This randomized clinical trial evaluates peer-comparison audit and feedback to primary care physicians with a high volume of antibiotic prescribing to assess the effect of targeted messaging on avoiding unnecessary antibiotic initiation and long-duration prescribing.
Key Points
Question
Does providing a single, mailed, peer-comparison letter on antibiotic use to high-prescribing primary care physicians targeting either initiation or duration of antibiotic treatment modify prescribing behavior?
Findings
In this randomized clinical trial of 3500 primary care physicians in Ontario, Canada, receipt of a letter targeting appropriate antibiotic durations resulted in a statistically significant 4.8% relative reduction in total antibiotic use.
Meaning
A single, peer-comparison, antibiotic-feedback letter to high-prescribing physicians can be effective and cost saving, especially if it includes targeted messaging on appropriate durations of antibiotic prescriptions.
Abstract
Importance
Antibiotic overuse contributes to adverse drug effects, increased costs, and antimicrobial resistance.
Objective
To evaluate peer-comparison audit and feedback to high-prescribing primary care physicians (PCPs) and assess the effect of targeted messaging on avoiding unnecessary antibiotic prescriptions and avoiding long-duration prescribing.
Design, Setting, and Participants
In this 3-arm randomized clinical trial, administrative data collected from IQVIA’s Xponent database were used to recruit the highest quartile of antibiotic-prescribing PCPs (n = 3500) in Ontario, Canada.
Interventions
Physicians were randomized 3:3:1 to receive a mailed letter sent in December 2018 addressing antibiotic treatment initiation (n = 1500), a letter addressing prescribing duration (n = 1500), or no letter (control; n = 500). Outliers at the 99th percentile at baseline for each arm were excluded from analysis.
Main Outcomes and Measures
The primary outcome was total number of antibiotic prescriptions over 12 months postintervention. Secondary outcomes were number of prolonged-duration prescriptions (>7 days) and antibiotic drug costs (in Canadian dollars). Robust Poisson regression controlling for baseline prescriptions was used for analysis.
Results
Of the 3465 PCPs included in analysis, 2405 (69.4%) were male, and 2116 (61.1%) were 25 or more years from their medical graduation. At baseline, PCPs receiving the antibiotic initiation letter and duration letter prescribed an average of 988 and 1000 antibiotic prescriptions, respectively; at 12 months postintervention, these PCPs prescribed an average of 849 and 851 antibiotic prescriptions, respectively. For the primary outcome of total antibiotic prescriptions 12 months postintervention, there was no statistically significant difference in total antibiotic use between PCPs who received the initiation letter compared with controls (relative risk [RR], 0.96; 97.5% CI, 0.92-1.01; P = .06) and a small statistically significant difference for the duration letter compared with controls (RR, 0.95; 97.5% CI, 0.91-1.00; P = .01). For PCPs receiving the duration letter, this corresponds to an average of 42 fewer antibiotic prescriptions over 12 months. There was no statistically significant difference between the letter arms. For the initiation letter, compared with controls there was an RR of 0.98 (97.5% CI, 0.93-1.03; P = .42) and 0.97 (97.5% CI, 0.92-1.02; P = .19) for the outcomes of prolonged-duration prescriptions and antibiotic drug costs, respectively. At baseline, PCPs who received the duration letter prescribed an average of 332 prolonged-duration prescriptions with $14 470 in drug costs. There was an 8.1% relative reduction (RR, 0.92; 97.5% CI, 0.87-0.97; P < .001) in prolonged-duration prescriptions, and a 6.1% relative reduction in antibiotic drug costs (RR, 0.94; 97.5% CI, 0.89-0.99; P = .01). This corresponds to an average of 24 fewer prolonged-duration prescriptions and $771 in drug cost savings per PCP over 12 months.
Conclusions and Relevance
In this randomized clinical trial, a single mailed letter to the highest-prescribing PCPs in Ontario, Canada providing peer-comparison feedback, including messaging on limiting antibiotic-prescribing durations, led to statistically significant reductions in total and prolonged-duration antibiotic prescriptions, as well as drug costs.
Trial Registration
ClinicalTrials.gov Identifier: NCT03776383
Introduction
Overuse of antibiotics is contributing to a public health crisis of antimicrobial resistance. It is estimated that drug-resistant infections will increase dramatically in the coming decades without interventions to curb unnecessary antibiotic use.1 Greater use of antibiotics is associated with an increase in patient-level and ecological-level drug resistance.2,3,4 Inappropriate antibiotic use is also associated with adverse drug effects, increased seeking of health care for self-limited illnesses, and increased costs.5,6 The majority of human antibiotic consumption occurs in the community and is prescribed by primary care physicians (PCPs), which makes this group of prescribers crucial stakeholders and partners in antimicrobial stewardship efforts.7 An estimated 25% to 50% of all antibiotics prescribed in primary care are unnecessary, with substantial geographical and prescriber variability.8,9,10,11,12 Furthermore, there is substantial antibiotic overuse through excessively prolonged prescribing durations, particularly in later-career PCPs.13,14
Peer-comparison audit and feedback can be an effective tool to modify PCP behavior and improve patient outcomes.15 The behaviors associated with unnecessary antibiotic treatment initiation are generally not related to knowledge gaps, nor are they responsive to education alone.16,17 Physicians frequently attribute unnecessary antibiotic prescribing to external factors, such as perceived patient expectations.18 Conversely, unnecessary prolonged-duration antibiotic prescribing is more likely related to a knowledge gap because research showing the noninferiority of shorter antibiotic courses compared with longer ones is relatively new clinical evidence.13,19,20,21,22,23,24 Social normative feedback can be a powerful motivator for change.25 Previous randomized clinical trials (RCTs) evaluating antibiotic peer-comparison audit and feedback have been mostly effective in reducing antibiotic use without causing harm.16,26,27,28 Further research is needed to elucidate the effective aspects of antibiotic audit and feedback on prescribing that build on advances in behavioral sciences and are scalable across health care systems.
