Abstract
Purpose
This study assessed whether the newly developed PET radioligand [11C]PS13, which has shown excellent in vivo selectivity in previous animal studies, could be used to quantify constitutive levels of cyclooxygenase-1 (COX-1) in healthy human brain.
Methods
Brain test-retest scans with concurrent arterial blood samples were obtained in 10 healthy individuals. The one- and unconstrained two-tissue compartment models, as well as the Logan graphical analysis were compared, and test-retest reliability and time-stability of total distribution volume (VT) were assessed. Correlation analyses were conducted between brain regional VT and COX-1 transcript levels provided in the Allen Human Brain Atlas.
Results
In the brain, [11C]PS13 showed highest uptake in the hippocampus and occipital cortex. The pericentral cortex also showed relatively higher uptake compared with adjacent neocortices. The two-tissue compartment model showed the best fit in all the brain regions, and the results from the Logan graphical analysis were consistent with those from the two-tissue compartment model. VT values showed excellent test-retest variability (range 6.0–8.5%) and good reliability (intraclass correlation coefficient range 0.74–0.87). VT values also showed excellent time-stability in all brain regions, confirming that there was no radiometabolite accumulation and that shorter scans were still able to reliably measure VT. Significant correlation was observed between VT and COX-1 transcript levels (r = 0.82, P = 0.007), indicating that [11C]PS13 binding reflects actual COX-1 density in the human brain.
Conclusions
These results from the first-in-human evaluation of the ability of [11C]PS13 to image COX-1 in the brain justifies extending the study to disease populations with neuroinflammation.
Clinical trial registration
NCT03324646 at https://clinicaltrials.gov/. Registered October 30, 2017. Retrospectively registered.
Keywords: Cyclooxygenase-1, Positron emission tomography, Inflammation, Test-retest reliability, Neuroimaging
Introduction
Cyclooxygenase (COX) is an essential enzyme in the synthesis process of pro-inflammatory prostanoids from arachidonic acid, where the COX system works as the rate-limiting step [1]. Two primary isoforms of COX have been characterized, i.e., COX-1 and COX-2. It is widely thought that COX-1 is constitutively expressed and that COX-2 is induced in response to inflammatory stimuli in most tissues. However, the distribution and action of COX isoforms in the brain is more complicated than this conventional dogma, and mixed findings have been observed in the literature. For instance, recent brain studies suggest that COX-2 is also constitutively expressed in neurons and that COX-1 is primarily localized in microglia, contributing to pro-inflammatory responses in neurodegenerative diseases [2-4]. Although the precise pro-inflammatory mechanism of COX-1 is unknown, it has been hypothesized that COX-1 expression is increased in activated microglia and that this, in turn, enhances the production of potent oxygen free radicals that facilitate cytotoxicity and neuronal loss in neurodegenerative diseases [5]. Thus, COX-1 has two important aspects as a potential biomarker for neuroinflammation, i.e., (1) co-localization with microglia and (2) pro-inflammatory activity.
Our laboratory recently developed [11C]PS13, a novel positron emission tomography (PET) radioligand for COX-1 [6, 7]. Notably, [11C]PS13 is the first PET radioligand that directly binds to COX-1 and is not a prodrug-type radioligand. Previous monkey studies from our laboratory demonstrated the excellent in vivo selectivity of this ligand in major organs such as the spleen, gastrointestinal tract, kidneys, and brain [8], justifying the extension of studies into healthy human individuals, an essential next step in developing [11C]PS13 as a useful neuroinflammatory biomarker in neurodegenerative diseases such as Alzheimer’s disease.
This study sought to evaluate [11C]PS13 in healthy human individuals. In particular, the kinetic modeling, test-retest reliability, and time-stability of [11C]PS13 imaging, as well as the postmortem correlation of its binding distribution in the brain, were observed.
Material and methods
Radiopharmaceutical preparation
[11C]PS13 was prepared as previously described [6], with a molar activity of 97.3 ± 36.6 GBq/μmol at the time of injection under our Investigational New Drug (IND) Application 136,241. Radiochemical purity was 99.3 ± 0.5%.
Participants
Ten individuals participated in this study, i.e., six male and four female, ages 29.3 ± 7.2 years, and body weight 79.9 ± 16.9 kg (mean ± SD). All participants were medically and psychiatrically healthy based on their medical history, physical examination, blood and urine laboratory testing, and electrocardiogram. None of the participants had taken any kind of nonsteroidal anti-inflammatory drugs for two weeks or aspirin for four weeks prior to the PET scans. Informed consent was obtained from all individual participants included in the study, in accordance with the National Institutes of Health (NIH) Combined Neurosciences Institutional Review Board (Protocol 17-M-0179).
