Abstract
Background
Alcohol consumption and related harm increase rapidly from the age of 12 years. We evaluated whether alcohol screening and brief intervention is effective and cost-effective in delaying hazardous or harmful drinking amongst low-risk or abstaining adolescents attending Emergency Departments (EDs).
Methods
This ten-centre, three-arm, parallel-group, single-blind, pragmatic, individually randomised trial screened ED attenders aged between 14 and 17 years for alcohol consumption. We sampled at random one third of those scoring at most 2 on AUDIT-C who had access to the internet and, if aged under 16, were Gillick competent or had informed consent from parent or guardian. We randomised them between: screening only (control intervention); one session of face-to-face Personalised Feedback and Brief Advice (PFBA); and PFBA plus an electronic brief intervention (eBI) on smartphone or web. We conducted follow-up after six and 12 months. The principal outcomes were alcohol consumed over the 3 months before 12-month follow up, measured by AUDIT-C; and quality-adjusted life-years.
Findings
Between October 2014 and May 2015, we approached 5,016 eligible patients of whom 3,326 consented to be screened and participate in the trial; 2,571 of these were low-risk drinkers or abstainers, consuming an average 0.14 units per week. We randomised: 304 to screening only; 285 to PFBA; and 294 to PFBA and eBI. We found no significant difference between groups, notably in weekly alcohol consumption: those receiving screening only drank 0.10 units (95% confidence interval 0.05 to 0.18); PFBA 0.12 (0.06 to 0.21); PFBA and eBI 0.10 (0.05 to 0.19).
Interpretation
While drinking levels remained low in this population, this trial found no evidence that PFBA with or without eBI was more effective than screening alone in reducing or delaying alcohol consumption.
Keywords: Alcohol, Alcohol screening, Brief intervention, Electronic brief intervention, Adolescent, Low risk, Emergency department, Randomised controlled trial, Effectiveness, Cost-effectiveness
Background and objectives
In adolescence alcohol is the most widely used substance and a major health concern (Adger & Saha, 2013). The burdens of disease, social costs and harms associated with its use are extensive and common in young adults. In addition, the development of later alcohol-related harms have been linked to early onset of drinking during adolescence (Lim et al., 2012).
Alcohol consumption often commences in early adolescence and increases rapidly to young adulthood (Heron et al., 2012). Adolescents who begin to drink at an early age are at higher risk for injury, illness, long-term alcohol misuse, or even death related to alcohol use (Bellis et al., 2009; Hingson, Heeren, & Winter, 2006). A previous study of alcohol screening in Emergency Departments (EDs) in England found the prevalence of ever drinking alcohol among adolescents was 4% for those aged 10 years and increased to 90% by age 17 with associations between alcohol consumption and earlier onset of drinking linked to poorer health and social functioning (Donoghue et al., 2017).
Several school-based, selective interventions that target non-drinking youths have delayed the onset of drinking behaviours and alcohol-related harms (Conrod, Castellanos, & Mackie, 2008; Spoth, Greenberg, & Turrisi, 2008). There is also emerging evidence that universal preventive interventions, or strategies implemented during adolescence, have the potential to delay the initiation, or decrease the rate, of alcohol consumption (McKay et al., 2018) or substance misuse and associated problems into young adulthood (Trudeau et al., 2016). A meta-analysis of these trials showed small but positive effects of interventions on adolescent alcohol use (Strøm, Adolfsen, Fossum, Kaiser, & Martinussen, 2014). But systematic reviews of the evidence are hampered by heterogeneity across interventions, populations and outcomes, and by poor reporting of the trials (Agabio et al., 2015; Foxcroft & Tsertsvadze, 2011). Hospital activity data suggests that adolescents are far more likely to seek health intervention from urgent care and emergency settings than traditional primary care settings (Gnani et al., 2014) and there is some evidence of an association between risk taking behaviour that results in an ED attendance and alcohol use (Donoghue et al., 2017). These factors combined with the fact attendance at ED creates a ‘teachable moment’ where attendees are more likely to respond to a behavioural intervention suggest ED may be an appropriate setting to deliver adolescent alcohol screening and intervention (Patton, Crawford & Touquet, 2003).
Alcohol and drugs prevention programmes facilitated by the internet using computers, tablets and smartphones are also becoming widely available (Hoeppner et al., 2017). A recent study of adolescents found lower rates of substance misuse initiation among those exposed to a web-based intervention (Walton et al., 2014). A previous review of these programmes found that they have the potential to reduce use of alcohol and other drugs and intention to use them in future (Donoghue, Patton, Phillips, Deluca, & Drummond, 2014). However, their effectiveness remains unclear, especially in adolescents (Crane, Garnett, Michie, West, & Brown, 2018). These findings, together with the implementation advantages and high fidelity associated with new technology, suggest that interventions facilitated by computers and the Internet offer a promising channel for large population-based interventions.
There is little research exploring the potential effects of different intervention approaches on alcohol consumption for those who are abstinent or low-risk users. Therefore, we designed this trial to evaluate the effectiveness and cost-effectiveness of face-to-face personalised feedback and brief advice (PFBA) and electronic brief intervention (eBI), compared with screening alone, on alcohol consumption by low-risk adolescents. A complementary trial estimated the effects of these interventions in high-risk adolescent drinkers (Deluca et al., 2020).
Our primary null hypothesis was: PFBA and eBI are no more effective than screening alone in delaying alcohol consumption, as measured 12-months after randomisation by the AUDIT-C.
Our secondary null hypotheses were:
-
1.
PFBA and eBI are no more cost-effective than screening alone in reducing alcohol consumed, as measured 12-months after randomisation assessed by AUDIT-C.
-
2.
PFBA and eBI are no more effective than screening alone in reducing alcohol-related problems and consequences in adolescents, as measured 12-months after randomisation.
Methods
Study design and baseline procedures
Our study was a three-arm, parallel-group, single-blind, pragmatic, individually randomised trial, stratified by ED and gender. We published the full trial protocol in 2015 (Deluca et al., 2015). However we later modified that protocol, with the agreement of the trial steering committee, to measure consumption by AUDIT-C rather than the Time Line Follow-Back 28-day method (TLFB28). This simplified data collection at baseline, enabled participants to complete follow up online, and thus minimised attrition.
We conducted the trial in accordance with the Declaration of Helsinki, achieved full NHS ethics approval (14/LO/0721), and registered it with the International Standard Controlled Trials Number Registry (ISRCTN45300218).
