Abstract
Depletion of nicotinamide adenine dinucleotide (NAD+), a central redox cofactor and the substrate of key metabolic enzymes, is the causative factor of a number of inherited and acquired diseases in humans. Primary deficiencies of NAD+ homeostasis are the result of impaired biosynthesis, while secondary deficiencies can arise due to other factors affecting NAD+ homeostasis, such as increased NAD+ consumption or dietary deficiency of its vitamin B3 precursors. NAD+ depletion can manifest in a wide variety of pathological phenotypes, ranging from rare inherited defects, characterized by congenital malformations, retinal degeneration, and/or encephalopathy, to more common multifactorial, often age‐related, diseases. Here, we discuss NAD+ biochemistry and metabolism and provide an overview of the etiology and pathological consequences of alterations of the NAD+ metabolism in humans. Finally, we discuss the state of the art of the potential therapeutic implications of NAD+ repletion for boosting health as well as treating rare and common diseases, and the possibilities to achieve this by means of the different NAD+‐enhancing agents.
Keywords: disease, metabolism, NAD+, NAD+ homeostasis, therapy
Subject Categories: Metabolism
This comprehensive review discusses pathological consequences of NAD+ metabolism alterations and the therapeutic potential of NAD+ enhancers.
Glossary
- Genetic disorder
A disease caused by changes (mutations) in the DNA sequence of an individual. These mutations can occur in one or in multiple genes.
- Leber Congenital Amaurosis (LCA)
Rare inherited eye disorder primarily affecting the retina. LCA symptoms include severe visual impairment, involuntary movement of the eyes (nystagmus), and/or increased sensitivity to light (photophobia).
- Mitochondria
Subcellular compartments (organelles) found in the cytoplasm of eukaryotic cells equipped with the enzymatic machinery to degrade fatty acids, sugar‐derived substrates, and amino acids to CO2 and H2O to synthesize ATP through aerobic respiration via a process called oxidative phosphorylation.
- NAD+ precursor
Molecule that can be converted into NAD+ through a series of reactions inside cells. To date, established NAD+ precursors are tryptophan, nicotinic acid (NA), nicotinamide (NAM), nicotinic acid riboside (NAR), and the oxidized and reduced forms of nicotinamide mononucleotide (NMN and NMNH) and nicotinamide riboside (NR and NRH).
- Niacin
General term used to define vitamin B3 and derivatives, including nicotinic acid, nicotinamide, and related compounds, such as nicotinamide riboside. Vitamin B3 is found in a wide variety of foods. Many countries have food fortification programs with niacin to prevent pellagra.
- Pellagra
A disease caused by deficient intake of vitamin B3 (niacin). Pellagra is also known as the disease of the four D’s, as it is clinically manifested by photosensitive dermatitis, diarrhea, dementia, and, eventually, death.
- NAD(H)
The oxidized (NAD+) and reduced (NADH) forms of nicotinamide adenine dinucleotide are essential molecules in cellular energy metabolism due to their ability to transfer electrons. NAD+ is also used as a substrate by several families of enzymes, the so‐called NAD+ consumers, which regulate major biological processes. The central role of NAD(H) in metabolism makes these molecules crucial for cellular proper function.
- Preiss–Handler pathway
This pathway, described in 1958 by Jack Preiss and Philip Handler, involves the conversion of nicotinic acid into NAD+ in three sequential enzymatic steps carried out by the enzymes NA phosphoribosyltransferase (NAPRT), NMN adenylyltransferases (NMNATs), and NAD+ synthase (NADSYN).
Introduction
Nicotinamide adenine dinucleotide exists in two forms, including an oxidized (NAD+) and a reduced (NADH) form, and plays a key role in intermediary metabolism, as obligatory partner in numerous oxidation/reduction reactions. Discovered over 100 years ago by Harden & Young (Harden & Young, 1906), NAD+ went through a period of relative anonymity until its renaissance 20 years ago, when it was reported as an essential substrate for the activity of sirtuins, a family of NAD+‐dependent deacetylases which play an essential role in the regulation of energy metabolism and mitochondrial function (Houtkooper et al, 2012). Besides sirtuins, other NAD+‐consuming enzymes have been identified during the last decades. This includes the poly(ADP‐ribose) polymerase (PARP) protein family (Leung, 2017) and the cyclic ADP‐ribose (cADPr) synthases, including CD38 (Aksoy et al, 2006) and CD157 (Ortolan et al, 2019). Together, these three families of enzymes regulate major biological processes in cells (Ansari & Raghava, 2010), making NAD+ homeostasis vital for proper cellular functioning.
The importance of NAD+ for human health and disease is exemplified by the existence of genetic diseases caused by defects in the biosynthesis of NAD+ with often devastating consequences in terms of the clinical signs and symptoms in patients. Furthermore, the cellular dependence on NAD+ also becomes evident if we consider the fact that NAD+ depletion is a common factor in numerous diseases (Okabe et al, 2019). Importantly, several reports have been published in which NAD+ repletion by means of supplementation with NAD+‐enhancing molecules was successful in ameliorating the outcomes of these conditions, including neurodegenerative disorders (Liu et al, 2013; Zhou et al, 2015; Schondorf et al, 2018), metabolic diseases (Revollo et al, 2007; Yoshino et al, 2011; Canto et al, 2012; Lee et al, 2015; Katsyuba et al, 2018), and age‐related complications (Mouchiroud et al, 2013b; Mills et al, 2016; Zhang et al, 2016).
Although most of the research on the implications of NAD+ in health and disease has been carried out in animal models, NAD+ deficiency has also been reported to cause a number of medical conditions in humans, which can be subdivided into two groups including the primary and secondary deficiencies of NAD+ homeostasis. The first group involves the genetically determined deficiencies of NAD+ synthesis, due to deleterious mutations in genes coding for enzymes directly involved in NAD+ biosynthesis, from both de novo and salvage pathways. In contrast, the second group of diseases involves alterations of NAD+ metabolism induced by other factors, such as increased NAD+ consumption and/or a dietary deficiency of NAD+ precursors.
In this review, we provide an overview of the current state of knowledge on human diseases caused by primary or secondary disturbances of NAD+ homeostasis and discuss about the therapeutic potential of NAD+ enhancers. However, other conditions affecting the balance between NAD+ and NADH, but which are not caused by a deficit in the NAD+ pool, i.e., the sum of NAD+ and NADH, are out of the scope of this review.
Cellular NAD+ homeostasis
The cellular pool of NAD+ and NADH is tightly regulated through a careful balance between its biosynthesis—de novo from tryptophan or via salvage pathways from precursors—and its breakdown by NAD+‐consuming enzymes. The relative rates of these two processes determine the cellular availability of NAD+ and NADH. While the ratio between NAD+ and NADH plays a major role in the maintenance of redox homeostasis, in this review we will predominantly focus on NAD+ metabolism. For more information about the implications of the NAD+:NADH balance, diseases affecting this balance, and innovative therapies in this domain, we refer the readers to several reviews dealing with this topic (Ying, 2008; Teodoro et al, 2013; Xiao et al, 2018).
NAD(H) biosynthesis and salvage
NAD+ can be synthesized de novo from tryptophan or through recycling of its precursors (Fig 1). In the de novo pathway, dietary tryptophan is taken up into cells by neutral amino acid transporters, such as SLC6A19 (Belanger et al, 2018), and converted into N‐formylkynurenine by the enzyme indoleamine 2,3‐dioxygenase (IDO) or tryptophan 2,3‐dioxygenase (TDO) (Bender, 1983), after which N‐formylkynurenine is converted into α‐amino‐β‐carboxymuconate‐ε‐semialdehyde (ACMS) in a sequence of enzymatic steps carried out by arylformamidase (AFMID), kynurenine 3‐monooxygenase (KMO), kynureninase (KYNU), and 3‐hydroxyanthranilate 3,4‐dioxygenase (HAAO) (Fig 1). ACMS is then either (i) enzymatically converted into α‐amino‐β‐muconate‐ε‐semialdehyde by the enzyme ACMS decarboxylase, after which the semialdehyde is subsequently degraded to completion via multiple enzymatic steps, or (ii) spontaneously cyclized to form quinolinic acid (QA). In the latter case, QA is condensed by the enzyme quinolinate phosphoribosyl transferase (QPRT) into nicotinic acid mononucleotide (NaMN). NaMN can also be synthesized via the Preiss–Handler pathway from nicotinic acid (NA; also known as niacin or vitamin B3), which is taken up into the cell via the NA transporters SLC5A8 and SCL22A13 (Fig 1). Either way, the fate of NaMN is to be adenylated by one of the nicotinamide mononucleotide adenylyl transferases (NMNATs)—convergent enzymes to all known NAD+ biosynthetic pathways—to nicotinic acid adenine dinucleotide (NaAD), which is the penultimate substrate finally used by the NAD+ synthase (NADSYN) in combination with glutamine and ATP, to produce NAD+ (Houtkooper et al, 2010) (Fig 1).
Figure 1. NAD+ synthesis pathways.
