Abstract
Pulmonary arterial hypertension (PAH) is a chronic and progressive disorder characterized by vascular remodeling of the small pulmonary arteries, resulting in elevated pulmonary vascular resistance and ultimately, right ventricular failure. Expanded understanding of PAH pathophysiology as it pertains to the nitric oxide (NO), prostacyclin (prostaglandin I2) (PGI2) and endothelin-1 pathways has led to recent advancements in targeted drug development and substantial improvements in morbidity and mortality. There are currently several classes of drugs available to target these pathways including phosphodiesterase-5 inhibitors (PDE5i), soluble guanylate cyclase (sGC) stimulators, prostacyclin class agents and endothelin receptor antagonists (ERAs). Combination therapy in PAH, either upfront or sequentially, has become a widely adopted treatment strategy, allowing for simultaneous targeting of more than one of these signaling pathways implicated in disease progression. Much of the current treatment landscape has focused on initial combination therapy with ambrisentan and tadalafil, an ERA and PDE5I respectively, following results of the AMBITION study demonstrating combination to be superior to either agent alone as upfront therapy. Consequently, clinicians often consider combination therapy with other drugs and drug classes, as deemed clinically appropriate, for patients with PAH. An alternative regimen that targets the NO and PGI2 pathways has been adopted by some clinicians as an effective and sometimes preferred therapeutic combination for PAH. Although there is a paucity of prospective data, preclinical data and results from secondary data analysis of clinical studies targeting these pathways may provide novel insights into this alternative combination as a reasonable, and sometimes preferred, alternative approach to combination therapy in PAH. This review of preclinical and clinical data will discuss the current understanding of combination therapy that simultaneously targets the NO and PGI2 signaling pathways, highlighting the clinical advantages and theoretical biochemical interplay of these agents.
Keywords: pulmonary arterial hypertension, combination PAH drug therapy, nitric oxide pathway, prostacyclin pathway
Introduction
Pulmonary arterial hypertension (PAH) is a subgroup of pulmonary hypertension that results in elevated pulmonary vascular resistance and eventually, right-side ventricular failure and death. 1 Symptoms of PAH include progressive dyspnea on exertion, fatigue, and exertional chest pain. 2 Although relatively rare, with an estimated prevalence in the United States of 10.6 cases per 1 million adults, PAH is a devastating disease with a median survival of approximately 7 years for patients receiving treatment. 3,4
The management of PAH has advanced in recent years due to an expanded understanding of PAH pathophysiology, specifically as it pertains to 3 biochemical pathways involved in pulmonary vascular homeostasis: the nitric oxide (NO), prostacyclin (prostaglandin I2; PGI2) and endothelin-1 pathways. These advancements have led to the development of several classes of drugs that target these pathways, which include phosphodiesterase-5 inhibitors (PDE5i), soluble guanylate cyclase (sGC) stimulators, prostacyclin class agents, and endothelin receptor antagonists (ERAs). Drug choice and route of administration are dependent on a variety of factors including side effect profile, clinical status of the patient, patient preference and lifestyle, as well as physician experience. Agents in each of these drug classes have demonstrated clinical benefit in their respective clinical trials, leading to FDA approval for treating PAH. 5 Furthermore, the AMBITION trial demonstrated initial combination therapy with ambrisentan and tadalafil, an ERA and PDE5I, respectively, to be superior to monotherapy with either agent alone, leading to increased focus on combination therapy in more recent drug trials. 6
Despite this combination therapy being incorporated into the European Respiratory Society, and CHEST guidelines as a recommended initial treatment for patients with World Health Organization functional class (WHO FC) II or III disease, not all patients with PAH may be appropriate for upfront initiation with these agents. 7,8 Additionally, results from the COMPASS-2 study make it unclear whether similar clinical benefit observed in the AMBITION trial could be achieved using other drugs in the ERA and PDE5i therapeutic classes, such as bosentan and sildenafil, respectively, which did not improve the time to first morbidity or mortality event in this trial. 9 Alternatively, it is possible that combination therapy with agents outside of the ERA and PDEi drug classes, when used early in disease progression, may yield similar or superior outcomes to the results demonstrated in the AMBITION study.
One specific combination for consideration is targeting of the NO and PGI2 pathways. Although frequently used in clinical practice, there is a paucity of prospective, controlled data showing the efficacy of this drug combination. The drug classes that target the NO pathway are PDE5i and sGC stimulators, while prostacyclin mimetics and IP receptor agonists target the PGI2 signaling pathway (Table 1). Here, we aim to describe the preclinical data, as well as results from secondary data analyses of clinical studies targeting the NO and PGI2 pathways in order to provide a narrative review and novel insights into this alternative combination as a reasonable and sometimes preferred approach to combination therapy in PAH.
Table 1.
Classes and Route of Administration of FDA Approved Agents Used in the Treatment of Pulmonary Arterial Hypertension.
| ERA | PDE5i | sGC Stimulator | Prostacyclin Mimetic | IP Receptor Agonist |
|---|---|---|---|---|
| Ambrisentan (oral) Bosentan (oral) Macitentan (oral) |
Sildenafil (oral) Tadalafil (oral) |
Riociguat (oral) | Epoprostenol (IV) Iloprost (Inhalation) Treprostinil (SC, IV, inhaled, oral) |
Selexipag (oral) |
Abbreviations: ERA, endothelin receptor antagonist; IP, prostacyclin receptor; IV, intravenous; PDE5i, phosphodiesterase-5 inhibitor; SC, subcutaneous; sGC, soluble guanylate cyclase.
