Key Points
Question
How do functional outcomes change from the acute to chronic phases of recovery after moderate to severe TBI (msTBI)?
Findings
In this cohort study of 484 participants with msTBI, by 12 months postinjury, approximately half of those with severe TBI and three-quarters of those with moderate TBI recovered the ability to function independently at home for at least 8 hours per day. Among participants in a vegetative state at 2 weeks, 77% recovered consciousness and 25% regained orientation by 12 months.
Meaning
In this study, the presence of acute severe impairment did not universally portend poor functional outcomes after msTBI; clinicians should refrain from making early, definitive prognostic generalizations about the likelihood of poor functional outcomes following moderate and severe TBI.
This cohort study assesses outcomes in major areas of life functions at 2 weeks and 3, 6, and 12 months after moderate to severe traumatic brain injury.
Abstract
Importance
Moderate to severe traumatic brain injury (msTBI) is a major cause of death and disability in the US and worldwide. Few studies have enabled prospective, longitudinal outcome data collection from the acute to chronic phases of recovery after msTBI.
Objective
To prospectively assess outcomes in major areas of life function at 2 weeks and 3, 6, and 12 months after msTBI.
Design, Setting, and Participants
This cohort study, as part of the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study, was conducted at 18 level 1 trauma centers in the US from February 2014 to August 2018 and prospectively assessed longitudinal outcomes, with follow-up to 12 months postinjury. Participants were patients with msTBI (Glasgow Coma Scale scores 3-12) extracted from a larger group of patients with mild, moderate, or severe TBI who were enrolled in TRACK-TBI. Data analysis took place from October 2019 to April 2021.
Exposures
Moderate or severe TBI.
Main Outcomes and Measures
The Glasgow Outcome Scale–Extended (GOSE) and Disability Rating Scale (DRS) were used to assess global functional status 2 weeks and 3, 6, and 12 months postinjury. Scores on the GOSE were dichotomized to determine favorable (scores 4-8) vs unfavorable (scores 1-3) outcomes. Neurocognitive testing and patient reported outcomes at 12 months postinjury were analyzed.
Results
A total of 484 eligible patients were included from the 2679 individuals in the TRACK-TBI study. Participants with severe TBI (n = 362; 283 men [78.2%]; median [interquartile range] age, 35.5 [25-53] years) and moderate TBI (n = 122; 98 men [80.3%]; median [interquartile range] age, 38 [25-53] years) were comparable on demographic and premorbid variables. At 2 weeks postinjury, 36 of 290 participants with severe TBI (12.4%) and 38 of 93 participants with moderate TBI (41%) had favorable outcomes (GOSE scores 4-8); 301 of 322 in the severe TBI group (93.5%) and 81 of 103 in the moderate TBI group (78.6%) had moderate disability or worse on the DRS (total score ≥4). By 12 months postinjury, 142 of 271 with severe TBI (52.4%) and 54 of 72 with moderate TBI (75%) achieved favorable outcomes. Nearly 1 in 5 participants with severe TBI (52 of 270 [19.3%]) and 1 in 3 with moderate TBI (23 of 71 [32%]) reported no disability (DRS score 0) at 12 months. Among participants in a vegetative state at 2 weeks, 62 of 79 (78%) regained consciousness and 14 of 56 with available data (25%) regained orientation by 12 months.
Conclusions and Relevance
In this study, patients with msTBI frequently demonstrated major functional gains, including recovery of independence, between 2 weeks and 12 months postinjury. Severe impairment in the short term did not portend poor outcomes in a substantial minority of patients with msTBI. When discussing prognosis during the first 2 weeks after injury, clinicians should be particularly cautious about making early, definitive prognostic statements suggesting poor outcomes and withdrawal of life-sustaining treatment in patients with msTBI.
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability in the US and globally.1,2 Survivors of moderate to severe TBI (msTBI) commonly experience long-term challenges in returning to work or school, social relationships, and activities of normal daily living.3 Over the long term, msTBI is also associated with increased risk for poorer health, cognitive decline, neurodegenerative disease, and increased need for personal care across the life span.4,5
Long-term follow-up studies,6,7 however, report considerable variability in recovery and degree of residual disability after msTBI. While both moderate TBIs (Glasgow Coma Scale8 [GCS] scores 9-12) and severe TBIs (GCS scores 3-8) can produce lifelong impairments, several long-term outcome studies9,10,11,12 indicate that even after severe TBI, as many as 20% of survivors will recover functional independence within 5 years of injury. Several studies have described long-term outcomes in patients with msTBI,4,6,13,14 yet few have involved early assessment or longitudinal follow-up of patients to track outcomes from the acute through chronic phases of recovery. Capturing the full longitudinal trajectory of outcomes after msTBI is critical to identifying both short-term and long-term variables that differentiate patients who will go on to favorable recovery from those at highest risk of poor long-term outcomes. This, in turn, informs interventions to reduce overall disability associated with msTBI.
The Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study15 is the largest prospective observational study in the US, to our knowledge, to comprehensively investigate the natural history of recovery after TBI from the acute phase (eg, hospital admission) to the chronic phase (eg, 12 months postinjury). Extending prior research focused on patients with msTBI, this study initiated systematic monitoring of functional outcomes early after injury (ie, at 2 weeks) and at 3, 6, and 12 months postinjury. Our analysis aimed to expand existing knowledge of the frequency and extent of recovery achievable in patients with msTBI, including those with disorders of consciousness.
Methods
Study Design
TRACK-TBI was a prospective, multicenter observational study conducted at 18 level 1 trauma centers in the US that enrolled patients with TBI between February 26, 2014, and August 8, 2018. The study was approved by the institutional review board of each TRACK-TBI site. Participants or their legally authorized representatives provided written informed consent to participate. Outcome assessments were completed at 2 weeks and 3, 6, and 12 months postinjury. Data were collected in accordance with the TBI Common Data Elements.16,17 All outcome measures were administered and scored by trained examiners certified on the TRACK-TBI outcome assessment battery. Injury severity was classified through direct administration of the GCS on hospital emergency department arrival. Assessments were performed in person at 2 weeks, 6 months, and 12 months or by telephone if in-person follow-up was not possible. All 3-month assessments were performed by telephone. When a participant was too impaired to complete the assessment, outcome measures were completed by a designated proxy.
Study Population
For inclusion in TRACK-TBI, patients with TBI were required to present to a participating trauma center within 24 hours of injury with clinical indications for obtaining a computed tomography scan under American College of Emergency Medicine/US Centers for Disease Control and Prevention criteria.18 TRACK-TBI inclusion and exclusion criteria are listed in eTable 1 in the Supplement.19 TRACK-TBI enrolled participants across all severity levels. The current analyses include participants 17 years and older with moderate TBI (GCS scores 9-12) or severe TBI (GCS scores 3-8) (eFigure in the Supplement).
