Abstract
The talc industry and Food and Drug Administration (FDA) have asserted that talc has been asbestos-free since 1976 when the industry created a voluntary specification for the asbestos content of cosmetic talc. However, recent evidence reveals that cosmetic talc is not and never was asbestos-free. This narrative review examines the talc industry’s role in delaying and ultimately blocking federal regulation of cosmetic talc from the 1970s to today. We review primary source material, including corporate documents released in recent litigation and FDA documents released in response to Freedom of Information Act requests. Our results indicate that the talc industry exerted considerable influence over three key areas: regulatory proceedings at the FDA; testing methods and the manipulation of test results (including undisclosed results); and press coverage and the medical literature. The talc companies’ actions and FDA indifference have had a lasting effect on consumer health, including the regulation of talc by other government agencies.
Keywords: regulation, deregulation, asbestos, talc, cosmetics industry, FDA
Introduction
Numerous authors have sought to categorize the various methods corporations use to influence scientific research, government regulation, and public discourse in an effort to avoid regulation.1–8 These methods rely on industry connections to and influence on regulatory agencies. They often include the production, interpretation, and dissemination of industry-approved science, the manipulation of safety standards, and the creation of strategic public relations campaigns that promote uncertainty about product safety. In the United States, instances of conflicts of interest in the Food and Drug Administration (FDA) and other regulatory bodies are well documented, including a “revolving door” of employment between government organizations and industry.9,10 However, few have published accounts of the behind-the-scenes negotiations between federal agencies, industry executives, and industry scientists. Here, we provide a case study on cosmetic talc that relies on industry documents, released through litigation, and FDA documents, acquired through Freedom of Information Act (FOIA) requests. This case study examines a nearly fifty-year industry campaign to avoid government regulation of cosmetic talc. It provides insights regarding the lasting impact on public health policy in the United States of decisions made in the 1970s, a contentious decade for government regulation.
Concerns about the health effects of cosmetic talc have recently come into the public sphere due to allegations that Johnson and Johnson (J&J) baby powder contains asbestos, causing both mesothelioma and ovarian cancer. 11 J&J has since stopped sales of talc-based baby powders in North America, 12 but there is still no regulatory oversight. Representatives from several talc mining and manufacturing companies (TM&MCs) have maintained that talc has been asbestos-free since 1976. In that year, the Cosmetic, Toiletry and Fragrance Association (CTFA), which represents the personal care products industry, claimed they implemented “stringent safety and quality control measures designed to ensure the absence of asbestos fibers from consumer talc products” (p. 218). 13 As part of these measures, the CTFA developed a test method for the determination of the asbestos content of talc, known as the J4-1 method. 14 CTFA is now known as the Personal Care Products Council.
As we show, the TM&MCs have long been aware that historical and current tests for asbestos in talc reveal that talc is not and never was asbestos-free. Moreover, the claim that talc is asbestos-free has had a lasting effect on the regulation of cosmetic talc by the FDA and the National Toxicology Program (NTP), as well as on the characterization of the risks associated with the use of talc in the medical literature. 15
The 1976 J4-1 method was part of a voluntary, unenforced industry self-regulation plan, implemented after the industry pressured the FDA to renounce regulatory control. We reviewed correspondence among the FDA, TM&MCs, and research institutions around key regulatory events prior to 1976. The TM&MCs, particularly J&J (which held the majority of the talc powder market share in 197616), exerted considerable influence over:
Regulatory proceedings at the FDA;
Testing methods and the manipulation of test results (including undisclosed results); and
Press coverage and the medical literature.
These efforts led to the continued sale of cosmetic talcum powders containing asbestos and fibrous talc (which the Occupational Safety and Health Administration has regulated as asbestos 17 ). There were significant downstream consequences well into the twenty-first century for scientific practice, regulation at agencies other than FDA, and public health. While concerted action on the part of industry to hide the asbestos content of talc over the course of fifty years is apparent, our review also questions the FDA’s role in sustaining scientific uncertainty around an important public health concern.
Methods
We reviewed primary source material consisting of corporate documents uncovered in litigation and government documents released through FOIA requests. Talc-related documents were deposited in a searchable database that was accessible to researchers during Gail Ingham, et al. v. Johnson & Johnson, et al., a St. Louis lawsuit regarding the use of commercial talcum powder. This document database included records produced by numerous TM&MCs. a In addition, we reviewed talc-related laboratory records. b We initially searched for asbestos-related terms, such as the names of asbestos minerals, “fiber(s),” “fibrous,” “acicular,” and “needle(s).” We then conducted additional searches based on themes that emerged within this initial data set, focusing on terms associated with testing protocols, quality control measures, test results, and specific talc mines and mills. The authors reviewed depositions of individuals connected to talc litigation. In addition, we conducted a review of the published literature on the asbestos and talc relationship, and the relationship of talc to ovarian cancer.
We provide a narrative review of meetings, events, newspaper reports, tests, research, and decisions by FDA and industry. The review is organized chronologically, with some exceptions to facilitate understanding of context. Unless otherwise specified, the word “talc” should be read as “talc used in cosmetics” and not “cosmetic talc” which, as we show, is a marketing construct. Because there is too much to cover in a single article, we have prepared a supplementary file with thirteen sections which include additional references and details on particular events and issues. Where relevant in this text, we refer to the supplementary file and section. So that readers can easily find cited statements from source documents, we have also included page numbers in parentheses to indicate where the information can be found in the source document.
In recent litigation, the CTFA, J&J, and Luzenac have claimed that many of the documents we cite were “confidential trade secrets.” The complete set of cited documents and other public documents are available at https://repository.library.brown.edu/ under “David Egilman Papers.” Our review of documents is limited to the documents made available to us, as well as those released to the public domain.
Results
Historical Background
An association between talc and asbestos was established as early as 1898. 18 Subsequent studies noted the presence of asbestos in talc deposits and the health effects of talc exposure.18–26 However, it was not until the late 1960s that scientists reported “fibrous talc” and probably asbestos in cosmetic talc products.27,28 Around that time, the research and advocacy efforts of Dr. Irving Selikoff at the Mt. Sinai School of Medicine in New York City were calling widespread public attention to the link between asbestos and cancer. Selikoff had organized a 1964 meeting of the New York Academy of Sciences which publicized the fact that there was no known safe level of asbestos exposure. 29 In 1971, The New York Post publicized the fact that researchers at Mt. Sinai had found asbestos in talcum powder. 30
According to Rosner et al., 31 authors of The Politics of Measurement of Asbestos in Talc, in the 1970s,
Skepticism of large institutions was burgeoning, with activist ire aimed at everything from major research universities to the military to large corporations. Advocacy for the interests of the everyday consumer, particularly around health and safety concerns, was exemplified by the attorney Ralph Nader, who became the public face of a revived consumer movement that thrived from the early 1960s into the 1980s.
The advocacy efforts of Ralph Nader, Irving Selikoff, and others as well as the establishment of government institutions such as Occupational Safety and Health Administration and the National Institutes of Occupational Safety and Health helped advance public, worker, and consumer health through regulatory action. This was met by a neoliberal backlash promoting industry self-regulation, privatization, and market liberalism. Scholars of this period have explained the long-term degradation of public health in the United States as a result of neoliberal structural adjustments and destabilization of the public sector.32,33
Rosner et al. 31 provide a summary of the testing methodology debate that followed Mt. Sinai’s disclosure of asbestos in talc products. They focus on exchanges between the CTFA and FDA, relying on many of the same sources as we do. We build on their narrative, drawing attention to the integral role that J&J played in blocking regulation.
Evidence of Asbestos in Talc: Early Public and FDA Knowledge (1970–1973)
After learning of Mt. Sinai’s findings, Jerome Kretchmer of New York City Environmental Protection Agency urged the FDA to take three actions with respect to talcum powder:
Sampling all available brands;
Recommend that manufacturers revise their formulations or take asbestos-containing product off the market; and
Perform a follow-up epidemiologic study. 34
On 3 August 1971, the FDA held a meeting with city and federal officials, the cosmetic companies, and researchers. 35 At the meeting, tensions between the regulatory agencies and the TM&MCs were evident. Participants debated about methods for identifying asbestos in talc and about “ … the medical significance of asbestos and other fibers, and the mineralogy of asbestos and talc ore deposits” (p. 2). 35 After the meeting, the FDA and the CTFA began to conduct a series of tests that would form the basis for a proposed specification for talc. 36
On 16 June 1972, The New York Times (NYT) reported on asbestos found in textured ceiling paint and talcum powder in the Mt. Sinai studies, revealing that two brands tested showed from 5 to 25 percent asbestos fibers and that “after repeated inquiries from newsmen, the [EPA] released a letter [ … ] naming Landers and Johnson & Johnson as the brands.” 37 The article reported that the FDA “was still awaiting results of tests on other brands, and that the agency suspected that ‘virtually every talcum powder contains some asbestos.’” 37 The article also reported Kretchmer’s advice that the public should “stop using talcum powder until suspicions about its asbestos content were cleared up.” 37
After J&J was named in the 1972 NYT article, Dr. Wilson Nashed, J&J’s Associate Director of Research, called the FDA, the New York City Environmental Protection Agency, and Mt. Sinai to request a correction to the story. 38 Dr. Arthur M. Langer, the researcher at Mt. Sinai who had tested the talc products, expressed to Nashed that he was not consulted about his “very, very preliminary findings” but maintained that J&J products contained traces of asbestos. 38 Nashed succeeded in convincing the FDA to assure the NYT that talc was safe to use. 38 The next day, the NYT published a correction, Talc Warning is Labeled False, quoting Langer as stating that the talc samples contained only “trace” amounts of asbestos and that J&J’s is the “most pure” of the ones they had examined. 39 The article also reported that the FDA spokesman did not believe a warning was required on talc.
