Abstract
Purpose:
Cases of chemotherapy-induced peripheral neuropathy (CIPN) under-reporting have been sporadically described in the literature, but no studies have focused on actively examining this behavior. Our primary aim was to identify women who purposefully under-reported CIPN, along with reasons for doing so. A secondary aim was to explore factors enabling or hindering communication of CIPN to clinicians.
Methods:
Semi-structured interviews were conducted with women with breast cancer who had received paclitaxel in a prospective observational study. The interview guide was developed based on factors hypothesized to influence side effect disclosure to clinicians. Interviews were recorded, transcribed verbatim, and thematically content analyzed.
Results:
Thirty-four women were interviewed. Three main themes emerged from the analysis: 1) enablers of CIPN reporting (e.g., positive relationships with the oncology team, sufficient appointment time, existence of alternative communication channels to office visits, expectation of CIPN as a side effect); 2) deterrents to CIPN reporting (e.g., perception of need to complete the full course of therapy, fear of treatment discontinuation, lack of knowledge of long-term consequences of CIPN); and 3) balancing survival versus functional impairment due to CIPN. Women prioritized efficacy over CIPN until physical functioning was meaningfully affected. No patients reported purposeful CIPN under-reporting, but three women admitted having considered doing so.
Conclusions:
Despite the lack of evidence of CIPN withholding, women considered both the effectiveness and the toxicity of paclitaxel treatment, as well as beliefs about treatment and long-term consequences of CIPN and relationship with the oncology team, when deciding whether to report CIPN symptoms.
Keywords: Breast cancer, Chemotherapy-Induced Peripheral Neuropathy, Qualitative Research [MH], Taxoids [MH]
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common side effect from several chemotherapeutic agents, including platins, vinca alkaloids, and taxanes [1,2]. Within the context of breast cancer treatment, taxanes are commonly used in the adjuvant setting and contribute to the development of CIPN in 20–60% of patients [3,4]. The experience of CIPN is multifaceted, with impairment at the sensory (numbness, tingling, pain, impaired sensation and hearing), motor (cramps in feet, limb loss of strength) and autonomic levels (blurred vision, dizziness when standing from sitting or lying position) [5,6]. CIPN experience in previous qualitative work has been analogized to “fingernails on a chalkboard” [7], walking on “hot coals” [8], “sandpaper” [9], “a rock on the bottom of your feet” [8], “walking in mud” [9], or described as “pain like needle being stuck in my toes” [7], “severe burning in fingertips” [10], or “feet being asleep” [9].
CIPN can last several months past treatment discontinuation and can, in some cases, become permanent [6,11,12], thus significantly affecting patients’ quality of life [13,14]. Activities of daily living, such as dressing, cooking, sewing, performing household tasks or leisure activities, can become challenging or even impossible. In some cases, walking, climbing stairs, driving, exercising, and socializing are severely compromised [7–10]. Loss of balance, falls, and injury due to CIPN have also been described [7,8]. These symptoms lead to feelings of frustration, embarrassment, isolation, anxiety, depression and loss of purpose among cancer patients [8].
Currently, limited therapeutic options for preventing or treating CIPN exist, with some evidence that duloxetine may be effective for treating painful neuropathy [15–18]. Thus, the strategy used in clinical practice to avoid progression of moderate CIPN symptoms to severe, life-altering CIPN consists of decreasing, delaying or discontinuing chemotherapy treatment, which happens in approximately one-quarter of patients with non-metastatic breast cancer receiving paclitaxel [19]. Treatment disruption is a decision made collaboratively between clinicians and patients by weighing the risks of CIPN progression, and its associated consequences, against the risk of compromising treatment effectiveness [20,21]. Therefore, patient-clinician communication is key, particularly because patients rely on clinicians to tell them how much CIPN they should endure before any treatment decisions are made [7]. Yet, some patients feel that health care professionals are skeptical or dismissive of their CIPN symptoms, and that they provide conflicting information with regard to CIPN severity and longevity [7,22]. This behavior causes feelings of anger and frustration among patients and may lead to symptom under-reporting [7,22]. Another aspect that may contribute to CIPN under-reporting is patients’ determination to complete the full course of treatment to maximize its effectiveness, at the risk of developing long-term or permanent toxicity [5,6]. CIPN has been regarded as an expected and accepted consequence of treatment [5,6], reflecting the trade-off between short-term dysfunction to avoid long-term regret of cancer recurrence [7].
No studies to date have assessed whether CIPN under-reporting is a common occurrence among women with breast cancer receiving paclitaxel. The primary aim of this study was to identify patients who purposefully under-reported CIPN symptoms to their oncologist, and if so, to explore their reasons for under-reporting. A secondary aim was to explore factors enabling or deterring communication of CIPN to clinicians.
