Abstract
Background
The symptom of heavy menstrual bleeding (HMB) diminishes quality-of-life for many mid-age women and imposes substantial societal burden. We investigated our hypothesis that HMB reflects impaired endometrial vasoconstriction due to endometrial glucocorticoid deficiency. Does reversing this deficiency, by short-term luteal-phase treatment with exogenous glucocorticoid (dexamethasone), ameliorate HMB?
Methods
In our Bayesian response-adaptive parallel-group placebo-controlled randomised trial, five pre-planned interim analyses used primary outcome data to adjust randomisation probabilities to favour doses providing most dose-response information. Participants with HMB, recruited from Lothian (Scotland) NHS clinics and via community invitations/advertisements, were aged over 18 years; reported regular 21–42 day menstrual cycles; and had measured menstrual blood loss (MBL) averaging ≥ 50 mL over two screening periods. Identically encapsulated placebo, or one of six Dexamethasone doses (0·2 mg, 0·4 mg, 0·5 mg, 0·6 mg, 0·75 mg, 0·9 mg), were taken orally twice-daily over five days in the mid-luteal phase of three menstrual cycles. Participants, investigators, and those measuring outcomes were masked to group assignment.
Primary outcome, change in average MBL from screening to ‘treatment’, was analysed by allocated treatment, for all with data.
Trial Registration
ClinicalTrials.gov NCT01769820; EudractCT 2012–003,405–98
Findings
Recruitment lasted 29/01/2014 to 25/09/2017; 176 were screened, 107 randomised and 97 provided primary outcome data (n = 24,5,9,21,8,14,16 in the seven arms, placebo to 1·8 mg total daily active dose). In Bayesian normal dynamic linear modelling, 1·8 mg dexamethasone daily showed a 25 mL greater reduction in MBL from screening, than placebo (95% credible interval 1 to 49 mL), and probability 0·98 of benefit over placebo. Adverse events were reported by 75% (58/77) receiving dexamethasone, 58% (15/26) taking placebo. Three serious adverse events occurred, two during screening, one in a placebo participant. No woman withdrew due to adverse effects.
Interpretation
Our adaptive trial in HMB showed that dexamethasone 1·8 mg daily reduced menstrual blood loss. The role of dexamethasone in HMB management deserves further investigation.
Funding
UK MRC DCS/DPFS grant MR/J003611/1.
Keywords: Heavy menstrual bleeding (HMB), Randomised controlled trial, Dexamethasone, Adaptive randomisation, Endometrium, Bayesian, Menorrhagia, Abnormal uterine bleeding (AUB)
RIC: Low dose dexamethasone as treatment for women with heavy menstrual bleeding: a response-adaptive randomised placebo-controlled dose-finding parallel group trial (DexFEM).
Evidence before this study
Amongst otherwise healthy women of reproductive age, the symptom of heavy menstrual bleeding (HMB), recurring monthly over many years, as is common, amounts to substantial cumulative individual morbidity. The adverse impact on quality of life and productivity is all too often underestimated by health care providers/policymakers.
In routine clinical practice, particularly primary care, first line treatment for the symptom of HMB is usually medical therapy (hormonal or non-hormonal). There have been no new medical approaches for HMB since the levonorgestrel-releasing intrauterine system (LNG-IUS), licensed in 2001. This locally-delivered hormonal treatment is highly effective in reducing MBL volume, and also provides contraceptive efficacy if that is required, but nearly a fifth of new users of LNG-IUS are dissatisfied with side-effects (often including unpredictable break-through bleeding/spotting), and it is unsuitable for women desiring pregnancy (as are systemic progestin therapies). A 2013 systematic review of non-surgical treatments for HMB found that non-hormonal medical treatments showed good percent reductions in MBL volumes, compared to baseline or placebo i.e. 26% to 54% reduction in blood loss volumes (MBL) for anti-fibrinolytics, 20% to 52% for non-steroidal anti-inflammatory drugs (NSAIDs). However when prescribed for HMB in routine clinical practice, these treatments are often reported as insufficiently effective, or to have become ineffective after a few months. The UK NHS HMB Audit in outpatient gynaecology clinics (2012–2013, n = 8183) found that 29% and 33% respectively of patients, first attending with symptom of HMB, received oral medication and LNG-IUS. It is salient that within the follow-up year, over half those starting with oral medication and one third starting with IUS had switched to a different treatment (often surgical).
This might be partly explained by the fact that a conventional entry criterion for trials of HMB treatments is ‘confirmation’ of HMB by objective assessment of baseline menstrual blood loss volume (MBL), as exceeding 80 mL average over at least two menstrual periods. In contrast, routine clinical practice almost never involves assessment of MBL volume, so many clinic/primary care recipients of these treatments are likely to have average MBL volumes considerably lower than the threshold for participation in the original research trials.
This highlights that for the debilitating symptom of HMB, as commonly encountered in clinical care, there is urgent need for a targeted medical treatment that is effective and acceptable, and that can be used while seeking to become pregnant. Alternative medical treatment options for HMB are needed if surgical intervention is to be avoided and fertility/ uterus preserved.
Our laboratory studies dissecting potential mechanistic pathways implicated in the symptom of HMB, suggested involvement of endometrial glucocorticoid deficiency. We hypothesised that the symptom of HMB could be ameliorated by administration of dexamethasone (a well-known cortisol surrogate) for 5 days in the luteal phase of the menstrual cycle, a time of endometrial blood vessel differentiation. Given dexamethasone is already licensed, there would be no need for safety/tolerability studies in healthy volunteers, offering the potential for accelerated translation from pre-clinical to clinical studies.
A small proof-of-concept animal study of our proposed therapeutic strategy provided highly supportive evidence, so the next step was a Bayesian adaptive randomised dose-finding trial of dexamethasone for treatment of HMB in women.
Added value of this study
The DexFEM study (Dexamethasone For Excessive Menstruation) provides the first evidence of therapeutic benefit of dexamethasone in heavy menstrual bleeding. The efficiency gains provided by the adaptive design enabled, for a given sample size, investigation of a larger number of potential doses compared to a conventional parallel group design. For dexamethasone dose 1•8 mg daily for 5 days in the luteal phase of each menstrual cycle, we found a mean reduction in MBL of 25 mL, consistent with our local endometrial glucocorticoid-deficiency hypothesis for HMB.
We included women with moderate sized fibroids and used a 50 mL MBL threshold for eligibility for our trial (rather than the commonly used 80 mL), so our trial reports treatment effect for women representative of those seeking clinical care for the symptom of HMB.
The trial raised no safety concerns but participation involved only three treatment cycles. It is thus still to be established that long-term use of dexamethasone treatment with 1•8 mg daily dose (but for only 5 days per menstrual cycle), would not induce the adverse effects known to be associated with chronic use of glucocorticoids.
Steroids are commonly prescribed in early pregnancy (for non-pregnancy related conditions) so if a woman with symptoms of HMB is seeking to conceive, Dexamethasone could be considered for amelioration of HMB.
Implication of all available evidence
Our findings support dexamethasone as a safe and effective medical therapy for HMB, making it a treatment option that might be welcomed by women who eschew surgical treatment, experience unacceptable side-effects with hormonal treatment, or wish to try for pregnancy.
Local delivery options (intra-vaginal/intra-uterine) should allow a lower dose than oral treatment, so could be considered to address concerns regarding cumulative glucocorticoid effects, as well as having the potential to be more convenient for users.