Methods
In order to comprehensively review the evidence of the combination of NO and PGI2 pathways in the treatment of PAH, we searched electronic databases, including PubMed, the Cochrane Library, Web of Science and MEDLINE. Only English language publications were included. All pertinent in vitro, animal and human studies, including original research, case reports, case series, meta-analyses and systematic reviews from the inception of each database to January 2020 were included. All authors have expertise in the clinical and preclinical subject matter and reviewed the papers identified in the search and came to a consensus on those publications to be included in the final manuscript.
This review of clinical and preclinical data will discuss the current understanding of combination therapy that simultaneously targets the NO and PGI2 signaling pathways, highlighting the clinical advantages and potential biochemical synergy of these agents targeting these 2 pathways.
The Prostacyclin (PGI2) Pathway in PAH
Prostacyclin (PGI2) is synthesized by vascular endothelial cells and is a natural ligand for the IP receptor, which is expressed throughout the body, including the heart, lungs, pulmonary arteries, peripheral arteries, nerves, and the gastrointestinal system. 10,11 Prostacyclin is part of the larger prostanoid family of signaling molecules, including prostaglandins (PGD2, PGE2, PGI2 and PGF2 α) and thromboxane A2 (TXA2). 12,13 Prostacyclins produce their effects by activating corresponding prostacyclin receptors throughout the body, which have been identified as the IP, EP1, EP2, EP3, EP4, DP1, FP and TP receptors. 14 IP, EP2, EP4, and DP1 receptor activation leads to increases in cyclic adenosine 3’,5’ monophosphate (cAMP) which stimulates pulmonary artery vasodilation, inhibits vascular smooth muscle cell proliferation, and inhibits platelet aggregation. 15,16 Contrarily, TXA2 is a potent pulmonary vasoconstrictor and an activator of platelet aggregation. 17 Prostacyclin synthase and thus PGI2 levels are decreased in the pulmonary arteries of patients with PAH. 18 It is this imbalance between the production of the vasoactive mediators (PGI2 and NO) and vasoconstrictor (endothelin-1 and TXA2) that may play a role in the pulmonary vascular remodeling seen in PAH. Endothelin-1 is produced and secreted into the circulation by endothelial cells of the pulmonary vessels following transformation from its precursor form big ET-1 by the ET-converting enzyme (ECE-1).
Early available prostacyclin therapies included intravenous epoprostenol and inhaled iloprost. Treprostinil, a synthetic prostacyclin mimetic, was developed to address the short in vivo half-life of both epoprostenol (∼3 min) and iloprost (∼20-30 min). 19 Several formulations of treprostinil now exist, and the drug can be administered SC, orally, IV, and by inhalation. Prostacyclin class agents are typically used as add-on therapy or reserved for patients with severe disease and are assumed to have a mechanism of action similar to that of endogenous PGI2, although this is a topic of active research. 12,20
Preclinical evidence supports that prostacyclin pathway agents have different binding affinities for various other prostanoid receptors, and these differences are thought to affect both the potency of the molecule as well as adverse events experienced by patients. 14 Like endogenous prostacyclin, all stable prostacyclin mimetics bind to the IP receptor. Epoprostenol, which is a synthetic version of endogenous PGI2, has poor selectivity of prostanoid receptors and binds to and activates EP1, EP3, and TP receptors. Iloprost is approximately equipotent at activating both the IP and EP1 receptors and has significant activity at the EP4 receptor. By contrast, treprostinil has a high affinity for the DP1 and EP2 receptor while having a 100-fold lower binding affinity for EP1. Selexipag differs from prostacyclin mimetics in that it is a pro-drug that is rapidly hydrolyzed in vivo to its active metabolite, which has a high affinity for the IP receptor alone. 21 The clinical ramifications of the different receptor affinities between prostacyclin class agents is an area of ongoing research.
The Nitric Oxide Pathway in PAH
Nitric oxide (NO), an endogenous vasodilator, is produced when arginine is converted by NO synthase in pulmonary vascular endothelial cells. NO catalyzes the formation of cyclic guanylate monophosphate (cGMP) by guanylate cyclase when it reaches vascular smooth muscle. 22,23 cGMP then stimulates smooth muscle relaxation and regulates cellular proliferation and inflammation in the vessel wall. 24 In PAH, a deficiency of NO synthase in pulmonary vascular endothelial cells results in reduced NO production which affects vascular tone and other cellular activities in the vessel wall. 25
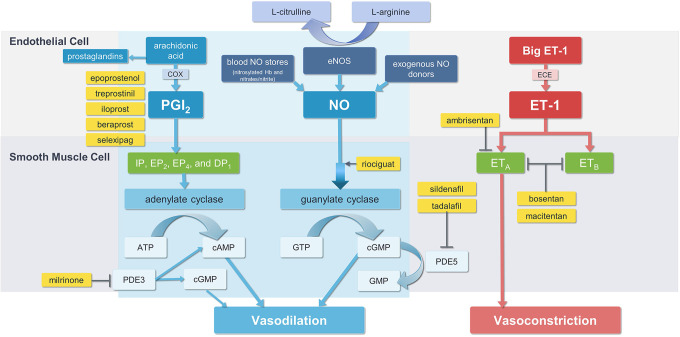
Since the 1990s, several agents targeting the NO pathway have been approved for the treatment of PAH including PDE5is (tadalafil, sildenafil) and sGC stimulators (riociguat). PDE5is are vasodilators that enhance and prolong cGMP action by selectively inhibiting the cGMP specific PDE5 isoenzyme that catalyzes the breakdown of cGMP. 26 Riociguat acts both in synergy with endogenous NO by sensitizing sGC to endogenous NO through stabilization of NO-sGC binding, and by directly stimulating sGC to produce cGMP independently of NO availability. 27 Figure 1 illustrates the NO pathway, along with the other known pathways targeted in the treatment of pulmonary arterial hypertension. 28
Figure 1.