The ten participating EDs covered the North-East of England, East Yorkshire and London (full list in Acknowledgements). We approached consecutive attenders between October 2014 and May 2015 after ED staff had cleared them. We sought to include: patients between their 14th and 17th birthdays; scoring at most 2 on the AUDIT-C; able and willing to provide informed consent or have consent provided by a responsible adult; alert and orientated; able to speak English sufficiently to complete assessments; resident within 20 miles of the ED; and having access to a smartphone or internet enabled device. We excluded participants: with a severe injury requiring immediate treatment; with a serious mental health problem or psychological issues, especially if referred to specialist services; grossly intoxicated; who had received alcohol or substance use treatment in the past 6 months; or currently participating in any other alcohol research study.
We screened eligible and consenting patients for alcohol consumption in a private area of the ED using the AUDIT-C on an electronic tablet. Based on earlier work which estimated that 25% of adolescents attending ED were high-risk drinkers, we randomly selected one third of participants eligible for the low risk trial. We thanked those not selected, gave them a £5 voucher, and returned them to the care of ED staff. Those selected for participation completed the rest of the baseline questionnaires, received their allocated intervention and a reminder that a researcher would follow them up 6 and 12 months later, and then returned to the care of ED staff. They completed those follow-ups on-line, by phone or by post, as they preferred.
Randomisation and blinding
We used simple random sampling embedded in the tablet software programmed independently of the research team to select one third of eligible participants. After confirming their eligibility, taking consent, and completing baseline assessments, we stratified the resulting sample by ED and gender, and allocated them at random with equal probabilities between three groups – due to receive screening only, PFBA or eBI. We used strings generated independently of the research team, encrypted for security, and embedded within the tablet to create and randomly permute random blocks of size 3 or 6.
Though keeping participants and interventionists blind to these allocated interventions was neither feasible nor desirable in a pragmatic trial, we blinded the researchers conducting follow-ups at 6 and 12 months, and the analytical team, until primary analysis was complete.
Procedures
Trained researchers were responsible for the delivery of the allocated intervention, which was facilitated by the tablet. Full details of the interventions including screenshots and tools are available elsewhere (Deluca et al., 2020) and a brief summary of intervention components provided in Table 1.
Table 1.
Summary of trial arm components.
| Component | Screening only | Personalised feedback and brief advice (PFBA) | Personalised feedback and brief advice and Electronic Brief Intervention (EBI) |
|---|---|---|---|
| Rational, theory or goal | Control condition | Brief motivational interview to maintain abstinence or low-level consumption. | Brief motivational interview to maintain abstinence or low-level consumption and access to an interactive electronic app. |
| Materials | None | Healthy Lifestyle Leaflet | Healthy Lifestyle Leaflet and smartphone app. |
| Procedure | Screening only using AUDIT-C. | Brief advice and discussion of alcohol use, covering feedback of screening result, recommended consumption levels, normalised consumption for age, strategies to maintain abstinence or low-level drinking and sources of additional support. | In addition to PFBA participants were introduced to a smartphone or PC based app designed to help maintain abstinence or low-level consumption. The app centred around a city with specific building where advise could be sought. Participants could create drinking diaries, create goals, receive personalised feedback and seek advice regarding risks associated with alcohol use. |
| Interventionist | ED nurse or researcher | ED nurse or researcher | ED nurse or researcher, app was self-directed |
| Delivery mode | Screening tool self-completed on IPAD | Face-to-face discussion | Interaction with app was self-directed |
| Location | Emergency Department | Emergency Department | PFBA and initial introduction to the app was in the Emergency Department, interaction with the app was at the participants discretion. |
| Session duration and frequency | 1 minute, one occasion | Up to 5 minutes, one occasion | PFBA and introduction to app up to 20 minutes on one occasion. Interaction with the app was not limited in terms of duration or frequency. |
Screening only group – ‘treatment as usual’
After completing the baseline assessment, control participants received our thanks for their participation, a £5 voucher, and reminders that researchers would contact them to arrange follow-up interviews after 6 and 12 months; they then returned to the care of ED staff.
Personalised feedback and brief advice (PFBA)
This was an age-specific version of the SIPS Brief Advice About Alcohol Risk (Coulton et al., 2009; Drummond et al., 2014). We based it on the FRAMES model for brief alcohol intervention; Feedback of screening, Responsibility to be the agent of change, Advice on how to achieve change, Menu of options, Empathic style and enhancing Self-Efficacy (Hester, 1995). The intervention covered feedback on screening results and their meaning, recommended levels of alcohol consumption for young people, normative comparisons, a summary of the risks associated with alcohol consumption, the benefits of avoiding or delaying drinking, and strategies to support doing so. Each participant also received a copy of a leaflet that included additional information about alcohol intoxication, alcohol poisoning, alcohol and the law, and contact details for further support. This took an average of 5 minutes to deliver.
Personalised feedback plus a smartphone- or web-based brief intervention (eBI)
SIPS City is a mobile web application co-produced with young people and capable of working offline. Though it can use a variety of platforms, we optimised it for recent iPhone and Android phones. It was developed for this research by the software developer Codeface Ltd. in collaboration with the research team. The content was similar to our PFBA but the eBI added gaming aspects to engage and motivate users to explore and learn (facts and figures) about alcohol, receive personalised feedback, and set goals.
When possible, our researchers installed SIPS City on participants’ smartphones while they were attending ED and encouraged them to use it. When they did not have access to their phone, researchers showed them the app and its components on a study iPad; and sent email and text messages within 24 hours with instructions on how to download and install the app on their smartphone at home. Our software sent two reminders to those who had not yet installed the app. To participants without a smartphone but with internet access through other devices, we provided access to a web version of the application.
Training and fidelity
We recruited researchers experienced in healthcare or research to recruit patients and deliver the interventions. We trained them in trial procedures and the interventions, notably with examples and role play.
During the trial we assessed fidelity to the interventions by audio-recording a random 20% sample of each researcher's sessions. A senior researcher assessed whether each recording had delivered key aspects of the interventions as planned; and gave feedback to improve fidelity when necessary.
