Key Points
Question
Has mechanical ventilation of preterm infants changed in response to increasing noninvasive ventilation use and increasing survival of infants at the limits of viability?
Findings
In this cohort study using 2 large US national data sets and including more than 1 million preterm infants, the use of mechanical ventilation in nonanomalous preterm infants in neonatal intensive care units decreased from 29.4% in 2008 to 18.5% in 2018. Nationally, these changes were associated with nearly 30 000 fewer infants receiving mechanical ventilation than expected during the study period.
Meaning
The findings of this study suggest that the use and duration of mechanical ventilation decreased significantly in preterm infants between 2008 and 2018.
Abstract
Importance
In preterm infants, mechanical ventilation (MV) is associated with adverse pulmonary and neurodevelopmental outcomes. Multiple randomized clinical trials over the past 2 decades have shown the effectiveness of early noninvasive ventilation (NIV) in decreasing the use of MV in preterm infants. The epidemiologic factors associated with respiratory support in US preterm infants and any temporal changes after these trials is unknown.
Objective
To evaluate temporal changes in MV and noninvasive respiratory support in US preterm infants.
Design, Setting, and Participants
In a cohort design, 2 large national data sets (Pediatrix Clinical Data Warehouse for the clinical cohort and National Inpatient Sample for the national cohort) were used to collect data on preterm infants (<35 weeks’ gestation) without congenital anomalies who received active intensive care and were discharged home or died in the birth hospital from January 1, 2008, to December 31, 2018. Data analysis was conducted from December 10, 2019, to December 16, 2020.
Exposure
Discharge year.
Main Outcome and Measures
In the clinical cohort, detailed respiratory support data were generated, including days of MV and NIV modalities, and temporal trends were evaluated using multivariable modified Poisson or negative binomial regression models with discharge year as a continuous variable. In the national cohort, observed and expected national MV use were calculated.
Results
Among 259 311 infants (47.2% female) in 359 neonatal intensive care units in the clinical cohort, decreases were noted in the use (from 29.4% of infants in 2008 to 18.5% in 2018, relative risk for annual change, 0.96; 95% CI, 0.95-0.96) and duration (mean days, from 10.3 in 2008 to 9.7 in 2018; rate ratio for annual change, 0.98; 95% CI, 0.97-0.98) of MV. Noninvasive ventilation use increased from 57.9% of infants in 2008 to 67.4% in 2018 (adjusted relative risk for annual change, 1.02; 95% CI, 1.02-1.03), and mean NIV duration increased by 3.2 days (95% CI, 2.9-3.6 days). With increased use of continuous positive airway pressure and nasal intermittent positive-pressure ventilation as the main factors in the increase, the mean duration of respiratory support increased from 13.8 to 15.4 days (adjusted rate ratio for annual change, 1.03; 95% CI, 1.02-1.04) from 2008 to 2018. Among 1 169 441 infants in the national cohort, MV use decreased from 22.0% in 2008 to 18.5% in 2018, with an estimated 29 700 fewer ventilated infants and 142 000 fewer days of MV than expected during this period.
Conclusions and Relevance
These findings suggest that preterm respiratory support changed significantly from 2008 to 2018, with decreased use and duration of MV, increased use and duration of NIV, and an overall increase in respiratory support duration.
This cohort study examines the changes in the use of mechanical ventilation in preterm infants during the past decade.
Introduction
Mechanical ventilation (MV) is a lifesaving therapy for preterm infants with respiratory failure but is associated with increased mortality,1 bronchopulmonary dysplasia,2 and neurodevelopmental impairment, including cerebral palsy and deafness.3,4,5 Mechanical ventilation exposes infants to higher rates of adverse events,6,7,8 painful procedures,9 and neurotoxic sedative medications10 compared with noninvasive ventilation (NIV) that does not require endotracheal intubation. Over the past 2 decades, randomized clinical trials showed that NIV instituted immediately after birth improved clinical outcomes and decreased the need for MV in preterm infants with respiratory distress syndrome.11,12,13 Following the publication of these trials, the early use of NIV in preterm infants became the focus of single-center14 and network-based15 initiatives to decrease bronchopulmonary dysplasia and invasive MV. Recent data from Canadian neonatal intensive care units (NICUs) reported that use of MV has decreased in extremely preterm infants and use of NIV has increased.16 However, published national rates of MV in US preterm infants are more than 20 years old,17,18 and recent national changes in NIV and MV in the US have not been described.
We hypothesized that use of MV in the US has decreased over recent decades. Furthermore, with a growing population of extremely preterm infants in NICUs, we hypothesized that use of MV has become more prevalent in infants at lower gestational ages (GAs). Our objective was to evaluate temporal changes in respiratory support practices in US preterm infants. In addition, we provide a contemporary estimate of the overall scope and costs of MV in preterm infants in the US.
Methods
Study Design and Data Sources
We performed a retrospective, descriptive cohort study using the Pediatrix BabySteps Clinical Data Warehouse (CDW) (referred to herein as the clinical cohort) and the National Inpatient Sample (NIS) (referred to herein as the national cohort). The CDW consists of prospectively collected clinical information used to document the health record of infants cared for in Pediatrix practices.15 The NIS is a nationally representative, all-payer database of a random 20% sample of US inpatient discharges compiled annually by the Agency for Healthcare Research and Quality. The NIS is constructed using a complex survey design with discharges weighted to permit nationally representative inferences.19 Detailed information about both data sources and their differences is provided in the eMethods 1 in the Supplement.