A further confirmatory trial is warranted to verify the efficacy of 1.8 mg daily dexamethasone dose, to explore treatment effect by aetiology and to investigate the criticality of timing of administration of treatment.
Alt-text: Unlabelled box
1. Introduction
The symptom of heavy menstrual bleeding (HMB) affects 20% to 52% of menstruating UK women [1], [2], [3], [4], and in developing countries is 4 to 27%, or more in older (multiparous) women [5]. HMB diminishes quality-of-life and has adverse impact on employment and family/caring roles [6], [7], [8]. In otherwise healthy mid-age women, the morbidity due to HMB is often underestimated, whereas the cumulative impact on quality of life (QOL), over 30 days per year, would be unacceptable in most other health conditions [7,9]. Menstruation is acknowledged globally as incurring costs that exacerbate poverty. Hence some recent government initiatives to encourage/fund provision of free menstrual protection [7,10].
Current management of symptom of HMB is generic and includes conservative, medical or surgical approaches (endometrial ablation and hysterectomy). There are diverse potential mechanisms for HMB [8,11], so identifying an effective treatment often has to be trial-and-error [8,9,12]. In routine clinical practice, particularly primary care, first line treatment is usually medical therapy (hormonal or non-hormonal) but this may be found to be ineffective, or may not be tolerated, for example on account of common side effects of hormonal treatments [6,9,12]. In the UK NHS HMB Audit (2012–2013) of hospital gynaecology clinic healthcare (n = 8183) it was reported that oral medication, LNG-IUS and surgery were received within the follow-up year by 29%, 33% and 43%, respectively, of patients newly attending hospital outpatient gynaecology clinics, while 18% received no treatment [13].
No novel medical treatment for HMB has been developed for near 20 years, no time-specific (non-hormonal) treatment for at least 30 years. An established highly effective treatment for HMB is the contraceptive levonorgestrel-releasing intrauterine system (LNG-IUS; available since 1995, licensed for HMB in 2001) [3,6,9,12]. However, the LNG-IUS is unsuitable for women desiring pregnancy (as are systemic progestin therapies) and 17% of women receiving LNG-IUS for HMB were dissatisfied with this treatment [3].
A 2013 systematic review of non-surgical treatments for HMB found that trials of non-hormonal medical treatments for HMB were completed in the main over two decades ago, before 1996, and generally imposed an entry criterion of objectively assessed average baseline menstrual blood loss volume (MBL) exceeding 80 mL [12]. The medical treatments reviewed showed good percent reductions in measured MBL volumes, for treatment compared to baseline or placebo (26% to 54% reduction for anti-fibrinolytics, 20% to 52% for NSAIDs) [12]. However, when prescribed for HMB in routine clinical practice, patients very often report these treatments as being or soon becoming insufficiently effective [6,9].
The 2012 UK NHS HMB Audit found that at one year after index attendance at gynaecology clinic, over a third of women (36%) were ‘unhappy’ or ‘very unhappy’ with their ongoing HMB symptoms [13]. Furthermore, by this point there had been a switch to different treatment (possibly surgical), by over half those starting with oral medication, and by one third starting with IUS [13]. Alternative treatment options for HMB are needed for surgical intervention to be avoided and fertility/ uterus preserved. This highlights the urgent need for targeted medical treatment for the debilitating symptom of HMB, that is effective, that does not have unacceptable side effects, and that can be used longish-term, even while seeking to become pregnant.
Endogenous glucocorticoids inhibit angiogenesis [14]. Regulation of endometrial blood vessel function is required to limit endometrial bleeding and menstrual blood loss [15]. Local endometrial glucocorticoid deficiency may, therefore, result in increased menstrual blood loss. We have demonstrated that endometrium from women with HMB has increased luteal phase expression of 11βhydroxysteroid dehydrogenase type 2 (11βHSD2), an enzyme which inactivates cortisol (the major glucocorticoid) [14]. We thus hypothesised that "rescue" of luteal phase endometrial glucocorticoid deficiency, at an early stage of endometrial repair/angiogenesis, would improve endometrial (spiral arteriole) blood vessel differentiation, augment local blood vessel vasoconstriction at onset of menses, and reduce volume of menstrual bleeding [14], [15], [16]. Dexamethasone was selected for use because it is not inactivated by 11βHSD2, but converted to 11-dehydro-Dex which remains bioactive.
We then conducted a small “proof of concept” animal study, which provided highly supportive evidence for reduction in volume of menstrual bleeding with dexamethasone (a cortisol surrogate/ glucocorticoid receptor agonist), administered in the luteal phase several days prior to expected menses. (see Supplement A)
This dose-finding study aimed to determine (i) if dexamethasone administered to women seeking treatment for the symptom of HMB is safe, acceptable and efficacious, and (ii) the optimal dose from those tested. A Bayesian adaptive trial was designed, requiring participation of substantially fewer women than a traditional multi-arm randomised trial [16,17].
2. Methods
2.1. Study design
The randomised controlled trial Dexamethasone For Excessive Menstruation (DexFEM), undertaken in Lothian, Scotland, had a Bayesian response-adaptive parallel group design, comparing six doses of oral dexamethasone treatment and placebo. The full protocol is available [dx.doi.org/10.17504/protocols.io.bpw3mpgn] and we have published details of the trial design (Study 3 in protocol) [16], and of preliminary simulation studies to develop the adaptive trial [17] (Or see supplements F, H, I).
2.2. Ethics
The trial received favourable ethical opinion from Scotland A Research Ethics Committee (12/SS/0147), clinical trial authorisation from the UK Medicines and Healthcare products Regulatory Agency (MHRA ref 01384/0226/001–0001), and was registered with ClinicalTrials.gov [NCT01769820] and EudractCT (2012–003405–98). All patients received full study information and opportunity for questions/discussion before providing written research consent.
2.3. Participants
The study population was women aged 18 years and over wishing treatment for the symptom of HMB. The main exclusion criteria were intending trying for pregnancy in the next five months, average cycle length outside 21 to 42 days, menstrual periods ‘very irregular’, breastfeeding, pregnancy ‘possible’ but unwilling to use contraception, diabetes mellitus, previous or current cancer of cervix/uterus/ovary/breast, and seven prohibited medications including taking systemic, inhaled or potent topical glucocorticoid treatments in the last month [16]. (See Supplement B.1) If a woman eligible as above wished to participate, a further eligibility criterion was average MBL over two screening menstrual periods equal to or exceeding 50 mL. MBL was assessed by objective validated laboratory measurement of collected used sanitary protection, by modified alkaline-haematin method [18].
We recruited from: National Health Service Lothian (NHSL) hospital gynaecology clinics and community gynaecology clinic; from Lothian primary care through mail-out by Scottish Primary Care Research Network (SPCRN) [16]; and by media advertising. Subsequently we added recruitment facilitated by SHARE (Scottish Health Research Register of over 200,000 people who had agreed to receive invitations for research studies selected as of potential interest using data in their NHS records), and via three study advertisements displayed on Facebook to women in the relevant age range and our geographical location.
Clinic recruitment involved clinicians informing women who reported symptoms of HMB, attending a NHSL community gynaecology clinic or gynaecology outpatient department, about the research, and if interested, providing full written information [16]. Similarly, any woman from other recruitment routes who contacted the research team about the ‘advertised’ research, was sent full study information. Women given study information were contacted by telephone after about a week, and if the decision was to participate, a research screening appointment was scheduled at Royal Infirmary Edinburgh gynaecology department. After informed consent had been confirmed by study doctor, or occasionally delegated hospital gynaecologist, eligibility was assessed, including a full menstrual, medical history and pelvic examination. This included ultrasound (USS) if uterine enlargement was found and there was no prior relevant USS (within six months). Classification of potential aetiologies underpinning the symptom of HMB was evaluated as part of this assessment [11].
