Abstract
Autophagy is a degradative pathway during which autophagosomes are formed that enwrap cytosolic material destined for turnover within the lytic compartment. Autophagosome biogenesis requires controlled lipid and membrane rearrangements to allow the formation of an autophagosomal seed and its subsequent elongation into a fully closed and fusion-competent double membrane vesicle. Different membrane remodeling events are required, which are orchestrated by the distinct autophagy machinery. An important player among these autophagy proteins is the small lipid-modifier Atg8. Atg8 proteins facilitate various aspects of autophagosome formation and serve as a binding platform for autophagy factors. Also Rab GTPases have been implicated in autophagosome biogenesis. As Atg8 proteins interact with several Rab GTPase regulators, they provide a possible link between autophagy progression and Rab GTPase activity. Here, we review central aspects in membrane dynamics during autophagosome biogenesis with a focus on Atg8 proteins and selected Rab GTPases.
Keywords: Autophagy, Atg8 proteins, Rab GTPases, Membrane dynamics
Highlights
-
•
Comprehensive overview of membrane processes during autophagosome formation
-
•
Detailed elucidation of the role of Atg8 proteins in autophagosome biogenesis
-
•
Insights on the function of Rab GTPases and their effector proteins in autophagy
1. Introduction
Autophagy is a highly conserved intracellular process responsible for the degradation of a diverse range of cellular components. During macroautophagy, which is the principal autophagy pathway, double membrane vesicles, called autophagosomes, are formed de novo and engulf cytoplasmic material that is destined for degradation within vacuoles in yeast and plants or lysosomes in higher eukaryotes. Depending on the mode of cargo selection, macroautophagy (hereafter termed autophagy for simplicity) can be a bulk, non-selective or a selective process. Nutrient depletion or reduced energy levels induce the uptake of random cytoplasmic material into autophagosomes during bulk autophagy. The subsequent turnover of the material into its building blocks and their recycling allows cells to maintain their energy and metabolite levels and, hence, cellular homeostasis. Selective autophagy also supports cell survival by removing distinct cargo, such as damaged organelles or protein aggregates as well as pathogens.
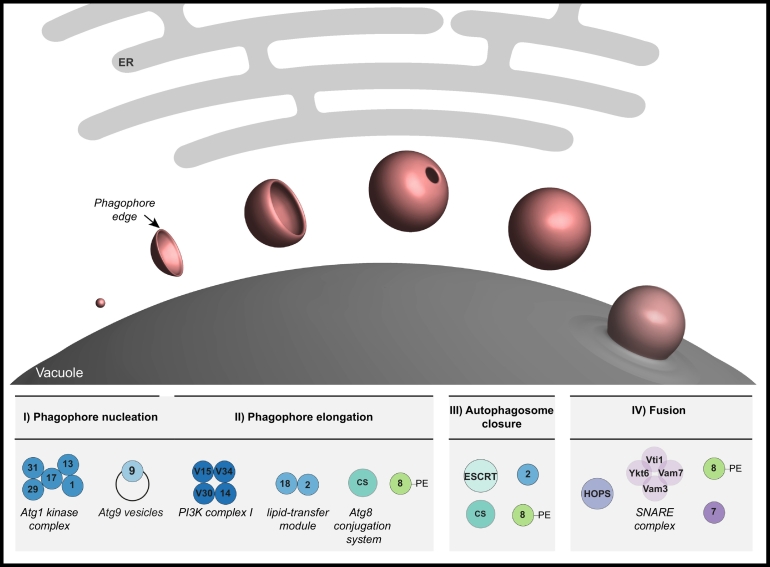
Autophagosome formation requires multiple steps: i) nucleation of an initial membrane, ii) controlled expansion of the nascent membrane (phagophore, also called isolation membrane) around the cargo material, iii) final closure of the double membrane vesicle and iv) subsequent vesicle fusion with the lytic compartment (Fig. 1). All these events require orchestrated membrane rearrangements. Many of these processes are initiated and controlled by so-called Atg (autophagy-related) proteins. More than 40 Atg proteins have been identified so far and of these around 20 core autophagy proteins regulate discrete steps during autophagosome biogenesis. The core autophagy proteins can be clustered into functional groups: the Atg1 kinase complex, the class III phosphatidylinositol-3 kinase complex I (PIK3C3-CI), the Atg9/Atg2-Atg18 lipid transfer machinery and the dual ubiquitin-like conjugation system (Fig. 1). Autophagosome formation relies on the concerted actions of these modules and is briefly summarized in the following for yeast during bulk autophagy.