Outcomes
Our primary outcome was the average alcohol consumption in standard UK units (equivalent to 8 grams of ethanol) per week over the previous three months, estimated from responses to AUDIT-C by multiplying drinking frequency per week by average consumption per drinking occasion (Alkhaldi et al., 2016). Our previous work showed good agreement between consumption assessed by the extended AUDIT-C and TLFB28 (Donoghue et al., 2014) and the AUDIT-C has demonstrated excellent responsiveness over time (Bradley et al., 1998). AUDIT-C also assesses whether participants are considered at low risk with a score of at most 2; or at higher risk with a score of at least 3 (Coulton et al., 2018). Participants completed AUDIT-C at baseline, 6 and 12 months.
We assessed alcohol-related problems and consequences by questions 19, 21 and 22 from the ESPAD study (Hibell, Guttormsson, Ahstom, et al., 2012). These measure the frequency of common alcohol-related problems such as fighting, problems with family, peers, school and police, injuries and accidents and engaging in regretted or unprotected sexual activity. Again, participants responded at baseline, 6 and 12 months. We assessed general psychological functioning at baseline and 12-months by the Strengths and Difficulties Questionnaire (Muris, Meesters, Eijkelenboom, & Vincken, 2004). All these measures have been validated for the target adolescent population.
Economic outcomes
Our primary outcome measure for economic evaluation was health utility, estimated by the EuroQol questionnaire with 5 Dimensions and 5 Levels (EQ-5D-5L) (Herdman et al., 2011; Janssen et al., 2013). This focuses on five dimensions of health: mobility; self-care; usual activities; pain or discomfort; and anxiety or depression. We converted scores on each dimension to health utilities ranging from 1 (perfect health) through 0 (as bad as dead) to – 0.285 (extreme problems on all dimensions, thus worse than dead) using a tariff derived by the EuroQol group from social preference surveys in the UK (Devlin, Shah, Feng, Mulhern, & van Hout, 2018). Our economic measures also included the service use questionnaire known as the Client Service Receipt Inventory (CSRI), adapted to the target adolescent population.
Sample size calculation
As we know of no evidence on the minimal clinically important difference for this population, we powered the trial to detect an effect size of 0.3 or greater, equivalent to 30% of the population standard deviation, generally regarded as a small effect. To yield 80% power of detecting this difference with a two-sided significance level of 5%, one needs to analyse 175 participants followed-to month 12 in each of the three groups. Our experience of studying brief interventions suggested that loss to follow-up at 12 months was unlikely to exceed 30%. So, we increased our target sample size to 250 in each group, and 750 in total. Our previous surveys of this population suggested that 25% of adolescents screened would score at least 3 on AUDIT-C and thus be ineligible (Coulton et al., 2018; Donoghue et al., 2017). As we conducted this trial alongside a complementary high-risk study (submitted herewith) we predicted that for every high-risk participant identified through screening we would identify 3 low-risk participants. Hence, we selected a random third of eligible low-risk patients as potential participants.
Statistical analysis
We used SAS to analyse the trial ‘by treatment allocated’, that is by analysing participants as members of their allocated group irrespective of the treatment they received. The analysis team stayed blind to participants’ allocated group until they completed the main analysis.
We knew that the primary outcome – weekly alcohol consumption at month 12 – was usually positively skewed in this population. So we checked this before transforming the resulting data by taking cube roots, and transformed back once analysis was complete. Analysis of the primary outcome used a fixed effects model for allocated groups, random effects for ED, and included gender, age and baseline alcohol consumption as covariates. We also undertook sensitivity analysis by using non-parametric Wilcoxon rank sum indices within a similar fixed effects model. We assessed the influence of missing data by using multiple imputation to assess the sensitivity of observed outcomes to missing data. We analysed secondary outcomes by similar mixed-effect models adjusted for gender, age and the appropriate baseline covariate. To assess the validity of the results, we estimated Bayes factors for the primary outcome, comparing PFBA with control and eBI with control. Exploratory secondary analysis used linear regression to identify prognostic factors at baseline that may affect the primary outcome at 12 months.
Economic analysis
We undertook cost-effectiveness analysis, measuring both the resources used (costs) and the resulting gain in utility (effectiveness). We used data from individual participants to estimate mean differences in costs between interventions and control; and converted their EQ-5D-5L utility scores at baseline, and 6 and 12 months to quality adjusted life years (QALYs) using the ‘area under the curve’. We adopted two distinct perspectives – societal and that of the National Health Service and Personal Social Services (NHS+PSS).
As these data are subject to sampling error, we used stochastic sensitivity analysis in the form of 1000 non-parametric bootstrapped replications of costs and effects to derive 95% confidence intervals of the incremental cost effectiveness ratio (ICER); and cost-effectiveness acceptability curves showing the probability that interventions were cost-effective over a range of willingness to pay (WTP), typically between £20,000 and £30,000 per QALY in the UK (Briggs, 2001; Drummond, Stoddart, & Torrance, 1999).
To cost service use at 2014 prices, we used local unit costs where possible, supplemented by published national costs31 and information from previous alcohol studies (Coulton et al., 2009; Coulton et al., 2008; Drummond et al., 2009). Since we incurred costs only over 12 months, discounting was not necessary. We estimated the cost of screening and of delivering the two interventions by measuring resource inputs to each arm of the trial at 6 and 12 months, including the time of trainers and trainees, their expenses, and the cost of materials. We estimated the effects on NHS and non-NHS costs from information gathered on participants’ contact with primary care, secondary care, specialist health services, social services and the criminal justice system through our adapted version of the CSRI.
We used multiple imputation to handle data missing from individual EQ-5D-5L questions, and derived utilities from the imputed variables. We assessed whether missing costs were truly missing or truly zero, and imputed truly missing costs from the average cost of each intervention.
Results
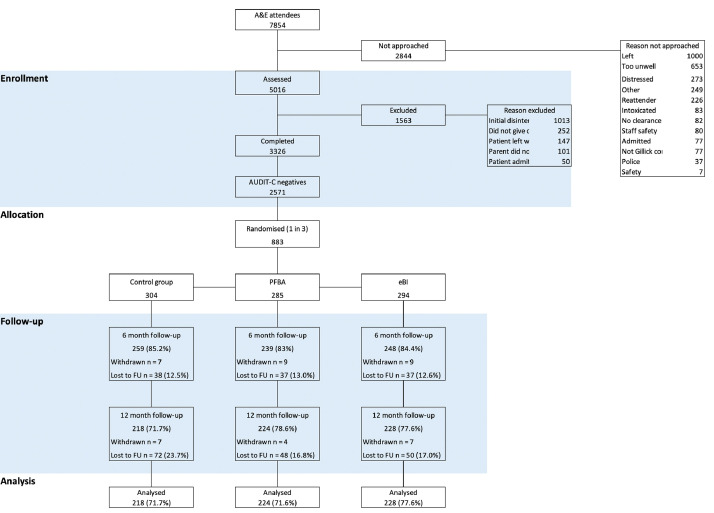
The CONSORT diagram in Fig. 1 shows the flow of patients through the trial.