2.4. Randomisation and masking
We planned to randomise 108 women. Allocation to placebo was 28.6% (2/7) throughout. The six dexamethasone doses (0·4 to 1·8 mg daily, split morning and evening) started with equal allocation probabilities (11·9% each). At each of five adaptations (triggered at 16, 32, 50, 66, and 84 randomisations), we used interim analysis, based on accumulating primary outcome data, to re-calculate allocation probabilities to favour dexamethasone doses which would provide most information on the underlying dose-response curve. A normal dynamic linear model (NDLM) of the primary outcome was used to predict, for each dexamethasone dose, the amount of information about the dose-response curve that would be gained if the next patient were randomised to that dose [17]. This was quantified as the reciprocal of the predicted variance (ie precision) of the primary outcome at the current estimate of the ED95 dexamethasone dose (the dose with 95% of the maximum efficacy). Subject to approval by the independent Data Monitoring Committee (DMC) who oversaw the trial, the set of dexamethasone dose randomisation probabilities was then updated, to be proportional to the predicted increase in information to be gained were a patient to be randomised to each dose. (See Supplements F, D.2)
Computer-generated pseudo-random numbers were used to generate the randomised allocation sequence. No stratification or blocking was used. Randomisation probabilities resulting from the adaptation analyses were communicated, by the unmasked statistician, only to the trials unit programmer responsible for updating the web-based randomisation system. Bulk supplies of encapsulated placebo and dexamethasone were held by NHSL hospital pharmacy who dispensed trial medication in bottles labelled with a unique randomisation number from the list provided by Edinburgh Clinical Trials Unit.
Encapsulation of placebo and dexamethasone in identical hard gelatine capsules ensured participants and clinical staff were masked to allocations. Enrolment and accessing the web-based randomisation system were undertaken by members of the study clinical research team, which ensured allocation sequence was concealed from researchers, clinical staff, and participants. Only the DMC, the trials unit statistician producing the DMC reports, and pharmacy staff dispensing the study medication were aware of treatment allocations during the trial.
2.5. Procedures
The intervention was placebo or one of 6 doses of (licensed) synthetic glucocorticoid dexamethasone (manufactured by Tayside Pharmaceuticals, Dundee), taken orally twice-daily for five days in the luteal phase of three menstrual cycles. It was judged that, for optimum vascular benefit, treatment should commence nine days before the next menstrual period, which generally starts 15 days after ovulation. Urine dipstick-testing was to be commenced day seven of the cycle, expecting a positive result (urine evidence of luteinizing hormone (LH) surge) to precede ovulation by about 12 h, and next menstrual period by 16 days. Each participant was given a supply of her allocated intervention at the start of cycle, notified the research team when positive dip-stick result occurred, and self-administered treatment regimen was scheduled to start seven days later. If no LH surge was detected, or if serial urine testing was not carried out, treatment start was calculated by counting back from cycle-date-history-predicted start of next period. (See also Supplement B.2) Clinical caution meant DexFEM participants had to conduct a pregnancy test each cycle. Women were reminded by text message when to start dipstick-testing, and when to start medication, including a reminder that treatment start was subject to a negative pregnancy test. Participants were also asked to record study medication intake and to return all unused study medication.
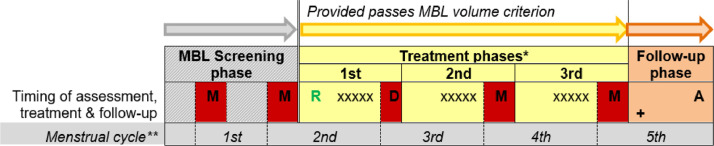
In addition to data collection at recruitment, Fig. 1 shows there was assessment of each study menstrual period, overview questionnaire assessment of treatment phase after the 3rd period, and a follow-up clinic appointment 30 days after last treatment.
Fig. 1.
Participant time-line in study – 5 to 6 months from screening appointment

2.6. Outcomes
Primary outcome was change from baseline in laboratory-assayed MBL i.e. given there was no MBL collection in first treatment phase, average MBL of second and third treatment phase periods minus average of two screening periods. Secondary outcomes included data collected via (i) Treatment Review Questionnaire completed after last treated menstrual period, assessing satisfaction with treatment, and (ii) diary reports women completed during and at end of each study period (two baseline, three treatment phase). The five diaries also allowed, for each treatment phase, calculation of change from baseline in semi-quantitative (pictogram-diary-derived) estimate of volume of menstrual period [19]. Subjective outcomes collected contemporaneously in treatment phase diaries were: heaviness of period, and comparison of this period to ‘before trial’ regarding: heaviness, period pain, feeling generally well, impact of period on daily activities, and period being a ‘problem’. The Treatment Review Questionnaire, completed after third treated period, collected overall treatment phase outcomes: self-reported ‘lighter’ or ‘much lighter bleeding’, generally 'feeling much better during periods', improvement in period pain ('less' or 'much less severe'), and acceptability of treatment.
2.7. Statistical analysis
A detailed statistical analysis plan (SAP) was written while blinded to randomised treatment allocations, prior to study database lock (see Supplement E). For all outcomes, analysis was by allocated groups, for all data provided. The dose-response curve for the primary outcome was analysed in WinBUGS using a Bayesian second order NDLM [17], adjusted for the screening MBL measurements of each woman. The NDLM is flexible and requires few assumptions about the shape of the underlying dose-response curve [17]. For each dexamethasone dose, a 95% credible interval (CrI) was then calculated for the mean difference in MBL change (dexamethasone minus placebo). Using non-informative priors, the posterior probability of efficacy against placebo was calculated for each dose. (See Supplement C). Change from baseline in menstrual diary pictogram scores (average of three treatment phase periods minus the average of two screening periods) was analysed as for primary outcome. Binary secondary endpoints were derived from Treatment Review Questionnaire ‘satisfaction with treatment’ items and from diary subjective reports, and these were analysed using Bayesian comparison of proportions for each dexamethasone dose versus placebo. A 95% credible interval was calculated for the difference in proportions (dexamethasone minus placebo).
On the basis of simulations, a sample size of 100 was estimated to have statistical power of 93·8%, assuming: within-patient standard deviation of 18 mL for MBL (unpublished data); mean MBL benefit for treatment over placebo is at most 16 mL; and a range of plausible shapes of dose-response curves (steep incline, slowly ascending, inverted U-shape). The target enrolment total was set at 108 to allow for withdrawals/loss to follow-up.
2.8. Role of funder
The funder of the study (MRC) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The writing group (including corresponding author) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
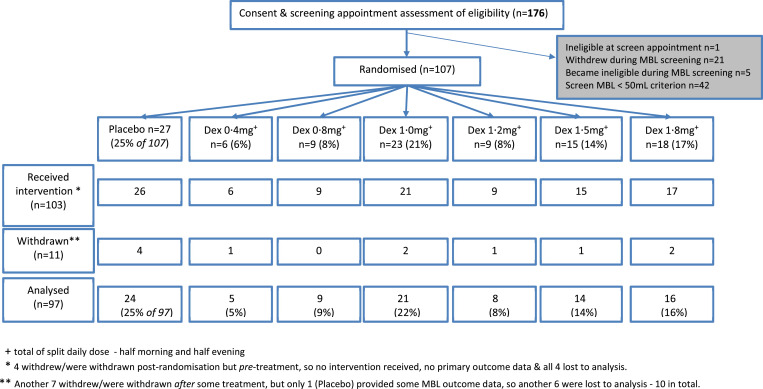
Recruitment (screening appointment) ran from 29/1/2014, to 25/9/2017, ensuing randomisations (to all groups) continued until November 2017, and final follow-up was March 2018. Fig. 2 presents the participant flow for the study. (See also Supplement D.1)
Fig. 2.