Primary Outcome Measure
The Glasgow Outcome Scale–Extended (GOSE)16,20,21,22 is to our knowledge the most widely used outcome measure in TBI studies23 and many clinical trials submitted to the US Food and Drug Administration.17,24 It is validated for in-person or telephone administration. Participants (or their informants) are asked to report functional difficulties in 6 major life domains (independence in the home, independence outside the home, work functioning, social/leisure functioning, relationship problems, and other problems that affect daily life) and endorse only problems that are worse than any preinjury difficulties. The GOSE overall score classifies outcome into 8 categories (Figures 1 and 2). Disability ratings on the GOSE were scored for problems reflecting the combined effects of TBI and associated peripheral injuries (GOSE-All). Every GOSE rating was centrally curated for logical inconsistencies, unusual score combinations, and missing values.
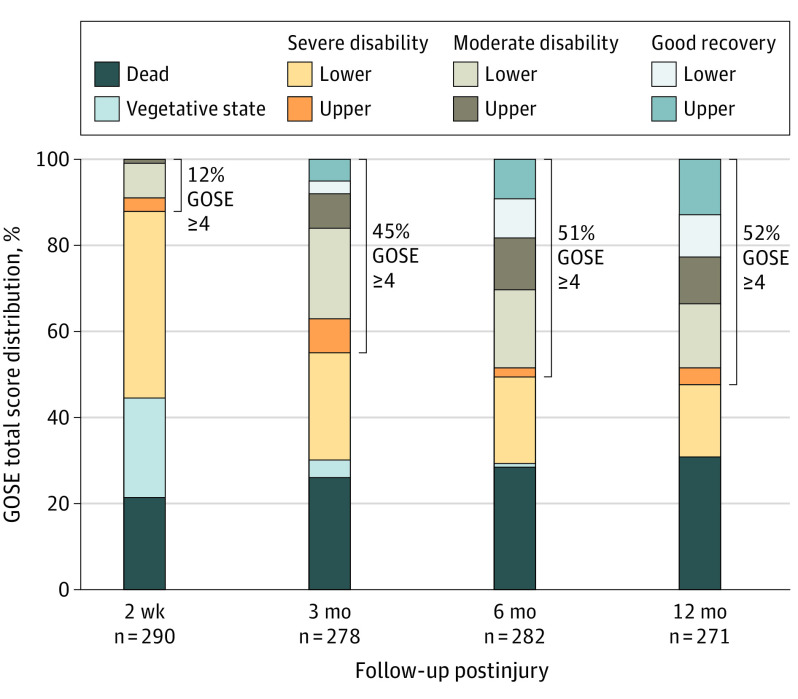
Figure 1. Glasgow Outcome Scale–Extended (GOSE) Total Score Distribution for Patients With Severe Traumatic Brain Injury at 2 Weeks and 3, 6, and 12 Months Postinjury.
Table 2 indicates 1 participant from the severe traumatic brain injury group (0.3%) was in a vegetative state (GOSE score, 2) at 12 months postinjury.
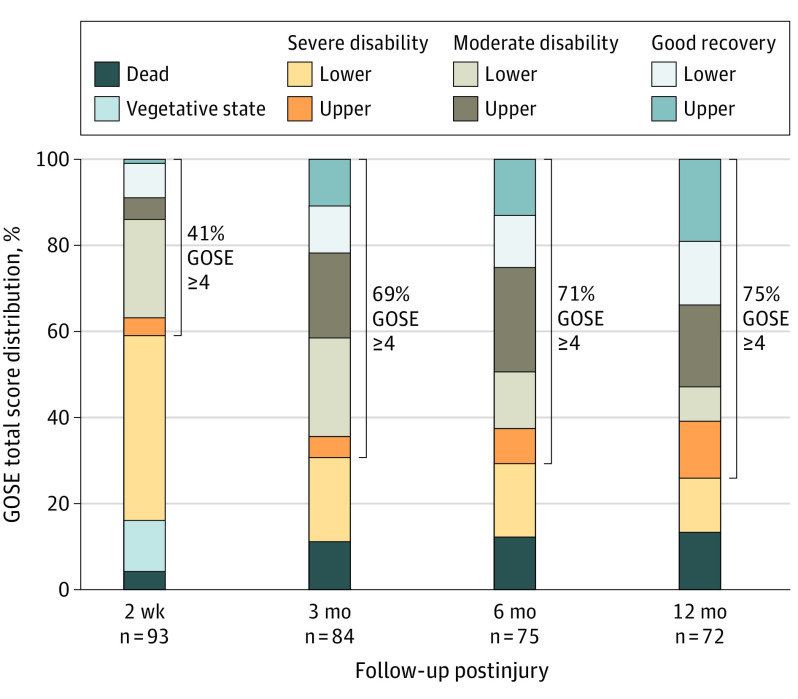
Figure 2. Glasgow Outcome Scale–Extended (GOSE) Total Score Distribution for Patients With Moderate Traumatic Brain Injury at 2 Weeks and 3, 6, and 12 Months Postinjury.
Based on prior TBI studies and clinical trials,12,25,26,27 GOSE outcome scores were dichotomized as a favorable outcome (GOSE scores 4-8) or an unfavorable outcome (GOSE scores 1-3). Those with a favorable outcome included survivors of TBI who were independent at home but may have required assistance for activities outside the home; an unfavorable outcome was characterized by dependence on daily assistance because of cognitive and/or physical disability. Our rationale for including the upper level of the severe disability category (ie, GOSE score 4) as a favorable outcome is based on the premise that participants who are able to function at home without supervision for more than 8 hours per day have considerable functional autonomy and relatives overseeing their care can maintain full-time employment outside the home.
Secondary Outcome Measures
The Disability Rating Scale (DRS) evaluates the degree of impairment in life function after TBI, using 4 domains: consciousness (ie, eye opening, communication ability, motor response); cognitive ability (ie, to feed, toilet, and groom oneself); overall level of functioning; and employability.28 The DRS differs from the GOSE in that only cognitive ability is considered for self-care ratings (ie, feeding, toileting, grooming). Global ratings of overall level of functioning and employability consider both physical and cognitive abilities. Total DRS scores range from 0 to 29, categorized by severity of disability (Figure 3). The DRS Post-Acute Interview29 was used in this study. The clinical outcome assessment battery included the Rivermead Post Concussion Symptoms Questionnaire,30 Brief Symptom Inventory 18-item,31 Satisfaction With Life Scale,32 and neuropsychological measures of verbal memory (Rey Auditory Verbal Learning Test33,34), processing speed (Wechsler Adult Intelligence Scale–Fourth Edition Processing Speed Index35), and executive functioning (Trail Making Test36).