Fig. 1.
Consort diagram.
Table 2 confirms that demographic and outcome variables were similar across all three groups at baseline. The mean age of trial participants was 15.1 years; 51% were female and 62% identified themselves as of white ethnicity. The mean age of their first drink was 13.8 years, and their mean weekly alcohol consumption was only 0.14 units of alcohol. The majority of participants attended ED because of a bodily injury or accident (59.2%), an infection (7.6%) or gastrointestinal complaint (6.1%). Table 3 shows adjusted least mean squares for main outcomes. Table 4 shows the mean differences and 95% confidence intervals of outcomes between PFBA and control and between eBI and control. Thus, we found no significant difference for any outcome. Our non-parametric sensitivity analysis confirmed this for the primary outcome. Furthermore, our multiple imputation models showed that missing data did not affect these findings. The Bayes factors comparing PFBA and eBI with control were 0.05 (SE 0.18) and 0.05 (SE 0.13) respectively, thus reinforcing the null findings for the primary outcome.
Table 2.
Demographic and baseline outcomes by allocated group.
| Control (n=304) | PFBA (n=285) | eBI (n=294) | |
|---|---|---|---|
| Mean age in years (SD) | 15.2 (1.1) | 15.1 (1.0) | 15.2 (1.0) |
| Mean age of first drink (SD) | 13.8 (1.7) | 13.6 (1.9) | 13.9 (1.8) |
| Male n (%) | 144 (47.4) | 145 (50.9) | 143 (48.6) |
| White n (%) | 187 (61.5) | 182 (64.0) | 180 (61.2) |
| Black n (%) | 53 (17.4) | 54 (18.9) | 51 (17.3) |
| Asian n (%) | 26 (8.6) | 20 (7.0) | 19 (6.5) |
| Other n (%) | 38 (12.5) | 29 (10.1) | 44 (15.0) |
| Smoker n (%) | 31 (10.3) | 20 (7.1) | 26 (8.7) |
| Alcohol use | |||
| Abstinent n (%) | 82 (27.0) | 73 (25.6) | 81 (27.6) |
| Mean weekly alcohol consumed (SD)a | 0.14 (0.28) | 0.14 (0.28) | 0.15 (0.29) |
| Mean AUDIT-C Score (SD) | 0.38 (0.66) | 0.40 (0.71) | 0.43 (0.72) |
| Monthly heavy episodic alcohol use n (%)b | 8 (2.6) | 21 (7.4) | 19 (6.4) |
| Ever intoxicated n (%)c | 105 (24.7) | 101 (35.4) | 102 (34.7) |
| Intoxicated in past 12 months n (%)c | 76 (25.0) | 76 (26.6) | 76 (26.0) |
| Intoxicated in past 30 days n (%)c | 18 (6.0) | 21 (7.5) | 24 (8.0) |
| Alcohol-related problems | |||
| Fighting n (%) | 33 (10.9) | 26 (9.2) | 16 (5.4) |
| Accident or injury n (%) | 51 (16.7) | 40 (14.1) | 32 (10.9) |
| Parent problems n (%) | 33 (10.9) | 20 (7.0) | 18 (6.1) |
| Peer problems n (%) | 31 (10.3) | 32 (11.3) | 20 (6.8) |
| School problems n (%) | 27 (9.0) | 26 (9.2) | 20 (6.8) |
| Victim of theft n (%) | 18 (5.8) | 6 (2.1) | 12 (4.1) |
| Police problems n (%) | 12 (3.8) | 14 (4.9) | 16 (5.4) |
| Hospitalised n (%) | 33 (10.8) | 23 (7.9) | 20 (6.8) |
| Unprotected sex n (%) | 14 (4.5) | 8 (2.8) | 18 (6.1) |
| Regretted sex n (%) | 8 (2.5) | 6 (2.1) | 10 (3.4) |
| Strengths and Difficulties | |||
| (Higher scores indicate more difficulties) | |||
| Mean overall score (SD) | 10.9 (5.7) | 10.7 (5.5) | 11.0 (5.7) |
| Mean emotional score (SD) | 3.0 (2.2) | 3.1 (2.3) | 3.2 (2.4) |
| Mean conduct score (SD) | 2.0 (1.7) | 1.9 (1.6) | 2.0 (1.6) |
| Mean hyperactivity score (SD) | 3.7 (2.3) | 3.8 (2.3) | 3.7 (2.2) |
| Mean Peer problem score (SD) | 2.1 (1.6) | 1.9 (1.6) | 2.1 (1.6) |
| Mean prosocial score (SD) | 7.8 (1.8) | 7.9 (1.8) | 7.8 (1.7) |
Measured as standard drinks where 1 standard drink = 8g ethanol.
Measured as 6 or more standard drinks in a single drinking episode.
Intoxication is self-defined as loss of control while drinking.
Table 3.
Proportion abstinent and adjusted least mean squares and 95% CI for outcomes at 6 and 12 months by allocated group.
| Control |
PFBA |
eBI |
||||
|---|---|---|---|---|---|---|
| Month 6Mean (95% CI)(n=259) | Month 12Mean (95% CI)(n=218) | Month 6Mean (95% CI)(n=239) | Month 12Mean (95% CI)(n=224) | Month 6Mean (95% CI)(n=248) | Month 12Mean (95% CI)(n=228) | |
| Alcohol use | ||||||
| Abstinent n (%) | 95 (36.4) | 100 (43.3) | 75 (33.1) | 92 (40.2) | 97 (38.8) | 100 (43.1) |
| Weekly alcohol consumed a | 0.06 (0.03; 0.10) | 0.10 (0.05; 0.18) | 0.04 (0.02; 0.07) | 0.12 (0.06; 0.21) | 0.05 (0.03; 0.09) | 0.10 (0.05; 0.19) |
| AUDIT-C Score | 0.14 (0.08; 0.22) | 0.22 (0.12; 0.36) | 0.08 (0.04; 0.14) | 0.21 (0.11; 0.35) | 0.06 (0.19; 0.21) | 0.21 (0.11; 0.35) |
| Strengths and Difficulties | ||||||
| Overall score | - | 10.8 (10.2; 11.4) | - | 10.2 (9.58; 10.8) | - | 10.4 (9.76; 11.0) |
| Emotional score | - | 3.32 (3.07; 3.57) | - | 3.06 (2.81; 3.30) | - | 3.14 (2.90; 3.39) |
| Conduct score | - | 1.58 (1.40; 1.77) | - | 1.59 (1.41; 1.77) | - | 1.75 (1.57; 1.93) |
| Hyperactivity score | - | 3.48 (3.21; 3.75) | - | 3.35 (3.08; 3.61) | - | 3.23 (2.97; 3.50) |
| Peer problem score | - | 2.41 (2.20; 2.61) | - | 2.17 (1.96; 2.37) | - | 2.29 (2.08; 2.49) |
| Prosocial score | - | 7.95 (7.71; 8.19) | - | 7.98 (7.74; 8.22) | - | 7.75 (7.51; 7.99) |
Measured as standard drinks where 1 standard drink = 8g ethanol.