By 3 August 1972, the FDA received a report from Dr. Seymour Lewin, a Professor of Chemistry at New York University, whom they had hired to test various commercial talc products purchased at retail outlets in the Northeast for asbestos. 40 Lewin reported that 43 out of 102 commercial talc product samples contained tremolite and/or chrysotile asbestos. The report found 5 percent chrysotile asbestos by weight in J&J’s Shower to Shower (see Table 1). 40 Several days later, the Associate Director of Technology for the FDA, Dr. Robert Schaffner, told Nashed of J&J that “the Naderites have been pressuring him to release Dr. Lewin’s report” as public information (p. 1). 44 Nashed was adamant that J&J’s private tests showed no asbestos in their products, which was incorrect, and contended “that Dr. Lewin’s technique may be inaccurate” (p. 2) 44 (see Table 3).
Table 1.
FDA Knowledge: Studies Conducted by the FDA Regarding J&J Products.
| Year | Consultant | Tested | Method | Results |
|---|---|---|---|---|
| 1972 | Lewin, New York University40,47 | J&J Shower to Shower; Baby Powder; Medicated Powder;102 total products (not all J&J) | XRD; supplemented with optical microscopy | “The analyses show that 59 of the products have no detectable amounts of any of the asbestiform minerals (by the technique employed, proportions by weight of 1–2% or less could escape detection), 20 had small but definite percentages of tremolite, 7 had small percentages of chrysotile, 9 had small percentages of both tremolite and chrysotile, and 7 had substantial percentages of one or both of these asbestiform minerals.”47 Shower to Shower—5% chrysotile. Lewin repeat tests on J&J products found 2-5 percent chrysotile in Baby Powder and Shower to Shower, and 4 percent tremolite in Medicated Powder. |
| 1972 | Sperry Rand41 | J&J Shower to Shower (Lewin sample) | Polarized light microscopy (PLM) | Fibers with length-to-width ratios of 10-to-1 to 50-to-1; diameter less than .05 micron; “characteristic of chrysotile and not tremolite” |
| 1973 | Lewin, New York University; FDA; Pfizer42 | J&J Shower to Shower; Baby Powder; Medicated Powder;195 total products (not all J&J) | XRD; supplemented by optical microscopy | 195 products tested; 13 had both tremolite and chrysotile, 6 had chrysotile only, and 24 had tremolite only (43 positives in total). Trace tremolite in J&J Medicated Powder. |
| 1974 | FDA—Dr. Stuart43 | J&J Shower to Shower | Optical microscopy | No chrysotile; found tremolite/actinolite fibers. |
Note. XRD = X-ray diffraction; FDA = Food and Drug Administration.
Table 3.
J&J Tests Not Presented to the FDA.
| Year | Consultant | Tested | Method | Results |
|---|---|---|---|---|
| 1958 | Battelle59 | Italian Talc No. 1 for Baby Powder | Microscopic examination | Fibrous tremolite |
| 1966 | J&J internal test60 | Historic baby powder | Microscopic examination | Tremolite in domestic talc, Italian talc and fibrous talc. |
| 1971 | Langer at Mt. Sinai61–63 | Ovarian tissue samples from the Tenovus Institute for Cancer Research. 64 | Electron diffraction | Talc and chrysotile |
| 1971 | Langer at Mt. Sinai61–63 | Johnson & Johnson Baby Powder. | Electron diffraction | Talc and chrysotile |
| 1971 | J&J internal test65 | Baby Powder | Microscope and XRD | Tremolite needles, actinolite |
| 1971 | McCrone66 | Grantham Ore; Shower to Shower; and Medicated Powder (344 L baby powder) | Electron diffraction; electron microprobe | Tremolite and chrysotile in Grantham Ore; chrysotile fibers in Medicated Powder and Shower to Shower (no asbestos in 344 L baby powder) |
| 1972 | Fred Pooley, a professor in the Department of Mineral Exploitation at the University College Cardiff67 | Val Chisone Talc (source for all J&J cosmetic talc powders 1946–1968 and 1981–1982) | Various | Tremolite fibers |
| 1972 | Dr. Thomas Hutchinson, professor at the University of Minnesota68 | Lewin’s sample of Shower to Shower | TEM | 5 “unmistakably chrysotile asbestos” fibers and 3 serpentine material in “perfect chrysotile patterns” |
| 1972 | McCrone69 | 108T and 109 T baby powder talc lots (Lewin samples 133 and 134) | XRD, light microscopy, TEM, electron diffraction | 0.2%–0.5% tremolite “rods” |
| 1972 | J&J/Sperry Rand internal test41 | Shower to Shower | SEM | Chrysotile fibers |
| 1972–1973 | Lewin, unfinished study, funds withdrawn70 | Unfinished | Unfinished | J&J privately funded Lewin to study asbestos content of talc in baby powder, including funding a stipend for a graduate student, Avriam Elkies, to work on the project. Elkies testified that midway through 1973, J&J withdrew the scholarship and took all his research results. These results have never been produced. |
| 1973 | Fred Pooley, a professor in the Department of Mineral Exploitation at the University College Cardiff71 | Vermont talc (source for J&J cosmetic talc powders from 1968 to 2003 and 2010) | Preconcentration of asbestos with XRD | Tremolite .05% in Vermont talc |
Note. XRD = X-ray diffraction; TEM = transmission electron microscopy; SEM = scanning electron microscopy.
Representatives of the CTFA and its member companies disputed Lewin’s results during a meeting with the FDA on 11 August 1972. They argued that other tests had failed to detect chrysotile, and they pressured Lewin to “confirm” his results using a different method.45,46 Lewin ultimately agreed to retest the samples. He said that if no asbestos was found, “the sample will be declared ‘no detectable asbestos’ notwithstanding the [original] X-ray finding” (p. 5). 45 However, the attendees at the meeting understood that this new method, light microscopy, was “not capable of detecting fine chrysotile fibers” (p. 5). Despite these concerns, Dr. Alfred Weissler, the Acting Director of Cosmetics at the FDA, acquiesced: “I understand that some samples will be passed even though they contain such fibers, but we are willing to live with it” (p. 5). 45 J&J did not inform the FDA that other consultants had already found chrysotile in their products (see Table 3). The FDA ultimately agreed that Lewin should “confirm” the results using light microscopy 45 (Supplement Section 1).
Lewin found 2–5 percent chrysotile in his repeat analysis of Johnson’s Baby Powder and Shower to Shower, and 4 percent tremolite asbestos in J&J Medicated Powder; J&J received a copy of Lewin’s report a little over a month later. 47 By this point, another FDA test confirmed the presence of chrysotile (see Table 1). At a September 21st meeting with the FDA, Lewin, and J&J’s consultants, Nashed again challenged Lewin’s chrysotile findings, presenting evidence of the absence of chrysotile in their Italian mine and talc products. 48 As the meeting began to “deteriorate into a non-fruitful pathway,” Nashed suggested a “compromise:” that Lewin work with J&J’s consultant laboratory, McCrone Associates Inc., to “establish the best microscopy technique to be followed” (p. 4). 48 After working with McCrone labs, Lewin admitted an error in his analysis of another company’s product but did not change his opinion on J&J products. 49
J&J personnel continued to pressure the FDA to not release Lewin’s “erroneous findings.” 49 Nashed insisted that Mt. Sinai had not found chrysotile in J&J’s Shower to Shower and continued to find reasons for possible errors in Dr. Lewin’s tests. 49 In a “frank, friendly talk” with Schaffner of the FDA on 1 November 1972, Fuller of J&J “concluded that unless [Dr. Schaffner] could assure me that the [Lewin] report would not be published I was instructed to make an appointment with FDA Commissioner Edwards and make our viewpoint known to him” (p. 2). 50 Schaffner replied that he was not happy with Lewin’s report, which “would be issued only over my dead body” (p. 3) but also noted his fear of “favoritism” toward J&J were their products to be cleared. 50 However, on 22 November 1972, Schaffner told J&J that he believed Shower to Shower was “off the hook” and the report “will not be issued ‘unless I drop dead.’” 51 The FDA stood by its word to J&J. The FDA has not produced Lewin’s original “report” in response to FOIA requests; we reviewed copies present in industry files.
However, pursuant to a FOIA request, the FDA produced a second version of Lewin’s results dated 7 December 1972. 52 Results for J&J products that had previously tested positive for chrysotile were reclassified as “n.d.” or “none detected” except for a trace of tremolite in Johnson’s Medicated Powder (which Lewin had previously reported contained 4% tremolite). These findings were based on a new analysis using acid treatment, 51 which can dissolve chrysotile. 53 On 8 December, the FDA relayed these findings to J&J. 54 Apparently satisfied, J&J “no longer considered it necessary” to meet with the FDA commissioner. 54
The FDA provided a report on 31 July 1973 that included the final tabulation of Lewin’s results, results from different laboratories on the same products Lewin used (see Table 1), and tests on an additional ninety-three products. 42 The FDA reported that of the 195 samples tested, 17 were positive for chrysotile and 36 were positive for tremolite asbestos. 42 Lewin’s tremolite findings in J&J products were left out. 42 The FDA assured the Environmental Defense Fund that this final information was accurate 55 (see Supplement Section 1).
CTFA and TM&MC Private Testing (1970–1974)
In the same time frame, the TM&MCs conducted numerous tests on their own products. They appear to have cherry-picked which tests they delivered to the FDA. On the basis of the reports summarized in Table 2, which were given to the FDA, Nashed argued to the FDA that the Lewin samples did not contain chrysotile. 58 However, the companies did not provide their own test results finding tremolite and chrysotile in numerous products, including baby powder, Shower to Shower, talc ore, and ovarian tissue, as we detail in Table 3. There is evidence that J&J and at least one of its consultant laboratories also manipulated the findings of tests delivered to the FDA, as evidenced by Figures 1 and 2 (see Supplement Section 2 for full details). 57
Table 2.
