Abstract
In response to the COVID-19 pandemic, several vaccines were developed and rolled out at unprecedented speed, and notwithstanding this rapid pace of development, the results from initial clinical trials involving tens of thousands of adult subjects generally indicated that most vaccines were remarkably effective and safe, with no major safety warnings noted. However, with more than 2 billion vaccination doses administered to date, reports of rare adverse events following immunization (AEFI) are beginning to emerge. In late February 2021, atypical thrombotic events following immunization with the adenoviral vector-based ChAdOx1 nCov-19 vaccine were first reported, and similar events have also been observed in recipients of the adenoviral vector-based Ad26.COV2.S vaccine and the mRNA-based BNT162b2 and mRNA-1273 vaccines. These manifestations of atypical thrombosis and thrombocytopenia following COVID-19 vaccine immunization are now collectively referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT). Although the reported incidence remains very low and does not affect the overall benefit of immunization, it is also true that if left untreated, VITT can be debilitating or even fatal. Therefore, this review seeks to provide a comprehensive overview regarding the incidence, pathogenesis, presentation, diagnosis, and treatment of VITT, as well as considerations for special populations, based on the currently available evidence in the literature. It is hoped that this will enhance awareness of this vaccine side effect, so that cases of VITT may be identified and treated in a timely and appropriate manner.
Keywords: Adverse events following immunization, COVID-19, Vaccine, Vaccine-induced immune thrombotic thrombocytopenia, Vaccine-induced prothrombotic immune thrombocytopenia, Vaccine side effects
INTRODUCTION
The COVID-19 pandemic spurred a global race to develop effective vaccines, and in an unprecedentedly short period of time, several vaccines entered large clinical trials involving tens of thousands of adult participants. Trial results generally showed these vaccines to be remarkably efficacious and safe, with no major safety issues reported.1-3 On the basis of these results, the United States Food and Drug Administration (FDA), European Medicines Agency (EMA), and other regulators around the world duly issued emergency use authorizations (EUA) to several COVID-19 vaccines, including the adenoviral vector-based ChAdOx1 nCov-19 (Vaxzevria: University of Oxford/AstraZeneca or CoviShield: Serum Institute of India; EMA-authorized) and Ad26.COV2.S (Janssen/Johnson & Johnson; FDA- and EMA-authorized) vaccines, and the mRNA-based BNT162b2 (Comirnaty: Pfizer-BioNTech; FDA- and EMA-authorized) and mRNA-1273 (Moderna; FDA- and EMA-authorized) vaccines. Vaccination remains the single most effective way to reduce severe or fatal disease in COVID-19 for now.4 However, with more than 2 billion vaccination doses administered to date, it is inevitable that cases of rare adverse events following immunization (AEFI) will begin to emerge. In late February 2021, atypical thrombotic events were first reported in a small number of individuals who received the ChAdOx1 nCov-19 vaccine,5 and further reports have emerged in recent months.6-8 Similar events have been reported for the Ad26.COV2.S vaccine,9,10 the BNT162b2 vaccine,1,8 and the mRNA-1273 vaccine1,8 as well. Afflicted individuals can display a diverse array of persistent (> 3 days) signs and symptoms, the most common of which include severe headache, dizziness, visual disturbances, fever, shortness of breath, or pain in the back, abdomen, or extremities.1,6-8,10,11 Upon clinical examination, thromboses at unusual sites such as the cerebral venous sinus (CVST) or splanchnic venous system (SVT), pulmonary embolism, deep vein thrombosis, or acute arterial thrombosis were noted.1,6-8,10,11 Although these events bear clinical resemblance to heparin-induced thrombocytopenia (HIT), the vast majority of cases had no known exposure to heparin before the onset of illness.1,6,7,12 These atypical manifestations of thrombosis and thrombocytopenia have now been collectively termed, vaccine-induced immune thrombotic thrombocytopenia (VITT),1,12-14 also known as vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) or thrombosis with thrombocytopenia syndrome (TTS).14
INCIDENCE
Due to limited clinical experience, diagnostic challenges, and variable reporting mechanisms and follow-up periods, the true incidence of VITT following COVID-19 vaccination remains unknown. Current figures are based on three types of sources: analysis of pharmacovigilance reporting results,1,8,10,15-19 prospective or retrospective analysis of vaccinated populations,20-23 or large-scale prospective post-marketing Phase IIIb or Phase IV studies.24,25 Table 1 presents incidence rates derived from these sources in the form of events per vaccinated people or events per administered vaccine doses, and it can be seen that incidence varies widely, even for the same vaccine. Initial reports suggested a higher incidence in young females,6,7 but this may simply reflect the demographics of early vaccinated populations, the majority of which were women.10,16,20,22,23 To make sense of this data, it is important to look at background or expected incidence rates of the thromboses and thrombocytopenia that constitute VITT, as well as incidence rates in COVID-19 patients.26 Current data suggest that background incidence rates may be comparable to or slightly higher than incidence rates reported after vaccination,10,18,19,27,28 although after this is taken into account, some studies still show increased thrombotic risk following ChAdOx1 nCov-19 vaccination.20,24 Reported incidence rates of suspected VITT following immunization with mRNA vaccines remains within expected background levels to date.18,19,28 However, incidence rates in COVID-19 patients, especially in hospitalized patients, significantly exceed both background and post-vaccination incidence rates for all four vaccines discussed in this review,16,23,27 and thus it should be stressed that the benefits of vaccination still exceed the risk of developing VITT.
