Introduction
Leukocytoclastic vasculitis (LCV) is a small-vessel vasculitis that presents with palpable purpura. The most common identified triggers of LCV are infection or exposure to a new medication. Herpes zoster (HZ) occurs as a result of the reactivation of the varicella-zoster virus (VZV). VZV is able to infect endothelial cells directly, producing a spectrum of vasculitides, including that of large and medium vessels. We report a case of a patient with synchronous HZ infection and segmental small-vessel vasculitis.
Case report
A 68-year-old female with a history of idiopathic pulmonary hypertension, diastolic heart failure, coronary artery disease, atrial fibrillation, and chronic kidney disease was admitted to our hospital for hypoxic respiratory failure. She required intensive care unit admission and intubation. She was found to have diffuse alveolar hemorrhage caused by cryptogenic organizing pneumonia. COVID-19 testing was negative. She was empirically treated with intravenous immunoglobulin and high-dose intravenous methylprednisolone. She improved and was transferred to the medical floor, at which point dermatology was consulted for a new, asymptomatic, cutaneous eruption on the lateral aspect of the right thigh.
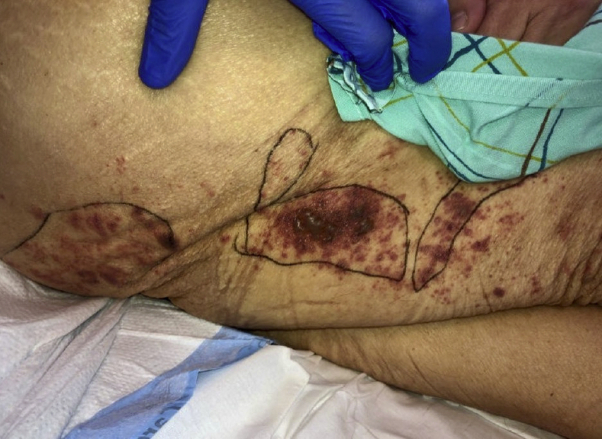
The patient was afebrile and hemodynamically stable. Purpuric macules coalescing into patches were present on the lower portion of the right extremity in a dermatomal distribution. Focal areas of involvement were palpable and had centrally located hemorrhagic vesicles and bullae (Fig 1). The cutaneous examination was otherwise unremarkable.
Fig 1.
Clinical morphology of a new-onset palpable purpuric eruption with hemorrhagic bullae, localized to a segmental distribution on the lower portion of the right extremity.
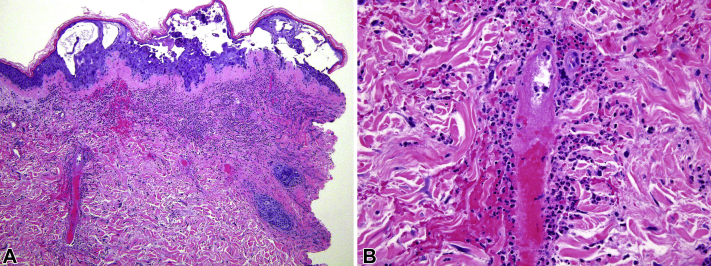
A skin biopsy of the right thigh was performed. It showed intra- and sub-epidermal vesicles and keratinocytes with acantholysis, multinucleation and nuclear margination. This observation was overlying a neutrophilic dermal infiltrate with leukocytoclasia, extensive necrosis of vessel walls, fibrin deposition and extravasated erythrocytes (Fig 2). Many of the endothelial cells and several dermal fibroblasts demonstrated enlarged nuclei with prominent nucleoli. Immunohistochemical staining for VZV was strongly positive in epidermal keratinocytes, endothelial cells and fibroblasts.
Fig 2.