The objective of this RCT was to evaluate the effect of notifying PCPs that they are in the highest antibiotic prescribing quartile compared with their peers. We hypothesized that social normative feedback would affect antibiotic initiation. We further hypothesized that focused educational content on appropriate initiation and duration would have additional effects on the volume of antibiotics prescribed or volume of prolonged-duration prescriptions, respectively.
Methods
Design
We performed a 3-arm parallel RCT with an allocation ratio of 3:3:1 to receipt of a single mailed feedback letter with recommendations on antibiotic initiation, receipt of a similar letter but with recommendations on prescribing duration, or no letter (control). We used an unequal allocation ratio to maximize any potential effect the intervention would have on antibiotic use in the population. The trial protocol (Supplement 1) was approved by the Ethics Review Board at Public Health Ontario, and a waiver for informed consent was granted owing to the minimal risk and burden to participants in receiving a single letter. A debrief letter was mailed to all PCPs at study completion.
Participants and Setting
All family physicians or general practitioners in Ontario, Canada (population, 14.4 million) were ranked by highest to lowest total number of antibiotic prescriptions based on prescribing data between March 1, 2017, and February 28, 2018 (regardless of patient volume). We only included oral antibiotics dispensed by outpatient pharmacies (eTable 1 in Supplement 2). The 3500 highest-prescribing PCPs (of 12 888) were identified from administrative data and included in the trial.
Data Collection
The data set used was from the Xponent database from IQVIA, which captures 61.3% of outpatient prescriptions dispensed by community pharmacies in Ontario. IQVIA then supplements this database with insurance and antibiotic sales data and applies a proprietary geospatial extrapolation algorithm to project to 100% of all prescriptions. IQVIA projection methodology is internally validated29 but not publicly available. We previously validated the Ontario Xponent database as a reliable data source in identifying high-volume antibiotic prescribers.30
Intervention
Each letter informed the PCP recipient that they were in the highest 25th percentile compared with their peers for antibiotics prescribed based on their total number of antibiotic prescriptions. The initiation letter provided a table on appropriate antibiotic initiation for common respiratory infections, with recommendations and tools adapted from Choosing Wisely Canada resources.31 The duration letter provided recommendations on appropriate antibiotic prescribing durations (eAppendix in Supplement 2). The control group did not receive a letter.
We designed the letters using an iterative process of stakeholder engagement. Family physicians from the executive committee of the Ontario Medical Association Section on General and Family Practice contributed to the letter text. The letters were cosigned by the chief of infection prevention and control at Public Health Ontario (G.G.) and the chair from the Ontario Medical Association Section on General and Family Practice. We engaged Ontario Health (Quality) and a convenience sample of practicing Ontario PCPs to help improve the wording of the letters.
The letters (eAppendix in Supplement 2) were mailed in early December 2018. A debrief letter was sent in December 2019 to all participants and included updated data. We telephoned a random sample of 135 PCPs between January 20 and February 4, 2020, to inquire if they recalled receiving the letter.
Allocation
Unrestricted randomization was used to allocate PCPs to 1 of the 3 study arms by using a random number generator in SAS, version 9.4 (SAS Institute). Of the 3500 enrolled PCPs, 1500 were assigned to each intervention arm, and 500 were assigned to the control arm. An epidemiologist at Public Health Ontario conducted the allocation and was not otherwise involved in the study or analysis. Other study team members were blinded to the randomized arm assignments.
Outcomes
We used 3 prespecified outcomes. These outcomes were based on routinely collected administrative data and could not be influenced by the study team. The primary outcome was total antibiotic volume based on number of unique prescriptions. Baseline data were collected in the 12 months prior to the intervention in December 2018. The primary outcome was based on the 12 months after. We used a simple count of antibiotic prescriptions. We initially planned to use a rate of antibiotic prescriptions per total prescriptions, because patient visits were not available in the data set for a denominator, but after consultation with methodologists, we decided not to adjust for total prescriptions because this variable is a postrandomization outcome. The initial trial registration listed enrollment into Ontario Health (Quality) practice reports as the primary outcome. We subsequently changed the primary outcome to focus on antibiotic prescribing. These decisions were made after trial registration but before inspection of the results.
The secondary outcomes were number of prolonged-duration antibiotic prescriptions (defined as >7 days) and total antibiotic costs (drug acquisition costs only, not including pharmacist dispensing fees, in Canadian dollars). We defined more than 7 days as prolonged because most community-managed infections can be effectively treated with 7 or fewer days of antibiotics.32
Sample Size
Because the sample size was fixed by all available PCPs in the top quartile of Ontario, we calculated the detectable difference given the available sample size. Using analysis of covariance, sample sizes of 1500 in each of the 2 intervention arms vs 500 in the control arm achieves 84% power to detect a mean difference of 70 prescriptions in at least 1 of the 2 intervention arms vs control using an F test at a 2-sided significance level of P = .025, corrected for 2 comparisons. We assumed a standard deviation of 700 prescriptions (obtained from baseline data) and a correlation with the baseline mean prescriptions of 0.8.