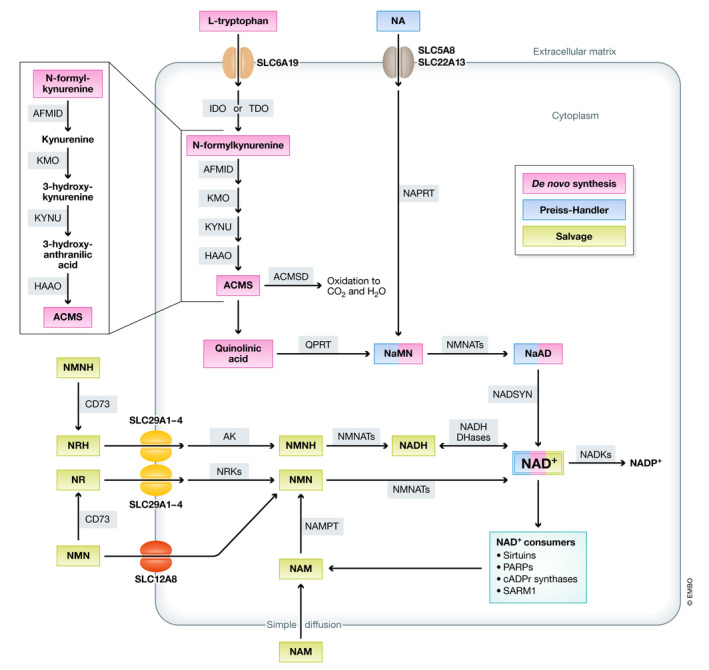
NAD+ can be synthesized de novo from tryptophan, through the Preiss–Handler pathway from NA or via salvage of the NAD+ precursors NMN, NR, NMNH, or NRH. ACMS: aminocarboxymuconate‐semialdehyde; ACMSD: aminocarboxymuconate‐semialdehyde decarboxylase; AFMID: arylformamidase; AK: adenosine kinase; CD73: cluster of differentiation 73 (5’‐nucleotidase); HAAO: 3‐hydroxyanthranilate 3,4‐dioxygenase; IDO: indoleamine 2,3‐dioxygenase; KMO: kynurenine 3‐monooxygenase; KYNU: kynureninase; NA: nicotinic acid; NADKs: NAD+ kinases; NaAD: nicotinic acid adenine dinucleotide; NAD+: nicotinamide adenine dinucleotide; NADP+: nicotinamide adenine dinucleotide phosphate; NADSYN: NAD+ synthase; NAM: nicotinamide; NaMN: nicotinic acid mononucleotide; NAPRT: nicotinate phosphoribosyltransferase; NMN: nicotinamide mononucleotide; NMNH: reduced nicotinamide mononucleotide; NMNAT: nicotinamide mononucleotide adenylyl transferase; NR: nicotinamide riboside; NRH: reduced nicotinamide riboside; NRK: nicotinamide riboside kinase; QPRT: quinolinate phosphoribosyltransferase; SLC: solute carrier transporter; and TDO: tryptophan 2,3‐dioxygenase.
Apart from the Preiss–Handler pathway, NAD+ salvage also occurs through recycling of other precursors, such as nicotinamide (NAM), which is generated by NAD+‐consuming enzymes, or the vitamin B3 derivative nicotinamide riboside (NR). After entering cells by simple diffusion in the case of NAM, or through the equilibrative nucleoside transporters (ENTs) SLC29A1‐4 in the case of NR, both molecules are directly converted into nicotinamide mononucleotide (NMN) by the enzyme NAM phosphoribosyltransferase (NAMPT) or the NR kinases (NRKs), respectively (Mouchiroud et al, 2013a). Interestingly, NMN can also act as an external NAD+ precursor via extracellular conversion to NR by the ectoenzyme 5’‐nucleotidase CD73 (Garavaglia et al, 2012; Grozio et al, 2013) or through specific transport via the recently described SLC12A8 transporter (Grozio et al, 2019).
Until very recently, six molecules were considered to act as extracellular NAD+ precursors including tryptophan, NA, NAM, nicotinic acid riboside (NaR), NMN, and NR (Tempel et al, 2007; Canto et al, 2015; Rajman et al, 2018). However, the current set of NAD+ precursors has recently been expanded with two new molecules, including the reduced forms of NMN (NMNH) and NR (NRH). In contrast to their oxidized counterparts, NMNH and NRH are efficiently converted to NAD+ via a new NAMPT and NRK‐independent pathway (Giroud‐Gerbetant et al, 2019; Yang et al, 2019; Zapata‐Pérez et al, 2021). In this new metabolic route, NMNH is cleaved by CD73 to NRH, which is then taken up by the same transporters (ENTs) as NR. Inside the cell, NRH is phosphorylated back to NMNH by adenosine kinase (AK), after which NMNH is finally converted into NADH by the NMNATs, which is then interconverted into NAD+ by the large variety of NADH dehydrogenases.
Finally, NAD+ can be subjected to one last phosphorylation step driven by the cytosolic NAD+ kinase NADK1 or the mitochondrial NADK2, both producing NADP+, which, together with NADPH, is essential for the maintenance of the redox balance and the biosynthesis of fatty and nucleic acids (Ying, 2008), among others (Fig 1).
Combined, the de novo, Preiss–Handler and NAD+ salvage pathways catalyze the formation of NAD(H), continuously counteracting the loss of NAD(H) by the three known families of NAD+‐consuming enzymes.
NAD+‐consuming enzymes
Three families of proteins are classically considered as the main consumers of NAD+ through their enzymatic reactions, i.e., (i) sirtuins (SIRTs), (ii) poly(ADP‐ribose) polymerases (PARPs), and (iii) cyclic ADP‐ribose (cADPr) synthases, all of them generating NAM as a result of NAD+ utilization (Figure 1). More recently, sterile alpha and TIR motif‐containing 1 (SARM1) has been identified as an enzyme with NAD+‐cleavage activity in neurons, establishing a new family of NAD+‐consuming enzymes (Essuman et al, 2017). Finally, the Nudix family of hydrolases might also play a role in NAD+ homeostasis in mammals, as they cleave NADH to NMNH and AMP (Abdelraheim et al, 2003, 2017). However, there is only in vitro evidence of their activity, and their role in maintaining NAD+ homeostasis under physiological conditions remains unknown.
Sirtuins are a family of NAD+‐dependent protein deacetylases that, in mammals, consists of seven members (SIRT1 to SIRT7) with different subcellular locations (Michishita et al, 2005; Houtkooper et al, 2012). Sirtuins act as metabolic regulators that trigger cellular adaptations in response to changes in the cellular energy status. In line with their central role in cellular metabolism, sirtuin activation by increased NAD+ bioavailability has proven beneficial in a number of preclinical studies (Yoshino et al, 2011; Canto et al, 2012; Cerutti et al, 2014; Pirinen et al, 2014; Gariani et al, 2016; Jukarainen et al, 2016; Ryu et al, 2016) and has focused attention on this family of enzymes as potential therapeutic targets, also in humans (Jukarainen et al, 2016; Pirinen et al, 2020; Remie et al, 2020).
Similar to sirtuins, the DNA damage‐activated poly(ADP‐ribose) polymerases (PARPs) consume NAD+ during transfer of ADP‐ribosyl moieties to other proteins (Leung, 2017). However, from the 17 members that comprise the PARP superfamily, PARP1 and PARP2 account for almost all PARP activity, and therefore, these two enzymes are considered the main NAD+ consumers in cells (Bai & Canto, 2012).
A third cluster of NAD+‐consuming enzymes involves the family of cyclic ADP‐ribose (cADPr) synthases CD38 and CD157. By generating the second messenger cADPr through NAD+ cleavage, cADPr synthases are able to influence intracellular calcium fluxes, cell cycle activity, insulin signaling, and the immune response (Aksoy et al, 2006; Malavasi et al, 2008; Wei et al, 2014; Ortolan et al, 2019).
SARM1 has been reported as an important NAD+ consumer, with special relevance in the regulation of neuronal cell death. In fact, in response to neuronal damage, SARM1 is activated, catalyzing the hydrolysis of NAD+ to ADPr, cADPr, and NAM and promoting cytoskeletal degradation and axon destruction (Gerdts et al, 2013; Essuman et al, 2017).
For more extensive coverage of NAD+‐consuming enzymes, we refer the readers to several excellent reviews with more detailed information on sirtuins (Houtkooper et al, 2012; Chang & Guarente, 2014), PARPs (Schreiber et al, 2006; Canto et al, 2013; Leung, 2017), and cADPr synthases (Quarona et al, 2013).
NAD(H) compartmentalization and maintenance of the redox balance
It is important to emphasize that cellular NAD+ homeostasis depends not only on its synthesis and utilization, but also on subcellular compartmentalization. This is not only true for the total content of NAD+ and NADH, but also applies to the NAD(H) redox state. Indeed, the nuclear and cytoplasmic NAD+:NADH ratio is 700:1, whereas that in mitochondria is much lower (8:1) (Williamson et al, 1967). Total NAD(H) content also differs between different cellular compartments. In fact, although NAD(H) levels are comparable between the nucleus and the cytosol (Cambronne et al, 2016), mitochondria can account for up to 70% of the total pool, depending on the mitochondrial volume in a particular cell type (Di Lisa & Ziegler, 2001). Although the mechanism behind this differential content of NAD+ plus NADH has remained unresolved, the recent identification of SLC25A51/MCART1 as an active transporter of NAD+ from the cytosol to mitochondria may well provide an explanation (Kory et al, 2020; Luongo et al, 2020). Furthermore, cellular NAD(H) distribution can be explained by a combination of (i) distinct subcellular expression patterns of NAD+ biosynthetic and consuming enzymes (Houtkooper et al, 2010); (ii) organelle‐specific transporters of NAD+ intermediates (Nikiforov et al, 2011; Gaudino et al, 2019); and (iii) a set of redox systems that interconvert NAD+ and NADH, such as the TCA cycle, complex I of the electron transport chain, various cytosolic dehydrogenases, or the malate‐aspartate shuttle (also known as the Borst cycle) (Canto et al, 2015; Yang & Sauve, 2016; Borst, 2020) (Figure 2). The presence of such a wide array of organelle‐specific mechanisms emphasizes the importance of maintaining a tight regulation of the NAD+ and NADH concentrations not only in the cytosol, but also at a subcellular level.
Figure 2. Maintenance of the interorganelle NAD(H) homeostasis.