Methods
Study Design
This was an exploratory descriptive qualitative study using semi-structured interviews. An interpretive phenomenological approach was selected because the focus was on understanding the lived experience of women who developed CIPN in light of the researcher’s interpretation, as well as their thought process when reporting CIPN to clinicians [23]. This study was approved by the University of Michigan Institutional Review Board (HUM00086259 and HUM00099924) and the University of Michigan Rogel Comprehensive Cancer Center (UMCCC 2014.002).
Participants and recruitment
Women with breast cancer who had enrolled in a prospective observational study of paclitaxel treatment delivered in the adjuvant or neo-adjuvant setting for curative intent, and who had concluded their paclitaxel treatment, were invited to take part in this qualitative study [24]. The prospective study enrolled 60 women, aged 18 years or older, with early stage breast cancer scheduled to receive weekly paclitaxel 80 mg/m2 infusions for 12 weeks at the University of Michigan Rogel Comprehensive Cancer Center. The primary objective of the observational study was to discover clinical and genetic predictors of paclitaxel-induced peripheral neuropathy (ClinicalTrials.gov registration number: NCT02338115). As part of the study, women completed a weekly patient reported CIPN questionnaire [25]. All 60 women who completed the prospective study were re-contacted (median=4.9, range 0.1–14.3 months after conclusion of paclitaxel) up to three times via e-mail or patient portal and invited for an interview with the researchers. This purposive sample of women represented information-rich cases due to their experience undergoing neurotoxic chemotherapy treatment and ability to provide the greatest insight into the research question [26]. Ultimately, 34 women were interviewed, with the remaining individuals not responding to the recruitment email or patient portal message. Informed consent was obtained from each participant prior to the interview.
Data collection
Individual face-to-face interviews were conducted in a private room at Michigan Medicine facilities, or via the telephone when in-person interviews were not feasible, between May and December 2016. No other individuals besides the participant and one of the researchers were present at the interview. Interviews were conducted by a PhD trained pharmacist with experience in qualitative research, who was a post-doctoral fellow at the University of Michigan, four doctor of pharmacy students at the same institution and a fellow who was a PharmD/MBA (all female). No relationship between individuals who conducted the interviews and participants existed prior to the study.
The interview guide was developed based on factors hypothesized to influence patient side effect disclosure to clinicians, namely: relationship with the oncologist and the oncology team; attitudes toward disclosure of side effects to clinicians; perception of risks and benefits of paclitaxel therapy and of continuing therapy after experiencing CIPN; characteristics of the CIPN experience during and after chemotherapy; and awareness of long-term consequences of CIPN (Supplemental material). The interview guide was thoroughly discussed by the research team until agreement with the final version and subsequently pre-tested with two volunteers from the University of Michigan Rogel Comprehensive Cancer Center Breast Cancer Advisory Advocate Committee to establish face validity [27]. Minor changes to the wording of the questions resulted from pre-testing. The guide was iteratively modified following the first couple of interviews to better guide the discussion with participants as the study unfolded.
Our sampling strategy aimed to maximize the potential for achieving data saturation during analysis, indicated by the redundancy of the information provided by respondents and the emergence of no new content to identified themes or additional themes [28]. We were uncertain how many interviews would be required to identify a sample of under-reporters sufficient to reach saturation in response to our research questions. During analysis, we found that among the 34 women interviewed, we were able to reach saturation related to factors enabling and hindering CIPN symptom reporting but were unable to reach saturation regarding aspects related with under-reporting.
Data Analysis
Interviews were audio-recorded and transcribed verbatim by a professional external party. Transcription accuracy was checked for 10% of interviews. An inductive thematic content analysis using ATLAS.ti version 7.5.18 was performed, where the themes emerged directly from the data rather than being imposed a priori [29]. An initial version of the codebook was created by a qualitative data analyst after reviewing the transcripts. The codebook was thoroughly discussed with one of the researchers who is a PhD trained pharmacist and modifications were made accordingly. A sample of five transcripts were then independently coded by the two researchers and discrepancies were discussed and reconciled through a consensus process. After final adjustments were made to the codes and respective definitions, a third research team member who is a Doctor of Pharmacy candidate coded the remaining interviews based on the updated codebook. Weekly meetings were held during the coding process to discuss coding-related questions. Whenever changes to the codebook resulted from these discussions, all transcripts previously coded were reviewed for accuracy. A process of analysis and comparison back to the original transcripts ultimately resulted in codes being grouped into major themes. The research team debated the major themes and subthemes, and reached consensus as to their organization and relationship, ensuring the reliability of the analysis.[30] The importance of a concept or theme identified during qualitative analysis is not directly related to frequency of coding. However, in reporting results below, we use phrases such as “most participants”, “the majority of participants” or “patients overwhelmingly reported” to indicate a high prevalence of the discussed idea among research participants, usually among greater than 50% of respondents.