Table 4.
Adjusted least mean squares difference versus control and 95% CI for outcomes at 6 and 12 months by allocated group.
| PFBA |
eBI |
|||
|---|---|---|---|---|
| Month 6Mean (95% CI) | Month 12Mean (95% CI) | Month 6Mean (95% CI) | Month 12Mean (95% CI) | |
| Alcohol use | ||||
| Weekly alcohol consumeda | -0.06 (-0.14; 0.06) | 0.03 (-0.07; 0.13) | -0.02 (-0.10; 0.06) | 0.01 (-0.10; 0.11) |
| AUDIT-C Score | -0.08 (-0.18; 0.02) | -0.01 (-0.12; 0.11) | -0.03 (-0.13; 0.07) | -0.01 (-0.12; 0.11) |
| Strengths and Difficulties | ||||
| Overall score | - | -0.58 (-1.45; 0.28) | - | -0.40 (-1.26; 0.46) |
| Emotional score | - | -0.27 (-0.62; 0.09) | - | -0.18 (-0.53; 0.17) |
| Conduct score | - | 0 (-0.25; 0.26) | - | 0.16 (-0.09; 0.42) |
| Hyperactivity score | - | -0.14 (-0.52; 0.24) | - | -0.25 (-0.63; 0.13) |
| Peer problem score | - | -0.24 (-0.50; 0.03) | - | -0.12 (-0.38; 0.15) |
| Prosocial score | - | 0.03 (-0.27; 0.33) | - | -0.20 (-0.51; 0.10) |
All participants allocated to control or PFBA received the intervention as allocated. Of those allocated to eBI, only 103 (35%) engaged with the intervention after leaving the ED. Our exploratory analysis of prognostic factors that affect alcohol consumption at month 12 identified as predictors: higher consumption at baseline; lower age of first drink; being older; being female; greater positive alcohol expectancy; and more alcohol-related problems. But we found no relationship in the eBI group between increased engagement with the intervention and alcohol consumption at 12 months.
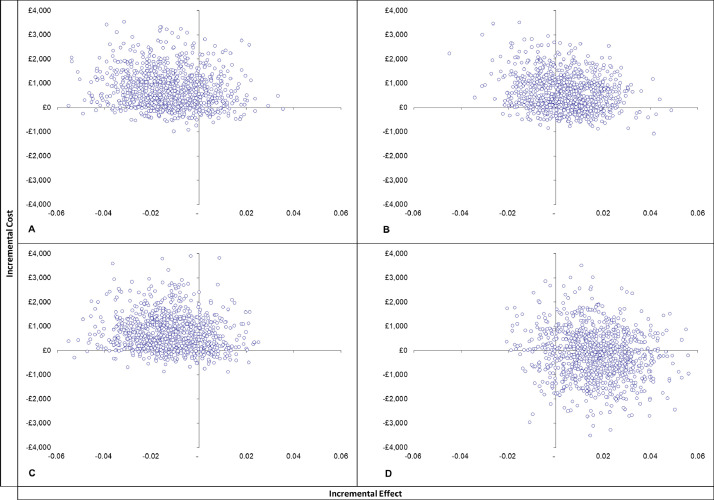
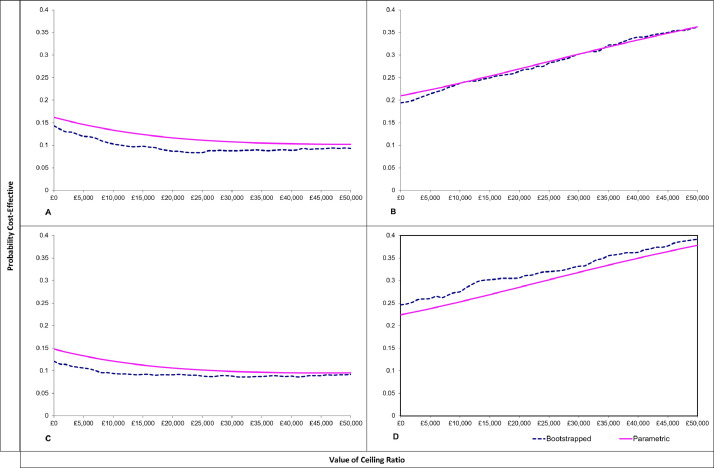
Cost-effectiveness analyses showed that, from both the societal perspective and the narrower NHS-PSS perspective, screening alone dominates the eBI intervention. The PFBA intervention generated large ICERs of £131k per QALY gained from the societal perspective and £121k from the NHS+PSS perspective (insert here cost-effectiveness planes & CEACs). Probabilistic sensitivity analysis (PSA) showed that from the societal perspective fewer than 9% of simulations for eBI versus control were cost effective at both £20,000 and £30,000 thresholds, while 26% and 30% of simulations for PFBA versus control were cost effective at the £20,000 and £30,000 thresholds respectively. From the NHS+PSS perspective PSA again showed that about 9% of simulations for eBI versus control were cost effective at both £20,000 and £30,000 thresholds, while 31% and 33% of simulations for PFBA versus control were cost effective at the £20,000 and £30,000 thresholds respectively (Figs. 2 and 3).
Fig. 2.
Cost effectiveness planes.
A. Control v EBI (societal perspective)
B. Control v PFBA (societal perspective)
C. Control V EBI (NHS/PSS perspective)
D. Control v PFBA (NHS/PSS perspective)
Fig. 3.
Cost Effectiveness Acceptability Curves.