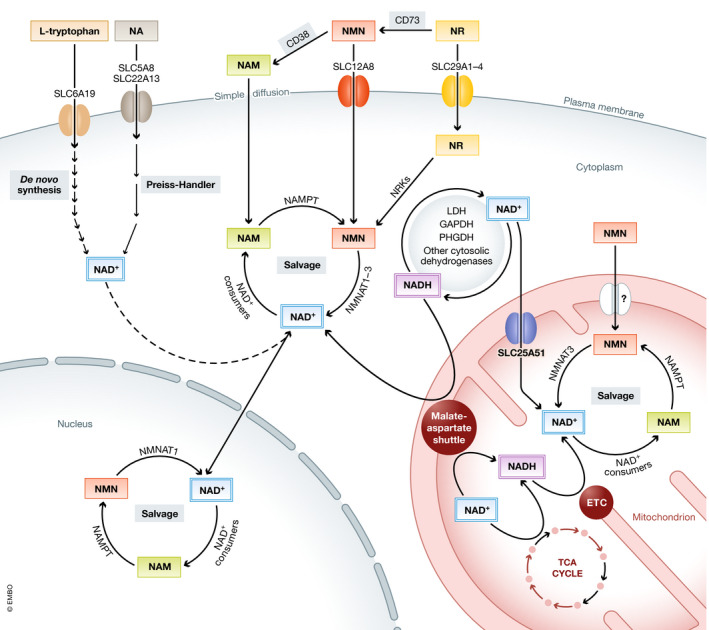
Several pathways and redox systems are involved in the maintenance of cellular NAD(H) homeostasis. The NAD+ precursors tryptophan, nicotinic acid (NA), nicotinamide (NAM), nicotinamide mononucleotide (NMN), or nicotinamide riboside (NR) are transported into the cell via specific transporters or simple diffusion and incorporated into the cytosolic or nuclear NAD+ pools. Via the reactions of the cytosolic dehydrogenases or the malate‐aspartate shuttle, NAD+ and NADH can then be interconverted to maintain the cytosolic and nuclear NAD+:NADH ratios. Inside mitochondria, the malate‐aspartate shuttle works in combination with the TCA cycle and the electron transport chain (ETC) to maintain this redox balance. Besides, the mitochondrial NAD(H) pool is maintained by salvage pathways and active NAD+ transport through the newly discovered transporter SLC25A51. ETC: electron transport chain; GAPDH: glyceraldehyde 3‐phosphate dehydrogenase; LDH: lactate dehydrogenase; and PHGDH: phosphoglycerate dehydrogenase.
NAD+ deficiency in human disease
A correct balance between NAD+ biosynthesis and breakdown is crucial to maintain cellular NAD+ homeostasis. Therefore, any imbalance between these two processes will ultimately lead to a net deficiency of intracellular NAD+ levels. This NAD+ depletion may arise from genetically determined deficiencies of NAD+ synthesis (primary NAD+ deficiencies) or from other factors that lead to a reduced NAD+ content (secondary NAD+ deficiencies). Given that putative secondary NAD+ deficiencies caused by deficient intake of niacin, such as pellagra, were already known to cause disease in humans long before the human genome had been sequenced and the genetic background of NAD+‐related disorders elucidated, we will start our discussion with these secondary deficiencies.
Secondary NAD+ deficiencies
Pellagra, also known as the disease of “the four D’s”: diarrhea, dermatitis, dementia, and, eventually, death, was the first human disease associated with a deficiency in NAD+ precursors. Pellagra was firstly considered to be an infectious disease and reached epidemic proportions in Europe and the United States between the 18th and 19th centuries (Bender, 1983). It was not until 1937 when the connection between pellagra and a deficit of NA and NAM intake due to poor nutrition was made (Elvehjem et al, 1937). To date, this is the only known human condition caused by a deficiency of an NAD+ precursor in the diet.
Secondary NAD+ deficiencies can also have a genetic origin. A good example is glutamine synthetase deficiency due to mutations in the encoding gene (GLUL) (Table 1). Homozygous missense mutations in GLUL lead to glutamine deficiency (Haberle et al, 2011), which impairs the activity of the glutamine‐dependent NAD+ synthase (NADSYN), causing a very rare disease characterized by neonatal onset of severe encephalopathy, NAD+ and glutamine depletion, and an overall poor prognosis in the absence of treatment (Hu et al, 2015).
Table 1.
Deleterious human variants of NAD+‐related genes.
| Gene | Variant 1 | Clinical condition | Reference |
|---|---|---|---|
| GLUL | 970C → A (HMZ) | Glutamine and NAD+ depletion, neonatal encephalopathy, hyperammonemia, poor prognosis | Hu et al, (2015) |
| SLC6A19 |
517G → A (HMZ) 718C → T (HMZ) 725T → C (HMZ) 1501G → A (HMZ) IVS8+1A (HMZ) IVS11+2G (HMZ) 908C → T; 1787_1788insG (C/HTZ) |
Hartnup disorder: pellagra‐like rash, photosensitivity, ataxia, tremors. | Cheon et al, (2010); Kleta et al, (2004); Seow et al, (2004) |
| HAAO |
483dupT (HMZ) 558G → A (HMZ) |
Congenital cardiac, renal, limb, and ear‐related defects | Shi et al, (2017) |
| KYNU |
170‐1G → T (HMZ) 468T → A; 1045_1051delTTTAAGC (C/HTZ) |
Congenital cardiac, renal, limb, and ear‐related defects | Shi et al, (2017) |
| NMNAT1 |
1A → G; 769G → A (C/HTZ) 25G → A (HMZ) 37G → A; 293T → G (C/HTZ) 37G → A; 468T → C (C/HTZ) 53A → G; 769G → A (C/HTZ) 53A → G; 472G → C (C/HTZ) 59T → A; 769G→ A (C/HTZ) 98A → G (HMZ) 161C → T; 293T → G (C/HTZ) 196C → T; 709C → T (C/HTZ) 199G → T; 769G → A (C/HTZ) 205A → G; 650T → A (C/HTZ) 205A → G; 769G → A (C/HTZ) 215T → A (HMZ) 253T → C; 769G → A (C/HTZ) 271G → A (HMZ) 319G → T; 709C → T (C/HTZ) 362delA; 769G → A (C/HTZ) 362delA; 532G → A (C/HTZ) 439G → C; 769G → A (C/HTZ) 439G+1G → C; 769G → A (C/HTZ) 466G → C; 769G → A (C/HTZ) 507G → A; 710G → T (C/HTZ) 507G → A (HMZ) 518A → G; 769G → A (C/HTZ) 542A → G; 769G → A (C/HTZ) 552A → G; 769G → A (C/HTZ) 595G → T; 769G → A (C/HTZ) 617A → G; 769G → A (C/HTZ) 619C → T; 769G → A (C/HTZ) 643G → T; 769G → A (C/HTZ) 709C → T; 565delG (C/HTZ) 716T → C; 769G → A (C/HTZ) 723delA; 769G → A (C/HTZ) 731T → C; 769G → A (C/HTZ) 769G → A (HMZ) 769G → A; 817A → G (C/HTZ) 752A → G; 769G → A (C/HTZ) 838T → C (HMZ) |
Early‐onset form of Leber congenital amaurosis (LCA): nystagmus, hyperopia, visual loss | Falk et al, (2012); Koenekoop et al, (2012); Nash et al, (2018); Perrault et al, (2012); Siemiatkowska et al, (2014) |
| NADSYN1 |
145T → C; 395G → T (C/HTZ) 735T → A; 1839C → G (C/HTZ) 1717G → A (HMZ) 1717G → A; 1819del (C/HTZ) |
Vertebral, cardiac, renal, and limb defects | Szot et al, (2020) |
| NAXE |
177C → A (HMZ) 196C → T; 516+1G → A (C/HTZ) 281C → A (HMZ) 653A → T; 743delC (C/HTZ) 804_807delinsA (HMZ) |
Acute‐onset ataxia, delayed development, respiratory insufficiency, skin lesions, poor prognosis. Aggravated by febrile periods. | Kremer et al, (2016); Spiegel et al, (2016) |
| NAXD |
44delG; 51_54delAGAA (C/HTZ) 51_54delAGAA (HMZ) 54_57delAAGA (HMZ) 101_102delTA; 318C → G (C/HTZ) 187G → A; 948_949insTT (C/HTZ) 308C → T (HMZ) 331C → T; 776T → G (C/HTZ) 839+1G → T; 922C → T (C/HTZ) |
Acute‐onset ataxia, muscular hypotonia, respiratory insufficiency, skin lesion, poor prognosis. Aggravated by febrile periods. | Borna et al, (2020); Van Bergen et al, (2019); Zhou et al, (2020) |
HMZ: homozygous and C/HTZ: compound heterozygous.
Heteroplasmic single or multiple deletions in mitochondrial DNA (mtDNA) often manifest as adult‐onset mitochondrial myopathy, a disease characterized by progressive weakness of the eye muscles, generalized muscle weakness, and fatigue (Ylikallio & Suomalainen, 2012). A very recent study has demonstrated that patients with mitochondrial myopathy also have disturbed NAD+ metabolism (Pirinen et al, 2020), with low NAD+ levels not only in muscle, but also in blood, pointing toward a systemic alteration of NAD+ metabolism. Interestingly, although no changes in the expression of PARPs, CD38, or sirtuins were detected, this study suggests that NAD+ depletion may be driven by impaired NAD+ salvage from NAM, enhanced NAM elimination, and increased activity of NAD+ consumers (Pirinen et al, 2020).
In humans, NAD+ deficiency can also occur during the natural process of aging (Fang et al, 2017). Although most of the research on the role of NAD+ depletion in aging has been carried out in animal models (Mouchiroud et al, 2013b; Yaku et al, 2018), mounting evidence suggests that these findings can be translated to humans. In fact, NAD+ levels are depleted in tissues from aged humans, such as skin (Massudi et al, 2012) and brain (Zhu et al, 2015). However, the mechanisms driving NAD+ depletion during human aging are not yet fully understood. In experimental model systems, it has been postulated that age‐related NAD+ deficiency is driven by a combination of (i) increased activity of NAD+‐consuming enzymes, especially PARP1 (Mandir et al, 1999; Liaudet, 2002; Mouchiroud et al, 2013b) and CD38 (Aksoy et al, 2006; Camacho‐Pereira et al, 2016), and (ii) compromised NAD+ biosynthesis, which is primarily caused by decreased NAMPT‐mediated NAD+ salvage (Revollo et al, 2007; Yoshino et al, 2011; Liu et al, 2012; Ghosh et al, 2014), but also by a decrease in the activity of the mitochondrial enzyme NMNAT3 (Son et al, 2016). Studies on the role of NAD+ biosynthetic and consuming enzymes in human aging are still in their infancy. In fact, only a few reports have shown impaired NAMPT activity or PARP1 overactivation in elderly or obese subjects (Barth et al, 2010; Dahl et al, 2010; Gaddipati et al, 2010; Jukarainen et al, 2016; Zhou et al, 2016), which has also been linked to thoracic aortic aneurysms (Watson et al, 2017). Moreover, NAMPT protein abundance decreases with age in human skeletal muscle and can be reverted by exercise training (de Guia et al, 2019), suggesting that physical activity may be important in the maintenance of NAD+ homeostasis in the aged muscle.