Fig. 1.
Autophagosome formation in yeast. A membrane precursor enwraps cargo material (not shown) by elongating into a closed double membrane vesicle, which finally fuses with the vacuole. The process of autophagosome formation is tightly regulated by an orchestrated Atg machinery (different blue/green shades) including the Atg1 kinase complex (composed of Atg1, Atg13, Atg17, Atg29, Atg31), Atg9 vesicles, the core PIK3C3-CI (composed of Vps34, Vps15, Vps30, Atg14), the Atg2-Atg18 module, and the Atg8 conjugation system including Atg4 (CS), which promotes Atg8 lipidation to PE. Some early Atg members also play a role during late autophagy steps such as the Atg8 conjugation system in autophagosome closure. This scission step is also facilitated by the ESCRT machinery (turquoise). Autophagosome-vacuole fusion is promoted by the SNARE proteins (light purple) Ykt6, Vti1, Vam7 and Vam3, the tethering complex HOPS (purple), and the Rab GTPase Ypt7 (dark purple). Lipidated Atg8 (green) is implicated in various steps throughout autophagosome biogenesis.
At the onset of autophagy, a phagophore assembly site (PAS, also called pre-autophagosomal structure) forms at the vacuole and in close proximity to the endoplasmic reticulum (ER) [1,2]. PAS formation is initiated by the assembly of Atg1 kinase complexes into dynamic high-molecular structures, which results in the clustering of the serine/threonine Atg1 kinase and its consequential activation, allowing the recruitment of other Atg proteins [[3], [4], [5]]. Subsequently, small Golgi-derived vesicles containing the transmembrane protein Atg9 are targeted to this early PAS structure, potentially constituting the nucleating membrane for the nascent phagophore [6]. A key modification at the early phagophore is the generation of phosphatidylinositol-3-phosphate (PI3P) by the PIK3C3-CI [[7], [8], [9], [10]]. PI3P serves to recruit further Atg factors like the Atg2-Atg18 module, which contributes to the transfer of lipids to the growing autophagosomal membrane. Phagophore elongation is additionally promoted by the covalent attachment of Atg8 to phosphatidylethanolamine (PE) present in the membrane. This ubiquitin-like protein plays a versatile role during autophagosome biogenesis [[11], [12], [13], [14]] and will be discussed in more detail throughout this review. Once the cargo material is completely enwrapped, the vesicle is sealed and most autophagy proteins are released from the outer membrane [15,16]. Lastly, the outer autophagosomal membrane fuses with the vacuole where the inner vesicle containing the cargo is degraded (Fig. 1). In general, these processes are largely conserved from yeast to mammals. Many yeast Atg proteins have more than one homologue in higher eukaryotes with different functional roles described, but the biological importance of this functional diversity remains largely open.