We used the CDW to generate detailed descriptions of respiratory support (including MV and NIV) in US NICUs and used the NIS to generate US population-level estimates of MV. We used similar inclusion criteria to query both data sources for hospital discharges from January 1, 2008, to December 31, 2018. Both data sets contain deidentified data at the discharge level with unique patient identifiers. Infants cannot be tracked across multiple hospitalizations and the 2 data sets cannot be linked. Therefore, infants transferred between centers may potentially appear in the data sets more than once. To ensure that infants were counted only once in each data set, we evaluated only birth hospitalizations. The Vanderbilt University Medical Center Institutional Review Board approved the study with a waiver of informed consent owing to the use of deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Identification of Infants
We identified infants who were inborn, preterm (GA 23 weeks 0 days to 34 weeks 6 days in the CDW and GA <35 weeks in the NIS), received care only in the birth hospital and were not transferred, and had no major congenital anomalies. We excluded infants who did not receive active intensive care, defined as death without MV on the day of birth or the next day. To identify our national cohort, we used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM),20,21 and ICD-10 Procedure Coding System (ICD-10-PCS) codes (depending on discharge date; eMethods 2 and eMethods 3 in the Supplement).
Identification of Respiratory Support Modalities
In the clinical cohort, we collected respiratory support data including days of MV (both conventional and high-frequency ventilation [HFV]); days of NIV modalities including nasal intermittent positive-pressure ventilation (NIPPV), continuous positive airway pressure (CPAP), and high-flow nasal cannula (HFNC); and overall days of positive-pressure respiratory support (MV, NIPPV, CPAP, or HFNC). We also identified the first respiratory support modality (termed first-intention support) and exogenous surfactant use for each infant. We were unable to determine the method of surfactant delivery in our data sets. In the national cohort, we identified infants who had an ICD-9-CM or ICD-10-PCS code for MV (eMethods 3 in the Supplement).
Statistical Analysis
For the clinical cohort, we calculated the percent of NICU admissions receiving each modality and the mean, median, and interquartile ranges for the duration of therapy in treated infants. We also calculated the absolute change and associated 95% CIs in each respiratory support practice in 2008 and 2018. To assess temporal trends in respiratory support, we calculated either the adjusted relative risk (aRR) for annual change, using Poisson regression models with robust covariance estimators22 (binary outcomes), or the adjusted rate ratio for annual change, using negative binomial regression models23 (count outcomes), with discharge year treated as a continuous variable. We adjusted models for center-level clustering and combinations of a priori–specified covariates: GA, sex, race, birth weight, antenatal corticosteroids, small for GA status, cesarean delivery, 5-minute Apgar score, and maximal oxygen requirement during days 0 to 2. To determine whether trends in MV were similar across all infants, we repeated analyses with infants stratified by week of GA, sex, race, and survival status. We also collected survival to discharge for each GA to provide context to respiratory support trends.
For the national cohort, we calculated quarterly and yearly MV use from 2008 to 2018 with the numerator as the number of infants meeting inclusion criteria with an MV code during the birth hospitalization and the denominator as all birth hospitalizations meeting inclusion criteria. We repeated these analyses for infants who met all inclusion criteria (national cohort), all preterm infants (GA<37 weeks), and those who met all inclusion criteria except if they were transferred to another center before discharge. To assess MV trends in the NIS, we calculated P value for trend across years using variance-weighted least-squares regression,24 treating year as a continuous variable.25,26 To quantify changes in MV practices from 2008 to 2018, we fit a multivariable logistic regression model with MV as the dependent variable to 2008 NIS data and used model coefficients to calculate expected MV cases from 2009 to 2018 based on 2008 practices. We further calculated the potential difference in MV days by applying GA-specific MV duration from the clinical cohort to expected and observed counts in the national cohort (eMethods 4 in the Supplement). In addition, to provide a contemporary estimate of the burden of MV in preterm infants, we calculated the hospital costs and total length of stay for the national cohort and all preterm infants in the 2018 NIS.
Analyses were based on infants with nonmissing values for covariates and outcomes and we did not use imputation in either data set. Less than 1% of infants in the clinical cohort had missing data. For all NIS analyses, we used methods accounting for the complex survey design (svy: suite in Stata) including survey weights and we report weighted counts and proportions. All analyses were performed in Stata, version 15.2 (StataCorp LLC). All tests were 2-sided and used a significance level of P < .05. Data analysis was conducted from December 10, 2019, to December 16, 2020.
Results
From January 1, 2008, to December 31, 2018, a total of 259 311 infants (47.2% female) in 359 NICUs in our clinical cohort and 1 169 441 infants in our national cohort met inclusion criteria (eFigure 1 in the Supplement). In the clinical cohort, 60 794 infants (23.4%) received MV and 175 295 (67.6%) received any positive-pressure support during their NICU admission. In the national cohort, 238 635 infants (20.4%) received MV during their birth hospitalization. Table 1 reports characteristics of the study cohorts in 2018.