A role for extracellular NAMPT (eNAMPT) in maintaining NAD+ homeostasis during aging has also been described recently. This extracellular protein, usually considered as a proinflammatory cytokine whose increased levels in circulation have been linked to several human pathologies (Garten et al, 2015), can also function as a systemic NAD+ biosynthetic enzyme (Revollo et al, 2007; Yoon et al, 2015). A recent study shows that eNAMPT is exclusively contained in extracellular vesicles and that the levels of this extracellular protein decline with age in mice and human plasma (Yoshida et al, 2019). These results suggest that extracellular NAMPT plays a role in the maintenance of NAD+ homeostasis in human aging, especially in tissues with low levels of iNAMPT.
Primary deficiencies of NAD+ synthesis
The primary NAD+ deficiencies include the inherited disorders due to mutations in genes coding for one of the enzymes involved in NAD+ biosynthesis, affecting either de novo or salvage pathways. A summary of the different diseases known late 2020 and the affected genes and tissues is provided in Table 1 and Fig 3.
Figure 3. Mutations in NAD+ biosynthesis‐related genes cause a variety of clinical manifestations.
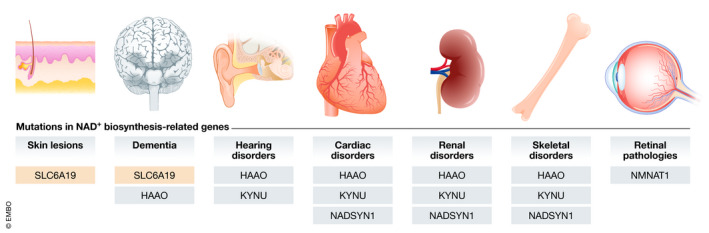
Impaired tryptophan transport through defects in the SLC6A19 transporter is manifested as skin lesions and dementia. Dementia is also present in patients with mutations in the HAAO gene. Impairment of NAD+ synthesis, either at its upstream enzymes (HAAO and KYNU) or in the distal part of the pathway (NADSYN1), leads to cardiac, renal, hearing, or skeletal (malformations) disorders. Mutations in NMNAT1 are the only known cause for retinal pathology associated with NAD+ deficiency in humans.
Deficient intake of the NAD+ precursors NA and NAM is the cause of pellagra, suggesting a role of downstream NAD+ synthesis pathways in the onset of this disease. Pellagra can also have a genetic basis. Indeed, homozygous or compound heterozygous mutations in the neutral amino acid transporter SLC6A19 (Table 1), which is required for tryptophan transport, cause a pellagra‐like disorder known as Hartnup disease (Kleta et al, 2004; Seow et al, 2004; Cheon et al, 2010), a syndrome with similar symptoms to niacin deficiency (Tahmoush et al, 1976). Indeed, expression of the wild‐type human SLC6A19 in Xenopus laevis oocytes showed that this transporter is able to incorporate neutral amino acids in a sodium‐dependent manner. In contrast, the expression of deleterious variants of SLC6A19 showed impaired leucine uptake, providing evidence that reduced amino acid transport through SLC6A19 is indeed the causative factor in Hartnup disease (Kleta et al, 2004; Seow et al, 2004).
Once tryptophan enters cells, it is converted into quinolinic acid (QA) in a process involving five enzymatic steps (Fig 1), two of which are carried out by the enzymes HAAO and KYNU. In a study carried out in four different families, it was reported that homozygous loss‐of‐function variants of the HAAO or KYNU genes, or compound heterozygous variants of KYNU (Table 1), led to reduced plasma NAD+ and NADH levels, and were associated with a number of clinical abnormalities, including defects in vertebral segmentation, renal hypoplasia, and other congenital malformations (Shi et al, 2017). The NAD+ decline was accompanied by large increases in the concentrations of the HAAO substrate 3‐hydroxyanthranilic acid in HAAO‐deficient patients, and the KYNU substrate 3‐hydroxykynurenine in KYNU‐deficient patients, which is in line with a block in the NAD+ synthesis pathway at the level of the two corresponding enzymes (Fig 1). Interestingly, follow‐up experiments in mice demonstrated that it is the loss of embryonic NAD+ and not a deficit in maternal NAD+ that leads to embryonic defects, and that niacin supplementation resolves such defects. These results provide further support suggesting that NAD+ homeostasis is essential for embryonic development, and emphasize the potential therapeutic benefit that niacin or other NAD+ precursors may have for humans suffering from these conditions.
The next step in the de novo synthesis of NAD+ is carried out by the enzyme QPRT, which converts QA, a potent excitotoxic and convulsant agent (Lugo‐Huitron et al, 2013), into NaMN. Although specific mutations affecting the QPRT gene have not yet been associated with human disease, upregulation of the kynurenine pathway and a consequent increase in QA levels have been identified in Alzheimer’s disease patients (el‐Defrawy et al, 1986; Guillemin et al, 2005). In addition, decreased QPRT activity, which also leads to QA accumulation, has been reported in brain tissue from individuals with epilepsy but not in normal human brain tissue (Feldblum et al, 1988), suggesting that some neurological disorders could be caused by impairment in QPRT function. However, whether QPRT impairment affects NAD+ levels in these patients has remained unknown.
From NaMN, the de novo pathway continues via adenylation to NaAD by the NMNATs, which are central enzymes also in the NAD+ salvage pathways (Fig 1). Among the three known NMNAT enzymes, mutations in the nuclear NMNAT isoform 1 have been identified in several studies as one of the many causes of an early‐onset and progressive form of Leber congenital amaurosis (LCA), a severe blinding retinal disease (Falk et al, 2012; Koenekoop et al, 2012; Perrault et al, 2012; Siemiatkowska et al, 2014; Nash et al, 2018). In these studies, individuals with LCA were found to carry homozygous or compound heterozygous nonsense, read‐through, or missense mutations predicted to cause partial loss of function of NMNAT1 (Table 1). Interestingly, these in silico predictions were validated through the detection of lower concentrations of NAD+ in red blood cells (Koenekoop et al, 2012) and fibroblasts (Falk et al, 2012) of affected individuals compared to controls, although in the latter case this difference was only borderline significant (P = 0.067). In these two studies, in vitro assays using wild‐type and mutant NMNAT1 proteins were also carried out, confirming a significantly reduced enzymatic activity of the most representative variants of NMNAT1 (Falk et al, 2012; Koenekoop et al, 2012). Interestingly, biochemical characterization of NMNAT1 mutants in a later study suggested that reduced NMNAT enzymatic activity may not be the leading cause of LCA onset since most of the LCA‐associated NMNAT1 variants did not lead to lower enzyme activities (Sasaki et al, 2015). At the same time, the secondary structure of many mutants was less stable, which led the authors to suggest that a combination of reduced activity and decreased protein stability under stressful conditions should be considered as the mechanism for retinal degeneration mediated by mutations in NMNAT1 (Sasaki et al, 2015).
The final step in the de novo NAD+ synthesis pathway is catalyzed by the glutamine‐dependent enzyme NADSYN. A study in five individuals with bi‐allelic deleterious variants in NADSYN1 (Table 1) reported the presence of vertebral, cardiac, renal, and limb defects derived from NAD+ deficiency (Szot et al, 2020). Via a genetic complementation approach in Saccharomyces cerevisiae lacking the NADSYN1 homolog QNS1, recombinant expression of wild‐type human NADSYN1 was able to rescue the growth rate deficiency and to restore NAD+ levels in the absence of the NAD+ precursor NR. In contrast, the NADSYN1 p.Ala573Thr variant, which represents the most common variant identified among the patients, failed to do so under the same conditions, suggesting that the p.Ala573Thr‐variant is truly causative. These results were further corroborated by measurement of NAD+ synthetase activity using affinity‐purified proteins. Interestingly, in vitro analysis showed that mutations in both the NAD+‐synthase and the glutaminase domains of the protein are expected to result in a reduced capacity to generate NAD+ in comparison with the wild type, clearly indicating that bi‐allelic loss‐of‐function variants of NADSYN1 are a cause of congenital NAD+ deficiency.
Impairment of de novo NAD+ biosynthesis at different levels is undoubtedly the cause of a variety of clinical conditions in humans. Therefore, it is tempting to speculate that loss‐of‐function mutations in other genes involved in NAD+ synthesis may also lead to similar conditions. These genes would include the different tryptophan transporters, such as the L‐amino acid transporters LAT1 and LAT2 (Kudo & Boyd, 2001; Vumma et al, 2011), or enzymes of the kynurenine pathway, such as IDO, TDO, AFMID, or KMO.
Importantly, loss of activity of NAD+ synthesizing enzymes may not be the only underlying cause of NAD+ deficiencies. Indeed, the high activity of α‐amino‐β‐carboxymuconate‐semialdehyde (ACMS) decarboxylase (ACMSD), an enzyme that decarboxylates ACMS, an upstream metabolite of quinolinic acid (QA), is considered a key regulator of the differences in dietary niacin requirements among species (Ikeda et al, 1965). Interestingly, overexpression of human ACMSD in mice generates a strong dependency on dietary niacin, which leads to NAD+ deficiency when these dietary requirements are not met (Palzer et al, 2018). Therefore, gain‐of‐function mutations in this enzyme might also contribute to reduced NAD+ synthesis in niacin‐deficient diets.
NAD+ salvage from precursors is also a main contributor to the total pool of NAD+ in cells (Houtkooper et al, 2010), although the only enzyme involved in NAD+ salvage that has been linked to human disease is NMNAT1, which also plays a role in the de novo NAD+ synthesis pathway. However, no specific mutations in other essential enzymes for NAD+ salvage have been reported to cause NAD+ deficiencies in humans so far. This includes (i) the NR, NMN, or NA transporters SLC29A1‐4, SLC12A8, SLC5A8, and SLC22A13, respectively, (ii) the NA, NAM, and NR‐recycling enzymes NAPRT, NAMPT, and NRK, (iii) the NRH‐processing enzyme AK, involved in NRH conversion to NAD+ (Giroud‐Gerbetant et al, 2019; Yang et al, 2020) (Fig 1), or (iv) the recently discovered mammalian mitochondrial NAD+ transporter SLC25A51 (Kory et al, 2020; Luongo et al, 2020) (Fig 2).