CONSORT diagram.
Of 149 women who completed MBL screening, 42 (28%) failed the 50 mL average MBL inclusion criterion, leaving 107 women to be randomised. After the final adaptation, the allocation across arms was as shown in the randomisation row of Fig. 2. (See also Supplement D.2) Ninety-six of 107 (90%) completed the trial, 97 (91%) providing primary outcome data. Across the three treatment phases adherence was 86% (89/103), 90% (88/98), and 79% (76/96), respectively. There were five protocol violations; one resulted in withdrawal, for ineligibility, before start of treatment. (See also supplement D.3).
Participants were aged 21 to 54 years and self-reported HMB had been a problem for six months to 37 years (Table 1). A quarter of women reported their periods lasting more than six days, 33% of women were nulliparous and 74% had painful periods. The predominantly white ethnicity (93%) reflects 2014 NHS Lothian Health Board data for the region, for females aged 18–49 years (92%). The participants in placebo and dexamethasone groups had very similar baseline characteristics and were generally comparable in terms of medical history. (See also Supplement D.4).
Table 1.
Participant characteristics at screening appointment.
| Placebo |
Dexamethasone |
|||
|---|---|---|---|---|
| N | 27 | 80 | ||
| Median [min, Q1, Q3, max]* | Median [min, Q1, Q3, max]* | |||
| Age (years) | 43 [21, 38, 46, 53] | 43 [21, 37·5, 47, 54] | ||
| Response when asked how long HMB has been a problem (years) | 5 [1, 3, 11, 33] | 5 [0·5, 2, 14, 37] | ||
| Minimum duration of menses in previous 3 months (days) ⁎⁎ | 5·5 [3, 5, 7, 15] | 5·0 [2, 4, 6, 14] | ||
| Maximum duration of menses in previous 3 months (days) ⁎⁎ | 7 [4, 6, 8, 21] | 7 [3, 5, 7, 15] | ||
| Mean screening menstrual blood loss (mL) | 112·0 [51, 63·5, 206, 677·5] | 136·0 [50, 84, 187, 479·5] | ||
| Approx. years since last pregnancy (if applicable⁎⁎⁎) | 6 [2, 5, 9, 24] | 11 [0, 6, 15, 31] | ||
| Weight (kg) | 69·2 [48, 60·9, 91, 129] | 74·4 [46, 64·8, 85·2, 124] | ||
| Units of alcohol consumed per week currently | 1 [0, 0, 6, 16] | 3 [0, 0·5, 10, 40] | ||
| Mean (SD) | Mean (SD) | |||
|---|---|---|---|---|
| Age at menarche (years) | 12·6 (1·4) | 12·8 (1·5) | ||
| Systolic blood pressure (mmHg) | 122·0 (13·4) | 124·9 (12·3) | ||
| Diastolic blood pressure (mmHg) | 76·1 (13·3) | 77·2 (10·1) | ||
| Minimum cycle length past 3 months (days) | 26·4 (2·7) | 26·0 (2·6) | ||
| Maximum cycle length past 3 months (days) | 30·5 (4·4) | 29·8 (3·5) | ||
| Placebo |
Dexamethasone |
|||
|---|---|---|---|---|
| N | 27 |
80 |
||
| n | % | n | % | |
| Ethnicity | ||||
| White | 26 | 96% | 73 | 91% |
| Mixed | 0 | 1 | 1% | |
| Asian | 0 | 3 | 4% | |
| African | 1 | 4% | 1 | 1% |
| Caribbean | 0 | 1 | 1% | |
| Not disclosed | 0 | 1 | 1% | |
| No. of births | ||||
| None | 10 | 37% | 25 | 31% |
| 1 | 3 | 11% | 16 | 20% |
| 2 | 9 | 33% | 22 | 27% |
| 3 | 4 | 15% | 13 | 16% |
| 4–6 | 1 | 4% | 4 | 5% |
| No. of miscarriages | ||||
| None | 17 | 63% | 59 | 74% |
| 1 | 7 | 26% | 15 | 19% |
| 2–4 | 3 | 11% | 6 | 8% |
| No. of terminations | ||||
| None | 22 | 81% | 63 | 79% |
| 1 | 3 | 11% | 11 | 14% |
| 2 | 2 | 7% | 5 | 6% |
| 3 | 0 | 0 | ||
| 4 | 0 | 1 | 1% | |
| Response when asked if has painful periods | ||||
| Yes | 21 | 78% | 58 | 73% |
| No | 6 | 22% | 22 | 27% |
| Fibroids present | ||||
| No recent USS/MRI | 1 | 4% | 5 | 6% |
| No fibroids detected | 16 | 59% | 41 | 51% |
| Fibroid < 3 cm | 8 | 30% | 25 | 31% |
| Fibroid >= 3 cm | 2 | 7% | 9 | 11% |
| Smoking history | ||||
| Current (1 to 20 per day) | 6 | 22% | 8 | 10% |
| Previous | 3 | 11% | 19 | 24% |
| Never | 18 | 67% | 53 | 66% |
min = minimum, Q1 = 1st quartile, Q3 = 3rd quartile, max = maximum.
2 with missing data, one Placebo, one dexamethasone.
78 women had been pregnant, with 19 allocated to placebo, and 58 to Dexamethasone.
For those with primary outcome data, treatment groups placebo (n = 24) and (overall) active (n = 73) had, respectively, medians of average screening MBL 105 and 132 mL (maxima 384, 480 mL).
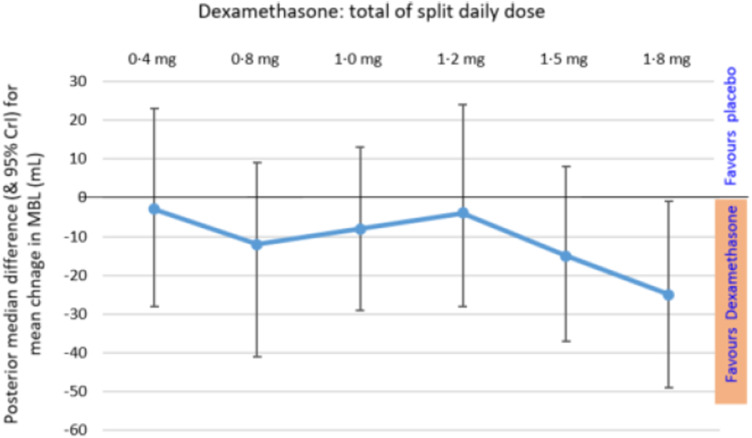
The lower half of Table 2 reports the NDLM results for primary outcome, separately by dose. Fig. 3 shows some treatment advantage in all dose groups, but that the greatest treatment benefit was in the 1·8 mg total daily dose – a treatment reduction in MBL of 25 mL, with 95% credible interval 1 to 49 mL. This dose had posterior probabilities, of 0·98 and 0·89 respectively, for any advantage over placebo, or at least a 10 mL advantage.