FDA Knowledge: J&J Disclosed Tests on Shower to Shower as Presented to FDA and Discussed September 21st, 1972. 56
| Year | Consultant | Tested | Method | Results |
|---|---|---|---|---|
| 1972 | Fred Pooley, J&J consultant, a professor in the Department of Mineral Exploitation at the University College Cardiff56 | Val Chisone Talc (source for Shower to Shower) | Various | Reported no evidence of chrysotile; Pooley found tremolite fibers, but J&J did not report this to FDA |
| 1972 | Dr. W. T. Caneer, The Colorado School of Mines56 | Shower to Shower | Step scanning XRD | No chrysotile; LOD 1% |
| 1972 | Professor Gordon Brown, Department of Geology of Princeton University56 | Shower to Shower | Step scanning XRD | No chrysotile; LOD 1% |
| 1972 | Walter McCrone56 | Shower to Shower | “Optical staining” techniques | None detected: LOD 1% The report found 0.2-0.5% tremolite, but this report was replaced by another version that reported finding “only a few isolated crystals” (see Figure 1and 2).57 |
| 1972 | Jack Sheltz56 | Shower to Shower | Differential thermal analysis | None detected; LOD 1% |
Note. LOD = Level of Detection; FDA= Food and Drug Administration; XRD = X-ray diffraction.
Figure 1.
Writing on McCrone Associates original report to J&J.
Figure 2.
A copy of McCrone Associates initial report to J&J with handwritten edits.
J&J further promoted the myth that their own talc was from a “good source” that they “ … subject to refining with multiple washing before we obtain the baby powder grade; this together with extensive studies by world experts assures its freedom from asbestos” (p. 3). 72 However, J&J knew that “asbestos-form particles” could not be completely removed from talc ore, a fact which they told the hearing clerk at the U.S. Department of Health, Education, and Welfare, 73 and that they could not rely on the “clean mine” (asbestos-free) approach to assure the absence of asbestos in talc. 74
A Debate Over Test Methodology (1973–1976)
The Center for Science in the Public Interest and the Environmental Defense Fund petitioned the FDA on 27 June 1973 for the “promulgation of regulations [ … ] to prohibit the adulteration of food and drugs with asbestos.” 75 On 28 September 1973, the FDA published a proposed regulation for asbestos in talc in the Federal Register, which called for a 99.9 percent purity for amphibole asbestos fiber and 99.99 percent for chrysotile and proposed the use of a polarized microscope. 75 The CTFA responded to the proposed regulation by completing a “critical review” of the FDA method through round robin testing of five different samples of cosmetic talc and one talc sample spiked with asbestos. 76 They informed the FDA that their proposed method was unreliable and should not be implemented, stating: “It results in both false-positive and false-negative findings. It is also tedious and may consume as much as one half day per sample” (p. 2). 76 They proposed that the TM&MCs work with the FDA to develop a “more reliable and more practical” method (p. 3) and summarized seven alternative test methods. 76 The CTFA determined that of the seven methods, transmission electron microscopy with electron diffraction “appears to offer the best, most reliable method and is probably capable of detecting chrysotile and tremolite (fibrous), both at a level of 0.1%” (p. 9). 76 However, some CTFA members expressed “grave concern” (p. 3) over the inclusion of transmission electron microscopy, which they said was too sensitive and cost prohibitive for small manufacturers. 77 J&J’s consultant had found chrysotile in Lewin’s J&J product samples using transmission electron microscopy (see Table 3). In a memo to file about a January 1974 meeting with the FDA, Nashed states that “Our very preliminary calculation indicates that substantial asbestos can be allowed safely in a baby powder” (p. 2). 78
Rosner et al. 31 offer some historical context for the CTFA’s confidence in pressuring the FDA:
The industry was willing to challenge the FDA since some privately believed that the “FDA is reluctant to take any legal action in any problems with industry.” The CTFA had been told that the FDA had “neither the money nor the manpower to pursue matters so that they will have airtight cases in scientific matters.”
In December 1974, J&J wrote to the CTFA concerned that they needed to preempt FDA’s adoption of more sensitive asbestos detection methods: “We believe it is critical for the CTFA to now recommend these methods to the FDA before the art advances to more sophisticated techniques with higher levels of sensitization [sic]” (p. 1). 79 J&J’s consultants had already developed a centrifugation method that would concentrate asbestos in talc so that it could be detected microscopically. 80 They deemed this method “too sensitive” 71 and noted in a memo: “we deliberately have not included a concentration technique as we felt it would not be in worldwide company interests to do this” (Supplement Section 3a). 81 There is no evidence that they shared the consultant recommendations on the concentration test method with the FDA or the CTFA. However, the FDA explored a similar method themselves in 1975, which one J&J representative described as “more disturbing” than other proposed methods 82 (Supplement Section 3b).
The CTFA ultimately recommended X-ray diffraction as the primary screening method for amphibole asbestos, to be used in the CTFA J4-1. 14 At best, this method could detect levels of asbestos above 0.5 percent. 14 The CTFA also convinced the FDA that the method did not need to test for chrysotile, the only non-amphibole form of asbestos, as they falsely claimed it had never been detected in talc (p. 3). 83 By this time, there was ample evidence of chrysotile in various companies’ products and talc mines (see Table 3). (See Supplement Section 4 on X-ray diffraction and Supplement Section 5 on chrysotile.)
In 197584 and 1976, 85 the CTFA sent the FDA updates on the latest approved CTFA Cosmetic Talc Specification as well as their internal analyses of talcs that were meant to illustrate the “responsibility of industry in monitoring its talc” and to “give [the FDA] assurance as to the freedom from contamination by asbestos form materials of cosmetic talc products” (p. 1). 85 Unfortunately, the letters contained multiple misrepresentations, notably the exclusion of positive results for chrysotile and tremolite (see Supplement Section 6a for details). After 1976, the FDA requested that the companies periodically report results of their own analyses on talc, but the companies resisted (Supplement Section 6b for exchange). 86 No subsequent test reports from industry were found in FDA records. J&J admitted they did not provide any test results to the FDA performed after 1973. 87
In March of 1975, the FDA announced that the proposed regulations for asbestos content in talc would be delayed, in part because the proposed method was “difficult to use, laborious, and not practical for its intended purpose.” 88 In 1976, Dr. Heinz Eiermann, the FDA commissioner in charge of cosmetics from 1973 to 1991, privately criticized the adequacy of the industry testing, particularly noting that their “analytical effort” was very small considering the “business volume” of the top cosmetic talc producers. 89 However, Eiermann publicly defended J&J, stating that their talc “has been found to be virtually free of asbestos” (p. 4) and that there is “no evidence” baby powder is hazardous, citing J&J’s own testing of talc (p. 3) 90 (see Supplement Section 7 for details on Eiermann).
The agency never issued a final regulation for asbestos in talc.
Final CTFA Specifications (1976–1977)
In July of 1976, the CTFA discussed a definition of “cosmetic talc” which did not require that talc be “asbestos-free” 91 :
Talc is an essentially white, odorless, fine powder which is ground from naturally occurring rock ore. It consists of a minimum of 90% hydrated magnesium aluminum silicate, having the ideal formula Mg6(Si8O20) · (OH)4, with the remainder consisting of naturally associated minerals such as calcite, chlorite, dolomite, kaolin, and magnesite, and containing no detectable asbestos minerals. [Emphasis added; p. 2]
The CTFA’s method, known as CTFA Method J4-1, was issued on 7 October 1976 for “the detection of amphibole minerals in cosmetic talc.” 14 This framing, “no detectable asbestos” essentially allowed for asbestos to be present in levels that were undetectable based on the test method; this meant that any amount of chrysotile— which was not included in J4-1— was permitted. CTFA Method J4-1 was an unenforced specification that the CTFA never formally codified; the organization omitted any, even voluntary, compliance requirements13,92 (see Supplement Section 8 for details).
In 1977, the CTFA coordinated a round robin and determined that the J4-1 method was not “accurate, reliable and practical” [emphasis in original, p. 1]. 93 Three of the open market products tested positive for more than 0.5 percent asbestos by two to three labs. 93 Those products with “inconsistent results” would be retested. 93 We could not locate the results of the second round robin, but four of the seven samples tested contained tremolite and/or anthophyllite at levels over 0.5 percent 94 (see Supplement Section 9 for details on round robin testing).
Mt. Sinai continued to find asbestos in various cosmetic talc products, ten of which were reported in a 1976 article in the NYT. 95 In 2019, the FDA detected chrysotile asbestos in J&J baby powder; this asbestos-containing J&J baby powder lot passed both the CTFA J4-1 method and J&J’s transmission electron microscopy method TM7024. 96
There is a safe and effective alternative to cosmetic talc. Cornstarch powders have been on the market since at least the 1890s (p. 37). 97 In 1977, J&J compared the qualities of corn starch and talc powders and found that cornstarch “overall is rated significantly higher by mothers” (p. 5). 98 There is no evidence that corn starch is a carcinogen of any kind.