Statistical Analysis
Analysis was by intention to treat. The unit of analysis was the PCP. A post hoc modification of the protocol was used to exclude the outliers at the 99th percentile at baseline from each arm after identifying implausibly high numbers of antibiotic prescriptions attributed to a small number of PCPs in the data set. A similar approach was taken in a previous trial26 with the justification that these PCPs were likely to be systematically different than the study population and subject to measurement error. We used a modified robust Poisson regression approach to analyze the repeated outcome data. Robust SEs were estimated using an exchangeable correlation structure.33 The model included a fixed effect for time per quarter year, as well as an interaction term for time and intervention group. The baseline differences between arms were constrained to zero.34 The primary a priori comparisons of interest were between the average antibiotic prescriptions over 12 months in each letter arm vs control, controlling for prescriptions over the 12 pretrial months. Pairwise least-square mean differences were expressed as relative risks (RRs) with 97.5% CIs. Statistical significance was assessed at the Bonferroni-corrected level of 2-sided P = .025 to account for 2 comparisons of each intervention arm vs control. Planned secondary comparisons were between (1) the average pooled across the 2 letter arms vs control and (2) the initiation letter vs the duration letter. Secondary analysis of the primary outcome used quarterly intervals to examine learning and decay effects. Analyses were conducted in SAS, version 9.4 (SAS Institute).
We prespecified subgroup analyses derived from the Xponent database: baseline prescribing levels (very high [≥90th percentile] vs high [<90th percentile]), years from medical graduation (early career [<11 years], midcareer [11-24 years], or late career [≥25 years]), PCP gender, practice location, and patient sex and age groupings (female, <18 years old; male, <18 years old, female, 18-64 years old; male, 18-64 years old; female, ≥65 years old; and male, ≥65 years old). We included interactions between each subgroup variable and time and the 3-way interaction between the subgroup variable, time, and intervention group to test statistically significant differences between subgroups.
Results
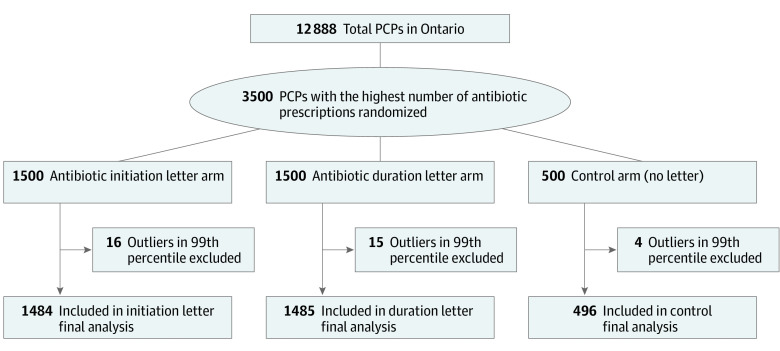
After excluding the 99th percentile outliers, there were 1484 PCPs who received the initiation letter, 1485 who received the duration letter, and 496 controls for analysis (Figure 1). All PCPs were included from baseline on December 1, 2017, and no PCPs were lost to follow-up at study completion of November 30, 2019. Of the random sample of 135 PCPs called to evaluate fidelity of receipt, 45 (33.3%) reported receiving the letter, 41 (30.3%) did not receive the letter, and 50 (37.0%) could not be reached after 2 attempts.
Figure 1. CONSORT Flowchart of Study Participants.
Primary care physicians (PCPs) include general practice or family physicians.
Groups were well balanced (Table 1). Of the 3465 PCPs included in analysis, 2405 (69.4%) were male, and 2116 (61.1%) were 25 or more years from their medical graduation. The median (interquartile range) baseline prescribing volumes were 734 (561-1134) antibiotic prescriptions in the initiation letter arm, 741 (557-1133) in the duration letter arm, and 747 (556-1159) in the control arm. Just fewer than 20% of prescriptions were dispensed to children younger than 18 years and more than 20% to adults 65 years or older (Table 1). Physicians included in this study were more likely to be male and 25 or more years from their medical graduation compared with lower antibiotic–prescribing PCPs in Ontario (eTable 2 in Supplement 2). Figure 2A and B demonstrate the volume of total and prolonged antibiotic prescriptions prescribed by this cohort of PCPs from 1 year before to 1 year after the letters were mailed.
Table 1. Baseline Characteristics of Included Primary Care Physicians.
| Characteristics | No. (%) | ||
|---|---|---|---|
| Initiation letter arm (n = 1484) | Duration letter arm (n = 1485) | Control arm (n = 496) | |
| Gender | |||
| Male | 1019 (68.7) | 1041 (70.1) | 345 (69.6) |
| Female | 465 (31.3) | 444 (29.9) | 151 (30.4) |
| Years from medical graduation | |||
| ≤10 | 180 (12.1) | 181 (12.2) | 61 (12.3) |
| 11-24 | 385 (25.9) | 391 (26.3) | 145 (29.2) |
| ≥25 | 919 (61.9) | 907 (61.1) | 290 (58.5) |
| Practice region | |||
| Rural | 87 (5.9) | 90 (6.1) | 19 (3.6) |
| Urban | 1397 (94.1) | 1395 (93.9) | 477 (96.4) |
| Baseline prescribing level | |||
| Very high (≥90th percentile) | 525 (35.4) | 554 (37.3) | 169 (34.1) |
| High (<75th-90th percentile) | 959 (64.6) | 931 (62.7) | 327 (65.9) |
| Antibiotic prescriptions in the previous 12 mo, median (IQR) | 734 (561-1143) | 741 (557-1133) | 747 (556-1159) |
| Proportion of antibiotics prescribed to patient groups by age and sex, % | |||
| <18 y | |||
| Male | 8.5 | 8.3 | 8.6 |
| Female | 8.7 | 8.5 | 8.8 |
| 18-64 y | |||
| Male | 21.4 | 21.8 | 21.7 |
| Female | 37.5 | 37.7 | 38.6 |
| ≥65 y | |||
| Male | 8.9 | 9.1 | 8.4 |
| Female | 14.9 | 14.8 | 13.9 |
Abbreviation: IQR, interquartile range.
