Abstract
Physiological linkage refers to the degree to which peoples’ physiological responses change in coordinated ways. Here we examine whether and how physiological linkage relates to incidents of shared emotion, distinguished by valence. Past research has used an “overall average” approach and characterized how physiological linkage over relatively long time periods (e.g., 10–15 minutes) reflects psychological and social processes (e.g., marital satisfaction, empathy). Here, we used a “momentary” approach and characterized whether physiological linkage over relatively short time periods (i.e., 15 seconds) reflects shared positive emotion, shared negative emotion, or both, and whether linkage during shared emotions relates to relational functioning. Married couples (156 dyads) had a 15-minute conflict conversation in the laboratory. Using behavioral coding, each second of conversation was classified into one of four emotion categories: shared positive emotion, shared negative emotion, shared neutral emotion, or unshared emotion. Using a composite of three peripheral physiological measures (i.e., heart rate, skin conductance, finger pulse amplitude), we computed momentary in-phase and anti-phase linkage to represent coordinated changes in the same or opposite direction, respectively. We found that shared positive emotion was associated with higher in-phase and lower anti-phase linkage, relative to the other three emotion categories. Greater in-phase physiological linkage during shared positive emotion was also consistently associated with higher-quality interactions and relationships, both concurrently and longitudinally (i.e., five to six years later). These findings advance our understanding of the nature of physiological linkage, the emotional conditions under which it occurs, and its possible associations with relational functioning.
Keywords: dyadic interaction, affective science, positive psychology, psychophysiology, positivity resonance theory
Humans are social animals—people need to connect to others to adapt and thrive. Individuals can become interpersonally “linked” during face-to-face social interactions in numerous ways, including through similar thoughts, behaviors, emotion, physiological responses, and brain activity (Kinreich, Djalovski, Kraus, Louzoun, & Feldman, 2017; Konvalinka et al., 2011; Levenson & Gottman, 1983; Levy, Goldstein, & Feldman, 2017; Parkinson, Kleinbaum, & Wheatley, 2018). The extent of this linkage is temporally dynamic, waxing and waning from moment to moment over the course of a given interaction (e.g., Di Mascio, Boyd, Greenblatt, & Solomon, 1955; Feldman, Magori-Cohen, Galili, Singer, & Louzoun, 2011; Wilson et al., 2018).
In this paper, we focus on physiological linkage and investigate its association with shared emotion. A common defining feature of emotion, across many theorists, is response coherence (Ekman, 1992; Lazarus, 1991; Levenson, 1994; Tompkins, 1962). At the individual level, physiological responses during emotion have been shown to rise and fall in step with behavioral and experiential responses (Brown, Van Doren, Ford, Mauss, Sze, & Levenson, 2019; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005). Response coherence during emotion has also been shown at the dyadic level (Butler, 2017; Feldman, 2007), which motivated us to examine how dynamic shifts in shared emotion, evident at behavioral or experiential levels, might relate to linkage evident at physiological levels. We also explore the degree to which physiological linkage during shared emotion reflects couples’ relational functioning. This work can illuminate both the nature of human social connection and pathways to improve connection quality, which may have important implications for individual and collective health and functioning (Timmons, Margolin, & Saxbe, 2015; Wilson et al., 2018).
In undertaking this research, we emphasize the importance of using a momentary approach to assess linkage and characterizing whether it reflects individuals’ physiological responses changing in the same direction (i.e., in-phase linkage) or opposing directions (i.e., anti-phase linkage). Our primary goal was to test three competing hypotheses about how physiological linkage might reflect shared emotion. Specifically, we examined whether physiological linkage characterizes incidents of shared emotion regardless of valence (Competing Hypothesis #1), or whether it is most prominent during incidents of shared negative emotion (Competing Hypothesis #2) or, alternatively, during incidents of shared positive emotion (Competing Hypothesis #3). We also explored the degree to which physiological linkage (both during short periods of time when emotions are shared and over longer periods of time without regard to shared emotion) is associated with the quality of couples’ interactions and relationships.
Physiological Linkage
Early Studies
Although a long history of research has examined linkage in different biological response systems (e.g., Chang, Livingstone, Bosnyak, & Trainor, 2017; Di Mascio et al., 1955; Konvalinka et al., 2011; Levenson & Gottman, 1983; Levy et al., 2017; Saxbe & Repetti, 2010; Waters, West, Karnilowicz, & Mendes, 2017), arguably the greatest focus has been given to linkage in autonomic nervous system (ANS) responses such as heart rate (HR), skin conductance (SC), and finger temperature measured from interacting social partners (Butler, 2015; Palumbo et al., 2016).
Early physiological linkage research was conducted primarily in clinical settings (e.g., examining linkage between therapists and clients during therapeutic interviews). In the earliest studies, positively and negatively correlated heart rates (HRs) were observed between a therapist and a client during psychotherapy interviews (Di Mascio, Boyd, & Greenblatt, 1957; Di Mascio et al., 1955). Similarly, synchronized electromyogram activity was reported between clients and psychologists when the therapist either praised or criticized a story the client told (Malmo, Boag, & Smith, 1957).
Physiological Linkage in Close Relationships
In 1983, the first study of physiological linkage during unrehearsed conversations between spouses was reported using a standardized laboratory procedure in which participants engaged in 15-minute face-to-face conversations about relationship issues (i.e., events of the day, an area of relationship conflict) and multiple physiological measures were obtained continuously from both interactants (Levenson & Gottman, 1983). Since that time, research on physiological linkage has been extended to include dyads in other types of close relationships, including parents and children (e.g., Feldman et al., 2011), friends (e.g., Järvelä, Kivikangas, Kätsyri, & Ravaja, 2013) and teammates (e.g., Henning, Boucsein, & Claudia Gil, 2001). The majority of these studies (e.g., Gates, Gatzke-Kopp, Sandsten, & Blandon, 2015; Wilson et al., 2018) were laboratory-based, using variants of the procedure developed by Levenson and Gottman (Levenson & Gottman, 1983). Field-based studies have also been conducted, in which researchers examined physiological linkage during brief communal events (e.g., Konvalinka et al., 2011) or over longer time periods of daily living (e.g., Saxbe & Repetti, 2010).
Most physiological linkage research has attempted to elucidate the specific psychological processes associated with physiological linkage and findings have been mixed (for reviews, see: Palumbo et al., 2016; Timmons et al., 2015). For example, one of the earliest and most studied topics has been the association between physiological linkage and qualities of the relationship or interaction. Levenson and Gottman (1983) found that greater physiological linkage (measured using HR, SC, finger pulse transmission time [FPT], and general somatic activity [ACT]) between spouses when discussing an area of conflict in their relationship was associated with lower levels of marital satisfaction. Other researchers have attempted to replicate these findings using similar or different physiological measures (e.g., respiratory sinus arrhythmia [RSA] or cortisol) in both laboratory and naturalistic settings. Among these studies, some observed similar effects, such that greater physiological linkage was associated with lower-quality interactions or relationships (Gates et al., 2015; Liu, Rovine, Cousino Klein, & Almeida, 2013; Saxbe & Repetti, 2010); others observed the opposite effect (i.e., greater physiological linkage was associated with better relationship/interaction quality; Helm, Sbarra, & Ferrer, 2014; Marci, Ham, Moran, & Orr, 2007; Marci & Orr, 2006); and some studies did not find any associations (Reed, Randall, Post, & Butler, 2013; Thomsen & Gilbert, 1998).
Physiological Linkage and Emotion
In our view, emotions are short-lived phenomena that can produce changes in multiple response systems (i.e., physiological, experiential, and behavioral; Levenson, 2014; Levenson et al., 2016). Although most theories of emotion suggest physiological activation is associated with emotion, theories differ markedly in the specific relationships between physiology and emotion that are postulated. These range from very general relationships (e.g., various emotions produce undifferentiated physiological arousal; Cannon, 1927), to more specific ones that link particular emotions (e.g., anger versus disgust; Ekman, Levenson, & Friesen, 1983) or particular families of emotions (e.g., negatively-valenced versus positively-valenced emotions; Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000) with different patterns of physiological arousal. Empirical evidence suggests both negative and positive emotions are associated with physiological activation (Ax, 1953; Ekman et al., 1983; Kreibig, 2010; Shiota et al., 2017; Shiota, Neufeld, Yeung, Moser, & Perea, 2011). Positive emotions have also been shown to undo or de-activate physiological responses activated by prior negative emotions (Fredrickson & Levenson, 1998; Fredrickson, Mancuso, Branigan, & Tugade, 2000; Yuan, McCarthy, Holley, & Levenson, 2010).
Whether physiological linkage emerges between individuals is a separate issue from the degree of specificity envisioned between emotion and physiology. Because linkage is typically quantified as positive correlations between interactants’ physiological responses, physiological linkage is thought to occur when interactants share any emotional states that produces similar physiological activation. From the perspective that emotions produce non-specific patterns of physiological activation (Cannon, 1927), linkage could even occur when two people experience different emotions that are in the same or even different families of emotions. For example, if one person is angry and the other is afraid, and both emotions produce elevations in HR, then HR linkage would increase as a result of two different emotions that are in the same emotion family (i.e., anger and fear are both negative-valence emotions). On the other hand, if one person is angry while the other person is laughing (which also increases HR), HR linkage would also increase, but as a result of two different emotions that are in different emotion families (i.e., mirthful laughing typically occurs during positive emotion such as amusement). For some emotional conditions that occur during dyadic interactions, linkage correlations would be expected to approach zero, such as incidents when neither person is experiencing an emotion, or incidents when one person experiences an emotion (that activates or deactivates their physiology) and the other person experiences no emotion. Thus, periods when interactants share emotions (even if not the same emotion) may be characterized by greater physiological linkage than periods in which only one, or neither interactant experiences emotion.
Numerous theories support the proposition that physiological linkage should increase during periods of shared emotion (Butler, 2017). Although, as described above, physiological linkage can plausibly reflect persons simultaneously experiencing different emotions, most of these theories imply that persons simultaneously experience the same emotion. For example, the framework of temporal interpersonal emotion systems (TIES) suggests that dyadic shared emotional states occur during interactions, and that these shared states give rise to simultaneous changes in multiple emotion response systems (including physiology) for each individual in the interaction (Butler, 2011, 2017). Similarly, the perception-action model (PAM) suggests that when an observer perceives the emotional and/or behavioral states of another, this perception automatically activates in the observer a shared emotional and/or behavioral state together with its associated physiological activity (Preston & de Waal, 2002). Additionally, affective process theory (APT) suggests that shared emotion occurs when individuals share the same appraisal of emotional stimuli (e.g., people laugh at the same joke or cry due to recalling the same sad story at the same time; Elfenbein, 2014). In this view, the shared appraisal leads to similar changes in physiology across individuals. Studies on the specific behaviors associated with linkage also suggest linkage is strongest during behaviors that reflect or create shared emotions (e.g., physical touch (Waters et al., 2017), mimicry (Semin & Cacioppo, 2008), vocal synchrony (Feldman et al., 2011), and empathy (Levenson & Ruef, 1992; Marci et al., 2007).
Some shared emotional states could produce greater linkage relative to other shared emotional states. Early linkage studies often emphasized the role that shared negative emotion had in producing physiological linkage (e.g., Levenson & Gottman, 1983). Indeed, classic findings of negativity bias in affective phenomena (Baumeister, Bratslavsky, Finkenauer, & Vohs, 2001; Ito, Larsen, Smith, & Cacioppo, 1998; Rozin & Royzman, 2001) imply that the linkage effects of shared negative emotion would exceed those of shared positive emotion, perhaps simply because negative emotions, although less frequent than positive emotions in daily life, are often experienced as more intense. Yet, recent linkage studies have given greater billing to shared positive emotion (Feldman et al., 2011; Marci et al., 2007). A contemporary framing of attachment theory, for instance, holds that physiological synchrony results from micro-level relational shifts undertaken to maintain shared positive emotion, which ultimately supports bond formation (Feldman, 2007). Complementing this perspective, positivity resonance theory (Fredrickson, 2016) predicts greater physiological linkage during shared positive emotion due to the contrasting effects of positive versus negative emotion on cognitive tendencies, such as broadened awareness and other-focus during pleasant affective states versus narrowed awareness and self-focus during unpleasant ones (Fredrickson, 2013a, 2013b). To the extent that other-focus during positive emotion entails eye contact, the simulation of smiles model (SIMS; Niedenthal, Mermillod, Maringer, & Hess, 2010) holds that a neurally-mediated embodied simulation ensues, which implies an increase in physiological linkage. Thus, while some perspectives point to greater physiological linkage when negative emotion is shared, others argue for greater linkage when positive emotion is shared. Research is needed to examine whether shared negative versus shared positive emotion differ in their degree (or form) of physiological linkage.
Methodological Issues
Comparing results across studies of physiological linkage is made difficult by significant differences in methodology. In this section, we highlight methodological issues that need to be considered in research on physiological linkage and emotion.
Assessment of Emotion
Emotion models.
Emotions can be assessed as discrete states (e.g., anger versus fear), types or families of emotion (e.g., negative-valence versus positive-valence emotions), or non-specific emotion (e.g., emotion versus no emotion). Emotions can also be assessed as dimensions (e.g., intensity of valence or arousal). Decisions about level of specificity in the assessment of emotion often reflect a combination of theoretical (e.g., which emotions or groups of emotion are thought to activate or deactivate physiology in differentiable ways), practical (e.g., time and expense involved with different ways of measuring emotion such as behavioral coding versus self-report), and participant (e.g., fatigue associated with repeated self-reports of emotional experience) considerations.
Emotion assessment approach.
In our initial study of physiological linkage and emotion (Levenson & Gottman, 1983), we developed a procedure for obtaining a continuous self-report of the valence of subjective emotional experience using a “rating dial.” For this approach, the dyadic interaction was video-recorded and then each partner watched a replay of the video and adjusted a dial so that it always indicated the way that she or he was feeling during the interaction using a nine-point scale (anchored by “very negative”, “neutral”, and “very positive”). This approach for obtaining continuous self-reports of emotion has since been widely used in emotion research, including in studies of physiological linkage in dyads (e.g., Reed et al., 2013). An alternative approach is to measure expressive behavior using behavioral coding. For example, we and others have used the Specific Affect Coding System (SPAFF; Coan & Gottman, 2007), which determines the presence of a number of specific positive and negative emotional behaviors based on a gestalt of verbal content, voice tone, context, facial expression, gestures, and body movement. Recent advances in machine learning and pattern recognition have led to computer programs that code specific emotional behaviors primarily based on facial expressions (e.g., Feldman et al., 2011). Although these computerized methods are far less time-consuming than traditional behavioral coding, questions remain about their reliability and validity (Barrett, Adolphs, Marsella, Martinez, & Pollak, 2019), especially when used with the complex dynamic emotional behaviors that occur during dyadic interactions.
Measuring Physiological Linkage
Selecting physiological measures.
The ANS produces changes in a large number of different organs (e.g., heart, blood vessels, sweat glands, stomach, pupils) via sympathetic (SNS) and/or parasympathetic (PNS) innervations. In addition, motor changes produced by the somatic nervous system (e.g., body movement) can be enormously important in studies of physiological linkage. Unless one believes that all of these physiological systems change together in one unified pattern of activation during all emotional states, the choice of measures can have important implications for findings (Waters et al., 2017). According to a recent literature review (Palumbo et al., 2016), approximately 60% of the previous research on physiological linkage has only examined a single physiological measure, with a particular focus on HR or SC. The remaining research has examined two or more physiological measures, with attempts to identify common (e.g., Reed et al., 2013) or specific (e.g., Waters et al., 2017) linkage patterns for each physiological measure. Physiological measures and the organ systems they index differ greatly in temporal dynamics, including rapidity of change (e.g., surface temperature changes much more slowly than HR), periodicity (e.g., respiratory inhalation and exhalation typically occurs at a rate of 9 to 24 cycles per minute; Brown, Beightol, Koh, & Eckberg, 1993; Hirsch & Bishop, 1981), and proneness to artifact (RSA is prone to respiratory artifacts during talking and laughing, Grossman, Karemaker, & Wieling, 1991; peripheral pulse measures are prone to movement artifacts, Murray & Foster, 1996). In our original study (Levenson & Gottman, 1983), we used an aggregated measure of physiological linkage that was based on three ANS (i.e., HR, SC, FPT) and one somatic nervous system measure (i.e., ACT). Although it is always important to report linkage findings using individual physiological measures, Palumbo et al. concluded that methods “combining multiple physiological measures [are] reasonable approaches for capturing a general autonomic pattern” (Palumbo et al., 2016, p. 104).