Image data acquisition
Brain PET scans were obtained on a Biograph mCT (Siemens Healthineers, Erlangen, Germany) scanner in three dimensions; transmission data for attenuation correction were acquired by a computed tomography (CT) scan before radioligand injection. Following a 1-m intravenous bolus injection of [11C]PS13 (690.2 ± 40.6 MBq), brain dynamic emission scans were obtained for 120 min in 33 frames (6 × 0.5 min, 3 × 1 min, 2 × 2 min, and 22 × 5 min). A head holder was used to minimize head motion between CT and PET scans as well as during PET acquisitions. PET images were reconstructed with ordered subset expectation maximization (OSEM) with time of flight (TOF) and resolution recovery using the following parameters: 3 iterations, 21 subsets, 200 matrix size, 2.0 zoom, Gaussian post-filter with 2.0 mm full width at half maximum (FWHM). For test-retest comparisons, two brain scans were obtained on the same day in each participant with the radioligand injections separated by more than 2.5 h.
Three-dimensional T1-weighted magnetic resonance images (MRI) were obtained in all participants within three months from the PET scans, using a 3-T Philips Achieva scanner (Philips Healthcare, Andover, MA, USA) with turbo field echo sequence (repetition time = 8.1 ms, echo time = 3.7 ms, flip angle = 8, matrix = 181 × 256 × 256, voxel size = 1 × 0.983 × 0.983 mm).
Blood analyses
To determine radioligand plasma concentrations, arterial blood samples were drawn from the radial artery concurrently with the PET scans. They were drawn continuously for the first 2.5 min (sampled at 5 mL/min) using a continuous sampling system (PBS-101, COMECER Netherlands, Joure, Netherlands), and discretely at 3, 5, 10, 15, 30, 45, 60, 90, and 120 min by manual sampling. The continuous blood data for the first 2.5 min and later discrete blood data were combined to form a whole blood activity curve that covered the entire duration of the scan. In each discrete sample, the concentration of parent radioligand was measured after separating plasma from the whole blood as previously described [9]. The total plasma to whole blood ratio and the plasma parent fraction (i.e., the ratio of parent radioligand radioactivity to total plasma radioactivity) measured at 3 min were applied to the earlier continuous whole blood data to calculate the total plasma and parent plasma concentrations for the first 2.5 min. Because the overall shape of the plasma parent fraction curve was not consistent with any previously reported conventional model [10], the raw value of plasma parent fraction measured at each time point was directly multiplied by the corresponding total plasma concentration to obtain the parent plasma concentration in each scan. This parent plasma concentration was fitted to a tri-exponential function after relative weighting.
Plasma-free fractions for [11C]PS13 were measured in triplicate for each scan and in pooled human arterial plasma (stored at −80 °C). The latter served as the standard for normalization across scans [11]. Measurements were performed by ultrafiltration through membrane filters as previously described [12].
Additional in vitro experiments using the whole blood were conducted to evaluate the potential effect of blood cells on [11C]PS13 distribution over time. Under the assumption of negligible species differences, the experiment on blood cell uptake was conducted using the monkey blood. The arterial blood obtained from monkey or human was anticoagulated using heparin. To assess blood cell uptake, 1.7 MBq of [11C]PS13 was mixed well with 6 mL of the monkey whole blood, and the blood samples were periodically removed to quantify the total radioactivity in the whole blood and the separated plasma. To assess blood cell release, 0.2 MBq of [11C]PS13 was mixed well with 15 mL of the human whole blood and incubated for 40 min to achieve equilibrium. After the blood cells were harvested by centrifugation and the radioactive plasma was replaced by the same volume of nonradioactive plasma, the blood samples were periodically removed to quantify the total radioactivity in the whole blood and the separated plasma. The experiment on blood cell uptake was conducted at room temperature and at 37 °C, while that on blood cell release was conducted at room temperature only. Hematocrits were determined by capillary centrifugation. Blood cell radioactivity concentrations were calculated as follows [13]:
Here, CBlood cells, Cwhole blood, and Cplasma indicate radioactivity concentrations (cpm/mL) in the blood cells, whole blood, and plasma, respectively, and Hct indicates hematocrit.
Tracer kinetic modeling and test-retest reliability in brain
Brain PET images were calibrated to Bq/mL and analyzed using PMOD (PMOD Technologies LLC, Zürich, Switzerland). PET images were reviewed and corrected for motion by realigning individual frames to an averaged image from 2 to 30 min post-injection using a rigid-matching algorithm. For region-of-interest (ROI)-based analyses, the realigned PET images were coregistered to the MRI by a rigid-matching procedure based on mutual information. Each coregistered PET image was reviewed and manually adjusted when the coregistration was not optimal. A set of 83 predefined regions from the Hammers N30R83 maximum probability atlas was adjusted to the MRI scan of individual participants using the PNEURO module of PMOD [14]. These were segmented from the spatially normalized individual MRIs and applied to the dynamic PET images. For better representation and noise reduction, the 83 ROIs were combined into nine gray matter–masked consolidated ROIs using the weighted average based on the number of voxels in each region. The nine ROIs were chosen based on the commonly used subdivision of the brain into the cortical lobes and subcortical structures.