As a means to ensure rigor and trustworthiness [31,32], the analysis was upheld to the following principles: 1) triangulation, by having multiple independent researchers analyze the data and discuss theme interpretation; 2) reflexivity, by having individuals from diverse backgrounds analyze the data, thus reducing bias stemming from investigators prior assumptions and experiences (a CIPN expert, a PhD trained pharmacist with prior oncology experience, a qualitative data analyst not familiar with the topic); 3) thick description, by presenting a thorough description of the methods used and the participants in the sample; and 4) attention to negative cases, by including subjects with a wide range of CIPN experiences. Exemplar quotes were extracted to support the analysis and to illustrate themes and subthemes. All quotes were used verbatim, with the exception of the word “taxol” which was replaced with “paclitaxel”. Study reporting followed the consolidated criteria for reporting qualitative research (COREQ) checklist [33].
Results
A total of 34 women were interviewed, of whom 30 reported experiencing various degrees of CIPN during treatment and 22 reported still experiencing CIPN at the time of the interview. Four women decreased or delayed paclitaxel dose and nine discontinued treatment before completing their 12 scheduled doses due to CIPN. Demographic and clinical characteristics of interview participants are presented in Table 1. Participants were representative of the overall cohort and there were no significant differences between interview participants and non-participants. The interviews lasted on average 33 minutes (range 9–70, shorter interviews corresponding to women who did not experience CIPN). Three main themes related to CIPN reporting emerged from the analysis: 1) enablers of CIPN reporting; 2) deterrents to CIPN reporting; and 3) balancing survival versus functional impairment due to CIPN. Figure 1 and Table 2 summarize the main findings and present exemplar quotes for each theme.
Table 1.
Demographic and clinical characteristics of interview participants.
| Characteristic | Participants n=34 |
|---|---|
| Age [Mean (SD), range] | 56.9 (8.8), 39–71 |
| Race [n (%)] | |
| Caucasian | 31 (91.2) |
| African American | 1 (2.9) |
| Other | 2 (5.9) |
| Diagnosis of diabetes [n (%)] | 1 (2.9) |
| Chemotherapy setting [n (%)] | |
| Adjuvant | 25 (73.5) |
| Neoadjuvant | 9 (26.5) |
| Receipt 12 doses paclitaxel [n (%)]a | 22 (64.7) |
Four women decreased (range between doses 7 and 12) or delayed (range between doses 3 and 11) paclitaxel dose; nine women discontinued treatment before completing their 12 scheduled doses (range between doses 6 and 10).
Fig. 1.
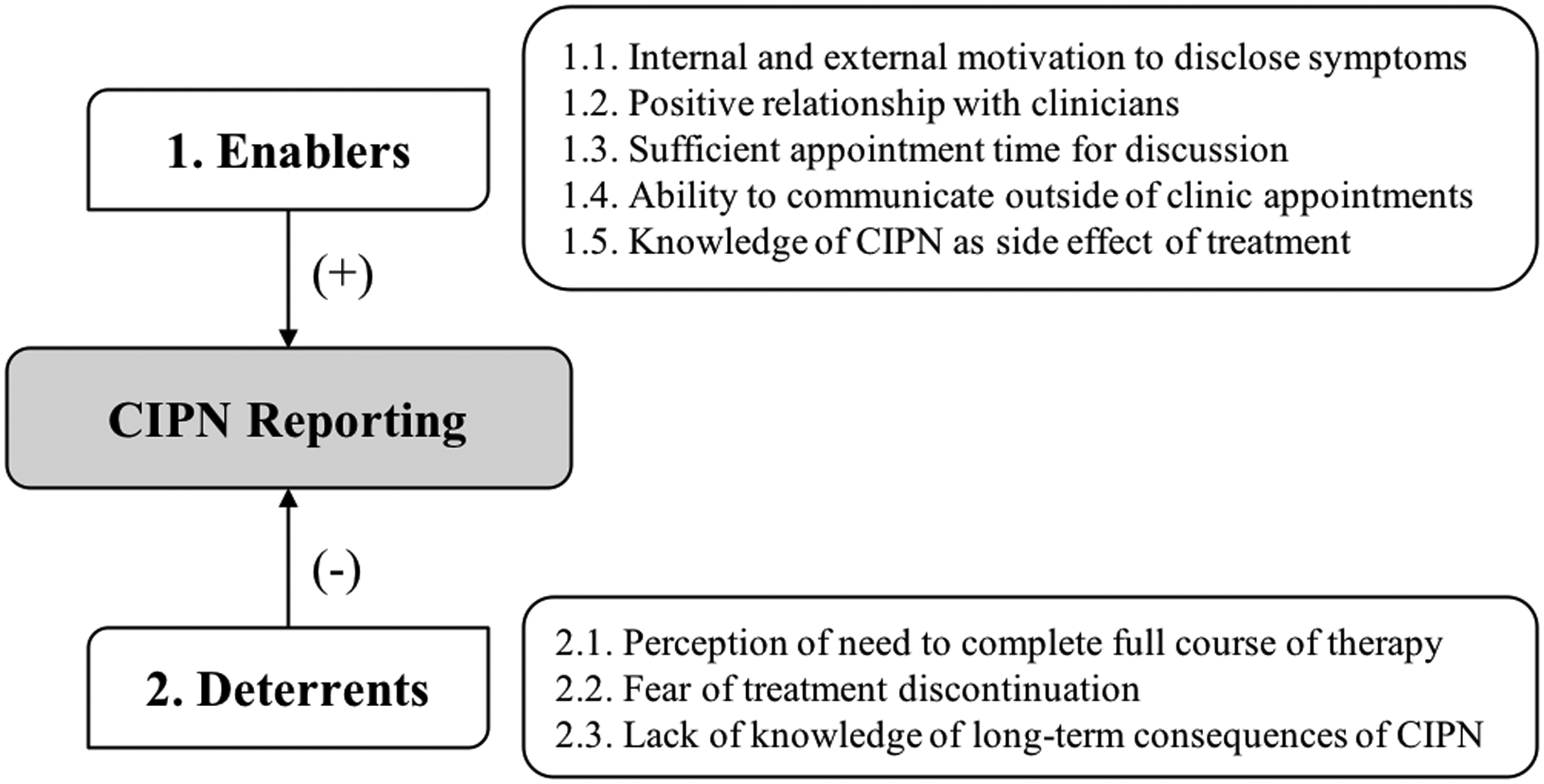
Summary of enablers and deterrents of CIPN reporting to clinicians. CIPN, chemotherapy-induced peripheral neuropathy.
Table 2.
Summary of themes and sub-themes identified from the qualitative analysis, along with exemplar quotes.
| Themes | Exemplar Quotes | |
|---|---|---|
| Theme 1: Enablers of CIPN reporting | 1.1. Internal and external motivation to disclose all symptoms |
Internal motivators
|
| 1.2. Positive relationship with clinicians |
|
|
| 1.3. Sufficient appointment time for discussion |
|
|
| 1.4. Ability to communicate outside of clinic appointments |
|
|
| 1.5. Knowledge of CIPN as side effect of treatment |
|
|
| Theme 2: Deterrents to CIPN reporting | 2.1. Perception of need to complete the full course of therapy |