Duration of linkage.
In our original study (Levenson & Gottman, 1983), the primary measure of physiological linkage for each couple was a single-value that resulted from a bivariate times-series analysis (Gottman, 1981) applied to 90 10-second averages of each of four physiological measures obtained from each spouse during a 15-minute interaction. This single-measure approach, which reflected overall linkage during the interaction, was dictated in part by the demands of the time-series analytic method used (which required the 90 data points) and the limits of memory storage that characterized laboratory computers of that era (it was simply not possible, for example, to store 900 one-second averages of multiple physiological variables obtained from two spouses on-line until computations could later be performed). With dramatic advances in computer technology and on-line storage capability, it is now feasible to study linkage at a much finer grain of measurement. Moreover, alternative computational approaches (e.g., correlations instead of bivariate time-series or Fourier analyses) require fewer data points to establish reliable indices. Thus, it is now feasible to compute measures of momentary physiological linkage (e.g., linkage calculated every 15-seconds, based on physiological data in short time intervals such as one second) reflecting the changes in linkage that occur throughout a longer interaction. This momentary approach enables measures of physiological linkage to approximate more closely the temporal dynamics of emotion during dyadic interactions, which change continuously over time (Chen, Aksan, Anderson, Grafft, & Chapleau, 2014; Levenson, 2014; Yuan et al., 2010). Although measures of overall linkage (which can be based on a single computation that includes all available data points or by averaging values computed using subsets of data points) are still used (e.g., Reed et al., 2013; Waters, West, & Mendes, 2014), the momentary approach offers advantages, especially when attempting to explore the particular states and/or behaviors that are associated with increases or decreases in physiological linkage.
Forms of linkage.
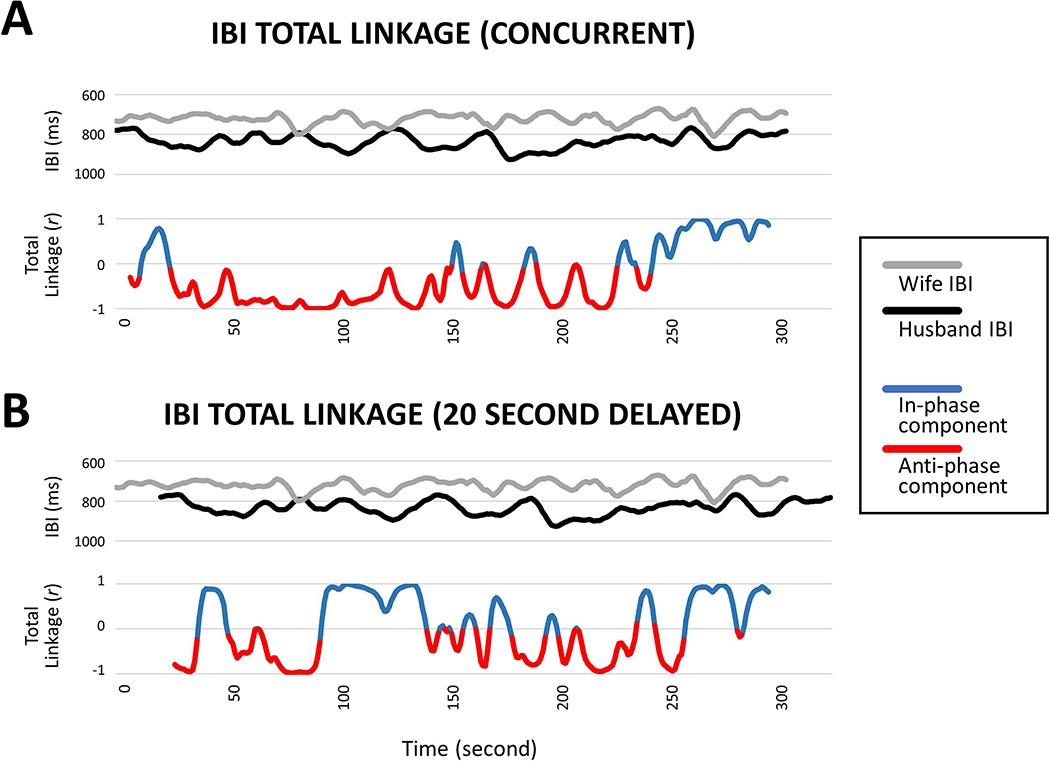
Although physiological linkage has most commonly been based on positive correlations (e.g., both interactants’ HRs rise and fall at the same time), linkage can take other forms. Linkage can also be manifested in negative correlations (e.g., one interactant’s HR rises while at the same time the other interactant’s HR falls). Butler and colleagues (Butler, 2015; Reed et al., 2013) have called these distinct patterns “in-phase” and “anti-phase” linkage, respectively. Negative correlational linkage could occur, for instance, when one interactant is in the throes of an emotion that increases physiological arousal (e.g., anger increases HR) while at the same time the other interactant is in the throes of an emotion that reduces arousal (disgust decreasing HR or contentment returning HR to baseline levels; Fredrickson & Levenson, 1998; Levenson, Ekman, & Friesen, 1990). In most linkage research, incidents of in-phase and anti-phase linkage have not been examined separately but rather have been allowed to contribute to indices of total linkage (e.g., see Fig. 1A). Although the specific meaning and conditions under which in-phase and anti-phase linkage occur remain unclear, some evidence suggests that they may be associated with different psychological processes (e.g., more in-phase linkage when one person tries to influence the other; more anti-phase linkage when people take turns in engaging and disengaging in talking or other social behaviors; Butler, 2015; Reed et al., 2013; Vallacher, Nowak, & Zochowski, 2005).
Figure 1.
Illustration of the relationships between total, in-phase, and anti-phase linkage, and linkage computed using the concurrent and delayed manners. (1A) Top: Time series of cardiac interbeat intervals (IBI) in a couple during face-to-face conversations for 5 minutes (300 seconds). (1A) Bottom: Time series of the couple’s concurrent total linkage computed using the same method of the current study (i.e., Pearson’s correlations with a 15-second rolling window; no time lag). Note that the total linkage time series can be conceptualized as being composed by an “in-phase” component (blue line), which represents the degree to which the couple’s IBI were positively correlated; and an “anti-phase” component (red line), which represents the degree to which the couple’s IBI were negatively correlated. (1B) Top: IBI time series in the same couple in which the husband’s data were realigned to the wife’s by adding a 20-second time delay. (1B) Bottom: Time series of the couple’s IBI total linkage based on the realigned data, which is conceptually equivalent to computing 20-second delayed IBI linkage scores based on the original IBI time series (without realignment; Fig. 1A Top). Note that in the delayed linkage time series, some of the original anti-phase components now become in-phase (e.g., from 90 to 135 seconds); some of the original in-phase components now become anti-phase (e.g., around 250 seconds).
Data analytic approach.
Although measures of linkage based on correlations have been most commonly used in the literature (e.g., Gates et al., 2015; Marci et al., 2007; Wilson et al., 2018), other ways of calculating linkage between the physiological responses of interactants exist. For example, in our original research (Levenson & Gottman, 1983), we used a bivariate time series approach (Gottman, 1981) that assessed the extent to which each interactant’s pattern of physiological responding accounted for variation in the other partner’s pattern of responding, beyond the variance accounted for by that partner’s own pattern of responding (thus controlling for autocorrelation or cyclicity). Another commonly used approach is multilevel modeling, which allows researchers to examine physiological linkage while also modeling the non-independence of interactants’ physiological data (e.g., Reed et al., 2013). More recently, coherence wavelet analysis (based on Fourier decompositions of physiological time-series data) has also been applied in the linkage research (e.g., Müller & Lindenberger, 2011; Quer, Daftari, & Rao, 2016). Although complex statistical approaches offer researchers increased flexibility in their research question, correlations nevertheless offer a parsimonious and statistically valid approach to capturing linkage across individuals.
Time lags.
People’s physiological responses can be linked concurrently (i.e., their physiological responses change at the same time) or in a delayed manner (i.e., one person’s physiological response changes after that of the other; Thorson, West, & Mendes, 2017). Several previous studies have systematically compared these two approaches, yet the findings have been mixed. For example, Reed et al. (2013) found that the concurrent approach was better for revealing the association between physiological linkage and social influence; in contrast, Messina et al. (2013) found that the delayed approach was better for revealing the association between physiological linkage and perceived empathy during the interaction. An important topic when using the delayed approach is the selection of time lag. Most previous research has selected a single, fixed time lag (e.g., 10 seconds) and applied it to the entire interaction for all dyads (Thorson et al., 2017). Alternatively, more recent research has considered the time lag itself to be dynamic, varying within and between any given dyadic social interaction. Using the method of “dynamic time warping,” the time-series of dyadic physiological data are dynamically re-aligned based on the maximum possibility of similarity (e.g., Kang & Wheatley, 2017). A common caveat for all research using time lags is that there is currently no way for researchers to know the actual time lags that have occurred. As a result, researchers typically assume that the most appropriate time lag is the one that reveals the strongest effects (e.g., Messina et al., 2013), or for which the dyadic physiological data become the most similar (e.g., Kang & Wheatley, 2017). However, both assumptions remain to be tested and may not hold across different types of interacting dyads and different interaction contexts. In addition, depending on the length of the time lag used, important linkage patterns, such as anti-phase linkage, could inadvertently be transformed into other patterns, such as in-phase linkage (e.g., Fig. 1B). Considering these issues, the concurrent approach seems least problematic and most justifiable.
The Present Study
In the present study, we examined associations among physiological linkage, emotion, and relationship experiences in an archival data set from a longitudinal study of couples in long-term marriages who engaged in 15-minute conversations about an area of relationship conflict. The study design reflected the methodological issues reviewed above. Regarding various emotion models, we focused on comparing positive versus negative emotion. Regarding the emotion assessment approach, our primary analyses were based on an observational coding system that identified distinct emotion-related behaviors ultimately grouped into positive and negative emotion categories. In secondary analyses, to establish generalizability, we drew on self-reported emotional valence derived from the rating dial procedure. Regarding selecting physiological measures, we chose a composite of three physiological measures representing the ANS for our primary analyses and also report secondary analyses that use these three physiological measures individually. Regarding duration of linkage, we used the momentary approach, measuring physiology and emotional behavior on a second-by-second basis and computing indices of linkage within rolling, 15-second time windows. Regarding forms of linkage, we systematically examined in-phase and anti-phase linkage. In preliminary data analyses, we also tested whether any emotion conditions would be best characterized as “no linkage.” Regarding data analytic approach, we used the correlational approach, computing Pearson’s correlations between second-by-second changes in spouse’s physiological activity within 15-second time windows. Regarding time lags, we focused on concurrent changes and thus did not include time lags.
We tested three competing and mutually exclusive hypotheses. Each reflects a different potential pattern of the degree of physiological linkage across four different emotion categories, namely, shared positive emotion, shared negative emotion, shared neutral emotion (i.e., both partners showed no emotion), and unshared emotion.
Competing Hypothesis #1:
Incidents of shared positive and shared negative emotion have a relatively equivalent degree of linkage that is higher than the linkage evident during incidents of shared neutral emotion and incidents when emotion is not shared. This first hypothesis reflects the widely-endorsed view that all emotions, regardless of valence, are characterized by changes in physiological responding (distinct or not); thus any emotion that is shared could produce greater linkage.
Additionally, based on views that emphasize differences between positive and negative emotion families, we tested two other competing hypotheses, each of which posits that degrees of physiological linkage would differ depending on whether positive versus negative emotion is shared.
Competing Hypothesis #2:
Incidents of shared negative emotion are associated with greater physiological linkage relative to incidents of shared positive emotion, shared neutral emotion, and incidents when emotion is not shared. This second hypothesis is based on evidence for negativity bias (e.g., Rozin & Royzman, 2001) and earlier findings on the role that shared negative emotion may play in producing heightened physiological linkage and marital distress (e.g., Levenson & Gottman, 1983).
Competing Hypothesis #3:
Incidents of shared positive emotion are associated with greater physiological linkage relative to incidents of shared negative emotion, shared neutral emotion, and incidents when emotion is not shared. This third hypothesis reflects contemporary models of attachment (Feldman, 2007) and positivity resonance theory (e.g., Fredrickson, 2013a, 2016), both of which contend that physiological responses become more linked when positive emotion is shared. Initial empirical data (Feldman et al., 2011) support this view.
Additionally, we recognize that emotion has the capacity to either activate or deactivate physiological arousal. Interestingly, past research has rarely examined whether observed linkage effects reflect a physiological activation or deactivation across interactants. To address this knowledge gap, we also characterized changes in physiological responding (e.g., increased or decreased physiological reactivity) when couples start to share positive, negative, or neutral emotion.
Finally, we explored how different forms of physiological linkage (i.e., in-phase, anti-phase, momentary, overall average) relate to couples’ relational functioning. We drew on two markers of couples’ perceptions of their relational functioning, one episodic and the other global: (a) quality of interactions: the overall affective tone of the conflict conversation, as derived from the rating dial procedure (i.e., how positive or negative spouses rated their experiences during the conversation), and (b) quality of relationships, the couples’ average marital satisfaction, as derived from two well-validated self-report surveys to assess relationship satisfaction in married couples.
Method
Participants
Data for this study were drawn from a longitudinal study of 156 couples in long-term marriages who were initially studied in 1989/1990. Computed study variables used herein are available on the Open Science Framework at https://osf.io/pvedh/?view_only=2cd92c803fd34fef8a71c70e5cdd6186. Participant recruitment (Carstensen, Gottman, & Levenson, 1995; Levenson, Carstensen, & Gottman, 1993) was designed so that the final sample was representative of the geographic area around the University of California, Berkeley in terms of ethnicity, socioeconomic status, and religion. The 156 couples included two age cohorts: (a) middle-aged couples, who had been married for at least 15 years, with the older partner between 40 and 50 years of age; and (b) older couples, who had been married for at least 35 years, with the older partner between 60 and 70 years of age. The sample was also selected to include an equal number of couples who were classified as satisfied or dissatisfied based on their reports of marital satisfaction. The demographic characteristics of the participants are shown in Table 1. Among these 156 couples, 132 returned to our laboratory and completed the same tasks in 1995/1996 (T2). The primary analyses reported in the present study were performed using T1 data. We then repeated these analyses using T2 data to determine whether our findings were reliable over time. In exploratory analyses, we also examined whether physiological linkage at T1 related to the quality of couples’ interactions and relationships both at T1 and T2.
Table 1.
Demographic characteristics of research participants.
| Total (n = 156) | ||||
|---|---|---|---|---|
|
|
||||
| Min | Max | Mean | SEM | |
|
| ||||
| Years of marriage | 13 | 49 | 30.42 | 0.82 |
| Age | ||||
| Husbands | 39 | 70 | 54.11 | 0.81 |
| Wives | 37 | 70 | 52.80 | 0.80 |
| Years of education | ||||
| Husbands | 10 | 20 | 16.48 | 0.22 |
| Wives | 8 | 20 | 15.26 | 0.20 |
|
| ||||
Among the 156 couples recruited for the T1 assessment, six couples were excluded because all of their physiological data were unusable. An additional 21 couples were excluded because their behavioral data did not allow classification into the four emotion categories of interest (see below). This occurred because one couple did not provide any valid behavioral data, 15 couples did not exhibit any shared positive emotion, and five couples did not exhibit any shared negative emotion. Excluded couples did not differ from included couples on demographic or linkage variables.1 Demographic information for the 129 couples in the analysis sample is shown in Supplemental Table S1. Among these 129 couples, three couples had two and 29 couples had one physiological measure missing due to procedural errors or artifacts. Therefore, although primary analyses that used the composite measure were performed with all 129 couples, follow-up analyses that used single physiological measures were performed with a smaller number of couples based on available data (e.g., n of dyads for skin conductance, one of the physiological channels = 116). The number of couples included in each analysis of this study is shown in the captions and legends of each table and figure.