The kinetic components of [11C]PS13 tissue uptake were initially explored using spectral analysis as previously described [15]. Then, total distribution volume (VT) in each ROI was calculated using the one-tissue compartment model [16], the two-tissue compartment model [17], and Logan graphical analysis [18]. In the processes of model fitting, brain data were weighted by assuming Poisson weighting. For the Logan graphical analysis, t* was automatically determined by allowing an error criterion of 20% (i.e., 9.0 ± 5.2 min in 20 scans). The relative performance of kinetic models was assessed via the Akaike information criterion (AIC) and F-tests [19]. To visualize COX-1 distribution in the brain, [11C]PS13 parametric images—where each voxel represents VT—were generated using the Logan graphical analysis in the PXMOD module of PMOD. Test-retest repeatability of the obtained outcome parameters was examined by calculating relative test-retest variability (TRV) and the absolute TRV (aTRV), and reliability was assessed using the intraclass correlation coefficient (ICC) of VT estimated using the two-tissue compartment model [20-22]. The TRV and aTRV were calculated as follows:
The time-stability of VT values was evaluated by fitting the regional time-activity curves for PET data with truncated acquisition times using the two-tissue compartment model, ranging from 120 to 20 min. The ratio of the regional VT values from the truncated scan to that from the 120-min measurement was computed for each ROI.
COX-1 transcript as a surrogate of target density
The messenger RNA (mRNA) transcription maps of PTGS1, the coding gene of COX-1 obtained from six donors, were available in the Allen Human Brain Atlas (https://human.brain-map.org/) [23]. Two probes related to the expression of PTGS1 were available for each donor: A_23_P216966 and A_24_P64167. Regional mRNA values were expressed in log2 scale. For easier comparison with the consolidated ROI-based VT values of [11C]PS13, the 26 regional transcription values provided in the maps were averaged into nine regional transcription values. The correlation between [11C] PS13 VT values from 10 participants and the PTGS1 transcription levels measured by each probe from six donors was analyzed using Spearman’s rank-order correlation (IBM SPSS Statistics 25; Chicago, IL, USA).
Results
Pharmacological effects
Participants received an injected chemical dose of 0.11 ± 0.06 nmol/kg [11C]PS13. This dose caused no pharmacological effects in any participant during the 120-min PET scan, as assessed by patient reports, blood pressure, pulse, temperature, respiratory rate, electrocardiogram, and basic laboratory tests.
Kinetic modeling and distribution of brain binding
When a representative high-performance liquid chromatography (HPLC) radiochromatogram was obtained from an arterial plasma sample 60 min post-[11C]PS13 injection, more than five radiometabolites were detected with clear separation from the parent peak (Fig. 1A). The plasma parent fraction of [11C]PS13 in the arterial blood decreased until 60 min post-intravenous administration and then slightly increased with higher variability at 90 or 120 min in both test and retest conditions (Fig. 1B). Despite the atypical shape of the plasma parent fraction curve, the overall measurement of [11C]PS13 plasma concentrations followed a typical tri-exponential curve with low variability (Fig. 1C, D, and Supplementary Fig. S1).
Fig. 1.
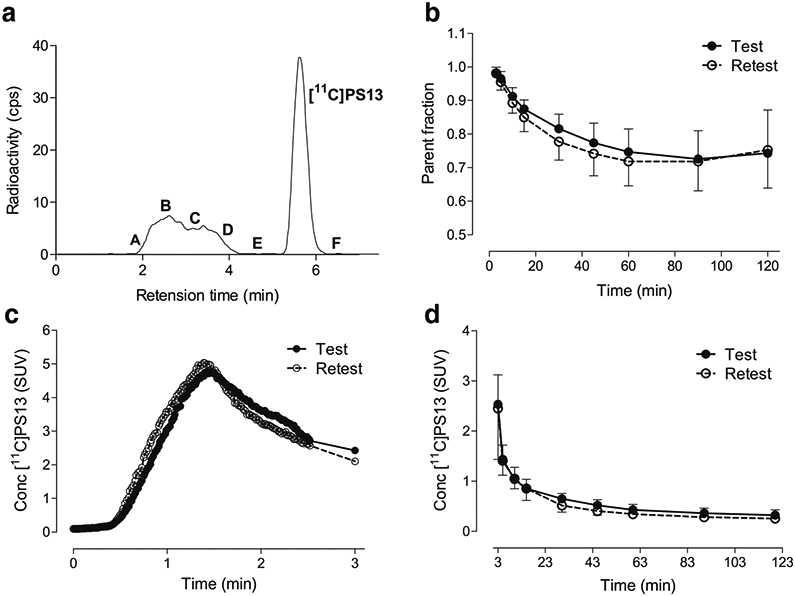