A. Control v EBI (societal perspective)
B. Control v PFBA (societal perspective)
C. Control V EBI (NHS/PSS perspective)
D. Control v PFBA (NHS/PSS perspective)
Thus it is highly unlikely that in low risk patients either of the two interventions is cost effective at either threshold when compared with screening alone.
Discussion
The aim of this trial was to evaluate the effectiveness and cost-effectiveness of two distinct brief interventions designed to delay the onset of drinking or reduce drinking in low-risk adolescent drinkers compared with screening in emergency departments. To do so, we achieved both our recruitment and retention targets. Hence the trial was well powered to detect differences in our primary outcome between either intervention and screening alone. However careful analysis detected no hint of statistically significant difference.
Furthermore, alcohol consumption remained stable across the 12 months of follow up and there were no significant differences between groups on secondary outcome measures. Posterior Bayes Factors supported the null hypothesis that both personalised feedback and brief advice and electronic brief interventions (eBI) are as effective in reducing alcohol consumption in low-risk drinkers as screening alone. We also found that higher alcohol consumption, older age, greater positive alcohol expectancy, and greater alcohol-related problems at baseline all predicted higher levels of drinking at 12 months, consistent with previous research findings.
Limitations
The main limitation of this study is that engagement with the eBI was low, with only one third of allocated participants engaging with that after leaving hospital. This probably reduced the effect of the eBI relative to screening alone. As this was a pragmatic trial, however, this is likely to reflect the engagement to be expected in the typical adolescent identified in ED.
Low engagement with electronic applications is a common issue. The vast majority of apps, and other online interventions, are not used beyond one month after they are downloaded (Kohl, Crutzen, & de Vries, 2013). We also know that patients are less likely to engage in interventions when the onus to engage is on themselves (Drummond et al., 2014; Kaner et al., 2013; Khadjesari et al., 2011).
Much of the literature on eBI has focused on the provision of websites rather than smartphone apps (Donoghue et al., 2014). Arguably the main issue in developing an effective eBI app is engaging participants enough for them to find it useful. Engagement has been defined as how a user interacts with and experiences the technology in question (Alkhaldi et al., 2016). For example engagement with smartphone apps is often measured by the pattern of downloads, number of page visits and average session lengths. Patterns of use can identify the most and least useful features of an app. Bewick et al. showed how participant engagement with a web-based electronic intervention reduced the consumption of alcohol (Bewick et al., 2010). There is also evidence on user preferences for content, features and style, and strategies to improve engagement (Alkhaldi et al., 2016; Crane, Garnett, Brown, West, & Michie, 2015; Milward et al., 2016). But two personalised alcohol interventions apps for young adults (Drinks Meter and OneTooMany) also found no evidence of impact on risky drinking (Davies, Lonsdale, Hennelly, Winstock, & Foxcroft, 2017).
Implication for policy and practice
Alcohol consumption by participants remained very low across the three arms of the study. But we found that PFBA and eBI were no more effective than screening alone in delaying the onset of drinking or reducing alcohol consumption in this low-risk group of adolescents attending emergency departments. Hence there is no case to roll out these interventions to adolescents at low risk.
This reinforces the findings of Strom et al. (2014) who found no evidence that universal prevention programmes affect alcohol use within schools. They concluded that, with current methods of implementation, such programmes are no better than the standard alcohol curriculum in schools.
Funding
This paper reports independent research funded by a National Institute for Health Research (NIHR) Programme Grant for Applied Research (RP-PG-0609-10162). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. CD, EK and JS hold NIHR Senior Investigator awards. CD and JS were partly funded by the NIHR Specialist Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King's College London. CD was partly funded also by the NIHR Collaboration for Leadership in Applied Health Research and Care, and the NIHR Applied Research Collaboration at King's College Hospital NHS Foundation Trust. TP was funded by a NIHR Clinical Doctoral Research Fellowship and is part funded by the NIHR Yorkshire and the Humber Clinical Research Network. EK is supported by the NIHR Applied Research Collaboration, North East and North Cumbria.
Declarations of Interest
CD, JS and TP have declared personal funding above. JS has also worked to develop new and improved treatments with pharmaceutical companies from whom he and his employer (King's College London) have received honoraria, fees and travel costs. Over the past 3 years, this has included work with Indivior, MundiPharma, Camurus and Accord; and trial medication supply from Camurus. JS has also been named in a patent registration by a pharma company as inventor of a concentrated nasal naloxone spray. King's College London has also registered intellectual property on a novel buccal naloxone formulation. EK is a senior scientist in the NIHR Schools of Primary Care Research and Public Health Research, and the UKCRC Centre of Excellence in Translation Public Health Research. All other authors have no conflicts of interest to report.
Acknowledgements
We would like to thank the over 100 researchers who worked tirelessly to recruit study participants across the 10 participating EDs. Ruth McGovern, Jen Bradley, Matt Breckons, Emma Simpson, Hayley Alderson, Kirsten Hall, Jen Birch,Paul Corrigan, Jamie Rea, Emily Clare, Carly Brown, Andrea Mulligan, Lisa McDougall, Kenn Walker,Jan Milner, Steph Ogilvie, Steven Elliott, Gillian Lathan, Ashley Lowe, Nicola Connor, Lisa Dingwall, Samantha Nesbitt, Joanne Firman, Alice Hunt, Amanpreet Banga, Amy Wolstenholme, Delah Akomah, Ellen McDonald, Hannah Rose, Jon Jezak, Jordan Quinn, Kim Mihaljevic, Lauren Schumacher, Melissa Ashe, Rebecca McDonald, Sadie Boniface, Saira Shamim, Sarah Feehan, Tajinder Rai, Vera Forjaz, Vicky Brooks, Michelle Lee, Abel Jalloh, Paul Burnett, Carol Taylor, Helen Garvey, Lydia Bromley, Kate Cheung, Paul Williams, Hilary Thornton, Thomas Phillips, Gayle Clifford, Madeleine Duffy, Lyndsey Dixon, Sue Leach, Deborah Smart, Liam Spencer, Anne Marie Ianzito, Louise Carr, Mohamed Pujeh, Sarah Chamberlin, Chidimma Onyejiaka, Tara Harvey, Antionette McNulty, Pat Daly, Val Dun-Toroosian, Lorraine O’Connell, Lesley Haley, Danielle Walker, Anthony Kennedy, Lesley Alderton, Sheila Blenkin, Jill Deane, Amanda Cowton, Tarn Nozedar, Emma Grey, Chloe Barclay, Mandy Porritt, Natasha Newell, Elaine Garett, Kirsty Banham, Julie Gray, Susan Crawford, Debbie Wilson, Wendy Cheadle, June Battram, Julie Colarossi, Claire Irish, Gabrielle Osborne, Heather Marley, Jason Pickering, Karolina Bogdanowicz, Catherine Elzerbi, Susan Kelsey, Hannah Kaner, Andrea Mulligan, Rebecca Reed, Stephanie Ogilvie, Erin Graybill, Sasha Taylor, Wendy Hall, Louise Tam, Naomi Bateman, Liz Jacques, Khatiba Raja, Tom Bramhall, Samantha Lovely and Simon Flynn. Participating EDs:
St Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH
King's College Hospital, Denmark Hill, London SE5 9RS
Ealing Hospital, Uxbridge Rd, Southall UB1 3HW
Croydon University Hospital, 530 London Rd, Croydon CR7 7YE
Hull Royal Infirmary, Anlaby Rd, Hull HU3 2JZ
Darlington Memorial Hospital, Hollyhurst Rd, Darlington DL3 6HX
Queen Elizabeth Hospital, Gateshead, Queen Elizabeth Ave, Gateshead NE9 6SX
North Tees Hospital, Holdforth Road, Hartlepool TS24 9AH
South Tyneside District Hospital, Harton Ln, South Shields NE34 0PL
Sunderland Royal Hospital, Kayll Rd, Sunderland SR4 7TP
Software developers:
We thank Richard McGregor and Danny Berzon of Codeface Ltd for developing SIPS City, the electronic application for the brief intervention, and that for trial management.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.drugpo.2021.103113.