At T2, 132 couples returned to our laboratory to repeat the T1 laboratory assessment. Seven of these couples were excluded because their physiological data were not usable. An additional 25 couples were excluded because we could not identify all four emotion categories of interests for data analyses (e.g., did not exhibit any shared positive or shared negative emotion). The analyses of T2 data were thus based on the remaining 100 couples (see Supplemental Table S1 for their demographic information).
Apparatus
Video recording.
The frontal views of each partner’s face and upper torso during the conversation were obtained using two remotely controlled video cameras, which were partially concealed behind darkened glass. The images from the two video cameras were combined into a single split-screen image using a special effects generator and were recorded on a VHS videocassette recorder. The voices of each partner during the conversation were recorded using two lavaliere microphones.
Rating dial.
Each partner provided continuous ratings of their own emotion during the conversation (while watching the video of their interaction; see below for details) using a rating dial that traversed an 180 degree path, with the dial pointer moving over a 9-point scale anchored by the legends “extremely negative” = 1, “neutral” = 5, and “extremely positive” = 9 (Ruef & Levenson, 2007). The rating dial produced an electrical signal proportional to dial position. This signal was sampled at 300 Hz using software developed by R.W. Levenson.
Physiological recording.
A system consisting of a Grass Model 7 12-channel polygraph and a DEC LSI 11/73 microcomputer was used to obtain2 (a) cardiac interbeat interval (IBI) – Beckman miniature electrodes with Redux paste were placed in a bipolar configuration on opposite sides of the participant’s chest and the interval between successive R-waves of the electrocardiogram was measured in milliseconds; (b) skin conductance level (SCL) – a device passed a small constant voltage between Beckman regular electrodes attached to the palmar surface of the middle phalanges of the first and third fingers of the nondominant hand using sodium chloride in Unibase as the electrolyte; (c) finger pulse amplitude (FPA) –finger pulse was measured using a photoplethysmograph attached to the middle finger of the nondominant hand; the trough-to-peak amplitude was used as an index of the amount of blood in the finger; and (d) general somatic activity (ACT)—an electromechanical transducer attached to a platform under each partner’s chair generated an electrical signal proportional to the amount of body movement in any direction. All physiological data were sampled at 330 Hz. In our primary analyses, we use an ANS composite measure of linkage that combines IBI, SCL, and FPT, and we examine whether effects of ANS linkage are due to linkage in somatic activity.
Marital satisfaction.
We used two well-validated self-report surveys to assess relationship quality (satisfaction) in married couples: (a) the Martial Adjustment Test (Locke & Wallace, 1959), which consists of 15 items (e.g., “Do you confide in your mate?”), and (b) the Marital Relationship Inventory (Burgess, Locke, & Thomes, 1971), which consists of 22 items (e.g., “How happy would you rate your marriage?”).
Procedure
At both the T1 and T2 assessments, couples completed a questionnaire package at home that included demographic and relationship quality questions. Couples came to the laboratory after having not spoken to each other for at least 8 hours. Electrodes for physiological recording were attached to both spouses and they engaged in three conversations: (a) events of the day (at T2 the topic was the events of the past five years); (b) problem area of continuing disagreement; (c) pleasant topic. Prior to the problem area and pleasant topic conversation couples completed questionnaires that helped them select a conversation topic. Each conversation lasted for 15 minutes and was preceded by a five-minute silent period. The present study focused on the discussion of a problem area because (a) this type of conversation has been the focus of most previous research on married couples (e.g., Gates et al., 2015; Levenson & Gottman, 1983; Thomsen & Gilbert, 1998; Wilson et al., 2018), making our results more comparable with previous findings, and (b) most couples exhibited a wide range of emotional behaviors when discussing areas of disagreement (Yuan et al., 2010), which allowed us to compare incidents of shared positive emotion with incidents of shared negative emotion.
Several days after the conversation, the couples returned to the laboratory and watched a video of their conversation. While watching the video, they provided continuous ratings of their own emotion during the conversation using the rating dial described above. All couples provided informed consent (approved by local Institutional Review Boards) before their research participation and received $150 for participating at each time point.
Data Reduction
Emotion data.
For our primary data analyses, video recordings were used to determine the presence of emotional behaviors during the conversation. In supplemental analyses, rating dial data were used to determine the presence of emotional experiences during the conversation.
Emotional behaviors.
Second-by-second positive and negative emotional behaviors for each partner were coded by a team of trained coders (blind to the research hypotheses) using the Specific Affect Coding System (SPAFF; Coan & Gottman, 2007). SPAFF uses verbal content, voice tone, context, facial expression, gestures, and body movement to code positive and negative emotional behaviors. For speakers, positive emotional codes are joy, humor, affection, interest, and validation; and negative emotion codes are contempt, disgust, defensiveness, belligerence, domineering, anger, whining, sadness, and fear/tension. For listeners, emotion codes are positive emotion, negative emotion, and stonewalling. Speaker and listener emotional behaviors were coded using a 3-point scale (0 = absent; 1 = low intensity; 2 = high intensity). For the T1 assessment, coders used a computerized dial to indicate each SPAFF code and intensity at every second of the interaction. The code that best described the emotion of each partner was indicated on the dial until a change in behavior occurred such that another code (either one of the emotion codes described above, or a neutral code) better reflected the emotional state of the partner. A “neutral” code (0 = absent; 1 = presence) for speakers and listeners was also given to seconds during which no positive or negative emotional behaviors were coded. At least two coders participated in behavioral coding, and inter-coder reliability was determined using the second-by-second agreement of coders throughout the 15-minute conversation. Inter-coder reliability was high (kappa = 0.64, z =19.25). Complete information about SPAFF coding and its reliability in this study has been published elsewhere (Carstensen et al., 1995). For the T2 assessment, the coding procedure was identical, except new software allowed coders to pause and rewind the video recording to assign codes (pausing and rewinding were not allowed at T1).
To test whether shared positive and negative emotion were associated with the same or different patterns of physiological linkage, we created four emotion categories. To do so, for each partner, we first computed a single second-by-second time series of emotional behaviors, in which +1 indicated that a positive SPAFF emotional behavior was coded in that second (either as a speaker or listener; regardless of intensity); −1 indicated that a negative SPAFF emotional behavior was coded in that second (again, either as a speaker or listener; regardless of intensity); and 0 indicated that neutral or no SPAFF emotional behavior was coded in that second. Fig. 2A presents an example of one couple’s emotional behaviors over the 15-minute conversation. Using these second-by-second SPAFF time series for each partner, we created a second-by-second time series of dyadic SPAFF for each couple in which each second of the conversation was classified into one of four mutually exclusive emotion categories: (a) shared positive emotion: both partners had a positive SPAFF code; (b) shared negative emotion: both partners had a negative SPAFF code; (c) shared neutral emotion (both partners showed no emotion): neither partner had a positive or negative SPAFF code (or both partners received a “neutral” SPAFF code); (d) unshared emotion: one partner had a positive or negative SPAFF code and the other either did not have a positive or negative SPAFF code or had a code that was not matched in valence (e.g., one partner had a positive SPAFF code and the other had a negative SPAFF code). The total time in each emotion category for each couple is shown in Supplemental Table S2. To better understand the role of specific emotional behaviors, within each emotion category we also computed the percentage of time that each participant was assigned each specific SPAFF code.
Figure 2.
Illustration of research methods (i.e., identifying presence of shared and unshared emotion using expressive behavioral data; computing physiological linkage measures on individual physiological channels and the ANS composite measure) using example data from one study couple. (2A) Emotional expressive behaviors: Positive values correspond to positive emotional behaviors; negative values correspond to negative emotional behaviors; 0 corresponds to no emotion. Areas shaded green, red, and blue represent examples of incidents of shared positive emotion, shared negative emotion, and shared neutral emotion, respectively. (2B) Physiological reactivity (husband in black, wife in gray) and physiological linkage (total linkage; in colors) by individual physiological measures. Note that physiological total linkage was computed using a 15-second rolling window, i.e., for any given second of the conversation (e.g., the two blue dots), a Pearson’s correlation coefficient was computed based on the 15 seconds of physiological data surrounding that specific second (the two blue boxes). (2C) Physiological linkage (total, in-phase linkage, and anti-phase linkage) of the ANS composite measure. Note. IBI = Cardiac interbeat intervals. SCL = Skin conductance level. FPA = Finger pulse amplitude.
Emotional experiences.
We used T1 rating dial data to determine whether our findings based on SPAFF coding of emotional behaviors would generalize to a different measure of emotion (i.e., reports of subjective emotional experience). Based on second-by-second averages of the rating dial position, we identified three emotion categories. Incidents of shared positive emotion were defined as one-second periods when both partners rated their own emotion above 5 (i.e., above “neutral” on the rating dial) and above the mean of their own ratings over the entire conversation (i.e., more positive than their typical emotion during the conversation); incidents of shared negative emotion were defined as one-second periods when both partners rated their own emotion below 5 (i.e., below “neutral” on the rating dial) and below the mean of their own ratings over the entire conversation (i.e., more negative than their typical emotion during the conversation); incidents of unshared emotion were defined as one-second periods that did not fall into either of the above two categories. Because the rating dial did not include a “no emotion” rating, we could not compute a “shared neutral emotion” category similar to our analyses with behavioral data. The total time in each emotion category for each couple is shown in Supplemental Table S2.
Separately for T1 and T2, we also used the rating dial data to derive an index of the overall affective tone of each conversation by computing each couple’s average rating of their experiences during the conversation across the entire 15-minutes. Higher scores indicate that couples rated the conversation to be more positive overall, which suggested better interaction quality.
Physiological data.
Data preprocessing.
All physiological data were averaged every second. Artifacts in physiological data (e.g., caused by movements or procedural errors) were first identified by trained research assistants and then either interpolated using adjacent clean data points (for artifacts shorter than 10 seconds) or coded as missing (for artifacts equal to or longer than 10 seconds). Any physiological measure with more than 25% missing data for a participant was not included in data analyses. Therefore, not all couples had all three physiological measures available for data analyses; Supplemental Table S2 shows a complete list of measures included in data analyses by couple. To reduce the impact of differences in the speed of responding across physiological measures, time series of all physiological measures were smoothed using a 10-second rolling time window (e.g., SCL changes are relatively slow compared to IBI changes, therefore by smoothing both signals using the same rolling time window, fast-changing IBI signals would become more comparable to slow-changing SCL signals; Chen et al., 2014; Dawson, Schell, & Filion, 2007). Fig. 2B shows an example of physiological data from one couple that has had artifacts removed or corrected and has been smoothed.
Physiological linkage.
For each physiological measure for each couple, we first computed a second-by-second time series of total linkage by calculating Pearson’s correlations between the two partners’ second-by-second physiological responses within 15-second rolling time windows (Marci et al., 2007; Marci & Orr, 2006). That is, for each second of the conversation (e.g., the two blue dots in the IBI total linkage panel of Fig. 2B), a Pearson’s correlation coefficient was computed based on the 15 seconds of physiological data surrounding that second (e.g., the two blue boxes in the IBI reactivity panel of Fig. 2B). For our primary analyses, we also computed a composite ANS linkage score. This was done by averaging, for each second, the linkage correlations that had been computed for IBI, SCL, and FPA. (Fig. 2C top).
Separately (for each individual physiological measure and the composite ANS measure), we also computed a time series of in-phase linkage and a time series of anti-phase linkage. For each second of the in-phase linkage time series, we either entered the correlation coefficient from the relevant linkage time series if it was positive, or entered a 0 if the correlation was 0 or negative (Fig. 2C middle). Similarly, for each second of the anti-phase linkage time series, we either entered the relevant correlation coefficient if it was negative, or entered 0 if it was 0 or positive. (Fig. 2C bottom). Prior to statistical analyses, correlations in the anti-phase linkage time series were multiplied by −1 so that higher positive values in both in-phase and anti-phase linkage time series reflected greater linkage. Because in-phase and anti-phase linkage were directly derived from the total linkage, the correlations between these two linkage components and total linkage were both high (in-phase: r = .86, p < .001; anti-phase: r = −.65, p < .001). Importantly, the in-phase and anti-phase linkages were only weakly correlated with each other (r = −.17, p = .035), suggesting that these two linkage components may reflect different processes (Supplemental Table S3).
Although our primary analyses use in-phase and anti-phase linkage (separately), we also repeated all analyses using total linkage values and report these in Supplemental Fig. S2.
Physiological activation/deactivation.
To determine whether the onsets of shared emotion were associated with increased (i.e., activation) or decreased (i.e., deactivation) physiological arousal for each individual, we computed a physiological reactivity composite time series measure for each individual by first normalizing the time series of IBI, SCL, and FPA; second, multiplying normalized IBI and FPA time series by −1 so that higher scores reflect greater ANS arousal; and third, averaging the three normalized (and inverted, for IBI and FPA) physiological time series for each second.
Next, we identified the onset of each group of seconds (referred to as “epoch”) for which both partners received a positive SPAFF code or both received a negative SPAFF code (as illustrated in Fig. 2A, these periods of shared emotion typically lasted for several seconds). For comparison, we also identified onsets of each group of seconds (or epoch) for which neither partner received a positive or negative SPAFF code (i.e., shared neutral emotion). For each of these epochs, for each partner, we calculated a change score for the physiological reactivity composite by subtracting the average response for the five seconds before the onset of the shared affect epoch from the average for the five seconds after the epoch began3. Thus, positive change scores represent physiological activation whereas negative change scores represent physiological deactivation. Finally, for each partner, we averaged the physiological reactivity change scores for all shared positive emotion epochs, all shared negative emotion epochs, and all epochs when neither partner expressed an emotion (i.e., shared neutral emotion).
Marital satisfaction data.
Consistent with past research (Carstensen et al., 1995; Levenson et al., 1993; Levenson & Gottman, 1983; Verstaen, Haase, Lwi, & Levenson, 2018) and to reduce Type I errors, we first computed the average, separately for husbands and wives, of their scores on the Martial Adjustment Test (Locke & Wallace, 1959) and Marital Relationship Inventory (Burgess et al., 1971) as an index of each partner’s overall relationship satisfaction. Measures showed high internal consistency (e.g., alpha range = .80-.86 at T1). Next, within each couple, we averaged the husband’s and wife’s overall relationship satisfaction scores (separately for T1 and T2) to index overall relationship quality for each couple. Higher scores indicate better relationship quality.
Results
Preliminary results
Percentage of time for each SPAFF code in shared positive and shared negative emotion.
Most analyses in the current study focus on the two shared emotion categories (i.e., shared positive emotion and shared negative emotion) defined according to expressive behaviors coded using SPAFF. To understand which specific SPAFF codes (e.g., anger, contempt, joy, etc.) composed these two shared emotion categories, in preliminary analyses, we first computed the percentage of time that each specific SPAFF code occurred in each partner, relative to the total time of each shared emotion category.
Results of these analyses are shown in Fig. 3. Regarding incidents of shared positive emotion, in both husbands and wives, listener’s positive emotion had largest percentage (45% for husbands and 49% for wives), followed by speaker’s humor (26% for husbands and 26% for wives), then validation (13% for husbands and 12% for wives) and affection (11% for husbands and 8% for wives). Regarding incidents of shared negative emotion, for both husbands and wives, listener’s negative emotion had largest percentage (34% for husbands and 37% for wives), followed by defensiveness (25% for husbands and 18% for wives) and fear (12% for husbands and 9% for wives).
Figure 3.
Percentage of time that specific emotional behaviors (i.e., SPAFF codes) were present during all incidents of shared positive emotion (top) and shared negative emotion (bottom) in husbands and wives. Mean ± 1 SEM. Note. Speaker = speaker code. Listener = listener code. Nonparametric paired samples Wilcoxon Tests were performed to compare scores between every two adjacent SPAFF codes. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
Test of normality.
To test the three competing hypotheses, we used T1 data to compute average ANS linkage scores for the four emotion categories (i.e., shared positive emotion, shared negative emotion, shared neutral emotion, and unshared emotion) derived from SPAFF coding. We performed parallel analyses (i.e., compared linkage scores across emotion categories; see below for details about these parallel analyses) to test (a) over-time reliability of the primary findings (using T2 data), (b) two possible explanations for observed differences, (c) generalizability of the primary findings across individual physiological measures, emotion categories defined using rating dial data, and age groups. We concluded with one set of analyses to examine physiological activation/deactivation associated with onsets of shared emotion, and another set to examine associations between linkage variables and relationship quality.