Figure 2. Monthly Mean Volume of Antibiotic Prescriptions and Prolonged Antibiotic Prescriptions in All Study Arms.
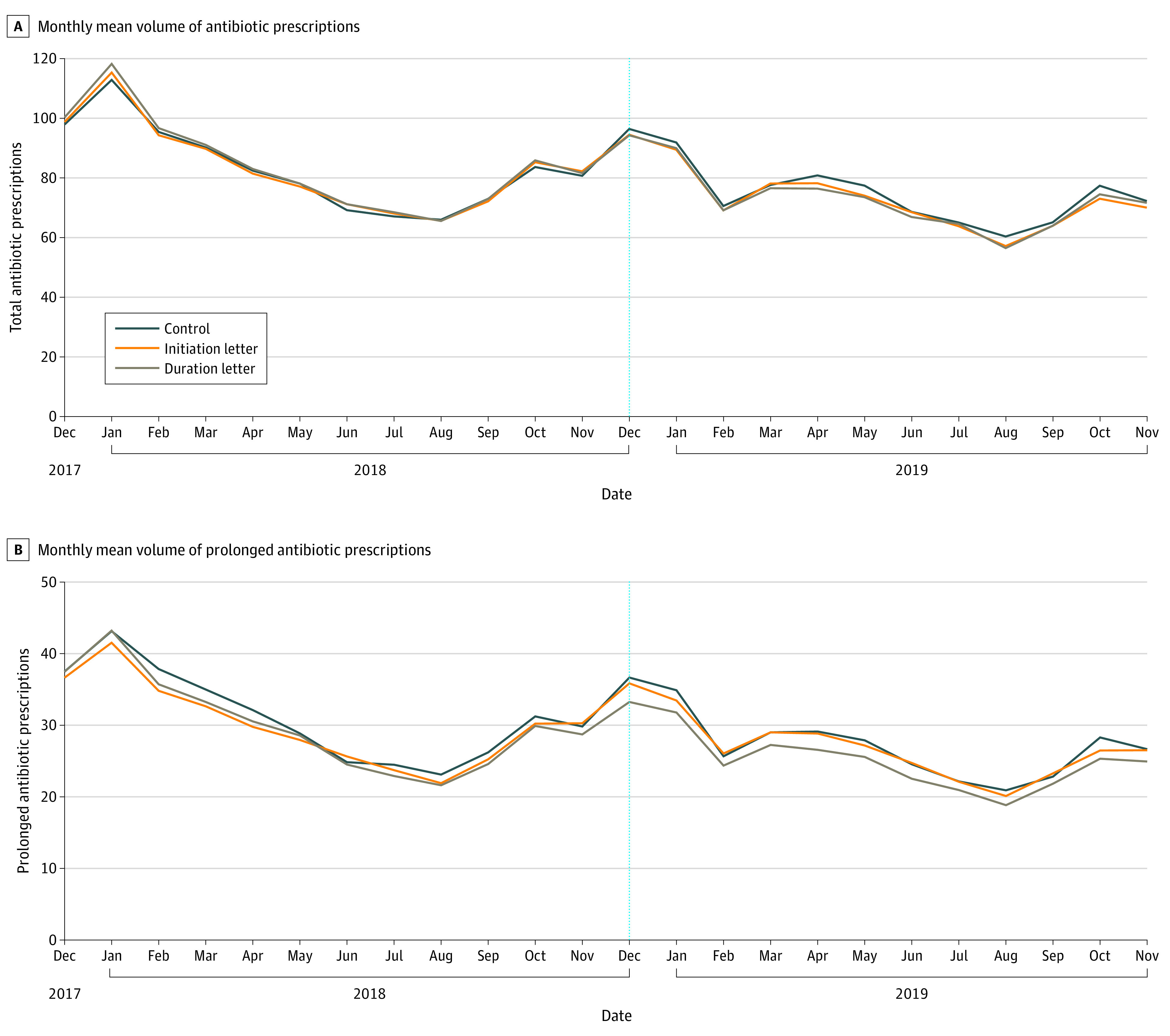
Prolonged antibiotic prescriptions are defined as more than 7 days’ duration.
At baseline, PCPs receiving the initiation letter prescribed a mean (SD) of 988 (732) antibiotic prescriptions per year, and the control PCPs prescribed 988 (723). At 12 months postintervention, PCPs who received the initiation letter prescribed a mean (SD) of 849 (730) antibiotic prescriptions per year, and the control PCPs prescribed 881 (695) (eTable 3 in Supplement 2). During the 12 months postintervention, there was no statistically significant difference in total antibiotic use between PCPs who received the initiation letter compared with controls (RR, 0.96; 97.5% CI, 0.92-1.01; P = .06) (Table 2 and eTable 6 in Supplement 2). At baseline, PCPs receiving the duration letter prescribed a mean (SD) of 1000 (743) antibiotic prescriptions per year, and at 12 months postintervention, these PCPs prescribed 851 (711) antibiotic prescriptions per year. There was a small statistically significant difference in total antibiotic use between PCPs receiving the duration letter compared with controls (RR, 0.95; 97.5% CI, 0.91-1.00; P = .01). The difference between the initiation letter arm and duration letter arm for total antibiotic use was not statistically significant (RR, 0.99; 97.5% CI, 0.96-1.02; P = .42). The receipt of either letter resulted in a statistically significant 4.2% relative difference in total antibiotic use compared with controls (RR, 0.96; 97.5% CI, 0.92-1.00; P = .02).