Analysis of arterial plasma after intravenous injection of [11C]PS13 in 10 healthy human individuals. (A) Reversed phase radio high-performance liquid chromatography (HPLC) analysis of plasma at 60 min in a participant. At least six radiometabolites were detected; A = 10.5%; B = 14.5%; C = 5%; D = 12.1%; E = 0.4%; Parent = 57.4%; F = 0.1%. (B) Change of plasma parent fraction over time. (C) Plasma parent concentration during the earliest 3 min after injection. (D) Plasma parent concentration from 3 min post-injection. Data are mean ± SD in B and D, and mean in C, where the SD was not displayed for the sake of clarity. SUV, standardized uptake value
To assess whether this atypical pattern of plasma parent fraction was associated with significant uptake and release of [11C]PS13 in blood cells, additional in vitro experiments using the monkey and human whole blood were conducted. [11C]PS13 uptake into blood cells was rapid, occurring within 5 min, followed by a slow and sustained release to plasma over 80 min; this suggests that blood cell–released [11C]PS13 may have contributed to the increase of plasma parent fraction after 60 min (Supplementary Fig. S2). Given that the plasma concentration of [11C]PS13 decreased tri-exponentially over time, the sustained release of [11C]PS13 from blood cells to plasma illustrated in Supplementary Fig. S2(B) could have resulted in increasing patterns of plasma parent fraction after 60 min due to the relatively low [11C]PS13 concentrations in plasma. However, this should not have affected measurements at earlier time points because the amount of [11C]PS13 released from blood cells was relatively negligible compared with the initially high [11C]PS13 concentrations in plasma.
While no significant differences were observed in the overall measure of [11C]PS13 plasma concentrations between test and retest conditions (Fig. 1 C and D), plasma-free fraction between test and retest conditions was highly variable (aTRV = 23.2%, Supplementary Fig. S3). Moreover, the triplicate measurements for each scan and in pooled human arterial plasma also showed high ranges of variability (percent coefficient of variation ranges 0.4–16.7% for individual participants and 0.6–8.7% for the pooled plasma).
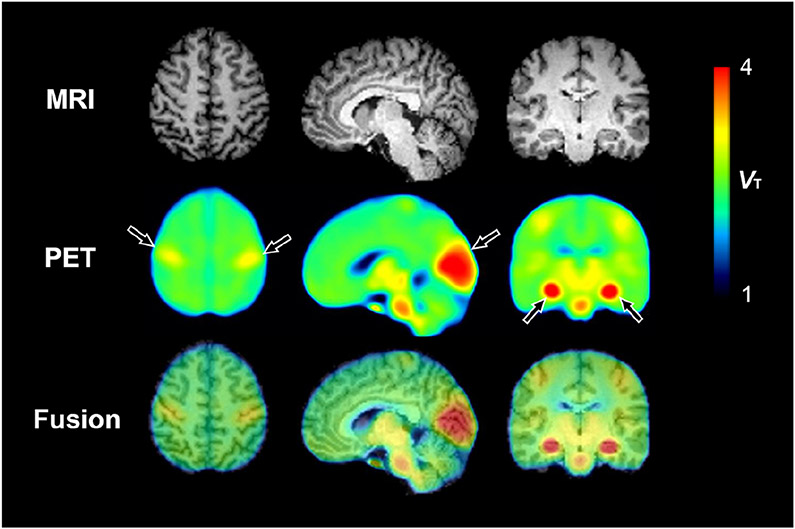
The highest binding of [11C]PS13 in the brain was observed in the hippocampus, occipital cortex, thalamus, and brainstem (Fig. 2 and Supplementary Table S1). Notably, the pericentral cortex also showed relatively higher binding than adjacent neocortical areas. Representative time-activity curves in brain regions with high or low uptake are depicted in Fig. 3. In spectral analysis, the majority of brain regions in all subjects displayed two equilibrating components. When compared by both F-test and the AIC, the two-tissue compartment model showed better fit than the one-tissue compartment model in all brain regions for all 20 scans (Supplementary Table S1). Because the results from the Logan graphical analysis at the regional level were consistent with those from the two-tissue compartment model (Supplemental Table S1), the voxel-based parametric maps of VT illustrated in Fig. 2 were generated using the Logan graphical analysis.
Fig. 2.
Parametric total distribution volume (VT) images calculated by the Logan graphical analysis method. MRI is from a representative participant, and PET images are averaged from 20 scans in 10 participants. Arrows represent notable [11C]PS13 binding in the pericentral cortex, occipital cortex, and hippocampus
Fig. 3.