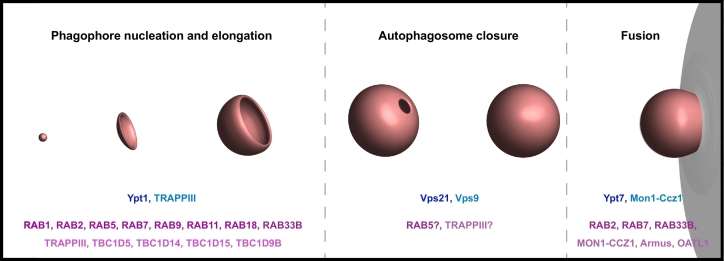
Recruitment and regulation of the different Atg modules depends on intermolecular signaling processes, which are still not well understood. Good candidates to mediate between the consecutive steps during the formation of autophagosomes are Rab GTPases, and many have been reported to control different steps during autophagosome biogenesis [[17], [18], [19], [20], [21], [22], [23], [24], [25]], (Fig. 2; Table 1, Table 2). The evolutionary highly conserved Rab GTPases are key regulators of intracellular membrane trafficking events including vesicle sorting, budding events, membrane tethering, and fusion and scission processes [26]. Their versatile role stems from their ability to be flexibly integrated into diverse intracellular membranes through C-terminal prenylation sites, and to interact with a huge variety of so-called effector proteins. Rab GTPases cycle between an inactive GDP-bound state in the cytosol, during which the prenyl-groups are shielded by a GDP dissociation inhibitor (GDI), and an active GTP-bound state at the membrane [26,27]. The exchange from GDP to GTP, facilitated by guanine exchange factors (GEFs), causes a conformational change within the Rab GTPases and functions like an off/on-switch. Consequently, this reduces the affinity to the GDI and stabilizes Rab GTPases at the membrane. Further, it allows protein-protein interactions between Rab GTPases and their effector proteins, which are then enabled to function in membrane remodeling processes [26,27]. Finally, the intrinsic GTPase activity of Rab GTPases, which is enhanced by GTPase activating proteins (GAPs), results in the hydrolysis of GTP to GDP and the abrogation of the signaling pathway. Interestingly, many Rab GTPases or their regulators interact with Atg8 proteins [28,29].
Fig. 2.
Rab GTPases in autophagy. Rab GTPases play essential roles throughout autophagy in yeast and mammals. The Rab GTPases (yeast: dark blue; metazoan: dark purple), GEF and GAP proteins (yeast: light blue; metazoan: light purple) included in this review are depicted in association with the autophagy steps they are proposed to function in.
Table 1.
Selected yeast Rab GTPases and their regulators in autophagy.
| Rab GTPase (yeast) | GEF | GAP | Function | Literature |
|---|---|---|---|---|
| Ypt1 | TrappIII | Gyp1a | Ypt1 and TrappIII associate with Atg9 vesicles and the PAS. TrappIII regulates Atg9 cycling. Ypt1 mediates Atg1 localization to the PAS. Gyp1 together with Atg8 regulates selective autophagy. |
[19,48,49,208,227] |
| Vps21 | Vps9 | Vps21 and Vps9 are involved in the regulation of ESCRT machinery for autophagosome closure. | [21,217,221] | |
| Ypt7 | Mon1-Ccz1a | Ypt7 and Mon1-Ccz1 mediate autophagosome-vacuole fusion. | [23,196.235] |
Direct interaction with Atg8 proteins.
Table 2.
Selected metazoan Rab GTPases and their regulators in autophagy.
| RAB GTPase (metazoan) | GEF | GAP | Function | Literature |
|---|---|---|---|---|
| RAB1 | TRAPPIII | RAB1 mediates the recruitment of the ULK1 complex from the cytosol and controls ATG9 cycling. ATG9 vesicles are positive for RAB1. | [18,[50], [51], [52]] | |
| RAB2 | OATL1 (also known as TBC1D25) | RAB2 interacts with ULK1 and mediates the association between ULK1 and ATG9. ATG9 vesicles are positive for RAB2. RAB2 stimulates ULK1 activity. RAB2 mediates autophagosome-lysosome fusion. |
[24,238] | |
| RAB5 | RAB5 stimulates the activity of PIK3C-CI as well as of PI(4)- and PI(5)-phosphatases. RAB5 activity stimulates ATG12-ATG5 conjugation. |
[[109], [110], [111], [112]] | ||
| RAB7 | MON1/CCZ1/C18orf8 |
Armusa (also known as TBC1D2A), TBC1D5a, TBC1D15a |
RAB7 mediates intracellular autophagosome trafficking and autophagosome-lysosome fusion. Further roles of RAB7 include regulation of membrane expansion during mitophagy. RAB7B regulates Atg4 activity. RAB7 GAPs are involved in ATG9 trafficking. The GEF MON1/CCZ1/C18orf8 mediates autophagosome-lysosome fusion. |
[17,22,56,57,197,201,[[203], [204], [205],237] |
| RAB9 | RAB9 mediates unconventional autophagy (independent of Atg8 conjugation machinery). | [87] | ||
| RAB11 |
TBC1D9Ba, TBC1D15a |
RAB11 acts on recycling endosomes which are ULK1 positive and function in ATG9 trafficking and further provide a phagophore assembly platform. RAB11A binds WIPI2 and enhances WIPI2 association with membranes. |
[20,51,55,58,206] | |
| RAB18 | RAB18 acts on lipid droplets and influences ATG9 trafficking. | [25] | ||
| RAB33B | OATL1a (also known as TBC1D25) | OATL1 is implicated in autophagosome-lysosome fusion. RAB33B binds ATG16L and enhances ATG16L association with membranes. |
[138,139,209] |
Direct interaction with Atg8 proteins.