Histopathology of skin biopsy from the right thigh demonstrated intra- and sub-epidermal vesicles, keratinocytes multinucleation, and a neutrophilic dermal infiltrate with leukocytoclasia, consistent with herpesvirus infection and small-vessel leukocytoclastic vasculitis. (Hematoxylin-eosin stain; original magnification: A, ×10; B, ×40)
Virologic assay using multiplex nucleic acid amplification of vesicular fluid was negative for the herpes simplex virus and positive for VZV. The patient had a normal complete blood count, coagulation studies, and negative inflammatory markers. Additionally, her autoimmune connective tissue disease workup was unremarkable, including testing of antineutrophil cytoplasmic antibodies, antiphospholipid antibodies, cryoglobulin, antibasement membrane antibody, any specific for lupus, Sjogren syndrome and scleroderma antibodies, complement levels. Mycobacterial, bacterial and fungal tissue cultures taken from the site of the eruption were also negative.
The patient was initiated on intravenous acyclovir which resulted in a progressive improvement in her purpuric eruption. She was transitioned to oral valacyclovir and completed a 10-day course with the ultimate resolution of the eruption.
Discussion
This patient's clinical presentation was initially concerning for bullous leukocytoclastic vasculitis. LCV encompasses a heterogeneous group of disorders with end-stage inflammation of the small capillaries and post-capillary venules of the skin. The most commonly identified triggers of LCV are infection or exposure to a new medication. However, an estimated 50% of cases have no identifiable cause.1 As this patient was found to have concomitant features of both LCV and herpes zoster infection clinically and pathologically, HZ was likely the pathophysiologic trigger of LCV in this case.
VZV may be associated with a spectrum of vasculitides ranging from focal capillaritis in immunocompetent hosts to a large vessel vasculitis and severe segmental thrombotic vasculopathy in the immunocompromised. Lymphocytic, eosinophilic, and granulomatous vasculitides have been described in different reports of VZV infection.2, 3, 4 Additionally, the virus can affect all vessel types, as small, medium, and large vessel vasculitides related to VZV infection have been described.2,5,6 While some rare reports demonstrate clinical signs of vasculitis prior to the classic VZV cutaneous eruption,7,8 the majority of reports demonstrate vasculitis after VZV infection.2,5 Evidence of LCV with a synchronous VZV infection has been rarely reported.6,7,9
Cell-mediated immunity is important in the local immune response to VZV, as such herpes zoster has greater morbidity in the elderly and the immunosuppressed. The host immunosuppression likely played a role in our case as the patient recovered from severe cryptogenic organizing pneumonia and received high-dose corticosteroids. Several theories have been put forth to explain the pathophysiologic mechanisms by which VZV damages blood vessels. Firstly, hematogenous viral spread to small cutaneous vessels is a possible mechanism for LCV induction.10 In our patient, LCV occurred simultaneously in the same dermatomal distribution as her HZ, making viremia an unlikely means of spread. Secondly, the direct spread of the virus to dermal vessels from overlying vesicles and bullae is another proposed mechanism. However, this possibility also seems unlikely in our patient as both her LCV and herpes zoster arose synchronously. Thirdly, on reactivation from sensory nerve ganglia, the direct spread of VZV to the dermal vessels from contiguous nerves has been proposed,3 and seems to be the most reasonable pathophysiologic mechanism for this patient's presentation. Given the dermatomal involvement, an infection of endothelial cells via adjacent nerves through the abluminal side of the vessel could have precipitated clinical signs of vasculitis.
