 (105) * |
| Total | 243 |
Fig. 2.
Total number of deer samples collected in eastern Australia between March 2018 and November 2019.
2.2. Sample collection methods
Blood was drawn from the jugular vein, heart or thoracic cavity and collected in plain and EDTA tubes (Becton Dickinson, Franklin Lakes, NJ, USA). EDTA tubes were inverted to mix and prevent clotting, forthwith kept under refrigerated conditions, and transported to the laboratory. Immediately upon reception, samples were centrifuged for 10 min at 2,000 g. Aliquots of blood pellet samples were stored at −20 °C.
2.3. Genomic DNA extraction from blood
Considering the relatively large number of whole blood samples processed in this study, a time- and cost-efficient DNA extraction method was required. We considered that a classical phenol-chloroform DNA extraction method (Chacon-Cortes and Griffiths, 2014) would provide an attractive low-cost advantage for a large number of samples, while a commercial bead-based approach (MagMAX™CORE Nucleic Acid Purification Kit) might provide a faster and more reproducible outcome. To undertake a method comparison, ten blood samples were randomly selected, and genomic DNA (gDNA) was extracted from an equal sample volume using both methods. The concentration and purity of the extracted gDNA were measured with an IMPLEN Nanophotometer (IMPLEN, Munich, Germany).
2.3.1. Classical phenol-chloroform method
200 μL of blood were lysed with 0.15% saponin at 4 °C for 10 min and centrifuged at 2,800 g for 10 min. The pellet was washed with PBS, resuspended in 700 μL of Lysis buffer (10 mM Tris pH 8, 1 mM EDTA pH 8, 0.4 M NaCl and 1% SDS) and incubated at 37 °C for 1 h. A minimum of two phenol-chloroform extractions were performed by adding 700 μL of phenol-chloroform-isoamyl alcohol mixture (cat#: 77617, Sigma-Aldrich, St. Louis, MO, USA) until the aqueous phase was clear. The DNA present in the aqueous phase was ethanol-precipitated and centrifuged at 9,000 g for 3 min at 4 °C. The supernatant was discarded, and the pellet washed with 70% ethanol. The DNA pellet was resuspended in TE buffer pH 8 (1 M Tris pH 8 and 0.5 M EDTA pH 8).
2.3.2. Bead-based automated kit
Genomic DNA was extracted from 200 μL of blood pellet samples with the bead-based automated kit MagMAX™CORE Nucleic Acid Purification Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's recommendations. The extraction was processed using the KingFisher™ Duo Prime Purification System (ThermoFisher Scientific, Pittsburgh, PA, USA). Genomic material was eluted in 90 μL of elution buffer and stored at −20 °C until required.
2.4. Detection of parasite DNA by PCR
Parasite genus DNA was investigated by PCR amplification of the conserved region of the 18S subunit of the ribosomal RNA gene (18S rRNA) of Trypanosoma, Sarcocystis, Babesia and Theileria genomes, and the cytochrome b conserved gene of Plasmodium. The corresponding conserved primers were obtained from the literature (Martin et al., 2016; Schaer et al., 2013; Thompson et al., 2013; Yang et al., 2014) and PCR conditions were modified accordingly (Table 2). PCR positive controls were generated using genomic DNA extracted from parasite in vitro cultures of Trypanosoma brucei, Plasmodium falciparum, Sarcocystis gigantea, Babesia bovis and Theileria orientalis. Non-template controls, which consisted of the PCR mix with nuclease-free water, were included in each assay as a negative control.
Table 2.
Conserved primers and PCR amplification conditions used in this study to amplify the 18S rRNA gene of Babesia, Theileria, Trypanosoma and Sarcocystis, as well as the Plasmodium cytochrome b gene.
| Parasite genus | Gene | Primer name | Primer sequence (5’ – 3′) | Amplicon | PCR conditions |
|
|---|---|---|---|---|---|---|
| annealing | extension | |||||
|
Babesia Theileria |
18 S rRNA | Piro1-S | CTTGACGGTAGGGTATTGGC | 1400 bp | 55 °C | 90 s |
| Piro3-AS | CCTTCCTTTAAGTGATAAGGTTCAC | |||||
| Trypanosoma | 18 S rRNA | S825F | ACCGTTTCGGCTTTTGTTGG | 950 bp | 60 °C | 60 s |
| SLIR | ACATTGTAGTGCGCGTGTC | |||||
| Sarcocystis | 18 S rRNA | cocc18SF | GAAAGTTAGGGGCTCGAAGA | 400 bp | 57 °C | 45 s |
| cocc18SR | CCCTCTAAGAAGTGATACA | |||||
| Plasmodium | cytochrome b | 3932 F | GGGTTATGTATTACCTTGGGGTC | 750 bp | 57 °C | 60 s |
| DW4 | TGTTTGCTTGGGAGCTGTAATCATAATGTG | |||||
PCR amplification was performed in a 25 μL reaction mixture containing 1 × Green GoTaq Flexi buffer, 2 mM MgCl2, 10 mM dNTPs, 0.2 μM of both forward and reverse primers (Table 2), 0.625 units of GoTaq G2 DNA polymerase (Promega, Madison, WI, USA), and 1 μL of total genomic DNA template. The PCR program consisted of an initial denaturation step at 95 °C for 2 min, followed by 35 cycles of denaturation at 95 °C for 45 s, annealing at 55–60 °C for 45 s and extension at 72 °C for 45–90 s, with a final extension of 5 min at 72 °C (Table 2). Amplification occurred in a T100 thermal cycler (BioRad, Hercules, CA, USA). Amplification products were visualised by gel electrophoresis, using a 2% agarose gel, RedSafe™ (iNtRON Biotechnology, Gyeonggi-do, Korea), and a high-resolution imaging system - ChemiDoc™ MP Imaging System (Bio-Rad, Hercules, CA, USA).
