Abstract
The restrictions adopted during the coronavirus disease 2019 (COVID-19) pandemic limiting direct medical consultations and access to healthcare centers reduced the participation of patients with chronic diseases, such as osteoporosis (OP), in screening and monitoring programs. This highlighted the need for new screening diagnostic tools that are clinically effective, but require minimal technical and time commitments, to stratify populations and identify who is more at risk for OP and related complications. This paper provides an overview of the potential use of blood-related factors, such as platelet (PLT)- and monocyte-related factors, as biomarkers able to quickly screen, detect, and monitor OP in both sexes. Such biomarkers might be of key importance not only during the COVID-19 pandemic but also, even more importantly, during periods of better global health stability.
Keywords: osteoporosis, COVID-19, platelets, monocyte, spontaneous osteoclastogenesis
The COVID-19 era
In just over a year, COVID-19 has impacted the world and spread like wildfire, transforming our societies from a local to a universal level [1]. In response to this global disaster, almost all countries developed extraordinary measures, including social isolation, travel bans, work restrictions, and nationwide lockdowns [2]. Although these stringent measures have been indispensable from a public health standpoint, they introduced challenges and difficulties in the management of many preexisting medical conditions that have suddenly altered common medical practices [2,3]. Currently, patients with chronic conditions could have difficulties in accessing health facilities and/or participating in monitoring and screening programs. The situation that has been unfolding in these past months is truly a ‘perfect storm’; we are facing an increasing aging population worldwide, particularly in Western countries, with related chronic diseases together with the COVID-19 pandemic (Box 1 ). This storm could lead to a worsening of the social impact of chronic and degenerative diseases considering the current difficulty with access to diagnostic tests and the ever-increasing inequality in the quality of health care worldwide.
Box 1. The Italian situation.
According to the numbers that emerged from a survey conducted for an Italian insurance broker by mUp Research and Norstat, it was found that 32.8 million Italians had their visits, exams, or operations scheduled for 2020 postponed if not canceled; specifically, about 27.9 million Italians (i.e., 73.6% of those who had scheduled an appointment at a health facility) underwent one or more referrals, while 13 million, equal to more than one in three patients (34.3%), had to deal with cancellation (https://www.tgcom24.mediaset.it/cronaca/tre-milionidi-italiani-hanno-rinunciato-a-cure-medicheper-difficolt-economiche-legate-al-covid_27827496-202102k.shtml).
Alt-text: Box 1
OP management in the COVID-19 era
The ‘COVID-19 era’ effect on chronic pathologies, such as OP, is truly dramatic if we consider how important it is to guarantee proper protocols for OP screening, diagnosis, treatment, and monitoring [4]. In this context, several centers activated telemedicine programs to continue OP management but limited studies on their role are currently available [5]. One study carried out at Women’s College Hospital in Toronto, Canada, reported that OP patients assessed by telemedicine presented a higher prevalence of fragility fractures, comorbidities, and need for allied health resources than those serviced by the outpatient clinic [6]. Considering that, worldwide, OP affects about 200 million people and causes more than 8.9 million fractures annually, resulting in an OP fracture every 3 seconds, appropriate protocols for patient screening, diagnosis, and monitoring in addition to telemedicine programs are mandatory [6,7]. This is particularly true in this moment [5,8], especially considering the unknown long-term effects of COVID-19 infection on bone metabolism [9,10].