Table 2.
Primary outcome change in MBL: data summaries and statistical modelling.
| Randomised Treatment |
||||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Dexamethasone | Total dose Dexamethasone (split twice daily) |
||||||
| (any dose) | 0·4 mg | 0·8 mg | 1·0 mg | 1·2 mg | 1·5 mg | 1·8 mg | ||
| Data Summaries | ||||||||
| N | 24 | 73 | 5 | 9 | 21 | 8 | 14 | 16 |
| Mean change in MBL*(mL) | −6·0 | −20·9 | −7·8 | −23·8 | −18·5 | 5·8 | −24·5 | −36·7 |
| SD | 51·3 | 44·0 | 54·3 | 41·7 | 37·8 | 50·6 | 31·5 | 53·8 |
| Minimum | −170 | −188 | −66·0 | −92·0 | −143 | −58·5 | −85·5 | −188 |
| Maximum | 97·0 | 89·5 | 75·5 | 37·0 | 47·5 | 89·5 | 35·0 | 27·0 |
| NDLM-modelled Treatment Effects | ||||||||
| Posterior median for difference (dexamethasone minus placebo) in mean MBL change (mL)⁎⁎ | −3 | −12 | −8 | −4 | −15 | −25 | ||
| 95% CrI⁎⁎⁎ (mL) | −28, 23 | −41, 9 | −29, 13 | −28, 24 | −37, 8 | −49,−1 | ||
| Posterior probability of at least a 10 mL dexamethasone advantage over placebo | 0·26 | 0·58 | 0·42 | 0·32 | 0·67 | 0·89 | ||
| Posterior probability of any advantage of dexamethasone over placebo | 0·64 | 0·86 | 0·78 | 0·63 | 0·90 | 0·98 | ||
Negative values for ‘mean change’ in primary outcome indicate treatment advantage over placebo. MBLs were laboratory-assessed in only the 2nd and 3rd treatment phases, but eight women provided only one MBL collection (six missing the 2nd treatment phase MBL, two the 3rd). For these the single MBL laboratory assessment obtained was analysed.
From NDML model of individual mean MBL change (from screening baseline to treatment phase), with adjustment for individual average baseline MBL.
CrI credible interval.
Fig. 3.
Primary outcome results: Posterior median difference, for each dexamethasone dose group minus placebo, in change in menstrual blood loss volume (mL), Rogue and 95% CrI Legend: Primary outcome is change in assayed MBL for an individual i.e. their treatment phase MBL– screening MBL. Normal dynamic linear model of change in MBL is adjusted for Screening MBL of each participant.
Secondary outcome menstrual volumes calculated from menstrual diaries[19], available for 25 women on placebo, 74 on dexamethasone, showed similar treatment effects to the primary MBL outcome. The estimated advantage for the 1·8 mg dose over placebo, for change in mean volume from screening to treatment, was a 32 mL reduction in blood loss (95% CrI 69 mL reduction to 4 mL increase), with posterior probability of any advantage over placebo of 0·96. (See also Supplement D.5)
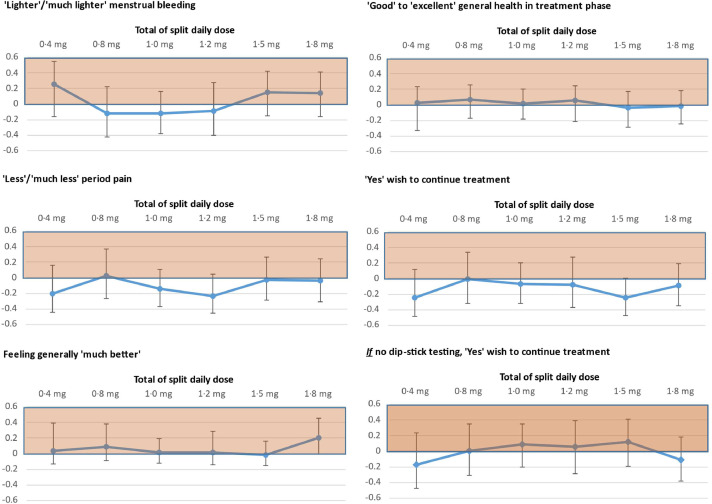
For women's secondary outcome menstrual diary subjective assessments for each treatment phase, see Supplements D.6-D.8. Regarding final overview assessment by Treatment Review Questionnaire, Fig. 4 shows, for dexamethasone dose versus placebo, posterior median differences in proportions reporting more favourable dichotomized responses (most recent period compared to before trial). Comparing placebo and dexamethasone groups, similar proportions expressed willingness to keep taking the treatment if it were available for HMB, but without the need for dip-stick testing: 44% (11/25) and 45% (32/71) respectively.
Fig. 4.
Treatment Review Questionnaire secondary outcome results: Difference in proportions responding as indicated - for each dexamethasone dose minus placebo - and 95% CrI
Legend: The left-panel items ask participant to compare their most recent menstrual period (3rd), to before the trial, while the right-panel items ask for 'absolute' judgements. Estimates plotted in shaded area favour dexamethasone, while those plotted in unshaded area, favour placebo.
Left panel n = 97, 95, 97 respectively, and right panel n = 97, 95, 96.
There were three serious adverse events (AEs): two in screening phase, and one post-randomisation, in a woman receiving placebo. There were no suspected unexpected serious adverse reactions. Overall, 73 women (71% of 103 receiving treatment) had at least one AE, with rates lower for placebo (15, 58%) than dexamethasone (58, 75%). No patient was withdrawn on account of AEs. (See also Supplement D.9).
4. Discussion
Our trial found greater reduction in menstrual blood loss volume with dexamethasone than placebo, especially at highest dose of 0·9 mg twice-daily, which showed mean reduction in MBL of 25 mL (CrI 1 mL to 49 mL) (Fig. 3). These findings are consistent with our glucocorticoid-deficiency hypothesis, that locally available endometrial cortisol may be necessary for optimal endometrial blood vessel maturation, to limit menstrual blood loss [14], [15], [16].
Methodological challenges in menstrual research are pertinent. Participants had thorough assessment of HMB symptoms and of eligibility, prior to recruitment. This included those with uterine fibroids not of size necessitating surgery because, while our study addresses local impact of glucocorticoid in the endometrium, there are no research findings to date suggesting that uterine structural features, for example, fibroids, disturb glucocorticoid signalling pathways.