To evaluate the detection limit of the PCR assay, six samples of defibrinated horse blood spiked with various amounts of Plasmodium falciparum-infected erythrocytes (101 - 106 parasites/mL) were prepared. Following the instructions of the MagMAX™CORE Nucleic Acid Purification Kit, gDNA was extracted from each of these samples and amplification of the P. falciparum cytochrome b gene (3932 F/DW4 primers, 750 bp amplicon) was performed (see Supplementary Figure 1). Further, gDNA of the other positive controls (Trypanosoma brucei, Sarcocystis gigantea, Babesia bovis and Theileria orientalis) was diluted in nuclease free water to obtain a concentration range and determine the limit of detection for the corresponding PCR. The PCR detection limit for each positive control ranged between 0.5 and 1 ng/μL (data not shown).
2.5. Testing for anti-Babesia bovis antibodies by immunofluorescence assay
Serum samples were screened for antibodies against Babesia bovis using the commercially available immunofluorescence kit MegaFLUO® B. bovis (MegaCor Diagnostik GmbH, Hörbranz, Austria) according to the manufacturer instructions. The immunofluorescence assay (IFA) kit is validated for the detection of B. bovis antibodies in cattle serum and is provided with protocol modifications for the detection of B. bovis antibodies in deer samples. The main modification consisted of the substitution of the conjugate antibody by anti-deer IgG (H + L) antibody FITC-labelled (Seracare, Milford, MA, USA) diluted at 1:500. Briefly, IFA slides, test sera and reagents were brought to room temperature. Test sera were diluted at 1:20 in phosphate-buffered saline (PBS). Diluted test sera were individually placed on the slides and incubated in a humidity chamber for 30 min at 37 °C. The slides were washed in PBS for 5 min, three times. Diluted anti-deer IgG-FITC conjugate was added and again incubated in a humidity chamber for 30 min at 37 °C. The slides were washed three times in PBS for 5 min removing any excess of unbound reagents. The slides were air dried, and coverslips mounted. Examination of the slides was performed with an Olympus BX53 microscope under a 40 × lens (total 400 × magnification), and each well was compared to the fluorescence pattern observed in the positive and negative controls.
2.6. Parasite prevalence and statistical analysis
Comparisons were performed using a non-parametric test for paired samples (Wilcoxon test) with α = 0.05. The analysis was performed using R version 4.0.0 (R Development Core Team, Vienna, Austria).
3. Results
3.1. Deer species and geographical distribution
During the sampling period, 243 blood samples were collected from four wild deer species (fallow, rusa, sambar and chital deer) across eastern states and territories of mainland Australia (Fig. 1 and Table 1). Most samples (69%) were collected between June and October (i.e., winter and spring) (Fig. 2). Among the four deer species included in this study, two accounted for more than 83% of the samples: fallow deer (57%) and rusa deer (26%). Of note, 69% of the specimens were collected in the state of New South Wales (Table 1). Similar numbers of females (n = 120) and males (n = 116) were sampled, with no sex information available for seven animals. Individuals were classified in three age categories based on morphological characteristics including body size, tooth wear, and antler growth: fawn (<1 year), yearling (1–2 years) and adult (≥2 years). Most samples were from adults (n = 149), followed by yearlings (n = 74) and fawns (n = 13), with age information unavailable for seven animals.
3.2. Comparison of DNA extraction methods
To undertake a method comparison, ten blood samples were randomly selected, and genomic DNA was extracted from an equal sample volume using a phenol-chloroform and a bead-based method. The mean DNA concentration obtained with the MagMAX™CORE extraction kit was lower (p = 0.002); however, these samples presented a similar A260/A230 ratio (p = 0.25) and a higher A260/A280 ratio (p < 0.0001) when compared to those obtained after a phenol-chloroform DNA extraction (Table 3). Thus, the DNA purity of the samples processed with the MagMAX extraction method was deemed superior and more consistent when compared to the phenol-chloroform method. Therefore, the bead-based extraction method was used henceforth to extract genomic DNA from all the remaining 233 whole blood samples.
Table 3.