Pathways targeted in the treatment of pulmonary arterial hypertension. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; COX, cyclooxygenase; ECE, endothelin-converting enzyme; cGMP, cyclic guanosine monophosphate; DP1, prostaglandin D2 receptor 1; eNOS, endothelial nitric oxide synthase; EP2, prostaglandin E2 receptor; EP4, prostaglandin E4 receptor; ET-1, endothelin-1; ETA, endothelin type A receptor; ETB, endothelin type B receptor; IP, prostaglandin I2 receptor; GTP guanosine triphosphate; NO, nitric oxide; PDE3, phosphodiesterase type 3; PDE5, phosphodiesterase type 5; PGI2, prostaglandin I2. Adapted from Nakamura 2019 https://www.mdpi.com/1422-0067/20/23/5885/htm. 28
Preclinical Evidence
Knebel and colleagues studied the cooperative mechanism between treprostinil and PDE5i in a human erythrocyte model which may provide rationale for their co-administration in PAH. 29 They postulated that because cAMP levels are regulated by phosphodiesterase 3 (PDE3), and PDE3 is inhibited by cGMP, increases in cGMP caused by selective PDE5 inhibition could increase cAMP levels. In their experiments, they observed that pre-treatment of healthy human erythrocytes with 2 different PDE5is, zaprinast and tadalafil, augmented cAMP-mediated ATP release stimulated by treprostinil administration. Similar results were observed in a subsequent study using erythrocytes of patients with PAH. 30 Treprostinil stimulated increases in cAMP and ATP release from erythrocytes of patients with PAH and pre-treatment with zaprinast and tadalafil led to greater increases in ATP release.
These observations could be extended to the cardiac myocyte, where the PDE3 enzyme is highly expressed and where inhibition of PDE3 enzyme with the PDE3 inhibitor, milrinone, is often used to produce a cardiac inotropic effect. 31 Thus, it is possible (if not likely) that the combination of an agent that increases cGMP, such as a PDE5i or sGC stimulator, and an agent that increases cAMP (i.e., PGI2) may have a synergistic effect in the vascular smooth muscle, as well as in the cardiac myocyte, via an interaction with the PDE3 enzyme.
There is also evidence for drugs targeting the NO and prostacyclin pathway to have synergistic effects on reducing cell proliferation, which is a key component of PAH disease pathology. 32,33 Patel and colleagues performed studies on the effects of various PAH drugs on the proliferation of pulmonary artery smooth muscle cells (PASMCs) derived from PAH patients. PASMCs exhibited increased proliferation when incubated with endothelin-1. Notably, all PAH drugs tested in these series of experiments reduced this proliferation, albeit to varying degrees. Interestingly, the antiproliferative effects of treprostinil were increased ≥10 fold when combined with the NO pathway drugs tested by the authors (riociguat and tadalafil) but decreased ≥3-fold when treprostinil was combined with ERAs. 32 The clinical implications of this study are not yet clear, but these data suggest that synergism by drugs targeting the NO and PGI2 pathways enhance cellular antiproliferation.
Clinical Evidence
There are numerous studies and published work in PAH patients that have demonstrated that the use of NO pathway drugs in combination with prostacyclin pathway agents results in therapeutic benefit for patients with PAH (Table 2). Below, we hig hlight a few studies where this combination of therapies has resulted in notable improvements in clinical markers of interest.
Table 2.
Studies and Series Evaluating Combination Therapy of Nitric Oxide and Prostacyclin Pathway Agents in PAH.