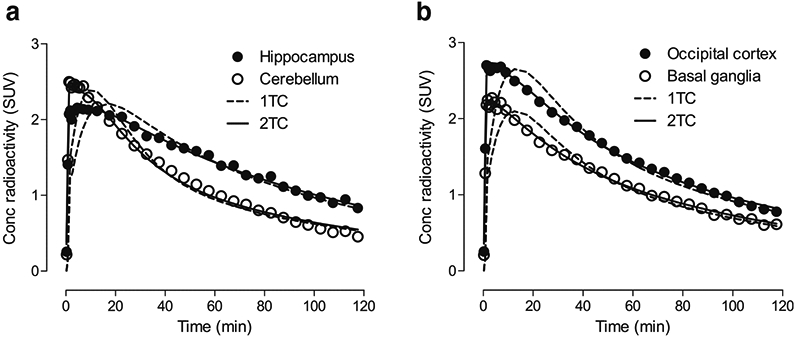
Time-activity curves of representative brain regions in a participant fitted with the two-tissue compartment model (2TC) and one-tissue compartment model (1TC)
Test-retest reliability and time-stability
Using the two-tissue compartment model, VT values in all brain regions showed excellent test-retest variability (aTRV range 6.0–8.5%) and good reliability (ICC range 0.74–0.87; Table 1).
Table 1.
Test-retest repeatability and reliability of total distribution volume (VT) of [11C]PS13 derived with the two-tissue compartment model from 120 min duration scans (n = 10)
| Region | VT (mL/cm3) | ||
|---|---|---|---|
| aTRV (%) | TRV (%) | ICC | |
| Frontal cortex | 6.6 | 3.4 | 0.84 |
| Hippocampus | 8.5 | 5.7 | 0.78 |
| Temporal cortex | 6.6 | 3.7 | 0.82 |
| Parietal cortex | 8.5 | 5.6 | 0.74 |
| Occipital cortex | 7.1 | 4.6 | 0.83 |
| Basal ganglia | 7.0 | 3.6 | 0.86 |
| Thalamus | 6.3 | 2.6 | 0.87 |
| Cerebellum | 6.0 | 2.9 | 0.86 |
| Brainstem | 7.6 | 3.4 | 0.81 |
| Whole brain | 6.4 | 3.8 | 0.84 |
VT, total distribution volume; aTRV, absolute test-retest variability; TRV, test-retest variability; ICC, intraclass correlation coefficient
To assess the time-stability of the outcome measures, the effect of the duration of the PET scan on VT estimated via the two-tissue compartment model was investigated. The normalized VT values to 120-min values were plotted as a function of the duration of image acquisition in Fig. 4, which shows that VT values for the hippocampus, occipital cortex, and cerebellum were within 10% of the 120-min values by 40–60 min in most scans.
Fig. 4.
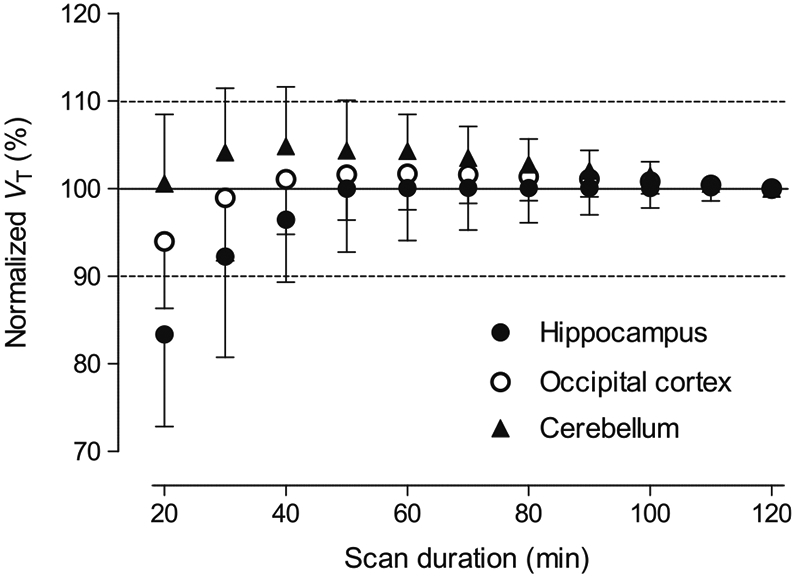
Time-stability analysis. Total distribution volume (VT) results were calculated by the two-tissue compartment model and normalized to the values measured by 120 min duration scans (n = 20). Data are mean ± SD
Correlation between [11C]PS13 binding and COX-1 transcript level
When VT values in nine brain regions were plotted as a function of the COX-1 transcript levels measured by PTGS1 mRNA expression, two mRNA probes provided different results. The levels measured by probe A_24_P64167 showed a high positive correlation with the [11C]PS13 VT values (r = 0.82, P = 0.007, Fig. 5); in contrast, those measured by probe A_23_P216966 showed no significant correlation (r = 0.37, P = 0.33), likely because of its low sensitivity for the target [24, 25]. When the data from probe A_24_P64167 were chosen for comparison, both [11C]PS13 VT values and COX-1 transcript levels showed consistently highest values in the hippocampus and lowest values in the cerebellum.