Downstream Implications of the 1976 Specification (1977–Present)
Continued industry self-regulation of talc despite citizen petitions to the FDA
The FDA received four citizen petitions filed in 1978, 99 1983, 100 1994, 101 and 2008, 101 which each asked the FDA regulate talc due to its possible carcinogenicity. They also received a FOIA request in 1977 (see Supplement Section 10a–e for details on each). 102 The FDA continued to rely on information provided by the industry, including contacting J&J or the CTFA for information on the industry products.102,103 The FDA denied the 1978 and 1986 petitions in 197999 and 1986, 104 respectively, citing inconclusive evidence and relying on tests provided by the industry. The FDA did not deny the 1994 and 2008 petitions, both from the Cancer Prevention Coalition, until 2014, a few months after a South Dakota jury found that J&J talc caused a plaintiff’s ovarian cancer.105,106 J&J used the 2014 FDA decision as part of its defense in an ongoing ovarian cancer trial in 2014 soon after the FDA released it. 107 In their reasons for denying the petitions, the FDA concluded that the epidemiologic data were insufficient to merit a cancer warning because the petition did not cite any evidence of “current” (post-1976) talc containing asbestos and that current talc comes from “asbestos-free” mines and can be purified. 105
The companies continued to provide the FDA with industry-influenced “scientific” support. For instance, in 1994, the FDA and industry cofunded a workshop, Talc: Consumer Uses and Health Perspectives, with the International Society of Regulatory Toxicology and Pharmacology. 108 International Society of Regulatory Toxicology and Pharmacology is financed in part by the tobacco, pharmaceutical, and chemical industries and had a direct influence on those in attendance at the workshop (see Supplement Section 11a for details on influence). 109 The TM&MCs used the workshop as a platform to attempt to discredit a recently-completed NTP animal study demonstrating talc lung carcinogenicity (see Supplement Section 11b for details on study and TM&MC influence). 110 In response to the 2008 petition, the CTFA offered the FDA111 a recently published narrative review and meta-analysis of talc and ovarian cancer, compiled by Drs. Michael S. Huncharek and Joshua E. Muscat, 112 which J&J and Luzenac had jointly funded via a law firm, Crowell & Moring, “so as to preserve the benefit of the attorney work product privilege, which is helpful in protecting confidentiality” (p. 2). 113 Huncharek and Muscat agreed that the funders could review the report and suggest changes before submission to the NTP and publication (see Supplement Section 10e). 114
Use of the 1976 specification to prevent talc from being labeled a carcinogen
The TM&MCs also influenced other regulatory agencies and standards-setting organizations, including influencing the NTP’s decision to not include talc as a carcinogen in their 10th Report on Carcinogens in the early 2000s. In October 2000, the NTP released a Draft Background Document citing evidence regarding the relationship between talc and cancer and recommending that both talc containing and not containing asbestiform fibers were “reasonably anticipated to be a human carcinogen.” 115 The Report on Carcinogens Subcommittee would meet in December 2000 to make a final determination on the carcinogenic status of talc.
Ahead of the meeting, Luzenac began to craft a campaign to “create a reasonable doubt” in the mind of the NTP’s Board of Scientific Counselors that “they may not be acting on the best advice from their consultants” (p. 2). 116 Luzenac had now taken the lead from J&J in defending the safety of talc (Luzenac was owned by Rio Tinto until 2011, when it was sold to Imerys). Luzenac retained the services of Dr. Alfred Wehner, who had served as a consultant to J&J, and sought the help of the Center for Regulatory Effectiveness (CRE), a private consulting firm and lobbyist group that helped clients oppose government regulation. 117 CRE claims they offer “independent analyses of agency regulations” on their website, but Luzenac retained their services to lobby against regulation of talc 118 (see Supplement Section 12a for details on CRE). Luzenac then “discovered” what they claimed was a “fatal flaw” in the NTP report: the report “assumed” that talc without specification of minerology or morphology may contain asbestos fibers due to the widespread contamination of talc with asbestiform minerals.119,120 CRE advanced the “fatal flaw” argument as their primary critique of the Draft Background Document: CRE argued that because the industry took steps to ensure that talc was “virtually free of asbestiform fibers” after 1976, and the available epidemiologic evidence did not differentiate between asbestiform and non-asbestiform talc before and after this date, there was not enough “scientific support” for the NTP’s “assumption” that there was a “widespread contamination” of talc. 120 As a second point, CRE attacked the adequacy of the NTP animal study, citing the International Society of Regulatory Toxicology and Pharmacology 1994 workshop. 120 The CTFA adopted a more polished version of the “fatal flaw” argument in their 2002 comments on the 10th Report on Carcinogens and used it again in their 2004 comments on the 12th Report on Carcinogens. 121 Their comments hinged on the distinction between “pure” cosmetic talc and talc containing asbestos, stating that while there was epidemiological evidence for a relationship between asbestos and ovarian cancer, “pure” or “asbestos-free” talc was “accepted in the medical community for decades” (p. 2). They then argued:
A review of the epidemiologic studies on ovarian cancer and talc exposure shows that a large portion of the exposures in all of the studies must have occurred prior to 1976. In addition, none of those studies were able to characterize the composition of the powders or identify brands. Thus, in addition to the analytical weaknesses discussed previously , the exposures might have involved exposure to asbestos , making the studies essentially lacking in utility and data quality for the purpose of evaluating the safety of present-day cosmetic talc. [ … ] Present-day cosmetic talc must be assumed to be free of asbestos , consistent with the CTFA specification and absent evidence to the contrary. (p. 4) [Emphasis Added]
Of course, as we have illustrated, the 1976 specification did not assure that talc was asbestos-free.
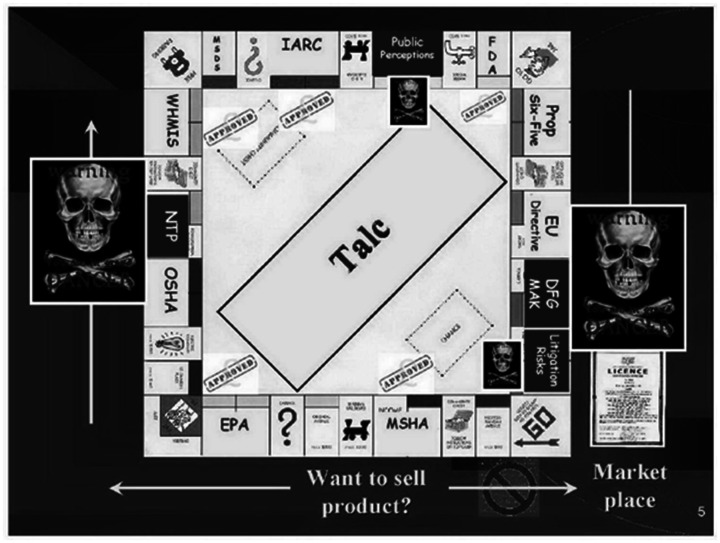
The NTP ultimately deferred voting on both asbestiform and non-asbestiform talc. 119 Rich Zazenski of Luzenac took credit for finding the “flaw,” asserting that this argument “would be our winning hand; if not during the NTP review process, certainly it would prevail in the courts … ” (p. 2). 119 He also attributed this early success with the NTP to the companies’ creation of “confusion” around the “fatal flaw” but suggested that the NTP might remove the “fatal flaw assumptions” (pp. 2–3) in a subsequent draft. 122 In response to this potential problem, his colleague Robert Bernstein noted: “Time to come up with more confusion!” 122 David Michaels further details these events as classic examples of the corporate production of scientific doubt. 8 However, he notes that the industry also relied on political clout and their connections to the Bush Administration to make this strategy work (see Michaels 8 and Supplement Section 12c). Luzenac would retrospectively, in a 2011 presentation, reflect on this experience with the NTP as a “regulatory challenge” that they “could not afford to lose,” (p. 2) and use the image shown in Figure 3 to compare the response to regulation to a monopoly game. 123
Figure 3.
Comparison of regulation to monopology game.
Industry legal consultants also promoted the “fatal flaw” argument to the International Agency for Research on Cancer (IARC) in 2005 and the Cosmetic Ingredient Review—an “independent” review body supported by the FDA, the Consumer Federation of America, and the Personal Care Products Council—in 2012.124–126 Both organizations accepted the representation that the CTFA’s voluntary standards ensured the purity of talc sold after 1976 (see Supplement Section 13a/b for details).
The 1976 talc specification has continued to mislead epidemiologists studying talc carcinogenicity. Of thirty-two epidemiologic studies of talc and ovarian cancer, twelve accepted the claim that cosmetic talc products have been asbestos-free since 1976. 15 Without an alternative mechanistic explanation, some researchers rejected causal associations between talc use and ovarian cancer. 15
Discussion
The documents reviewed indicate that the CTFA, J&J, and other industry representatives exerted considerable influence in three key areas in the 1970s: (1) regulatory proceedings at the FDA; (2) testing methods and the manipulation of test results (including undisclosed results); and (3) press coverage and the medical literature. After 1976, when the industry succeeded in preventing government regulation of cosmetic talc products, their influence continued. The actions undertaken by the CTFA and J&J reflect what David Michaels and others have called manufacturing uncertainty or doubt about the harmful effects of a product.5,8
Our review also indicates that the industry successfully pressured the FDA to ignore evidence that talc contained asbestos and be “willing to live” with inadequacies of the J4-1 method which would not assure the absence of asbestos in talc. 42 We shed light on the insidious nature of corporate influence over regulatory bodies, and public health policy in particular. For this reason, it is worth noting the several cases of revolving-door employment in this case study. The FDA commissioner in charge of cosmetics from 1973 to 1991, Dr. Heinz Eiermann, worked for J&J and Shulman, two talc manufacturers, before coming to the FDA, and both Dr. John Wenninger, deputy director of the FDA Division of Cosmetics and Technology in the 1970s and 1980s, and Dr. John Bailey, the director of the FDA Office of Cosmetics and Color from 1992 to 2002, went on to work for CTFA.127–130 Bailey spent nine years with the CTFA eventually serving as the Executive Vice-President of Science; he currently works for EAS, a consulting company focused on lobbying the FDA and testifies as an expert witness for J&J and Colgate in talc litigation.128,131 Wenninger coauthored the updated 1992 and 2002 CTFA Cosmetic Ingredient Handbooks (he had also served on the editorial advisory board of the 1977 Handbook).132,133 The industry also understood who to target in the FDA. For instance, the Senior Vice President of Science for the CTFA, Dr. Norman Estrin, once noted that Weissler was “ … a weak member of the FDA group and he will probably bury the [asbestos test] methodology in a lot of paper.” 134
As Historians Rosner et al. describe, the 1970s witnessed a political and economic battle surrounding public health and regulation. While significant traction was made in the early 1970s in terms of establishing government organizations to protect worker, consumer, and public health, by 1980, a conservative backlash ushered in what David Harvey calls an “emphatic turn towards neoliberalism:” “Deregulation, privatization, and withdrawal of the state from many areas of social provision have been all too common” (pp. 2–3). 32 While historically we can situate this case study within this contentious period, the downstream effects of deregulation (i.e. industry self-regulation) of talc on public health are apparent. Further, as neoliberalism has become the dominant political-economic rationale of the current era, scholars have called into question the influence of neoliberalism on numerous public health concerns. 33
In a similar review of corporate and government documents, Hessari et al.’s 9 analysis of conversations between the CDC and Coca-Cola similarly points out the inappropriateness of “allowing conflicted corporate actors to engage in well-established tactics to further commercial goals” for “an organization established to protect public health.” Corporate interests must be prevented from wielding power over regulatory proceedings and decision-making. Otherwise, regulatory bodies become complicit in protecting industry profits rather than public health. By its own definition, the FDA is first and foremost “responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.” 12 As criminologists Lynch et al. 135 state, the agency should be recognized as central to policing corporate crime in addition to protecting the public’s health. Our review indicates that a number of government and public interest groups including the New York City Environmental Protection Agency, the Cancer Prevention Coalition, and private citizens regularly petitioned the FDA (from 1971 to 2008) to address concerns around talc carcinogenicity. In each instance, the FDA adopted industry’s profit-driven position over that of public health.