|
| 2.2. Fear of treatment discontinuation |
|
|
| 2.3. Lack of knowledge of long-term consequences of CIPN |
|
|
| Theme 3: Balancing effectiveness versus functional impairment due to CIPN | 3.1 CIPN tolerability associated with symptom reporting |
|
| 3.2 Potential CIPN under-reporting |
|
|
CIPN, chemotherapy-induced peripheral neuropathy
Theme 1: Enablers of CIPN reporting
1.1. Internal and external motivation to disclose all symptoms
Most women believed that it was critical to be completely honest about their CIPN and other side effects, in order for physicians to provide the best possible care. For those aware of the long-term consequences of CIPN, fear of permanent functional impairment was also an internal motivator to disclose symptoms.
The oncology team and family members were important external motivators to disclose symptoms. Patients were frequently warned that CIPN could become highly debilitating and were asked about CIPN during visits, to the extent that some women considered there to be too much emphasis on CIPN in detriment of other side effects (e.g., pain). In addition, family members who had prior knowledge of the long-term effects of CIPN, insisted that CIPN be reported.
1.2. Positive relationship with clinicians
Patients overwhelmingly reported a positive relationship with their oncologist during treatment. Reasons cited included a sense of openness, comfort, encouragement, caring, listening to the patient, availability to answer questions, and explaining the process using patient-friendly terms. Patients trusted their oncologists’ expertise and judgement.
A few exceptions felt that, at times, their concerns were not given full consideration – “I think sometimes some of my concerns were kind of not pushed off, but it was like oh don’t worry about that, don’t worry about that kind of thing” –, or that oncologists focused more on the cancer itself than on the impact of treatment on patients’ quality of life.
Positive relationships with other members of the healthcare team were also widely reported. Women highlighted that the team worked unified and communicated effectively, allowing patients to experience continuity of care without having to constantly repeat their medical history. The positive attitudes of all members of the team and the stress-free atmosphere of the clinic and staff were also emphasized. Nurse practitioners were often perceived as more available than oncologists, thus contributing to the strong patient-nurse relationship voiced by many women.
1.3. Sufficient appointment time for discussion
The majority of interviewees perceived their appointment time with the clinicians to be adequate and conducive to asking questions, without feeling rushed or dismissed. For some, the visits with the oncologist were less frequent, which made them more conscious of the oncologist’s time. Despite feeling that all their questions were answered, women found that nurse practitioners often had more time to speak with patients.
1.4. Ability to communicate outside of clinic appointments
To contact the oncology team in-between appointments, patients called the office, messaged through the patient portal, or emailed specific members of the team. Most patients were pleased with both the answers received and the response time. Problems were also escalated, if necessary, by the team member receiving the question through practices, such as consulting the oncologist or scheduling an in-person meeting.