In this review, we will discuss how the different Atg modules and their interactors in yeast and mammals regulate membrane events during autophagosome formation, with a special focus on the small modifier Atg8, as well as selected Rab GTPases.
2. Membrane sources during autophagosome nucleation and elongation
Compared to canonical vesicle formation during which vesicles bud from an existing membrane, autophagosome biogenesis is considered a de novo process. Nonetheless, it still occurs at membrane sites or in close proximity to membrane structures. Autophagosome nucleation and elongation in yeast takes place at the vacuolar surface in close vicinity to the ER. The Atg1 complex member Atg13 interacts with the vacuolar-rim resident protein Vac8, thereby directing PAS assembly and subsequent recruitment of further Atg proteins to the vacuole [2].
In mammalian cells, autophagosome formation occurs at the ER. The initial phagophore is found in close contact with a ring-shaped ER subdomain denominated omegasome, with which the nascent autophagosomal structure interacts dynamically [30]. Forming mammalian autophagosomes can also have simultaneous contacts with other organelles, including late endosomes, Golgi and mitochondria [[30], [31], [32]].
In yeast, clustering and consequential activation of the Atg1 kinase is crucial for autophagosome initiation both in bulk and in selective autophagy [[3], [4], [5]] and the same appears to hold true for the mammalian Atg1 homologue ULK1 [[33], [34], [35]]. Thus, the large membrane surface of the vacuole or ER are optimal to serve as platforms for the formation of the supramolecular protein assemblies needed. While in yeast it is unclear whether the only function of the vacuole is to allow the assembly of the early PAS proteins or whether this membrane site has other functions during phagophore formation, in mammals, the ER and its contact sites with mitochondria are not only of particular importance during the recruitment of the ATG machinery, but both also supply lipids to nascent autophagosomes during starvation [[36], [37], [38], [39]].
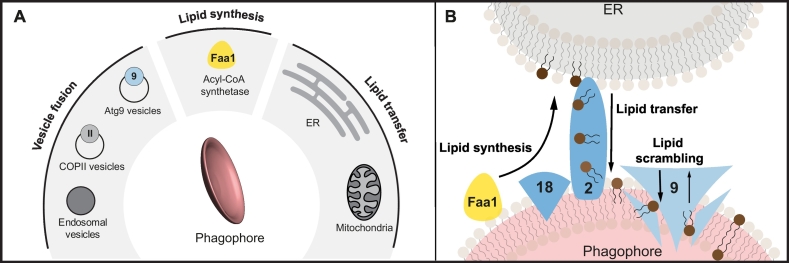
Once autophagosome formation is initiated, the nascent phagophore undergoes rapid elongation, resulting in the engulfment of the cargo within a few minutes [30]. This suggests that pre-existing membrane structures form the nucleating phagophore and that efficient lipid supply is available for the expansion of the autophagosomal membrane. The possible membrane sources of the autophagosome have been a matter of debate during the last decades, with all the main membranous compartments (e.g. ER, mitochondria, Golgi, endocytic vesicles and the plasma membrane) reported as possible suppliers for autophagosome biogenesis (Fig. 3A) [32].
Fig. 3.
Membrane sources for autophagosome formation. A) Possible sources of lipids. B) Proposed molecular mechanism of autophagosome growth. Once the precursor membrane is formed, Faa1 produces new lipids at autophagosome-ER contact sites, which are transferred to the growing phagophore by the Atg2-Atg18 complex. The scrambling activity of Atg9 might redistribute the lipids to both sides of the phagophore lipid bilayer.