Table 1. Reported VITT incidence in COVID-19 vaccination.
| Analysis of pharmacovigilance reporting results | |||||
| Vaccine | Country (study population) | VITT cases | Incidence* | Key findings | Ref. |
| ChAdOx1 nCov-19 | Europe EudraVigilance (34,000,000) | 222 (VTE: 222) | 1/153,153 | Incidence lower than estimated rates for the general population, but unusual thromboses were listed as a rare side effect of the ChAdOx1 nCov-19 vaccine. | 10 |
| ChAdOx1 nCov-19 | Germany (2,700,767) | 31 (VTE: 31) | 1/87,121 | Germany suspended the ChAdOx1 nCov-19 vaccine for regular use in persons under 60. | 15 |
| ChAdOx1 nCov-19 | United Kingdom (21,200,000) | 77 (VTE: 77) | 1/275,325 | Thrombotic risk after vaccination lower than that observed for hospitalized COVID-19 patients. | 16 |
| Ad26.COV2.S | United States (7,200,000) | 12 (VTE: 12) | 1/600,000 | A warning on rare clotting events was added to the Ad26.COV2.S vaccine. | 17 |
| BNT162b2 | Europe EudraVigilance (54,000,000) | 35 (VTE: 35) | 1/1,542,857 | Currently undergoing safety review by the EMA. | 1 |
| BNT162b2 | United States VAERS (18,841,309) | 15 | 1/1,256,087# | Observed case numbers are not greater than those expected for the general population. | 18 |
| mRNA-1273 | Europe EudraVigilance (4,000,000) | 5† (VTE: 5) | 1/800,000 | Currently undergoing safety review by the EMA. | 1 |
| mRNA-1273 | United States VAERS (16,260,102) | 13 | 1/1,250,777# | Observed case numbers are not greater than those expected for the general population. | 18 |
| BNT162b2mRNA-1273 | United States (20,000,000) | 20 (VTE: 20) | 1/1,000,000 | Incidence may be comparable or lower than coincidental rates, but asymptomatic and mild cases may be underreported. | 19 |
| ChAdOx1 nCov-19, BNT162b2, mRNA-1273 | Global WHO Vigibase (361,734,967) | 2,169 (VTE: 795; ATE: 1,374) | 1/166,775 | ATE and VTE were comparable for the ChAdOx1 nCov-19 vaccine, but 2-3 times more ATE than VTE were reported for the mRNA vaccines. | 8 |
| Retrospective analysis of vaccinated populations | |||||
| Vaccine | Country (study population; female percentage) | VITT cases | Incidence* | Key findings | Ref. |
| ChAdOx1 nCov-19 | Denmark (148,792; 80%), Norway (132,472; 78%) | 142 (VTE: 59; ATE: 83) | 1/1,981 | Increased rates of VTE were observed following vaccination, and Denmark and Norway have since suspended use of the ChAdOx1 nCov-19 vaccine. | 20 |
| ChAdOx1 nCov-19 | Nepal (5,591; N/A) | 1 | 1/5,591 | Potential deep vein thrombosis was observed in a serious case. | 21 |
| ChAdOx1 nCov-19 | South Korea (5,589; 77%) | 0 | 0 | No VITT cases were noted. | 22 |
| BNT162b2 | South Korea (277; 77%) | 0 | 0 | No VITT cases were noted. | 22 |
| BNT162b2mRNA-1273 | United States (489,871; 59.1%) | 24 | 1/20,411 | Thrombosis rates remain lower than that in COVID-19 patients. | 23‡ |
| Prospective large-scale post-marketing phase IIIb or phase IV studies | |||||
| Vaccine | Country (study population) | VITT cases | Incidence* | Key findings | Ref. |
| ChAdOx1 nCov-19 | Scotland (1,707,962) | 4,319 (VTE: 893; ATE: 3,288) | 1/395 | An association was found between ChAdOx1 nCov-19 vaccination and higher risk of ITP and ATE. | 24 |
| Ad26.COV2.S | South Africa (288,368) | 0 | 0 | Five thrombotic events were reported in subjects with known thromboembolism risk factors, but no VITT cases were noted. | 25 |
| BNT162b2 | Scotland (821,052) | 2,084 (VTE: 421; ATE: 1,603) | 1/394 | No associations with ITP, VTE, or ATE were noted for BNT162b2 vaccination. | 24 |
* Incidence is presented as VITT events per vaccinated people, except # reflects VITT events per vaccination doses. † Unvetted numbers at time of publication. ‡ The data in this reference was cited from a pre-print study by Taquet M et al. Cerebral venous thrombosis: a retrospective cohort study of 513,284 confirmed COVID-19 cases and a comparison with 489,871 people receiving a COVID-19 mRNA vaccine. Available from: URL: https://osf.io/a9jdq/.