Table 2. Results of Between-Arm Differences 12 Months Postintervention.
| Comparator | Total antibiotic prescriptions | Prolonged-duration prescriptions (>7 d) | Antibiotic drug costs (CAN$) | |||
|---|---|---|---|---|---|---|
| RR (97.5% CI) | P value | RR (97.5% CI) | P value | RR (97.5% CI) | P value | |
| Initiation letter vs control | 0.96 (0.92-1.01) | .06 | 0.98 (0.93-1.03) | .42 | 0.97 (0.92-1.02) | .19 |
| Duration letter vs control | 0.95 (0.91-1.00)a | .01 | 0.92 (0.87-0.97)a | <.001 | 0.94 (0.89-0.99)a | .01 |
| Duration vs initiation letter | 0.99 (0.96-1.02) | .42 | 0.94 (0.90-0.98) | .001 | 0.97 (0.93-1.00) | .03 |
| Either letter vs control | 0.96 (0.92-1.00) | .02 | 0.95 (0.91-1.00) | .02 | 0.96 (0.91-1.00) | .03 |
Abbreviation: RR, relative risk.
Statistically significant at P < .025.
At baseline, PCPs receiving the duration letter prescribed a mean (SD) of 332 (393) prolonged antibiotic prescriptions per year (eTable 4 in Supplement 2); the control PCPs prescribed 347 (430) prolonged-duration prescriptions. There was a statistically significant 8.1% relative difference in prolonged-duration prescriptions between the duration letter arm and controls (RR, 0.92; 97.5% CI, 0.87-0.97; P < .001). Receipt of the initiation letter had no statistically significant effect, but there was a statistically significant difference between the duration letter arm and initiation letter arm on prolonged-duration prescriptions (RR, 0.94; 97.5% CI, 0.90-0.98; P = .001) (Table 2). At baseline, the mean (SD) drug costs per PCP receiving the duration letter was $14 470 ($11 459) (eTable 5 in Supplement 2). Receipt of the duration letter significantly reduced drug costs by 6.1% (RR, 0.94; 97.5% CI, 0.89-0.99; P = .01) (Table 2). Compared with controls, receipt of the duration letter resulted in 42 fewer antibiotic prescriptions, 24 fewer prolonged-duration prescriptions, and $771 in drug cost savings on average per PCP over 12 months.
In the quarterly analysis, there was minimal change over all 4 quarters from receipt of either the initiation or duration letter on total antibiotic prescriptions (eFigures 1 and 2 in Supplement 2). There was a small decay effect in the duration letter arm (but not the initiation letter arm) on prolonged antibiotic prescriptions decreasing from a RR of 0.93 (95% CI, 0.86-1.01) at 0 to 3 months to an RR of 0.96 (95% CI, 0.85-1.09) at 9 to 12 months postintervention (eFigures 3 and 4 in Supplement 2).
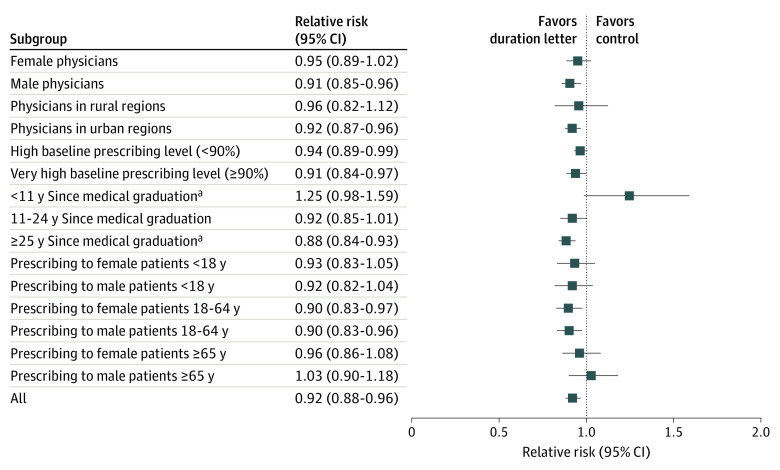
In the planned subgroup analyses, the overall effect was consistent across major subgroups. A significant difference was observed for receipt of the duration letter compared with control, as PCPs 25 or more years from their medical graduation, compared with PCPs fewer than 11 years from their medical graduation, were statistically significantly more likely to prescribe fewer prolonged-duration prescriptions after receiving the letter (RR, 1.25; 95% CI, 0.98-1.59; P = .006; Figure 3). Other subgroup comparisons are presented in eAppendix and eFigures 5 through 11 in Supplement 2.
Figure 3. Forest Plot Showing Subgroup Analysis of Duration Letter Arm Compared With Control for the Outcome of Prolonged Antibiotic Duration.
Error bars indicate 95% CIs.
aP = .006 for interaction.
If all 3500 of the highest-prescribing PCPs in Ontario received the duration letter, this would have resulted in a cumulative reduction at 12 months of 147 000 fewer antibiotic prescriptions (10 per 1000 population), 84 000 fewer antibiotic prescriptions of more than 7 days’ duration (6 per 1000 population), and a $2 700 000 reduction in drug costs ($193 per 1000 population).
Discussion
Mailing a single letter to PCPs notifying them that they are among the highest quartile of antibiotic prescribers compared with their peers resulted in a 4.2% relative difference in overall ambulatory antibiotic prescribing and $1.7 million in drug cost savings.A letter focusing on appropriate antibiotic durations resulted in a relative 8.1% fewer prolonged-duration prescriptions, in addition to 4.8% fewer total antibiotic prescriptions.