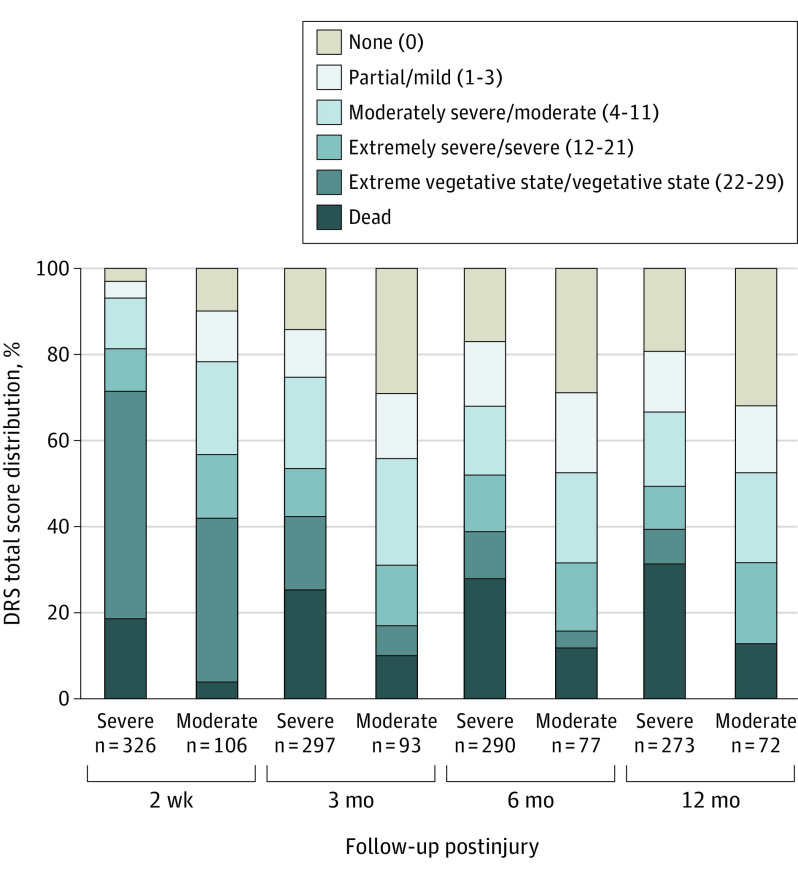
Figure 3. Disability Rating Scale (DRS) Total Score Distribution for Patients With Moderate or Severe Traumatic Brain Injury at 2 Weeks and 3, 6, and 12 Months Postinjury.
Numbers in parentheses in the key indicate DRS score ranges.
Statistical Analysis
Differences in patient and injury characteristics between the severe and moderate TBI groups were analyzed using Mann-Whitney tests for continuous variables and Fisher exact tests for categorical variables.37 All reported P values are 2 tailed with a significance threshold of .05. Participants were included in this analysis if they had at least 1 GOSE or DRS score. Performance on the GOSE and DRS was summarized for each severity group by reporting the frequencies of disability by severity, while counting those who were deceased or in a vegetative state (VS) as disabled in every area. To determine if any bias caused by missing outcomes affected the findings, separate propensity models were constructed at each point for the presence or absence of GOSE and DRS outcomes. These models were based on study site and most of the demographic and injury characteristics in Table 1, with resulting propensities for having the outcome determined by the Toolkit for Weighting and Analysis of Nonequivalent Groups–boosted regression algorithm (rand.org).38 The weights for the analysis were then calculated by inverting the propensity scores and standardizing such that the mean weight corresponding to each type of outcome measure remained equal to 1. These sensitivity analyses showed that the missing outcomes affected the results to a negligible degree; thus, only the unweighted analyses are reported. The study had at least 80% power to detect differences of 18 percentage points in the cumulative GOSE or DRS for each domain. Additional information on statistical analyses related to the GOSE and DRS outcomes are in eTables 2-7 in the Supplement; the neuropsychological test data are in eTable 8 in the Supplement. Statistical analysis was carried out using SPSS version 26 (IBM) and SAS version 9.4 (SAS Institute) from October 2019 to April 2021.
Table 1. Study Population and Acute-Injury Characteristics for Severe Traumatic Brain Injury (TBI) and Moderate TBI Groups.
| Characteristic | Participants, No. (%) | P valuec | |
|---|---|---|---|
| With severe TBIa (n = 362) | With moderate TBIb (n = 122) | ||
| Age, median (IQR), y | 35.5 (25-53) | 38 (25-53) | .67 |
| Unknown, No. | 1 | 0 | |
| Male | 283 (78.2) | 98 (80.3) | .70 |
| Female | 79 (21.8) | 24 (19.7) | |
| Race | |||
| White | 275 (76.0) | 92 (75.4) | .64 |
| Black | 49 (13.5) | 20 (16.4) | |
| Other/unknown | 38 (10.5) | 10 (8.2) | |
| Hispanic ethnicity | |||
| No | 286 (82.2) | 89 (73.0) | .04 |
| Yes | 62 (17.8) | 33 (27.0) | |
| Unknown, No. | 14 | 0 | |
| Insurance | |||
| Commercial | 184 (58.8) | 63 (58.9) | .72 |
| None | 53 (16.9) | 15 (14.0) | |
| Medicare/other | 76 (24.3) | 29 (27.1) | |
| Unknown, No. | 49 | 15 | |
| Education, mean (SD), y | 12.6 (2.6) | 12.9 (2.8) | .36 |
| Unknown, No. | 54 | 12 | |
| Employment | |||
| Full-time | 173 (54.2) | 63 (56.3) | .30 |
| Part-time | 38 (11.9) | 20 (17.9) | |
| Occasional, special, or unemployed | 34 (10.7) | 11 (9.8) | |
| Retired, disabled, or not working | 49 (15.4) | 14 (12.5) | |
| Other | 25 (8) | 4 (4) | |
| Unknown, No. | 43 | 10 | |
| Living situation | |||
| Independent living | 224 (70.0) | 81 (71.1) | .84 |
| Living with others | 88 (27.5) | 29 (25.4) | |
| Homeless | 3 (0.9) | 2 (1.8) | |
| Other | 5 (1.6) | 2 (1.8) | |
| Unknown, No. | 42 | 8 | |
| Previous TBI | |||
| No | 246 (85.4) | 81 (79.4) | .16 |
| Yes | 42 (14.6) | 21 (20.6) | |
| Unknown, No. | 74 | 20 | |
| Neurologic disorder other than TBI | |||
| No | 325 (90.0) | 109 (89.3) | .86 |
| Yes | 36 (10.0) | 13 (10.7) | |
| Unknown, No. | 1 | 0 | |
| Neurodevelopmental disorder | |||
| No | 342 (94.7) | 116 (95.1) | >.99 |
| Yes | 19 (5.3) | 6 (4.9) | |
| Unknown, No. | 1 | 0 | |
| Mental health history | |||
| No | 280 (77.3) | 99 (81.1) | .45 |
| Yes | 82 (22.7) | 23 (18.9) | |
| Unknown, No. | 0 | 0 | |
| Headache history | |||
| No | 358 (99.2) | 119 (97.5) | .17 |
| Yes | 3 (0.8) | 3 (2.5) | |
| Unknown, No. | 1 | 0 | |
| Migraine history | |||
| No | 354 (98.1) | 118 (96.7) | .48 |
| Yes | 7 (1.9) | 4 (3.3) | |
| Unknown, No. | 1 | 0 | |
| Cause of injury | |||
| Motor vehicle crash, occupant | 100 (27.6) | 30 (24.6) | .15 |
| Motorcycle crash | 53 (14.6) | 14 (11.5) | |
| Motor vehicle crash, cyclist or pedestrian | 52 (14.4) | 17 (13.9) | |
| Fall | 84 (23.2) | 32 (26.2) | |
| Assault | 21 (5.8) | 16 (13.1) | |
| Other/unknown | 52 (14.4) | 13 (10.7) | |
| Loss of consciousness | |||
| No | 3 (0.9) | 9 (8.0) | <.001 |
| Yes | 337 (99.1) | 103 (92.0) | |
| Unknown, No. | 22 | 10 | |
| Posttraumatic amnesia | |||
| No | 19 (8.3) | 3 (3.0) | .14 |
| Yes | 210 (91.7) | 92 (97.0) | |
| Unknown, No. | 133 | 27 | |
| Peripheral injuryd | |||
| No | 35 (9.8) | 10 (8.3) | .72 |
| Yes | 322 (90.2) | 111 (91.7) | |
| Unknown, No. | 5 | 1 | |
| Glasgow Coma Scale score in emergency department | |||