The FDA’s 2014 rejection of the 2008 citizen’s petition and J&J’s subsequent use of this rejection in a trial is also concerning. Industries often use the FDA’s product approval as a defense against lawsuits. 136 In the case of pharmaceuticals, Kesselheim and Avorn 136 warn that if this defense is accepted in the majority of courts, “FDA approval of a drug would absolve companies of responsibility for failing to adequately evaluate or report the risks associated with their products.” In this case, the FDA chose to leave talc unregulated and J&J used this account of FDA inaction to support its defense of talc to the public and to juries in court.
Large industries continue to influence scientific research, specifications, standards, and other forms of safety regulation with the motivation of protecting sales and preventing lawsuits, often when other, safer substitutes are available. Industry wealth and networks of power can undermine adequate government oversight.
Conclusion
The FDA and TM&MC assertion that cosmetic talc has been “asbestos-free” since 1976 relies on the publication of the 1976 CTFA J4-1 method not on actual test results. This test method replaced a proposed FDA regulation on talc, was voluntary, and was never formally codified. 14 In addition to being unenforceable, the 1976 CTFA J4-1 specification was also defective: it permitted the presence of the carcinogens (including chrysotile and fibrous talc) and only detected amphiboles at levels over 0.5 percent.14,94 The TM&MCs suppressed evidence of asbestos in talc products and withheld information on superior testing methods from the FDA and the scientific community. As a result, epidemiological and other studies that seek to define the relationship between talc and various cancers also repeat the misleading notion that talc has been asbestos-free since 1976. 15
Representatives from several TM&MCs have maintained—and still maintain—that in 1976 the industry implemented “stringent safety and quality control measures designed to ensure the absence of asbestos fibers from consumer talc products.” 13 J&J’s website, for instance, as of December 2020, falsely states that: “JOHNSON’s talc products do not contain asbestos. A frequent misperception is that JOHNSON’s baby powder contains talc made with asbestos, a substance classified as cancer-causing. Since the 1970s, talc used in consumer products has been required to be asbestos-free.” 137 However, “no detectable asbestos” is not the same as “asbestos-free.” As Rosner et al. 31 state on this issue: “The difference in these methodologies meant that potentially billions of asbestos fibers could be released into the air when babies were powdered or adults powdered themselves” (p. 1). In 1974, J&J told the FDA that they would follow a precautionary principle,
Dr. Fuller stressed Johnson & Johnson’s policy of full cooperation with FDA and that if the results of any scientific studies show any question of safety of talc, Johnson & Johnson will not hesitate to take it off the market. (p. 3) 78
However, in practice, at every crossroad, J&J and the FDA interpreted “doubt” as a basis for inaction rather than a basis for protection of the public’s health. The FDA has still not initiated any regulatory process for talc. J&J discontinued sales of talc-based baby powder in North America in April 2020, shortly after the FDA found asbestos in J&J baby powder. 12 They did not issue a recall and continued to sell products that were on store shelves.
Since the 1960s, doctors had advised against the use of cosmetic talc for the undisputed harms they cause, asphyxiation and talcosis, and the increase risk of cancer and support legislation that bans the use of talc in cosmetic powders. 138 It is crucial that researchers continue to investigate and publish ongoing examples of regulatory oversight and internal conversations so that methods to improve government agencies become more apparent. Physicians, policy makers, and regulatory body themselves must become adept at identifying industry influence over issues that may cause harm to human and environmental health. We must work to curb instances of corporate influence on the FDA and other regulatory bodies. In the very least, we need independent, democratic watchdogs with science boards of researchers who do not collaborate in any way with the related industries, keeping industry and the government in check.
Limitations
Due to the elaborate nature of these events, this review is not exhaustive and much detail is omitted. Our review of documents is further limited to the documents made available to us, as well as those released to the public domain.
Supplemental Material
Supplemental material, sj-pdf-1-new-10.1177_1048291121996645 for A Review of the Talc Industry’s Influence on Federal Regulation and Scientific Standards for Asbestos in Talc by Tess Bird, Joan E. Steffen, Triet H. Tran and David S. Egilman in NEW SOLUTIONS: A Journal of Environmental and Occupational Health Policy
Author Biographies
Tess Bird is an interdisciplinary social science researcher and medical anthropologist. She is a current research affiliate at the Institute of Social and Cultural Anthropology, University of Oxford and a former Mellon Fellow in Writing in the Social Sciences at Wesleyan University.
Joan E. Steffen is a second-year student at Harvard Law School. She is a policy director for the Harvard Prison Legal Assistance Project and a copresident of Harvard’s chapter of the National Lawyers Guild. Prior to attending law school, Joan worked at Never Again Consulting.
Triet H. Tran is a graduate of Brown University in Statistics and Economics. He works at Never Again Consulting.
David S. Egilman is a clinical professor of Family Medicine at Brown University and owner of Never Again Consulting. He also runs a non-profit that funds community-oriented primary care training programs in developing countries.
Notes
TM&MCs include: Avon, BASF, Chanel, Colgate-Palmolive, CTFA, Cyprus, Imerys/Rio Tinto Materials/Luzenac, J&J, Johns-Manville, Pfizer, R.T. Vanderbilt, Shulton, and Whittaker, Clark & Daniels.
Laboratories include: ES Laboratories, RJ Lee, National Institutes of Occupational Safety and Health, the Colorado School of Mines, McCrone Associates Inc., Dartmouth, Bain Environmental, Mount Sinai Hospital, and the Mine Safety and Health Administration.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David S. Egilman serves as an expert witness in litigation at the request of people who claim injuries resulting from the use of talcum powders. Triet H. Tran works for David S. Egilman. Joan E. Steffen and Tess Bird worked for David S. Egilman during the initial phases of research and writing. Dr. Egilman was not compensated for work on this article. Triet H. Tran, Joan E. Steffen, and Tess Bird were not compensated by law firms for work on this article. No party to these litigations reviewed this commentary or had input into its content. A copy of the article was sent to lawyers representing Johnson & Johnson for comments prior to publication.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Tess Bird https://orcid.org/0000-0003-2997-6798
David S. Egilman https://orcid.org/0000-0003-0280-163X
Supplemental material: Supplemental material for this article is available online.
References
- 1.Egilman D, Bohme S. Over a barrel: corporate corruption of science and its effects on workers and the environment. Int J Occup Environ Health 2005; 11: 331–337. [DOI] [PubMed] [Google Scholar]
- 2.Ong EK, Glantz SA. Constructing ‘sound science’ and ‘good epidemiology’: tobacco, lawyers, and public relations firms. Am J Public Health 2001; 91: 1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moodie R. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet 2013; 381: 670–679. [DOI] [PubMed] [Google Scholar]
- 4.Michaels D. Doubt is in their product: how industry’s assult on science threatens your health. Oxford: Oxford University Press, 2008. [Google Scholar]
- 5.Michaels D, Monforton C. Manufacturing uncertainty: contested science and the protection of the public’s health and environment. Am J Public Health 2005; 95: S39–S48. [DOI] [PubMed] [Google Scholar]
- 6.Stuckler D, McKee M, Ebrahim S, et al. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med 2012; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulman S, Abend K, Meyer A, et al. Smoke, mirrors, and hot air: how exxonmobil uses big tobacco’s tactics to manufacture uncertainty on climate science . Cambridge, MA: Union of Concerned Scientists, 2007. [Google Scholar]
- 8.Michaels D. The triumph of doubt: dark money and the science of deception. Oxford: Oxford University Press, 2020. [Google Scholar]
- 9.Hessari N, Ruskin G., McKee M, et al.. Public meets private: conversations between Coca-Cola and the CDC. Milbank Q 2019; 97: 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piller C. FDA’s revolving door: companies often hire agency staffers who managed their successful drug reviews. Science Magazine, 5 July 2018, https://www.sciencemag.org/news/2018/07/fda-s-revolving-door-companies-often-hire-agency-staffers-who-managed-their-successful (accessed 9 February 2021).
- 11.Shoot B. Jury awards $29 million in latest Johnson & Johnson cancer-causing baby powder lawsuit. Fortune Magazine, 15 March 2019, http://www.fortune.com/2019/03/15/cancer-asbestos-johnson-jnj-baby-talcum-powder-trial/ (accessed 9 February 2021).