| Study name/Lead author | Number of patients | NO pathway agent | PGI2 pathway agent | Primary study endpoint | Result |
|---|---|---|---|---|---|
| PACES 34 | 267 | Sildenafil | Epoprostenol IV | Change from baseline to week 16 in 6MWD | Placebo-adjusted increase of 28.8 m (95% CI, 13.9 to 43.8 meters) in 6MWD-minute walk distance in sildenafil group. |
| PATENT-1 27 | 443 | Riociguat | Oral Prostanoid | Change from baseline to week 12 in 6MWD | At week 12, 6MWD increased by a mean of 30 m in 2.5 mg—maximum group and decreased by mean of 6 m in placebo. Riociguat improved 6MWD in patients receiving no other treatment; ERA + riociguat resulted in 23 m mean increase in 6MWD; oral prostanoid + riociguat resulted in 56 m mean increase. |
| TRIUMPH 9 | 235 | Sildenafil | Inhaled Treprostinil | Peak 6MWD at 12 weeks. | Change from baseline in peak 6MWD was 19 m at week 6 (P <0.0001) and 20 m at week 12 (P = 0.0004). Change from baseline in trough 6MWD at week 12 was 14 m (P = 0.0066). |
| GRIPHON 35 | 1156 | PDE5 inhibitor | Selexipag | Composite of death from any cause or a complication related to PAH | A primary end-point event occurred in 397 patients, 41.6% of those in the placebo group and 27.0% of those in the selexipag group. There were no differences when evaluating subgroups based on PAH therapy at baseline (P value for interaction 0.9518). |
| FREEDOM-C 36 | 39 | PDE5 inhibitor | Oral Treprostinil | Change from baseline to week 16 in 6MWD | Patients receiving PDE inhibitor background therapy experienced a 6MWD improvement of 17.0 m compared to 5.0 m for those on background ERA, or 10.0 m for those on ERA and a PDE5 inhibitor. |
| FREEDOM-C2 37 | 310 | PDE5 inhibitor | Oral Treprostinil | Change from baseline to week 16 in 6MWD | Patients receiving PDE5 inhibitor background therapy experienced almost twice 6MWD treatment effect (15.0 m) as those receiving background ERA (7.7 m). |
| FREEDOM-EV 38 | 690 | PDE5 inhibitor | Oral Treprostinil | Time to first clinical worsening event | Clinical worsening occurred in 26% of the oral treprostinil group compared with 36% of placebo participants. No significant difference in the time to clinical worsening when subgroups analyzed based on background therapy |
| Wilkens 2001 39 | 5 | Sildenafil | Inhaled Iloprost | Change in mean PAP and PVR | Combination of sildenafil plus iloprost lowered mean PAP significantly more than iloprost alone (13.8 ± 1.4 versus 9.4 ± 1.3 mm Hg; P<0.009). Increase in cardiac output was observed without any significant changes in heart rate or systemic arterial pressure. |
| Ruiz 2006 40 | 20 | Sildenafil | Epoprostenol IV Treprostinil SC Aerosolized iloprost |
NYHA FC, exercise capacity (6MWD), signs of right ventricular failure, and echocardiography | Significant and persistent improvement in functional class and exercise capacity (6MWD) increase by 79 m at 1 year and 105 m at 2 years; sustained clinical stabilization after 1 and 2 years of adjunct sildenafil. |
| Ghofrani 2002 41 | 30 | Sildenafil | Inhaled Iloprost | Systemic and pulmonary arterial pressure, pulmonary arterial
occlusion pressure, cardiac output, central venous pressure,
peripheral arterial oxygen saturation, and arterial
and mixed venous blood gases were measured |
Patients who received 50 mg of sildenafil plus iloprost, maximum change in pulmonary vasodilatory potency was −44.2% (95% CI, −49.5% to −38.8%), compared with −14.1% (CI, −19.1% to −9.2%) in response to NO. With 50 mg sildenafil plus iloprost, area under the curve for reduction in PVR surpassed that of administration of 50 mg of sildenafil alone and iloprost alone combined, vasodilatory effect lasted longer than 3 hours, and systemic arterial pressure and arterial oxygenation were maintained. |
| Ghofrani 2003 42 | 14 | Sildenafil | Inhaled Iloprost | Exercise capacity (6MWD), pulmonary hemodynamics, WHO FC | At week 12, deterioration in 6MWD (decline to 256 m over 18 months from initial improvement to 305 m with iloprost treatment) was reversed, increasing to 346 m, sustained up to 12 months. Distribution of functional class improved, and favorable change in all hemodynamic variables. |
| Voswinckel 2008 43 | 28 | Inhaled NO; Sildenafil | Inhaled Treprostinil |
PVR, PAP, CO | Inhaled NO reduced PVR to 87.3% ± 5.1% of baseline, reduced
mean PAP to 89.7% ± 3.5% and increased CO to 102.4% ± 2.9%.
Sildenafil reduced PVR to 80.1% ± 5.0%, mPAP to 86.5% ± 2.9%
and increased CO to 103.8% ± 3.2%. Treprostinil, inhaled 1 h
after sildenafil, reduced PVR to 66.3% ± 3.8%, mPAP to 77.8%
± 3.3%, and increased CO to 107.1% ±
3.3%. Treprostinil after sildenafil, induced additional pulmonary vasodilatation, and the PVR reduction of the combination was significantly stronger than the effect of NO. |
| Antoniou 2013 44 | 8 | Inhaled NO | Inhaled Iloprost | PVR, mPAP | Significant improvement in right ventricular
function—tricuspid annular velocity increased by 1.7 cm/s
and TAPSE augmented by 1.8 mm Significant decreases in PVR (decrease in PVR of 169 dynes·s·cm-5 and mPAP decreased by 10 mmHg |
| Bendayan 2008 45 | 4 | Tadalafil | Treprostinil SC (3) Epoprostenol (1) |
Exercise capacity (6MWD) Change in functional class |
After 3 months of therapy, all patients improved clinically
and showed an increase in mean 6MWD from 214 to 272
m Three patients improved functional class from IV to III |
Abbreviations: PAH, Pulmonary arterial hypertension; PVR, Pulmonary vascular resistance; PAP, Pulmonary arterial pressure; 6MWD, 6-minute walk distance; WHO FC, World Health Organization functional class; NYHA FC, New York Heart Association functional class; CO, Cardiac output; NO, nitric oxide; PDE5, phosphodiesterase-5.