| Mean (SD) | 4.3 (1.8) | 10.4 (1.1) | NA |
| Median (IQR) | 3 (3-6) | 10 (9-12) | |
| Abbreviated Injury Scale head and neck score | |||
| Mean (SD) | 4.0 (1.2) | 3.4 (1.3) | <.001 |
| Median (IQR) | 4 (3-5) | 4 (3-4) | |
| Unknown, No. | 13 | 5 | |
| Injury Severity Score total score | |||
| Mean (SD) | 25.6 (11.5) | 20.1 (9.4) | .001 |
| Median (IQR) | 26 (17-33) | 20 (13-27) | |
| Unknown, No. | 13 | 5 | |
| Injury Severity Score peripheral score | |||
| Mean (SD) | 8.2 (9.4) | 5.7 (7.4) | .02 |
| Median (IQR) | 5 (1-14) | 1 (1-9) | |
| Unknown, No. | 13 | 5 | |
| Highest level of care | |||
| Emergency department | 0 | 0 | .003 |
| Inpatient unit | 7 (2.0) | 10 (8.4) | |
| Intensive care unit | 351 (98.0) | 109 (91.6) | |
| Unknown, No. | 4 | 3 | |
| Interventions | |||
| External ventricular drain | |||
| No | 261 (72.1) | 97 (79.5) | .12 |
| Yes | 101 (27.9) | 25 (20.5) | |
| Shunt | 10 (3) | 1 (1) | |
| No | 352 (97.2) | 121 (99.2) | .31 |
| Yes | 10 (2.8) | 1 (0.8) | |
| Craniectomy | 102 (28) | 25 (20) | |
| No | 260 (71.8) | 97 (79.5) | .10 |
| Yes | 102 (28.2) | 25 (20.5) | |
| Craniotomy | 42 (12) | 11 (9) | |
| No | 320 (88.4) | 111 (91.0) | .51 |
| Yes | 42 (11.6) | 11 (9.0) | |
| Length of hospitalizatione | |||
| Mean (SE) | 25.9 (2.4) | 15.6 (1.4) | <.001 |
| Median (SE) | 17.8 (0.8) | 16.6 (0.8) | |
| Unknown, No. | 9 | 6 | |
Glasgow Coma Scale scores of 3 to 8.
Glasgow Coma Scale scores of 9 to 12.
P value from Mann-Whitney and Fisher exact tests as appropriate.
Peripheral injury was defined as either having a peripheral (below the neck) Injury Severity Score greater than 0 or ever reporting peripheral injuries on a Glasgow Outcome Scale–Extended or Disability Rating Scale assessment.
Days to patients being discharged alive (survival analysis with deaths censored). Length-of-stay analysis treats all of the deaths as censored values, with unknown values reflecting incomplete admission or discharge information.
Results
Patient and Acute Injury Characteristics
The TRACK-TBI study enrolled 2697 patients, of whom 484 had moderate or severe TBI and were included in this analysis. The severe TBI group (n = 362 [13.7% of those in the TBI-TRACK study]; median [interquartile range (IQR)] age, 35.5 [25-53] years; 283 men [78.2%]; 275 White individuals [76.0%]) and the moderate TBI group (n = 122 [4.5% of those in the TBI-TRACK study]; median [IQR] age, 38 [25-53] years; 98 men [80.3%]; 92 White individuals [75.4%]) were highly comparable in demographics, causes of trauma, and short-term neurosurgical interventions (Table 1). Missingness on GOSE and DRS scores was 20.9% (n = 101) and 10.5% (n = 51), respectively, at 2 weeks postinjury, with both rates increasing to 29% (GOSE: 141 [29.1%]; DRS: 138 [28.5%]) by 12 months. The mean (median; IQR) GCS score was 4.3 (3; 3-6) in the severe TBI group and 10.4 (10; 9-12) in the moderate TBI group. Participants with severe TBI were more frequently admitted to the intensive care unit (relative risk, 1.07 [95% CI, 1.01-1.13]; P = .001) and had a lengthier mean hospital stay (means [SEs]: severe TBI, 29.5 [2.4] days; moderate TBI, 15.6 [1.4]; days; difference, 10.3 days; P < .001) than participants with moderate TBI. Over the 12-month follow-up period, of those with available data (357 of 484 [73.7%]), 60.2% (215 of 357) received inpatient rehabilitation and 256 of 357 (71.7%) received either inpatient or outpatient rehabilitation services.
Primary Outcomes at 2 Weeks and 3, 6, and 12 Months Postinjury
Figures 1 and 2 illustrate the distribution of GOSE total scores for the severe TBI and moderate TBI groups, respectively, at 2 weeks and 3, 6, and 12 months postinjury. The 12-month mortality rate was 30.6% in the severe TBI group (n = 83 of 271) and 13% in the moderate TBI group (n = 9 of 72). Of 92 deaths, 30 (33%) occurred within 3 days of injury, 48 (52%) within the first week, and 64 (70%) within the first 2 weeks.
At 2 weeks postinjury, 36 of 290 (12.4%) in the severe TBI group had achieved a favorable outcome (GOSE scores 4-8) and only 2 of 290 (0.7%) made a good recovery (defined as a GOSE score of 7 or 8) (Figure 1). The percentage of participants with severe TBI and GOSE scores of 4 or more nearly quadrupled from 2 weeks (36 of 290 [12.4%]) to 3 months (125 of 278 [45.0%]) and reached 52.4% (n = 142 of 271) by 12 months postinjury. Furthermore, the percentage of participants with severe TBI and good recovery (GOSE scores 7-8) increased to 8.3% (n = 23 of 278) at 3 months, 18.4% (n = 52 of 282) at 6 months, and 22.9% (n = 62 of 271) at 12 months, including 12.5% (n = 34 of 271) who achieved a full recovery (GOSE score 8).
In the moderate TBI group, 38 of 93 (41%) had a favorable outcome (GOSE scores ≥4) by 2 weeks postinjury, which increased to 58 of 84 (69%) at 3 months, 53 of 75 (71%) at 6 months, and 54 of 72 (75%) by 12 months (Figure 2). By 1 year, 25 of 72 participants with moderate TBI (35%) had achieved a good recovery (GOSE scores 7-8), including 14 of 72 (19%) who had a complete recovery (GOSE score 8). No participants declined from a favorable to an unfavorable outcome on the GOSE from 2 weeks to 12 months postinjury.