Table 1. Demographic and Clinical Characteristics of Study Cohort Infants in 2018.
| Characteristic | Clinical cohort (n = 23 530), No. (%) | National cohort (n = 108 325), weighted % (95% CI)a | ||
|---|---|---|---|---|
| No MV (n = 19 173) | Any MV (n = 4357) | No MV (n = 88 330) | Any MV (n = 19 995) | |
| Sex | ||||
| Male | 9966 (52.0) | 2400 (55.1) | 52.5 (51.8- 53.2) | 54.8 (53.2- 56.4) |
| Female | 9204 (48.0) | 1955 (44.9) | 47.5 (46.8-48.2) | 45.2 (43.6-46.8) |
| Race | ||||
| White | 8557 (44.6) | 1864 (42.8) | 44.7 (42.9-46.6) | 44.7 (41.9-47.4) |
| Black | 4330 (22.6) | 1155 (26.5) | 22.8 (21.3-24.4) | 25.6 (23.3-28.1) |
| Hispanic | 3670 (19.1) | 791 (18.2) | 19.3 (17.8-21.0) | 17.5 (15.4-19.9) |
| Asian | 662 (3.5) | 132 (3.0) | 5.0 (4.4-5.8) | 4.2 (3.4-5.2) |
| Otherb | 1954 (10.2) | 415 (9.5) | 8.1 (7.2-9.1) | 8.0 (6.8-9.4) |
| Estimated GA, median (IQR), wk | 33 (32-34) | 28 (26-31) | 33 (32-34) | 29 (26-32) |
| Cesarean delivery | 12 016 (63.4) | 3301 (76.8) | 59.5 (58.5-60.4) | 73.8 (72.3-75.3) |
| Multiple births | 5306 (27.7) | 1027 (23.6) | 26.5 (25.8-27.2) | 24.5 (23.2-25.9) |
| Birth weight, median (IQR), g | 1945 (1610-2250) | 1080 (760-1610) | NAd | NAd |
| Antenatal corticosteroidsc | 16 093 (83.9) | 3706 (85.1) | NAd | NAd |
| Small for GA | 1825 (9.5) | 582 (13.4) | NAd | NAd |
| Apgar score, median (IQR) | ||||
| 1 min | 8 (7-8) | 5 (2-7) | NAd | NAd |
| 5 min | 9 (8-9) | 7 (6-8) | NAd | NAd |
| Primary payer | NAd | NAd | ||
| Public | NA | NA | 51.5 (50.0-53.0) | 55.9 (53.7-58.2) |
| Private | NA | NA | 42.2 (40.7-43.7) | 39.2 (37.1-41.4) |
| Self-pay | NA | NA | 3.0 (2.7-3.4) | 1.7 (1.2-2.2) |
| Other | NA | NA | 3.4 (2.7-4.1) | 3.2 (2.5-4.2) |
Abbreviations: GA, gestational age; IQR, interquartile range; MV, mechanical ventilation; NA, not applicable; NIV, noninvasive ventilation.
All counts except estimated GA in the National Inpatient Sample represent the weighted counts. Data on sex, estimated GA, and payer were missing in less than 1% and race was missing in 9% of infants in the national cohort.
Variable reported as “other” in data set without further categorization.
Defined as receiving at least 1 dose at any time before birth.
Variables were not available in this data set.
In the clinical cohort, use of MV decreased from 29.4% of infants in 2008 to 18.5% in 2018 (aRR for annual change, 0.96; 95% CI, 0.95-0.96) with the largest absolute decreases from 2010 to 2016 (Table 2). Similarly, the duration of MV in ventilated infants decreased slightly over the study period from a mean of 10.3 days in 2008 to 9.7 days in 2018 (aRR for annual change, 0.98; 95% CI, 0.97-0.98). The overall use of HFV decreased during the study period, primarily due to decreased use in infants greater than 25 weeks’ GA. When stratified by GA, MV use decreased in all infants greater than 25 weeks’ GA, and MV duration decreased in infants 24 to 32 weeks’ GA (eTable 1 and eTable 2 in the Supplement), despite increasing survival rates in extremely preterm infants (eTable 3 in the Supplement). When stratified by sex and race, both the absolute and relative decreases in MV were similar across the various groups. The largest absolute reduction in MV duration was seen in infants who were of Black race (mean days, −2.3; 95% CI, −4.0 to −0.8), although nearly all groups showed similar relative decreases in MV duration after adjustment (eTable 4 in the Supplement).
Table 2. Changes in Respiratory Support Use in Nonanomalous Preterm Infants (<35 weeks) in NICUs in the Pediatrix Clinical Data Warehouse From 2008-2018a.