1.5. Knowledge of CIPN as side effect of treatment
All except one of the participants were aware that CIPN was a side effect from treatment with paclitaxel. At the initial appointment, clinicians provided patients with a description of common CIPN symptoms such as ‘numbness’ or ‘tingling’. In some cases, the description tended to be simplistic with no mention of possible effect on function, which could have affected women’s understanding. In other cases, clinicians used allegories, such as ‘walking on marbles’, that resulted in patients not being able to recognize CIPN symptoms and thus not reporting them.
Theme 2: Deterrents to CIPN reporting
2.1. Perception of need to complete the full course of therapy
Most patients agreed that completing all 12 paclitaxel doses was critical to maximize therapeutic success and minimize risk of cancer recurrence, and women felt very strongly about completing the full course of therapy. Over a third (n=9) were not aware that treatment discontinuation was an option, even in the case of severe CIPN. Patients voiced that they preferred to have known upfront that discontinuation was an option.
2.2. Fear of treatment discontinuation
When faced with the need to discontinue treatment due to CIPN severity, women expressed concern and fear that not receiving the 12 doses would jeopardize long-term survival. Some women also perceived treatment discontinuation as a sign of weakness and giving up. In these cases, oncologists played a critical role explaining the risk/benefit relationship of paclitaxel.
Despite some resistance to accept treatment discontinuation, several women experienced a sense of relief for not needing additional treatment.
2.3. Lack of knowledge of long-term consequences of CIPN
While a majority of women were aware of the long-term consequences of CIPN prior to starting treatment, having received information from the oncology team and other patients or from searching the web, others were unaware altogether that CIPN could be permanent. For those who experienced CIPN during treatment, they expected symptoms to go away once treatment was concluded.
Theme 3: Balancing effectiveness versus functional impairment due to CIPN
3.1. CIPN tolerability associated with symptom reporting
When deciding whether to interrupt paclitaxel treatment (delay or discontinuation) due to CIPN, patients prioritized efficacy over CIPN until physical functioning was affected to a certain degree, at which point patients prioritized avoiding further worsening of CIPN. The effect of this phenomenon on symptom disclosure depended on patient prioritization at the time: prioritizing efficacy would likely discourage symptom reporting, while prioritizing adverse effect mitigation would likely encourage reporting.
When asked to think back at what they would have done differently, women currently experiencing long-term CIPN articulated that they would have asked to discontinue treatment early, had they known that it was an option, or asked for CIPN preventative measures.
3.2. Potential CIPN under-reporting
No patients in our study admitted to having purposefully under-reported CIPN to their clinicians. Three patients suggested circumstances in which they contemplated under-reporting CIPN symptoms to their clinicians but, ultimately, decided not to. The main reason for considering an incomplete disclosure of symptoms was fear of treatment discontinuation, which could compromise long-term therapeutic success.
An inclination to conceal side effects was also identified in a patient who experienced a severe rash – I did have that feeling where I don’t want anybody to you know say anything or notice anything that’s going to make them have to stop the treatment, because I really didn’t want to do that.
Discussion
Women in our study did not purposefully under-report CIPN to their clinicians, but three admitted they considered doing so. Factors enabling full symptom disclosure included positive relationships with the oncologist and the oncology team, sufficient appointment time to discuss treatment and side effects, existence of alternative communication channels to office visits, and expectation of CIPN as a side effect from chemotherapy. Factors potentially contributing to incomplete symptom reporting were the perception of need to complete the full course of therapy, fear of treatment discontinuation, and lack of knowledge of long-term consequences of CIPN.
Treatment effectiveness and avoidance of cancer recurrence was an absolute priority for these patients. As reported in our study and elsewhere, women expected and accepted CIPN as a side effect from treatment [7,34]. While participants in other studies considered CIPN to be of minimal importance within the context of a cancer diagnosis [7,8], referring to it as “background noise” [7], women in our study prioritized survival over functional impairment, only up to a certain threshold. Despite not having observed CIPN under-reporting in our sample, women held many of the same concerns that lead other women to not disclose symptoms, including fear of cancer recurrence and wanting to complete the full course of therapy. Under-reporting can also result from changes in patients’ motivations and tolerance of CIPN over time. What may be considered a side effect in the beginning, may be perceived as a “new normal” later on, as described in patients with colorectal cancer receiving oxaliplatin [35]. This underscores the importance of iterative, rather than one-time, patient-clinician discussions to account for a shift in CIPN reporting priorities over time.
All patients in our study reported having a positive relationship with the team, but this may not be true for patients receiving care at different institutions. In previous studies, patients worried that the healthcare team would not understand or believe them [7]. Similarly, some women in our study felt that their concerns were not completely addressed and that there was a greater emphasis on treating the cancer than on the impact of treatment on patients’ quality of life. Previous research suggests that patients must reach a “threshold trust” in their physician before they seek relief for an immediate concern [36]. Likewise, the way a physician communicates with patients also impacts how willingly patients participate in the interaction, including reporting symptoms and concerns [37]. Our findings show that patients had positive interactions with and easy access to the oncology team, both during and outside clinic visits.