The mixed-model analysis confirmed that the GOSE improved over time through the first year, with approximately 1 (95% CI, 0.82-1.11) point of improvement between 2 weeks and 3 months, decreasing to an improvement of 0.2 (95% CI, 0.05-0.34) points between 6 and 12 months (each P < .001; eTable 2 in the Supplement). Similar analysis on ranked data (ie, percentiles) confirmed that the results were not driven by the noninterval nature of the GOSE score.
Of the 79 participants in a VS at 2 weeks postinjury, 17 (22%) died within 12 months of injury. Of the 62 survivors, 52 (84%) recovered consciousness by 3 months, 58 (94%) by 6 months, and all 62 participants (100%) by 12 months. By 12 months, 14 of 56 survivors (25%) with available data went on to regain orientation and achieve a favorable outcome by 12 months (eTable 4 in the Supplement). Table 2 shows 1 participant in VS at 12 months postinjury who had declined and was not in a VS at 2 weeks.
Table 2. Frequencies Within Each Glasgow Outcome Scale–Extended (GOSE) Domain for Severe and Moderate Traumatic Brain Injury (TBI) Groups at 2 Weeks and 3, 6, and 12 Months Postinjury.
| GOSE domain severity in unweighted analyses | No. (%)a | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 wk | 3 mo | 6 mo | 12 mo | |||||
| Severe (n = 290) | Moderate (n = 93) | Severe (n = 278) | Moderate (n = 84) | Severe (n = 282) | Moderate (n = 75) | Severe (n = 271) | Moderate (n = 72) | |
| Vegetative state and death | ||||||||
| Vegetative state | 68 (23.4) | 11 (12) | 10 (3.6) | 0 | 4 (1.4) | 0 | 1 (0.4) | 0 |
| Died | 60 (20.7) | 4 (4) | 73 (26.3) | 9 (11) | 78 (27.7) | 9 (12) | 83 (30.6) | 9 (13) |
| Independence in the home | ||||||||
| No assistance | 31 (10.7) | 36 (39) | 115 (41.4) | 55 (65) | 139 (49.3) | 51 (68) | 137 (50.6) | 50 (69) |
| Infrequent assistance | 5 (1.7) | 2 (2) | 9 (3.2) | 3 (4) | 6 (2.1) | 2 (3) | 5 (1.8) | 4 (6) |
| Frequent assistance | 126 (43.4) | 40 (43) | 71 (25.5) | 17 (20) | 55 (19.5) | 13 (17) | 45 (16.6) | 9 (13) |
| Independence in shopping | ||||||||
| No assistance | 33 (11.4) | 36 (39) | 115 (41.4) | 54 (64) | 140 (49.6) | 48 (64) | 135 (49.8) | 47 (65) |
| Assistance | 128 (44.3) | 42 (45) | 80 (28.8) | 21 (25) | 60 (21.3) | 18 (24) | 52 (19.2) | 16 (22) |
| Independence in traveling | ||||||||
| No assistance | 33 (11.4) | 34 (37) | 110 (39.6) | 54 (64) | 139 (49.3) | 47 (63) | 133 (49.1) | 46 (64) |
| Assistance | 128 (44.1) | 44 (47) | 85 (30.6) | 21 (25) | 61 (21.6) | 19 (25) | 54 (19.9) | 17 (24) |
| Workb | ||||||||
| No deficit | 2 (1.0) | 8 (10) | 25 (11.8) | 21 (29) | 57 (26.9) | 23 (37) | 70 (34.0) | 30 (49) |
| Reduced capacity | 3 (1.4) | 4 (5) | 25 (11.8) | 12 (17) | 26 (12.3) | 12 (19) | 20 (9.7) | 7 (11) |
| Limited or unable to work | 133 (63.3) | 55 (70) | 125 (59.2) | 34 (47) | 94 (44.3) | 22 (35) | 79 (38.3) | 19 (31) |
| Social and leisure functioning | ||||||||
| No deficit | 11 (3.8) | 16 (17) | 56 (20.1) | 34 (40) | 92 (32.6) | 34 (45) | 98 (36.2) | 34 (47) |
| A bit less | 9 (3.1) | 7 (8) | 23 (8.3) | 10 (12) | 24 (8.5) | 6 (8) | 28 (10.3) | 9 (13) |
| Much less | 17 (5.9) | 11 (12) | 51 (18.3) | 15 (18) | 38 (13.5) | 15 (20) | 27 (10.0) | 13 (18) |
| Unable | 124 (42.9) | 43 (47) | 65 (23.4) | 16 (19) | 46 (16.3) | 11 (15) | 34 (12.5) | 7 (10) |
| Psychological or interpersonal problems | ||||||||
| No disruption | 101 (34.9) | 38 (41) | 122 (43.9) | 47 (56) | 114 (40.4) | 34 (45) | 109 (40.2) | 35 (49) |
| Occasional | 6 (2.1) | 4 (4) | 16 (5.8) | 5 (6) | 25 (8.9) | 6 (8) | 13 (4.8) | 7 (10) |
| Frequent | 16 (5.5) | 14 (15) | 37 (13.3) | 13 (15) | 40 (14.2) | 18 (24) | 43 (15.9) | 15 (21) |
| Constant | 38 (13.1) | 21 (23) | 20 (7.2) | 10 (12) | 21 (7.4) | 8 (11) | 22 (8.1) | 6 (8) |
| Other disabling symptoms | ||||||||
| No impact | 38 (13.1) | 12 (13) | 49 (17.6) | 14 (17) | 52 (18.4) | 15 (20) | 53 (19.6) | 18 (25) |
| Some impact | 123 (42.6) | 66 (71) | 146 (52.5) | 60 (72) | 148 (52.5) | 51 (68) | 134 (49.4) | 45 (63) |
Percentages within each domain sum to 100% after adding percentages of individuals in vegetative state and death categories for the group at each time, with the exception of the work domain, which excludes those who did not work preinjury.
Work among those who worked preinjury; denominators for these calculations were 210 for those with severe injury at 2 weeks, 79 for those with moderate injury at 2 weeks, 211 for those with severe injury at 3 months, 72 for those with moderate injury at 3 months, 212 for those with severe injury at 6 months, 62 for those with moderate injury at 6 months, 206 for those with severe injury at 1 year, and 7 for those with moderate injury at 1 year.
Table 2 details specific GOSE score frequencies within each domain for the severe TBI and moderate TBI groups across all points. eTable 5 in the Supplement provides cumulative frequencies and risk ratios for the 2 groups at each assessment point. The severe TBI group had greater disability than the moderate TBI group in all GOSE domains. In both groups, the percentage of participants requiring assistance progressively declined from 2 weeks to 12 months postinjury. Although 259 of 290 participants (89.3%, including those who died and those in VS) in the severe TBI group required some level of assistance with routine life activities at 2 weeks postinjury, by 12 months, approximately 50% required no assistance at home (137 of 271 [50.6%]) or with shopping (135 [49.8%]) and traveling (133 [49.1%]), and 70 of 271 workers (34%) reported being fully functional at work. At 12 months, most participants with moderate TBI required no assistance at home (50 of 72 [69%]) or with shopping (47 [65%]) and traveling (46 [64%]).