Appendix. Supplementary materials
References
- Adger H., Jr., Saha S. Alcohol use disorders in adolescents. Pediatrics in Review. 2013;34(3):103–114. doi: 10.1542/pir.34-3-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agabio R., Trincas G., Floris F., Mura G., Sancassiani F., Angermeyer M.C. A systematic review of school-based alcohol and other drug prevention programs. Clinical Practice and Epidemiology in Mental Health: CP & EMH. 2015;11(Suppl 1 M6):102–112. doi: 10.2174/1745017901511010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhaldi G., Hamilton F.L., Lau R., Webster R., Michie S., Murray E. The effectiveness of prompts to promote engagement with digital interventions: A systematic review. Journal of Medical Internet Research. 2016;18(1):e6. doi: 10.2196/jmir.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis M.A., Phillips-Howard P.A., Hughes K., Hughes S., Cook P.A., Morleo M. Teenage drinking, alcohol availability and pricing: A cross-sectional study of risk and protective factors for alcohol-related harms in school children. BMC Public Health. 2009;9 doi: 10.1186/1471-2458-9-380. 380-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick B.M., West R., Gill J., O’May F., Mulhern B., Barkham M. Providing web-based feedback and social norms information to reduce student alcohol intake: A multisite investigation. Journal of Medical Internet Research. 2010;12(5):e59. doi: 10.2196/jmir.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K.A., McDonell M.B., Bush K., Kivlahan D.R., Diehr P., Fihn S.D. The AUDIT alcohol consumption questions: reliability, validity, and responsiveness to change in older male primary care patients. Alcoholism: Clinical and Experimental Research. 1998;22(8):1842–1849. doi: 10.1111/j.1530-0277.1998.tb03991.x. [DOI] [PubMed] [Google Scholar]
- Briggs A. Handling uncertainty in economic evaluation and presenting the results. In: McGuire M.D.A.A., editor. Economic evaluation in health care: Merging theory with practice. Oxford University Press; Oxford: 2001. [Google Scholar]
- Conrod P.J., Castellanos N., Mackie C. Personality-targeted interventions delay the growth of adolescent drinking and binge drinking. Journal of Child Psychology and Psychiatry. 2008;49(2):181–190. doi: 10.1111/j.1469-7610.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- Coulton S., Alam M.F., Boniface S., Deluca P., Donoghue K., Gilvarry E. Opportunistic screening for alcohol use problems in adolescents attending emergency departments: An evaluation of screening tools. Journal of Public Health. 2018:1–8. doi: 10.1093/pubmed/fdy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton S., Bland M., Cassidy P., Deluca P., Drummond C., Gilvarry E. Screening and brief interventions for hazardous alcohol use in accident and emergency departments: A randomised controlled trial protocol. BMC Health Services Research. 2009;9:114. doi: 10.1186/1472-6963-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton S., Watson J., Bland M., Drummond C., Kaner E., Godfrey C. The effectiveness and cost-effectiveness of opportunistic screening and stepped care interventions for older hazardous alcohol users in primary care (AESOPS) - A randomised control trial protocol. BMC Health Services Research. 2008;8:129. doi: 10.1186/1472-6963-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane D., Garnett C., Brown J., West R., Michie S. Behavior change techniques in popular alcohol reduction apps: Content analysis. Journal of Medical Internet Research. 2015;17(5):e118. doi: 10.2196/jmir.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane D., Garnett C., Michie S., West R., Brown J. A smartphone app to reduce excessive alcohol consumption: Identifying the effectiveness of intervention components in a factorial randomised control trial. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-22420-8. 4384-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E.L., Lonsdale A.J., Hennelly S.E., Winstock A.R., Foxcroft D.R. Personalized digital interventions showed no impact on risky drinking in young adults: A pilot randomized controlled trial. Alcohol and Alcoholism. 2017;52(6):671–676. doi: 10.1093/alcalc/agx051. [DOI] [PubMed] [Google Scholar]
- Deluca P., Coulton S., Alam M.F., Boniface S., Donoghue K., Gilvarry E. Adolescent alcohol use disorders presenting through emergency departments: Development and randomised controlled trial of age-specific alcohol screening and brief interventions (SIPS Junior Research Programme) Programme Grants for Applied Research. 2020;8(2) doi: 10.3310/pgfar08020. [DOI] [Google Scholar]
- Deluca P., Coulton S., Alam M.F., Cohen D., Donoghue K., Gilvarry E. Linked randomised controlled trials of face-to-face and electronic brief intervention methods to prevent alcohol related harm in young people aged 14-17 years presenting to Emergency Departments (SIPS junior) BMC Public Health. 2015;15:345. doi: 10.1186/s12889-015-1679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin N.J., Shah K.K., Feng Y., Mulhern B., van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Economics. 2018;27(1):7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K., Patton R., Phillips T., Deluca P., Drummond C. The effectiveness of electronic screening and brief intervention for reducing levels of alcohol consumption: A systematic review and meta-analysis. Journal of Medical Internet Research. 2014;16(6):e142. doi: 10.2196/jmir.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue K., Rose H., Boniface S., Deluca P., Coulton S., Alam M.F. Alcohol consumption, early-onset drinking, and health-related consequences in adolescents presenting at emergency departments in England. Journal of Adolescent Health. 2017;60(4):438–446. doi: 10.1016/j.jadohealth.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Drummond C., Coulton S., James D., Godfrey C., Parrott S., Baxter J. Pilot study of the effectiveness and cost-effectiveness of stepped care intervention for alcohol use disorders in primary care - STEPWICE trial. British Journal of Psychiatry. 2009 doi: 10.1192/bjp.bp.108.056697. In press. [DOI] [PubMed] [Google Scholar]