Other alterations of NAD+ metabolism
Whereas research on NAD+ metabolism and human disease has been mainly focused on its biosynthesis and degradation, the pathological implications of the damage and repair mechanisms of NAD+ metabolites have only recently been considered. NADH and NADPH can be damaged by hydration of one of the double bonds of the nicotinamide ring under specific stress conditions, such as increased temperature or acidic pH, leading to the formation of the aberrant forms NADHX and NADPHX (Fig 4). NADHX can also be formed by the activity of glyceraldehyde 3‐phosphate dehydrogenase (Rafter et al, 1954). NAD(P)HX can spontaneously react to cyclic NAD(P)HX in an irreversible way (Fig 4). The resulting damaged/aberrant cofactors cannot act as electron carriers, and inhibit key dehydrogenase enzymes, at least in vitro (Yoshida & Dave, 1975; Prabhakar et al, 1998). To prevent their toxic effects, NADHX and NADPHX are detoxified by the sequential activity of the NAD(P)HX epimerase (NAXE), which converts the R‐NAD(P)HX epimers to their S forms, and the NAD(P)HX dehydratase (NAXD), which converts S‐NAD(P)HX back to NAD(P)H in an ATP‐dependent manner.
Figure 4. NAD(P)HX production and repair systems.
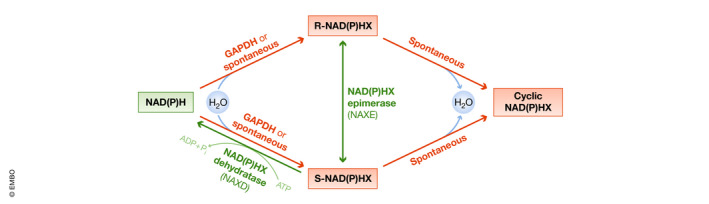
Via the activity of GAPDH or spontaneously under mild acidic conditions or high temperatures, NADH and NADPH can be hydrated to the R or S forms of their toxic metabolites NAD(P)HX, which can be irreversibly converted to cyclic NAD(P)HX. To avoid the accumulation of these potentially harmful intermediates, R‐NAD(P)HX are epimerized to their S forms through the activity of NAXE. S‐NAD(P)HX is then detoxified to NAD(P)H via the ATP‐dependent activity of NAXD.
Inherited deficiencies of NAXE or NAXD cause fever‐induced, severe multisystemic disorders, including ataxia, cerebellar edema, respiratory insufficiency, and skin lesions, followed by rapid neurological deterioration and premature demise (Kremer et al, 2016; Spiegel et al, 2016; Van Bergen et al, 2019; Borna et al, 2020; Zhou et al, 2020). In two different studies with a total of eleven subjects, homozygous or compound heterozygous mutations in NAXE caused devastating neurological disorders (Kremer et al, 2016; Spiegel et al, 2016). While NAD(H) levels were unaltered in NAXE‐deficient fibroblasts, the R and S epimers of NADHX were found to accumulate, together with its cyclic form, for which no repair system is known (Kremer et al, 2016). This was accompanied by reduced activity of respiratory chain complex I and pyruvate oxidation in isolated mitochondria from fresh muscle biopsies (Kremer et al, 2016). Together with the accumulation of lactate measured in cerebrospinal fluid, these results clearly point toward impaired mitochondrial energy metabolism in NAXE deficiency.
Mutations in the NAXD gene have also been associated with accumulation of aberrant metabolites and neurological disorders. Three independent studies have demonstrated the consequences of NAXD deficiency (Van Bergen et al, 2019; Borna et al, 2020; Zhou et al, 2020), which resemble those found in patients with NAXE deficiency. These include accumulation of NADHX but unaltered NAD(P)H levels (Van Bergen et al, 2019), complex I deficiencies (Van Bergen et al, 2019; Borna et al, 2020), and reduced cytochrome c oxidase activity (Van Bergen et al, 2019). Interestingly, lentiviral transduction of the cytosolic or mitochondrial forms of NAXD completely reversed NADHX accumulation in patient fibroblasts.
In vitro, mutated variants of NAXD showed reduced protein expression (Borna et al, 2020) and loss of enzymatic activity, especially at temperatures higher than 30 °C (Van Bergen et al, 2019), suggesting that protein instability at relatively high temperatures may be the molecular mechanism behind fever episodes as common inductors of these diseases.
Altogether, the different studies presented in this review illustrate the importance of maintaining NAD+ and NADH homeostasis not only through a careful balance between their synthesis, consumption, and interconversion, but also through essential repairing mechanisms that detoxify their aberrant forms.
Therapeutic strategies aimed at alleviating NAD+ deficiency in humans
The identification of NAD+ deficiency as the underlying cause of several human pathologies makes NAD+ metabolism an appealing therapeutic target. In fact, during the last decade, strategies to increase NAD+ levels to fight disease have attracted the attention of the research community (Houtkooper & Auwerx, 2012; Katsyuba & Auwerx, 2017; Connell et al, 2019).
Strategies to boost NAD+ levels fall into two categories: (i) inhibition of NAD+ consumption, and (ii) stimulation of NAD+ biosynthesis. Inhibition of NAD+ consumption has been proven effective in raising NAD+ levels in preclinical studies (Bai et al, 2011; Escande et al, 2013; Pirinen et al, 2014; Haffner et al, 2015). However, in humans this approach has mainly focused on the use of non‐specific CD38 inhibitors, such as quercetin, to improve cardiometabolic parameters (Pfeuffer et al, 2013; Dower et al, 2015; Menezes et al, 2017), or the administration of PARP inhibitors for the treatment of ovarian and breast cancers (Murthy & Muggia, 2019), but their NAD+‐boosting effects are still to be determined. The approach that has attracted most attention in clinical research involves stimulation of NAD+ synthesis by supplementation of NAD+ precursors. In fact, a large number of clinical trials have been carried out with the NAD+ precursors NA, NAM, NR, and, to a much lesser extent, NMN (Connell et al, 2019; Katsyuba et al, 2020).
The therapeutic effects of NA and NAM in humans have long been known, as they were established as the curative agents for pellagra and the pellagra‐like disorder Hartnup disease (Smith et al, 1937; Elvehjem et al, 1974; Patel & Prabhu, 2008). Apart from its role as an NAD+ precursor, the metabolic effects triggered by NA can also occur through its interaction with the Gi protein‐coupled receptor GPR109A (HM74A in humans). Through this mechanism, NA and its derivative acipimox act as lipid‐ and cholesterol‐lowering agents in humans (Sirtori et al, 1981; Taskinen & Nikkila, 1988; Berge & Canner, 1991; Brown et al, 2001; Westphal et al, 2006). Moreover, these molecules have been described to have potential benefits for chronic kidney disease (Restrepo Valencia & Cruz, 2008; Jin Kang et al, 2013) and the physiological functioning of mitochondria in muscle of subjects with type 2 diabetes (van de Weijer et al, 2015). NA supplementation has also been proposed to alleviate the relapse of the skin lesions in NAXD deficiency, although this study was limited to one subject and a 4‐month follow‐up period (Zhou et al, 2020). Interestingly, NA also restored systemic NAD+ deficiency, leading to improved muscle performance in patients with mitochondrial myopathy (Pirinen et al, 2020). In fact, the latter study is the first to demonstrate that induction of NAD+ levels improves muscle performance in human subjects.
The therapeutic potential of the NAD+ precursors NR and NMN has also been explored in a variety of clinical trials, especially in the case of NR. In contrast to NA, which induces a painful flushing reaction (Benyo et al, 2005), neither NMN nor NR have been reported to cause any adverse effect (Conze et al, 2019), even after a single oral dose of 0.5 g of NMN (Irie et al, 2020) or supplementation with NR for 3 months at doses as high as 2 g per day (Dollerup et al, 2018). However, the promising therapeutic effects observed in preclinical animal models have so far been difficult to translate to humans, where the beneficial effects of NMN and NR are mild. In fact, although NR lowers circulating inflammatory cytokines (Elhassan et al, 2019), increases acetylcarnitine concentrations in skeletal muscle, and increases the sleeping metabolic rate to some extent (Remie et al, 2020), it has no effects on overall activity, exercise performance, motor function, or mitochondrial bioenergetics (Dollerup et al, 2018; Martens et al, 2018; Elhassan et al, 2019; Dollerup et al, 2020). Furthermore, NR proved unable to improve glucose tolerance, β‐cell secretory capacity, and incretin hormone secretion in response to a glucose challenge in nondiabetic obese individuals (Dollerup et al, 2019). This lack of beneficial effects in humans may be in part related to the inability of NMN and NR to enhance NAD+ levels in human tissues. Admittedly, although an increase in whole blood NAD+ content was reported upon NR administration (Trammell et al, 2016; Airhart et al, 2017), supplementation with this precursor has failed to increase NAD+ in other tissues, such as muscle (Elhassan et al, 2019; Dollerup et al, 2020), even after administration of 1 g per day during 6 weeks (Remie et al, 2020). Such inefficacy of NMN and NR in enhancing NAD+ levels in humans could be due to the duration of the studies, which may be insufficient to achieve a clinical benefit, or with the experimental setup, which has been mainly focused on healthy subjects with normal basal NAD+ levels.
Conclusions and future directions
The recognition that NAD+ deficiency is linked to human disease has led to a marked interest in this molecule and burgeoning of this broad and exciting field, even though the full translational and therapeutic power of repletion therapies remains to be explored. We expect that a considerable number of human diseases caused by monogenic defects in NAD+ metabolism remain to be discovered. Also for common diseases, it seems likely that additional pathologies in which reduced NAD+ plays a role in the pathophysiology will be discovered. Such findings would give way to new therapeutic strategies, such as supplementation with NAD+ precursors.
Future studies will need to elucidate the therapeutic potential and effects of NAD+ replenishment therapies for NAD+ deficiency conditions, especially now that NAD+ has arisen as the underlying cause of a number of different human diseases. Available preclinical and clinical data are promising though, as NA has already been proven to ameliorate systemic NAD+ deficiency in humans caused by mitochondrial myopathy (Pirinen et al, 2020) and to counteract the embryogenic defects of pathogenic variants of Haao and Kynu in mice (Shi et al, 2017). NAD+ enhancers are, therefore, a very interesting therapeutic option for those patients in whom impaired NAD+ metabolism is detected. This could apply not only to HAAO‐ and KYNU‐deficient patients, but also to other disorders such as those caused by a deficiency of GLUL (Hu et al, 2015) or NADSYN (Szot et al, 2020), which could be overcome by leveraging the NADSYN1‐independent recycling pathway from NAM, NMN, or NR.