| Characteristic | No. (% of total) | Change, 2008-2018 (95% CI)b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |||
| Admissions, No. | 23 427 | 23 235 | 23 489 | 23 628 | 23 784 | 23 597 | 23 919 | 24 242 | 23 341 | 23 119 | 23 530 | ||
| Any MV | 6876 (29.4) | 6634 (28.6) | 6380 (27.2) | 6070 (25.7) | 5805 (24.4) | 5414 (22.9) | 5236 (21.9) | 5010 (20.7) | 4576 (19.6) | 4436 (19.2) | 4357 (18.5) | −10.8 (−11.6 to −10.1) | |
| Any HFV | 1638 (7.0) | 1667 (7.2) | 1646 (7.0) | 1526 (6.5) | 1526 (6.4) | 1393 (5.9) | 1396 (5.8) | 393 (5.8) | 1272 (5.5) | 1300 (5.6) | 1347 (5.7) | −1.3 (−1.7 to −0.8) | |
| MV days in ventilated infants | −0.6 (−1.3 to 0.1) | ||||||||||||
| Mean | 10.3 | 10.2 | 10.2 | 9.7 | 10.0 | 10.0 | 10.0 | 9.9 | 9.5 | 9.7 | 9.7 | NA | |
| Median (IQR) | 3 (2-9) | 3 (2-9) | 3 (2-9) | 3 (2-9) | 3 (2-10) | 3 (2-9) | 3 (2-10) | 3 (2-10) | 3 (2-9) | 3 (2-9) | 3 (2-10) | ||
| Any NIVc | 13 568 (57.9) | 13 760 (59.2) | 14 292 (60.9) | 14 826 (62.8) | 15 133 (63.6) | 15 256 (64.7) | 15 532 (64.9) | 16 112 (66.5) | 15 441 (66.2) | 15 417 (66.7) | 15 868 (67.4) | 9.5 (8.7 to 10.4) | |
| NIV days in NIV treated infantsc | 3.2 (2.9 to 3.6) | ||||||||||||
| Mean | 10.1 | 10.3 | 10.6 | 11.2 | 12.0 | 12.3 | 12.4 | 12.8 | 13.0 | 13.3 | 13.4 | NA | |
| Median (IQR) | 4 (2 to 10) | 4 (2 to 10) | 4 (2 to 11) | 4 (2 to 12) | 5 (2 to 13) | 5 (2 to 14) | 5 (2 to 14) | 5 (3 to 15) | 5 (2 to 15) | 5 (3 to 16) | 5 (3 to 16) | ||
| Any respiratory supportd | 15 096 (64.4) | 15 091 (65.0) | 15 457 (65.8) | 15 840 (67.0) | 15 984 (67.2) | 16 067 (68.1) | 16 318 (68.2) | 16 855 (69.5) | 16 066 (68.8) | 16 050 (69.4) | 16 471 (70.0) | 5.6 (4.7 to 6.4) | |
| Respiratory support days in treated infantsb | 1.6 (1.1 to 2.1) | ||||||||||||
| Mean | 13.8 | 13.9 | 14.0 | 14.2 | 15.0 | 15.0 | 15.0 | 15.2 | 15.2 | 15.5 | 15.4 | ||
| Median (IQR) | 4 (2-12) | 4 (2-12) | 4 (2-14) | 5 (2-14) | 5 (2-15) | 5 (2-15) | 5 (2-15) | 5 (3-16) | 5 (3-16) | 6 (3-17) | 6 (3-17) | ||
Abbreviations: HFV, high-frequency ventilation; IQR, interquartile range; MV, mechanical ventilation; NA, not applicable; NICU, neonatal intensive care unit; NIV, noninvasive ventilation.
Total of 259 311 infants in 359 NICUs.
Absolute change refers to mean duration when unit of measurement is days.
Includes nasal intermittent positive-pressure ventilation (NIPPV), continuous positive airway pressure (CPAP), and high-flow nasal cannula (HFNC).
Respiratory support Includes HFV, conventional MV, NIPPV, CPAP, or HFNC.
Concomitant with decreases in MV, NIV use increased significantly (Table 2). Use of NIV increased from 57.9% of infants in 2008 to 67.4% of infants in 2018 (aRR for annual change, 1.02; 95% CI, 1.02-1.03). For infants who received NIV, the mean duration of NIV increased by 3.2 days (95% CI, 2.9-3.6 days) from 2008 to 2018 (aRR for annual change, 1.04; 95% CI, 1.04-1.05). Both the use and duration of NIV increased at nearly all GAs (eTable 5 and eTable 6 in the Supplement) owing to increased use of NIPPV and CPAP (eTable 7 in the Supplement). First-intention NIPPV or CPAP also increased, growing from 39.6% to 64.8% in infants receiving respiratory support from 2008 to 2018; first-intention MV decreased by nearly half (39.0% to 21.2%) during that period. First-intention HFNC remained relatively stable from 2008 to 2013 but decreased in all years after 2013 (Figure 1). Use of exogenous surfactant decreased from 30.8% to 24.8% of infants between 2008 and 2018, with the percent of infants receiving surfactant without MV increasing from 24.6% in 2008 to 39.1% in 2018 (eTable 7 in the Supplement).
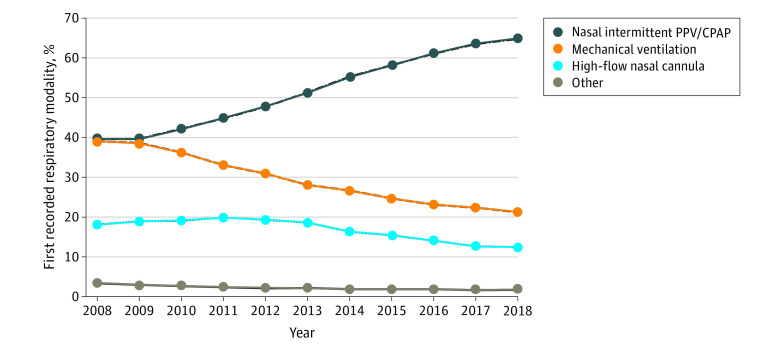
Figure 1. First Reported Respiratory Support Modality in Infants Receiving Positive-Pressure Ventilation in the Pediatrix Clinical Data Warehouse From 2008-2018.
The use of nasal intermittent positive-pressure ventilation (PPV) or continuous positive airway pressure (CPAP) as the initial respiratory modality increased in all years after 2009 in concert with decreased use of mechanical ventilation. Other modalities included low-flow nasal cannula, oxygen hood, or room air.