There has been an increasing number of trials evaluating audit and feedback in recent years; however, the field has not improved on past successes.35 Additional research is needed to elucidate which aspects of audit and feedback result in the most desirable outcomes. Hallsworth et al26 conducted a large-scale antibiotic peer-comparison trial in the United Kingdom notifying PCP practices that they were in the highest 20th percentile compared with their peers. This resulted in a 3.3% (95% CI, 2.3%-4.3%) relative reduction in antibiotic items dispensed per 1000 population. Meeker et al27 conducted a cluster RCT in the United States on 3 behavioral interventions and found that peer-comparison feedback on unnecessary prescribing resulted in an absolute reduction of 5.2% (95% CI, 1.6%-6.9%) and was the only intervention that had a sustained effect.36 Gulliford et al28 conducted a cluster RCT providing practice-level peer-comparison reports in the United Kingdom, which resulted in a relative reduction in antibiotic prescribing rates for respiratory tract infections of 12% (95% CI, 1%-22%) and did not result in patient harms from increased serious bacterial infections. A multiarm RCT conducted in Australia16 demonstrated an effect from a single mailed letter on total antibiotic use, as well as the effect of personalizing the feedback and including a central figure demonstrating the results, but this study was not peer reviewed. Hemkens et al37 sent physicians in Switzerland quarterly feedback metrics resulting in no statistically significant effect in antibiotic use. The explanation for the lack of success in this trial was likely in part because of the busy report that failed to direct the participants’ attention to the social normative aspect.38
One of the strengths of this study is that we provided feedback using unadjusted counts of total antibiotic prescriptions to the highest-prescribing PCPs. The use of total antibiotics may be more accessible and administratively simpler to implement. We demonstrated that a single mailed letter identifying PCPs as a high prescriber resulted in small but statistically significant changes in prescribing, which has implications for jurisdictions without access to visit denominator data or case-mix data for adjustment. Furthermore, we demonstrated a reduction in antibiotic prescriptions of more than 7 days’ duration by providing targeted education on appropriate antibiotic durations, further reducing likely unnecessary antibiotic exposure. The observed effect on duration was driven by those performing most poorly at baseline. We have previously demonstrated that later-career PCPs (≥25 years after medical graduation) more often prescribe prolonged-duration antibiotics.13 In the present trial, we demonstrated that these same PCPs were considerably more likely to change their behavior in response to feedback.
Behavior-change interventions that reduce unnecessary prescribing in patients are critical to antimicrobial stewardship efforts. However, this study highlights that focusing on education to target inappropriate antibiotic initiation may be less effective. Future interventions should address barriers that cause a gap between what prescribers know and what they do. Prescribers may need help better balancing emotionally salient factors driving unnecessary prescribing, such as perceived patient expectations, time constraints, habits, and fear of patients worsening, with less emotionally salient but potentially more clinically important factors, such as antimicrobial resistance and the risk of adverse drug reactions.39 An emotional response may be invoked through identifying PCPs as high prescribers compared with their peers, thereby nudging the PCP toward the more desirable behavior; additional approaches may include reframing the decision.40,41 Given the large sample in this trial and previous literature showing similar results, these findings are likely to be applicable to other high-income countries and settings for conducting peer-comparison audit and feedback in primary care.
Limitations
This study has some limitations. First, we were unable to adjust for practice type or size, or population denominators, which can result in less acceptance of audit and feedback.42 Some PCPs who prescribe lower amounts of antibiotics per patient population compared with their peers may have been included as high prescribers based on patient volume; however, high and unnecessary prescribing are highly correlated.43 One of the strengths of this study is demonstrating the effect of simpler and readily available data, but it is possible that adjusted data could result in more substantial changes in prescribing. Second, as part of the posttrial evaluation, we could only determine that an estimated one-third of PCPs remembered receiving the letter. Receiving feedback more than once is likely more effective than a single mailing,15 and future studies should consider strategies to improve engagement and durability of the response to a single letter. Third, we were unable to assess the appropriateness of individual antibiotic prescriptions or patient outcomes, such as serious bacterial infection risk, adverse drug events, or antimicrobial resistance. We defined prolonged antibiotic duration as more than 7 days because most outpatient-treated infections should be treated with 7 or fewer days of antibiotics; however, there are some instances where longer durations are warranted. Fourth, the effect size was modest but likely effective on a population level. Additional strategies aimed to optimize antibiotic use in primary care are needed in conjunction with audit and feedback. Fifth, the IQVIA data involves an extrapolation algorithm that we have previously validated but may effect some PCPs differentially.30 Sixth, we quantified the effect of the intervention on drug costs but did not have data on other costs, such as pharmacy dispensing fees and downstream effects of antibiotic overuse. Finally, we adjusted the statistical significance using a Bonferroni correction; however, the statistical significance of the secondary outcomes and strata should be interpreted cautiously.
Conclusions
In this RCT, peer-comparison antibiotic audit and feedback to PCPs that identify them as high prescribers is modestly effective at reducing antibiotic use. More marked reduction in inappropriate antibiotic prescribing will require additional strategies. We demonstrated that a single peer-comparison letter on antibiotic prescribing can reduce drug costs and was successfully implemented across an entire health care system. The addition of a resource on appropriate antibiotic durations resulted in substantial reductions in prolonged-duration prescriptions in addition to reduced overall antibiotic use.