We performed Kolmogorov-Smirnov tests (K-S) for all planned analyses to assess whether the averaged physiological linkage/reactivity scores for each emotion category were normally distributed. These analyses revealed that the majority of these scores were not normally distributed, e.g., for the primary analyses, K-S’s D for the two ANS linkage measures (i.e., in-phase and anti-phase) ranged between .04 and .23 (see Supplemental Table S4 for complete results). Therefore, nonparametric statistical tests were used for all remaining analyses. Because analyses relied on archival data, our sample size was predetermined. However, for our primary analysis (comparing the degree of linkage across 4 emotion categories), using an asymptotic relative efficiency factor to determine power with an estimated small effect size of .1, alpha of .05, and a correlation of .5 among the repeated measures, power in our sample of 129 is 98%.
Test of the “existence of linkage”.
Before moving to primary data analyses that compared the four emotion categories in terms of the associated in-phase and anti-phase linkage, we first examined whether averaged linkage scores during each of these emotion categories were significantly different from zero. One-sample Wilcoxon signed rank tests were performed. To control for Type I error in these and all subsequent analyses, multiple comparisons were adjusted using the Bonferroni method. These tests revealed that for all four emotion categories, averaged in-phase (Zs > 9.66, ps < .001) and anti-phase (Zs > 9.10, ps < .001) linkage scores were significantly greater than zero.4
Primary findings: Associations between physiological linkage and shared positive and/or negative emotion
Based on the ANS linkage composite and four emotion categories (i.e., shared positive emotion, shared negative emotion, shared neutral emotion, and unshared emotion)5 defined using SPAFF behavioral coding, we examined whether the in-phase linkage and anti-phase linkage differed between emotion categories. This allowed us to test our three competing research hypotheses:
Competing Hypothesis #1:
Incidents of shared positive and shared negative emotion have a relatively equivalent degree of linkage that is higher than the linkage evident during incidents of shared neutral emotion and incidents when emotion is not shared.
Competing Hypothesis #2:
Incidents of shared negative emotion are associated with greater physiological linkage relative to incidents of shared positive emotion, shared neutral emotion, and incidents when emotion is not shared.
Competing Hypothesis #3:
Incidents of shared positive emotion are associated with greater physiological linkage relative to incidents of shared negative emotion, shared neutral emotion, and incidents when emotion is not shared.
For all analyses reported below, we performed both a Friedman test and a Kendall’s coefficient of concordance (W) to examine between-category differences and effect sizes, respectively. Significant emotion category effects were decomposed using post hoc pairwise Wilcoxon signed-rank tests.
Regarding the in-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 57.12, p < .001, W = 0.15. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with greater in-phase linkage than the other three emotion categories, ps < .001 (Fig. 4A left). In addition, there were non-significant trending effects such that incidents of shared negative emotion were associated with lower in-phase linkage, as compared to incidents of unshared emotion and incidents of shared neutral emotion (ps < .10). Regarding the anti-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 66.76, p < .001, W = 0.17. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with lower anti-phase linkage than the other three emotion categories, ps < .001 (Fig. 4A right).
Figure 4.
4A: Primary findings: The associations between physiological linkage and shared positive/negative emotion. Analyses tested the effects of emotion categories (shared positive emotion, shared negative emotion, shared neutral emotion, and unshared emotion) on two measures of physiological linkage (i.e., in-phase and anti-phase linkage) in the ANS composite measure in T1 (1989/1990). 4B: These effects observed in T1 were reliably observed in a subgroup of couples who returned to our laboratory and completed the same tasks five to six years later (T2; 1995/1996). Brackets indicate performed between-category post hoc comparisons (n of comparison = 6 for each linkage measure). Annotations indicate statistically significant or trending effects. Mean ± 1 SEM. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
These findings6,7,8 support Competing Hypothesis #3, as only incidents of shared positive emotion (but not incidents of shared negative emotion) were associated with greater physiological linkage (in-phase) as compared to incidents of shared negative emotion, shared neutral emotion, and unshared emotion.
Reliability of the primary findings over time
To determine whether findings from analyses using T1 data were reliable over time, we repeated the analyses using T2 data. Regarding the in-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 55.40, p < .001, W = 0.19. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with greater in-phase linkage than the other three emotion categories, ps < .001 (Fig. 4B left). Regarding the anti-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 36.90, p < .001, W = 0.12. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with lower anti-phase linkage than the other three emotion categories, ps < .01 (Fig. 4B right). In summary, these results demonstrated that support for Competing Hypothesis #3 was reliable over time.
Possible alternative explanations: Time spent in each emotion category and linkage in somatic activity
Because couples discussed a problem area, the total time that they exhibit shared positive emotion ought to be less than the total time that they exhibit shared negative emotion or unshared emotion. This was indeed the case (see Supplemental Table S2). Additionally, changes in peripheral physiology, particularly increased cardiac and vascular responses, can be driven by metabolic demands created by somatic activity (Levenson, 2014; Obrist, Webb, Sutterer, & Howard, 1970). To determine the extent to which any observed linkage was driven by time differences (i.e., linkage scores computed from fewer time samples could be less reliable compared to linkage scores computed from a larger number of time samples) or body movements that typically accompany emotion, additional analyses (i.e., adjusting for total time or degree of partners’ movement linkage) were performed to test these two possible explanations. Again, because most of the adjusted linkage measures were not normally distributed (Supplemental Table S4), we used nonparametric tests.
Time spent in each emotion category.
We first tested whether our primary findings would remain statistically significant when adjusted for the total time of each emotion category (by computing residual scores of linkage in which time in each emotion category was regressed out). Regarding the in-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 64.76, p < .001, W = 0.17. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with greater in-phase linkage than the other three emotion categories, ps < .001. In addition, incidents of shared negative emotion were associated with lower in-phase linkage than incidents of shared neutral emotion (p < .05) and incidents of unshared emotion (p < .001; Fig. 5A left). Regarding the anti-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 46.72, p < .001, W = 0.12. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with lower anti-phase linkage than the other three emotion categories, ps < .001. In addition, incidents of shared negative emotion were associated with greater anti-phase linkage than incidents of unshared emotion (p < .01; Fig. 5A right).
Figure 5.
Testing two possible explanations: Time spent in each emotion category and linkage in somatic activity. Analyses tested the effects of emotion categories on the ANS in-phase and anti-phase linkage composite, after the total time periods of four emotion categories (5A) and husband-wife linkage in general somatic activities (5B) were adjusted. Brackets indicate performed between-category post hoc comparisons (n of comparison = 6). Annotations indicate statistically significant or trending effects. Mean ± 1 SEM. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
Linkage in somatic activity.
We next tested whether our primary findings would remain statistically significant when the analyses adjusted for the degrees of each couple’s ACT linkage in each emotion category (using the same residual procedure described above). Before the analyses, we first computed averaged in-phase and anti-phase ACT linkage for the four emotion categories (see Supplemental Fig. S5 for the linkage scores). We then repeated the primary analyses while adjusting the couple’s ACT linkage associated with each emotion category.9 Regarding the in-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 10.99, p < .05, W = 0.03. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with greater in-phase linkage than incidents of shared negative emotion (p < .001) and incidents of unshared emotion (p < .01). However, incidents of shared positive emotion were not associated with greater in-phase linkage than incidents of shared neutral emotion (Fig. 5B left). Regarding the anti-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(3) = 10.75, p < .05, W = 0.03. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with lower anti-phase linkage than the other three emotion categories, ps < .05; Fig. 5B right).
In summary, analyses failed to support either of these two alternative explanations. For time spent in each emotion category, the associations between incidents of shared positive emotion and increased physiological linkage observed in the primary analyses could not be explained by differences in the time spent in each emotion category.10 That is, all associations found in prior primary analyses remained statistically significant and effect sizes remained moderate in all analyses that adjusted for the time spent in each emotion category. For linkage in somatic activity, although results suggest that partners’ simultaneous movements may have had some effect on linkage, movement linkage was not the sole source for the physiological linkage effects observed in the primary analyses. After we adjusted for couple’s ACT linkage, effect sizes for in-phase and anti-phase linkage dropped from small-moderate (0.15 and 0.17) to small (0.03 and 0.03). Even so, most associations between shared positive emotion and greater in-phase linkage remained statistically significant.
Generalizability of the primary findings: Across individual physiological measures, emotion response systems, and age cohorts
In the primary analyses, we focused on the ANS composite and used expressive behaviors (i.e., SPAFF codes) to define incidents of shared and unshared emotion. In addition, the study sample (T1) included couples from different age cohorts (i.e., middle-aged and older age). In the following analyses, we tested whether the primary findings would generalize to: (a) the three individual physiological measures that composed the ANS composite measure; (b) emotion categories defined based on subjective experience (i.e., from rating dial data); and (c) different age cohorts.
Individual physiological measures.
We first tested whether our primary findings, which were based on the ANS composite measure, would generalize to linkage in each individual physiological measure. Regarding the in-phase linkage, Friedman tests revealed significant effects of emotion categories for all physiological measures: IBI, χ2(3) = 49.70, p < .001, W = 0.15; SCL, χ2(3) = 28.99, p < .001, W = 0.08; FPA, χ2(3) = 11.19, p < .05, W = 0.03. Pairwise post hoc comparisons revealed effects consistent with prior analyses for each physiological measure, such that incidents of shared positive emotion were associated with greater in-phase linkage than the other three emotion categories at statistically significant levels (i.e., for all comparisons in IBI, SCL, and two comparisons in FPA; ps < .05) or non-significant trending levels (for the difference between shared positive emotion and shared neutral emotion in FPA; p < .10; Fig. 6A–C, left). Regarding anti-phase linkage, Friedman tests revealed significant effects of emotion categories for all three physiological measures: IBI, χ2(3) = 84.42, p < .001, W = 0.26; SCL, χ2(3) = 20.92, p < .001, W = 0.06; FPA, χ2(3) = 25.03, p < .001, W = 0.07. Pairwise post hoc comparisons revealed effects consistent with prior analyses for each physiological measure, such that incidents of shared positive emotion were associated with lower anti-phase linkage than the other three emotion categories, ps < .05 (Fig. 6A–C right).
Figure 6.
Generalizability of the primary findings from the ANS composite measure to individual physiological measures. Analyses tested the effects of emotion categories on physiological linkage in (6A) cardiac interbeat interval or IBI, (6B) skin conductance level or SCL, and (6C) finger pulse amplitude or FPA. Brackets indicate performed between-category post hoc comparisons (n of comparison = 6). Annotations indicate statistically significant or trending effects. Mean ± 1 SEM. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
Subjective experience of emotion.
Next, we tested whether our primary findings for the four emotion categories based on expressive behaviors would generalize to the three emotion categories that we could compute based on subjective experience.11 Regarding the in-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(2) = 22.65, p < .001, W = 0.09. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with greater in-phase linkage than the other two emotion categories, ps < .001 (Fig. 7 left). Regarding the anti-phase linkage, a Friedman test revealed significant effects of emotion categories, χ2(2) = 20.60, p < .001, W = 0.08. Pairwise post hoc comparisons indicated that incidents of shared positive emotion were associated with lower anti-phase linkage than the other two emotion categories, ps < .05 (Fig. 7 right).
Figure 7.
Generalizability of the primary findings from emotion categories defined by expressive behaviors to categories defined using subjective experience (rating dial) data. Analyses tested the effects of emotion categories on physiological linkage in the ANS composite measure. Brackets indicate performed between-category post hoc comparisons (n of comparison = 6). Annotations indicate statistically significant or trending effects. Mean ± 1 SEM. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
Age cohorts.
We divided our research sample into middle-aged and older subgroups to test whether our primary findings would be separately found in each age subgroup. Regarding the in-phase linkage, Friedman tests revealed significant effects of emotion categories for both middle-aged couples, χ2(3) = 28.33, p < .001, W = 0.19, and older couples, χ2(3) = 36.64, p < .001, W = 0.15. Pairwise post hoc comparisons again revealed similar effects for both age subgroups, such that incidents of shared positive emotion were associated with greater in-phase linkage compared to the other three emotion categories, ps < .05 (Fig. 8 left). In middle-aged couples, the in-phase linkage was lower during incidents of shared negative emotion compared to incidents of shared neutral emotion and unshared emotion (ps < .05). Regarding the anti-phase linkage, Friedman tests revealed significant effects of emotion categories for both middle-aged couples, χ2(3) = 35.64, p < .001, W = 0.18, and older couples, χ2(3) = 32.35, p < .001, W = 0.17. Pairwise post hoc comparisons revealed similar effects for both age subgroups, such that incidents of shared positive emotion were associated with lower anti-phase linkage than the other three emotion categories, ps < .01 (Fig. 8, right).
Figure 8.
Generalizability of the primary findings across two different age cohorts. Analyses tested the effects of emotion categories on physiological linkage in the ANS composite measure separately within 65 middle-age couple (8A) and 64 older couples. Brackets indicate performed between-category post hoc comparisons (n of comparison = 6). Annotations indicate statistically significant or trending effects. Mean ± 1 SEM. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
In summary, findings from these generalizability analyses together suggest that our primary findings were robust, because they consistently emerged across different physiological measures (i.e., the composite or individual physiological measures), emotion response systems (i.e., emotion categories defined using expressive behaviors or subjective experience), and age cohorts. Interestingly, regarding the generalizability to individual physiological measures, the emotion category effect sizes varied markedly across physiological measures, with the largest occurring for IBI (0.15 and 0.26, for in-phase and anti-phase linkage, respectively), followed by SCL (0.08 and 0.06), and the smallest occurring for FPA (0.03 and 0.07). Regarding the generalizability to subjective experience of emotion, we also noticed that while all effects were statistically significant, the effect sizes were relatively small (0.09 and 0.08), as compared to the moderate effect sizes (0.15 and 0.17) found in the primary analyses, which used expressive behaviors to determine emotion categories.
Physiological activation/deactivation in both partners
To characterize patterns of physiological reactivity (i.e., activation or deactivation) that may contribute to increased in-phase linkage during incidents of shared positive emotion (as suggested by our primary findings), we focused on the onsets of shared positive emotion, shared negative emotion, and shared neutral emotion epochs and compared change scores in the physiological reactivity composite between these epochs. Again, because most of these reactivity change scores were also not normally distributed (Supplemental Table S4), we continued with nonparametric tests.
Friedman tests revealed significant effects of emotion categories for both husbands, χ2(2) = 78.16, p < .001, W = 0.31, and wives, χ2(2) = 121.55, p < .001, W = 0.48. Pairwise post hoc comparisons revealed that for both husbands and wives, onsets of shared positive emotion epochs were associated with a greater increase in physiological activity compared to onsets of shared negative emotion epochs and shared neutral emotion epochs (ps < .001). In addition, again in both husbands and wives, onsets of shared negative emotion epochs were associated with a greater increase in physiological activity than onsets of shared neutral emotion epochs (ps < .001), Fig. 9. In summary, these findings reveal increased physiological activation in both partners12 when they started to share either positive or negative emotion, and also that such activation is greatest for shared positive emotion.
Figure 9.
Determining physiological activation or deactivation at onsets of shared positive and negative emotion. In both husbands (9A) and wives (9B), there was a greater increase in physiological activity (indexed by a composite of IBI, SCL, and FPA; in normalized scores) at the onsets of shared positive emotion, relative to onsets of shared negative and shared neutral emotion. Brackets indicate performed between-category post hoc comparisons (n of comparison = 3). Annotations indicate statistically significant or trending effects. Mean ± 1 SEM. †p<.10. *p<.05. **p<.01. ***p<.001. n. s. = effects not significant or trending.