Although MV use decreased, the overall combined use of positive-pressure respiratory support (invasive or noninvasive) increased throughout the study period (Table 2). From 2008 to 2018, the percent of infants who received positive-pressure respiratory support increased from 64.4% to 70.0% (aRR for annual change, 1.02; 95% CI, 1.01-1.02) and the mean duration of respiratory support increased from 13.8 to 15.4 days (aRR for annual change, 1.03; 95% CI, 1.02-1.04). The largest absolute increases in the duration of positive-pressure respiratory support occurred in infants from 27 to 29 weeks’ GA (Figure 2; eFigures 2-4 in the Supplement).
Figure 2. Mean Duration of Respiratory Support in Surviving Infants in the Pediatrix Clinical Data Warehouse From 2008 to 2018.

Duration of respiratory support in infants of 23 to 25 weeks’ gestational age (GA) (A), 26 to 28 weeks’ GA (B), 29 to 31 weeks’ GA (C), and 32 to 34 weeks’ GA (D). Note that the y-axis scale changes for each GA group. Only data from infants who received any type of positive-pressure support are shown. Noninvasive ventilation included nasal intermittent positive-pressure ventilation, continuous positive airway pressure, and high-flow nasal cannula. Total duration of positive-pressure respiratory support increased at nearly all GAs, mainly due to increased duration of nasal intermittent positive-pressure ventilation and continuous positive airway pressure.
The use of MV in the national cohort increased from 21.5% during quarter 1 of 2008 to a maximum of 24.4% during quarter 1 of 2010 before decreasing during the remainder of the study period (Figure 3). Annual MV use decreased by 3.5%, from 22.0% in 2008 to 18.5% in 2018 (P < .001 for trend) during the entire study period and by 4.7% (P < .001 for trend) from the highest rate in 2010 (eTable 8 in the Supplement). Nationally, the mean GA of ventilated infants decreased during the study period as the number of infants less than 29 weeks’ GA increased (eTable 9 in the Supplement). Although rates were higher in the national cohort, we observed similar trends in MV when the entire US preterm population was considered (eFigure 5 in the Supplement). From 2008 to 2018, 13% to 17% of all infants who met study inclusion criteria in the NIS (except transfer status) were transferred from the birth hospital to another center before discharge. These infants had higher rates of MV than nontransferred infants (national cohort), peak MV rates in 2010, and a decrease in rates from 2010 to 2016 (eFigure 6 in the Supplement).
Figure 3. Observed vs Expected Mechanical Ventilation Use During the Birth Hospitalization in National Cohort Infants in the National Inpatient Sample From 2008 to 2018.
Observed mechanical ventilation use (solid line) decreased after 2010, but expected mechanical ventilation (gray circles) remained stable. Given these differences, an estimated 29 700 fewer infants were ventilated in the national cohort during the birth hospitalization than expected based on 2008 practice patterns. Expected mechanical ventilation was calculated by fitting a logistic regression model to 2008 data and applying model coefficients to data from subsequent years. Shaded area indicates 95% CI for observed mechanical ventilation use.
Estimated National Changes in MV
We estimate that approximately 29 700 fewer infants received MV in our national cohort from 2009 to 2018 than expected from MV practices in 2008 (Figure 3). We estimate this lower number of infants amounted to approximately 142 000 fewer days of MV in US NICUs from 2009 to 2018, with most occurring after 2013, coinciding with increasing NIV use.
National Burden of Preterm MV in 2018
In 2018, an estimated 19 995 infants in the national cohort received MV. These infants accounted for an estimated 168 000 days of MV, total hospital length of stay of 1 064 300 days, and in-hospital costs of $2.2 billion. When all preterm infants (<37 weeks’ GA), regardless of diagnosis or transfer status, were included, we estimate that 37 480 infants received MV during their birth hospitalization. These infants accounted for a total hospital length of stay of 1 771 070 days and in-hospital costs of $3.9 billion during their birth hospitalizations.
Discussion
Using 2 large national data sets, we provide a comprehensive description of respiratory support use in US preterm infants. We identified several important changes in respiratory care practices for preterm infants since 2008. First, use and duration of MV have decreased significantly as NIV use has increased. This decrease in MV was seen in all sexes, races, and at nearly all GAs and was associated with nearly 30 000 fewer infants receiving MV in our national cohort from 2009 to 2018 compared with 2008 practice patterns. Second, expanded use of NIV was associated with an increase in duration of positive-pressure respiratory support at nearly all GAs. Third, although use of MV has decreased, preterm respiratory failure continues to consume substantial national health care resources.
We found a trend toward decreasing use of invasive respiratory care, including MV and surfactant, during the study period. Our finding of decreasing MV is consistent with previous reports from North American NICUs over the past 3 decades.27,28 A report from the Neonatal Research Network noted that MV use in infants less than 29 weeks’ GA decreased from 90% in 2002 to 85% in 2012.27 We saw some difference between our 2 cohorts, likely related to slight differences in populations. In the clinical cohort, which captured only infants admitted to NICUs, MV use decreased each year of the study period, with the largest decrease after 2009. However, in the national cohort, which captured all infants discharged from hospitals in the NIS regardless of care unit, MV use increased from 2008 to 2010 before decreasing in the latter half of 2010. This decrease in MV and the rapid increase in first-intention NIPPV and CPAP use corresponded temporally to the publication of large randomized clinical trials evaluating CPAP as the initial respiratory modality in preterm infants with respiratory distress.12,13 In parallel with decreased MV use, surfactant use also decreased. We speculate that the expanded use of CPAP and/or NIPPV in US NICUs as the initial respiratory strategy in preterm infants was the main factor of decreasing MV from 2010 to 2018. Although earlier reports from the Neonatal Research Network and the Vermont Oxford Network suggested that surfactant use significantly increased in the 1990s and 2000s,27,28 our data suggest that increasing adoption of NIV has reversed this change.