ATE, arterial thrombotic events; EMA, European Medicines Agency; ITP, idiopathic thrombocytopenic purpura; N/A, not available; Ref., reference number; VAERS, Vaccine Adverse Event Reporting System; VITT, vaccine-induced immune thrombotic thrombocytopenia; VTE, venous thrombotic events; WHO, World Health Organization.
POTENTIAL MECHANISMS OF VITT PATHOGENESIS
The pathogenesis of VITT has not been well-established thus far, but it bears clinical and biochemical similarities to HIT.1,6,7,12 VITT and the clinically similar HIT have a common denominator in the presence of high-titer anti-PF4-polyanion antibodies,6,7,29 which can cause platelet activation in functional assays.7 However, it should be noted that the detection of these antibodies alone is not an indication or predictor of VITT. A Norwegian study of 492 health care workers who received a first dose of the ChAdOx1 nCov-19 vaccine found 6 individuals with anti-PF4-polyanion antibodies, but these antibodies did not have platelet-activating properties, and no cases of VITT developed.30 Other studies have failed to find anti-PF4-polyanion antibodies in suspected cases of VITT,10 and it is possible that several different mechanisms may be involved in VITT pathogenesis.
PF4 (platelet factor 4, also known as CXCL4) is one of the most abundant chemokines released upon platelet activation, and is a positively-charged tetrameric protein that binds to glycosaminoglycans.31 When released from platelet α-granules, PF4 binds to polyanions and undergoes conformational changes that can induce a strong immune response, leading to the production of antibodies that activate platelets and cause them to clump together, resulting in clot formation and decreased platelet levels.29 Ultimately, such marked stimulation of the coagulation system can induce clinically significant thromboembolic complications, as in HIT.32 Moreover, PF4 is also a chemotactic protein for immune effector cells such as monocytes and neutrophils, and the prothrombotic action of anti-PF4 antibodies likely involves antibody-mediated platelet activation through IgG-FcγR interactions and similar FcR-mediated engagement of immune effector cells.10,29,32 Activation of these other cell types will further increase thrombotic risk.32
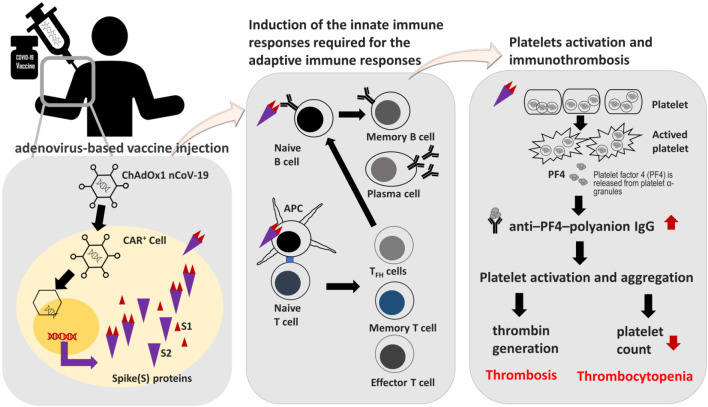
In view of this, several potential mechanisms involving platelets and PF4 have been proposed for VITT to date. It should be noted that the cause of VITT may not be the same for all cases, and thus different mechanisms should be widely assessed and explored. One proposed mechanism involves direct interaction between platelets and SARS-CoV-2 spike proteins produced after vaccination (Figure 1). It is known that SARS-CoV-2 can directly activate platelets via interactions between the virus spike protein and the abundant angiotensin-converting enzyme 2 (ACE2) receptors on platelet surfaces.33 Following immunization with adenoviral vector-based vaccines, adenoviruses in the vaccines will enter coxsackievirus and adenovirus receptor-positive (CAR+) cells, and these cells will then produce and present SARS-CoV-2 spike proteins to trigger an immune response. It is possible that in rare instances, spike proteins may be overexpressed or released into the bloodstream to interact with platelets, thus inducing platelet activation, PF4 release, and anti-PF4 antibody formation, ultimately resulting in thrombosis and thrombocytopenia (Figure 1). Incidentally, platelets and their precursor megakaryocytes are also CAR+ cells,33 and it is worth investigating whether adenoviruses in the vaccines can enter or interact with these cells, as well as the impact of this on platelet activation and anti-PF4 antibody formation.