Comparison of DNA extraction methods for yield and purity parameters. SD: Standard deviation.
| Phenol-chloroform method | MagMAX™CORE extraction kit | ||
|---|---|---|---|
|
DNA concentration (ng/μL) |
Mean | 215.3 | 80.4 |
| SD | 35.2 | 81.2 | |
| range | 156–286 | 28–283 | |
|
A260/A230 ratio |
Mean | 1.70 | 1.70 |
| SD | 0.10 | 0.04 | |
| range | 1.49–1.80 | 1.64–1.76 | |
|
A260/A280 ratio |
Mean | 1.45 | 1.90 |
| SD | 0.02 | 0.08 | |
| range | 1.42–1.48 | 1.77–2.00 | |
3.3. Detection of parasite DNA by PCR
Primers previously published targeting an internal fragment (400–1400 bp) from the 18S rRNA gene of Babesia and Theileria (Piro1-S/Piro1-AS), Trypanosoma (S825F/SLIR), and Sarcocystis (cocc18SF/cocc18SR), and a 750 bp amplicon from the cytochrome b gene of Plasmodium (3932F/DW4) were used in this study (Table 2). The PCR detection limit for the amplification of Plasmodium parasites was determined to be at 103 parasites/mL, corresponding to a gDNA concentration of 0.55 ng/μL (see Supplementary Figure 1). For each PCR amplification experiment, an amplicon of the expected size was successfully amplified using 5–10 ng/μL of purified Babesia bovis, Theileria orientalis, Trypanosoma brucei, Sarcocystis gigantea and Plasmodium falciparum DNA templates as the positive controls. However, no amplicon was obtained for any of the 243 deer blood samples processed for either the Babesia, Theileria, Trypanosoma, and Sarcocystis 18S rRNA gene or the Plasmodium cytochrome b gene.
Despite our best efforts to obtain whole blood samples from Queensland, where the warm climate may favour the (potential) vectors of these parasites, only four specimens were accessed and all returned negative results for Babesia, Theileria, Trypanosoma, Sarcocystis and Plasmodium DNA (described in section 3.3). However, wild chital deer serum samples (n = 105) were obtained from the tropical region of Queensland (Fig. 1). As detection of haemoparasites in serum samples has previously been demonstrated, including for detection of Plasmodium (Bharti et al., 2007; Pomari et al., 2020) and Trypanosoma (Melo et al., 2015) parasites, we decided to investigate the presence of five parasitic genera on a larger number of chital deer serum samples. Genomic DNA was extracted from serum samples (n = 50) and PCR amplification of the Babesia, Theileria, Trypanosoma, and Sarcocystis 18S rRNA gene and of the Plasmodium cytochrome b gene was performed (Table 2). Amplicons of the expected size were successfully obtained for the positive control with Babesia bovis, Theileria orientalis, Trypanosoma brucei, Sarcocystis gigantea and Plasmodium falciparum DNA template. However, no amplicons of the five parasitic genera screened were obtained in the chital deer serum samples.
3.4. Testing for anti-Babesia bovis antibodies by immunofluorescence assay
Babesia DNA was not detected in the four whole blood or 50 of the serum samples obtained from Queensland, excluding the possibility of current infections. Therefore, we investigated the presence of anti-Babesia antibodies as an indication of past infections. Exposure to Babesia bovis infections in the Queensland chital deer serum samples (n = 105) was investigated by IFA using the commercial kit MegaFLUO® B. bovis. While the kit positive control provided a bright fluorescent signal, no fluorescence was observed for any of the 105 serum samples processed indicating the absence of anti-Babesia antibodies in these chital deer serum samples.
4. Discussion
4.1. Australian wild deer appear to be free of haemoparasite and Sarcocytis infections
The present study assessed the prevalence of four vector-borne protozoan blood parasites (Trypanosoma, Plasmodium, Babesia and Theileria) and the coccidian parasite Sarcocystis in four wild deer species (fallow, rusa, sambar and chital) across eastern Australia. Parasites of interest were selected based on evidence of infection of deer species overseas, or parasite detection in Australian livestock or wildlife (Supplementary Table 1).
The presence or absence of the five parasitic genera was inferred through PCR amplification of conserved genes (18S rRNA, cytochrome b). No evidence of infection with Trypanosoma, Plasmodium, Babesia, Theileria or Sarcocystis parasites was found in any of the tested blood samples. Further, no evidence of past Babesia infection was found via serology testing. This study represents the first large-scale investigation of haemoparasitic and Sarcocystis infection in Australian wild deer. The data presented here suggest that Australian wild deer are unlikely to be reservoir hosts for Trypanosoma, Plasmodium, Babesia, Theileria, and Sarcocystis parasites.
4.2. Theileria and Babesia infections
Theileria and Babesia parasites are endemic in livestock in Queensland and northern NSW, with B. bovis causing more than 80% of the reported outbreaks of tick fever in cattle in Queensland and northern New South Wales (Bock et al., 2006; Jenkins, 2018). Moreover, the cattle tick, Rhipicephalus (Boophilus) microplus, which is the vector of B. bovis, has widely spread in Queensland (Cutulle et al., 2009). These factors, together with the fact that Babesia spp. were detected in cervids from Europe (Remesar et al., 2019; Zanet et al., 2014), Asia (Zamoto-Niikura et al., 2018), and America (Cantu et al., 2009; da Silveira et al., 2011), make it possible that wild Australian deer might carry some of these parasites. While the small number of whole blood samples collected from chital deer in Queensland (n = 4), limited our opportunities for detecting infected animals, we explored the presence of antibodies against B. bovis in a group of serum samples from the same site and deer species. These analysis suggested that chital deer are not exposed to Babesia parasites. However, these serum samples were collected in a relatively small geographical area (75 km radius) and further investigations would be needed to confirm that Babesia spp. are absent from wild deer in Queensland.