Our findings highlight priorities for neonatal respiratory care research. First, although the proportion of preterm infants in the US who received MV decreased from 2008 to 2018, the absolute number of extremely preterm infants (<28 weeks) who received MV increased, with the largest increases seen in infants less than 25 weeks’ GA. This finding is consistent with reports over the past 2 decades showing increasing intervention and improving survival for periviable infants.27,29,30 However, because most MV and NIV trials have excluded these infants, few data exist to inform evidence-based respiratory care practices in this population.11,12,31 Because these immature infants consume an increasing proportion of NICU resources, research to support the development of respiratory care strategies tailored to this stage of pulmonary development are important. Second, increased use and duration of NIV were observed at nearly all GAs. Our data do not allow us to identify the exact reason for this increased use. Although use of NIV is associated with improved respiratory outcomes compared with MV,12 the few studies evaluating strategies for how and when to discontinue NIV support in preterm infants have had conflicting results.32,33,34 Studies are needed to identify infants who can be safely weaned from NIV and the optimal method to do so.35 Future work should also explore the financial outcomes associated with increased NIV use, especially in more mature preterm infants.
Limitations
Our study has several important limitations. First, to achieve study objectives, we chose to use 2 separate data sources. Although the CDW contains detailed data on the use of respiratory support modalities in US NICUs, it did not allow us to make national inferences. Conversely, although the NIS allowed us to draw accurate national inferences for MV use, it does not provide detailed data about the duration and/or type of respiratory support. We were also unable to link the 2 data sets owing to the unique patient identifiers used in each data source. We attempted to address this limitation by using similar inclusion criteria in each database, and baseline demographics were similar between the cohorts. However, we cannot be certain that the populations within the 2 data sets are entirely comparable. Second, for analyses in the NIS, we depended on administrative codes. Because the sensitivity and specificity of many of these codes have not been reported, we cannot be certain that our population estimates are accurate. Third, because of the unique patient identifiers used in each data set, we were unable to track infants across multiple hospitalizations in both data sources and therefore limited our main analyses to infants during the birth hospitalization. This limitation introduces the potential that changes in transfer patterns over the course of the study period could have biased our findings. We were also unable to know whether our observations about respiratory support duration held true in transferred infants. Given that transferred infants have higher rates of MV, we likely underreported the true burden of preterm respiratory failure in the US.
Conclusions
The findings of this study suggest that respiratory support practices in preterm infants in the US changed significantly from 2008 to 2018, with decreased use and duration of MV, increased use and duration of NIV with NIPPV and CPAP, and an overall increase in respiratory support. We estimate that these changes were associated with nearly 30 000 fewer infants in the US receiving MV over the past decade, although more infants received NIV support and the long-term effects of this increased support are not known. Because evidence indicates that MV is injurious to preterm lungs and extended NIV may result in improved pulmonary outcomes, including increased functional residual capacity at hospital discharge,32 these changes might represent significant improvements in neonatal respiratory care over the past decade.
eMethods 1. Detailed Descriptions of Study Data Sources
eMethods 2.ICD-9-CM and ICD-10-CM Codes Used to Identify Infants With Major Anomalies or Syndromes in the National Inpatient Sample
eMethods 3. Detailed Description of Data Fields Used in the National Inpatient Sample to Identify Study Cohort and Outcomes
eMethods 4. Description of Study Procedures Used to Calculate Expected Use and Expected Duration of Mechanical Ventilation in the National Cohort from 2009-2018
eFigure 1. Flow Diagram for Construction of the Clinical and National Cohorts
eFigure 2. Average Duration of Respiratory Support Modalities in Surviving Infants in the Pediatrix Clinical Data Warehouse from 2008-2018 (23-26 weeks)
eFigure 3. Average Duration of Respiratory Support Modalities in Surviving Infants in the Pediatrix Clinical Data Warehouse from 2008-2018 (27-30 weeks)
eFigure 4. Average Duration of Respiratory Support Modalities in Surviving Infants in the Pediatrix Clinical Data Warehouse from 2008-2018 (31-34 weeks)
eFigure 5. Mechanical Ventilation Use During the Birth Hospitalization in All Preterm Infants (<37 Weeks’ Gestational Age) in the National Inpatient Sample From 2008-2018
eFigure 6. Mechanical Ventilation Use During the Birth Hospitalization in Transported Preterm Infants (<34 Weeks’ Gestational Age) in the National Inpatient Sample From 2008-2018