MBL assessment is the preferred outcome for HMB drug trials, despite requiring laboratory infrastructure and larger research budget, and that it may deter participation. HMB treatment trials generally utilise an entry criterion of 80 mL mean screening MBL, a value that represents the upper 5% point of the distribution of MBL volumes in a Scandinavian community study undertaken in 1960, which is not necessarily an appropriate contemporary threshold for HMB warranting clinical intervention [20]. In a large US trial this threshold was failed by 71% of the 711 potential HMB trial participants [21]. Furthermore, laboratory measured MBL assessment is very rarely undertaken in routine clinical practice, so many women requesting treatment for HMB might not have average measured MBL exceeding 80 mL over two/three periods, as used in HMB trials. Thus it is unknown how well the trial-established evidence for contending medical treatments applies to many women being treated for problematic HMB. Diverse aetiologies are hypothesized for HMB [8,11], and HMB research is beset with many outcome measures [22]. HMB is seldom the only troubling symptom being experienced so there are heterogeneous presentations of HMB [1,18,23,24]. Therefore reduction in MBL volume might not necessarily be a sufficient indicator of treatment ‘success’ [20]. Subjective assessment of treatment effect might be more pertinent, or assessments (plural) might allow a more holistic picture and give more interpretable results [20]. Treatment effect reported as absolute reduction in MBL volume might not be informative for women. Lukes et al. found that while the threshold for patient-perceived meaningful absolute reduction differed with baseline MBL (i.e. 20 mL threshold for baseline MBLs 80 to 160 mL, but 56 mL for baseline MBL over 160 mL), a meaningful relative reduction was 22% of baseline MBL regardless of baseline volume [25]. Reliability of measurement of ‘objective‘ MBL depends on completeness of menstrual collection, and optimum laboratory protocols for assay method. Pictogram diary assessment is more acceptable to participants, but its validity and reliability depends on conscientious and careful diary completion. HMB trials seldom use diaries to assess menstrual volume, very rarely diaries only, perhaps due to concern that performance observed in method validation studies (such as [19]) would not be achieved without parallel MBL assessment.
Some strengths of our trial are as follows. We have demonstrated the feasibility of an adaptive randomisation trial for HMB. Adaptive designs require substantial preliminary development work but they enable efficient learning about dose response, and they estimate treatment effects with improved precision [26,27]. MBL data is available within 2 weeks of luteal phase treatment, and hence well-suited to adaptive trials, which work best where outcome data is available soon after treatment, for earliest possible inclusion into the next adaptation analysis [26]. There was excellent retention through five menstrual cycles of screening and treatment, despite the demands on participants of MBL collection, and urine dip-stick testing. Support of participants by experienced research nurses, via mobile phone contacts, in particular regarding start dates, ensured provision of primary outcome data by 91% of participants (97/107). Our lower MBL eligibility threshold (50 mL) means DexFEM trial participants are more representative of patients with clinical need for treatment of HMB, than in most published HMB trials. Outcome assessment included both objective and semi-quantitative assessment of MBL, and subjective assessment of effect of treatment on a range of aspects of periods, including volume. The observed dose-response pattern for pictogram-estimated MBLs was very similar to that for objectively assessed MBL, albeit less marked. These diary MBL assessments ensured we had some assessment of volume of the first treatment phase period, where there was no laboratory MBL assessment, and will allow further methodological analyses. Given dexamethasone is already licensed, there is no need for safety/tolerability studies in healthy volunteers, offering the potential for accelerated translation from pre-clinical to clinical studies. In our trial there were only 3 serious adverse events, none occurring on/after active treatment, and no patient was withdrawn on account of adverse events.
Some limitations in DexFEM are as follows. The trial time-line had to be extended to achieve target recruitment, so adding new sources of participants means there was potential for case mix change across time, which could raise concerns in relation to ‘adapting’ (changing) randomisation probabilities. However, the randomisation process ensured contemporaneous (placebo) controls across the entire study. Typical within-woman variation in cycle lengths, particularly amongst those aged over 45 years, means that counting cycle days to schedule treatment is unreliable, while mid-cycle daily dipstick-testing to identify LH surge is burdensome. Experience tells us that a proportion of women decline trial participation if submission of used menstrual protection is required, which could distort the study sample. Diversity in mean baseline MBL (which for DexFEM participants ranged from 50 to 677·5 mL) may reduce overall precision of estimation of dexamethasone treatment effect. Similarly, diversity in concomitant menstrual symptoms could distort subjective assessment of treatment effect, for specific subgroups.
We used a lower than usual MBL threshold for eligibility (50 mL), and only 28% of participants recruited for DexFEM failed this criterion. The DexFEM trial sample was therefore representative of patients with clinical need for treatment of HMB i.e. heterogeneous in terms of presentation, concomitant menstrual symptoms, baseline MBL, duration of problem, parity, and in some cases presence of moderate-sized uterine fibroids.
Our findings support a glucocorticoid-deficiency hypothesis for HMB, but it is possible dexamethasone may be having a pharmacological effect independent of endogenous glucocorticoid action. A dose of 0·75 mg Dexamethasone once every day is considered to provide physiological replacement that is similar, for example, to prednisolone 5 mg once daily. Dexamethasone was well tolerated, with 79% to 90% of participants taking all of their allocated treatment in each treatment phase, and no serious AEs occurred with active treatment. However, chronic use of glucocorticoids at supra-physiological doses is associated with a higher incidence of cardiovascular disease [28] and fracture [29] as well as risks from suppression of endogenous cortisol secretion. There is thus a tension between reversing an hypothesised local endometrial deficiency in cortisol (to ensure adequate luteal phase local glucocorticoid for endometrial blood vessel maturation necessary to limit menstrual blood loss) and minimising cumulative systemic glucocorticoid dose. Our regimen comprised very short-term administration of Dexamethasone, for just five days during the luteal phase of each menstrual cycle.
One potential advantage of dexamethasone management of HMB is that it might be licensed for women with HMB seeking to control symptoms while trying for pregnancy. In the general population, women may conceive while using regular steroid medications for (non-HMB) medical indications, often at higher doses than used in DexFEM. The administration of steroids in very early pregnancy has been reviewed [30] including administration early for the treatment of recurrent miscarriage. The daily doses typically used for prevention of miscarriage, usually for the entire first trimester, are equivalent to twice to three times the daily doses used in DexFEM, and HMB treatment as per DexFEM would persist for only five days of the single cycle in which conception occurs.
The dose of dexamethasone with the maximum effect on MBL was 0·9 mg (twice daily). This showed an average relative reduction in MBL volume of 19% of individual screening MBL, which is close to the published finding that 22% of baseline MBL is a ‘meaningful’ relative reduction [25]. However, we found dexamethasone had no apparent beneficial effect on period pain (Fig. 4), a symptom strongly associated with HMB complaint [1,18].
Dexamethasone treatment for the symptom of HMB shows considerable promise as a treatment option, one that might be welcomed by women who eschew surgical treatment, experience unacceptable side-effects with hormonal treatment, or wish to try for pregnancy. Future longer-term studies will be required to establish that the systemic dexamethasone regimen used would not induce the known adverse effects of dexamethasone use. Local delivery of dexamethasone#, targeted to the required site of action, could reduce the dose required, and hence systemic levels, allaying concerns regarding cumulative doses of dexamethasone across time. Intrauterine delivery of dexamethasone could be achieved by adapting existing intrauterine delivery methods, although this would mean continuous administration. Intravaginal modes of site-targeted delivery would allow short-term intervention preceding onset of menses which could substantially reduce dose delivered. Further investigations (e.g. N-of–one studies) could aid investigation of these alternative regimes, and of critical timings for effective dexamethasone administration. Studies exploring consistency of dexamethasone benefit according to presentation and underlying aetiology for the HMB would be invaluable in enhancing understanding of mechanisms.
HMB has substantial adverse impact on the quality-of-life of individuals affected, their families and society[1,2,[4], [5], [6], [7], [8],10,24], and HMB can exacerbate anaemia and iron deficiency [6,18]. Women often have years of quality-of-life deficit before seeking professional help for HMB[1], because they do not wish to take hormone treatment and decline surgical treatments which may impair future fertility. Amongst DexFEM participants the median duration of HMB, so far, was five years. In the past 20 years there have been no new medical approaches for HMB treatment, yet the options currently available are all too often found wanting [3,6,9,12,13]. There remains unmet need for medical therapies that have fewer unacceptable side effects.