4.3. Trypanosoma and Plasmodium infections
Australian livestock are currently free of Trypanosoma and Plasmodium infections, but the presence of suitable vectors, anopheline mosquitos for Plasmodium (Cooper et al., 1996) and day-feeding midges for Trypanosoma (Thompson et al., 2014) constitute a risk for livestock and other animals should a deer-infecting trypanosome species be introduced into Australia (Reid, 2002). Infections with Plasmodium parasites have been described in a variety of Australian wildlife, including mammal, such as Leadbeater's possum (Gymnobelideus leadbeateri) (Scheelings et al., 2016), birds and reptiles (Spratt and Beveridge, 2018). Trypanosoma infections are widely described in Australian marsupials, rodents and bats (Thompson et al., 2014). While Trypanosoma and Plasmodium species identified in reptiles, birds and marsupials in Australia are considered unlikely to infect eutherian species such as deer and livestock, particular interest is focussed on T. evansi, given its high risk of spreading to northern Australia from the islands of Indonesia or Papua New Guinea (Reid, 2002). It may therefore be a major threat to Australian wildlife and livestock (Aregawi et al., 2019; Desquesnes et al., 2013). Importantly, infection of deer with T. evansi has been reported in Asia (Malaysia and Thailand), offshore islands in the Indian Ocean (Mauritius), and South America (Brazil). T. evansi was detected in sambar and chital deer in Thailand (Desquesnes et al., 2013) and outbreaks associated with high morbidity and mortality have been also reported in this country in rusa and hog deer (Aregawi et al., 2019).
4.4. Sarcocystis infections
Sarcocystis parasites are generally located in striated muscle tissues and the central nervous system of the intermediate hosts. However, the opportunistic nature of the sampling and the specific field conditions of this study proved to be a challenge in obtaining and preserve tissue samples. Sarcocystis parasites penetrate endothelial cells of blood vessels and migrate to muscles tissues through the blood (Dubey et al., 2016). Therefore, Sarcocystis parasites can be found in the blood and a recent study has successfully detected Sarcocystis parasites in blood samples from domestic llamas (Lama glama) in Argentina (Martin et al., 2016). Thus, in the absence of muscle tissue, blood was considered a suitable sample for our study and used to investigate the presence of Sarcocystis parasites in wild deer. In Australia, the presence of Sarcocystis has been reported in cattle (Savini et al., 1992), sheep (Savini et al., 1993), alpacas (Gabor et al., 2010) and native terrestrial mammals including kangaroos (Macropus and Osphranter spp.) and wallabies (Petrogale and Macropus spp.) (Ladds, 2009). However, a previous report of histological examination of 72 muscle samples collected from rusa, sambar, fallow and hog deer in Australia resulted negative for the presence of Sarcocystis parasites (Munday et al., 1978). Deer are recognised as intermediate hosts for numerous species of Sarcocystis, but it is unknown whether Sarcocystis organisms are host-specific for deer species (Dubey et al., 2016). Therefore, the need for intensive surveillance of Sarcocystis infection including a variety of tissue samples and multiple detection methods remains.
4.5. The importance of reporting lack of evidence of parasitic infections
Although the limited knowledge of parasitic infections in Australian wild deer populations is restricted to helminths (Cripps et al., 2019; Jenkins et al., 2020; McKenzie et al., 1985; Moriarty, 2004), the literature shows that deer species currently present in Australia are susceptible to the pathogens screened in the present survey, except for Plasmodium (also see Supplementary Material). The report of research findings, including negative results, is important because they contribute to understanding where and when deer are potentially infected by these parasites. Moreover, null findings provide checks and balances against positive results and are important for robust meta-analyses (Fanelli et al., 2017). Numerous reviews have identified bias towards publication of positive results, and hence it is important to report negative finding (Fanelli et al., 2017; Mlinaric et al., 2017). This is of critical importance in wildlife research, where there are substantial logistical and financial constraints on extensive testing and hence any additional information is important.
It is also important to note that meta-analyses are often used in wildlife research due to the difficulties in field data collection (Aregawi et al., 2019; Raboisson et al., 2020). Further to this, in the context of animal health, wildlife disease surveillance is an important tool to obtain information on morbidity and mortality, changes in patterns of disease occurrence over time, and early detection of disease outbreaks (Grogan et al., 2014). This is particularly true for species such as the four deer species that were screened in this study, because they could play an important role in biosecurity. To this end, we recommend the implementation of a passive pathogen surveillance program for wildlife (Duncan et al., 2008). For certain pathogens, this could be relatively easy to implement. For instance, hunters could be trained to identify, report, and collect samples of lesions compatible with Sarcocystis infections (Diefenbach et al., 2004).
4.6. Limitations of detection of parasites in deer blood samples
Lack of evidence of a parasitic infection can be interpreted in various ways. The most obvious one is the absolute absence of the pathogen(s) investigated. Small sample size (e.g., in tropical environment such as Queensland) may also limit the detection of infected individuals. False negative results could have arisen due to the season of sampling, low parasitaemia at the time of sample collection or fluctuation of parasitaemia during the parasite's life cycle.