Associations between physiological linkage and the quality of couples’ interactions and relationships
In exploratory analyses, we examined whether the degrees of physiological linkage (in-phase and anti-phase) during different types of shared emotion were associated with two markers of relational functioning (i.e., quality of interactions and quality of relationships), each assessed concurrently (at T1) as well as longitudinally, five to six years later (at T2, albeit with ~40 fewer couples, see Table 2 for exact N per analysis).13 Specifically, we conducted linear regression analyses with four dependent variables in turn: first, couples’ T1 and T2 average ratings of affective valence across their entire 15-minute conflict conversation (as an index of the quality of interactions), and second, their T1 and T2 average marital satisfaction (as an index of the quality of relationships). In each analysis, T1 in-phase and anti-phase linkage during incidents of shared positive emotion, shared negative emotion, and shared neutral emotion were the independent variables. Because most linkage scores were not normally distributed, we rank-ordered all variables before analyses. Results (Table 2, Model 1) indicated that greater in-phase linkage at T1 during shared positive emotion was associated, both concurrently and longitudinally, with higher interaction quality (T1: β = .33, p = .004; T2: β = .28, p = .043), and higher relationship quality (T1: β = .28, p = .013; T2: β = .28, p = .030).14 Interestingly, greater T1 anti-phase linkage during shared positive emotion was also concurrently associated with higher T1 relationship quality (T1: β = .27, p = .016); however, this effect was not evident longitudinally. Although some significant associations emerged with T1 anti-phase linkage as the predictor (e.g., with T2 relationship quality), these were less consistent across time and dependent variables.
Table 2.
Associations between T1 physiological linkage and T1 and T2 perceived quality of couples’ interactions and relationships.
| Quality of interactions |
Quality of relationships |
|||||||
|---|---|---|---|---|---|---|---|---|
| T1 (126 dyads) |
T2 (86 dyads) |
T1 (129 dyads) |
T2 (88 dyads) |
|||||
| Beta | p | Beta | p | Beta | p | Beta | p | |
|
| ||||||||
| Model 1 | ||||||||
| T1 In-phase linkage during shared positive emotion | 0.33 | 0.004 | 0.28 a | 0.043 | 0.28 | 0.013 | 0.28 | 0.030 |
| T1 Anti-phase linkage during shared positive emotion | 0.19 | 0.090 | 0.20 | 0.162 | 0.27 | 0.016 | 0.12 | 0.346 |
| T1 In-phase linkage during shared negative emotion | −0.11 | 0.369 | 0.08 | 0.596 | 0.01 | 0.918 | −0.13 | 0.361 |
| T1 Anti-phase linkage during shared negative emotion | −0.22 | 0.082 | −0.03 | 0.846 | −0.24 | 0.053 | −0.36 | 0.019 |
| T1 In-phase linkage during shared neutral emotion | −0.03 | 0.805 | 0.01 | 0.948 | −0.04 | 0.705 | 0.07 | 0.584 |
| T1 Anti-phase linkage during shared neutral emotion | 0.24 | 0.036 | 0.13 | 0.391 | 0.15 | 0.192 | 0.42 | 0.003 |
|
| ||||||||
| Model 2 | ||||||||
| T1 In-phase linkage during shared positive emotion | 0.36 | 0.004 | 0.19 | 0.191 | 0.29 | 0.018 | 0.32 | 0.033 |
| T1 Anti-phase linkage during shared positive emotion | 0.20 | 0.077 | 0.14 | 0.323 | 0.25 | 0.029 | 0.10 | 0.473 |
| T1 Overall in-phase linkage during entire conversation | −0.06 | 0.582 | 0.19 | 0.137 | 0.01 | 0.896 | −0.01 | 0.954 |
| T1 Overall anti-phase linkage during entire conversation | 0.05 | 0.585 | 0.13 | 0.267 | 0.05 | 0.575 | 0.20 | 0.086 |
|
| ||||||||
Note. All significant effects (bolded) remained statistically significant (p < .05) or trending (p < .10) when either (a) years of marriage at T1/T2 or (b) total time that couples exhibited shared positive emotion at T1 was included in the analyses as a covariate, except for the association between T1 in-phase linkage during shared positive emotion and T2 quality of the interactions (annotated with superscripted “a”). Although that sole effect was in the same direction (β = .21), it was not significant (p = .149) when the total time that couples exhibited shared positive emotion at T1 was included as a covariate.
We next explored how our momentary approach to calculating physiological linkage compared to an overall average approach. For momentary physiological linkage, we focused on in-phase linkage during incidents of shared positive emotion because, as shown in Table 2, Model 1, this measure was most consistently related to high-quality interactions and relationships (assessed both concurrently and longitudinally). We compared momentary linkage during shared positive emotion to couples’ overall average linkage computed over the entire 15-minute conversation, each computed from T1 physiological data. We again conducted linear regression analyses with the same four dependent variables used in Model 1. In Model 2, however, independent variables included T1 linkage during incidents of shared positive emotion and overall linkage, averaged across the entire conversation without regard for emotion (each with both in-phase and anti-phase forms, and again, rank ordered). Results (Table 2, Model 2) revealed that the pattern of effects for T1 linkage during shared positive emotion largely replicated the pattern evident for Model 1. By contrast, no significant effects emerged for overall linkage variables.15
In summary, results converge to suggest that (a) greater in-phase linkage during incidents of shared positive emotion, and (b) physiological linkage computed using the momentary, but not the overall approach, were associated with higher-quality concurrent and future interactions and relationships.
Discussion
In this study, we examined the association between physiological linkage and shared emotion in long-term married couples (using a momentary analytic approach) to test three mutually exclusive, competing hypotheses. Our findings unambiguously rejected Competing Hypotheses #1 and #2 and supported Competing Hypothesis #3. That is, two spouses’ physiological activities did not become more linked (in-phase) during shared emotion in general, regardless of valence (rejecting Competing Hypothesis #1), nor did they reflect negativity bias by becoming more linked during shared negative emotion (rejecting Competing Hypothesis #2). Rather, in-phase physiological linkage was greatest during incidents of shared positive emotion, relative to all other emotion categories (supporting Competing Hypothesis #3). Follow-up analyses showed the robustness of this effect, as it (a) was reliable across two time points (five to six years apart); (b) could not be solely explained by total time spent in each emotion category (positive and negative) across the target conversation, (c) could not be solely explained by the influence of somatic activity (although effect sizes reduced from moderate-small to small when analyses adjusted for couples’ linkage in somatic activity); and (d) generalized across different physiological measures, emotion response systems (e.g., expressive behavior, subjective experience), and age cohorts. We also characterized the pattern of physiological reactivity associated with the observed linkage effects and found significant physiological activation (vs. deactivation) in both husbands and wives when epochs of shared positive emotion began (as compared to epochs of shared negative emotion or shared neutral emotion, which also differed significantly from one another). Finally, we explored the associations between momentary physiological linkage and both concurrent and future relational functioning (i.e., quality of interactions and quality of relationships). We found that couples who had greater in-phase linkage during incidents of shared positive emotion in the T1 conversation also rated their conversation more positively and reported higher marital satisfaction, both at T1 and five to six years later, at T2. We did not find similar associations when using an overall average approach to compute physiological linkage.
Physiological linkage during incidents of shared positive emotion
The clear and robust support for Competing Hypothesis #3 aligns with contemporary models of attachment (Feldman, 2007) and positivity resonance theory (e.g., Fredrickson, 2013a, 2016). It also aligns with prior empirical findings, such as those showing that (a) therapists’ and clients’ SCL were mostly synchronized during positive but not negative social emotional interactions (Marci et al., 2007), (b) mothers’ and infants’ HRs became more synchronized when their positive emotion was linked (Feldman et al., 2011; note that shared negative emotion was not examined in this study), and (c) mothers’ and infants’ PNS activities were more linked during positive emotion contagion (Waters et al., 2017; note that in this study greater linkage in SNS activity was found during negative emotion contagion).
Interestingly, after statistically adjusting for couple’s linkage in somatic activity, the effect size (W) reduced considerably, from 0.15 (moderate-small) to 0.03 (small), and the difference between incidents of shared positive emotion and shared neutral emotion became non-significant. This pattern of results suggests that couples’ linkage in somatic activity during shared positive emotion may be one of the main sources of the observed physiological linkage effects. Increased somatic activity in both partners during shared positive emotion may reflect the presence of high arousal positive emotion such as humor. Humor often elicits laughter (Weisfeld, 1993), which is reliably contagious (Bachorowski & Owren, 2001; Provine, 1992) and typically associated with increased somatic (e.g., body movements) and respiratory activity (Filippelli et al., 2001; Lloyd, 1938; Svebak, 1975). Somatic activity mobilizes ANS activity, especially in the cardiovascular system (Obrist, 1981). Therefore, it is highly probable that the increased in-phase linkage we observed during incidents of shared positive emotion may have been most frequently caused by shared humor and any associated laughter, which activated somatic and ANS responses in both spouses at about the same time. Supporting this interpretation, we found that humor was the most frequent specific emotional behavior coded in both husbands and wives during incidents of shared positive emotion (Fig. 3).
We note, however, that although effect sizes were markedly reduced when adjusting for linkage in somatic activity, in-phase linkage remained significantly greater during incidents of shared positive emotion than during incidents of unshared emotion (in addition, all effects for total linkage remained statistically significant when adjusting for linkage in somatic activity; Supplemental Fig. S2D). This suggests that the somatic influence is not the sole source of our findings. As shown in Fig. 3, during incidents of shared positive emotion, husbands and wives also exhibited other positive emotional behaviors, including validation and affection, which are not typically accompanied by increased somatic activity. Given the unique interpersonal functions of positive emotion (Fredrickson, 2001, 2013a; Prochazkova & Kret, 2017; Sanchez & Vazquez, 2014), shared positive emotion may lead to increased physiological linkage by widening the couple’s awareness and increasing their focus on each other, which in turn may support a unified attention to and understanding of one another’s emotional states (Niedenthal et al., 2010). For example, when partners show affection or validate each other’s emotion, they may increase their eye contact, nod, use more common language, and eventually generate shared appraisals and mutual understanding. This heightened attention towards an interaction partner and connection with that partner’s emotional state may be necessary for shared positive emotion to give rise to changes in each individual’s physiology and engender in-phase physiological linkage. Future research is needed to test these speculations about the behavioral mechanisms through which shared positive emotion may produce in-phase linked physiological responses.
Our findings are inconsistent with previous studies that found no association between shared positive emotion and physiological linkage (e.g., Levenson & Gottman, 1983; Reed et al., 2013), and also inconsistent with Waters et al. (2017), who found that mother’s and infant’s SNS activities were not linked during positive emotion contagion (which is in contrast to our findings that incidents of shared positive emotion were associated with increased in-phase linkage in SCL and FPA, two physiological measures primarily sensitive to the SNS). We note, however, that most of these previous studies took the traditional “overall average” approach by computing physiological linkage over the time course of the entire dyadic interaction. In addition, these studies also quantified physiological activity and positive emotion over longer time periods (e.g., 10 seconds or longer), which is in contrast to our study in which physiological activity and emotion were both quantified on a second-by-second basis. Specific psychological (e.g., humor) and behavioral (e.g., laughter) processes that appear likely to have contributed to the increased in-phase linkage we found during shared positive emotion may occur rapidly and only last for a few seconds. As a result, the association between shared positive emotion and physiological in-phase linkage may be more likely to be observed when short time intervals and the momentary approach are used (e.g., Feldman et al., 2011; Marci et al., 2007).
Physiological linkage during incidents of shared negative emotion
Competing Hypotheses #1 and #2 stated that incidents of shared negative emotion would be jointly (alongside incidents of shared positive emotion) or solely associated with greater physiological linkage (either in-phase or anti-phase), as compared to incidents of shared neutral emotion or unshared emotion. In failing to support these hypotheses, our results are consistent with findings of Levenson and Gottman’s 1983 study in which no associations were found between physiological linkage and shared negative (and positive) emotion (Levenson & Gottman, 1983). Our results, however, are inconsistent with previous findings that mothers’ and infants’ SNS activities became more in-phase linked (i.e., synchronized) during a reunion after mothers’ exposure to a stressful task (Waters et al., 2017; Waters et al., 2014).
Although many differences between studies might contribute to these disparate findings, one possible difference is the research sample. Waters et al. studied dyads of mothers and infants (Waters et al., 2017; Waters et al., 2014), whereas we and Levenson and Gottman (1983) studied married adult couples. Unlike infants, adults can be well-equipped and motivated to interrupt or avoid the exchange of negative emotion using interpersonal emotion regulation strategies (Riediger & Klipker, 2014), which may reduce or lower linkage. Future studies should examine how the degree of physiological linkage and shared negative emotion during interactions varies with age, and examine the emotion regulation strategies used during dyadic interactions.
Another major difference between studies is the degree of negative emotion that is exchanged across partners. The Waters et al. study used a paradigm in which mothers’ negative emotion unidirectionally passed to their infants during the reunion (Waters et al., 2017; Waters et al., 2014). The “unidirectionality” of the flow of the negative emotion from one person to the other may explain why in-phase linkage was found in their studies. In our study and the study of Levenson and Gottman (1983), negative emotion expressed by couples during conflict involved bidirectional and reciprocal exchange. For example, during incidents of shared negative emotion, one partner may experience an offense-oriented negative emotion like anger while the other partner experiences a defense-oriented emotion like fear. These different negative emotions may alternate between partners, such as when one partner eventually feels angry after being attacked by the other for a period of time. This idea is supported by our findings that defense-oriented emotion (e.g., defensiveness, fear) and offense-oriented emotion (e.g., anger, contempt, domineering, belligerence) occurred equally frequently during the incidents of shared negative emotion (e.g., in wives, 28% and 25%, respectively, Fig. 3). Because our SPAFF coding focused on specific emotions only for speakers but not for listeners, we do not know whether these defense-oriented and offense-oriented behaviors occurred simultaneously within the dyad. Future studies should consider coding specific emotional behaviors for both speakers and listeners.
In the same vein, we also observed the interesting pattern that anti-phase linkage tended to be greater during incidents of shared negative emotion than during incidents of other emotion categories. Although this pattern was observed in most of our analyses, it only exceeded statistical significance levels in analyses that adjusted for the total time that each emotion category occurred. Patterns of anti-phase linkage have been suggested to occur during turn-taking (Reed et al., 2013; Vallacher et al., 2005), which is consistent with the idea that partners took turns defending themselves and offending their partner. The observed trends regarding the associations between shared negative emotion and anti-phase linkage suggest an important area for future study, which may require a bigger sample size and/or a more fine-grained determination of different specific defensive and offensive negative emotions within the overarching category of “negative emotion.”
Another difference between the current and past studies is the analytic approach. Our study used a momentary approach, in which physiological and emotional data were analyzed in short time intervals (i.e., 1 second) and physiological linkage was computed separately for the incidents during which shared positive and negative emotion was either present or not present. Other studies, by contrast, used an overall average approach in which physiological and emotional data were analyzed in longer time intervals (e.g., 30 seconds) and then the overall average of physiological linkage was computed over the entire conversation. Under an overall average approach, rapid and potentially short-lasting dyadic physiological patterns (e.g., one person’s HR increases while the other person’s HR decreases within a 10- or 30-second time window) are not registered. This analytic difference may explain why shared negative emotion appears to be associated with anti-phase linkage in our study, but rather was associated with in-phase linkage in the Waters’ studies (Waters et al., 2017; Waters et al., 2014), and not associated with either in- or anti-phase linkage in the Levenson and Gottman’s study (1983).
In each of the emotion categories, including shared neutral emotion, levels of linkage significantly differed from zero. Significant linkage that occurred during incidents of shared neutrality may conceivably reflect that such incidents fell in close proximity to periods of shared emotion (partners synchronously returned to baseline physiological levels). Perhaps more likely, however, this linkage may reflect non-emotional factors during interactions, such as similar respiration patterns or synchronized shifts in posture, gaze or attention. Future research should examine the potential role of non-emotional factors in generating physiological linkage between individuals during interactions.
Physiological activation at onsets of shared positive emotion
In theory, increased in-phase physiological linkage may occur either when shared positive emotion activates or deactivates physiological arousal simultaneously in both partners. For example, when partners laugh together or simultaneously show affection toward the same target or one another, they may increase their shared somatic activities, behavioral tendencies (i.e., approach), and/or subjective experiences in ways that may cause each partners’ physiological responses to be activated at the same time. Similarly, previous studies have found that positive emotions can facilitate an “undoing,” or deactivation effect when they follow a negative emotion (Fredrickson & Levenson, 1998; Fredrickson et al., 2000; Yuan et al., 2010). In the context of a conflict discussion, shared positive emotion may thus simultaneously quell prior physiological responses activated by shared negative emotion in both partners. In this study, we characterized the changes in physiological reactivity evident in both husbands and wives at the onsets of epochs of shared emotion. We found that as shared positive epochs began, significant increases in physiological arousal emerged in both interacting partners (as compared to onsets of epochs of shared negative emotion and shared neutral emotion, which also differed from one another, with shared negative epochs showing significantly greater increases in physiological arousal relative to shared neutral epochs). This pattern of results suggests that physiological activation (rather than deactivation) characterizes epochs of shared positive emotion. In past research, increased physiological activation has been found during mirthful, genuine laughter (Buchowski et al., 2006; Fry & Rader, 1977; Langevin & Day, 1972; Sahakian & Firshman, 2007). Our finding of physiological activation in both partners thereby provides additional support to the idea that shared humor and laughter may be a major contributor to the in-phase linkage evident during incidents of shared positive emotion.