The first study demonstrating the potential advantages of adding sildenafil to potentiate and prolong the vasodilatory properties of prostanoids was a small, randomized, open-label trial of 30 patients with severe pulmonary hypertension (mPAP >40 mm Hg) that evaluated the safety and effectiveness of oral sildenafil, alone and in combination with inhaled iloprost in patients with PAH. 41 Oral administration of sildenafil 50 mg plus inhaled iloprost produced a maximum change in PVR that was nearly twice the vasodilatory response of either single agent alone. Importantly, the authors noted a decrease in the ratio of pulmonary to systemic vascular resistance in the combination-therapy groups, indicative of preferential pulmonary vasodilatation over systemic vasodilatation without a worsening of arterial oxygenation. Although this study was limited by a small sample size and a lack of long-term clinical outcomes, it was one of the first studies to suggest that sildenafil in combination with an inhaled PGI2 had additive hemodynamic effects. An additional analysis of reduction in PVR as measured by area under the curve (AUC) signaled that patients who received both oral sildenafil and inhaled iloprost had an AUC for PVR reduction that significantly exceeded the summed AUCs of patients who received the 2 agents alone as monotherapy (P < 0.001, ANOVA). This unique AUC analysis of PVR provides evidence that the complementary effects of these 2 therapies on hemodynamics may be more than the sum of the individual treatments.
More recently, the FDA approved riociguat, a sGC stimulator targeting the NO pathway, for the treatment of PAH. Riociguat makes sGC more sensitive to endogenous NO by stabilizing NO-sGC binding, and riociguat also directly stimulates sGC to produce cGMP independently of NO availability. 27 In the PATENT-1 study, 443 patients with PAH were randomized to either riociguat or placebo. 27 After 12 weeks, patients receiving riociguat had a significant improvement in 6MWD with a +36.0 m placebo-corrected change (P < 0.001). In a secondary analysis, authors assessed the effects of riociguat by subgroups based on background PAH therapy and found that riociguat improved 6MWD by 101.0 m (95% CI 27-176; n = 27) in patients pretreated with non-IV prostanoids compared to 26.0 m in patients on an ERA and 38.0 m in patients who were treatment-naïve. 46 In addition, improvement in mean N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were more pronounced in the treatment-naïve (−443 ± 1233 pg/mL) and prostanoid subgroup (−203 ± 872 pg/mL) than in the ERA subgroup (+90 ± 2212 pg/mL).
In PATENT-1, the apparent increased treatment effect in patients pretreated with prostanoids compared to patients receiving no other treatment for PAH was somewhat unexpected. The magnitude of response in treatment naïve patients is usually higher than the response among patients already on background PAH therapy. However, the 6MWD improvement in the prostanoid pretreated subgroup was nearly 3-fold that of the treatment-naïve population. Although only a small subgroup (6%, N = 28) of PATENT-1 patients were pretreated with prostanoids, this secondary data analysis adds to the growing body of evidence that there might be an enhanced clinical benefit with combination therapy targeting the NO and PGI2 pathways. It is also notable that the use of prostanoids in combination with riociguat was not associated with an increase in the incidence of adverse events. This may be due to the favorable adverse effect profile of inhaled iloprost, the most commonly used prostanoid in PATENT-1.
A number of earlier studies confirm the findings found in PATENT-1. Voswinkel and colleagues investigated the safety and hemodynamic effects of combination oral sildenafil and inhaled treprostinil in patients with PAH (n = 28), in patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH; n = 17) and patients with pulmonary fibrosis associated pulmonary hypertension (n = 5). 43 The combination induced additive, acute pulmonary selective vasodilatation without significant side effects. Patients were treated sequentially with inhaled NO (20 ppm), sildenafil (50 mg) and inhaled treprostinil (15 mg or 30 mg) during right heart catheterization. They found that treprostinil inhalation after sildenafil administration induced additional pulmonary vasodilatation and that the PVR reduction of the combination was approximately double the effect of NO alone. The investigators suggested that the observed additive effects clinically demonstrate the postulated benefits of parallel activation of the cAMP and cGMP pathways.
The results of combination therapy using PDE5i and treprostinil in the registration trials for oral treprostinil also support a cooperative effect between the NO and PGI2 pathways. 37,36 The investigators noted more profound improvements in 6MWD in patients who received oral treprostinil on top of background PDE5i therapy as compared to background therapy of an ERA alone or ERA with a PDE5i. In FREEDOM-C, patients receiving background PDE5i therapy had a 6MWD improvement of 17.0 m compared to 5.0 m in patients on an ERA and 10.0 m in patients on an ERA and a PDE5i. 36 In FREEDOM-C2, patients receiving oral treprostinil with PDE5i background therapy had a 6MWD improvement of 15.0 m compared to 7.7 m in those receiving background ERA therapy and 4.0 m for patients receiving both ERA and PDE5i background therapy. 37
However, not all studies support this interplay between agents targeting the NO and prostacyclin pathways. The TRIUMPH-1 study assessed the efficacy and safety of inhaled treprostinil in 235 patients with PAH on background bosentan or sildenafil. 9 The authors concluded that for those patients that remained symptomatic while on bosentan or sildenafil, the addition of inhaled treprostinil increased exercise capacity, improved quality of life, and was safe and well-tolerated. Patients in the sildenafil subgroup, however, did not achieve a significant improvement in 6WMD (9.0 m) whereas patients on background bosentan experienced a significant 6MWD improvement of 22.0 m at week 12. Based on earlier reports of the potential synergy between the cAMP and cGMP pathways with sildenafil and prostanoids, the 6MWD result was unexpected and investigators speculated that it may have been a function of sample size, a smaller number of patients taking sildenafil, as well as the variable sildenafil dose allowed at baseline (any prescribed dose ≥20 mg 3 times daily).