Protocol and statistical analysis plan
eAppendix. Initiation and Duration Letters
eTable 1. Oral Outpatient Antibiotic Drugs Included in the Various Antibiotic Classes
eTable 2. Comparison of primary care physicians in Ontario, Canada included and not included in this intervention
eTable 3. Absolute mean number of total antibiotic prescriptions and marginal differences compared to controls
eTable 4. Absolute mean number of prolonged duration (>7 days) antibiotic prescriptions and marginal differences compared to controls
eTable 5. Absolute mean antibiotic costs (CAN$) and marginal differences compared to controls
eTable 6. Regression model coefficients
eFigure 1. Change in total antibiotic prescriptions over time between the initiation letter and control
eFigure 2. Change in total antibiotic prescriptions over time between the duration letter and control
eFigure 3. Change in prolonged duration prescriptions over time between the initiation letter and control
eFigure 4. Change in prolonged duration prescriptions over time between the duration letter and control
eFigure 5. Forest plot showing subgroup analysis of the duration letter compared to the initiation letter for the outcome of total antibiotic use
eFigure 6. Forest plot showing subgroup analysis of duration letter compared to the initiation letter for the outcome of prolonged antibiotic duration
eFigure 7. Forest plot showing subgroup analysis of the initiation letter compared to control for the outcome of total antibiotic use
eFigure 8. Forest plot showing subgroup analysis of initiation letter compared to control for the outcome of prolonged antibiotic duration
eFigure 9. Forest plot showing subgroup analysis of duration letter compared to control for the outcome of total antibiotic use
eFigure 10. Forest plot showing subgroup analysis of the initiation or duration letters compared to control for the outcome of total antibiotic use
eFigure 11. Forest plot showing subgroup analysis of the initiation or duration letters compared to control for the outcome of prolonged antibiotic duration
Data Sharing Statement
References
- 1.O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. Review on Antimicrobial Resistance. May 2016. Accessed May 24, 2021. https://www.biomerieuxconnection.com/wp-content/uploads/2018/04/Tackling-Drug-Resistant-Infections-Globally_-Final-Report-and-Recommendations.pdf
- 2.Low M, Neuberger A, Hooton TM, et al. Association between urinary community-acquired fluoroquinolone-resistant Escherichia coli and neighbourhood antibiotic consumption: a population-based case-control study. Lancet Infect Dis. 2019;19(4):419-428. doi: 10.1016/S1473-3099(18)30676-5 [DOI] [PubMed] [Google Scholar]
- 3.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14(1):13. doi: 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 5.Nelson RE, Hatfield KM, Wolford H, et al. National estimates of healthcare costs associated with multidrug-resistant bacterial infections among hospitalized patients in the United States. Clin Infect Dis. 2021;72(suppl 1):S17-S26. doi: 10.1093/cid/ciaa1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA. 2016;316(20):2115-2125. doi: 10.1001/jama.2016.16201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Antimicrobial Resistance Surveillance System—update 2020. Public Health Agency of Canada . Updated July 9, 2020. Accessed May 24, 2021. https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-2020-report.html
- 8.Schwartz KL, Langford BJ, Daneman N, et al. Unnecessary antibiotic prescribing in a Canadian primary care setting: a descriptive analysis using routinely collected electronic medical record data. CMAJ Open. 2020;8(2):E360-E369. doi: 10.9778/cmajo.20190175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864-1873. doi: 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 10.Chua KP, Fischer MA, Linder JA. Appropriateness of outpatient antibiotic prescribing among privately insured US patients: ICD-10-CM based cross sectional study. BMJ. 2019;364:k5092. doi: 10.1136/bmj.k5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz KL, Brown KA, Etches J, et al. Predictors and variability of antibiotic prescribing amongst family physicians. J Antimicrob Chemother. 2019;74(7):2098-2105. doi: 10.1093/jac/dkz112 [DOI] [PubMed] [Google Scholar]
- 12.Hicks LA, Taylor THJ Jr, Hunkler RJUS. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368(15):1461-1462. doi: 10.1056/NEJMc1212055 [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Lazaro CI, Brown KA, Langford BJ, Daneman N, Garber G, Schwartz KL. Late-career physicians prescribe longer courses of antibiotics. Clin Infect Dis. 2019;69(9):1467-1475. doi: 10.1093/cid/ciy1130 [DOI] [PubMed] [Google Scholar]
- 14.King LM, Hersh AL, Hicks LA, Fleming-Dutra KE. Duration of outpatient antibiotic therapy for common outpatient infections, 2017. Clin Infect Dis. 2020;ciaa1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6(6):CD000259. doi: 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nudge vs superbugs: a behavioural economics trial to reduce the overprescribing of antibiotics. Australian Government, Department of Health, Department of the Prime Minister and Cabinet . June 2018. Accessed May 24, 2021. https://behaviouraleconomics.pmc.gov.au/sites/default/files/projects/report-nudge-vs-superbugs.pdf
- 17.King LM, Fleming-Dutra KE, Hicks LA. Advances in optimizing the prescription of antibiotics in outpatient settings. BMJ. 2018;363:k3047. doi: 10.1136/bmj.k3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough AR, Rathbone J, Parekh S, Hoffmann TC, Del Mar CB. Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother. 2015;70(9):2465-2473. doi: 10.1093/jac/dkv164 [DOI] [PubMed] [Google Scholar]
- 19.Altamimi S, Khalil A, Khalaiwi KA, Milner RA, Pusic MV, Al Othman MA. Short-term late-generation antibiotics versus longer term penicillin for acute streptococcal pharyngitis in children. Cochrane Database Syst Rev. 2012;(8):CD004872. doi: 10.1002/14651858.CD004872.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265. doi: 10.1001/jamainternmed.2016.3633 [DOI] [PubMed] [Google Scholar]
- 21.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696-705. doi: 10.1016/S1473-3099(14)70737-6 [DOI] [PubMed] [Google Scholar]
- 22.Kim DK, Kim JH, Lee JY, et al. Reappraisal of the treatment duration of antibiotic regimens for acute uncomplicated cystitis in adult women: a systematic review and network meta-analysis of 61 randomised clinical trials. Lancet Infect Dis. 2020;20(9):1080-1088. doi: 10.1016/S1473-3099(20)30121-3 [DOI] [PubMed] [Google Scholar]