Although it was only a minority, some women did not understand that CIPN could continue long-term. When weighing long-term treatment effectiveness against long-term toxicity, patients with a lack of understanding of long-term CIPN could be heavily leaning toward prioritizing effectiveness and, thus, be less prone to report CIPN. Consequently, treatment continuation could result in further CIPN progression and increased risk of long-term/irreversible symptoms. In a previous study, rectal cancer survivors articulated that knowledge of the long-term effects of symptoms on function prior to starting treatment might have altered their initial treatment decisions [38]. In our study, women voiced that they would likely hold different perceptions of the risk/benefit of treatment had their CIPN severely impacted long-term function. More rarely, women were not aware that CIPN could be a side effect from chemotherapy, which has also been previously reported [7,10,34,39].
As implications for practice, our study highlights the need for further work to optimize the communication between clinicians and patients about CIPN at the start of and throughout treatment. It is important that clinicians convey that CIPN can have long-term consequences and that paclitaxel treatment can be modified or discontinued to avoid causing severe, potentially irreversible, CIPN. Despite existing evidence that early discontinuation decreases effectiveness [20,21,40], conversations about the benefits/risks of treatment are necessary. Furthermore, there is a need for clinicians to describe CIPN symptoms considering its multifaceted nature [5,6] to avoid misinterpretation of CIPN symptoms by patients, as verified in our sample. Perhaps incorporating peer-to-peer education where patients share, first-hand, their CIPN experience with newly diagnosed patients as they begin treatment could be beneficial [41,42]. Patients appreciate serving in supporting and advocacy roles [43]. Further analysis of how the dialogue between patients and clinicians unfolds when discussing CIPN throughout treatment, by recording these interactions in a clinical setting, would be insightful to unveil further details about the communication flow and style.
Our study has some limitations. First, our sample was very homogenous with regard to education level (high) and race (White), and originated from a single institution; thus, the experience with care received and access to specific resources (e.g., nurse line, patient portal) was similar across participants. The lack of diversity among patients and oncologists may also have accounted for similar perspectives – previous research showed that racial bias among oncologists results in shorter interactions with patients and less patient-centered and supportive communication [44]. Therefore, investigating potential under-reporting in a more diverse sample is imperative. Patients in the prospective study completed weekly patient-reported CIPN questionnaires, which may have made them more aware of symptoms and prompted them to report during the clinic visit. We also included only cases of adjuvant/curative breast cancer, so their perspectives may differ from those of patients with metastatic breast cancer or other tumor types with different efficacy/toxicity balance. Even though our study focused on CIPN, under-reporting can be an issue for other symptoms as well. Some interviews were conducted over the telephone, rather than face-to-face, which could have affected rapport building between participants and the interviewers. Additionally, the amount of information obtained from participants who were willing to be interviewed for a long period of time differed from those whose interviews were brief. Finally, data saturation is a theoretical concept and for that reason the chance of misinterpretation of the saturation point should be acknowledged.
In conclusion, we did not find evidence of CIPN symptom withholding, but women had considerations on both the effectiveness and the toxicity of paclitaxel treatment, as well as other factors that make CIPN reporting to clinicians more or less likely. Future work should focus on identifying true CIPN symptom withholders and understanding their rationale, so that patient-clinician communication and treatment outcomes can be improved. Another avenue for research may encompass the creation of a survey instrument based on these findings to be used in a larger, more diverse sample of patients from multiple clinic sites (including community-based practices).
Supplementary Material
Supplemental Fig. Flow chart of study participants included in the interview.
Acknowledgements.
We thank all the women who accepted to participate in this qualitative study. We also acknowledge the contribution of Dr. Brittney M. Nobles, PharmD, MBA in assisting with two interviews.
Funding.
Financial support was received from the National Center for Advancing Translational Sciences under award number KL2TR000434 and 2UL1TR000433 (PI: Hertz), and the Michigan Institute for Clinical & Health Research (MICHR) Pilot Grant Program Seed Grants at the University of Michigan under award number U050294.
Conflict of interest.