- Drummond C., Deluca P., Coulton S., Bland M., Cassidy P., Crawford M., ... The effectiveness of alcohol screening and brief intervention in emergency departments: a multicentre pragmatic cluster randomized controlled trial. PLoS One. 2014;9(6):e99463. doi: 10.1371/journal.pone.0099463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M., Stoddart G., Torrance G. Oxford University Press; Oxford: 1999. Methods for the economic evaluation of health care programmes. [Google Scholar]
- Foxcroft D.R., Tsertsvadze A. Universal school-based prevention programs for alcohol misuse in young people. Cochrane Database of Systematic Reviews. 2011;(5) doi: 10.1002/14651858.CD009113. [DOI] [PubMed] [Google Scholar]
- Gnani S., McDonald H., Islam. S, Ramzan F., Davidson M., Ladbrooke T. Patterns of healthcare use among adolescents attending an urban general practitioner-led urgent care centre. Emergency Medical Journal. 2014;31:630–636. doi: 10.1136/emermed-2012-202017. [DOI] [PubMed] [Google Scholar]
- Herdman M., Gudex C., Lloyd A., Janssen M., Kind P., Parkin D. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Quality of Life Research. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron J., Macleod J., Munafò M.R., Melotti R., Lewis G., Tilling K. Patterns of alcohol use in early adolescence predict problem use at age 16. Alcohol and Alcoholism. 2012;47(2):169–177. doi: 10.1093/alcalc/agr156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R.K., Miller W.R. 2nd ed. Boston MA; 1995. Handbook of alcoholism treatment approaches. [Google Scholar]
- Hibell B, Guttormsson, U., Ahstom, S., et al. (2012). The 2011 ESPAD report; substance use among students in 36 European countries. http://www.espad.org/uploads/espad_reports/2011/the_2011_espad_report_full_2012_10_29.pdf (last accessed 26/01/2018): The Swedish Council for Information on Alcohol and Other Drugs; 2011.
- Hingson R.W., Heeren T., Winter M.R. Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine. 2006;160(7):739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Hoeppner B.B., Schick M.R., Kelly L.M., Hoeppner S.S., Bergman B., Kelly J.F. There is an app for that – Or is there? A content analysis of publicly available smartphone apps for managing alcohol use. Journal of Substance Abuse Treatment. 2017;82:67–73. doi: 10.1016/j.jsat.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Janssen M.F., Pickard A.S., Golicki D., Gudex C., Niewada M., Scalone L. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Quality of life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2013;22(7):1717–1727. doi: 10.1007/s11136-012-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner E., Bland M., Cassidy P., Coulton S., Dale V., Deluca P. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): Pragmatic cluster randomised controlled trial. BMJ. 2013;346:e8501. doi: 10.1136/bmj.e8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadjesari Z., Murray E., Kalaitzaki E., White I.R., McCambridge J., Thompson S.G. Impact and costs of incentives to reduce attrition in online trials: two randomized controlled trials. Journal of Medical Internet Research. 2011;13(1):e26. doi: 10.2196/jmir.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl L.F., Crutzen R., de Vries N.K. Online prevention aimed at lifestyle behaviors: A systematic review of reviews. Journal of Medical Internet Research. 2013;15(7):e146. doi: 10.2196/jmir.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., ... A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay M., Agus A., Cole J., Doherty P., Foxcroft D., Harvey S. Steps towards alcohol misuse prevention programme (STAMPP): A school-based and community-based cluster randomised controlled trial. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward J., Khadjesari Z., Fincham-Campbell S., Deluca P., Watson R., Drummond C. User Preferences for content, features, and style for an app to reduce harmful drinking in young adults: Analysis of user feedback in app stores and focus group interviews. JMIR mHealth uHealth. 2016;4(2):e47. doi: 10.2196/mhealth.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Meesters C., Eijkelenboom A., Vincken M. The self-report version of the Strengths and Difficulties Questionnaire: Its psychometric properties in 8- to 13-year-old non-clinical children. British Journal of Clinical Psychology. 2004;43(4):437–448. doi: 10.1348/0144665042388982. [DOI] [PubMed] [Google Scholar]
- Patton R., Crawford M.J., Touquet R. Impact of health consequences feedback on patients acceptance of advice about alcohol consumption. Emergency Medical Journal. 2003;20:451–452. doi: 10.1136/emj.20.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R., Greenberg M., Turrisi R. Preventive interventions addressing underage drinking: State of the evidence and steps toward public health impact. Pediatrics. 2008;121(Suppl 4(Suppl 4)):S311–S336. doi: 10.1542/peds.2007-2243E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm H.K., Adolfsen F., Fossum S., Kaiser S., Martinussen M. Effectiveness of school-based preventive interventions on adolescent alcohol use: A meta-analysis of randomized controlled trials. Substance Abuse Treatment, Prevention, and Policy. 2014;9(1):48. doi: 10.1186/1747-597X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau L., Spoth R., Mason W.A., Randall G.K., Redmond C., Schainker L. Effects of adolescent universal substance misuse preventive interventions on young adult depression symptoms: Mediational modeling. Journal of Abnormal Child Psychology. 2016;44(2):257–268. doi: 10.1007/s10802-015-9995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M.A., Resko S., Barry K.L., Chermack S.T., Zucker R.A., Zimmerman M.A. A randomized controlled trial testing the efficacy of a brief cannabis universal prevention program among adolescents in primary care. Addiction. 2014;109(5):786–797. doi: 10.1111/add.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.