- 12.Hsu T, Rabin R. Johnson & Johnson to end talc-based baby powder sales in North America. The New York Times, 19 May 2020, http://www.nytimes.com/2020/05/19/business/johnson-baby-powder-sales-stopped.html (accessed 9 February 2021).
- 13.Zazenski R, Ashton W, Briggs D, et al. Talc: occurrence, characterization, and consumer applications. Regul Toxicol Pharmacol 1995; 21: 218–229. [DOI] [PubMed] [Google Scholar]
- 14.CTFA Method J4-1: asbestiform amphibole minerals in cosmetic talc. Washington, DC: CTFA, https://repository.library.brown.edu/studio/item/bdr:841542/ (1976, accessed 9 February 2021).
- 15.Tran T, Steffen J, Clancy K, et al. Talc, asbestos, and epidemiology: corporate influence and scientific incognizance. Epidemiology 2019; 30: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee G. Letter to Dr. Norman Estrin (CTFA). Raritan, N.J.: J&J, 5 October 1976, https://repository.library.brown.edu/studio/item/bdr:858421/ (accessed 9 February 2021).
- 17.Appendix A to § 1926.55—1970 American Conference of Governmental Industrial Hygienists’ threshold limit values of airborne contaminants threshold limit values of airborne contaminants for construction OSHA, https://http://www.osha.gov/laws-regs/regulations/standardnumber/1926/1926.55AppA (accessed 9 February 2021).
- 18.Dana E. A textbook of mineralogy. New York: John Wiley & Sons, 1898. [Google Scholar]
- 19.Schulz R, Williams C. Commercial talc: animal and mineralogical studies. J Ind Hyg Toxicol 1942; 24: 75–79. [Google Scholar]
- 20.Siegal W, Amith A, Greenburg L. The dust hazard in tremolite talc mining, including roentgenological findings in talc workers. AJR Am J Roentgenol 1943; 49: 11–29. [Google Scholar]
- 21.Pask J, Warner P. Fundamentatl studies of talc: I, constitution of talcs. J Am Ceram Soc 1954; 37: 118–128. [Google Scholar]
- 22.Gillson J. Industrial minerals and rocks. New York: The American Institute of Mining, Metallurgical, and Petroleum Engineers, 1960.
- 23.Fyfe W. On the relative stability of talc, anthophyllite, and ensteatite. Am J Sci 1962; 260: 460–466. [Google Scholar]
- 24.Hunt AC. Massive pulmonary fibrosis from the inhalation of talc. Thorax 1956; 11: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porro F, Patton J. Pneumoconiosis in the talc industry. AJR Am J Roentgenol 1942; 518: 47. [Google Scholar]
- 26.Waldermar D. Effects of certain silicate dusts on the lungs. J Ind Hyg 1933; 15: 66–78. [Google Scholar]
- 27.Gross P, deTreville RTP, Cralley LJ, et al. Pulmonary ferruginous bodies: development in response to filamentous dusts and a method of isolation and concentration. Arch Path 1968; 85: 539–546. [PubMed] [Google Scholar]
- 28.Cralley L, Key M, Groth D, et al. Fibrous and mineral content of cosmetic talcum products. Am Ind Hyg Assoc J 1968; 29: 350–354. [DOI] [PubMed] [Google Scholar]
- 29.Selikoff IJ. Biological effects of asbestos. New York: New York Academy of Sciences Section of Biological and Medical Sciences, 1964. [Google Scholar]
- 30.Lawrence S. Asbestos in talcum? FDA to test. The New York Post, 13 August 1971, https://repository.library.brown.edu/studio/item/bdr:841666/ (accessed 9 February 2021).
- 31.Rosner D, Markowitz G, Chowkwanyun M. Nondetected”: the politics of measurement of asbestos in talc, 1971–1976. Am J Public Health 2019; 109: 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey D. A brief history of neoliberalism. Oxford: Oxford University Press, 2005. [Google Scholar]
- 33.Schrecker T, Bambra C. How politics make us sick: neoliberal epidemics. London: Palgrave Macmillan, 2015. [Google Scholar]
- 34.Kretchmer J. Letter to E. Richardson (Department of Health, Education, and Welfare). Washington, DC: Environmental Protection Agency, 28 June 1971, https://repository.library.brown.edu/studio/item/bdr:858524/ (accessed 9 February 2021).
- 35.Weissler A. Discussion session on "asbestos and talc". Washington, DC: FDA, 3 August 1971, https://repository.library.brown.edu/studio/item/bdr:1104405/ (accessed 9 February 2021).
- 36.Sandland G. Report of CTFA talc subcommittee on method to detect chrysotile and tremolite in talc. Washington, DC: CTFA, 10 December 1973, https://repository.library.brown.edu/studio/item/bdr:925786/ (accessed 9 February 2021).
- 37.Lichtenstein G. High levels of asbestos found in 3 paints and 2 talcums here. The New York Times, 16. June 1972, https://www.nytimes.com/1972/06/16/archives/high-levels-of-asbestos-found-in-3-paints-and-2-talcums-here.html (accessed 9 February 2021).
- 38.Nashed W. Memo to file, talc/asbestos. New Brunswick, N.J.: J&J, 17 June 1972, https://repository.library.brown.edu/studio/item/bdr:858453/ (accessed 9 February 2021).
- 39.Lichtenstein G. Doctor admits he may have been mistaken. The New York Times, 17 June 1972, https://repository.library.brown.edu/studio/item/bdr:858557/ (accessed 9 February 2021).
- 40.Lewin S. L T. Alfred Weissler (FDA), with attached tabulation of X-ray diffraction analyses of commercial products containing talc. Department of Chemistry, New York University, 3 August 1972, https://repository.library.brown.edu/studio/item/bdr:858455/ (accessed 9 February 2021).
- 41.Nashed W. Memo to Fuller (J&J), talc/asbestos, Shower to Shower talc. New Brunswick, N.J.: J&J, 24 August 1972, https://repository.library.brown.edu/studio/item/bdr:858458/ (accessed 9 February 2021).
- 42.Weissler A. Memo to Schaffner, summary and comments on Prof. Lewin’s analytical results for asbestos in talc. Washington, DC: FDA, 31 July 1973, https://repository.library.brown.edu/studio/item/bdr:858452/ (accessed 9 February 2021).
- 43.Stuart JW. Analyst work sheets (Lewin samples). Washington, DC: FDA, 23 April 1974, https://repository.library.brown.edu/studio/item/bdr:1104409/ (accessed 9 February 2021).
- 44.Nashed W. Memo to Fuller (J&J), confidential, talc/asbestos. New Brunswick, N.J.: J&J, 8 August 1972, https://repository.library.brown.edu/studio/item/bdr:858457/ (accessed 9 February 2021).
- 45.Nashed W. Memo to file, talc/asbestos FDA meeting August 11, 1972. New Brunswick, N.J.: J&J, 14 August 1972, https://repository.library.brown.edu/studio/item/bdr:841504/ (accessed 9 February 2021).
- 46.Wenniger J. Memorandum of meeting. Washington, D.C.: FDA, August 11, 1972, https://repository.library.brown.edu/studio/item/bdr:858459/ (accessed 9 February 2021).
- 47.Nashed W. Memo to fuller (J&J), talc/asbestos, with attached findings on Johnson & Johnson products from a report by Dr. S. Lewin (consultant to the FDA). New Brunswick, N.J.:, 26 September 1972, https://repository.library.brown.edu/studio/item/bdr:858464/ (accessed 9 February 2021).
- 48.Nashed W. Memo to file, Shower to Shower/asbestos, FDA meeting, September 21, 1972. New Brunswick, N.J.: J&J, 25 September 1972, https://repository.library.brown.edu/studio/item/bdr:1078410/ (accessed 9 February 2021).
- 49.Nashed W. Memo to fuller (J&J), talc/asbestos, FDA meeting, October 19, 1972. New Brunswick, N.J.: J&J, 20 October 1972, https://repository.library.brown.edu/studio/item/bdr:858467/ (accessed 9 February 2021).
- 50.Fuller R. Talc file, meeting November 1, 1972 with Dr. Schaffner - F.D.A. New Brunswick, N.J.: J&J, 13 December 1972, https://repository.library.brown.edu/studio/item/bdr:858470/ (accessed 9 February 2021).
- 51.Fuller R. Talc file, phone call - Dr. Schaffner (on November 22, 1972). New Brunswick, N.J.: J&J, 13 December 1972, https://repository.library.brown.edu/studio/item/bdr:858472/ (accessed 9 February 2021).
- 52.Weissler A. Handwitten note, Lewin results on J&J products. Washington, DC: FDA, 7 December 1972, https://repository.library.brown.edu/studio/item/bdr:1104408/ (accessed 9 February 2021).
- 53.Addison J, Davies LS. Analysis of amphibole asbestos in chrysotile and other minerals. Ann Occup Hyg 1990; 34: 159–175. AprPubMed PMID: 2169219. [DOI] [PubMed] [Google Scholar]
- 54.Fuller R. Memo to Nashed (J&J), Dr. Schaffner - F.D.A. New Brunswick, N.J.: J&J, 8 December 1972, https://repository.library.brown.edu/studio/item/bdr:858474/ (accessed 9 February 2021).
- 55.Weissler A. L T. Adamson and Lang (Environmental Defense Fund). Washington, DC: FDA, 2 March 1973, https://repository.library.brown.edu/studio/item/bdr:858475/ (accessed 9 February 2021).
- 56.Buerger M. The Washington meeting of September 21, 1972 of Johnson and Johnson with the Food and Drug Administration and conclusions to be drawn from the meeting. New Brunswick, NJ: J&J, 7 October 1972, https://repository.library.brown.edu/studio/item/bdr:858478/ (accessed 9 February 2021).