Sampling for this study occurred year-round except for summer months. It was not possible to sample deer during the summer months due to animal welfare, safety, and logistical reasons. As cold weather conditions negatively influence the transmission rates of most vector-borne diseases (Caminade et al., 2019), it is possible that sampling predominantly during autumn, winter and spring reduces the probability of detecting infected animals. Further, as our samples were provided by culling programs (i.e., lethal sampling), each sample was collected at a single time point (i.e., no serial sampling of animals), which can also minimise the probability of detecting parasites. An additional aspect to consider relates to the extremely low parasite load previously reported in wild deer. P. odocoilei, for example, is estimated to infect ~0.0015% red blood cells in white-tailed deer (Martinsen et al., 2016; Templeton et al., 2016a, 2016b), and Plasmodium parasitaemia levels (i.e., percentage of infected red blood cells) in cervids have been determined to be as low as 0.003% (Martinsen et al., 2016; Templeton et al., 2016a).
5. Conclusions
This study suggests that Australian wild deer are currently not significant host reservoirs of Trypanosoma, Plasmodium, Babesia, Theileria, and Sarcocystis infections. This survey represents the first large-scale molecular study of its type in Australian deer and provides important baseline information about the infection status of wild deer in eastern Australia.
Financial support
This work was supported by the Centre for Invasive Species Solutions under Grant PO1-L-002.
Authors’ contributions
Funding acquisition was carried out by Carlo Pacioni, David M. Forsyth, Anthony Pople, Karla J. Helbig. and Teresa G. Carvalho. Study conception and design was carried out by Carlo Pacioni, Karla J. Helbig and Teresa G. Carvalho. Sample collection was performed by David M. Forsyth, Anthony Pople and Jordan O. Hampton. Laboratory testing was conducted by Jose L. Huaman. Data were analysed by Jose L. Huaman, Carlo Pacioni and Teresa G. Carvalho The manuscript was drafted by Jose L. Huaman, Carlo Pacioni and Teresa G. Carvalho. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We thank Richard Francis (ABZECO), Jake Haddad (VPAC), Kirk Stone (Strathbogie Wildlife), Brendan Cowled and Edwina Leslie (AusVet), Andrew Bengsen, Troy Crittle and Quentin Hart (New South Wales Department of Primary Industries), Bob McKinnon and Amy Sheridan (North West Local Land Services), Michael Brennan and Matt Amos (Biosecurity Queensland), and staff from Parks Victoria for assisting with sample collection. Kim O'Riley and Peter Mee provided technical support. Professor Peter Irwin (Murdoch University, WA), A/Prof Darren Creek (Monash Institute for Pharmaceutical Sciences, VIC), Professor Brian Cooke (Monash University, VIC) and Dr Abdul Jabbar (University of Melbourne, VIC) provided genomic DNA samples of the parasites used as PCR positive controls in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2021.06.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aregawi W.G., Agga G.E., Abdi R.D., Buscher P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasites Vectors. 2019;12:67. doi: 10.1186/s13071-019-3311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada M., Takeda M., Tomas W.M., Pellegrin A., de Oliveira C.H.S., Barbosa J.D., da Silveira J.A.G., Braga E.M., Kaneko O. Close relationship of Plasmodium sequences detected from South American pampas deer (Ozotoceros bezoarticus) to Plasmodium spp. in North American white-tailed deer. Int. J. Parasitol. Parasites Wildl. 2018;7:44–47. doi: 10.1016/j.ijppaw.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government Department of Agriculture . Animal pests and diseases; 2020. Water and the Environment.https://www.agriculture.gov.au/pests-diseases-weeds/animal [Google Scholar]
- Australian Government Department of Agriculture . Livestock; 2020. Water and the Environment.https://www.agriculture.gov.au/export/controlled-goods/live-animals/livestock [Google Scholar]
- Bharti A.R., Patra K.P., Chuquiyauri R., Kosek M., Gilman R.H., Llanos-Cuentas A., Vinetz J.M. Polymerase chain reaction detection of Plasmodium vivax and Plasmodium falciparum DNA from stored serum samples: implications for retrospective diagnosis of malaria. Am. J. Trop. Med. Hyg. 2007;77:444–446. [PubMed] [Google Scholar]
- Bock R.E., de Vos A.J., Molloy J.B. Australian and New Zealand Standard Diagnostic Procedures. 2006. Tick-borne diseases of cattle; pp. 1–29. [Google Scholar]