Out of necessity, we are running fast toward the concept of hospital 4.0 without being fully prepared for the concept, where hospitals have the main task of treating acute patients, while chronic patients must be managed in their home with limited access to places of care [8]. With suspended and/or limited dual-energy X-ray absorptiometry (DXA) services in an effort to avoid exposing patients to clinical spaces, bone density (BMD) assessment for OP patients could be complex [11]. Thus, it is fundamental to stabilize the primary needs of OP patients and not leave them without proper support, since OP is a largely silent disease with a significant impact on patient’s quality of life [7]. Expert groups have already sounded the alarm on the public health emergency of OP; however, given the current times, there is a lapse of data on OP management during COVID-19, and these data are predominantly recommendations based on expert opinions [4]. What is clear is that, at present, there is an increased dependence on fracture risk calculators, such as fracture risk assessment tool (FRAX), that, however, are not based on BMD values [12]. FRAX uses a white female database for the calculation of 10-year fracture risk for the hip and other major OP fractures [4,12]. However, FRAX may identify fewer men for treatment and includes a concise set of clinical risk factors that does not take into consideration dietary factors and other chronic diseases that can potentially affect bone mass and quality [4,12,13]. Additionally, the usage of bone turnover markers has several drawbacks; although they are useful in differential diagnosis and early evaluation of patient response to treatment, they cannot be used for OP diagnosis [14,15]. Thus, the choice of the proper approach for OP patients in the COVID-19 era would be of key importance to stratify the population to identify those individuals more at risk for OP disease and related complications. Clinicians and researchers have always been devoted to the pursuit of innovative strategies able to detect and monitor OP in a simple, efficient, and effective way. Recent breakthroughs have highlighted different methods starting from noncoding RNAs [microRNAs (miRNAs) and long noncoding RNAs (lncRNAs)], proteins, enzymes, or hormones [15., 16., 17.]. However, what is really needed is a fast, predictable, easy to perform, minimally invasive method that is feasible in every laboratory and in every country and able to quickly screen, detect, and monitor OP in both female and male subjects. In other words, an appropriate method is required that involves minimal technical and time commitments, yet is capable of having great clinical impact.
Since in the COVID-19 era, OP should not become an unintended casualty, and every minor aspect could matter, here we give an overview on the potential use of blood markers, such as PLT- and monocyte-related factors (i.e., spontaneous osteoclastogenesis), as alternative and advanced methods for OP management. This paper provides great insight on these practical and quickly responsive methods that could facilitate physicians in the management of OP patients both during this new pandemic scenario and in the near future when we should be prepared for the new concept of hospital 4.0.
PLT-related factors as OP biomarkers
Osteoclasts (OCs), bone cells mainly responsible for bone resorption, originate from hematopoietic stem cells, while osteoblasts, the bone cells responsible for bone formation, control the survival and differentiation of hematopoietic stem cells [18]. Thus, it is straightforward to understand the presence of a close relationship between hematopoiesis and bone remodeling, considering that several studies found that PLTs, the cytoplasmic fragments derived from megakaryocytes, have a critical role in skeletal homeostasis, modulating bone formation and resorption [19,20]. An increasing number of clinical studies evaluated the relationship between PLTs and OP status based on BMD, considering and comparing healthy, osteopenic, and OP subjects [21., 22., 23., 24., 25., 26., 27., 28., 29.]. Additionally, a recent systematic review further underlined the strong correlation between PLT-related parameters and bone mineralization, finding a positive link between PLT size, distribution width, volume changes, and low BMD due to OP [30]. The option to use PLTs and PLT-related parameters, simply obtained from routine hematological investigation, to diagnose and correlate a specific pathological condition is unquestionably attractive. In this context, it was demonstrated that PLT count was associated with OP in a patient with chronic graft-versus-host disease [21], and, subsequently, the link between PLT count and OP status was further underlined, finding that PLT counts correlate with BMD in OP patients [22]. These studies demonstrated that PLT count may affect bone metabolism and particularly bone remodeling in middle-aged and elderly adults, detecting a positive association between PLT count and BMD in OP patients [21,22]. However, since these studies did not evaluate PLT volume (MPV) and PLT distribution width (PDW), which are also easily obtainable with routine hematological analyses, they cannot definitively conclude that the altered PLT counts reflect an altered PLT activation. Examining the relationship between MPV and PDW, specific early markers of PLT activation, and OP, other clinical studies demonstrated that the levels of these markers were negatively associated with OP and that they correlated with lumbar spine and femoral neck BMD [23., 24., 25.]