We have completed an adaptive randomised controlled trial, of a new medical therapy for the symptom of heavy menstrual bleeding, the first for over two decades. The findings, generalisable to the range of women seeking treatment for HMB, show that 0·9 mg of dexamethasone twice daily for five days in the luteal phase, reduces objectively-measured MBL volume. Further developmental and confirmatory research is warranted.
Contributors
Warner P: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualisation, Supervision, Project administration, Funding acquisition; Whitaker LHR: Investigation, Resources, Data curation, Writing - review & editing; Parker RA: Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing original draft, Writing review & editing, Visualisation, Supervision; Weir CJ: Conceptualization, Methodology, Software, Validation, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition; Douglas A: Resources, Data curation, Writing - original draft, Writing - review & editing, Project administration; Hansen CH: Methodology, Software, Validation, Formal analysis, Data curation, Writing - review & editing; Madhra M: Investigation, Resources, Writing - review & editing; Hillier SG: Conceptualization, Writing - review & editing, Funding acquisition; Saunders PTK: Conceptualization, Writing - review & editing, Funding acquisition; Iredale JP: Conceptualization, Writing - review & editing, Funding acquisition; Semple S: Conceptualization, Writing - review & editing, Funding acquisition; Slayden OD: Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - review & editing, Visualisation, Supervision; Walker BR: Conceptualization, Writing - original draft, Writing - review & editing, Funding acquisition; Critchley HOD: Conceptualization, Investigation, Resources, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
PW, CHH, AD, LW & MM, PTKS, SGH, ODS, CJW and RAP have no competing interests to disclose. Other disclosures, all outside the current research study, are:
BRW is a paid consultant for Actinogen Medical, an inventor on patents owned by the University of Edinburgh relating to manipulating and monitoring glucocorticoid action; receives royalties as editor of Davidson's Principles & Practice of Medicine published by Elsevier; and an honorarium as Chair of the MRC Population & Systems Medicine.
SS receives support from GlaxoSmithKline for his research group.
JPI is supported by the UK National Institute of Health Research (NIHR) and is Director of the University Hospitals Bristol Trust and University of Bristol NIHR Biomedical Research Centre.
HODC reports clinical research support for laboratory consumables and staff from Bayer AG and she has also provided consultancy advice (but with no personal remuneration) for Bayer AG; Vifor Pharma; Gedeon Richter; Myovant. She receives royalties from Up-to-Date for article on Abnormal Uterine Bleeding.
Acknowledgements
Direct project funding for the research was received from the UK Medical Research Council DCS/DPFS scheme, grant number: MR/J003611/1 (https://mrc.ukri.org/) [HODC is CI for the grant, while PW, CJW, BRW, SGH, PTKS, SS and JPI were all co-applicants. The grant funded employment on the project of co-authors CHH, AD, RAP, LW & MM].
DexFEM was also indirectly supported by core funding to various affiliate institutions:
• UoE MRC Centre for Reproductive Health [MRC core grants G1002033 2011–2016)PIs PTKS and HODC); MR/N022556/1 2016–2021 (renewal of core grant, no named PIs) - both https://mrc.ukri.org/]
• UoE Centre for Inflammation Research [MRC core grants from G0901697 2011–2017 (PI JPI); G9900991 2005–2010 (no DexFEM PI); both https://mrc.ukri.org/]
• Oregon National Primate Research Facility https://www.ohsu.edu/onprc [core grants over the time from ODS undertaking proof-of-concept study, until present - National Institutes of Health RR000163 PI J E Robertson, NIH P51 OD011092 PI P Bar-Gillespie (https://www.nih.gov/grants-funding)]
• SPCRN is supported by the Chief Scientist Office of the Scottish Government
• SHARE is supported by NHS Research Scotland, the Universities of Scotland and the Chief Scientist Office of the Scottish Government
None of the funders listed above had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
BRW is a Wellcome Trust Senior Investigator (grant 107049/Z/15/Z https://wellcome.org/grant-funding) and has received programmatic grant support from British Heart Foundation.
SS has been supported by a British Heart Foundation Centre of Research Excellence (2009–2014)
CJW and RAP are partly supported by NHS Lothian grant to the UoE Edinburgh Clinical Trials Unit.
We are grateful for the valuable contributions to DexFEM of:
The women who participated in the trial
DexFEM research team: Catherine Murray, Sharon McPherson, Moira Nicol, Alison Murray; Sheila Milne, Anne Houghton, Barbara Hamilton
NHS Lothian Pharmacy Services: Hazel Milligan, Ruaridh Buchan
Tayside Pharmaceuticals: Baxter Millar
ACCORD (sponsor) – Ray French, Marise Bucukoglu, Bernadette Gallagher, Liz Craig, Heather Charles
UoE Edinburgh Clinical Trials Unit (ECTU): Gina Cranswick, Garry Milne, Jacqueline Stephen, Michelle Steven and other ECTU staff
NHS Lothian: Gerry Beattie, Ailsa Gebbie, Sharon Cameron;
Scottish Primary Care Research Network (SPCRN) [now rebranded to NRS Primary Care Network]: Ellen Drost; Morag Place
SHARE: Shobna Vasishta, Louise Dow
UoE Technology Transfer Office: Giles Dudley, Emma Mickley
Trial Steering Committee (TSC): Mary Ann Lumsden, Siladitya Bhattacharya, Dharani Hapangama;
Data Monitoring Committee (DMC): Justin Clark, Ertan Saridogan, Rebecca Reynolds, Adrian Mander
Data sharing: The full study database will be available, subject to approval of requests/proposals by a DexFEM user group including HODC, PW, CJW. Data will be fully anonymised (including removal of exact dates) and full supporting documentation will be provided (e.g. CRF, PIS, protocol, SAP, data dictionary). Access will be via Edinburgh DataShare https://datashare.is.ed.ac.uk/ and approved requests will be issued with a time-limited, renewable, data sharing agreement.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103434.
Appendix. Supplementary materials
Supplement A. DexFEM Proof-of-concept: Exploratory non-human primate study of the effect of dexamethasone on menstrual blood loss.
Supplement B. Additional material for DexFEM trial Methods.
Supplement C. Introduction to Bayesian concepts and definitions, and adaptive trial design.
Supplement D. Additional DexFEM trial Results.
DexFEM Statistics Analysis Plan.
DexFEM Protocol for 3 studies in grant.
DexFEM CONSORT checklists for Bayesian adaptive trial ABSTRACT and PAPER.
Supplement H. Links to papers already published from DexFEM.