A number of herpes viruses have been associated with leukocytoclastic vasculitis, including herpes simplex virus, cytomegalovirus, human herpesvirus 6, and VZV. While it is not uncommon to see lymphocytic vasculitis histopathologically in cases of VZV, the simultaneous clinical occurrence of cutaneous vasculitis and HZ is exceedingly rare. To our knowledge, only one other report of clinically coincident, localized LCV and VZV has been reported. In the case described by Singh and Deng,11 the patient presented with hemorrhagic papulovesicular lesions with central confluence and vesicle formation. A biopsy revealed intraepidermal vesiculation with profound acantholysis, multinucleated giant cells, and intranuclear viral inclusions as well as fibrinoid degeneration of vessels, extravasation of red blood cells, inflammatory infiltrate with neutrophils and leukocytoclasia. The patient was immunosuppressed in the setting of a liver transplant. Another case report demonstrates a similar phenomenon in a patient presenting clinically with disseminated zoster and classic bilateral LCV.12 Additionally, dermatomal LCV prior to the diagnosis of zoster infection has also been reported.8 Our case demonstrates the onset of localized bullous HZ and coincident small-vessel LCV to the same dermatomal distribution, raising our awareness of such a synchronous clinical manifestation even in a setting of short-term immunosuppression.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Bolognia J., Jorizzo J., Schaffer J. 3rd ed. Elsevier Saunders; Philadelphia London: 2012. Dermatology. [Google Scholar]
- 2.Aram G., Rohwedder A., Nazeer T., Shoss R., Fisher A., Carlson J.A. Varicella-zoster-virus folliculitis promoted clonal cutaneous lymphoid hyperplasia. Am J Dermatopathol. 2005;27(5):411–417. doi: 10.1097/01.dad.0000178005.34515.7f. [DOI] [PubMed] [Google Scholar]
- 3.Erhard H., Rünger T.M., Kreienkamp M., Müller J., Müller-Hermelink H.K., Bröcker E.B. Atypical varicella-zoster virus infection in an immunocompromised patient: result of a virus-induced vasculitis. Am J Dermatopathol. 1995;32(5 Pt 2):908–911. doi: 10.1016/0190-9622(95)91560-5. [DOI] [PubMed] [Google Scholar]
- 4.Carlson J.A., Chen K.R. Cutaneous vasculitis update: neutrophilic muscular vessel and eosinophilic, granulomatous, and lymphocytic vasculitis syndromes. Am J Dermatopathol. 2007;29(1):32–43. doi: 10.1097/01.dad.0000245198.80847.ff. [DOI] [PubMed] [Google Scholar]
- 5.Elgoweini M., Blessing K., Jackson R., Duthie F., Burden A.D. Coexistent granulomatous vasculitis and leukaemia cutis in a patient with resolving herpes zoster. Clin Exp Dermatol. 2011;36(7):749–751. doi: 10.1111/j.1365-2230.2011.04085.x. [DOI] [PubMed] [Google Scholar]
- 6.Cury-Martins J., Bellesso M., Sotto M.N., Sanches J.A. Atypical herpes vasculitis in a leukemic patient: an unusual presentation. Hematol Transfus Cell Ther. 2019;41:95–98. doi: 10.1016/j.htct.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollina U., Schönlebe J. Segmental leukocytoclastic vasculitis in herpes zoster. Int J Dermatol. 2012;51(11):1351–1352. doi: 10.1111/j.1365-4632.2011.05167.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgard B., Smola S., Vogt T., Müller C.S.L. Small Vessel vasculitis in herpes zoster-discussion of current aspects of varicella zoster virus vasculopathy. Am J Dermatopathol. 2018;40(8):602–604. doi: 10.1097/DAD.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 9.Cohen C., Trapuckd S. Leukocytoclastic vasculitis associated with cutaneous infection by herpesvirus. Am J Dermatopathol. 1984;6(6):561–565. doi: 10.1097/00000372-198412000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Satyaprakash A.K., Tremaine A.M., Stelter A.A. Viremia in acute herpes zoster. J Infect Dis. 2009;200(1):26–32. doi: 10.1086/599381. [DOI] [PubMed] [Google Scholar]
- 11.Singh N., Deng J.-S. Answer to photo quiz I. Clin Infect Dis. 1998;26(4):981. doi: 10.1086/513931. [DOI] [PubMed] [Google Scholar]
- 12.Álvarez-Salafranca M., Garcés-Horna V., García-García M., Ara-Martín M. Atypical vasculopathic varicella-zoster infection mimicking cutaneous small-vessel vasculitis. Int J Dermatol. 2020;59(6):e214–e216. doi: 10.1111/ijd.14816. [DOI] [PubMed] [Google Scholar]