Fig. 5.
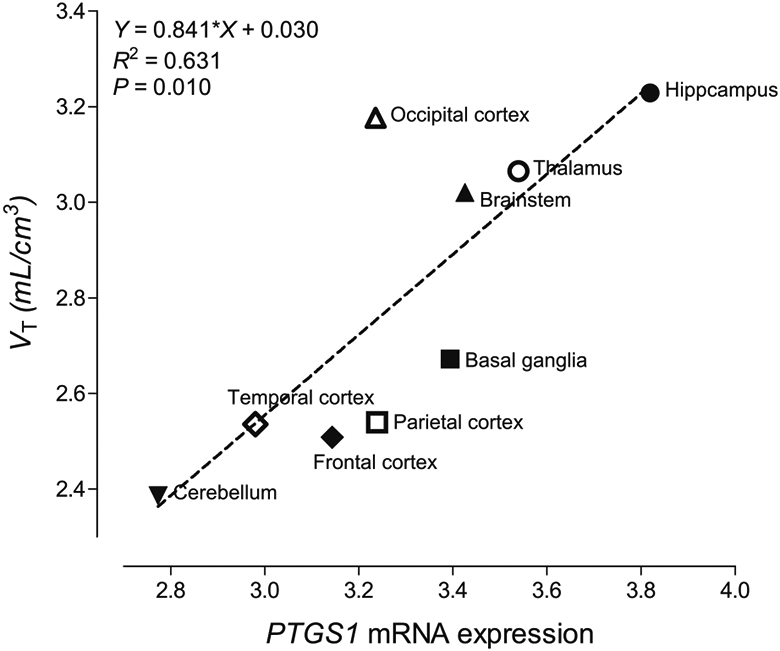
Correlation between in vivo brain total distribution volume (VT) of [11C]PS13 (n = 10) and ex vivo PTGS1 mRNA expression levels measured by probe A_24_P64167 provided in the Allen Human Brain Atlas (n = 6). Please note that, although [11C]PS13 binding is higher in pericentral cortices, the VT values of the parietal and frontal regions are not particularly high because they derive from a larger region encompassing the whole lobe
Discussion
The present study is the first-in-human evaluation of the ability of [11C]PS13 to image COX-1 in the brain. Kinetic modeling indicated that the two-tissue compartment model was preferable, and its results were consistent with those obtained with the Logan graphical analysis. VT of [11C]PS13 estimated via the two-tissue compartment model showed excellent test-retest reliability and time-stability, and also correlated well with the ex vivo mRNA transcript levels of COX-1 in the human brain.
An unexpected increase of plasma parent fraction 60 min post-radioligand injection was observed when plasma [11C]PS13 levels were measured. This trend was consistently observed across different individuals for both test and retest conditions, although the extent of the increase varied among individuals. It is possible that this was due to the significant uptake and release of [11C]PS13 in blood cells—most likely in platelets, which have high levels of COX-1—as hypothesized in our previous study [8] and also supported by the in vitro results (Supplementary Fig. S2). However, it is unclear whether this re-distribution of [11C]PS13 from blood cells to plasma happens before or after the blood is drawn, which also makes it uncertain whether the phenomenon should be taken into account in the kinetic modeling of plasma input function or not. Other potential explanations exist, including the more rapid clearance of radiometabolites versus parent radioligand at later time points, which could also have resulted in increased plasma parent fraction. Further investigations in larger populations with a variety of physiologic and pathologic conditions are required to better interpret these results.
Another notable finding was the extremely low free fraction in plasma of [11C]PS13 compared with other radioligands (0.003 ± 0.001, Supplementary Fig. S3). Such low free fraction measures tend to have quite high variability between test and retest conditions, as opposed to the small variability observed in other parameters such as VT, plasma parent concentration, and brain standardized uptake value (SUV, calculated by dividing measured radioactivity concentration by injected radioactivity and body weight). Therefore, the data were not corrected for free fraction under the assumption that such a small value with high variability could induce unnecessary variance and perhaps even bias in the analyses of test-retest reliability. The contributing factors for this variability in free fraction measurements are largely unknown, although potential factors such as physiological fluctuation of plasma composition due to diurnal variation or diet may have contributed to the results. Limited technical precision for measuring such a small value without significant noise may have contributed to the results as well, being suggested by the wide range of coefficient of variation in the measurements of each triplicate. It is speculated that this excessively high variability in the measurement of free fraction outweighs the biological change of free fraction in certain pathologic conditions, although further validation in patient populations compared with the healthy population is required.