The FREEDOM-EV study randomized 690 PAH patients, stratified by background monotherapy of either a PDE5i/sGC stimulator or ERA, to receive oral treprostinil or placebo. Overall, the addition of oral treprostinil to monotherapy treatment significantly reduced the time to clinical worsening. 38 However, investigators did not find any significant difference in this primary endpoint when evaluating subgroups based on background therapy, likely due to the study not being powered sufficiently to detect a difference (P value for interaction 0.904). 38
Similar results were observed in the GRIPHON trial, the pivotal study for selexipag, which was an event-driven trial assessing the effect of selexipag vs. placebo on a composite endpoint of death from any cause or a complication related to PAH. 35 Selexipag was found to significantly lower the risk of this composite endpoint but there were no differences when evaluating subgroups based on PAH therapy at baseline (P value for interaction 0.9518). 35
Summary and Clinical Application
As demonstrated in the AMBITION trial, upfront combination therapy with ambrisentan and tadalafil, an ERA and PDE5i respectively, is superior to either monotherapy alone in the treatment of PAH. 6 This important trial has emphasized the importance of initial combination therapy for patients with PAH. This systematic review summarizes the evidence for combination therapy using agents targeting the NO and PGI2 pathways to assist healthcare providers who may be considering an alternative regimen for patients for whom this drug combination may be a reasonable and, in some cases, preferred treatment option.
In selecting a prostacyclin class agent to combine with a PDE5i or sGC stimulator it is important to note the potential for systemic hypotension when combining either agent, in particular riociguat, with a parenteral prostanoid. This is often addressed by simply discontinuing a patient’s other antihypertensive agents or allowing a patient to acclimate to the prostanoid; alternatively, midodrine may be prescribed to increase systemic blood pressure without increasing pulmonary pressures. As noted, inhaled prostanoid formulations appear to be best tolerated in terms of blood pressure and side effect profile. Patients often prefer the convenience of oral prostacyclin class agents but oral treprostinil and selexipag are often associated with higher incidences of gastrointestinal side effects. SC and IV prostacyclin mimetics are viewed as the gold standard treatments for PAH patients but there is a high burden placed on patients who need to wear a continuous infusion pump. Treatment decisions must be carefully weighed based on the individual patient and disease characteristics. For example, the authors have anecdotal experience that PAH associated with connective tissue disease is often more aggressive and less responsive to oral non-prostacyclin therapies therefore SC or IV prostanoid treatment may be required earlier for this subgroup. To date, no specific PAH patient subgroups have been identified as being more responsive than another to NO pathway and PGI2 combination therapy.
Titration of a prostacyclin class agent in combination with a PDE5i or sGC stimulator in patients with PAH also needs to be individualized and grounded on patient risk stratification as assessed by the European Society of Cardiology (ESC) risk assessment model. When using NO pathway agents with a prostanoid, it is not uncommon for sGC stimulator or PDE5i dosing to be maximized prior to the addition of the second agent to minimize adverse events such as hypotension and headache. Conversely, prostacyclin class agents, in particular parenteral prostanoids, are often considered for initial treatment in patients with severe PAH or rapidly progressing disease. 20 If a patient is not meeting clinical goals or exhibits clinical worsening, an NO pathway drug can then be added.
Often, clinical decisions related to the sequence of addition may be influenced by drug formulary status at a particular site, with initiation of the first drug in the combination being one that is approved and accessible. The selection of subsequent treatments may also be influenced by payor-factors. Concerning cost, AMBITION therapy using ambrisentan and tadalafil is available in generic form making them desirable from a payor perspective.
Currently, it is unclear whether the cooperative effects between NO and prostacyclin pathway agents allows for lower doses of drugs to be used without compromising efficacy. As mentioned by other researchers, this theoretical biochemical synergism may translate clinically into lower doses of prostacyclin class agents combined with a NO pathway drug. 29 This could reduce prostacyclin adverse effects, such as headache, flushing, jaw pain, nausea, and vomiting, which often limit dose escalation and may result in discontinuation of an otherwise efficacious treatment for PAH.
NO donors, in particular NO gas by inhalation, has been shown to be beneficial to patients with pulmonary hypertension, particularly in pediatric patients. 47,48 Unfortunately, delivery of inhaled NO gas is challenging from a technical perspective and has thus far been limited to use in the hospital setting. Several studies are underway investigating the use of portable NO delivery systems in patients with PAH and this could represent an additional avenue to target the NO pathway in a more practical manner. 49,50 Oral nitrates and nitrites have been used with some success to treat pulmonary hypertension in animal models and are also a theoretical modality for enhancing the NO pathway. 51,52 Molsidomine, an NO prodrug administered orally, has been shown to reduce pulmonary pressure in patients with chronic obstructive lung disease as well as patients with pulmonary hypertension secondary to Takayasu’s arteritis. 53,54 L-arginine is a nitrogen donor for the synthetic pathway of nitric oxide and has been used with success in patients with pulmonary hypertension. 55,56 Further research on these latter 2 compounds is warranted to investigate their therapeutics benefits in combination with prostacyclin agents.
We acknowledge the potential limitations of a narrative review, such that despite search efforts to identify all relevant studies and previous publications, some may have not been included, as well, the restriction to English language literature may have also been a limitation of the work.
The pathophysiology of PAH is complex and a thorough understanding of mechanisms of action of different agents is necessary to evaluate their use in combination therapy. Prospective and controlled trials are needed to confirm and compare the efficacy of different modes of combination therapy, and treatment decisions need to be individualized to each patient’s clinical features and preferences. Although there are clinical nuances to combination therapy and managing side-effect profiles, the clinical and preclinical data summarized here provide a strong rationale for the use of combination therapy simultaneously targeting the NO and PGI2 signaling pathways to treat patients with PAH.