- Botero A., Thompson C.K., Peacock C.S., Clode P.L., Nicholls P.K., Wayne A.F., Lymbery A.J., Thompson R.C. Trypanosomes genetic diversity, polyparasitism and the population decline of the critically endangered Australian marsupial, the brush tailed bettong or woylie (Bettongia penicillata) Int. J. Parasitol. Parasites Wildl. 2013;2:77–89. doi: 10.1016/j.ijppaw.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminade C., McIntyre K.M., Jones A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019;1436:157–173. doi: 10.1111/nyas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu C.A., Ortega S.J., Garcia-Vazquez Z., Mosqueda J., Henke S.E., George J.E. Epizootiology of Babesia bovis and Babesia bigemina in free-ranging white-tailed deer in northeastern Mexico. J. Parasitol. 2009;95:536–542. doi: 10.1645/GE-1648.1. [DOI] [PubMed] [Google Scholar]
- Chacon-Cortes D., Griffiths L.R. Methods for extracting genomic DNA from whole blood samples: current perspectives. J. Biorepository Sci. Appl. Med. 2014;2:1–9. [Google Scholar]
- Cooper R.D., Frances S.P., Waterson D.G., Piper R.G., Sweeney A.W. Distribution of anopheline mosquitoes in northern Australia. J. Am. Mosq. Contr. Assoc. 1996;12:656–663. [PubMed] [Google Scholar]
- Cripps J.K., Pacioni C., Scroggie M.P., Woolnough A.P., Ramsey D.S.L. Introduced deer and their potential role in disease transmission to livestock in Australia. Mamm Rev. 2019;49:60–77. [Google Scholar]
- Cutulle C., Jonsson N.N., Seddon J. Population structure of Australian isolates of the cattle tick Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 2009;161:283–291. doi: 10.1016/j.vetpar.2009.01.005. [DOI] [PubMed] [Google Scholar]
- da Silveira J.A., Rabelo E.M., Ribeiro M.F. Detection of Theileria and Babesia in brown brocket deer (Mazama gouazoubira) and marsh deer (Blastocerus dichotomus) in the state of minas gerais, Brazil. Vet. Parasitol. 2011;177:61–66. doi: 10.1016/j.vetpar.2010.10.044. [DOI] [PubMed] [Google Scholar]
- Davis N.E., Bennett A., Forsyth D.M., Bowman D.M.J.S., Lefroy E.C., Wood S.W., Woolnough A.P., West P., Hampton J.O., Johnson C.N. A systematic review of the impacts and management of introduced deer (family Cervidae) in Australia. Wildl. Res. 2016;43:515–532. [Google Scholar]
- de Las Cuevas G.E.D., Prakas P., Strazdaite-Zieliene Z., Martinez-Gonzalez M., Rudaityte-Lukosiene E., Butkauskas D., Serviene E., Habela M.A., Calero-Bernal R. Sarcocystis morae (apicomplexa) in fallow deer (Dama dama) from Spain: ultrastructure and new host record. J. Parasitol. 2019;105:813–815. [PubMed] [Google Scholar]
- Desquesnes M., Holzmuller P., Lai D.H., Dargantes A., Lun Z.R., Jittaplapong S. Trypanosoma evansi and surra: a review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. BioMed Res. Int. 2013;2013:194176. doi: 10.1155/2013/194176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach D.R., Rosenberry C.S., Boyd R.C. From the field: efficacy of detecting Chronic Wasting Disease via sampling hunter-killed white-tailed deer. Wildl. Soc. Bull. 2004;32:267–272. [Google Scholar]
- Dubey J.P., Calero-Bernal R., Rosenthal B.M., Speer C.A., Fayer R. second ed. CRC Press; Boca Raton: 2016. Sarcocystosis of Animals and Humans; pp. 195–216. [Google Scholar]
- Duncan C., Backus L., Lynn T., Powers B., Salman M. Passive, opportunistic wildlife disease surveillance in the Rocky Mountain Region, USA. Transbound Emerg. Dis. 2008;55:308–314. doi: 10.1111/j.1865-1682.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- Duncan R.B., Jr., Fox J.H., Lindsay D.S., Dubey J.P., Zuccaro M.E. Acute sarcocystosis in a captive white-tailed deer in Virginia. J. Wildl. Dis. 2000;36:357–361. doi: 10.7589/0090-3558-36.2.357. [DOI] [PubMed] [Google Scholar]
- Fanelli D., Costas R., Ioannidis J.P. Meta-assessment of bias in science. Proc. Natl. Acad. Sci. U.S.A. 2017;114:3714–3719. doi: 10.1073/pnas.1618569114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A.C., Schuster G., Cobb W.J., James A.M., Cooper S.M., Perez de Leon A.A., Holman P.J. Molecular characterization of Trypanosoma (Megatrypanum) spp. infecting cattle (Bos taurus), white-tailed deer (Odocoileus virginianus), and elk (Cervus elaphus canadensis) in the United States. Vet. Parasitol. 2013;197:29–42. doi: 10.1016/j.vetpar.2013.04.037. [DOI] [PubMed] [Google Scholar]
- Forsyth D.M., Stamation K., Woodford L. Distributions of fallow deer, red deer, hog deer and chital deer in Victoria. 2016. https://www.parliament.vic.gov.au/images/stories/committeees/enrc/Invasive_Animals_on_Crown_land/210Q._2016.09.13_Attachment_17_-_Fallow_Deer_Red_Deer_Hog_Deer_and_Chital_Deer_in_Victoria.pdf 11 March 2021.