Figure 1.
Schematic depicting the mechanism of action of adenoviral vector-based COVID-19 vaccines (with the ChAdOx1 nCov-19 vaccine as an example) and a hypothetical model of how this could induce vaccine-induced immune thrombotic thrombocytopenia. Adenoviruses in the vaccines infect coxsackievirus and adenovirus receptor-positive (CAR+) cells after intramuscular injection, and these CAR+ cells subsequently produce SARS-CoV-2 spike protein, which is taken up by antigen-presenting cells (APC) for stimulation of T cells, resulting in the activation of adaptive immune responses. However, the SARS-CoV-2 spike proteins may also activate platelets, leading to the release of platelet factor 4 (PF4) from platelet α-granules, and ultimately resulting in stimulation of the coagulation system and clinically significant thromboembolic complications. Further research is needed to confirm this hypothetical model. TFH cells, T follicular helper cells.
Another proposed mechanism concerns the cross-reactivity of adenoviral antibodies with PF4, as studies have shown that antibodies to adenoviruses in the vaccine are also generated following immunization with ChAdOx1 nCov-19, and can persist at stable levels for almost 3 months.33 It is possible that rare types of such antibodies may inadvertently cross-react with PF4 to trigger platelet activation and clotting mechanisms, but further research is needed to confirm this.
Yet another proposed mechanism involves interactions between PF4 and specific components of COVID-19 vaccines. Like heparin, both double-stranded DNA and single-stranded mRNA are negatively-charged, and can form highly immunogenic complexes with PF4.29,33 If double-stranded DNA borne by adenoviral vector-based COVID-19 vaccines, such as ChAdOx1 nCov-19, inadvertently comes into contact with PF4 due to microtrauma and microbleeding at the injection site, the resulting DNA-PF4 complexes may subsequently trigger an immune reaction involving antigen-presenting cells, memory B cells, and potentially primary T cells, resulting in the formation of anti-PF4-polyanion antibodies that can activate platelets and cause thrombosis and thrombocytopenia. A similar risk may exist for mRNA vaccines, but due to modifications that dampen pathogen-associated molecular pattern sensing mechanisms, such as the replacement of uridine with N1-methyl-pseudouridine (m1Ψ),34 immunogenic risk may be reduced, thus resulting in lower rates of VITT observed thus far for mRNA vaccines. Other negatively-charged substances such as ethylenediaminetetraacetic acid (EDTA), which is used in the ChAdOx1 nCov-19 vaccine, may also interact with PF4 in rare circumstances to trigger immune thrombotic thrombocytopenia.33 However, more research is needed to confirm the exact vaccine components, if any, that can interact with PF4 to induce VITT.
CLINICAL ASPECTS AND DIAGNOSIS
To date, most cases of VITT have been reported after the first dose of the ChAdOx1 nCov-19 vaccine, with fewer cases reported for mRNA vaccines or after the second vaccine dose,13 although this may change as more pharmacovigilance data is collected. Onset remains largely within a window of 5-42 days post-vaccination, with no cases reported within 1-2 days or > 6 weeks after vaccination.14 VITT risk appears to be higher for younger females under the age of 60,13,14 but this may simply mirror the demographics of early vaccinated populations. VITT can be an aggressive syndrome, with reports indicating fatal outcomes in 20-50% of those affected.13 Therefore, clinicians should be particularly alert when examining patients who have received an adenoviral vector-based COVID-19 vaccine within the past 28 days,13 and who display key clinical features of VITT, including:
• Symptom onset between 4 and 28 days after COVID-19 vaccination;
• Chest pain and/or shortness of breath;
• Severe abdominal pain or persistent nausea and vomiting;
• Leg pain, redness or swelling, pallor or coldness;
• New neurologic symptoms, such as severe, recurrent, or persistent headaches, seizures, blurry or double vision, focal weakness, or aphasia;
• Aches or pain in the back or extremities;
• Petechiae or purpura.