Central in phagophore formation are Atg9-containing vesicles. Atg9 is a transmembrane protein found in numerous mobile cytosolic vesicles [6,40]. In yeast, these vesicles are recruited to the PAS via their interaction with the Atg1 complex members Atg17, Atg13 and Atg11 [[41], [42], [43], [44]] and an assembly of around three Atg9 vesicles is suggested to contribute to the initial phagophore [6,45,46]. These PAS-localized Atg9 vesicles would likely require fusion factors if they formed the initial phagophore by homotypic vesicle fusion [6]. Interestingly, yeast Atg9 vesicles have been found to be positive for the Rab GTPase Ypt1 and the TRAPPIII complex member Trs85, which are important to allow Atg9 vesicle shuttling to the PAS [19], however, they also might recruit further vesicular components to promote homotypic vesicle fusion [47]. As Ypt1 also associates with the Atg1 complex members Atg1, Atg11 and Atg17, it might support PAS formation [48,49]. In mammals, the Ypt1 homologue RAB1A/B promotes autophagy initiation by targeting the ULK1 complex to the phagophore formation site and by mediating Atg9 trafficking [[50], [51], [52]].
In mammals, the precursor membrane evolves from the ER [30] and has been shown to depend on ATG9 vesicles [53]. The localization of ATG9 at the autophagosome precursor appears to be only transient [54], making homotypic fusion events unlikely. Atg9 vesicle trafficking in higher eukaryotes is regulated by many Rab GTPases like RAB1, RAB2, RAB7, RAB11, RAB18 and/or its regulators like the TRAPPIII complex, the Rab GAPs TBC1D9B, TBC1D14 or TBC1D5 [18,24,25,51,52,[55], [56], [57]] (Table 2). These proteins are later often found to be present at the phagophore where they have been implicated in autophagy initiation. RAB1 and RAB2, for example, regulate ULK1 complex assembly or ULK1 translocation to the autophagosome formation site [24,50]. RAB11 has been implicated in the early assembly of Atg proteins and to recruit the mammalian Atg18 homologue WIPI2 to forming autophagosomes [58]. Thus, Atg9 vesicles do not only provide a membrane source but also shuttle relevant factors to the initiation site, which regulate autophagosome biogenesis.
Once the precursor membrane is formed, its elongation depends on lipid supply from different membrane sources [46,59]. These lipids are delivered by direct translocation from close membrane pools, or from distant sources through vesicular transport. Recent work from independent groups has shown that yeast and mammalian Atg2 can tether adjacent membranes and promote lipid transfer between two close membranous structures in vitro [38,39,[60], [61], [62]]. Thus, Atg2, in association with yeast Atg18 or mammalian PROPPIN family member WIPI4 and Atg9, forms a contact site between the ER and the nascent autophagosome to transport phospholipids to the growing phagophore [38,39,[61], [62], [63]]. This process likely only occurs at the edge of the nascent autophagosome, where these proteins localize [64]. Next to the ER, mitochondria have also been implicated in phagophore expansion [32,37]. In this line, mammalian ATG2 localizes to ER-mitochondria contact sites by interacting with the outer mitochondrial membrane proteins TOM70 and TOM40. ATG2 then together with ATG9 vesicles but independent of WIPI4 promotes phagophore growth [65]. Also Atg9 vesicles likely contribute to phagophore elongation in yeast and mammals, explaining the requirement of continuous Atg9 trafficking from different intracellular membrane sources [20,40,[66], [67], [68], [69]].
Mechanistically, Atg2 belongs to the recently identified chorein_N family of lipid transporters, which are characterized by their ability to accommodate several phospholipids at once and to form ‘tunnels’, through which a rapid lipid flow can be achieved [39,70] (Fig. 3B). This differs from traditional slower lipid transport modes, in which proteins work as ‘shuttles’, transferring one lipid at a time [71]. Such a mechanism by which only lipids are pumped into the forming autophagosome from donor organelles could explain why autophagosomes largely lack membrane proteins. If phagophore growth would solely occur by fusion of pre-existing membrane sources, the autophagosome would likely contain a higher abundance of membrane proteins, similar to the membrane supplier [72,73].