Physiological linkage and relational functioning
In exploratory analyses, we tested whether various forms of T1 physiological linkage were associated with couples’ relational functioning, both concurrently (at T1), and five to six years later (at T2). At each time point, we used two distinct markers of couples’ perceptions of their relational functioning: (a) quality of interactions, an episodic marker drawn from couples’ average evaluation of the overall affective tone of their conflict conversation (from the rating dial), and (b) quality of relationships, a global marker drawn from couples’ average marital satisfaction scores (from surveys).
These exploratory analyses showed that, among all types of T1 momentary physiological linkage (i.e., in-phase and anti-phase during shared positive, shared negative, or shared neutral emotion), in-phase linkage during shared positive emotion was significantly and consistently associated with both markers of relational functioning. The associations emerged for both the quality of interactions and the quality of relationships, both concurrently and longitudinally (see Table 2, Model 1). And with one exception, these associations also held when T1 in-phase and anti-phase linkage during shared positive emotions were compared to overall in-phase and anti-phase linkage during the entire conversation (see Table 2, Model 2). The degree to which couples’ physiological responses become linked when they each express positive emotion thus appears to be another marker of high-quality relational functioning, those in which both partners’ report relatively more pleasant affect, even when asked to discuss on ongoing disagreement, and those in which partners’ are more satisfied with their marriage. Even though these associations emerged both concurrently and longitudinally (over five to six years) and remained significant when controlling for years of marriage or total time spent sharing positive emotions, certain null results give us pause. We did not find, for instance, that T1 in-phase linkage predicted changes over time in relationship quality (see Footnote 13) and we did not find that the concurrent associations for T1 reported in Table 2 were clearly replicated at T2 (see Supplemental Table S6). However, statistical limitations, such as over time stability and restrictions of range, may have affected those analyses. Future research is needed to replicate these exploratory findings to discern whether in-phase linkage during shared positive emotion is indeed a reliable indicator of high-quality relational functioning.
Alongside the findings for in-phase linkage during shared positive emotion, we also found a consistent effect for anti-phase linkage, also during shared positive emotion. Specifically, greater anti-phase physiological linkage during shared positive emotion was associated with higher concurrent relationship quality, and this effect emerged for both Model 1 and Model 2 using T1 data (see Table 2) and also using T2 data (see Supplemental Table S6). Thus, even though anti-phase physiological linkage was significantly lower during incidents of shared positive emotion compared to other emotion categories at both T1 (see Figure 4A) and T2 (see Figure 4B), the extent to which it did occur was reliably associated with the concurrent quality of relationships. This effect of anti-phase linkage during shared positive emotion did not, however, extend to the longitudinal predictions or to the other marker of relational functioning (i.e., quality of the interactions).
Our exploratory analyses also showed that T1 anti-phase linkage during other emotional categories may forecast longitudinal trajectories of relationship quality, with the direction of the effect contingent on emotion category. Specifically, greater anti-phase physiological linkage during shared negative emotion predicted couples having lower quality of relationships five to six years later, whereas greater anti-phase physiological linkage during shared neutral emotion predicted couples having higher quality of relationships five to six years later (see Table 2, Model 1). Although anti-phase linkage may reflect turn-taking, the content of the turns taken may represent dysfunctional cycles of attack and defense during shared negative emotion, and more benign cycles of speaking and listening during shared neutral states. We speculate that the affective tone of turn-taking determines whether anti-phase physiological linkage bodes ill or well for future relationship quality. These and other exploratory findings require replication. We note, however, that these observations would be obscured by analyses that that combined anti-phase linkage with in-phase linkage, or that disregarded emotion, as would occur in computations of overall average linkage across entire conversations, which are likely to include incidents of shared positive, shared negative, and shared neutral emotions.
Theoretical implications
Findings from our study have several theoretical implications. First, our findings suggest that physiological linkage reflects the degree of interpersonal coordination in other emotion response systems, including expressive behavior and subjective experience, which we used as proximal measures of emotion (an inferred construct). Accordingly, it may be that physiological in-phase linkage, shared positive expressive behavior, and shared positive subjective experience are each one feature of a shared positive emotional state. This idea is consistent with theories that emphasize the coherence across multiple response systems when emotion occurs (Levenson, 2014; Levenson et al., 2016; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005), and support interpersonal emotion models (e.g., TIES; Butler, 2011; Butler, 2017) that suggest that shared emotional states are a source of activation across multiple response systems within each individual. Additionally, we also acknowledge that physiological in-phase linkage may be a product of shared positive emotion—more specifically, a product of the shared behavioral responses, such as laughter, that accompany shared positive emotion. This idea is somewhat supported by our finding that statistically controlling for the degree of couples’ linkage in somatic activity (which may reflect shared humor and the associated behaviors such as mutual laughter) reduced the effect sizes of the associations between in-phase physiological linkage and incidents of shared positive emotion. Although more research is needed to tease apart the interpersonal nature of emotion and physiological linkage, the current findings provide compelling evidence that shared positive emotional states are associated with a higher degree of coherent and interpersonal linkage than unshared emotional states.
Second, as noted earlier, our findings are consistent with Fredrickson’s positivity resonance theory (Fredrickson, 2016). Building on attachment theory, including the work of Feldman and colleagues (Feldman, 2007; Feldman et al., 2011), positivity resonance theory suggests that incidents of shared positive emotion are associated with increased biobehavioral synchrony, which—together with momentary mutual care and concern—represents the enactment of the positive emotion of love, which ultimately functions to build and strengthen high-quality relationships. A long history of research links positive emotion, particularly shared positive emotion, with interpersonal connectedness and prosocial (caring) behavior (Fredrickson, 2016; Fredrickson & Cohn, 2007; Gable & Reis, 2010; Gable, Reis, Impett, & Asher, 2004; Kurtz & Algoe, 2015; Major, Le Nguyen, Lundberg, & Fredrickson, 2018). Research has also found that cross-person synchronization in behavior, physiology, and neural activation is likewise associated with interpersonal connectedness, mutual understanding, and prosocial (caring) behavior (Bernieri, 1988; Feldman, 2012; Konvalinka et al., 2011; Marci et al., 2007; Parkinson, Kleinbaum, & Wheatley, 2017; Piazza, Hasenfratz, Hasson, & Lew-Williams; Valdesolo & DeSteno, 2011). The present study is the first to observe that these key components of positive social connection (i.e., physiological linkage, shared positive expressive behaviors, shared positive emotional experience) co-occur during the naturalistic social interactions of married couples and thus builds the case that these core features cohere within a holistic experience of positivity resonance. Additional support for positivity resonance theory comes from the exploratory evidence that greater in-phase physiological linkage during shared positive emotion was associated with higher-quality interactions and relationships, both concurrently and longitudinally. Such associations would be expected to the extent that momentary and recurrent experiences of love (i.e., positivity resonance) over time function to build and strengthen enduring social bonds (Fredrickson, 2016; Major et al., 2018; Otero et al., 2019). Future experimental research is needed, however, to test the causal direction implied by the theory.
Methodological implications
In this study, we focused on an ANS composite measure that consisted of three distinct physiological measures (i.e., IBI, SCL, and FPA), while also examining the associations between shared emotion and physiological linkage using each individual measure. Across all physiological measures, we observed consistent effects regarding the associations between incidents of shared positive emotion and greater in-phase linkage. However, we also found that the size of these effects varied considerably between measures. For in-phase linkage, the effect size was greatest for the composite measure (W = 0.15; median-small) and IBI (W = 0.15; median-small), followed by SCL (W = .08; small), and lowest for FPA (W =.03; small). For anti-phase linkage, effect size was greatest for IBI (W = .26; large), followed by the composite measure (W=.17; median-small), then FPA (W = .07; small), and lowest for SCL (W = .06; small). These findings suggest that some physiological measures (e.g., IBI and the ANS composite) may be more sensitive to momentary changes in dyadic emotion than other physiological measures. Because IBI, with its larger effect sizes, is responsive to both SNS and PNS influence, whereas SCL and FPA are primarily influenced by the SNS (but differ in responding times and acting receptors: SCL-slower, cholinergic, FPA-faster, alpha/adrenergic; Berntson, Quigley, Norman, & Lozano, 2016; Dawson et al., 2007), we speculate that the composite measure may reflect both SNS and PNS influences. Although future research is needed to test this speculation, our pattern of findings provide a possible explanation for why previous research using different physiological measures have observed mixed findings (Palumbo et al., 2016).
We also systematically evaluated the in-phase and anti-phase linkage, two distinct components of the total linkage (e.g., r between the in-phase and anti-phase linkage was −0.17). Our findings suggest that the in-phase linkage may highly overlap with the total linkage, as the correlations between them were high (r = .86) and across most analyses that compared physiological linkage between emotion categories, these measures revealed very similar effects (e.g., Fig. 4A and Supplemental Fig. S2A). On the other hand, our findings suggest that the anti-phase linkage may reveal additional information beyond the total linkage. Although preliminary and in need of replication, we observed an interesting trend that anti-phase linkage tended to be greater during incidents of shared negative emotion than during incidents of other emotion categories.
Finally, our findings, along with findings from many other studies (e.g., Feldman et al., 2011; Marci et al., 2007), suggest the merit of using the momentary approach, including quantifying physiological activity using short time intervals (i.e., one second) and computing physiological linkage using short time windows (i.e., 15 seconds). Using this momentary approach, robust effects of greater in-phase linkage during incidents of shared positive emotion emerged, alongside a trend of greater anti-phase linkage during incidents of shared negative emotion. Neither of these effects were found in Levenson and Gottman (1983) which used the same research paradigm but took the traditional overall average analytic approach. In addition, our study quantified the presence of emotion using either the highly time-precise expressive behavioral data (for primary analyses) or subjective experience data (for generalizability analyses), the latter of which changes relatively slowly compared to the former. Although both analyses revealed similar results for the association between incidents of shared positive emotion and greater in-phase linkage (i.e., Fig. 4A and Fig. 7), effects sizes were generally greater for the analyses that used expressive behavior to quantify emotion (e.g., for in-phase linkage, W = .15 versus W = .09). Importantly, when we explored whether physiological linkage during incidents of shared positive emotion was associated with the perceived quality of the interactions and the relationships, we found that the momentary approach outperformed an overall average approach. Future studies should continue to compare these different analytic approaches systematically, and to evaluate how short versus long time intervals for emotion and physiological data impact findings.
Strengths and limitations
Strengths of the present study include: (a) studying physiological linkage in a large sample of naturalistic dyadic interactions; (b) utilizing a novel approach by examining the momentary associations between shared emotion and dyadic physiology with a high level of temporal resolution; (c) systematically examining two forms of physiological linkage (i.e., in-phase and anti-phase linkage) and two types of shared emotion (i.e., shared positive and shared negative); (d) including incidents of shared neutral emotion and unshared emotion as comparison emotion categories; (e) establishing reliability of the effects over the span of 5–6 years, (f) rejecting an alternative explanation that effects can be explained by the duration of each emotion category; (g) determining the role of somatic activity as an influence on observed linkage effects; (h) establishing the generalizability of the primary findings to individual physiological measures (i.e., IBI, SCL, FPA), different emotion response systems (i.e., expressive behavior, subjective experience), and age cohorts; (i) characterizing the pattern of physiological reactivity (i.e., physiological activation versus deactivation) within each of the interactants at the onsets of shared positive, shared negative, and shared neutral emotion epochs; and (j) exploring connections between physiological linkage and relational functioning, using both an episodic marker of interaction quality and a global marker of relationship quality.
Our study also has important limitations, including: (a) focusing on a conflict conversation and not examining other social interaction contexts; (b) focusing on emotion and not examining other important social interaction behaviors such as emotion regulation; (c) focusing on valence and not examining other important aspects of shared emotion (e.g., shared intensity or arousal16); (d) SPAFF (i.e., the behavioral coding system we used) does not code specific emotions for listeners, so our analyses are limited and cannot test the effects of sharing specific types of emotion (e.g., shared humor, shared anger); (e) we did not include any measure that is exclusively sensitive to the PNS, so our findings cannot to speak to differentiating between the two branches of the ANS. As mentioned, although RSA can be computed from IBI, we did not take this approach because when people talk and laugh, their respiration patterns can be profoundly altered, which precludes an accurate estimation of PNS activities using IBI (Grossman et al., 1991); (f) we did not examine the causes of shared emotion (e.g., emotional mimicry, social influence, turn taking, shared memory), which may have important implications for momentary increases in physiological linkage. (e.g., the observed effects may also reflect the presence of these psychological processes that precede shared positive emotion; Feldman et al., 2011; Reed et al., 2013; Semin & Cacioppo, 2008; Waters et al., 2017); and (g) although our “second-by-second” analytic approach is highly temporally precise and allows us to detect rapid changes in dyadic emotional behaviors and physiology, it may be less capable of detecting patterns that take longer times to develop (e.g., responses of husbands and wives that may slowly converge or diverge over the time course of the interaction).
Conclusions
Past research has used the “overall average” approach to relate physiological linkage to psychological processes (e.g., Reed et al., 2013; Waters et al., 2017) and qualities of interpersonal relationships (e.g., Levenson & Gottman, 1983) with mixed results (Palumbo et al., 2016). In this study, we used a “momentary” approach to relate two types of physiological linkage (i.e., in-phase and anti-phase) and two types of shared emotion (i.e., shared positive and shared negative emotion) during dyadic interactions between long-term married couples.
Based on theories of emotion and physiology (e.g., Ax, 1953; Cannon, 1927; Ekman et al., 1983; Fredrickson, 2013b, 2016; Levenson, 2014; Levenson et al., 2016; Shiota et al., 2017) and past research on emotion and physiological linkage (e.g., Feldman et al., 2011; Levenson & Gottman, 1983), we tested three competing and mutually exclusive hypotheses that differentially predicted whether physiological linkage would be most prominent during incidents of shared negative emotion, shared positive emotion, or both. We uncovered robust and reliable evidence that incidents of shared positive emotion were characterized by greater in-phase physiological linkage relative to all other emotional incidents (i.e., shared negative emotion, shared neutral emotion, and unshared emotion). These linkage effects largely (but not solely) coincided with linkage in couples’ somatic activity, and simultaneous activation (versus deactivation) of each partners’ ANS responses, patterns plausibly related to shared laughter. Exploratory analyses also showed that in-phase physiological linkage during shared positive emotion was associated with relational functioning, both concurrently and longitudinally, as reflected in the overall affective tone of couples’ conversations in the laboratory (i.e., quality of interactions) and their marital satisfaction more generally (i.e., quality of relationships). This work helps to disentangle long-debated questions regarding the nature and relevance of physiological linkage during social interactions. Findings underscore that shared positive emotion connects marriage partners (physiologically) more than any other emotional states, shared or unshared, and that such linkage may reflect positive relational processes.
Supplementary Material
Acknowledgments
Computed study variables are available on the Open Science Framework at https://osf.io/pvedh/?view_only=2cd92c803fd34fef8a71c70e5cdd6186. This research was supported by National Institute on Aging (R37-AG017766 to Robert W. Levenson; K99-AG059947 to Kuan-Hua Chen). We thank Dr. Emilio Ferrer and Dr. Kevin Grimm for advice on statistical analyses; Deepak Paul for help with data management and technical support; all of our research assistants for their assistance with data collection, data cleaning and the coding of facial expressions of emotion. We also thank reviewers of the paper for their feedback and recommendations, and all couples who donated their time so generously to participate in this research over the span of many years.