Secondary Outcomes at 2 Weeks and 3, 6, and 12 Months Postinjury
Figure 3 illustrates the distribution of disability classification based on DRS total scores for severe TBI and moderate TBI groups. eTable 6 in the Supplement details DRS score frequencies within each domain across all time points. eTable 7 in the Supplement provides cumulative frequencies and risk ratios for degree of disability in the 2 groups at each assessment point. The severe TBI group had greater disability than the moderate TBI group in all domains on the DRS at all points.
At 2 weeks, 303 of 326 in the severe TBI group (92.9%) and 84 of 106 in the moderate TBI group (79.2%) had moderate disability or worse (DRS scores ≥4). By 1 year, 52 of 273 participants with severe TBI (19.0%) and 23 of 72 participants with moderate TBI (32%) reported no disability (DRS score 0) from their TBI; another 38 of 273 in the severe TBI group (13.9%) and 11 of 72 in the moderate TBI group (15%) reported only mild disability on the DRS.
eTable 8 in the Supplement compares the severe TBI and moderate TBI groups on clinical outcome measures at 12 months postinjury. In both groups, results indicate impaired cognitive performance relative to the normative standardization sample for these measures and high rates of symptom endorsement. When including all participants able to complete all or part of neuropsychological testing, effect sizes for the difference between the severe and moderate TBI groups on cognitive and patient-reported outcomes were small and not statistically significant. Larger effect sizes were observed between the groups, however, when also accounting for the participants missing because of death or severity of TBI-associated impairment that precluded neuropsychological assessment (eg, the Rey Auditory Verbal Learning Test Immediate Memory test: Cohen d, −0.46 [95% CI, −0.75 to −0.17]; the Trail Making Test part A assessment of executive functioning: Cohen d, 0.53 [95% CI, 0.24-0.83]).
Discussion
Traumatic brain injury is a life-changing event that can produce significant, lasting disability. An abundance of literature has characterized the prevalence of long-term impairments and their impacts on quality of life for survivors of msTBI.6,7,9 Results from this large, prospective observational study, however, provide a more encouraging view. A large proportion of the participants with msTBI showed major improvement in life functioning, with many regaining independence between 2 weeks and 12 months after msTBI. These findings should not be misinterpreted to imply an overly optimistic picture of outcomes after msTBI, because a high proportion of participants were left with considerable disabilities. However, these data further inform the natural history of recovery after msTBI and, in particular, wield important translational implications for early clinical management of patients with msTBI.
Although prior studies have investigated functional outcome after msTBI, few have prospectively tracked recovery from the acute through chronic phases, and most have relied only on the GOSE. Thus, the extent to which acute impairment is associated with long-term functional outcomes after msTBI has not been entirely clear. Our data indicate that the degree of impairment evident during the first 2 weeks postinjury should not be taken as a definitive indicator of unfavorable long-term functional outcome. At 2 weeks postinjury, 94% of the severe TBI group and 79% of the moderate TBI group had moderate to severe disability or worse (DRS scores ≥4) and approximately 80% required assistance in basic aspects of everyday function (GOSE scores 1-3). By 12 months, however, half of the severe TBI group and three-quarters of the moderate TBI group were able to function independently at home for at least 8 hours per day (GOSE scores ≥4). While prior investigators have observed that a substantial proportion of patients with severe TBI demonstrate functional improvement on the GOSE in the postacute phase,11,12,39 we obtained more precise disability ratings using the DRS. Among the severe TBI group, we found that 19% had no disability (DRS score 0) and another 14% had only mild disability (DRS scores 1-3) at 12 months postinjury. Perhaps most compelling, among participants who were in a VS at 2 weeks and survived, all recovered consciousness and more than 25% regained orientation by 12 months. These previously unreported findings attest to both the frequency and extent of recovery possible during the first year postinjury.
Prior studies of severe TBI have shown a stepwise increase in favorable outcome, based on GOSE score, from 3 months to 24 months postinjury. Data from those studies suggest that two-thirds of participants with severe TBI convert from an unfavorable outcome at 3 months to a favorable outcome at 2 years postinjury.12 Findings from the current study suggest that the trajectory of clinically significant functional improvement is evident earlier than 3 months postinjury. The percentage of participants with severe TBI and a favorable outcome nearly quadrupled from 12% at 2 weeks to 45% at 3 months, compared with an increase from 41% to 70% in the moderate TBI group. These improvements likely reflect the combined influence of naturally occurring spontaneous recovery and neurorehabilitative interventions.
The complex and heterogeneous nature of brain injury renders predicting outcomes challenging across the TBI spectrum, particularly for patients with more severe injuries. This challenge most often falls on critical care professionals charged with counseling families to make vital decisions, including whether to continue or withdraw life-sustaining treatment, based on information they believe is relevant to anticipating long-term functional outcomes.40 Prior studies have reported that, during the acute phase, trauma specialists overestimate the likelihood of poor outcome and underestimate the probability of good outcome in patients with severe injuries. Those clinician biases often influence key decision-making about patient care, even within the first 24 hours of injury.41,42 The VS is generally viewed as a dire outcome and an influential driver in clinical decision-making and goals of care. Our results clearly demonstrate the potential for recovery from this condition. All but 1 of the 79 participants in VS at 2 weeks postinjury who survived up to 1 year recovered at least basic communication ability, and 25% were fully oriented. Greater knowledge of the natural history of recovery from traumatic VS is critically important to clinical practice in combating the pervasive nihilism associated with this diagnosis. We believe it is essential that clinicians, particularly those in neurocritical care, recognize that traumatic VS is a dynamic condition that evolves over the first year. Recent evidence suggests that key behavioral benchmarks presaging later recovery often do not emerge in patients with traumatic disorders of consciousness until after 6 weeks postinjury.43
Withdrawal of life-sustaining treatment based on early prognostication of poor outcome accounts for most deaths in patients hospitalized for severe TBI.44,45 One-third of deaths in our study occurred within the first 72 hours after injury, half within the first week, and nearly three-quarters by 2 weeks. Separate studies have shown that a sizable percentage of patients with grave impairment, including the inability to follow commands at 2 weeks postinjury or even at admission to inpatient rehabilitation, go on to achieve favorable outcomes several months to years later,9,10 leading some authors to caution against early withdrawal of life-sustaining treatment.46 In view of existing evidence, the recently published American Academy of Neurology–American Congress of Rehabilitation Medicine–National Institute on Disability, Independent Living, and Rehabilitation Research Practice Guidelines on Disorders of Consciousness47 strongly recommended that clinicians avoid statements suggesting that patients with disorders of consciousness who are within 28 days of injury have a universally poor prognosis.
Limitations
In addition to its methodological strengths, some limitations of the current study should be recognized. First, our estimates of favorable outcomes may be lower than other studies that reported outcomes only in those who survived the acute hospital setting and were assessed longer after injury. In that sense, results of this study provide a more fully representative overall trajectory of recovery and outcome after msTBI, which accounts for mortality. Although the proportion of participants we enrolled with msTBI approximates epidemiologic estimates worldwide48 and in the US,49 we did not track the total number of patients with msTBI admitted to all participating sites. Thus, we cannot rule out the possibility that our findings were influenced to some extent by selection bias. Our methods to dichotomize favorable and unfavorable outcomes on GOSE scores was based, in large part, on the level of caregiver assistance required. This dichotomization should not be misinterpreted to mean that those who had favorable outcomes achieved full independence after TBI. Undoubtedly, the definition of a favorable outcome varies in every case; what one patient or family considers favorable may be highly unfavorable for another. Still, the distinction of being able to function independently at home for 8 hours per day is meaningful to both patients and caregivers. The DRS, which provides specific parameters for grading the level of function, offers some corroboration of the GOSE findings.