Syverson owns stock in Pfizer Inc. and AbbVie Inc. Farris is a consultant and received remuneration from Quio Technologies. Henry received support to her institution for pharma-sponsored clinical trials from Abbvie, Pfizer, and Innocrin Pharmaceuticals. All other authors declare that they have no conflict of interest.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Michigan Institutional Review Board (HUM00086259 and HUM00099924) and University of Michigan Rogel Comprehensive Cancer Center (UMCCC 2014.002), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Data availability statement. The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G (2012) Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol 82 (1):51–77. [DOI] [PubMed] [Google Scholar]
- 2.Miltenburg NC, Boogerd W (2014) Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev 40 (7):872–882. [DOI] [PubMed] [Google Scholar]
- 3.Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, Lunet N (2015) Neurological complications of breast cancer: A prospective cohort study. Breast 24 (5):582–587. [DOI] [PubMed] [Google Scholar]
- 4.Reyes-Gibby CC, Morrow PK, Buzdar A, Shete S (2009) Chemotherapy-induced peripheral neuropathy as a predictor of neuropathic pain in breast cancer patients previously treated with paclitaxel. J Pain 10 (11):1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanay MA, Armes J, Ream E (2017) The experience of chemotherapy-induced peripheral neuropathy in adult cancer patients: a qualitative thematic synthesis. Eur J Cancer Care (Engl) 26 (5):e12443. [DOI] [PubMed] [Google Scholar]
- 6.Chan CW, Cheng H, Au SK, Leung KT, Li YC, Wong KH, Molassiotis A (2018) Living with chemotherapy-induced peripheral neuropathy: Uncovering the symptom experience and self-management of neuropathic symptoms among cancer survivors. Eur J Oncol Nurs 36:135–141. [DOI] [PubMed] [Google Scholar]
- 7.Bakitas MA (2007) Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res 56 (5):323–331. [DOI] [PubMed] [Google Scholar]
- 8.Tofthagen C (2010) Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs 14 (3):E22–28. [DOI] [PubMed] [Google Scholar]
- 9.Speck RM, DeMichele A, Farrar JT, Hennessy S, Mao JJ, Stineman MG, Barg FK (2012) Scope of symptoms and self-management strategies for chemotherapy-induced peripheral neuropathy in breast cancer patients. Support Care Cancer 20 (10):2433–2439. [DOI] [PubMed] [Google Scholar]
- 10.Boehmke MM, Dickerson SS (2005) Symptom, symptom experiences, and symptom distress encountered by women with breast cancer undergoing current treatment modalities. Cancer Nurs 28 (5):382–389. [DOI] [PubMed] [Google Scholar]
- 11.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin L, Fallon M (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155 (12):2461–2470. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Unger JM, Crew KD, Till C, Greenlee H, Minasian LM, Moinpour CM, Lew DL, Fehrenbacher L, Wade JL, Wong SF, Fisch MJ, Lynn Henry N, Albain KS (2018) Two-Year Trends of Taxane-Induced Neuropathy in Women Enrolled in a Randomized Trial of Acetyl-L-Carnitine (SWOG S0715). J Natl Cancer Inst 110 (6):669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckhoff L, Knoop A, Jensen MB, Ewertz M (2015) Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer 51 (3):292–300. [DOI] [PubMed] [Google Scholar]
- 14.Beijers A, Mols F, Dercksen W, Driessen C, Vreugdenhil G (2014) Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Community Support Oncol 12 (11):401–406. [DOI] [PubMed] [Google Scholar]
- 15.Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, Tkaczuk K, Edelman M, Bao T (2014) Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus 3:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C (2008) Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 44 (11):1507–1515. [DOI] [PubMed] [Google Scholar]
- 17.Vahdat L, Papadopoulos K, Lange D, Leuin S, Kaufman E, Donovan D, Frederick D, Bagiella E, Tiersten A, Nichols G, Garrett T, Savage D, Antman K, Hesdorffer CS, Balmaceda C (2001) Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res 7 (5):1192–1197. [PubMed] [Google Scholar]
- 18.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB, Paice J, Schneider B, Smith ML, Smith T, Terstriep S, Wagner-Johnston N, Bak K, Loprinzi CL, American Society of Clinical O (2014) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32 (18):1941–1967. [DOI] [PubMed] [Google Scholar]
- 19.Speck RM, Sammel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, DeMichele A (2013) Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract 9 (5):e234–240. [DOI] [PubMed] [Google Scholar]
- 20.de Morree ES, Vogelzang NJ, Petrylak DP, Budnik N, Wiechno PJ, Sternberg CN, Doner K, Bellmunt J, Burke JM, Ochoa de Olza M, Choudhury A, Gschwend JE, Kopyltsov E, Flechon A, van As N, Houede N, Barton D, Fandi A, Jungnelius U, Li S, Li JS, de Wit R (2017) Association of Survival Benefit With Docetaxel in Prostate Cancer and Total Number of Cycles Administered: A Post Hoc Analysis of the Mainsail Study. JAMA Oncol 3 (1):68–75. [DOI] [PubMed] [Google Scholar]