eTable 1. Temporal Trends in Mechanical Ventilation Use in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 2. Temporal Trends in Mechanical Ventilation Duration in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 3. Survival to Hospital Discharge in Clinical Cohort Infants in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 4. Temporal Trends in Mechanical Ventilation in US NICUs in the Pediatrix Clinical Data Warehouse by Patient Characteristics From 2008-2018
eTable 5. Noninvasive Ventilation Use in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 6. Noninvasive Ventilation Duration in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 7. Temporal Trends in Noninvasive Respiratory Support and Exogenous Surfactant in US NICUs in the Pediatrix Clinical Data Warehouse From 2008-2018
eTable 8. National Estimates of Mechanical Ventilation During the Birth Hospitalization for Preterm Infants in the National Inpatient Sample From 2008-2018
eTable 9. Temporal Trends in the Use of Mechanical Ventilation by Gestational Age in Preterm Infants in the National Inpatient Sample From 2008-2018
References
- 1.Vendettuoli V, Bellù R, Zanini R, Mosca F, Gagliardi L; Italian Neonatal Network . Changes in ventilator strategies and outcomes in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2014;99(4):F321-F324. doi: 10.1136/archdischild-2013-305165 [DOI] [PubMed] [Google Scholar]
- 2.Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015;169(11):1011-1017. doi: 10.1001/jamapediatrics.2015.2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laptook AR, O’Shea TM, Shankaran S, Bhaskar B; NICHD Neonatal Network . Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115(3):673-680. doi: 10.1542/peds.2004-0667 [DOI] [PubMed] [Google Scholar]
- 4.Tsai WH, Hwang YS, Hung TY, Weng SF, Lin SJ, Chang WT. Association between mechanical ventilation and neurodevelopmental disorders in a nationwide cohort of extremely low birth weight infants. Res Dev Disabil. 2014;35(7):1544-1550. doi: 10.1016/j.ridd.2014.03.048 [DOI] [PubMed] [Google Scholar]
- 5.Guillot M, Guo T, Ufkes S, et al. Mechanical ventilation duration, brainstem development, and neurodevelopment in children born preterm: a prospective cohort study. J Pediatr. 2020;S0022-3476(20)30653-3. doi: 10.1016/j.jpeds.2020.05.039 [DOI] [PubMed] [Google Scholar]
- 6.Snijders C, van Lingen RA, Klip H, Fetter WP, van der Schaaf TW, Molendijk HA; NEOSAFE study group . Specialty-based, voluntary incident reporting in neonatal intensive care: description of 4846 incident reports. Arch Dis Child Fetal Neonatal Ed. 2009;94(3):F210-F215. doi: 10.1136/adc.2007.135020 [DOI] [PubMed] [Google Scholar]
- 7.Snijders C, van Lingen RA, van der Schaaf TW, Fetter WP, Molendijk HA; NEOSAFE study group . Incidents associated with mechanical ventilation and intravascular catheters in neonatal intensive care: exploration of the causes, severity and methods for prevention. Arch Dis Child Fetal Neonatal Ed. 2011;96(2):F121-F126. doi: 10.1136/adc.2009.178871 [DOI] [PubMed] [Google Scholar]
- 8.Hatch LD, Grubb PH, Lea AS, et al. Endotracheal intubation in neonates: a prospective study of adverse safety events in 162 infants. J Pediatr. 2016;168:62-66.e6. doi: 10.1016/j.jpeds.2015.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300(1):60-70. doi: 10.1001/jama.300.1.60 [DOI] [PubMed] [Google Scholar]
- 10.Carbajal R, Eriksson M, Courtois E, et al. ; EUROPAIN Survey Working Group . Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir Med. 2015;3(10):796-812. doi: 10.1016/S2213-2600(15)00331-8 [DOI] [PubMed] [Google Scholar]
- 11.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators . Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700-708. doi: 10.1056/NEJMoa072788 [DOI] [PubMed] [Google Scholar]
- 12.Finer NN, Carlo WA, Walsh MC, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970-1979. doi: 10.1056/NEJMoa0911783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group . Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069-e1076. doi: 10.1542/peds.2010-3848 [DOI] [PubMed] [Google Scholar]
- 14.Kakkilaya V, Jubran I, Mashruwala V, et al. Quality improvement project to decrease delivery room intubations in preterm infants. Pediatrics. 2019;143(2):e20180201. doi: 10.1542/peds.2018-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellsbury DL, Clark RH, Ursprung R, Handler DL, Dodd ED, Spitzer AR. A multifaceted approach to improving outcomes in the NICU: the Pediatrix 100 000 Babies Campaign. Pediatrics. 2016;137(4):e20150389. doi: 10.1542/peds.2015-0389 [DOI] [PubMed] [Google Scholar]
- 16.Weisz DE, Yoon E, Dunn M, et al. ; Canadian Neonatal Network Investigators . Duration of and trends in respiratory support among extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2021;106(3):286-291. doi: 10.1136/archdischild-2020-319496 [DOI] [PubMed] [Google Scholar]
- 17.Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med. 2001;164(7):1154-1160. doi: 10.1164/ajrccm.164.7.2012126 [DOI] [PubMed] [Google Scholar]
- 18.Wilson A, Gardner MN, Armstrong MA, Folck BF, Escobar GJ. Neonatal assisted ventilation: predictors, frequency, and duration in a mature managed care organization. Pediatrics. 2000;105(4 Pt 1):822-830. doi: 10.1542/peds.105.4.822 [DOI] [PubMed] [Google Scholar]
- 19.Healthcare Cost and Utilization Project (HCUP) . December 2019. Agency for Healthcare Research and Quality. Overview of the national (nationwide) inpatient sample (NIS). Accessed August 20, 2020 https://www.hcup-us.ahrq.gov/nisoverview.jsp
- 20.Centers for Disease Control and Prevention . International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Accessed August 10, 2020. https://www.cdc.gov/nchs/icd/icd10cm.htm