. Thus, an accurate measurement of PLT count, MPV, and PDW in OP patients can be key factors for screening, diagnostic, and therapeutic purposes. In addition to these simple and easily obtainable markers, the PLT/lymphocyte ratio and PLT-activating factor (PAF) were also found to correlate with low BMD, particularly in reference to the femoral and lumbar district [26., 27., 28.]. In fact, elevated levels of these PLT markers were detected in OP patients in comparison to healthy subjects [26., 27., 28.]. PAF promotes osteoclastic bone resorption by the activation of PAF receptor signaling. However, the studies mentioned earlier on MPV indirectly confirmed this result on PAF by using a simpler and more inexpensive marker of PLT activation [23., 24., 25.]. Furthermore, since PLTs have vitamin D receptors, which are important in bone remodeling, it was found that PLT vitamin D receptor was less expressed in OP patients, thus reinforcing the concept that PLTs contribute to OP pathogenesis [29]. All together, these studies highlighted that specific PLT alterations characterize OP pathogenesis and specifically correlate with BMD value. Thus, OP leads to morphological and functional changes of PLTs, which can be detected in the blood by merely measuring the concentration of PLTs as well as defining and specifying PLT morphological alterations. It is also important to emphasize that these blood analyses did not require any additional tests and can be directly carried out with routine analyses, which can also be easily performed at home, particularly for elderly individuals, disabled individuals, or individuals with limited mobility. Although the exact mechanism involving PLT changes and alterations during OP has not yet been well elucidated, several potential mechanisms can be hypothesized: (i) chronic inflammation, which leads to an activation of PLTs, influencing osteoclastogenesis via prostaglandin and receptor activator of nuclear factor-κB ligand (RANKL) signaling; (ii) increased oxidative stress, which leads to PLT activation through the regulation of megakaryocyte proliferation, differentiation, and maturation and through the modification of adenosine diphosphate-induced PLT aggregation; and (iii) interaction between 25-hydroxyvitamin D with the vitamin D receptor [29,31., 32., 33.].
Monocyte-related factors: spontaneous osteoclastogenesis as OP biomarkers
OCs, multinucleated cells derived from cells of the monocyte/macrophage lineage present in bone marrow and in peripheral blood, play a key role during OP, where excessive OC generation and activation is present [34]. Peripheral blood mononuclear cells (PBMCs) are of critical importance during osteoclastogenesis since they act as OC precursors and secrete osteoclastogenesis-related factors [35]. Potentially, all skeletal OCs are mainly derived from monocytes that migrate to bone via the peripheral circulation; when monocytes reach the bone, they differentiate and fuse into immature multinuclear OCs, and, subsequently, at sites of bone resorption, they are activated to become mature OCs [35,36]. The activity and formation of OCs are regulated by several growth factors and cytokines produced in the human body by a wide range of cells [37]. The main critical factors for OC differentiation are macrophage colony-stimulating factor (M-CSF), RANKL, and the inhibitor of OC differentiation (i.e., osteoprotegerin), all cytokines released by osteocytes and bone lining cells/osteoblasts [37]. Additionally, RANKL can also be produced by T cells [38,39]. Since the role of these cytokines is in OC differentiation and activity, common in vitro culture methods of monocytes use specific exogenous stimulating factors (e.g., M-CSF and RANKL) to maintain cell viability and to induce osteoclastogenesis [35., 36., 37., 38., 39., 40.]. However, two recent systematic literature reviews highlighted that during OP, monocytes can maintain their vitality and differentiate into multinucleated OCs in the absence of added osteoclastogenesis-stimulating factors [41,42]. This phenomenon was referred by the authors as ‘spontaneous’, ‘un-stimulated’, or ‘self-stimulatory’ osteoclastogenesis [40., 41., 42.]. These studies are based on preclinical and clinical studies that suggest an activation of OCs by inflammatory mediators present in the plasma of animals/patients affected by OP or an intrinsic change of cells toward more osteoclastic differentiation [43., 44., 45., 46.]. From a methodological point of view, spontaneous osteoclastogenesis was simply tested by culturing PBMCs from OP female animals (ovariectomized) or from female and male OP patients for a few days without any addition of OC-stimulating factors and evaluating the ‘true’ spontaneous ability of PBMCs to maintain their viability and to generate OCs [43., 44., 45., 46.]. The results also revealed that the number, time, and speed of OC differentiation were higher for OP males than for OP females [44]. This evidence is in line with previous observations that showed a higher resorptive activity in male OCs [47., 48., 49., 50.]. All these observations suggest that spontaneous osteoclastogenesis could be a useful tool for screening, monitoring, and diagnosing OP, not only in female patients but also in male patients. This tool is based on a simple, rapid, and inexpensive test that requires only 2–3 ml of peripheral blood. Methodologically, the peripheral blood must be in vitro cultured to isolate PBMCs (time needed, ~1 h) and subsequently evaluated (starting from 2–3 days later), only by light microscopy, to assess the ability of monocytes to maintain their viability and subsequently to spontaneously differentiate into OCs. In a preliminary phase of the use of this new OP marker, based on the spontaneous osteoclastogenesis outcome, patients could or could not be directed toward additional diagnostic investigations.