Although the GOSE and DRS are among the most widely used measures of global outcome in TBI research, they may lack granularity to precisely differentiate specific levels of consciousness and disability, suggesting that they should not be used in isolation for outcome assessment. Indeed, our detailed clinical outcome assessment battery detected substantial cognitive impairment and high prevalence of persistent symptoms in both the moderate TBI and severe TBI groups. Additionally, the 2-week GOSE and DRS outcome ratings may have been affected by sedating medications; it was not possible to precisely align the time sedating medications were administered or held with the period reflected in the GOSE and DRS scores, and it is also not possible to differentiate the direct effects of the TBI from the effects of sedating medications on level of responsiveness. Finally, data for this study were collected at predominantly urban level 1 trauma centers with well-established clinical neurotrauma systems of care, which may limit the generalizability of our findings to patients with msTBI who receive acute care in other (eg, non–level 1) settings.
Conclusions
Results from this prospective, longitudinal study indicate that many patients who sustain msTBI demonstrate major improvement in life functioning and retain the potential to recover functional independence by 12 months postinjury. While a substantial proportion of patients die at high rates or incur considerable lasting disability, there is growing evidence that severe acute impairment, including the diagnosis of VS, does not portend uniformly poor long-term functional outcomes. Accordingly, clinicians should be cautious about suggesting a high likelihood of permanent severe disability within the first 2 weeks postinjury. Ongoing efforts by TRACK-TBI investigators are underway to identify clinical variables that improve prognostic accuracy in patients with msTBI. In the interim, early prognostic counseling and decision-making about withdrawal of life-sustaining care should acknowledge the limitations of prognostic certainty.
eTable 1. TRACK-TBI INCLUSION/EXCLUSION CRITERIA
eTable 2. Results of mixed model analysis on Glasgow Outcome Scale Extended (GOSE) score
eTable 3. Glasgow Outcome Scale Extended (GOSE) frequencies at 3, 6, and 12 months for Severe TBI and Moderate TBI participants in vegetative state at 2 weeks post-injury
eTable 4. Disability Rating Scale (DRS) frequencies at 3, 6, and 12 months for Severe TBI and Moderate TBI participants in vegetative state at 2 weeks post-injury
eTable 5. Cumulative frequencies and risk ratios within each Glasgow Outcome Scale Extended (GOSE) Domain for Severe TBI and Moderate TBI groups at 2 weeks, 3, 6, and 12 months post-injury
eTable 6. Frequencies within each Disability Rating Scale (DRS) domain for Severe TBI and Moderate TBI groups at 2 weeks and 3, 6, and 12 months post-injury
eTable 7. Cumulative frequencies and risk ratios within each Disability Rating Scale (DRS) domain for Severe TBI and Moderate TBI groups at 2 weeks, 3 months, 6 months and 12 months post-injury
eTable 8. Group differences on clinical outcome assessment measures at 12 months post-injury
eFigure. STROBE diagram of participant enrollment and follow-up for Moderate TBI (GCS 9-12) and Severe TBI (GCS 3-8) groups
References
- 1.Centers for Disease Control and Prevention, US Department of Health and Human Services . Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2014. Published 2019. Accessed June 3, 2021. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf
- 2.Johnson WD, Griswold DP. Traumatic brain injury: a global challenge. Lancet Neurol. 2017;16(12):949-950. doi: 10.1016/S1474-4422(17)30362-9 [DOI] [PubMed] [Google Scholar]
- 3.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602-615. doi: 10.1097/00001199-199912000-00009 [DOI] [PubMed] [Google Scholar]
- 4.Kumar RG, Ketchum JM, Corrigan JD, Hammond FM, Sevigny M, Dams-O’Connor K. The longitudinal effects of comorbid health burden on functional outcomes for adults with moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2020;35(4):E372-E381. doi: 10.1097/HTR.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigurdardottir S, Andelic N, Røe C, Schanke AK. Trajectory of 10-year neurocognitive functioning after moderate-severe traumatic brain injury: early associations and clinical application. J Int Neuropsychol Soc. 2020;26(7):654-667. doi: 10.1017/S1355617720000193 [DOI] [PubMed] [Google Scholar]
- 6.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil. 2013;94(6):1199-1201. doi: 10.1016/j.apmr.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 7.Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil. 2003;84(10):1449-1457. doi: 10.1016/S0003-9993(03)00287-9 [DOI] [PubMed] [Google Scholar]
- 8.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81-84. doi: 10.1016/S0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 9.Hammond FM, Giacino JT, Nakase Richardson R, et al. Disorders of consciousness due to traumatic brain injury: functional status ten years post-injury. J Neurotrauma. 2019;36(7):1136-1146. doi: 10.1089/neu.2018.5954 [DOI] [PubMed] [Google Scholar]
- 10.Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. 2012;29(1):59-65. doi: 10.1089/neu.2011.1829 [DOI] [PubMed] [Google Scholar]
- 11.Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1-4 year follow-up. Prog Brain Res. 2009;177:73-88. doi: 10.1016/S0079-6123(09)17707-5 [DOI] [PubMed] [Google Scholar]
- 12.Wilkins TE, Beers SR, Borrasso AJ, et al. Favorable functional recovery in severe traumatic brain injury survivors beyond six months. J Neurotrauma. 2019;36(22):3158-3163. doi: 10.1089/neu.2018.6153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson L, Stewart W, Dams-O’Connor K, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16(10):813-825. doi: 10.1016/S1474-4422(17)30279-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.OʼNeil-Pirozzi TM, Ketchum JM, Hammond FM, Philippus A, Weber E, Dams-OʼConnor K. Physical, cognitive, and psychosocial characteristics associated with mortality in chronic TBI survivors: a National Institute on Disability, Independent Living, and Rehabilitation Research traumatic brain injury model systems study. J Head Trauma Rehabil. 2018;33(4):237-245. doi: 10.1097/HTR.0000000000000365 [DOI] [PubMed] [Google Scholar]
- 15.Yue JK, Vassar MJ, Lingsma HF, et al. ; TRACK-TBI Investigators . Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30(22):1831-1844. doi: 10.1089/neu.2013.2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maas AI, Harrison-Felix CL, Menon D, et al. Common data elements for traumatic brain injury: recommendations from the interagency working group on demographics and clinical assessment. Arch Phys Med Rehabil. 2010;91(11):1641-1649. doi: 10.1016/j.apmr.2010.07.232 [DOI] [PubMed] [Google Scholar]
- 17.Wilde EA, Whiteneck GG, Bogner J, et al. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch Phys Med Rehabil. 2010;91(11):1650-1660.e17. doi: 10.1016/j.apmr.2010.06.033 [DOI] [PubMed] [Google Scholar]