- 21.Loibl S, Skacel T, Nekljudova V, Luck HJ, Schwenkglenks M, Brodowicz T, Zielinski C, von Minckwitz G (2011) Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC Cancer 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binkley JM, Harris SR, Levangie PK, Pearl M, Guglielmino J, Kraus V, Rowden D (2012) Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer 118 (8 Suppl):2207–2216. [DOI] [PubMed] [Google Scholar]
- 23.Smith J, Osborn M (2008) Interpretive phenomenological analysis. In: Smith J (ed) Qualitative psychology: a practical guide to research methods. Sage, London, [Google Scholar]
- 24.Hertz DL, Kidwell KM, Vangipuram K, Li F, Pai MP, Burness M, Griggs JJ, Schott AF, Van Poznak C, Hayes DF, Lavoie Smith EM, Henry NL (2018) Paclitaxel Plasma Concentration after the First Infusion Predicts Treatment-Limiting Peripheral Neuropathy. Clin Cancer Res 24 (15):3602–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R (2005) The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 41 (8):1135–1139. [DOI] [PubMed] [Google Scholar]
- 26.Patton MQ (1990) Qualitative evaluation and research methods. 2nd edn. Sage, Newbury Park, CA [Google Scholar]
- 27.Whiteley AM, Whiteley J (2006) The familiarization study in qualitative research: from theory to practice. Qual Res J 6 (1):69–85. [Google Scholar]
- 28.Bowen GA (2008) Naturalistic inquiry and the saturation concept: a research note. Qual Res 8 (1):137–152. [Google Scholar]
- 29.Jacelon CS, O’Dell KK (2005) 102 - Case and grounded theory as qualitative research methods. Urol Nurs 25 (1):49–52. [PubMed] [Google Scholar]
- 30.Miles MB, Huberman MA (1994) Qualitative Data Analysis: An Expanded Sourcebook. 2nd edn. Sage Publications, Inc., [Google Scholar]
- 31.Hadi MA, Jose Closs S (2016) Ensuring rigour and trustworthiness of qualitative research in clinical pharmacy. Int J Clin Pharm 38 (3):641–646. [DOI] [PubMed] [Google Scholar]
- 32.Mays N, Pope C (2000) Qualitative research in health care. Assessing quality in qualitative research. BMJ 320 (7226):50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong A, Sainsbury P, Craig J (2007) Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 19 (6):349–357. [DOI] [PubMed] [Google Scholar]
- 34.Tofthagen C (2010) Surviving chemotherapy for colon cancer and living with the consequences. J Palliat Med 13 (11):1389–1391. [DOI] [PubMed] [Google Scholar]
- 35.Drott J, Starkhammar H, Kjellgren K, Bertero C (2016) The trajectory of neurotoxic side effects’ impact on daily life: a qualitative study. Support Care Cancer 24 (8):3455–3461. [DOI] [PubMed] [Google Scholar]
- 36.Caterinicchio RP (1979) 101 - Testing plausible path models of interpersonal trust in patient-physician treatment relationships. Soc Sci Med Med Psychol Med Sociol 13a (1):81–99. [DOI] [PubMed] [Google Scholar]
- 37.Street RL Jr., Gordon HS, Ward MM, Krupat E, Kravitz RL (2005) 94 - Patient participation in medical consultations: why some patients are more involved than others. Med Care 43 (10):960–969. [DOI] [PubMed] [Google Scholar]
- 38.Sanoff HK, Morris W, Mitcheltree AL, Wilson S, Lund JL (2015) Lack of Support and Information Regarding Long-Term Negative Effects in Survivors of Rectal Cancer. Clin J Oncol Nurs 19 (4):444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brauer ER, Long EF, Melnikow J, Ravdin PM, Ganz PA (2019) Communicating Risks of Adjuvant Chemotherapy for Breast Cancer: Getting Beyond the Laundry List. J Oncol Pract 15 (2):e98–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Yu Q, Wu XC, Hsieh MC, Loch M, Chen VW, Fontham E, Ferguson T (2018) Impact of chemotherapy relative dose intensity on cause-specific and overall survival for stage I-III breast cancer: ER+/PR+, HER2- vs. triple-negative. Breast Cancer Res Treat 169 (1):175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers JS (2012) Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum 39 (1):E31–40. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed S, Gotlieb WH, Erez G, Loiselle CG Pilot implementation of a person-centered e-health platform in gynecological cancer. Belong.Life. https://belong.life/implementation-of-a-person-centered-e-health-platform/. [Google Scholar]
- 43.Ho MY, McBride ML, Gotay C, Grunfeld E, Earle CC, Relova S, Tsonis M, Ruan JY, Chang JT, Cheung WY (2016) A qualitative focus group study to identify the needs of survivors of stage II and III colorectal cancer. Psychooncology 25 (12):1470–1476. [DOI] [PubMed] [Google Scholar]
- 44.Penner LA, Dovidio JF, Gonzalez R, Albrecht TL, Chapman R, Foster T, Harper FW, Hagiwara N, Hamel LM, Shields AF, Gadgeel S, Simon MS, Griggs JJ, Eggly S (2016) The Effects of Oncologist Implicit Racial Bias in Racially Discordant Oncology Interactions. J Clin Oncol 34 (24):2874–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. Flow chart of study participants included in the interview.