As for PLT-related factors, currently, the exact mechanism linked to spontaneous osteoclastogenesis is not completely clear. A different production of pleiotropic cytokines able to regulate OC differentiation and function can be hypothesized. Since OCs derive from CD14+ cells, the PBMCs cultured in vitro allow for the coculture of both lymphocytes and monocytes [51]. Additionally, tumor necrosis factor-α (TNF-α) and interleukin-1α (IL-1α), cytokines mainly originating from monocytes and macrophages, are able to stimulate OC formation from PBMCs [51., 52., 53.]; further, T lymphocytes release pleiotropic cytokines, several of which regulate OC differentiation and function [53]. Thus, it is possible that adding the exogenous cytokines, always used to induce osteoclastogenesis in vitro, does not lead to the alteration of the endogenous differences in the release of these cytokines.
Discussion
COVID-19 has strongly influenced, and will likely continue to have a profound impact on, our lifestyles and healthcare systems. Staying at home, avoiding access to hospitals, and limiting medical interventions as much as possible are just some of the indications for reducing infection spread. The impact of these indications could lead to an increased risk of depression and domestic accidents, to a decreased absorption of vitamin D, and to a reduction of medical treatments in patients with preexistent comorbidities and/or patients already subjected to polytherapy. Since all these conditions could enhance OP risk and exacerbation, it is currently necessary to provide the best screening, diagnosing, and monitoring strategies possible for these patients as well as to reconsider and apply new advanced approaches. Evidently, this would require a reorientation of healthcare systems to meet the challenge set forth by OP, ensuring adequate care, health promotion, and treatment equity and avoiding the fragmentation of the clinical route. Over time, different biomarkers have been proposed for OP, among which those most discussed are specific multifunctional proteins (e.g., Annexin A2 and prolyl 4-hydroxylase) and circulating miRNAs (e.g., miR-223-3p, miR-148a-3p, miR-125b-5p, and miR-124-3p) [16,54,55]. However, the information related to these biomarkers during OP are still limited due to the different types of samples (serum, circulating monocytes, bone marrow, and bone tissue) obtained from patients of different sexes and different ethnic groups with low BMD or bone fractures and compared with healthy or osteoarthritis patients [48,49].