Acknowledgment
We thank April Ingram and Julie Ulloa (Upstart Medical Communications) for their expertise in manuscript preparation.
Footnotes
Author Contributions: SM, MB, AN, ES, and HC collected data and conceived of the manuscript. All authors made significant contributions to the drafing of the manuscript. SM, GK, HO, and HC provided expert input and offered critical feedback. MB and ES coordinated the contributions of all authors to this paper. All authors were responsible for critically revising the paper. All authors approved the final version of this paper for submission.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SM, GK, HO, and HC declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. MB, AN, and ES are employees of United Therapeutics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by United Therapeutics Corporation.
ORCID iDs: Stacy Mandras  https://orcid.org/0000-0003-4700-4749
https://orcid.org/0000-0003-4700-4749
Eric Shen  https://orcid.org/0000-0001-5621-6570
https://orcid.org/0000-0001-5621-6570
References
- 1. Montani D, Günther S, Dorfmüller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis. 2013;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levinson AT, Klinger JR. Combination therapy for the treatment of pulmonary arterial hypertension. Ther Adv Respir Dis. 2011;5(6):419–e430. [DOI] [PubMed] [Google Scholar]
- 3. McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21(123):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25 suppl):D51–D59. [DOI] [PubMed] [Google Scholar]
- 5. Sitbon O, Gaine S. Beyond a single pathway: combination therapy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25(142):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galiè N, Barberà JA, Frost AE, et al. Initial use of Ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. [DOI] [PubMed] [Google Scholar]
- 7. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 8. Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the chest guideline and expert panel report. Chest. 2019;155(3):565–586. [DOI] [PubMed] [Google Scholar]
- 9. McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55(18):1915–1922. [DOI] [PubMed] [Google Scholar]
- 10. Norel X. Prostanoid receptors in the human vascular wall. Sci World J. 2007;7:1359–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Narumiya S. Physiology and pathophysiology of prostanoid receptors. Proc Jpn Acad Ser B Phys Biol Sci. 2007;83(9-10):296–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clapp LH, Abu-Hanna JHJ, Patel JA. Diverse pharmacology of prostacyclin mimetics: implications for pulmonary hypertension. In: Nakanishi T, Baldwin H, Fineman J, Yamagishi H, eds. Molecular Mechanism of Congenital Heart Disease and Pulmonary Hypertension. Springer; 2020. [Google Scholar]
- 13. Lang IM, Gaine SP. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respir Rev. 2015;24(138):630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clapp LH, Gurung R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015;120:56–71. [DOI] [PubMed] [Google Scholar]
- 15. Mubarak KK. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir Med. 2010;104(1):9–21. [DOI] [PubMed] [Google Scholar]
- 16. Whittle BJ, Silverstein AM, Mottola DM, Clapp LH. Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: treprostinil is a potent DP1 and EP2 agonist. Biochem Pharmacol. 2012;84(1):68–75. [DOI] [PubMed] [Google Scholar]
- 17. Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327(2):70–75. [DOI] [PubMed] [Google Scholar]
- 18. Tuder RM, Cool CD, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6):1925–1932. [DOI] [PubMed] [Google Scholar]
- 19. Deshpande SP, Mazzeffi MA, Strauss E, Hollis A, Tanaka KA. Prostacyclins in cardiac surgery: coming of age. Semin Cardiothorac Vasc Anesth. 2018;22(3):306–323. [DOI] [PubMed] [Google Scholar]
- 20. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest. 2014;146(2):449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuwano K, Hashino A, Asaki T, et al. 2-[4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy]-N-(methylsulfonyl)acetamide (NS-304), an orally available and long-acting prostacyclin receptor agonist prodrug. J Pharmacol Exp Ther. 2007;322(3):1181–1188. [DOI] [PubMed] [Google Scholar]
- 22. Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411(2-3):334–350. [DOI] [PubMed] [Google Scholar]
- 23. Cary SP, Winger JA, Marletta MA. Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP. Proc Natl Acad Sci U S A. 2005;102(37):13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klinger JR. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin Chest Med. 2007;28(1):143–167, ix. [DOI] [PubMed] [Google Scholar]
- 25. Swisher JW, Elliott D. Combination therapy with riociquat and inhaled treprostinil in inoperable and progressive chronic thromboembolic pulmonary hypertension. Respir Med Case Rep. 2016;20:45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogdan M, Humbert M, Francoual J, et al. Urinary cGMP concentrations in severe primary pulmonary hypertension. Thorax. 1998;53(12):1059–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura K, Akagi S, Ejiri K, et al. Current treatment strategies and nanoparticle-mediated drug delivery systems for pulmonary arterial hypertension. Int J Mol Sci. 2019;20(23):5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knebel SM, Elrick MM, Bowles EA, et al. Synergistic effects of prostacyclin analogs and phosphodiesterase inhibitors on cyclic 3’,5’ monophosphate accumulation and adenosine 3’,5’ triphosphate release from human erythrocytes. Exp Biol Med. 2013;238(9);1069–1074. [DOI] [PubMed] [Google Scholar]