Taken together, the results indicate that [11C]PS13 VT values in all brain regions showed excellent test-retest repeatability and reliability without correction for free fraction. Although the within-subject variability was exceptionally low (range 6.0–8.5%), none of the brain regions showed ICC higher than 0.9, perhaps due to the small number of participants and the relatively low between-subject variability, as has been observed with other radioligands [20]. The low between-subject variability may be related to the general characteristics of COX-1 in the healthy brain, including that COX-1 is constitutively expressed and contributes to physiological functions and homeostasis, which may require relatively constant expression levels across individuals. Similarly, the between-subject variability measured in a patient population with varying levels of COX-1 upregulation would be expected to be higher, particularly in brain regions with increased inflammatory activity; this, in turn, would have resulted in higher ICC than those measured in the current sample of healthy human adults. Therefore, it would be worthwhile to also assess test-retest reliability in a representative population of neuroinflammatory diseases such as Alzheimer’s disease.
Evaluation of the time-stability of VT measured by the two-tissue compartment model confirmed that no radiometabolite accumulated in the brain and that shorter scans (up to 40–60 min) still reliably measured VT. Moving forward, these characteristics may represent a significant advantage, given that [11C]PS13 is a candidate PET radioligand for evaluating neurodegenerative disorders, and that patients with neurodegenerative disorders such as Alzheimer’s disease are generally expected to be less tolerant of longer duration scans than healthy individuals.
In the present study, the highest binding of [11C]PS13 in the brain was observed in the hippocampus, occipital cortex, thalamus, and brainstem. The pericentral cortices adjacent to the central sulcus also showed relatively high binding compared with other frontal or parietal cortices. This pattern of distribution was quite consistent across healthy individuals regardless of their age or sex, suggesting that these brain regions express the highest levels of COX-1 under non-inflammatory conditions. This is strongly supported by the excellent correlation between the VT of [11C]PS13 and COX-1 transcript levels measured by PTGS1 mRNA expression. Although the transcript data from one of the two mRNA probes showed no significant correlation with VT, different levels of reliability between probes measuring the same gene have been well characterized in microarray data like those provided in the Allen Human Brain Atlas [25-27].
The present PET imaging results also reproduced the postmortem findings in the human brain observed in a previous study, which reported high COX-1 mRNA and protein levels measured in CA3 and CA4 hippocampal neurons as well as in microglia distributed in the hippocampus, basal ganglia, temporal and parietal neocortex, and white matter tracts [28]. In addition, relatively high expression of COX-1 in the primary visual and somatosensory cortices was suggested in a postmortem study of the ovine brain [29] as well as in the Allen Human Brain Atlas (https://human.brain-map.org/microarray/search/show?search_type=user_selections&user_selection_mode=2), echoing the present findings of high [11C]PS13 binding in the occipital and pericentral cortices relatively to adjacent neocortical areas (Fig. 2). The higher level of mRNA expression in the pericentral cortices found with the Allen Human Brain Atlas is not readily apparent from the VT values of the frontal and parietal lobes in Fig. 5 because these come from larger regions that encompass a wider area. Although the physiological role of COX-1 in neurons and microglia is unclear, it can be postulated that COX-1 contributes to the maintenance of synaptic integrity either directly in neurons or indirectly in microglia, which survey their microenvironment and have a tight interaction with neuronal synapses [30]. Given that increased numbers of COX-1-positive reactive microglia have been observed in the frontal and temporal cortex of postmortem human brain with Alzheimer’s disease [28, 31], the distribution of [11C]PS13 brain binding in patients with Alzheimer’s disease and other disorders marked by neuroinflammation is expected to differ from the patterns observed in the present study with healthy individuals.
One thing should be noted is that, in the present study, automatic coregistration had to be adjusted manually for some of the scans. In this context, a hybrid PET/MRI machines may allow a more straightforward coregistration, especially for radioligands with overall low cortical binding, such as [11C]PS13.
Taken together, the results from this first-in-human evaluation of the ability of [11C]PS13 to image COX-1 in the brain showed excellent test-retest reliability and time-stability in measuring the target density. The findings justify extending use of this radioligand to the study of populations with neuroinflammatory diseases such as Alzheimer’s disease and other neurodegenerative disorders.
Supplementary Material
Acknowledgments
The authors thank the NIH Clinical PET Center for the successful completion of the studies and Ioline Henter (NIMH) for the invaluable editorial assistance.