Although attempts to replenish NAD+ content with NA in fibroblasts from a patient with Leber congenital amaurosis caused by NMNAT1 mutations were unsuccessful (Falk et al, 2012), the potential of other NAD+‐boosting molecules warrants further research. Indeed, patients suffering from this disorder could benefit from this therapy, as supplementation with NAD+ precursors or NAD+‐consumption inhibitors could ensure enough substrate for NMNAT1 to overcome its reduced enzymatic function.
In light of the current evidence on the use of NAD+ replenishment therapies to treat human disease, future clinical trials should aim at establishing which precursor is best for each of these conditions. Surely, there is still a long way to go until a definitive cure for NAD+ deficiencies is found, but all the odds are on our side.
Pending issues
Identifying underlying NAD+ deficiencies in common and/or NAD+ metabolism‐related pathologies could lead to new therapeutic strategies for these diseases.
NAD+‐replenishment therapies should be tested in patients with suspected or confirmed NAD+ deficiency, which includes HAAO‐ and KYNU‐deficient patients
The pathophysiological role of many genes involved in NAD+ metabolism has not yet been elucidated. New studies attempting to associate mutations in these genes to human disease would be specially interesting.
New therapeutic avenues aimed to alleviate the consequences of NAD(P)HX accumulation should be explored, e.g., through gene replacement therapies.
Author contributions
RZ‐P, RJAW, CDMvK, and RHH contributed to the conception, the writing, and the editing of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
RZ‐P was supported by a postdoctoral grant from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement number 840110. Work in the Houtkooper group is financially supported by an ERC Starting grant (no. 638290) and a VIDI grant from ZonMw (no. 91715305).
EMBO Mol Med (2021) 13: e13943.
See the Glossary for abbreviations used in this article.
References
- Abdelraheim SR, Spiller DG, McLennan AG (2003) Mammalian NADH diphosphatases of the Nudix family: cloning and characterization of the human peroxisomal NUDT12 protein. Biochem J 374: 329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelraheim SR, Spiller DG, McLennan AG (2017) Mouse Nudt13 is a mitochondrial nudix hydrolase with NAD(P)H pyrophosphohydrolase activity. Protein J 36: 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, Tian R, Shen DD, O'Brien KD (2017) An open‐label, non‐randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One 12: e0186459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy P, White TA, Thompson M, Chini EN (2006) Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun 345: 1386–1392 [DOI] [PubMed] [Google Scholar]
- Ansari HR, Raghava GP (2010) Identification of NAD interacting residues in proteins. BMC Bioinform 11: 160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper R et al (2011) PARP‐1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13: 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai P, Canto C (2012) The role of PARP‐1 and PARP‐2 enzymes in metabolic regulation and disease. Cell Metab 16: 290–295 [DOI] [PubMed] [Google Scholar]
- Barth S, Klein P, Horbach T, Dotsch J, Rauh M, Rascher W, Knerr I (2010) Expression of neuropeptide Y, omentin and visfatin in visceral and subcutaneous adipose tissues in humans: relation to endocrine and clinical parameters. Obes Facts 3: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger AM, Przybylska M, Gefteas E, Furgerson M, Geller S, Kloss A, Cheng SH, Zhu Y, Yew NS (2018) Inhibiting neutral amino acid transport for the treatment of phenylketonuria. JCI Insight 3: e121762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender DA (1983) Biochemistry of tryptophan in health and disease. Mol Asp Med 6: 101–197 [DOI] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K, Offermanns S (2005) GPR109A (PUMA‐G/HM74A) mediates nicotinic acid‐induced flushing. J Clin Invest 115: 3634–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge KG, Canner PL (1991) Coronary drug project: experience with niacin. Coronary Drug Project Research Group. Eur J Clin Pharmacol 40(Suppl 1): S49–S51 [PubMed] [Google Scholar]
- Borna NN, Kishita Y, Abe J, Furukawa T, Ogawa‐Tominaga M, Fushimi T, Imai‐Okazaki A, Takeda A, Ohtake A, Murayama K et al (2020) NAD(P)HX dehydratase protein‐truncating mutations are associated with neurodevelopmental disorder exacerbated by acute illness. Brain 143: e54 [DOI] [PubMed] [Google Scholar]
- Borst P (2020) The malate‐aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life 72: 2241–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BG, Zhao X‐Q, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P et al (2001) Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 345: 1583–1592 [DOI] [PubMed] [Google Scholar]
- Camacho‐Pereira J, Tarrago MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A et al (2016) CD38 dictates age‐related NAD decline and mitochondrial dysfunction through an SIRT3‐dependent mechanism. Cell Metab 23: 1127–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne XA, Stewart ML, Kim D, Jones‐Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH (2016) Biosensor reveals multiple sources for mitochondrial NAD(+). Science 352: 1474–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Houtkooper R, Pirinen E, Youn D, Oosterveer M, Cen Y, Fernandez‐Marcos P, Yamamoto H, Andreux PA, Cettour‐Rose P et al (2012) The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high‐fat diet‐induced obesity. Cell Metab 15: 838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Sauve AA, Bai P (2013) Crosstalk between poly(ADP‐ribose) polymerase and sirtuin enzymes. Mol Asp Med 34: 1168–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Menzies KJ, Auwerx J (2015) NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 22: 31–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve A, Li W, Leoni V, Schon E, Dantzer F, Auwerx J et al (2014) NAD(+)‐dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab 19: 1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25: 138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon CK, Lee BH, Ko JM, Kim HJ, Yoo HW (2010) Novel mutation in SLC6A19 causing late‐onset seizures in Hartnup disorder. Pediatr Neurol 42: 369–371 [DOI] [PubMed] [Google Scholar]
- Connell NJ, Houtkooper RH, Schrauwen P (2019) NAD(+) metabolism as a target for metabolic health: have we found the silver bullet? Diabetologia 62: 888–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze D, Brenner C, Kruger CL (2019) Safety and metabolism of long‐term administration of NIAGEN (Nicotinamide Riboside Chloride) in a randomized, double‐blind, placebo‐controlled clinical trial of healthy overweight adults. Sci Rep 9: 9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl TB, Haukeland JW, Yndestad A, Ranheim T, Gladhaug IP, Damas JK, Haaland T, Loberg EM, Arntsen B, Birkeland K et al (2010) Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down‐regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 95: 3039–3047 [DOI] [PubMed] [Google Scholar]
- Di Lisa F, Ziegler M (2001) Pathophysiological relevance of mitochondria in NAD(+) metabolism. FEBS Lett 492: 4–8 [DOI] [PubMed] [Google Scholar]
- Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, Stødkilde‐Jørgensen H, Møller N, Brenner C, Treebak JT et al (2018) A randomized placebo‐controlled clinical trial of nicotinamide riboside in obese men: safety, insulin‐sensitivity, and lipid‐mobilizing effects. Am J Clin Nutr 108: 343–353 [DOI] [PubMed] [Google Scholar]
- Dollerup OL, Trammell SAJ, Hartmann B, Holst JJ, Christensen B, Moller N, Gillum MP, Treebak JT, Jessen N (2019) Effects of nicotinamide riboside on endocrine pancreatic function and incretin hormones in nondiabetic men with obesity. J Clin Endocrinol Metab 104: 5703–5714 [DOI] [PubMed] [Google Scholar]
- Dollerup OL, Chubanava S, Agerholm M, Søndergård SD, Altıntaş A, Møller AB, Høyer KF, Ringgaard S, Stødkilde‐Jørgensen H, Lavery GG et al (2020) Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin‐resistant men. J Physiol 598: 731–754 [DOI] [PubMed] [Google Scholar]
- Dower JI, Geleijnse JM, Gijsbers L, Zock PL, Kromhout D, Hollman PC (2015) Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double‐blind, placebo‐controlled, crossover trial. Am J Clin Nutr 101: 914–921 [DOI] [PubMed] [Google Scholar]
- el‐Defrawy SR, Boegman RJ, Jhamandas K, Beninger RJ (1986) The neurotoxic actions of quinolinic acid in the central nervous system. Can J Physiol Pharmacol 64: 369–375 [DOI] [PubMed] [Google Scholar]
- Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, Cartwright DM, Oakey L, Burley CV, Jenkinson N et al (2019) Nicotinamide riboside augments the aged human skeletal muscle NAD(+) metabolome and induces transcriptomic and anti‐inflammatory signatures. Cell Rep 28: 1717–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvehjem CA, Madden RJ, Strong FM, Woolley DW (1937) Relation of nicotinic acid and nicotinic acid amide to canine black tongue. J Am Chem Soc 59: 1767–1768 [Google Scholar]
- Elvehjem CA, Madden RJ, Strong FM, Woolley DW (1974) The isolation and identification of the anti‐black tongue factor. Nutr Rev 32: 48–50 [DOI] [PubMed] [Google Scholar]
- Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O'Neil L, White TA, Sinclair DA, Chini EN (2013) Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes 62: 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J (2017) The SARM1 Toll/Interleukin‐1 receptor domain possesses intrinsic NAD(+) cleavage activity that promotes pathological axonal degeneration. Neuron 93: 1334–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MJ, Zhang Q, Nakamaru‐Ogiso E, Kannabiran C, Fonseca‐Kelly Z, Chakarova C, Audo I, Mackay DS, Zeitz C, Borman AD et al (2012) NMNAT1 mutations cause Leber congenital amaurosis. Nat Genet 44: 1040–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Lautrup S, Hou Y, Demarest TG, Croteau DL, Mattson MP, Bohr VA (2017) NAD(+) in aging: molecular mechanisms and translational implications. Trends Mol Med 23: 899–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum S, Rougier A, Loiseau H, Loiseau P, Cohadon F, Morselli PL, Lloyd KG (1988) Quinolinic‐phosphoribosyl transferase activity is decreased in epileptic human brain tissue. Epilepsia 29: 523–529 [DOI] [PubMed] [Google Scholar]
- Gaddipati R, Sasikala M, Padaki N, Mukherjee RM, Sekaran A, Jayaraj‐Mansard M, Rabella P, Rao‐Guduru V, Reddy‐Duvvuru N (2010) Visceral adipose tissue visfatin in nonalcoholic fatty liver disease. Ann Hepatol 9: 266–270 [PubMed] [Google Scholar]