- Gabor M., Gabor L.J., Srivastava M., Booth M., Reece R. Chronic myositis in an Australian alpaca (Llama pacos) associated with Sarcocystis spp. J. Vet. Diagn. Invest. 2010;22:966–969. doi: 10.1177/104063871002200620. [DOI] [PubMed] [Google Scholar]
- Gortázar C., Acevedo P., Ruiz-Fons F., Vicente J. Disease risks and overabundance of game species. Eur. J. Wildl. Res. 2006;52:81–87. [Google Scholar]
- Gortazar C., Diez-Delgado I., Barasona J.A., Vicente J., De La Fuente J., Boadella M. The wild side of disease control at the wildlife-livestock-human interface: a review. Front. Vet Sci. 2015;1:1–12. doi: 10.3389/fvets.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan L.F., Berger L., Rose K., Grillo V., Cashins S.D., Skerratt L.F. Surveillance for emerging biodiversity diseases of wildlife. PLoS Pathog. 2014;10:5. doi: 10.1371/journal.ppat.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg A.M., Sayler K.A., Wisely S.M., John A.R.O. Natural history of Plasmodium odocoilei malaria infection in farmed white-tailed deer. mSphere. 2018;3:2. doi: 10.1128/mSphere.00067-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter S.M., Cordray C., Gorchakov R., Du I., Dittmar B., Brown E.L., Murray K.O., Nolan M.S. Identification of white-tailed deer (Odocoileus virginianus) as a novel reservoir species for Trypanosoma cruzi in Texas, USA. J. Wildl. Dis. 2018;54:814–818. doi: 10.7589/2017-09-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman P.J., Carroll J.E., Pugh R., Davis D.S. Molecular detection of Babesia bovis and Babesia bigemina in white-tailed deer (Odocoileus virginianus) from Tom Green County in central Texas. Vet. Parasitol. 2011;177:298–304. doi: 10.1016/j.vetpar.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Hornok S., Sugar L., Horvath G., Kovacs T., Micsutka A., Gonczi E., Flaisz B., Takacs N., Farkas R., Meli M.L., Hofmann-Lehmann R. Evidence for host specificity of Theileria capreoli genotypes in cervids. Parasites Vectors. 2017;10:473. doi: 10.1186/s13071-017-2403-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaman J.L., Pacioni C., Forsyth D.M., Pople A., Hampton J.O., Carvalho T.G., Helbig K.J. Serosurveillance and molecular investigation of wild deer in Australia reveals seroprevalence of Pestivirus infection. Viruses. 2020;12:116. doi: 10.3390/v12070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C. Bovine theileriosis in Australia: a decade of disease. Microbiol. Aust. 2018;39:215–219. [Google Scholar]
- Jenkins D., Baker A., Porter M., Shamsi S., Barton D. Wild fallow deer (Dama dama) as definitive hosts of Fasciola hepatica (liver fluke) in alpine New South Wales. Aust. Vet. J. 2020;98:546–549. doi: 10.1111/avj.13001. [DOI] [PubMed] [Google Scholar]
- Ladds P. CSIRO Publishing; Collingwood, Australia: 2009. Pathology of Australian Native Wildlife; pp. 197–230. [Google Scholar]
- Martin M., Decker Franco C., Romero S., Carletti T., Schnittger L., Florin-Christensen M. Molecular detection of Sarcocystis aucheniae in the blood of llamas from Argentina. Rev. Argent. Microbiol. 2016;48:200–205. doi: 10.1016/j.ram.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Martinsen E.S., McInerney N., Brightman H., Ferebee K., Walsh T., McShea W.J., Forrester T.D., Ware L., Joyner P.H., Perkins S.L., Latch E.K., Yabsley M.J., Schall J.J., Fleischer R.C. Hidden in plain sight: cryptic and endemic malaria parasites in North American white-tailed deer (Odocoileus virginianus) Sci. Adv. 2016;2:2. doi: 10.1126/sciadv.1501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie R.A., Green P.E., Thornton A.M., Chung Y.S., MacKenzie A.R., Cybinski D.H., St George T.D. Diseases of deer in south eastern Queensland. Aust. Vet. J. 1985;62:424. doi: 10.1111/j.1751-0813.1985.tb14129.x. [DOI] [PubMed] [Google Scholar]
- Melo M.F., Moreira O.C., Tenorio P., Lorena V., Lorena-Rezende I., Junior W.O., Gomes Y., Britto C. Usefulness of real time PCR to quantify parasite load in serum samples from chronic Chagas disease patients. Parasites Vectors. 2015;8:154. doi: 10.1186/s13071-015-0770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.S., Farnsworth M.L., Malmberg J.L. Diseases at the livestock-wildlife interface: status, challenges, and opportunities in the United States. Prev. Vet. Med. 2013;110:119–132. doi: 10.1016/j.prevetmed.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinaric A., Horvat M., Supak Smolcic V. Dealing with the positive publication bias: why you should really publish your negative results. Biochem. Med. 2017;27:3. doi: 10.11613/BM.2017.030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty A. The Royal National Park. University of Western Sydney; Sydney: 2004. Ecology and Enviromental Impact of Javan Rusa Deer (Cervus Timorensis Russa) [Google Scholar]
- Munday B.L., Mason R.W., Hartley W.J., Presidente P.J., Obendorf D. Sarcocystis and related organisms in Australian wildlife: I. Survey findings in mammals. J. Wildl. Dis. 1978;14:417–433. doi: 10.7589/0090-3558-14.4.417. [DOI] [PubMed] [Google Scholar]