Footnotes
Nonparametric Mann-Whitney U tests confirm that T1 couples included versus excluded in data analyses did not differ in their demographic characteristics, including husbands’ age (Z = .35, p = .72), wives’ age (Z = −.55, p = .58), husbands’ years of education (Z = 1.17, p = .24), wives’ years of education (Z = .22, p = .83), and years of marriage (Z = .72, p = .47). Similarly, included versus excluded couples did not differ in their physiological linkage scores computed over the entire conversation (disregarding emotion categories): averaged total linkage (Z = .20, p = .84), in-phase linkage (Z = .33, p = .74), and anti-phase linkage (Z = .04, p = .97). See below for methods to compute averaged linkage scores. Similarly, at T2, no significant differences emerged between couples included versus excluded in data analyses (Zs < 1.03; ps > .30).
We initially collected six ANS measures (cardiac interbeat interval [IBI], skin conductance level [SCL], finger pulse amplitude [FPA], pulse transmission time to the finger [FPT], pulse transmission time to the ear [EPT], finger temperature [TEM]) and general somatic activity (ACT). For our primary analyses, we used IBI, SCL, and FPA. These measures have been used in previous studies of linkage, thus enabling our findings to be more readily comparable to those of others. In the main text we examine whether linkage in somatic activity accounts for the effects of linkage in ANS measures, and secondary analyses were conducted to determine whether findings obtained using IBI, SCL, and FPA could be generalized to FPT and EPT. Results of these analyses are presented in Supplemental Fig. S1.
Epochs that started at the first and last five seconds of the 15-minute conversation were not analyzed due to insufficient samples to compute five second response mean). Thus, two husbands and one wife were excluded from data analyses because there was no enough epoch for all three emotion categories after excluding epochs occurring at the first and last five seconds for the conversation.
Similar analyses were performed for the total linkage. Results indicate that for all four emotion categories, averaged total linkage scores were significantly greater than zero (Zs > 3.87 ps < .001).
See Supplemental Table S5 for the total time that shared positive emotion, shared negative emotion, shared neutral, and unshared emotion occurred in couples included in data analyses.
To ensure the above results based on nonparametric analyses were robust, we performed additional parametric repeated-measures analyses (i.e., ANOVA and post-hoc t tests) based on linkage scores for the four emotion categories after z-score transformations (within each couple). Very similar results were found, such that incidents of shared positive emotion were associated with greater in-phase linkage and lower anti-phase linkage as compared to incidents of the other three emotion categories (ps < .001).
Here, physiological linkage was operationalized as a continuous measure. Based on the total linkage, we derived in-phase and anti-phase linkage, which were also continuous measures with scores ranging from 0 to 1. Our approach, however, did not consider “no linkage”, another possible form of physiological linkage, during which couple’s physiological responses were not meaningfully linked to each other. To address this gap, we performed additional analyses in which each second of the conversation was re-characterized by one of the three following linkage forms based on the associated Pearson’s correlation coefficient: (a) in-phase linkage, when correlation coefficient was greater than 0.1; (b) no linkage, when correlation coefficient was between 0.1 and −0.1 (including ± 0.1), and (c) anti-phase linkage, when correlation coefficient was lower than −0.1. We then computed the percentage of time that the couples exhibited in-phase linkage, no linkage, and anti-phase linkage relative to the total time of each emotion category. Results, as shown in Supplemental Fig. S3A middle and right, revealed very similar patterns for no linkage and anti-phase linkage (i.e., both no linkage and anti-phase linkage occurred less frequently during incidents of shared positive emotion than during incidents of other emotion categories, ps < .001). Similar to our primary findings (i.e., Fig. 4A), results also revealed that in-phase linkage computed using this categorical approach occurred more frequently during incidents of shared positive emotion than other emotion categories (ps< .001; Fig. S3A left). To further confirm these effects, we repeated these analyses and changed the cutoff from ± 0.1 to ± 0.2 and ± 0.3. Very similar results were revealed, ps< .01, Supplemental Fig. S3B, S3C.
To ensure our findings are robust even when a dynamic time lag is applied, we repeated our primary analyses and replaced the original linkage scores (i.e., Pearson’s correlation coefficient) with scores computed using cross-correlations with time lags. More specifically, for each second of the conversation, we performed a 10-lag cross-correlation (therefore range of time lag = −10 to +10 seconds) using the same 15 second rolling window. We then used the maximum and minimum correlation coefficients from the cross-correlation to represent in-phase and anti-phase linkage, respectively. Results of these analyses are very similar to our primary findings, such that incidents of shared positive emotion were associated with greater “maximum linkage,” as compared to incidents of other emotion categories, ps < .001, Supplemental Fig. S4.
Analyses of in-phase linkage adjusted for ACT in-phase linkage. Similarly, analyses of anti-phase physiological linkage adjusted for ACT anti-phase linkage.
To further confirm this conclusion, we performed additional analyses in which we repeated the primary analyses (as those for Fig. 4A) but only focused on a subgroup of couples (n of dyads = 48) who had at least 20 seconds for each of the four emotion categories. We also performed the same analyses on another subgroup of couples (n of dyads = 66) who exhibited shared positive emotion equal to or less than 20 seconds. The results, as shown in Supplemental Fig. S6, revealed that all the significant effects observed in previous primary analyses remained statistically significant (ps < .05).
See Supplemental Table S5 for the averaged time that shared positive emotion, shared negative emotion, and shared neutral emotion occurred (based on rating dial data) for the couples included in data analyses.
To ensure the observed effects based on the physiological reactivity composite were robust, we repeated the analyses for each individual physiological measure (i.e., IBI, SCL, and FPA). Very similar results were found in each of these measures (Supplemental Fig. S7). To further ensure the observed effects were robust, we performed additional analyses in which we excluded epochs (a) lasting less than five seconds, and (b) immediately following incidents in which either of the partners expressed the same target emotion (e.g., a husband already expressed a positive emotion before the onset of the husband’s and wife’s shared positive emotion). These analyses revealed very similar results (Supplemental Fig. S8). To ensure that physiological activation occurred in both partners at the same time (i.e., at the same emotion epochs), on an epoch-by-epoch basis, we further computed the percentage of time that the husband’s and wife’s physiological activity both increased for the three emotion categories (i.e., the number of epochs that this case occurred divided by the total number of epochs for each emotion category). As shown in Supplemental Fig. S9, the percentage of time that the couple both had an increased physiological activity was significantly greater at the onsets of shared positive emotion, relative to shared negative emotion and shared neutral emotion (ps < .001).
For completeness, we also examined concurrent associations between physiological linkage and relational functioning (i.e., quality of interactions and quality of relationships) within T2, using its smaller subset of couples, and report these in Supplemental Table S6. Likewise, we also explored associations between linkage variables and changes from T1 to T2 in the couples’ relational functioning (i.e., by controlling for T1 relational functioning in regression equations, or using difference scores for dependent variables). No significant effects emerged in analyses of change. We note, however, that not only were measures of relationship quality highly stable from T1 to T2 (r = .82, p < .001), but also that couples who returned for T2 had significantly higher relationship quality at T1 than those who did not return (on each of the two measures of marital satisfaction). We thus speculate that a restriction of range may have impacted some analyses.
Alternative explanations for the associations between physiological linkage during incidents of shared positive emotion and relational functioning may reflect years of marriage or the total time that couples exhibited shared positive emotion during the conversation (e.g., Otero et al., 2019). To address these possibilities, we performed additional linear regression analyses with covariates and found that, at statistically significant or trending levels, (a) for interaction quality, greater in-phase linkage during shared positive emotion remained associated with higher interaction quality at T1 when either of these variables was included as a covariate (βs > .249, ps < .033), and at T2 when years of marriage was included as a covariate (β = .25, p = .075); (b) for relationship quality, greater in-phase linkage during shared positive emotion remained associated with higher relationship quality at T1 (βs > .255, ps < .026) and at T2 (βs > .24, ps < .064) when either of these variables was included as a covariate.
We performed additional linear regression analyses with covariates (as in footnote #14) for the comparisons between the momentary and overall average linkage approaches. When including either years of marriage or the total time that couples exhibited shared positive emotion at T1 as a covariate, the momentary in-phase linkage during shared positive emotion was still associated with higher interaction quality at T1 (βs > .282, ps < .018), and higher relationship quality at T1 and T2 (βs > .285, ps < .055); the momentary anti-phase linkage during shared positive emotion was still associated with higher relationship quality at T1(βs > .21, ps < .064), at either statistically significant or trending levels. With the inclusion of covariates, in-phase and anti-phase overall average linkage still showed no significant associations with interaction or relationship quality at both T1 and T2.
We note that shared emotion “intensity” is not synonymous with shared emotion “arousal” (e.g., high intensity affection may not reflect high arousal). Although emotion intensity was coded in SPAFF (1 = low, 2 = high), descriptive analyses suggested insufficient distribution of intensities required for analyses, i.e., incidents of shared high-intensity emotion were only evident in 22 of 150 dyads, with a mode of only 9 incidents. Thus, the available data were not well-suited to examine alternative hypotheses about physiological linkage related to incidents of shared arousal or shared intensity.
References
- Ax AF (1953). The physiological differentiation between fear and anger in humans. Psychosomatic Medicine, 15(5), 433–442. [DOI] [PubMed] [Google Scholar]
- Bachorowski J-A, & Owren MJ (2001). Not all laughs are alike: Voiced but not unvoiced laughter readily elicits positive affect. Psychological Science, 12(3), 252–257. doi: 10.1111/1467-9280.00346 [DOI] [PubMed] [Google Scholar]
- Barrett LF, Adolphs R, Marsella S, Martinez AM, & Pollak SD (2019). Emotional Expressions Reconsidered: Challenges to Inferring Emotion From Human Facial Movements. Psychological Science in the Public Interest, 20(1), 1–68. doi: 10.1177/1529100619832930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, & Vohs KD (2001). Bad is Stronger than Good. Review of General Psychology, 5(4), 323–370. doi: 10.1037/1089-2680.5.4.323 [DOI] [Google Scholar]
- Bernieri FJ (1988). Coordinated movement and rapport in teacher-student interactions. Journal of Nonverbal Behavior, 12(2), 120–138. doi: 10.1007/bf00986930 [DOI] [Google Scholar]
- Berntson GG, Quigley KS, Norman GJ, & Lozano DL (2016). Cardiovascular Psychophysiology. In Berntson GG, Cacioppo JT, & Tassinary LG (Eds.), Handbook of Psychophysiology (4 ed., pp. 183–216). Cambridge: Cambridge University Press. [Google Scholar]
- Brown TE, Beightol LA, Koh J, & Eckberg DL (1993). Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol (1985), 75(5), 2310–2317. doi: 10.1152/jappl.1993.75.5.2310 [DOI] [PubMed] [Google Scholar]
- Buchowski MS, Majchrzak KM, Blomquist K, Chen KY, Byrne DW, & Bachorowski JA (2006). Energy expenditure of genuine laughter. International Journal Of Obesity, 31, 131. doi: 10.1038/sj.ijo.0803353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess EW, Locke HJ, & Thomes MM (1971). The family: From traditional to companionship. NY: Van Nostrand Reinhold Company. [Google Scholar]
- Butler EA (2011). Temporal Interpersonal Emotion Systems. Personality and Social Psychology Review, 15(4), 367–393. doi: 10.1177/1088868311411164 [DOI] [PubMed] [Google Scholar]
- Butler EA (2015). Interpersonal affect dynamics: It takes two (and time) to tango. Emotion Review, 7(4), 336–341. doi: 10.1177/1754073915590622 [DOI] [Google Scholar]
- Butler EA (2017). Emotions are temporal interpersonal systems. Current Opinion in Psychology, 17, 129–134. doi: 10.1016/j.copsyc.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion. Handbook of emotions, 2, 173–191. [Google Scholar]
- Cannon WB (1927). The James-Lange theory of emotions: A critical examination and an alternative theory. The American journal of psychology, 39(1/4), 106–124. [PubMed] [Google Scholar]
- Carstensen LL, Gottman JM, & Levenson RW (1995). Emotional Behavior in Long-Term Marriage. Psychology and Aging, 10(1), 140–149. Retrieved from 10.1037/0882-7974.10.1.14010.1037/0882-7974.10.1.140http://www.lib.utexas.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pdh&AN=pag101140&site=ehost-livehttp://www.lib.utexas.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pdh&AN=pag101140&site=ehost-live [DOI] [PubMed] [Google Scholar]
- Chang A, Livingstone SR, Bosnyak DJ, & Trainor LJ (2017). Body sway reflects leadership in joint music performance. Proceedings of the National Academy of Sciences, 114(21), E4134–E4141. doi: 10.1073/pnas.1617657114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-H, Aksan N, Anderson SW, Grafft A, & Chapleau MW (2014). Habituation of parasympathetic-mediated heart rate responses to recurring acoustic startle. Frontiers in Psychology, 5. doi: 10.3389/fpsyg.2014.01288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Gottman JM (2007). The specific affect coding system (SPAFF). In Coan JA & Allen JJB (Eds.), Handbook of emotion elicitation and assessment (pp. 267–285). New York, NY: Oxford. [Google Scholar]
- Dawson ME, Schell AM, & Filion DL (2007). The electrodermal system. In Cacioppo JT, Tassinary LG, & Berntson G (Eds.), Handbook of Psychophysiology (3 ed., pp. 159–181). New York: Cambridge University Press. [Google Scholar]
- Di Mascio A, Boyd RW, & Greenblatt M (1957). Physiological Correlates of Tension and Antagonism During Psychotherapy: A Study of” Interpersonal Physiology”. Psychosomatic medicine, 19(2), 99–104. [DOI] [PubMed] [Google Scholar]
- Di Mascio A, Boyd RW, Greenblatt M, & Solomon HC (1955). The psychiatric interview: a sociophysiologic study. Dis Nerv Syst, 16(1), 4–9. [PubMed] [Google Scholar]
- Ekman P, Levenson RW, & Friesen WV (1983). Autonomic nervous system activity distinguishes among emotions. Science, 221(4616), 1208–1210. [DOI] [PubMed] [Google Scholar]
- Elfenbein HA (2014). The many faces of emotional contagion: An affective process theory of affective linkage. Organizational Psychology Review, 4(4), 326–362. doi: 10.1177/2041386614542889 [DOI] [Google Scholar]
- Feldman R (2007). Parent–Infant Synchrony: Biological Foundations and Developmental Outcomes. Current Directions in Psychological Science, 16(6), 340–345. doi: 10.1111/j.1467-8721.2007.00532.x [DOI] [Google Scholar]
- Feldman R (2012). Parent–infant synchrony: A Biobehavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development, 77(2), 42–51. doi: 10.1111/j.1540-5834.2011.00660.x [DOI] [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, & Louzoun Y (2011). Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development, 34(4), 569–577. doi: 10.1016/j.infbeh.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Filippelli M, Pellegrino R, Iandelli I, Misuri G, Rodarte JR, Duranti R, … Scano G (2001). Respiratory dynamics during laughter. Journal of Applied Physiology, 90(4), 1441–1446. doi: 10.1152/jappl.2001.90.4.1441 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology: The broaden-and-build theory of positive emotions. American Psychologist, 56(3), 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL (2013a). Love 2.0: How our supreme emotion affects everything we feel, think, do, and become. NY: Plume. [Google Scholar]