For suspected cases of VITT, a complete blood count that includes the following items should be ordered, with results indicative of VITT shown as follows:12-14,35-37
• Complete blood cell count: thrombocytopenia with normal white blood cell and hemoglobin levels;
• Platelet count: Count < 150,000/μL,37 with nadirs of 9,000/μL to 107,000/μL reported;
• Prothrombin time (PT)/activated partial thromboplastin time (APTT): abnormal parameters may be indicative of early VITT, while normal parameters are suggestive of immune thrombocytopenia (ITP);36
• D-dimer test: Marked elevation14,35,36 or > 4 times upper limit of normal;37
• Fibrinogen test: low levels of fibrinogen have been noted in VITT;
• PF4-heparin enzyme-linked immunosorbent assay (ELISA):13,35-37 positive result. Note that ELISA tests should be used as other antibody assays (such as chemiluminescence35 or latex-enhanced immunoassays13) have been found to be negative in cases of VITT.
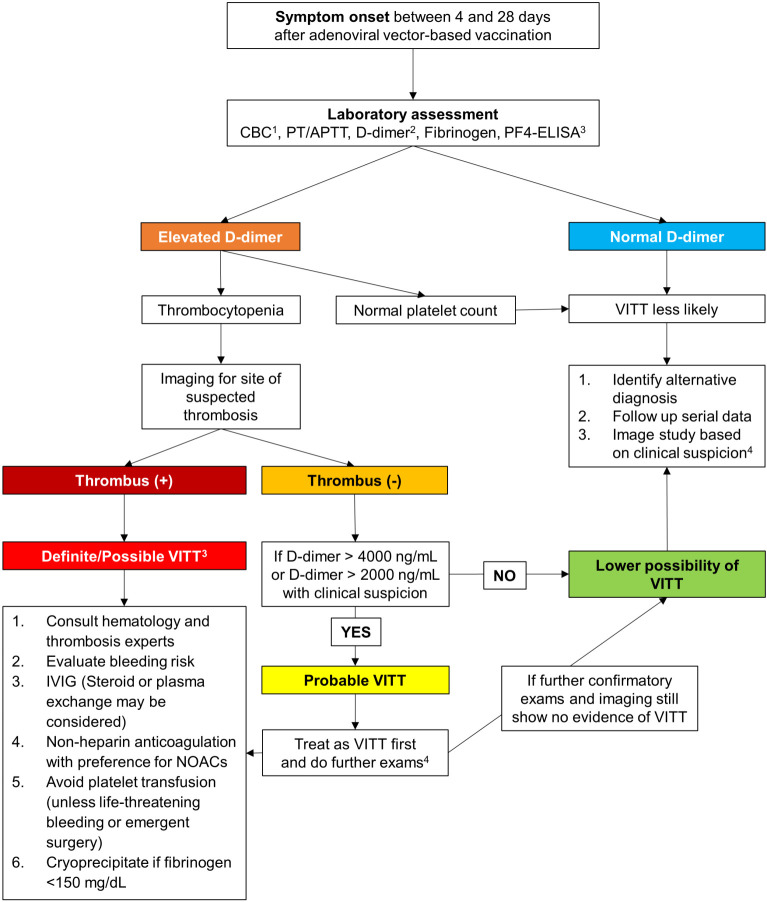
The German Society of Thrombosis and Haemostasis Research (GTH) recommends the complete blood count as the first test for VITT to be performed in their diagnostic algorithm,14 and in most reported cases with no heparin exposure, their serum will demonstrate vigorous reactivity on the PF4-heparin ELISA test. If the ELISA test is low positive, patients are recommended to undergo a confirmatory PF4 platelet activation assay.14,35,36 In addition to the complete blood count, computer tomography (CT) or magnetic resonance (MRI) imaging of appropriate areas in accordance with symptoms, such as imaging of the head for symptoms consistent with CVST, can also assist with diagnosis.14,35,37 A management algorithm for the diagnosis and treatment of VITT is presented in Figure 2. Please note that patients who experience major arterial or venous thrombosis with thrombocytopenia following immunization with the ChAdOx1 nCov-19 vaccine should not receive a second dose of the vaccine.8,10,13
Figure 2.