References
- 1.Santer M., Warner P., Wyke S. A Scottish postal survey suggested that prevailing clinical pre-occupation with heavy periods does not reflect the epidemiology of reported symptoms and problems. J Clin Epidemiol. 2005;58:1206–1210. doi: 10.1016/j.jclinepi.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Shapley P., Jordan K., Croft P.R. An epidemiological survey of symptoms of menstrual loss in the community. BJGP. 2004;54:359–363. [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharya S., Middleton L., Tsourapas A., Lee A., Champaneria R., Daniels J. Hysterectomy, endometrial ablation and Mirena(R) for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess. 2011;15:1–252. doi: 10.3310/hta15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Royal College of Obstetricians and Gynaecologists . RCOG Press; London: 2011. London school of hygiene & tropical medicine, ipsos MORI. national heavy menstrual bleeding audit: first annual report. [Google Scholar]
- 5.Harlow S.D., Campbell O.M. Epidemiology of menstrual disorders in developing countries: a systematic review. BJOG. 2004;111:6–16. doi: 10.1111/j.1471-0528.2004.00012.x. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Clinical Excellence . NICE Clinical Guideline CG44; 2007. Heavy menstrual bleeding. [Google Scholar]
- 7.Anand E., Kumar P., Unisa S., Singh J. Neglect of menstrual disorders in reproductive health care in India: a population-based survey. Women's Reprod. Health. 2018;5(4):287–300. doi: 10.1080/23293691.2018.1523116. [DOI] [Google Scholar]
- 8.Sriprasert I., Pakrashi T., Kimble T., Archer D.F. Heavy menstrual bleeding diagnosis and medical management. Contracept Reprod Med. 2017;2:20. doi: 10.1186/s40834-017-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaloo P., Davies S. Case discussion: heavy menstrual bleeding in primary care and beyond. Br J Family Med. 2014;2 https://www.bjfm.co.uk/case-discussion-heavy-menstrual-bleeding-in-primary-care-and-beyond [Google Scholar]
- 10.Period poverty Scotland https://www.economist.com/britain/2020/02/27/free-period-products-in-scotland, Accessed Sep 2020
- 11.Munro M.G., Critchley H.O.D., Fraser I.S., for the FIGO Menstrual Disorders Committee The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393–408. doi: 10.1002/ijgo.12666. [DOI] [PubMed] [Google Scholar]
- 12.K A Matteson D., D Rahn T.L., Wheeler I.I., Casiano E., Siddiqui N.Y., Harvey H.S., for the Society of Gynecologic Surgeons Systematic Review Group Non-surgical management of heavy menstrual bleeding: a systematic review and practice guidelines. Obstet Gynecol. 2013;121(3):632–643. doi: 10.1097/AOG.0b013e3182839e0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royal College of Obstetricians and Gynaecologists . RCOG Press; London: 2013. London school of hygiene & tropical medicine, IPSOS MORI. National heavy menstrual bleeding audit: third annual report. [Google Scholar]
- 14.Rae M., Mohamad A., Price D., Hadoke P.W., Walker B.R., Mason J.I. Cortisol inactivation by 11beta-hydroxysteroid dehydrogenase-2 may enhance endometrial angiogenesis via reduced thrombospondin-1 in heavy menstruation. J Clin Endocrinol Metab. 2009;94:1443–1450. doi: 10.1210/jc.2008-1879. [DOI] [PubMed] [Google Scholar]
- 15.Critchley H.O., Maybin J.A., Armstrong G.M., Williams A.R.W. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;100(3):1149–1179. doi: 10.1152/physrev.00031.2019. Epub Feb 7. [DOI] [PubMed] [Google Scholar]
- 16.Warner P., Weir C.J., Hansen C.H., Douglas A., Madhra M., Hillier S.G. Low-dose dexamethasone as a treatment for women with heavy menstrual bleeding: protocol for response-adaptive randomised placebo-controlled dose-finding parallel group trial (DexFEM) BMJ Open. 2015;5(1) doi: 10.1136/bmjopen-2014-006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen C.H., Warner P., Parker R.A., Walker B.R., Critchley H.O.D., Weir C.J. Development of a Bayesian response-adaptive trial design for the Dexamethasone for Excessive Menstruation study. Stat Methods Med Res. 2017;26:2681–2699. doi: 10.1177/0962280215606155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner P.E., Critchley H.O., Lumsden M.A., Campbell-Brown M., Douglas A., Murray G.D. Menorrhagia I: measured blood loss, clinical features, and outcome in women with heavy periods: a survey with follow-up data. Am J Obstet Gynecol. 2004;190:1216–1223. doi: 10.1016/j.ajog.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Wyatt K.M., Dimmock P.W., Walker T.J., O'Brien P.M.S. Determination of total menstrual blood loss. Fertil Steril. 2001;76:125–131. doi: 10.1016/s0015-0282(01)01847-7. [DOI] [PubMed] [Google Scholar]
- 20.Warner P.E., Critchley H.O., Lumsden M.A., Campbell-Brown M., Douglas A., Murray G.D. Menorrhagia II: is the 80-mL blood loss criterion useful in management of complaint of menorrhagia? Am J Obstet Gynecol. 2004;190:1224–1229. doi: 10.1016/j.ajog.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Lukes A.S., Moore K.A., Muse K.N., Gersten J.K., Hecht B.R., Edlund M. Tranexamic acid treatment for heavy menstrual bleeding A randomized controlled trial. Obstet Gynecol. 2010;116:865–875. doi: 10.1097/AOG.0b013e3181f20177. [DOI] [PubMed] [Google Scholar]
- 22.Rahn D.D., Abed H., Sung V.W., Matteson K.A., Rogers R.G., Morrill M.Y. Systematic review highlights difficulty interpreting diverse clinical outcomes in abnormal uterine bleeding trials. J Clin Epidemiol. 2011;64:293–300. doi: 10.1016/j.jclinepi.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warner P., Critchley H.O., Lumsden M.A., Campbell-Brown M., Douglas A., Murray G. Referral for menstrual problems: cross sectional survey of symptoms, reasons for referral, and management. BMJ. 2001;323:24–28. doi: 10.1136/bmj.323.7303.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santer M., Wyke S., Warner P. What aspects of periods are most bothersome for women reporting heavy menstrual bleeding? Community survey and qualitative study. BMC Womens Health. 2007;7:8. doi: 10.1186/1472-6874-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukes A.S., Muse K.N., Richter H.E., Moore K.A., Patrick D.L. Estimating a meaningful reduction in menstrual blood loss for women with heavy menstrual bleeding. Curr Med Res Opin. 2010;26:2673–2678. doi: 10.1185/03007995.2010.526098. [DOI] [PubMed] [Google Scholar]
- 26.Krams M., Sharma A., Dragalin V., Burns D.D., Fardipour P., Padmanabhan S.K. Adaptive approaches in clinical drug development. opportunities and challenges in design and implementation. Pharm Med. 2009;23(3):139–148. [Google Scholar]
- 27.Chataway J., Nicholas R., Todd S., Miller D.H., Parsons N., Valdes-Marquez E. A novel adaptive design strategy increases the efficiency of clinical trials in secondary progressive multiple sclerosis. Mult Scler. 2011;17:81–88. doi: 10.1177/1352458510382129. [DOI] [PubMed] [Google Scholar]
- 28.Souverain P.C., Berard A., van Staa T.P., Cooper C., Leufkens H.G.M., Walker B.R. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population-based case-control study. Heart. 2004;90:859–865. doi: 10.1136/hrt.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Staa T.P., Leufkens H.G.M., Abenhaim L., Zhang B., Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 30.Kemp M.W., Newnham J.P., Challis J.G., Jobe A.H., Stock S.J. The clinical use of steroids in pregnancy. Hum. Reprod. Update. 2016;22(2):240–259. doi: 10.1093/humupd/dmv047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement A. DexFEM Proof-of-concept: Exploratory non-human primate study of the effect of dexamethasone on menstrual blood loss.
Supplement B. Additional material for DexFEM trial Methods.
Supplement C. Introduction to Bayesian concepts and definitions, and adaptive trial design.
Supplement D. Additional DexFEM trial Results.
DexFEM Statistics Analysis Plan.
DexFEM Protocol for 3 studies in grant.
DexFEM CONSORT checklists for Bayesian adaptive trial ABSTRACT and PAPER.
Supplement H. Links to papers already published from DexFEM.