- 21.Centers for Disease Control and Prevention . International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Accessed August 11, 2020. https://www.cdc.gov/nchs/icd/icd9cm.htm
- 22.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 23.Hilbe J.Negative Binomial Regression. 2nd ed.Cambridge University Press; 2011. doi: 10.1017/CBO9780511973420 [DOI] [Google Scholar]
- 24.Weisburg S.Applied Linear Regression. 4th ed.John Wiley & Sons Inc; 2014. [Google Scholar]
- 25.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934-1940. doi: 10.1001/jama.2012.3951 [DOI] [PubMed] [Google Scholar]
- 26.Houchens RL, Ross D, Elixhauser A. Using the HCUP national inpatient sample to estimate trends. 2015. HCUP methods series report 2006-05 ONLINE. January 4, 2016. US Agency for Healthcare Research and Quality. Accessed December 1, 2019. https://www.hcup-us.ahrq.gov/reports/methods/methods.jsp
- 27.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi: 10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soll RF, Edwards EM, Badger GJ, et al. Obstetric and neonatal care practices for infants 501 to 1500 g from 2000 to 2009. Pediatrics. 2013;132(2):222-228. doi: 10.1542/peds.2013-0501 [DOI] [PubMed] [Google Scholar]
- 29.Younge N, Goldstein RF, Bann CM, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376(7):617-628. doi: 10.1056/NEJMoa1605566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins PL, Dagle JM, Bell EF, Colaizy TT. Outcomes at 18 to 22 months of corrected age for infants born at 22 to 25 weeks of gestation in a center practicing active management. J Pediatr. 2020;217:52-58.e1. doi: 10.1016/j.jpeds.2019.08.028 [DOI] [PubMed] [Google Scholar]
- 31.Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT; Neonatal Ventilation Study Group . High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. N Engl J Med. 2002;347(9):643-652. doi: 10.1056/NEJMoa012750 [DOI] [PubMed] [Google Scholar]
- 32.Lam R, Schilling D, Scottoline B, et al. The effect of extended continuous positive airway pressure on changes in lung volumes in stable premature infants: a randomized controlled trial. J Pediatr. 2020;217:66-72.e1. doi: 10.1016/j.jpeds.2019.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen CF, Sellmer A, Ebbesen F, et al. Sudden vs pressure wean from nasal continuous positive airway pressure in infants born before 32 weeks of gestation: a randomized clinical trial. JAMA Pediatr. 2018;172(9):824-831. doi: 10.1001/jamapediatrics.2018.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todd DA, Wright A, Broom M, et al. Methods of weaning preterm babies <30 weeks gestation off CPAP: a multicentre randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F236-F240. doi: 10.1136/adc.2011-300133 [DOI] [PubMed] [Google Scholar]
- 35.Gentle SJ, Ambalavanan N, Carlo WA. Oxygen saturation histograms predict nasal continuous positive airway pressure–weaning success in preterm infants. Pediatr Res. 2020;88(4):637-641. doi: 10.1038/s41390-020-0772-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Detailed Descriptions of Study Data Sources
eMethods 2.ICD-9-CM and ICD-10-CM Codes Used to Identify Infants With Major Anomalies or Syndromes in the National Inpatient Sample
eMethods 3. Detailed Description of Data Fields Used in the National Inpatient Sample to Identify Study Cohort and Outcomes
eMethods 4. Description of Study Procedures Used to Calculate Expected Use and Expected Duration of Mechanical Ventilation in the National Cohort from 2009-2018
eFigure 1. Flow Diagram for Construction of the Clinical and National Cohorts
eFigure 2. Average Duration of Respiratory Support Modalities in Surviving Infants in the Pediatrix Clinical Data Warehouse from 2008-2018 (23-26 weeks)
eFigure 3. Average Duration of Respiratory Support Modalities in Surviving Infants in the Pediatrix Clinical Data Warehouse from 2008-2018 (27-30 weeks)
eFigure 4. Average Duration of Respiratory Support Modalities in Surviving Infants in the Pediatrix Clinical Data Warehouse from 2008-2018 (31-34 weeks)
eFigure 5. Mechanical Ventilation Use During the Birth Hospitalization in All Preterm Infants (<37 Weeks’ Gestational Age) in the National Inpatient Sample From 2008-2018
eFigure 6. Mechanical Ventilation Use During the Birth Hospitalization in Transported Preterm Infants (<34 Weeks’ Gestational Age) in the National Inpatient Sample From 2008-2018
eTable 1. Temporal Trends in Mechanical Ventilation Use in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 2. Temporal Trends in Mechanical Ventilation Duration in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 3. Survival to Hospital Discharge in Clinical Cohort Infants in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 4. Temporal Trends in Mechanical Ventilation in US NICUs in the Pediatrix Clinical Data Warehouse by Patient Characteristics From 2008-2018
eTable 5. Noninvasive Ventilation Use in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 6. Noninvasive Ventilation Duration in US NICUs in the Pediatrix Clinical Data Warehouse by Gestational Age From 2008-2018
eTable 7. Temporal Trends in Noninvasive Respiratory Support and Exogenous Surfactant in US NICUs in the Pediatrix Clinical Data Warehouse From 2008-2018
eTable 8. National Estimates of Mechanical Ventilation During the Birth Hospitalization for Preterm Infants in the National Inpatient Sample From 2008-2018
eTable 9. Temporal Trends in the Use of Mechanical Ventilation by Gestational Age in Preterm Infants in the National Inpatient Sample From 2008-2018