- Garavaglia S, Bruzzone S, Cassani C, Canella L, Allegrone G, Sturla L, Mannino E, Millo E, De Flora A, Rizzi M (2012) The high‐resolution crystal structure of periplasmic Haemophilus influenzae NAD nucleotidase reveals a novel enzymatic function of human CD73 related to NAD metabolism. Biochem J 441: 131–141 [DOI] [PubMed] [Google Scholar]
- Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V et al (2016) Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatol 63: 1190–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W (2015) Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11: 535–546 [DOI] [PubMed] [Google Scholar]
- Gaudino F, Manfredonia I, Manago A, Audrito V, Raffaelli N, Vaisitti T, Deaglio S (2019) Subcellular characterization of nicotinamide adenine dinucleotide biosynthesis in metastatic melanoma by using organelle‐specific biosensors. Antioxid Redox Signal 31: 1150–1165 [DOI] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J (2013) Sarm1‐mediated axon degeneration requires both SAM and TIR interactions. J Neurosci 33: 13569–13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Levault KR, Brewer GJ (2014) Relative importance of redox buffers GSH and NAD(P)H in age‐related neurodegeneration and Alzheimer disease‐like mouse neurons. Aging Cell 13: 631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud‐Gerbetant J, Joffraud M, Giner MP, Cercillieux A, Bartova S, Makarov MV, Zapata‐Pérez R, Sánchez‐García JL, Houtkooper RH, Migaud ME et al (2019) A reduced form of nicotinamide riboside defines a new path for NAD(+) biosynthesis and acts as an orally bioavailable NAD(+) precursor. Mol Metab 30: 192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Mills KF, Yoshino J, Bruzzone S, Sociali G, Tokizane K, Lei HC, Cunningham R, Sasaki Y, Migaud ME et al (2019) Slc12a8 is a nicotinamide mononucleotide transporter. Nat Metab 1: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, Bruzzone S (2013) CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866‐treated tumor cells. J Biol Chem 288: 25938–25949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guia RM, Agerholm M, Nielsen TS, Consitt LA, Sogaard D, Helge JW, Larsen S, Brandauer J, Houmard JA, Treebak JT (2019) Aerobic and resistance exercise training reverses age‐dependent decline in NAD(+) salvage capacity in human skeletal muscle. Physiol Rep 7: e14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM (2005) Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropatol Appl Neurobiol 31: 395–404 [DOI] [PubMed] [Google Scholar]
- Haberle J, Shahbeck N, Ibrahim K, Hoffmann GF, Ben‐Omran T (2011) Natural course of glutamine synthetase deficiency in a 3 year old patient. Mol Genet Metab 103: 89–91 [DOI] [PubMed] [Google Scholar]
- Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR et al (2015) Discovery, synthesis, and biological evaluation of Thiazoloquin(az)olin(on)es as potent CD38 inhibitors. J Med Chem 58: 3548–3571 [DOI] [PubMed] [Google Scholar]
- Harden A, Young WJ (1906) The alcoholic ferment of yeast‐juice. Proc R Soc Lond B 77: 405–420 [Google Scholar]
- Houtkooper RH, Canto C, Wanders RJ, Auwerx J (2010) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 31: 194–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Auwerx J (2012) Exploring the therapeutic space around NAD+. J Cell Biol 199: 205–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Ibrahim K, Stucki M, Frapolli M, Shahbeck N, Chaudhry FA, Görg B, Häussinger D, Penberthy WT, Ben‐Omran T et al (2015) Secondary NAD+ deficiency in the inherited defect of glutamine synthetase. J Inherit Metab Dis 38: 1075–1083 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O (1965) Studies on the biosynthesis of nicotinamide adenine dinucleotide. Ii. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals. J Biol Chem 240: 1395–1401 [PubMed] [Google Scholar]
- Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, Yamashita K, Shigaki S, Ono T, Yukioka H et al (2020) Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J 67: 153–160 [DOI] [PubMed] [Google Scholar]
- Jin Kang H, Kim DK, Mi Lee S, Han Kim K, Hee Han S, Hyun Kim K, Eun Kim S, Ki Son Y, An WS (2013) Effects of low‐dose niacin on dyslipidemia and serum phosphorus in patients with chronic kidney disease. Kidney Res Clin Pract 32: 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukarainen S, Heinonen S, Ramo JT, Rinnankoski‐Tuikka R, Rappou E, Tummers M, Muniandy M, Hakkarainen A, Lundbom J, Lundbom N et al (2016) Obesity Is associated with low NAD(+)/SIRT pathway expression in adipose tissue of BMI‐discordant monozygotic twins. J Clin Endocrinol Metab 101: 275–283 [DOI] [PubMed] [Google Scholar]
- Katsyuba E, Auwerx J (2017) Modulating NAD(+) metabolism, from bench to bedside. EMBO J 36: 2670–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L, Matilainen O, Liscio P et al (2018) De novo NAD(+) synthesis enhances mitochondrial function and improves health. Nature 563: 354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsyuba E, Romani M, Hofer D, Auwerx J (2020) NAD+ homeostasis in health and disease. Nat Metab 2: 9–31 [DOI] [PubMed] [Google Scholar]
- Kleta R, Romeo E, Ristic Z, Ohura T, Stuart C, Arcos‐Burgos M, Dave MH, Wagner CA, Camargo SRM, Inoue S et al (2004) Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet 36: 999–1002 [DOI] [PubMed] [Google Scholar]
- Koenekoop RK, Wang H, Majewski J, Wang X, Lopez I, Ren H, Chen Y, Li Y, Fishman GA, Genead M et al (2012) Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat Genet 44: 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory N, uit de Bos J, van der Rijt S, Jankovic N, Güra M, Arp N, Pena IA, Prakash G, Chan SH, Kunchok T et al (2020) MCART1/SLC25A51 is required for mitochondrial NAD transport. Sci Adv 6: eabe5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer LS, Danhauser K, Herebian D, Petkovic Ramadža D, Piekutowska‐Abramczuk D, Seibt A, Müller‐Felber W, Haack TB, Płoski R, Lohmeier K et al (2016) NAXE mutations disrupt the cellular NAD(P)HX repair system and cause a lethal neurometabolic disorder of early childhood. Am J Hum Genet 99: 894–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA (2001) Characterisation of L‐tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J Physiol 531: 405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Hong YS, Jun W, Yang SJ (2015) Nicotinamide riboside ameliorates hepatic metaflammation by modulating NLRP3 inflammasome in a rodent model of type 2 diabetes. J Med Food 18: 1207–1213 [DOI] [PubMed] [Google Scholar]
- Leung AKL (2017) PARPs. Curr Biol 27: R1256–R1258 [DOI] [PubMed] [Google Scholar]
- Liaudet L (2002) Poly(adenosine 5'‐diphosphate) ribose polymerase activation as a cause of metabolic dysfunction in critical illness. Curr Opin Clin Nutr Metab Care 5: 175–184 [DOI] [PubMed] [Google Scholar]
- Liu LY, Wang F, Zhang XY, Huang P, Lu YB, Wei EQ, Zhang WP (2012) Nicotinamide phosphoribosyltransferase may be involved in age‐related brain diseases. PLoS One 7: e44933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Jiang H, Lee JH, Zhang G, Chen X, Kawamoto EM, Mattson MP (2013) Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging 34: 1564–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo‐Huitron R, Ugalde Muniz P, Pineda B, Pedraza‐Chaverri J, Rios C, Perez‐de la Cruz V (2013) Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev 2013: 104024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo TS, Eller JM, Lu M‐J, Niere M, Raith F, Perry C, Bornstein MR, Oliphint P, Wang L, McReynolds MR et al (2020) SLC25A51 is a mammalian mitochondrial NAD(+) transporter. Nature 588: 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 88: 841–886 [DOI] [PubMed] [Google Scholar]
- Mandir AS, Przedborski S, Jackson‐Lewis V, Wang ZQ, Simbulan‐Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM (1999) Poly(ADP‐ribose) polymerase activation mediates 1‐methyl‐4‐phenyl‐1, 2,3,6‐tetrahydropyridine (MPTP)‐induced parkinsonism. Proc Natl Acad Sci USA 96: 5774–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR (2018) Chronic nicotinamide riboside supplementation is well‐tolerated and elevates NAD(+) in healthy middle‐aged and older adults. Nat Commun 9: 1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ (2012) Age‐associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One 7: e42357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes R, Rodriguez‐Mateos A, Kaltsatou A, Gonzalez‐Sarrias A, Greyling A, Giannaki C, Andres‐Lacueva C, Milenkovic D, Gibney ER, Dumont J et al (2017) Impact of flavonols on cardiometabolic biomarkers: a meta‐analysis of randomized controlled human trials to explore the role of inter‐individual variability. Nutrients 9: 117 [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I (2005) Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell 16: 4623–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K et al (2016) Long‐term administration of nicotinamide mononucleotide mitigates age‐associated physiological decline in mice. Cell Metab 24: 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Auwerx J (2013a) NAD(+) metabolism: a therapeutic target for age‐related metabolic disease. Crit Rev Biochem Mol Biol 48: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper R, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo Y‐S, Viswanathan M, Schoonjans K et al (2013b) The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154: 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy P, Muggia F (2019) PARP inhibitors: clinical development, emerging differences, and the current therapeutic issues. Cancer Drug Resist 2: 665–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash BM, Symes R, Goel H, Dinger ME, Bennetts B, Grigg JR, Jamieson RV (2018) NMNAT1 variants cause cone and cone‐rod dystrophy. Eur J Hum Genet 26: 428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov A, Dolle C, Niere M, Ziegler M (2011) Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem 286: 21767–21778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K, Yaku K, Tobe K, Nakagawa T (2019) Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci 26: 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolan E, Augeri S, Fissolo G, Musso I, Funaro A (2019) CD157: From immunoregulatory protein to potential therapeutic target. Immunol Lett 205: 59–64 [DOI] [PubMed] [Google Scholar]