- 57.Stewart I. Letter to Goudie (J&J). Chicago, IL: McCrone Associates, 15 November 1972, https://repository.library.brown.edu/studio/item/bdr:841514/ (accessed 9 February 2021).
- 58.Nashed W. L T. Robert Schaffer (FDA). New Brunswick, NJ: J&J, 29 November 1972, https://repository.library.brown.edu/studio/item/bdr:841522/ (accessed 9 February 2021).
- 59.Smith WL. Progress report on further studies on the measurement and correlation of the physical properties of talc. Columbus, OH: Battelle Memorial Institute, 9 May 1958, https://repository.library.brown.edu/studio/item/bdr:841507/ (accessed 9 February 2021).
- 60.Ashton WH. Microscopic examination museum baby powder samples. New Brunswick, NJ: J&J, 13 July 1966, https://repository.library.brown.edu/studio/item/bdr:841508/ (accessed 9 February 2021).
- 61.Langer AM. Letter to Henderson (Tenovus Institute). New York, NY: Mount Sinai School of Medicine, 22 August 1971, https://repository.library.brown.edu/studio/item/bdr:858491/ (accessed 9 February 2021).
- 62.Hildick-Smith GL. Letter to Dr. Selikoff (Mt. Sinai School of Medicine). New Brunswick, NJ: J&J , 9 August 1971, https://repository.library.brown.edu/studio/item/bdr:858526/ (accessed 9 February 2021).
- 63.Langer A. Letter to Hildick-Smith (J&J). New York, NY: Mt. Sinai School of Medicine, 10 November 1971, https://repository.library.brown.edu/studio/item/bdr:858480/ (accessed 9 February 2021).
- 64.Henderson WJ, Joslin CA, Turnbull AC, et al. Talc and carcinoma of the ovary and cervix. J Obstet Gynaecol Br Commonw 1971; 78: 266–272. March 1PubMed PMID: 5558843. Epub 1971/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 65.Ashton WH. Letter to Hildick-Smith (J&J), assay of talc in baby powder (p.16). New Brunswick, NJ: J&J, 14 May 1971, https://repository.library.brown.edu/studio/item/bdr:858469/ (accessed 9 February 2021).
- 66.Grieger G. Preliminary report on examination of Grantham ore, medicated talcum powder, and shower to shower talcum powder. Chicago, IL: McCrone Associates, 3 September 1971, https://repository.library.brown.edu/studio/item/bdr:841528/ (accessed 9 February 2021).
- 67.Pooley FD, Kingston GA, Lightfoot J. An examination of Italian mine samples and relevant powders. Cardiff, Wales: Department of Mineral Exploitation, University of Cardiff, 8 September 1972, https://repository.library.brown.edu/studio/item/bdr:1161225/ (accessed 9 February 2021).
- 68.Hutchinson T. Investigation of possible asbestos contaminations in talc samples. Minneapolis, MN: University of Minnesota Space Science Center, 1972, https://repository.library.brown.edu/studio/item/bdr:1104412/ (accessed 9 February 2021).
- 69.Stewart I. Examination of Johnson and Johnson's baby powder (labeled “Do Not Use This Report”). Chicago, IL: McCrone Associates, 27 October 1972, https://repository.library.brown.edu/studio/item/bdr:1104411/ (accessed 9 February 2021). [Google Scholar]
- 70.Elkies A. Deposition in Swann vs. J&J in the circuit court of the city of St. Louis state of Missouri case no. 1422-cc-09326 (15 December 2016), https://repository.library.brown.edu/studio/item/bdr:858414/ (accessed 9 February 2021).
- 71.Rolle R. Memo to Shelley (J&J), proposed specs for analyzing talc for asbestos (p. 3). New Brunswick, NJ: J&J, 16 May 1973, https://repository.library.brown.edu/studio/item/bdr:858501/ (accessed 9 February 2021).
- 72.Nashed W. Memo to Fuller (J&J), talc/asbestos. New Brunswick, NJ: J&J, 31 January 1973, https://repository.library.brown.edu/studio/item/bdr:858434/ (accessed 9 February 2021).
- 73.Nashed WL. Letter to the Hearing Clerk, Department of Health, Education, and Welfare. New Brunswick, NJ: J&J, 11 October 1972, https://repository.library.brown.edu/studio/item/bdr:858466/ (accessed 9 February 2021).
- 74.Petterson D. Memo to DD Johnston (J&J), subject: Windsor Minerals and Talc. New Brunswick, NJ: J&J, 26 April 1973, https://repository.library.brown.edu/studio/item/bdr:841512/ (accessed 9 February 2021).
- 75. Asbestos Particles in Food and Drugs. Notice of proposed rulemaking. Fed Regist 1973; 38: 27976–27981. [Google Scholar]
- 76.Sandland G. Letter to FDA Hearing Clerk, proposed order method for asbestos in talc published in the federal register. Washington, DC: CTFA, 26 December 1973, https://repository.library.brown.edu/studio/item/bdr:858497/ (accessed 9 February 2021).
- 77.Sandland G. Meeting notes of CTFA talc subcomittee. Washington, DC: CTFA, 4 December 1974, https://repository.library.brown.edu/studio/item/bdr:858498/ (accessed 9 February 2021).
- 78.Nashed W, Hildick-Smith G. Memo to file, meeting with Commissioner Schmidt (FDA) January 16, 1974. J&J, 18 January 1974, https://repository.library.brown.edu/studio/item/bdr:1151835/ (accessed 9 February 2021).
- 79.Steinberg W. Letter to Estrin (CTFA) et al., Talc. New Brunswick, NJ: J&J, 17 December 1974, https://repository.library.brown.edu/studio/item/bdr:858495/ (accessed 9 February 2021).
- 80.Scheltz J. Memo to R. Rolle, review of experimental techniques for the concentration of asbestos minerals in talc - Project #0503-00. New Brunswick, NJ: J&J, 27 November 1974, https://repository.library.brown.edu/studio/item/bdr:1082590/ (accessed 9 February 2021).
- 81.Sloan IW. Memo to R. Rolle. New Brunswick, NJ: J&J, 18 February 1975, https://repository.library.brown.edu/studio/item/bdr:858547/ (accessed 9 February 2021).
- 82.Ashton WH. Memo to G. Lee. New Brunswick, NJ: J&J, 24 November 1976, https://repository.library.brown.edu/studio/item/bdr:858439/ (accessed 9 February 2021).
- 83.Estrin NC. Talc taskforce minutes. Washington, DC: CTFA, 7 February 1975, https://repository.library.brown.edu/studio/item/bdr:841518/ (accessed 9 February 2021).
- 84.Estrin N. L T. Schaffner (FDA). Re: summary of analyses of talcs from the United States and other parts of the world. CTFA, 23 September 1975, https://repository.library.brown.edu/studio/item/bdr:1104416/ (accessed 9 February 2021).
- 85.Estrin N. L T. Eiermann (FDA). Washington, DC: CTFA, 15 March 1976, https://repository.library.brown.edu/studio/item/bdr:841459/ (accessed 9 February 2021).
- 86.Estrin N. CTFA talc subcommittee minutes. Washington, DC: CTFA, 31 March 1976, https://repository.library.brown.edu/studio/item/bdr:841677/ (accessed 9 February 2021).
- 87.Videotable Deposition of Susan Nicholson, MD. Superior Court of New Jersey, Law Division: Middlesex County. Brody Deposition Services (6 March 2019), https://repository.library.brown.edu/studio/item/bdr:925795/ (accessed 9 February 2021).
- 88.Rules and regulations. Fed Regist 1975; 40: 11865–11869.
- 89.Eiermann H. Memo to Schaffner, asbestos in talc - summary company tests. Washington, DC: FDA, 18 March 1976, https://repository.library.brown.edu/studio/item/bdr:858441/ (accessed 9 February 2021). [Google Scholar]
- 90.Walcott J. Talc tests wanting, FDA experts admit. Record Bergen County, N.J., 11 March 1976, https://repository.library.brown.edu/studio/item/bdr:1082643/ (accessed 9 February 2021).
- 91.Estrin N. Minutes: CTFA talc subcommittee. CTFA, 8 July 1976, https://repository.library.brown.edu/studio/item/bdr:1078416/ (accessed 9 February 2021).
- 92.Brenard C. Email to T McCarthy, K Wylie, et al. (J&J), URGENT: talc in cosmetic products. Rio Tinto Minerals, 25 April 2009, https://repository.library.brown.edu/studio/item/bdr:841519/ (accessed 9 February 2021).
- 93.Minutes: CTFA Task Force on Round Robin Testing of consumer talcim [sic] products for asbestiform amphibole minerals. Washington, DC: CTFA, 17 May 1977, https://repository.library.brown.edu/studio/item/bdr:841544/ (accessed 9 February 2021).
- 94.Scheltz J. Memo to George Lee (J&J), NF Estrin (CTFA) and G. Sandland (Bristol-Myers Products) Re: CTFA Task Force On Round Robin Testing of Consumer Talcum Products. New Brunswick, NJ: J&J, 29 September 1977, https://repository.library.brown.edu/studio/item/bdr:841520/ (accessed 9 February 2021).
- 95.Asbestos found in ten powders. The New York Times, 10 March 1976, http://www.nytimes.com/1976/03/10/archives/asbestos-found-in-ten-powders.html (accessed 9 February 2021).
- 96.Steffen J, Tran T, Yiman M, et al. Serous ovarian cancer caused by exposure to asbestos and fibrous talc in cosmetic talc powders – a case series. J Occup Environ Med 2020; 62: ệ-e77. [DOI] [PubMed] [Google Scholar]
- 97.J&J powder management (presentation). New Brunswick, NJ: J&J, 12 January 2000, https://repository.library.brown.edu/studio/item/bdr:925812/ (accessed 9 February 2021).