Proposed algorithm for the management of vaccine-induced immune thrombotic thrombocytopenia. 1Thrombocytopenia is defined as platelet count < 150,000 μL, with typical reported nadirs ranging from 9,000 μL to 107,000 μL. 2Normal plasma levels of D-dimers are < 500 ng/mL, and the application of age-adjusted cut-offs (age × 10 ng/mL when above 50 years) for the exclusion of thrombosis can improve specificity. 3If available, anti-PF4-heparin ELISA testing and functional platelet activation testing are recommended for confirmed VITT cases (definite VITT). 4Further exams for probable VITT include sending serum samples for PF4 antibody assays (HIT ELISA); abdominal ultrasonography/CT imaging for portal or splanchnic vein thrombosis (even in the absence of symptoms); or imaging for CVST, which should be examined closely as initial images may be negative but positive results seen in subsequent testing.14,35 APTT, activated partial thromboplastin time; CBC, complete blood count; CT, computer tomography; CVST, cerebral venous sinus thrombosis; ELISA, enzyme-linked immunosorbent assay; HIT, heparin-induced thrombocytopenia; IVIG, intravenous immunoglobulin; NOAC, novel oral anticoagulant; PF4, platelet factor 4; PT, prothrombin time; VITT, vaccine-induced immune thrombotic thrombocytopenia.
TREATMENT
In cases of definite/possible VITT or probable VITT following diagnostic procedures, and where imaging reveals the presence of thrombosis, it is recommended that patients should be hospitalized, and treatment initiated with non-heparin anticoagulants.14,35,37 Platelet transfusions should be avoided.14,35 It should be noted that a significant proportion of VITT cases also had intracerebral hemorrhage, which may complicate initial management.14 For acute or severe cases, adjunctive therapies such as intravenous immunoglobulin (IVIG), plasma exchange, or mechanical thrombectomy may be helpful.14,35,37,38 For bleeding complications, IVIG and/or prednisone may also be suggested to improve platelet counts, and cryoprecipitate and/or fresh frozen plasma (FFP) may be used for correction of coagulopathy.14 Once patients recover, continuing oral anticoagulation is recommended.14,35 If imaging reveals no overt thrombosis, but thrombocytopenia with marked D-dimer elevation is present, thromboprophylaxis with non-heparin anticoagulants should be considered.14,35,36 Management recommendations are also presented in Figure 2.
Recently, two reports of successful treatment of VITT following immunization with the ChAdOx1 nCoV-19 vaccine have been published, in which non-heparin anticoagulation with short-acting danaparoid-sodium, high-dose IVIG, and prednisone,39 and non-heparin anticoagulation with endovascular treatment,40 respectively resulted in successful treatment outcomes without further complications. However, it should be noted that these treatment recommendations are based on the best evidence currently available, and may be revised or updated as new evidence emerges. Readers are encouraged to follow the latest updates and guidance from the FDA, EMA, United States Centers for Disease Control (CDC), and professional societies such as the International Society on Thrombosis and Haemostasis, American Heart Association/American Stroke Association, the American Society of Hematology, and the British Society of Haematology.
SPECIAL POPULATIONS
For patients receiving anti-platelet agents, it is recommended that:
• Anti-platelet therapy does not represent a therapeutic preventive strategy for VITT in native patients.
• Once undergoing anti-platelet therapy, it is suggested that current medication should be maintained.
Antiplatelet agents are commonly used in patients at risk for cerebrovascular events, acute coronary syndrome, or peripheral arterial disease or after percutaneous coronary or vascular interventions with stenting. Patients are at high risk for complications such as stent thrombosis if anti-platelet therapy is discontinued prematurely. Considering that atherosclerosis is the major reason for the use of anti-platelet agents, and that the potential mechanism of VITT differs from the process of atherosclerosis and stent failure, it is recommended to maintain anti-platelet therapy without interruption during the period of immunization with COVID-19 vaccines. For the same reasons, there is no evidence to recommend the avoidance of adenoviral vector-based vaccines for patients in this group. Importantly, there is a lack of evidence regarding a role for aspirin in the prevention of VITT, and in vitro evidence shows that aspirin does not prevent platelet activation by anti-PF4 antibodies.14,35 Aspirin is not efficacious in preventing HIT antibodies from activating platelets, and can also increase the risk of bleeding in TTS.
For patients receiving anticoagulants, it is recommended that:
• For VITT treatment, the choice of non-heparin anticoagulant depends on the patient’s clinical status and anticipated need to stop anticoagulation (based on risk of bleeding or need for an invasive procedure).
• Once undergoing anticoagulant therapy, it is suggested that current medication should be maintained.
Anticoagulants are mostly used in patients with a history of venous thromboembolism, atrial fibrillation with high embolic risk, any thrombotic events, intracardiac thrombus, and those with mechanical heart valve implants. Maintaining anticoagulant therapy without interruption is recommended during the period of immunization with COVID-19 vaccines, and there is currently no evidence contraindicating the use of adenoviral vector-based vaccines for patients in this group. Nevertheless, some points should be considered. First, before receiving any COVID-19 vaccine, the underlying etiology regarding TTS/HIT should undergo a detailed and comprehensive review. Second, platelet counts should be checked to ascertain whether there were any bleeding events following immunization with an adenoviral vector-based vaccine. Minor bleeding after vaccination, manifesting as bruising or petechiae, may be similar to that experienced while receiving anticoagulants, and thus could be overlooked by patients. Third, novel oral anticoagulants (NOACs) may be preferred over warfarin for general conditions, particularly in Asian patients; however, warfarin remains indicated in special groups such as patients with mechanical heart valve implants, and due to the higher bleeding risk associated with warfarin, it should be used with caution when thrombocytopenia is present.