- Palzer L, Bader JJ, Angel F, Witzel M, Blaser S, McNeil A, Wandersee MK, Leu NA, Lengner CJ, Cho CE et al (2018) Alpha‐amino‐beta‐carboxy‐muconate‐semialdehyde decarboxylase controls dietary niacin requirements for NAD(+) synthesis. Cell Rep 25: 1359–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AB, Prabhu AS (2008) Hartnup disease. Indian J Dermatol 53: 31–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Hanein S, Zanlonghi X, Serre V, Nicouleau M, Defoort‐Delhemmes S, Delphin N, Fares‐Taie L, Gerber S, Xerri O et al (2012) Mutations in NMNAT1 cause Leber congenital amaurosis with early‐onset severe macular and optic atrophy. Nat Genet 44: 975–977 [DOI] [PubMed] [Google Scholar]
- Pfeuffer M, Auinger A, Bley U, Kraus‐Stojanowic I, Laue C, Winkler P, Rufer CE, Frank J, Bosch‐Saadatmandi C, Rimbach G et al (2013) Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr Metab Cardiovasc Dis 23: 403–409 [DOI] [PubMed] [Google Scholar]
- Pirinen E, Auranen M, Khan NA, Brilhante V, Urho N, Pessia A, Hakkarainen A, Kuula J, Heinonen U, Schmidt MS et al (2020) Niacin cures systemic NAD(+) deficiency and improves muscle performance in adult‐onset mitochondrial myopathy. Cell Metab 31: 1078–1090 [DOI] [PubMed] [Google Scholar]
- Pirinen E, Cantó C, Jo Y, Morato L, Zhang H, Menzies K, Williams E, Mouchiroud L, Moullan N, Hagberg C et al (2014) Pharmacological Inhibition of poly(ADP‐ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab 19: 1034–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar P, Laboy JI, Wang J, Budker T, Din ZZ, Chobanian M, Fahien LA (1998) Effect of NADH‐X on cytosolic glycerol‐3‐phosphate dehydrogenase. Arch Biochem Biophys 360: 195–205 [DOI] [PubMed] [Google Scholar]
- Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F (2013) CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom 84: 207–217 [DOI] [PubMed] [Google Scholar]
- Rafter GW, Chaykin S, Krebs EG (1954) The action of glyceraldehyde‐3‐phosphate dehydrogenase on reduced diphosphopyridine nucleotide. J Biol Chem 208: 799–811 [PubMed] [Google Scholar]
- Rajman L, Chwalek K, Sinclair DA (2018) Therapeutic potential of NAD‐boosting molecules: the in vivo evidence. Cell Metab 27: 529–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remie CME, Roumans KHM, Moonen MPB, Connell NJ, Havekes B, Mevenkamp J, Lindeboom L, de Wit VHW, van de Weijer T, Aarts SABM et al (2020) Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am J Clin Nutr 112: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo Valencia CA, Cruz J (2008) Safety and effectiveness of nicotinic acid in the management of patients with chronic renal disease and hyperlipidemia associated to hyperphosphatemia. Nefrologia 28: 61–66 [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR et al (2007) Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6: 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D, Zhang H, Ropelle ER, Sorrentino V, Mazala D, Mouchiroud L, Marshall PL, Campbell MD, Ali AS, Knowels GM et al (2016) NAD+ repletion improves muscle function in muscular dystrophy and counters global PARylation. Sci Transl Med 8: 361ra139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Margolin Z, Borgo B, Havranek JJ, Milbrandt J (2015) Characterization of leber congenital amaurosis‐associated NMNAT1 mutants. J Biol Chem 290: 17228–17238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöndorf DC, Ivanyuk D, Baden P, Sanchez‐Martinez A, De Cicco S, Yu C, Giunta I, Schwarz LK, Di Napoli G, Panagiotakopoulou V et al (2018) The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson's disease. Cell Rep 23: 2976–2988 [DOI] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, de Murcia G (2006) Poly(ADP‐ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7: 517–528 [DOI] [PubMed] [Google Scholar]
- Seow HF, Broer S, Broer A, Bailey CG, Potter SJ, Cavanaugh JA, Rasko JE (2004) Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet 36: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN et al (2017) NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med 377: 544–552 [DOI] [PubMed] [Google Scholar]
- Siemiatkowska AM, van den Born LI, van Genderen MM, Bertelsen M, Zobor D, Rohrschneider K, van Huet RA, Nurohmah S, Klevering BJ, Kohl S et al (2014) Novel compound heterozygous NMNAT1 variants associated with Leber congenital amaurosis. Mol Vis 20: 753–759 [PMC free article] [PubMed] [Google Scholar]
- Sirtori CR, Gianfranceschi G, Sirtori M, Bernini F, Descovich G, Montaguti U, Fuccella LM, Musatti L (1981) Reduced triglyceridemia and increased high density lipoprotein cholesterol levels after treatment with acipimox, a new inhibitor of lipolysis. Atherosclerosis 38: 267–271 [DOI] [PubMed] [Google Scholar]
- Smith DT, Ruffin JM, Smith SG (1937) Pellagra successfully treated with nicotinic acid: a case report. JAMA 109: 2054–2055 [Google Scholar]
- Son MJ, Kwon Y, Son T, Cho YS (2016) Restoration of mitochondrial NAD(+) levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cells 34: 2840–2851 [DOI] [PubMed] [Google Scholar]
- Spiegel R, Shaag A, Shalev S, Elpeleg O (2016) Homozygous mutation in the APOA1BP is associated with a lethal infantile leukoencephalopathy. Neurogenetics 17: 187–190 [DOI] [PubMed] [Google Scholar]
- Szot JO, Campagnolo C, Cao Y, Iyer KR, Cuny H, Drysdale T, Flores‐Daboub JA, Bi W, Westerfield L, Liu P et al (2020) Bi‐allelic mutations in NADSYN1 cause multiple organ defects and expand the genotypic spectrum of congenital NAD deficiency disorders. Am J Hum Genet 106: 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmoush AJ, Alpers DH, Feigin RD, Armbrustmacher V, Prensky AL (1976) Hartnup disease. Clinical, pathological, and biochemical observations. Arch Neurol 33: 797–807 [DOI] [PubMed] [Google Scholar]
- Taskinen MR, Nikkila EA (1988) Effects of acipimox on serum lipids, lipoproteins and lipolytic enzymes in hypertriglyceridemia. Atherosclerosis 69: 249–255 [DOI] [PubMed] [Google Scholar]
- Tempel W, Rabeh WM, Bogan KL, Belenky P, Wojcik M, Seidle HF, Nedyalkova L, Yang T, Sauve AA, Park H‐W et al (2007) Nicotinamide riboside kinase structures reveal new pathways to NAD+. PLoS Biol 5: e263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro JS, Rolo AP, Palmeira CM (2013) The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol Mech Methods 23: 297–302 [DOI] [PubMed] [Google Scholar]
- Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C (2016) Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7: 12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bergen NJ, Guo Y, Rankin J, Paczia N, Becker‐Kettern J, Kremer LS, Pyle A, Conrotte J‐F, Ellaway C, Procopis P et al (2019) NAD(P)HX dehydratase (NAXD) deficiency: a novel neurodegenerative disorder exacerbated by febrile illnesses. Brain 142: 50–58 [DOI] [PubMed] [Google Scholar]
- Vumma R, Johansson J, Lewander T, Venizelos N (2011) Tryptophan transport in human fibroblast cells‐a functional characterization. Int J Tryptophan Res 4: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Nong Z, Yin H, O'Neil C, Fox S, Balint B, Guo L, Leo O, Chu MWA, Gros R et al (2017) Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration, and is suppressed in human thoracic aortic aneurysm disease. Circ Res 120: 1889–1902 [DOI] [PubMed] [Google Scholar]
- Wei W, Graeff R, Yue J (2014) Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca(2+) signaling pathway. World J Biol Chem 5: 58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, Nowotny P, Sparks LM, Paglialunga S et al (2015) Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 64: 1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal S, Borucki K, Taneva E, Makarova R, Luley C (2006) Adipokines and treatment with niacin. Metabolism 55: 1283–1285 [DOI] [PubMed] [Google Scholar]
- Williamson DH, Lund P, Krebs HA (1967) The redox state of free nicotinamide‐adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Wang RS, Handy DE, Loscalzo J (2018) NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal 28: 251–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaku K, Okabe K, Nakagawa T (2018) NAD metabolism: implications in aging and longevity. Ageing Res Rev 47: 1–17 [DOI] [PubMed] [Google Scholar]
- Yang Y, Sauve AA (2016) NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim Biophys Acta 1864: 1787–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mohammed FS, Zhang N, Sauve AA (2019) Dihydronicotinamide riboside is a potent NAD(+) concentration enhancer in vitro and in vivo. J Biol Chem 294: 9295–9307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang N, Zhang G, Sauve AA (2020) NRH salvage and conversion to NAD+ requires NRH kinase activity by adenosine kinase. Nat Metab 2: 364–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10: 179–206 [DOI] [PubMed] [Google Scholar]
- Ylikallio E, Suomalainen A (2012) Mechanisms of mitochondrial diseases. Ann Med 44: 41–59 [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, Nakagawa T, Yoshino J, Imai S (2015) SIRT1‐mediated eNAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab 21: 706–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Dave V (1975) Inhibition of NADP‐dependent dehydrogenases by modified products of NADPH. Arch Biochem Biophys 169: 298–303 [DOI] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S (2011) Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet‐ and age‐induced diabetes in mice. Cell Metab 14: 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Wong M, Apte RS, Imai SI (2019) Extracellular vesicle‐contained eNAMPT delays aging and extends lifespan in mice. Cell Metab 30: 329–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata‐Pérez R, Tammaro A, Schomakers BV, Scantlebery AML, Denis S, Elfrink HL, Giroud‐Gerbetant J, Cantó C, López‐Leonardo C, McIntyre RL et al (2021) Reduced nicotinamide mononucleotide is a new and potent NAD+ precursor in mammalian cells and mice. FASEB J 35: e21456 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R et al (2016) NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352: 1436–1443 [DOI] [PubMed] [Google Scholar]
- Zhou M, Ottenberg G, Sferrazza GF, Hubbs C, Fallahi M, Rumbaugh G, Brantley AF, Lasmezas CI (2015) Neuronal death induced by misfolded prion protein is due to NAD+ depletion and can be relieved in vitro and in vivo by NAD+ replenishment. Brain 138: 992–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF et al (2016) Hepatic NAD(+) deficiency as a therapeutic target for non‐alcoholic fatty liver disease in ageing. Br J Pharmacol 173: 2352–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li J, Stenton SL, Ren X, Gong S, Fang F, Prokisch H (2020) NAD(P)HX dehydratase (NAXD) deficiency: a novel neurodegenerative disorder exacerbated by febrile illnesses. Brain 143: e8 [DOI] [PubMed] [Google Scholar]
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W (2015) In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci USA 112: 2876–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]