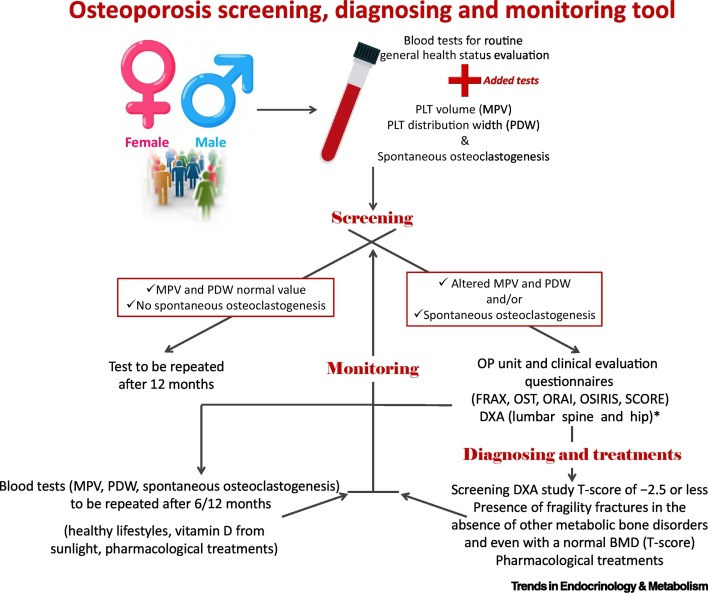
In this paper we propose the potential use of simple, readily available, and easy methods to perform OP screening, diagnosing, and monitoring in common clinical practice [i.e., PLT count and PLT-related parameters (MPV and PDW)] and spontaneous osteoclastogenesis. This paper highlighted how PLT count, PLT-related parameters, and spontaneous osteoclastogenesis could potentially be used to screen, diagnose, and monitor the presence of altered bone remodeling due to OP (Figure 1 ). Obviously, these data should be further validated by clinical trials for diagnostic tests able to demonstrate the precision, sensitivity, and specificity of these blood biomarkers, and non-osteoporotic patients should also be considered, in whom there are no altered PLT values and no spontaneous osteoclastogenesis; furthermore, eventual differences or biases related to age and/or sex or to other concurrent pathologies (e.g., cancers, osteolytic bone metastases, chronic inflammatory diseases, and viral infections and/or specific medications/drugs, such as chemotherapy, radiotherapy, anticoagulants, and anticonvulsants) that can affect PLTs and spontaneous osteoclastogenesis should also be evaluated [42,56]. However, these biomarkers could be complementary to existing traditional diagnostic and prognostic methods, since they could precede standard tests (i.e., DXA), discerning between patients who need these more exhaustive instrumental investigations. This aspect could also have important implications in terms of reduced health care costs, considering that the incidence of OP is expected to significantly enhance over the next decades due to the increase in life expectancy [57]. Furthermore, the idea of using blood biomarker panels alone and/or associated with other measurements and data from standard diagnostic methods has been attracting increasing interest considering the recent technological advances related to artificial intelligence, which already appears to be a promising technology capable of advancing research in the field of OP [58,59].
Figure 1.
Schematic representation to perform osteoporosis (OP) screening, diagnosing, and monitoring in the clinical practice using platelet (PLT) count and PLT-related parameters and spontaneous osteoclastogenesis.
*Despite that dual-energy X-ray absorptiometry (DXA) is the gold standard in screening and diagnosing OP, not everyone has access to bone density (BMD) testing by DXA [60]. An audit directed by the International Osteoporosis Foundation (IOF) on OP in Asia Pacific found that while Australia, Hong Kong, Japan, New Zealand, Republic of Korea, and Singapore were well resourced with 12–24 DXA machines per million individuals, China, India, Indonesia, Pakistan, Philippines, Sri Lanka, and Vietnam had less than 1 DXA machine per million [61]. Similarly, the IOF, in association with the European Federation of Pharmaceutical Industry Associations (EFPIA), evaluated DXA in the European Union and found that Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Italy, Portugal, and Slovenia had at least 11 DXA machines per million, while other countries (i.e., Bulgaria, Czech Republic, Hungary, Latvia, Lithuania, Luxembourg, Poland, Romania, and the United Kingdom) have insufficient DXA provisions [62]. It was also detected that the Latin American countries with the greatest access to DXA were Brazil and Chile, with 10 DXA machines per million, while other countries ranged from 0.9 to 6.7 per million [63].
Clearly the use of PLT count, PLT-related parameters, and spontaneous osteoclastogenesis do not solve all the problems related to OP patients during and after the COVID-19 lockdown; however, this could be a start in the right direction, giving clinicians immediately applicable and easily interpretable strategies to use for OP patients. The use of these complementary biomarkers is also attractive since they require only a blood sample that, with the strengthening of home-based health services due to the aging of the population and the ongoing COVID-19 pandemic, can be collected from patients directly in their own home, potentially helping to unburden overwhelmed healthcare systems.