- 18.Jagoda AS, Bazarian JJ, Bruns JJ Jr, et al. ; American College of Emergency Physicians; Centers for Disease Control and Prevention . Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52(6):714-748. doi: 10.1016/j.annemergmed.2008.08.021 [DOI] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of Royal Statistical Society, Series B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 20.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44(4):285-293. doi: 10.1136/jnnp.44.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. doi: 10.1089/neu.1998.15.573 [DOI] [PubMed] [Google Scholar]
- 22.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480-484. doi: 10.1016/S0140-6736(75)92830-5 [DOI] [PubMed] [Google Scholar]
- 23.Pettigrew LE, Wilson JT, Teasdale GM. Assessing disability after head injury: improved use of the Glasgow Outcome Scale. J Neurosurg. 1998;89(6):939-943. doi: 10.3171/jns.1998.89.6.0939 [DOI] [PubMed] [Google Scholar]
- 24.McMillan T, Wilson L, Ponsford J, Levin H, Teasdale G, Bond M. The Glasgow Outcome Scale—40 years of application and refinement. Nat Rev Neurol. 2016;12(8):477-485. doi: 10.1038/nrneurol.2016.89 [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson PJ, Kolias AG, Timofeev IS, et al. ; RESCUEicp Trial Collaborators . Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119-1130. doi: 10.1056/NEJMoa1605215 [DOI] [PubMed] [Google Scholar]
- 26.Vahedi K, Hofmeijer J, Juettler E, et al. ; DECIMAL, DESTINY, and HAMLET investigators . Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215-222. doi: 10.1016/S1474-4422(07)70036-4 [DOI] [PubMed] [Google Scholar]
- 27.Jüttler E, Unterberg A, Woitzik J, et al. ; DESTINY II Investigators . Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091-1100. doi: 10.1056/NEJMoa1311367 [DOI] [PubMed] [Google Scholar]
- 28.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma: coma to community. Arch Phys Med Rehabil. 1982;63(3):118-123. [PubMed] [Google Scholar]
- 29.Malec JF, Hammond FM, Giacino JT, Whyte J, Wright J. Structured interview to improve the reliability and psychometric integrity of the Disability Rating Scale. Arch Phys Med Rehabil. 2012;93(9):1603-1608. doi: 10.1016/j.apmr.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 30.King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587-592. doi: 10.1007/BF00868811 [DOI] [PubMed] [Google Scholar]
- 31.Derogatis LR, ed. Brief Symptom Inventory 18 (BSI-18): Administration, Scoring and Procedures Manual. Pearson; 2001. [Google Scholar]
- 32.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71-75. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 33.Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Archives de Psychologie. 1941;28:215-285. [Google Scholar]
- 34.Schmidt M. Rey Auditory Verbal Learning Test: a Handbook. Western Psychological Services; 1996. [Google Scholar]
- 35.Wechsler D. Wechsler Adult Intelligence Scale (WAIS-IV). 4th ed. Pearson; 2008. [Google Scholar]
- 36.Reitan RM. Trail making test results for normal and brain-damaged children. Percept Mot Skills. 1971;33(2):575-581. doi: 10.2466/pms.1971.33.2.575 [DOI] [PubMed] [Google Scholar]
- 37.Altman DG. Practical statistics for medical research. Chapman and Hall; 1991. [Google Scholar]
- 38.Griffin BA, Ridgeway G, Morral AR, et al. Toolkit for weighting and analysis of nonequivalent groups (TWANG). Published 2014. Accessed June 1, 2021. https://www.rand.org/statistics/twang
- 39.Kowalski RG, Hammond FM, Weintraub AH, et al. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. 2021;78(5):548-557. doi: 10.1001/jamaneurol.2021.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson T, Ryser MD, Ubel PA, et al. Withdrawal of life-supporting treatment in severe traumatic brain injury. JAMA Surg. 2020;155(8):723-731. doi: 10.1001/jamasurg.2020.1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufmann MA, Buchmann B, Scheidegger D, Gratzl O, Radü EW. Severe head injury: should expected outcome influence resuscitation and first-day decisions? Resuscitation. 1992;23(3):199-206. doi: 10.1016/0300-9572(92)90003-U [DOI] [PubMed] [Google Scholar]
- 42.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies through withdrawal of care: do they exist in traumatic brain injury, too? Neurocrit Care. 2013;19(3):347-363. doi: 10.1007/s12028-013-9925-z [DOI] [PubMed] [Google Scholar]
- 43.Giacino JT, Sherer M, Christoforou A, et al. Behavioral recovery and early decision making in patients with prolonged disturbance in consciousness after traumatic brain injury. J Neurotrauma. 2020;37(2):357-365. doi: 10.1089/neu.2019.6429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. [PubMed] [Google Scholar]
- 45.Turgeon AF, Lauzier F, Simard JF, et al. ; Canadian Critical Care Trials Group . Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581-1588. doi: 10.1503/cmaj.101786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vedantam A, Robertson CS, Gopinath SP. Clinical characteristics and temporal profile of recovery in patients with favorable outcomes at 6 months after severe traumatic brain injury. J Neurosurg. 2018;129(1):234-240. doi: 10.3171/2017.3.JNS162720 [DOI] [PubMed] [Google Scholar]
- 47.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness, report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):450-460. doi: 10.1212/WNL.0000000000005926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cassidy JD, Carroll LJ, Peloso PM, et al. ; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury . Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(43)(suppl):28-60. doi: 10.1080/16501960410023732 [DOI] [PubMed] [Google Scholar]
- 49.Langlois Orman J. Epidemiology. In: Silver J, McAllister T, Yudofsky S, eds. Textbook of Traumatic Brain Injury. 2nd ed. American Psychiatric Publishing, Inc; 2011. doi: 10.1176/appi.books.9781585624201.js01 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. TRACK-TBI INCLUSION/EXCLUSION CRITERIA
eTable 2. Results of mixed model analysis on Glasgow Outcome Scale Extended (GOSE) score
eTable 3. Glasgow Outcome Scale Extended (GOSE) frequencies at 3, 6, and 12 months for Severe TBI and Moderate TBI participants in vegetative state at 2 weeks post-injury
eTable 4. Disability Rating Scale (DRS) frequencies at 3, 6, and 12 months for Severe TBI and Moderate TBI participants in vegetative state at 2 weeks post-injury
eTable 5. Cumulative frequencies and risk ratios within each Glasgow Outcome Scale Extended (GOSE) Domain for Severe TBI and Moderate TBI groups at 2 weeks, 3, 6, and 12 months post-injury
eTable 6. Frequencies within each Disability Rating Scale (DRS) domain for Severe TBI and Moderate TBI groups at 2 weeks and 3, 6, and 12 months post-injury
eTable 7. Cumulative frequencies and risk ratios within each Disability Rating Scale (DRS) domain for Severe TBI and Moderate TBI groups at 2 weeks, 3 months, 6 months and 12 months post-injury
eTable 8. Group differences on clinical outcome assessment measures at 12 months post-injury
eFigure. STROBE diagram of participant enrollment and follow-up for Moderate TBI (GCS 9-12) and Severe TBI (GCS 3-8) groups