- 98.Hielle-Tucker L. Memo to C.E. LaRosa (J&J), JOHNSON'S baby powder with corn starch U/A analysis. New Brunswick, NJ: J&J, 18 July 1977, https://repository.library.brown.edu/studio/item/bdr:925704/ (accessed 9 February 2021).
- 99.Kennedy D. Letter to Dr. SM Wolfe and Dr. B Gordon (public citizen). Washington, DC: FDA, 11 January 1979, https://repository.library.brown.edu/studio/item/bdr:1104345/ (accessed 9 February 2021).
- 100.Douilett P. Petition for labeling of warning of the hazardous effects produced by asbestos in cosmetic talc. 8 November 1983, https://repository.library.brown.edu/studio/item/bdr:858510/ (accessed 9 February 2021).
- 101.Cashen J, Epstein SS. FDA citizen petitions from cancer prevention coalition, 1994 and 2008. 17 November 1994; 13 May 2008, https://repository.library.brown.edu/studio/item/bdr:1161226/ (accessed 9 February 2021).
- 102.Waggoner W. FDA call file, subject: FOI and Johnson's baby powder. New Brunswick, NJ: J&J, 20 June 1977, https://repository.library.brown.edu/studio/item/bdr:936011/ (accessed 9 February 2021).
- 103.Kavanaugh E. Letter to the FDA, Re: FDA Docket No. 94P-0420/CP 1. Washington, DC: CTFA, 16 June 1995, https://repository.library.brown.edu/studio/item/bdr:841681/ (accessed 9 February 2021).
- 104.Swanson J. Letter to Phillippe Douilett (citizen petition). Washington, DC: FDA, 11 July 1986, https://repository.library.brown.edu/studio/item/bdr:841680/ (accessed 9 February 2021).
- 105.Musser SM. Letter to Samuel Epstein (cancer prevention coalition). Re: RE: Docket Numbers 94P-0420 and FDA-2008-P-0309-000IlCP. Silver Spring, MD: FDA, 1 April 2014, https://repository.library.brown.edu/studio/item/bdr:841690/ (accessed 9 February 2021).
- 106.Leader A. South Dakota jury ties talc powder to cancer risk. Rapid City Journal, 5 October 2013, https://rapidcityjournal.com/news/south-dakota-jury-ties-talc-powder-to-cancer-risk/article_78bd7792-b78c-5adb-b684-e6b2c2db10ed.html (accessed 9 February 2021).
- 107.Hegarty M. Defendants Johnson & Johnson and Johnson & Johnson Consumers Inc.'s first amended responses to plaintiffs’ first request for production. In: Ingham et al. v. Johnson & Johnson, et al. Circuit Court of the City of St. Louis, State of Missouri, 27 May 2016, https://repository.library.brown.edu/studio/item/bdr:927172/ (accessed 9 February 2021).
- 108.Kavanaugh E. CTFA response statement: talc. Washington, DC: CTFA, 17 November 1994, https://repository.library.brown.edu/studio/item/bdr:925809/ (accessed 9 February 2021).
- 109.Velicer C. Tobacco papers and tobacco industry ties in regulatory toxicology and pharmacology. J Public Health Pol 2018; 39: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baker RD, Ducasse B. Workshop on talc: consumer uses and health perspectives. Imerys, 11 February 1994, https://repository.library.brown.edu/studio/item/bdr:841684/ (accessed 9 February 2021).
- 111.Memorandum of meeting at FDA. Imerys, 8 May 2009, https://repository.library.brown.edu/studio/item/bdr:858443/ (accessed 9 February 2021).
- 112.Muscat JE, Huncharek MS. Perineal talc use and ovarian cancer: a critical review. Eur J Cancer Prev 2008; 17: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hall R. Email to S. Mann (J&J). NTP proposal to list talc (SFXE30.pdf). Crowell & Moring LLP, 1 February 2005, https://repository.library.brown.edu/studio/item/bdr:858515/ (accessed 9 February 2021).
- 114.Huncharek M. Fax to Robert Glenn (Crowell & Morning). Talc projects letter of agreement. Meta-Analysis Research Group, 6 October 2004, https://repository.library.brown.edu/studio/item/bdr:858517/ (accessed 9 February 2021).
- 115.Technology Planning and Management Corporation. Draft report on carcinogens background document for talc asbestiform and non-asbestiform. Meeting of the NTP Board of Scientific Counselors Report on Carcinogens Subcommittee. U.S. Department of Health and Human Services, 13–14 December 2000, https://repository.library.brown.edu/studio/item/bdr:841468/ (accessed 9 February 2021).
- 116.Turner E. Email to Rich Zazenski, drafting of EUROTALC submission to NTP. Luzenac, 13 November 2000, http://www.toxicdocs.org/d/3JmOnNyr5wqdb683EMBV4yagE (accessed 9 February 2021).
- 117.Zazenski R. Email to Kent Cutler, Jerry Gauntt (Luzenac), Fwd: Talc/NTP Update. Luzenac, 1 November 2001, https://repository.library.brown.edu/studio/item/bdr:858552/ (accessed 9 February 2021).
- 118.CRE Invoices. Multinational Business Services, Inc., 2001-2015, https://repository.library.brown.edu/studio/item/bdr:1153278/ (accessed 9 February 2021).
- 119.Zazenski R, Memo to D. Harris, et al., 2001. major objectives or goals. Luzenac, 17 May 2001, https://repository.library.brown.edu/studio/item/bdr:1153282/ (accessed 9 February 2021).
- 120.Comments by CRE on the listing proposed for talc not containing asbestiform fibers in the report on carcinogens, tenth edition. Washington, DC: CRE, 29 November 2000, https://repository.library.brown.edu/studio/item/bdr:1153283/ (accessed 9 February 2021).
- 121.McEwen G. Letter to K. Olden (NTP), review of cosmetic talc for listing in the report on carcinogens, twelfth edition (69 Federal Register 28940). Washington, DC: CTFA, 18 March 2004, https://repository.library.brown.edu/studio/item/bdr:841466/ (accessed 9 February 2021).
- 122.Zazenski R. Email to Eric Turner, Robert Bernstein (Luzenac), summary of CRE meeting, time to come up with more confusion. Luzenac, 2 January 2001, https://repository.library.brown.edu/studio/item/bdr:841483/ (accessed 9 February 2021).
- 123.Identifying/managing product stewardship issues: “license to market.” Presentation slides. Luzenac, 2011, https://repository.library.brown.edu/studio/item/bdr:1161056/ (accessed 9 February 2021).
- 124.Kelly W. Draft of Letter to Dr. Baan (IARC). Imerys, 20 December 2005, https://repository.library.brown.edu/studio/item/bdr:858446/ (accessed 9 February 2021).
- 125.Deposition of William Kelly. Leavitt and McElroy v. Johnson & Johnson, et al. Superior Court of California, County of Alameda (5 November 2018), https://repository.library.brown.edu/studio/item/bdr:938039/ (accessed 9 February 2021).
- 126.Kelly W. Letter to Alan Andersen (CIR). Initial comments on CIR draft scientific literature review for “talc as used in cosmetics”. Washington, DC: CRE, 19 October 2012, https://repository.library.brown.edu/studio/item/bdr:858536/ (accessed 9 February 2021).
- 127.Draft minutes talc interested party task. Washington, DC: CTFA, 21 July 1993, https://repository.library.brown.edu/studio/item/bdr:858433/ (accessed 9 February 2021).
- 128.John B. Ph.D. Colors and cosmetics: EAS Consulting Group, https://easconsultinggroup.com/about-us/professionals/independent-advisors/john-bailey/ (2018, accessed 14 Jan 2021).
- 129.Obituary For Heinz Josef Eiermann, Louden Funeral Home, http://www.loudounfuneralchapel.com/obituaries/Heinz-Eiermann/ (2012, accessed 14 Jan 2021).
- 130.Gettings S. Scientific Advisory Executive Committee Minutes. Personal Care Products Council, 11. April 1995, https://repository.library.brown.edu/studio/item/bdr:925804/ (accessed 9 February 2021).
- 131.Bailey J, Expert Report of Dr. John E. Bailey, Jr. for Colgate-Palmolive Company in Hayes v. Colgate-Palmolive, et al. (29 July 2018), https://repository.library.brown.edu/studio/item/bdr:1104316/ (accessed 9 February 2021).
- 132.Estrin N, editor. CTFA cosmetic ingredient dictionary. Washington, DC: CTFA, 1977. [Google Scholar]
- 133.Wenniger J, McEwan G. CTFA cosmetic ingredient handbook. Washington, DC: CTFA, 1992. [Google Scholar]
- 134.Nashed W. Memo to JC Walcott (J&J), Special talc project No. 503, CTFA phone call. New Brunswick, NJ: J&J, 17 July 1971, https://repository.library.brown.edu/studio/item/bdr:936887/ (accessed 9 February 2021).
- 135.Lynch M, Burns R., Holcomb J.. Food for thought: an investigation of food and drug administration reporting practices, 1995-1999. Crim Justice Rev 2005; 30: 293–311. [Google Scholar]
- 136.Kesselheim AS, Avorn J. The role of litigation in defining drug risk. JAMA 2007; 297: 308–311. [DOI] [PubMed] [Google Scholar]
- 137.The facts about talc safety, http://www.jnj.com/our-products/the-facts-about-talc-safety (2016, accessed 23 December 2020).
- 138.Hughes W, Kalmer T. Massive talc aspiration: successful treatment with dexamethasone. Am J Dis Child 1966; 111: 653–654. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-new-10.1177_1048291121996645 for A Review of the Talc Industry’s Influence on Federal Regulation and Scientific Standards for Asbestos in Talc by Tess Bird, Joan E. Steffen, Triet H. Tran and David S. Egilman in NEW SOLUTIONS: A Journal of Environmental and Occupational Health Policy