Concluding remarks
As the rate of COVID-19 infection plateaus in much of the world, now is the moment to reflect on the ‘lessons’ learned that could, and almost certainly should, change the models that have been applied for OP management until recently. OP management is frequently, probably too frequently, carried out in a standardized way, and vulnerable populations, especially elderly patients and high-comorbidity patients, are usually on the passive receiving end of these standardized methods. Thus, we think that the use of PLT count, PLT-related parameters, and spontaneous osteoclastogenesis as more widely and simply available OP biomarkers could be of key importance not only in these times but also, even more importantly, during periods of better global health stability, which we anticipate will return soon (see Outstanding questions).
Outstanding questions.
Are there suitable biomarkers for evaluating an osteoporotic condition?
What could be the threshold levels to consider for each marker?
Which reservations should be considered in cases of comorbidities that could alter the validity of the dosages?
Are there biomarkers valid for diagnosis and monitoring and possibly evaluation of treatments over time?
Are there sex-related differences that might need to be highlighted?
What will the long-term consequences of the COVID-19 pandemic be on the screening, diagnosis, and follow-up programs of osteoporotic patients?
Alt-text: Outstanding questions
Acknowledgments
Acknowledgments
This work was supported by grants from IRCCS Istituto Ortopedico Rizzoli (Ricerca Corrente).
Declaration of interests
Two authors (F.S. and M.F.) of the present manuscript patented the spontaneous osteoclastogenesis method (European Patent granted on 21 March 2018 n. 3008470 deriving from the regional phase of the international application published in WO2014/199331 A1) that was described in this manuscript. The other authors have no interests to declare.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin M., et al. Preparing for COVID-19 exit strategies. Ann. Med. Surg. (Lond.) 2020;61:88–92. doi: 10.1016/j.amsu.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubulotta F., et al. Technologies to optimize the care of severe COVID-19 patients for health care providers challenged by limited resources. Anesth. Analg. 2020;131:351–364. doi: 10.1213/ANE.0000000000004985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu E.W., et al. Osteoporosis management in the era of COVID-19. J. Bone Miner. Res. 2020;35:1009–1013. doi: 10.1002/jbmr.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka M.J., et al. Telemedicine in the era of COVID-19: the virtual orthopaedic examination. J. Bone Joint Surg. Am. 2020;102 doi: 10.2106/JBJS.20.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston R., et al. Osteoporosis patients assessed by telemedicine: a unique high-risk cohort. J. Bone Miner. Res. 2015;30:S1. [Google Scholar]
- 7.Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 8.Afferni P., et al. Proceedings of IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS) 2018. Hospital 4.0 and its innovation in methodologies and technologies; pp. 333–338. Karlstad, Sweden. [Google Scholar]
- 9.Terpos E., et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95:834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artero A., et al. The adverse effects of estrogen and selective estrogen receptor modulators on hemostasis and thrombosis. Semin. Thromb. Hemost. 2012;38:797–807. doi: 10.1055/s-0032-1328883. [DOI] [PubMed] [Google Scholar]
- 11.The Lancet India under COVID-19 lockdown. Lancet. 2020;395:1315. doi: 10.1016/S0140-6736(20)30938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanis J.A., et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2020;31:801. doi: 10.1007/s00198-020-05303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippisley-Cox J., Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFracture Scores. BMJ. 2009;339:b4229. doi: 10.1136/bmj.b4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer D.C., et al. Pretreatment levels of bone turnover and the antifracture Efficacy of alendronate: the fracture intervention trial. J. Bone Miner. Res. 2006;21:292–299. doi: 10.1359/JBMR.051018. [DOI] [PubMed] [Google Scholar]
- 15.Eastell R., Pawel Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5:908–923. doi: 10.1016/S2213-8587(17)30184-5. [DOI] [PubMed] [Google Scholar]