Funding information
This study was funded by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (ZIAMH002795 and ZIAMH002793).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional research committee (the National Institutes of Health Combined Neurosciences Institutional Review Board, Protocol 17-M-0179) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00259-020-04855-2) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–81. 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. Cyclooxygenase in normal human tissues–is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med. 2009;13:3753–63. 10.1111/j.1582-4934.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasojima K, Schwab C, McGeer EG, McGeer PL. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999;830:226–36. [DOI] [PubMed] [Google Scholar]
- 5.Depboylu C, Weihe E, Eiden LE. COX1 and COX2 expression in non-neuronal cellular compartments of the rhesus macaque brain during lentiviral infection. Neurobiol Dis. 2011;42:108–15. 10.1016/j.nbd.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh P, Shrestha S, Cortes-Salva MY, Jenko KJ, Zoghbi SS, Morse CL, et al. 3-Substituted 1,5-diaryl-1 H-1,2,4-triazoles as prospective PET radioligands for imaging brain COX-1 in monkey. Part 1: synthesis and pharmacology. ACS Chem Neurosci. 2018;9:2610–9. 10.1021/acschemneuro.8b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrestha S, Singh P, Cortes-Salva MY, Jenko KJ, Ikawa M, Kim MJ, et al. 3-Substituted 1,5-diaryl-1 H-1,2,4-triazoles as prospective PET radioligands for imaging brain COX-1 in monkey. Part 2: selection and evaluation of [(11)C]PS13 for quantitative imaging. ACS Chem Neurosci. 2018;9:2620–7. 10.1021/acschemneuro.8b00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Shrestha SS, Cortes M, Singh P, Morse C, Liow JS, et al. Evaluation of two potent and selective PET radioligands to image COX-1 and COX-2 in rhesus monkeys. J Nucl Med. 2018;59: 1907–12. 10.2967/jnumed.118.211144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, et al. PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47:520–7. [PubMed] [Google Scholar]
- 10.Tonietto M, Rizzo G, Veronese M, Fujita M, Zoghbi SS, Zanotti-Fregonara P, et al. Plasma radiometabolite correction in dynamic PET studies: insights on the available modeling approaches. J Cereb Blood Flow Metab. 2016;36:326–39. 10.1177/0271678x15610585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abi-Dargham A, Gandelman M, Zoghbi SS, Laruelle M, Baldwin RM, Randall P, et al. Reproducibility of SPECT measurement of benzodiazepine receptors in human brain with iodine-123-iomazenil. J Nucl Med. 1995;36:167–75. [PubMed] [Google Scholar]
- 12.Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radio-tracers: beta-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83: 1014–9. 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 13.Kanegawa N, Collste K, Forsberg A, Schain M, Arakawa R, Jucaite A, et al. In vivo evidence of a functional association between immune cells in blood and brain in healthy human subjects. Brain Behav Immun. 2016;54:149–57. 10.1016/j.bbi.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47. 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- 16.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest. 1948;27:476–83. 10.1172/jci101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–27. 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 18.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7. 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 19.Glatting G, Kletting P, Reske SN, Hohl K, Ring C. Choosing the optimal fit function: comparison of the Akaike information criterion and the F-test. Med Phys. 2007;34:4285–92. 10.1118/1.2794176. [DOI] [PubMed] [Google Scholar]
- 20.Finnema SJ, Nabulsi NB, Mercier J, Lin SF, Chen MK, Matuskey D, et al. Kinetic evaluation and test-retest reproducibility of [(11)C]UCB-J, a novel radioligand for positron emission tomography imaging of synaptic vesicle glycoprotein 2A in humans. J Cereb Blood Flow Metab. 2018;38:2041–52. 10.1177/0271678x17724947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. 2000;20:1111–33. 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner R, Joshi A, Feng D, Zanderigo F, Ogden RT. Statistical evaluation of test-retest studies in PET brain imaging. EJNMMI Res. 2018;8:13. 10.1186/s13550-018-0366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–9. 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzo G, Veronese M, Expert P, Turkheimer FE, Bertoldo A. MENGA: a new comprehensive tool for the integration of neuroimaging data and the Allen Human Brain Transcriptome Atlas. PLoS One. 2016;11:e0148744. 10.1371/journal.pone.0148744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnatkevic Iute A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–67. 10.1016/j.neuroimage.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 26.Jaksik R, Iwanaszko M, Rzeszowska-Wolny J, Kimmel M. Microarray experiments and factors which affect their reliability. Biol Direct. 2015;10:46. 10.1186/s13062-015-0077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Bebu I, Li X. Microarray probes and probe sets. Front Biosci (Elite Ed). 2010;2:325–38. 10.2741/e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yermakova AV, Rollins J, Callahan LM, Rogers J, O'Banion MK. Cyclooxygenase-1 in human Alzheimer and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–46. [DOI] [PubMed] [Google Scholar]
- 29.Breder CD, Smith WL, Raz A, Masferrer J, Seibert K, Needleman P, et al. Distribution and characterization of cyclooxygenase immunoreactivity in the ovine brain. J Comp Neurol. 1992;322:409–38. 10.1002/cne.903220309. [DOI] [PubMed] [Google Scholar]
- 30.Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A, et al. Roles of microglia in brain development, tissue maintenance and repair. Brain. 2015;138:1138–59. 10.1093/brain/awv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoozemans JJ, Rozemuller AJ, Janssen I, De Groot CJ, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol. 2001;101:2–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.