- Fredrickson BL (2013b). Positive Emotions Broaden and Build. In Devine P & Plant A (Eds.), Advances in Experimental Social Psychology (Vol. 47, pp. 1–53): Academic Press. [Google Scholar]
- Fredrickson BL (2016). Love: Positivity resonance as a fresh, evidence-based perspective on an age-old topic. In Barrett LF, Lewis M, & Haviland-Jones JM (Eds.), Handbook of Emotions (pp. 847–858). : New York: Guilford Press. [Google Scholar]
- Fredrickson BL, & Cohn M (2007). Positive emotions. In Lewis MD, Haviland-Jones JM, & Barrett LF (Eds.), Handbook of emotions (pp. 777–796). New York, NY, US: Guilford Press. [Google Scholar]
- Fredrickson BL, & Levenson RW (1998). Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion, 12(2), 191–220. doi: 10.1080/026999398379718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, & Tugade MM (2000). The undoing effect of positive emotions. Motivation and emotion, 24(4), 237–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry WF, & Rader C (1977). The respiratory components of mirthful laughter. Journal of Biological Psychology, 19(2), 39–50. [Google Scholar]
- Gable SL, & Reis HT (2010). Good news! Capitalizing on positive events in an interpersonal context. Advances in Experimental Social Psychology, 42, 195–257. doi: 10.1016/S0065-2601(10)42004-3 [DOI] [Google Scholar]
- Gable SL, Reis HT, Impett EA, & Asher ER (2004). What do you do when things go right? The Intrapersonal and interpersonal benefits of sharing positive events. Journal of Personality and Social Psychology: Interpersonal Relations and Group Processes, 87(2), 228–245. doi: 10.1037/0022-3514.87.2.228 [DOI] [PubMed] [Google Scholar]
- Gates KM, Gatzke-Kopp LM, Sandsten M, & Blandon AY (2015). Estimating time-varying RSA to examine psychophysiological linkage of marital dyads. Psychophysiology, 52(8), 1059–1065. doi: 10.1111/psyp.12428 [DOI] [PubMed] [Google Scholar]
- Gottman JM (1981). Time-series analysisa: A comprehensive introduction for social scientists. New York: Cambridge University Press. [Google Scholar]
- Grossman P, Karemaker J, & Wieling W (1991). Prediction of Tonic Parasympathetic Cardiac Control Using Respiratory Sinus Arrhythmia: The Need for Respiratory Control. Psychophysiology, 28(2), 201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x [DOI] [PubMed] [Google Scholar]
- Helm JL, Sbarra DA, & Ferrer E (2014). Coregulation of respiratory sinus arrhythmia in adult romantic partners. Emotion, 14(3), 522–531. doi: 10.1037/a0035960 [DOI] [PubMed] [Google Scholar]
- Henning RA, Boucsein W, & Claudia Gil M (2001). Social–physiological compliance as a determinant of team performance. International Journal of Psychophysiology, 40(3), 221–232. doi: 10.1016/S0167-8760(00)00190-2 [DOI] [PubMed] [Google Scholar]
- Hirsch JA, & Bishop B (1981). Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am J Physiol, 241(4), H620–629. doi: 10.1152/ajpheart.1981.241.4.H620 [DOI] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, & Cacioppo JT (1998). Negative information weighs more heavily on the brain: The negativity bias in evaluative categorizations. Journal of Personality and Social Psychology, 75(4), 887–900. doi: 10.1037/0022-3514.75.4.887 [DOI] [PubMed] [Google Scholar]
- Järvelä S, Kivikangas JM, Kätsyri J, & Ravaja N (2013). Physiological Linkage of Dyadic Gaming Experience. Simulation & Gaming, 45(1), 24–40. doi: 10.1177/1046878113513080 [DOI] [Google Scholar]
- Kang O, & Wheatley T (2017). Pupil dilation patterns spontaneously synchronize across individuals during shared attention. Journal of Experimental Psychology: General, 146(4), 569–576. doi: 10.1037/xge0000271 [DOI] [PubMed] [Google Scholar]
- Kinreich S, Djalovski A, Kraus L, Louzoun Y, & Feldman R (2017). Brain-to-Brain Synchrony during Naturalistic Social Interactions. Scientific Reports, 7(1), 17060. doi: 10.1038/s41598-017-17339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinka I, Xygalatas D, Bulbulia J, Schjødt U, Jegindø E-M, Wallot S, … Roepstorff A (2011). Synchronized arousal between performers and related spectators in a fire-walking ritual. Proceedings of the National Academy of Sciences, 108(20), 8514–8519. doi: 10.1073/pnas.1016955108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. doi: 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Kurtz LE, & Algoe SB (2015). Putting laughter in context: Shared laughter as behavioral indicator of relationship well-being. Personal Relationships, 22(4), 573–590. doi: 10.1111/pere.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin R, & Day H (1972). Physiological correlates of humor. The psychology of humor: Theoretical perspectives and empirical issues, 129–142. [Google Scholar]
- Levenson RW (2014). The Autonomic nervous system and emotion. Emotion Review, 6(2), 100–112. doi: 10.1177/1754073913512003 [DOI] [Google Scholar]
- Levenson RW, Carstensen LL, & Gottman JM (1993). Long-Term Marriage : Age, Gender, and Satisfaction. Psychology and Aging, 8(2), 301–313. Retrieved from 10.1037/0882-7974.8.2.30110.1037/0882-7974.8.2.301http://www.lib.utexas.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pdh&AN=pag82301&site=ehost-livehttp://www.lib.utexas.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pdh&AN=pag82301&site=ehost-live [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, & Friesen WV (1990). Voluntary Facial Action Generates Emotion-Specific Autonomic Nervous System Activity. Psychophysiology, 27(4), 363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x [DOI] [PubMed] [Google Scholar]
- Levenson RW, & Gottman JM (1983). Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology, 45(3), 587–597. Retrieved from 10.1037/0022-3514.45.3.58710.1037/0022-3514.45.3.587http://www.lib.utexas.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pdh&AN=psp-45-3-587&site=ehost-livehttp://www.lib.utexas.edu:2048/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=pdh&AN=psp-45-3-587&site=ehost-live [DOI] [PubMed] [Google Scholar]
- Levenson RW, Lwi SJ, Brown CL, Ford BQ, Otero MC, & Verstaen A (2016). Emotion. In Berntson GG, Cacioppo JT, & Tassinary LG (Eds.), Handbook of Psychophysiology (4 ed., pp. 444–464). Cambridge: Cambridge University Press. [Google Scholar]
- Levenson RW, & Ruef AM (1992). Empathy: A physiological substrate. Journal of Personality and Social Psychology, 63(2), 234–246. doi: 10.1037/0022-3514.63.2.234 [DOI] [PubMed] [Google Scholar]
- Levy J, Goldstein A, & Feldman R (2017). Perception of social synchrony induces mother–child gamma coupling in the social brain. Social Cognitive and Affective Neuroscience, 12(7), 1036–1046. Retrieved from https://search.proquest.com/docview/1964617084/abstract/embedded/AJCWEKS5CQGFYAL7?source=fedsrch [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Cousino Klein L, & Almeida DM (2013). Synchrony of diurnal cortisol pattern in couples. Journal of Family Psychology, 27(4), 579–588. doi: 10.1037/a0033735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd EL (1938). The respiratory mechanism in laughter. The Journal of General Psychology, 19(1), 179–189. [Google Scholar]
- Locke HJ, & Wallace KM (1959). Short Marital-Adjustment and Prediction Tests: Their Reliability and Validity. Marriage and Family Living, 21(3), 251–255. doi: 10.2307/348022 [DOI] [Google Scholar]
- Major BC, Le Nguyen KD, Lundberg KB, & Fredrickson BL (2018). Well-being correlates of perceived positivity resonance: Evidence from trait and episode-level assessments. Personality and Social Psychology Bulletin, 44(12), 1631–1647. doi: 10.1177/0146167218771324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmo RB, Boag TJ, & Smith AA (1957). Physiological study of personal interaction. Psychosomatic Medicine, 19, 105–119. doi: 10.1097/00006842-195703000-00004 [DOI] [PubMed] [Google Scholar]
- Marci CD, Ham J, Moran E, & Orr SP (2007). Physiologic correlates of perceived therapist empathy and social-emotional process during psychotherapy. The Journal of nervous and mental disease, 195(2), 103–111. [DOI] [PubMed] [Google Scholar]
- Marci CD, & Orr SP (2006). The effect of emotional distance on psychophysiologic concordance and perceived empathy between patient and interviewer. Applied Psychophysiology and Biofeedback, 31(2), 115–128. doi: 10.1007/s10484-006-9008-4 [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, & Gross JJ (2005). The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion, 5(2), 175–190. doi: 10.1037/1528-3542.5.2.175 [DOI] [PubMed] [Google Scholar]
- Messina I, Palmieri A, Sambin M, Kleinbub JR, Voci A, & Calvo V (2013). Somatic underpinnings of perceived empathy: The importance of psychotherapy training. Psychotherapy Research, 23(2), 169–177. doi: 10.1080/10503307.2012.748940 [DOI] [PubMed] [Google Scholar]
- Müller V, & Lindenberger U (2011). Cardiac and Respiratory Patterns Synchronize between Persons during Choir Singing. PLOS ONE, 6(9), e24893. doi: 10.1371/journal.pone.0024893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray WB, & Foster PA (1996). The peripheral pulse wave: Information overlooked. Journal of Clinical Monitoring, 12(5), 365–377. doi: 10.1007/BF02077634 [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Mermillod M, Maringer M, & Hess U (2010). The Simulation of Smiles (SIMS) model: Embodied simulation and the meaning of facial expression. Behavioral and Brain Sciences, 33(6), 417–433. doi: 10.1017/S0140525X10000865 [DOI] [PubMed] [Google Scholar]
- Obrist PA, Webb RA, Sutterer JR, & Howard JL (1970). The cardiac-somatic relationship: some reformulations. Psychophysiology, 6(5), 569–587. doi: 10.1111/j.1469-8986.1970.tb02246.x [DOI] [PubMed] [Google Scholar]
- Otero MC, Wells JL, Chen K-H, Brown CL, Connelly DE, Levenson RW, & Fredrickson BL (2019). Behavioral indices of positivity resonance associated with long-term marital satisfaction. Emotion. doi: 10.1037/emo0000634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo RV, Marraccini ME, Weyandt LL, Wilder-Smith O, McGee HA, Liu S, & Goodwin MS (2016). Interpersonal autonomic physiology: A Systematic review of the literature. Personality and Social Psychology Review, 21(2), 99–141. doi: 10.1177/1088868316628405 [DOI] [PubMed] [Google Scholar]
- Parkinson C, Kleinbaum AM, & Wheatley T (2017). Spontaneous neural encoding of social network position. 1, 0072. doi:10.1038/s41562-017-007210.1038/s41562-017-0072https://www.nature.com/articles/s41562-017-0072#supplementary-informationhttps://www.nature.com/articles/s41562-017-0072#supplementary-information [Google Scholar]
- Parkinson C, Kleinbaum AM, & Wheatley T (2018). Similar neural responses predict friendship. Nature Communications, 9(1), 332. doi: 10.1038/s41467-017-02722-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza EA, Hasenfratz L, Hasson U, & Lew-Williams C Infant and Adult Brains Are Coupled to the Dynamics of Natural Communication. Psychological Science, 0(0), 0956797619878698. doi: 10.1177/0956797619878698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, & de Waal FB (2002). Empathy: Its ultimate and proximate bases. Behav Brain Sci, 25(1), 1–20; discussion 20–71. doi: [DOI] [PubMed] [Google Scholar]
- Prochazkova E, & Kret ME (2017). Connecting minds and sharing emotions through mimicry: A neurocognitive model of emotional contagion. Neuroscience & Biobehavioral Reviews, 80, 99–114. doi: 10.1016/j.neubiorev.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Provine RR (1992). Contagious laughter: Laughter is a sufficient stimulus for laughs and smiles. Bulletin of the Psychonomic Society, 30(1), 1–4. doi: 10.3758/bf03330380 [DOI] [Google Scholar]
- Quer G, Daftari J, & Rao RR (2016). Heart rate wavelet coherence analysis to investigate group entrainment. Pervasive and Mobile Computing, 28, 21–34. doi: 10.1016/j.pmcj.2015.09.008 [DOI] [Google Scholar]
- Reed RG, Randall AK, Post JH, & Butler EA (2013). Partner influence and in-phase versus anti-phase physiological linkage in romantic couples. International Journal of Psychophysiology, 88(3), 309–316. doi: 10.1016/j.ijpsycho.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Riediger M, & Klipker K (2014). Emotion Regulation in Adolescence. In Gross JJ (Ed.), Handbook of emotion regulation (2 ed., pp. 187–202). New York: The Guilford Press. [Google Scholar]
- Rozin P, & Royzman EB (2001). Negativity Bias, Negativity Dominance, and Contagion. Personality and Social Psychology Review, 5(4), 296–320. doi: 10.1207/s15327957pspr0504_2 [DOI] [Google Scholar]
- Ruef AM, & Levenson RW (2007). Continuous measurement of emotion. Handbook of emotion elicitation and assessment, 286–297. [Google Scholar]
- Sahakian A, & Firshman WH (2007). Humor and the cardiovascular system. Alternative Therapies in Health & Medicine, 13(4), 56–58. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=106145731&site=ehost-live [PubMed] [Google Scholar]
- Sanchez A, & Vazquez C (2014). Looking at the eyes of happiness: Positive emotions mediate the influence of life satisfaction on attention to happy faces. The Journal of Positive Psychology, 9(5), 435–448. doi: 10.1080/17439760.2014.910827 [DOI] [Google Scholar]
- Saxbe D, & Repetti RL (2010). For better or worse? Coregulation of couples’ cortisol levels and mood states. Journal of personality and social psychology, 98(1), 92. [DOI] [PubMed] [Google Scholar]
- Semin GR, & Cacioppo JT (2008). Grounding Social Cognition. In Smith ER & Semin GR (Eds.), Embodied Grounding: Social, Cognitive, Affective, and Neuroscientific Approaches (pp. 119–147). Cambridge: Cambridge University Press. [Google Scholar]
- Shiota MN, Campos B, Oveis C, Hertenstein MJ, Simon-Thomas E, & Keltner D (2017). Beyond happiness: Building a science of discrete positive emotions. American Psychologist, 72(7), 617–643. doi: 10.1037/a0040456 [DOI] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, & Perea EF (2011). Feeling good: Autonomic nervous system responding in five positive emotions. Emotion, 11(6), 1368–1378. doi: 10.1037/a0024278 [DOI] [PubMed] [Google Scholar]
- Svebak S (1975). Respiratory patterns as predictors of laughter. Psychophysiology, 12(1), 62–65. doi: 10.1111/j.1469-8986.1975.tb03062.x [DOI] [PubMed] [Google Scholar]
- Thomsen DG, & Gilbert DG (1998). Factors characterizing marital conflict states and traits: physiological, affective, behavioral and neurotic variable contributions to marital conflict and satisfaction. Personality and Individual Differences, 25(5), 833–855. doi: 10.1016/S0191-8869(98)00064-6 [DOI] [Google Scholar]
- Thorson KR, West TV, & Mendes WB (2017). Measuring Physiological Influence in Dyads: A Guide to Designing, Implementing, and Analyzing Dyadic Physiological Studies. Psychol Methods. doi: 10.1037/met0000166 [DOI] [PubMed] [Google Scholar]
- Timmons AC, Margolin G, & Saxbe DE (2015). Physiological linkage in couples and its implications for individual and interpersonal functioning: A literature review. Journal of Family Psychology, 29(5), 720–731. doi: 10.1037/fam0000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdesolo P, & DeSteno D (2011). Synchrony and the social tuning of compassion. Emotion, 11(2), 262–266. doi: 10.1037/a0021302 [DOI] [PubMed] [Google Scholar]
- Vallacher RR, Nowak A, & Zochowski M (2005). Dynamics of social coordination: The synchronization of internal states in close relationships. Interaction Studies: Social Behaviour and Communication in Biological and Artificial Systems, 6(1), 35–52. [Google Scholar]
- Verstaen A, Haase CM, Lwi SJ, & Levenson RW (2018). Age-related changes in emotional behavior: Evidence from a 13-year longitudinal study of long-term married couples. Emotion. doi: 10.1037/emo0000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SF, West TV, Karnilowicz HR, & Mendes WB (2017). Affect contagion between mothers and infants: Examining valence and touch. J Exp Psychol Gen, 146(7), 1043–1051. doi: 10.1037/xge0000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters SF, West TV, & Mendes WB (2014). Stress contagion: Physiological covariation between mothers and infants. Psychological Science, 25(4), 934–942. doi: 10.1177/0956797613518352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisfeld GE (1993). The adaptive value of humor and laughter. Ethology and Sociobiology, 14(2), 141–169. doi: 10.1016/0162-3095(93)90012-7 [DOI] [Google Scholar]
- Wilson SJ, Bailey BE, Jaremka LM, Fagundes CP, Andridge R, Malarkey WB, … Kiecolt-Glaser JK (2018). When couples’hearts beat together: Synchrony in heart rate variabilityduring conflict predicts heightened inflammation throughout the day. Psychoneuroendocrinology, 93, 107–116. doi: 10.1016/j.psyneuen.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JW, McCarthy M, Holley SR, & Levenson RW (2010). Physiological down-regulation and positive emotion in marital interaction. Emotion, 10(4), 467–474. doi: 10.1037/a0018699 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.