- 16.Muraca M., Cappariello A. The role of extracellular vesicles (EVs) in the epigenetic regulation of bone metabolism and osteoporosis. Int. J. Mol. Sci. 2020;21:8682. doi: 10.3390/ijms21228682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciuffi S., et al. Circulating microRNAs as novel biomarkers for osteoporosis and fragility fracture risk: is there a use in assessment risk? Int. J. Mol. Sci. 2020;21:6927. doi: 10.3390/ijms21186927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto K., et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J. Exp. Med. 2011;208:2175–2181. doi: 10.1084/jem.20101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciovacco W.A., et al. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J. Cell. Biochem. 2010;109:774–781. doi: 10.1002/jcb.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kacena M.A., et al. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39:991–999. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Pirsl F., et al. Characterization and risk factor analysis of osteoporosis in a large cohort of patients with chronic graft-versus host disease. Biol. Blood Marrow Transplant. 2016;22:1517–1524. doi: 10.1016/j.bbmt.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.L., et al. The positive association between peripheral blood cell counts and bone mineral density in postmenopausal women. Yonsei Med. J. 2011;52:739–745. doi: 10.3349/ymj.2011.52.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.S., et al. Mean platelet volume is negatively associated with bone mineral density in postmenopausal women. J. Bone Miner. Metab. 2012;30:660–665. doi: 10.1007/s00774-012-0362-4. [DOI] [PubMed] [Google Scholar]
- 24.Aypak C., et al. Association between mean platelet volume and bone mineral density in postmenopausal women. J. Phys. Ther. Sci. 2016;28:1753–1758. doi: 10.1589/jpts.28.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbal A., et al. Mean platelet volume and platelet distribution width can be related to bone mineralization. Osteoporos. Int. 2014;25:2291–2295. doi: 10.1007/s00198-014-2764-8. [DOI] [PubMed] [Google Scholar]
- 26.Eroglu S., Karatas G. Platelet/lymphocyte ratio is an independent predictor for osteoporosis. Saudi Med. J. 2019;40:360–366. doi: 10.15537/smj.2019.4.24009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H., et al. Higher plasma platelet-activating factor levels are associated with increased risk of vertebral fracture and lower bone mineral density in postmenopausal women. J. Bone Miner. Metab. 2015;33:701–707. doi: 10.1007/s00774-014-0634-2. [DOI] [PubMed] [Google Scholar]
- 28.Koseoglu S.B. Bone loss & platelet-to-lymphocyte ratio. Biomark. Med. 2017;11:5–10. doi: 10.2217/bmm-2016-0188. [DOI] [PubMed] [Google Scholar]
- 29.D’Amelio P., et al. Platelet vitamin D receptor is reduced in osteoporotic patients. Panminerva Med. 2012;54:225–231. [PubMed] [Google Scholar]
- 30.Salamanna F., et al. Platelet features and derivatives in osteoporosis: a rational and systematic review on the best evidence. Int. J. Mol. Sci. 2020;21:1762. doi: 10.3390/ijms21051762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koupenova M., et al. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018;122:337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Q., et al. Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis. Markers. 2016;2016 doi: 10.1155/2016/7067984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvagno F., et al. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodan G., Martin T.J. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Solal M.E., et al. Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokines. J. Clin. Endocrinol. Metab. 1993;77:1648–1653. doi: 10.1210/jcem.77.6.8263153. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson G.C., et al. Induction of osteoclasts from CD14-positive human peripheral blood mononuclear cells by receptor activator of nuclear factor κB ligand (RANKL) Clin. Sci. (Lond.) 2000;99:133–140. [PubMed] [Google Scholar]
- 37.Kudo O., et al. Proinflammatory cytokine (TNFα/IL-1α) induction of human osteoclast formation. J. Pathol. 2002;198:220–227. doi: 10.1002/path.1190. [DOI] [PubMed] [Google Scholar]
- 38.Kong Y.Y., et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 39.Colucci S., et al. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood. 2004;104:3722–3730. doi: 10.1182/blood-2004-02-0474. [DOI] [PubMed] [Google Scholar]
- 40.Brunetti G., et al. T cells support osteoclastogenesis in an in vitro model derived from human periodontitis patients. J. Periodontol. 2005;76:1675–1680. doi: 10.1902/jop.2005.76.10.1675. [DOI] [PubMed] [Google Scholar]