For patients with previous venous thromboembolism, it is recommended that:
• The benefits of vaccination continue to outweigh the risk, but careful consideration is advised for those at higher risk of specific types of blood clots and with background medical conditions.
• HIT, coincident with thrombocytopenia and venous thrombotic events (VTE) in unusual sites: as this shares similar features with VITT, adenoviral vector-based vaccines should be avoided in such patients.
• Other traditional provoking factors: as these have a different mechanism from VITT, adenoviral vector-based vaccines may be considered if no other option is available.
Venous thromboembolism is a common cardiovascular condition, but rare cases simultaneously present with thrombocytopenia. However, no evidence exists to show an association with higher risk of VITT for previous history of VTE or the presence of traditional provoking factors such as thrombotic event history, use of birth control or other hormone medication, autoimmune disease, low platelet count or other platelet disorders, or pregnant women, although this may potentially be due to the limited number of cases reported to date. Still, it is also true that VITT may not develop through the same mechanisms as traditional bleeding or clotting problems. Importantly, people with a history of HIT, which has clinical similarities to VITT, were reported to be at increased risk of VITT, and thus they are advised to receive other types of COVID-19 vaccines, rather than an adenoviral vector-based vaccine.10,32 As with HIT, VITT can occur at unusual sites such as the splanchnic (splenic, portal, mesenteric) veins, adrenal veins, and the cerebral and ophthalmic veins, and although the pathophysiology for these unusual sites of thrombosis is still unknown,6,7,36 it is recommended that patients with a history of thrombosis occurrence at atypical sites should receive other COVID-19 vaccines instead of an adenoviral vector-based vaccine. Nevertheless, it should be noted that thrombotic risk in COVID-19 patients, especially for hospitalized patients, remains far higher than that for VITT,16,27,28 and thus patients with a history of venous thromboembolism remain at higher risk for VTE following COVID-19 infection as compared to the risk of VITT after vaccination.
CONCLUSIONS
Following the widespread rollout of COVID-19 vaccines, rare cases of VITT have begun to emerge, particularly in relation to the adenoviral vector-based ChAdOx1 nCov-19 and Ad26.COV2.S vaccines. It should be noted that the risk of VITT remains far lower than the risk of thrombotic complications stemming from COVID-19 infection, especially for severe hospitalized cases, and thus the benefit of vaccination still outweighs the risks. Nevertheless, for the rare occurrences of VITT that do emerge after immunization, a standardized management procedure for diagnosis and treatment would be helpful for early detection and timely treatment of such cases, and could facilitate better outcomes. A management algorithm is accordingly proposed in Figure 2. This is based on the best available evidence at present, but changes may be warranted as new evidence and research emerges. In summary, this review provides a comprehensive overview of the incidence, possible mechanisms, diagnosis, and treatment of VITT as described in the literature to date, and hopefully this will facilitate the appropriate management of VITT after vaccination.