- 23.Stolbrink M, Amiry J, Blakey JD. Does antibiotic treatment duration affect the outcomes of exacerbations of asthma and COPD? a systematic review. Chron Respir Dis. 2018;15(3):225-240. doi: 10.1177/1479972317745734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford BJ, Quirk J, Carey S, Daneman N, Garber GE. Influencing duration of antibiotic therapy: a behavior change analysis in long-term care. Am J Infect Control. 2019;47(12):1409-1414. doi: 10.1016/j.ajic.2019.05.020 [DOI] [PubMed] [Google Scholar]
- 25.Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285(22):2871-2879. doi: 10.1001/jama.285.22.2871 [DOI] [PubMed] [Google Scholar]
- 26.Hallsworth M, Chadborn T, Sallis A, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet. 2016;387(10029):1743-1752. doi: 10.1016/S0140-6736(16)00215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. doi: 10.1001/jama.2016.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ. 2019;364:l236. doi: 10.1136/bmj.l236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308-1316. doi: 10.1093/cid/civ076 [DOI] [PubMed] [Google Scholar]
- 30.Schwartz KL, Chen C, Langford BJ, et al. Validating a popular outpatient antibiotic database to reliably identify high prescribing physicians for patients 65 years of age and older. PLoS One. 2019;14(9):e0223097. doi: 10.1371/journal.pone.0223097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Using antibiotics wisely. Choosing Wisely Canada . Accessed May 24, 2021. https://choosingwiselycanada.org/campaign/antibiotics/
- 32.Spellberg B, Rice LB. Duration of antibiotic therapy: shorter is better. Ann Intern Med. 2019;171(3):210-211. doi: 10.7326/M19-1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661-670. doi: 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
- 34.Hooper R, Forbes A, Hemming K, Takeda A, Beresford L. Analysis of cluster randomised trials with an assessment of outcome at baseline. BMJ. 2018;360:k1121. doi: 10.1136/bmj.k1121 [DOI] [PubMed] [Google Scholar]
- 35.Ivers NM, Sales A, Colquhoun H, et al. No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention. Implement Sci. 2014;9(1):14. doi: 10.1186/1748-5908-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linder JA, Meeker D, Fox CR, et al. Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA. 2017;318(14):1391-1392. doi: 10.1001/jama.2017.11152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemkens LG, Saccilotto R, Reyes SL, et al. Personalized prescription feedback to reduce antibiotic overuse in primary care: rationale and design of a nationwide pragmatic randomized trial. BMC Infect Dis. 2016;16(1):421. doi: 10.1186/s12879-016-1739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CR, Doctor JN, Goldstein NJ, Meeker D, Persell SD, Linder JA. Details matter: predicting when nudging clinicians will succeed or fail. BMJ. 2020;370:m3256. doi: 10.1136/bmj.m3256 [DOI] [PubMed] [Google Scholar]
- 39.Mehrotra A, Linder JA. Tipping the balance toward fewer antibiotics. JAMA Intern Med. 2016;176(11):1649-1650. doi: 10.1001/jamainternmed.2016.6254 [DOI] [PubMed] [Google Scholar]
- 40.Hrisos S, Eccles M, Johnston M, et al. Developing the content of two behavioural interventions: using theory-based interventions to promote GP management of upper respiratory tract infection without prescribing antibiotics #1. BMC Health Serv Res. 2008;8:11. doi: 10.1186/1472-6963-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bui TC, Krieger HA, Blumenthal-Barby JS. Framing effects on physicians’ judgment and decision making. Psychol Rep. 2015;117(2):508-522. doi: 10.2466/13.PR0.117c20z0 [DOI] [PubMed] [Google Scholar]
- 42.Ivers N, Barnsley J, Upshur R, et al. “My approach to this job is...one person at a time”: perceived discordance between population-level quality targets and patient-centred care. Can Fam Physician. 2014;60(3):258-266. [PMC free article] [PubMed] [Google Scholar]
- 43.Kitano T, Langford BJ, Brown KA, et al. The association between high and unnecessary antibiotic prescribing: a cohort study using family physician electronic medical records. Clin Infect Dis. 2021;72(9):e345-e351. doi: 10.1093/cid/ciaa1139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol and statistical analysis plan
eAppendix. Initiation and Duration Letters
eTable 1. Oral Outpatient Antibiotic Drugs Included in the Various Antibiotic Classes
eTable 2. Comparison of primary care physicians in Ontario, Canada included and not included in this intervention
eTable 3. Absolute mean number of total antibiotic prescriptions and marginal differences compared to controls
eTable 4. Absolute mean number of prolonged duration (>7 days) antibiotic prescriptions and marginal differences compared to controls
eTable 5. Absolute mean antibiotic costs (CAN$) and marginal differences compared to controls
eTable 6. Regression model coefficients
eFigure 1. Change in total antibiotic prescriptions over time between the initiation letter and control
eFigure 2. Change in total antibiotic prescriptions over time between the duration letter and control
eFigure 3. Change in prolonged duration prescriptions over time between the initiation letter and control
eFigure 4. Change in prolonged duration prescriptions over time between the duration letter and control
eFigure 5. Forest plot showing subgroup analysis of the duration letter compared to the initiation letter for the outcome of total antibiotic use
eFigure 6. Forest plot showing subgroup analysis of duration letter compared to the initiation letter for the outcome of prolonged antibiotic duration
eFigure 7. Forest plot showing subgroup analysis of the initiation letter compared to control for the outcome of total antibiotic use
eFigure 8. Forest plot showing subgroup analysis of initiation letter compared to control for the outcome of prolonged antibiotic duration
eFigure 9. Forest plot showing subgroup analysis of duration letter compared to control for the outcome of total antibiotic use
eFigure 10. Forest plot showing subgroup analysis of the initiation or duration letters compared to control for the outcome of total antibiotic use
eFigure 11. Forest plot showing subgroup analysis of the initiation or duration letters compared to control for the outcome of prolonged antibiotic duration
Data Sharing Statement