- 41.de Vries T.J., et al. What are the peripheral blood determinants for increased osteoclast formation in the various inflammatory diseases associated with bone loss? Front. Immunol. 2019;10:505. doi: 10.3389/fimmu.2019.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salamanna F., et al. Peripheral blood mononuclear cells spontaneous osteoclastogenesis: mechanisms driving the process and clinical relevance in skeletal disease. J. Cell. Physiol. 2016;231:521–530. doi: 10.1002/jcp.25134. [DOI] [PubMed] [Google Scholar]
- 43.Salamanna F. In vitro method for the screening and monitoring of estrogen-deficiency osteoporosis by targeting peripheral circulating monocytes. Age (Dordr.) 2015;37:9819. doi: 10.1007/s11357-015-9819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salamanna F., et al. Spontaneous osteoclastogenesis: hypothesis for gender-unrelated osteoporosis screening and diagnosis. Med. Hypotheses. 2017;109:70–72. doi: 10.1016/j.mehy.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 45.D'Amelio P., et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 46.D'Amelio P., et al. Spontaneous osteoclast formation from peripheral blood mononuclear cells in postmenopausal osteoporosis. FASEB J. 2005;19:410–412. doi: 10.1096/fj.04-2214fje. [DOI] [PubMed] [Google Scholar]
- 47.Jevon M., et al. Gender- and age-related differences in osteoclast formation from circulating precursors. J. Endocrinol. 2002;172:673–681. doi: 10.1677/joe.0.1720673. [DOI] [PubMed] [Google Scholar]
- 48.Merrild D.M., et al. Pit- and trench-forming osteoclasts: a distinction that matters. Bone Res. 2015;3:15032. doi: 10.1038/boneres.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michelsen J., et al. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone. 2013;57:399–404. doi: 10.1016/j.bone.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Minisola S., et al. Gender differences in serum markers of bone resorption in healthy subjects and patients with disorders affecting bone. Osteoporos. Int. 2002;13:171–175. doi: 10.1007/s001980200009. [DOI] [PubMed] [Google Scholar]
- 51.Shalhoub V., et al. Characterization of osteoclast precursors in human blood. Br. J. Haematol. 2000;111:501–512. doi: 10.1046/j.1365-2141.2000.02379.x. [DOI] [PubMed] [Google Scholar]
- 52.Mundy G.R. Cytokines and growth factors in the regulation of bone remodeling. J. Bone Miner. Res. 1993;8:S505–S510. doi: 10.1002/jbmr.5650081315. [DOI] [PubMed] [Google Scholar]
- 53.Kotake S., et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:1003–1012. doi: 10.1002/1529-0131(200105)44:5<1003::AID-ANR179>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 54.Bottani M., et al. The clinical potential of circulating miRNAs as biomarkers: present and future applications for diagnosis and prognosis of age-associated bone diseases. Biomolecules. 2020;10:589. doi: 10.3390/biom10040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donati S., et al. Circulating miRNAs: a new opportunity in bone fragility. Biomolecules. 2020;10:927. doi: 10.3390/biom10060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panday K., et al. Medication-induced osteoporosis: screening and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2014;6:185–202. doi: 10.1177/1759720X14546350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewiecki E.M., et al. Treat-to-target for osteoporosis: is now the time? J. Clin. Endocrinol. Metab. 2013;98:946–953. doi: 10.1210/jc.2012-3680. [DOI] [PubMed] [Google Scholar]
- 58.Ferizi U., et al. Artificial intelligence applied to osteoporosis: a performance comparison of machine learning algorithms in predicting fragility fractures from MRI data. J. Magn. Reson. Imaging. 2019;49:1029–1038. doi: 10.1002/jmri.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim H.K., et al. Prediction of femoral osteoporosis using machine-learning analysis with radiomics features and abdomen-pelvic CT: a retrospective single center preliminary study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clynes M.A., et al. Bone densitometry worldwide: a global survey by the ISCD and IOF. Osteoporos. Int. 2020;31:1779–1786. doi: 10.1007/s00198-020-05435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mithal A., et al. The Asia-Pacific regional audit-epidemiology, costs, and burden of osteoporosis in India 2013: a report of International Osteoporosis Foundation. Indian J. Endocrinol. Metab. 2014;18:449–454. doi: 10.4103/2230-8210.137485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernlund E., et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harvey N.C., et al. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos. Int. 2007;28:1507–1529. doi: 10.1007/s00198-016-3894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]