Acknowledgments
The authors would like to thank Vercentrys for providing editing support for the manuscript. This review was supported from the Ministry of Science and Technology of Taiwan, MOST grant MOST 109-2327-B-006-005- and D110-G2512 from Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Cines DB, Bussel JB. SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan ZP, Yang M, Lai CL. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals (Basel) 2021;14:406. doi: 10.3390/ph14050406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Dowling WE, Roman RG, et al. Status report on COVID-19 vaccines development. Curr Infect Dis Rep. 2021;23:9. doi: 10.1007/s11908-021-00752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghadas SM, Vilches TN, Zhang K, et al. The impact of vaccination on COVID-19 outbreaks in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konstantinides S. Thrombotic complications of vaccination against SARS-CoV-2: what pharmacovigilance reports tell us – and what they don’t. Eur Respir J. 2021 doi: 10.1183/13993003.01111-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smadja DM, Yue QY, Chocron R, et al. Vaccination against COVID-19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021 doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douxfils J, Favresse J, Dogné JM, et al. Hypotheses behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination. Thromb Res. 2021;203:163–171. doi: 10.1016/j.thromres.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchini M, Liumbruno GM, Pezzo M. COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021 doi: 10.1111/ejh.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021 doi: 10.1055/a-1469-7481. [DOI] [PubMed] [Google Scholar]
- 13.Sholzberg M, Arnold DM, Laupacis A. Recognizing, managing and reporting vaccine-induced immune thrombotic thrombocytopenia. CMAJ. 2021;193:E913–E915. doi: 10.1503/cmaj.210882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes GD, Cuker A, Piazza G, Siegal D. Vaccine-induced thrombotic thrombocytopenia (VITT) and COVID-19 vaccines: what cardiovascular clinicians need to know. Cardiology Magazine. 2021 [Google Scholar]
- 15.von Hundelshausen P, Lorenz R, Siess W, Weber C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): targeting pathomechanisms with bruton tyrosine kinase inhibitors. Thromb Haemost. 2021 doi: 10.1055/a-1481-3039. [DOI] [PubMed] [Google Scholar]
- 16.Bikdeli B, Chatterjee S, Arora S, et al. Cerebral venous sinus thrombosis in the US population, after adenovirus-based SARS-CoV-2 vaccination, and after COVID-19. J Am Coll Cardiol. 2021 doi: 10.1016/j.jacc.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021 doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh KJ, Baumblatt J, Chege W, et al. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2021;39:3329–3332. doi: 10.1016/j.vaccine.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021 doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottegård A, Lund LC, Karlstad O, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrestha S, Devbhandari RP, Shrestha A, et al. Adverse events following the first dose of ChAdOx1 nCoV-19 (COVISHIELD) vaccine in the first phase of vaccine roll out in Nepal. J Patan Acad Health Sci. 2021;8:9, 17. [Google Scholar]
- 22.Bae S, Lee YW, Lim SY, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torjesen I. Covid-19: risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. BMJ. 2021;373:n1005. doi: 10.1136/bmj.n1005. [DOI] [PubMed] [Google Scholar]
- 24.Simpson CR, Shi T, Vasileiou E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021 doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takuva S, Takalani A, Garrett N, et al. Thromboembolic events in the South African Ad26.COV2.S vaccine study. N Engl J Med. 2021 doi: 10.1056/NEJMc2107920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black SB, Law B, Chen RT, et al. The critical role of background rates of possible adverse events in the assessment of COVID-19 vaccine safety. Vaccine. 2021;39:2712–2718. doi: 10.1016/j.vaccine.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenner WJ, Gorog DA. Incidence of thrombotic complications in COVID-19: on behalf of ICODE: The International COVID-19 Thrombosis Biomarkers Colloquium. J Thromb Thrombolysis. 2021 doi: 10.1007/s11239-021-02475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Østergaard SD, Schmidt M, Horváth-Puhó E, et al. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021;397:1441–1443. doi: 10.1016/S0140-6736(21)00762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGonagle D, De Marco G, Bridgewood C. Mechanisms of immunothrombosis in vaccine-induced thrombotic thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection. J Autoimmun. 2021;121:102662. doi: 10.1016/j.jaut.2021.102662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sørvoll IH, Horvei KD, Ernstsen SL, et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J Thromb Haemost. 2021 doi: 10.1111/jth.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gleissner CA, von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol. 2008;28:1920–1927. doi: 10.1161/ATVBAHA.108.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arepally GM, Padmanabhan A. Heparin-induced thrombocytopenia: a focus on thrombosis. Arterioscler Thromb Vasc Biol. 2021;41:141–152. doi: 10.1161/ATVBAHA.120.315445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rzymski P, Perek B, Flisiak R. Thrombotic thrombocytopenia after COVID-19 vaccination: in search of the underlying mechanism. Vaccines (Basel) 2021;9:559. doi: 10.3390/vaccines9060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettini E, Locci M. SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines (Basel) 2021;9:147. doi: 10.3390/vaccines9020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furie KL. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia: on behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke. 2021 doi: 10.1161/STROKEAHA.121.035564. [DOI] [PubMed] [Google Scholar]
- 36.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19:1585–1588. doi: 10.1111/jth.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FACME multidisciplinary working group on the management of cerebral venous sinus thrombosis associated with COVID-19 vaccination. Neurologia (Engl Ed) 2021 doi: 10.1016/j.nrleng.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021 doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaler J, Ay C, Gleixner KV, et al. Successful treatment of vaccine-induced prothrombotic immune thrombocytopenia (VIPIT). J Thromb Haemost. 2021 doi: 10.1111/jth.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umbrello M, Brena N, Vercelli R, et al. Successful treatment of acute spleno-porto-mesenteric vein thrombosis after ChAdOx1 nCoV-19 vaccine. A case report. J Crit Care. 2021;65:72–75. doi: 10.1016/j.jcrc.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]