- Pomari E., Silva R., Moro L., La Marca G., Perandin F., Verra F., Bisoffi Z., Piubelli C. Droplet Digital PCR for the detection of Plasmodium falciparum DNA in whole blood and serum: a comparative analysis with other molecular methods. Pathogens. 2020;9:478. doi: 10.3390/pathogens9060478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboisson D., Ferchiou A., Pinior B., Gautier T., Sans P., Lhermie G. The use of meta-analysis for the measurement of animal disease burden: losses due to clinical mastitis as an example. Front. Vet. Sci. 2020;7:149. doi: 10.3389/fvets.2020.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel M.P. Neospora caninum infections in Australia and New Zealand. Aust. Vet. J. 2000;78:258–261. doi: 10.1111/j.1751-0813.2000.tb11751.x. [DOI] [PubMed] [Google Scholar]
- Reid S.A. Trypanosoma evansi control and containment in Australasia. Trends Parasitol. 2002;18:219–224. doi: 10.1016/s1471-4922(02)02250-x. [DOI] [PubMed] [Google Scholar]
- Remesar S., Diaz P., Prieto A., Markina F., Cao J.M.D., Lopez-Lorenzo G., Fernandez G., Lopez C.M., Panadero R., Diez-Banos P., Morrondo P. Prevalence and distribution of Babesia and Theileria species in roe deer from Spain. Int. J. Parasitol. Parasites Wildl. 2019;9:195–201. doi: 10.1016/j.ijppaw.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savini G., Dunsmore J.D., Robertson I.D., Seneviratna P. The epidemiology of Sarcocystis spp. in cattle of Western Australia. Epidemiol. Infect. 1992;108:107–113. doi: 10.1017/s0950268800049554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savini G., Dunsmore J.D., Robertson I.D., Seneviratna P. Sarcocystis spp in Western Australian sheep. Aust. Vet. J. 1993;70:152–154. doi: 10.1111/j.1751-0813.1993.tb06112.x. [DOI] [PubMed] [Google Scholar]
- Schaer J., Perkins S.L., Decher J., Leendertz F.H., Fahr J., Weber N., Matuschewski K. High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa. Proc. Natl. Acad. Sci. U.S.A. 2013;110:17415–17419. doi: 10.1073/pnas.1311016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheelings T.F., McLaren P.J., Tatarczuch L., Slocombe R.F. Plasmodium infection in a Leadbeater's possum (Gymnobelideus leadbeateri) Aust. Vet. J. 2016;94:299–303. doi: 10.1111/avj.12466. [DOI] [PubMed] [Google Scholar]
- Siembieda J.L., Kock R.A., McCracken T.A., Newman S.H. The role of wildlife in transboundary animal diseases. Anim. Health Res. Rev. 2011;12:95–111. doi: 10.1017/S1466252311000041. [DOI] [PubMed] [Google Scholar]
- Spratt D.M., Beveridge I. Wildlife parasitology in Australia: past, present and future. Aust. J. Zool. 2018;66:286–305. [Google Scholar]
- Templeton T.J., Asada M., Jiratanh M., Ishikawa S.A., Tiawsirisup S., Sivakumar T., Namangala B., Takeda M., Mohkaew K., Ngamjituea S., Inoue N., Sugimoto C., Inagaki Y., Suzuki Y., Yokoyama N., Kaewthamasorn M., Kaneko O. Ungulate malaria parasites. Sci. Rep. 2016;6:23230. doi: 10.1038/srep23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton T.J., Martinsen E., Kaewthamasorn M., Kaneko O. The rediscovery of malaria parasites of ungulates. Parasitology. 2016;143:1501–1508. doi: 10.1017/S0031182016001141. [DOI] [PubMed] [Google Scholar]
- Thompson C.K., Botero A., Wayne A.F., Godfrey S.S., Lymbery A.J., Thompson R.C. Morphological polymorphism of Trypanosoma copemani and description of the genetically diverse T. vegrandis sp. nov. from the critically endangered Australian potoroid, the brush-tailed bettong (Bettongia penicillata (Gray, 1837)) Parasites Vectors. 2013;6:121. doi: 10.1186/1756-3305-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C.K., Godfrey S.S., Thompson R.C. Trypanosomes of Australian mammals: a review. Int. J. Parasitol. Parasites Wildl. 2014;3:57–66. doi: 10.1016/j.ijppaw.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R., Reiss A., Cox-Witton K., Grillo T., Peters A. The importance of wildlife disease monitoring as part of global surveillance for zoonotic diseases: the role of Australia. Trav. Med. Infect. Dis. 2019;4:29. doi: 10.3390/tropicalmed4010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley M.J., Quick T.C., Little S.E. Theileriosis in a white-tailed deer (Odocoileus virginianus) fawn. J. Wildl. Dis. 2005;41:806–809. doi: 10.7589/0090-3558-41.4.806. [DOI] [PubMed] [Google Scholar]
- Yang J.F., Li Y.Q., Liu Z.J., Liu J.L., Guan G.Q., Chen Z., Luo J.X., Wang X.L., Yin H. Molecular evidence for piroplasms in wild Reeves' muntjac (Muntiacus reevesi) in China. Parasitol. Int. 2014;63:713–716. doi: 10.1016/j.parint.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Zamoto-Niikura A., Tsuji M., Qiang W., Morikawa S., Hanaki K.I., Holman P.J., Ishihara C. The Babesia divergens asia lineage is maintained through enzootic cycles between Ixodes persulcatus and sika deer in Hokkaido, Japan. Appl. Environ. Microbiol. 2018;84:7. doi: 10.1128/AEM.02491-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanet S., Trisciuoglio A., Bottero E., de Mera I.G.F., Gortazar C., Carpignano M.G., Ferroglio E. Piroplasmosis in wildlife: Babesia and Theileria affecting free-ranging ungulates and carnivores in the Italian Alps. Parasites Vectors. 2014;7:70. doi: 10.1186/1756-3305-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.