- 30. Bowles EA, Moody GN, Yeragunta Y, Stephenson AH, Ellsworth ML, Sprague RS. Phosphodiesterase 5 inhibitors augment UT-15C-stimulated ATP release from erythrocytes of humans with pulmonary arterial hypertension. Exp Biol Med (Maywood). 2015;240(1):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Movsesian M, Ahmad F, Hirsch E. Functions of PDE3 isoforms in cardiac muscle. J Cardiovasc Dev Dis. 2018;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel J, Abraham D, Nelson A, Silverstein A, Clapp L. Comparison of current therapies against endothelin-induced growth in pulmonary smooth arterial muscle cells (PASMCs) derived from patients with pulmonary arterial hypertension. Eur Respir J. 2014;44(suppl 58):P2355. [Google Scholar]
- 33. Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):13S–24S. [DOI] [PubMed] [Google Scholar]
- 34. Simonneau G, Rubin LJ, Galiè N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149(8):521–530. [DOI] [PubMed] [Google Scholar]
- 35. Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373(26):2522–2533 (and Supplemental Appendix, Figure S5). [DOI] [PubMed] [Google Scholar]
- 36. Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest. 2012;142(6):1383–1390. [DOI] [PubMed] [Google Scholar]
- 37. Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144(3):952–958. [DOI] [PubMed] [Google Scholar]
- 38. White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. FREEDOM-EV investigators. Combination therapy with oral treprostinil for pulmonary arterial hypertension: a double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2020;201(6):707–717 (and Supplemental Appendix, Figure E2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkens H, Guth A, König J, et al. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation. 2001;104(11):1218–1222. [DOI] [PubMed] [Google Scholar]
- 40. Ruiz MJ, Escribano P, Delgado JF, et al. Efficacy of sildenafil as a rescue therapy for patients with severe pulmonary arterial hypertension and given long-term treatment with prostanoids: 2-year experience. J Heart Lung Transplant. 2006;25(11):1353e7–1357. [DOI] [PubMed] [Google Scholar]
- 41. Ghofrani HA, Wiedemann R, Rose F, et al. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med. 2002;136(7):515–522. [DOI] [PubMed] [Google Scholar]
- 42. Ghofrani HA, Rose F, Schermuly RT, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42(1):158–164. [DOI] [PubMed] [Google Scholar]
- 43. Voswinckel R, Reichenberger F, Enke B, et al. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm Pharmacol Ther. 2008;21(5):824–832. [DOI] [PubMed] [Google Scholar]
- 44. Antoniou T, Koletsis EN, Prokakis C, et al. Hemodynamic effects of combination therapy with inhaled nitric oxide and iloprost in patients with pulmonary hypertension and right ventricular dysfunction after high-risk cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(3):459–466. [DOI] [PubMed] [Google Scholar]
- 45. Bendayan D, Shitrit D, Kramer MR. Combination therapy with prostacyclin and tadalafil for severe pulmonary arterial hypertension: a pilot study. Respirology. 2008;13(6):916e8. [DOI] [PubMed] [Google Scholar]
- 46. Humbert M, Galiè N, Ghofrani HA, et al. Efficacy of Riociguat In Pretreated Versus Treatment-Naive Patients with Pulmonary Arterial Hypertension (PAH) In the Phase III PATENT-1 Study. ATS Annual Conference. May 2013. New York, NY. [Google Scholar]
- 47. Roberts JD, Fineman JR, Morin FC, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The inhaled nitric oxide study group. N Engl J Med. 1997;336(9):605–610. [DOI] [PubMed] [Google Scholar]
- 48. Channick RN, Newhart JW, Johnson FW, et al. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests. Chest. 1996;109(6):1545–1549. [DOI] [PubMed] [Google Scholar]
- 49. Inhaled Nitric Oxide/INOpulse DS for Pulmonary Arterial Hypertension (PAH). Clinicaltrials.gov identifier NCT01457781. Accessed February 16, 2021. https://clinicaltrials.gov/ct2/show/NCT01457781
- 50. Study in Subjects With PAH and PH Secondary to IPF Using Inhaled GeNOsyl. (PHiano). Clinicaltrials.gov identifier NCT01265888. Accessed February 16, 2021. https://clinicaltrials.gov/ct2/show/NCT01265888
- 51. Malikova E, Carlström M, Kmecova Z, et al. Effects of inorganic nitrate in a rat model of monocrotaline-induced pulmonary arterial hypertension. Basic Clin Pharmacol Toxicol. 2020;126(2):99–109. [DOI] [PubMed] [Google Scholar]
- 52. Baliga RS, Milsom AB, Ghosh SM, et al. Dietary nitrate ameliorates pulmonary hypertension: cytoprotective role for endothelial nitric oxide synthase and xanthine oxidoreductase. Circulation. 2012;125(23):2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lampert E, Tuo N, Frans A, Lonsdorfer J. Disappearance of molsidomine effects on pulmonary circulation of patients with chronic obstructive pulmonary disease after a three week treatment. Pathol Biol (Paris). 1991;39(1):29–33. [PubMed] [Google Scholar]
- 54. Lee SD, Kim DS, Shim TS, et al. Nitric oxide and molsidomine in the management of pulmonary hypertension in Takayasu’s arteritis. Chest. 2001;119(1):302–307. [DOI] [PubMed] [Google Scholar]
- 55. Nagaya N, Uematsu M, Oya H, et al. Short-term oral administration of L-arginine improves hemodynamics and exercise capacity in patients with precapillary pulmonary hypertension. Am J Respir Crit Care Med. 2001;163(4):887–891. [DOI] [PubMed] [Google Scholar]
- 56. Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with L-arginine in pulmonary hypertension. Circulation. 1995;92(6):1539–1545. [DOI] [PubMed] [Google Scholar]