The recently solved crystal structure of human and yeast Atg9 has shown that this transmembrane protein forms a homotrimer with ramified cavities [[74], [75], [76]]. These cavities might allow shuttling of phospholipids across the membrane [[74], [75], [76]]. Indeed, Atg9 from both species behaves as a lipid scramblase in vitro, allowing the ATP-independent bidirectional transport of lipids [74,75]. As incoming lipids from Atg2 will be integrated into the cytoplasmic side of the membrane bilayer and would need to be transported to the luminal side to avoid aberrant membrane formation, an Atg9 scrambling action could allow such a redistribution of lipids. How Atg9 could prioritize transfer from the cytosolic to the luminal side of the phagophore membrane is unclear. It has been suggested that the unique asymmetric structure of Atg9 facilitates a unidirectional lipid transfer (Fig. 3B). In addition, Atg9 might act together with a regulatory partner. Phosphorylation of Atg9 by Atg1 is required for the initial phagophore formation and growth [77]. If Atg1 regulates Atg9 lipid transfer directionality remains to be addressed. In mammals, Atg9 vesicles are described to only transiently interact with the nascent autophagosomes and not to become an integral part. Whether here ER-resident scramblases, a novel group of proteins recently discovered [78,79], contribute in the lipid redistribution of the phagophore, or whether Atg9 still plays a direct role in lipid redistribution, requires further investigation. As hardly any autophagosomal membranes are formed in Atg9 scramblase mutants [75], the scramblase activity of Atg9 could alternatively influence upstream Atg9 trafficking.
Organelle membranes rather integrate newly synthesized than recycled phospholipids. Thus, it has been hypothesized for several years that de novo phospholipid biogenesis contributes to the expansion of the autophagosomal membrane [72]. The yeast Acyl-CoA synthetase Faa1 is recruited to nascent autophagosomes. It assists in supplying lipids by providing activated fatty acids to adjacent ER regions, where de novo synthesis of phospholipids takes place [80]. A direct connection between Faa1-mediated synthesis of phospholipids in the phagophore-contacting ER regions and the Atg2 lipid transfer from the ER to the phagophore has not yet been shown. It seems however likely that both processes work cooperatively, promoting an ‘on-demand’ synthesis of lipids on the ER, that are redirected to the growing autophagosomal membrane. The local synthesis of lipids in the neighboring ER regions promoted by Faa1 could contribute to the unidirectional transfer of lipids from the ER to the phagophore, which would otherwise be energetically unfavorable [75] (Fig. 3B). The human Faa1 homologue ACSL4 can complement Faa1 defects in yeast, which suggests a functional conservation [80]. Moreover, the ULK1 complex localizes to ER subdomains positive for several enzymes involved in lipid synthesis such as phosphatidylinositol synthase [53]. This synthase is involved in PI formation and thereby possibly contributes to autophagosome biogenesis [53].
Another possible lipid source for autophagosome elongation are COPII-coated vesicles, which control the transport of lipids and proteins from the ER to the Golgi apparatus [81]. The close proximity between the phagophore and the ER exit sites in yeast makes it likely that these vesicles can be redirected to the phagophore during autophagy induction [28,64]. However, the degree in which these membranes contribute to the phagophore elongation remains to be addressed. In mammals, COPII vesicles are formed from the ER-Golgi intermediate compartment (ERGIC) during autophagy. They seem to contribute rather to autophagosome precursor formation than to membrane elongation [82,83].
Further organelles have been implicated in autophagosome formation. The plasma membrane has been proposed to contribute in higher eukaryotes, as both ATG9 and ATG16L are incorporated into distinct pools of clathrin-coated vesicles originating from the plasma membrane [69]. These vesicles traffic along the endocytic pathway and fuse to contribute to phagophore formation when autophagy is induced [69,84]. A possible role of the nuclear membrane has so far only been described for unconventional autophagy in mammals [85] and yeast [86]. Unconventional autophagy can also rely on membranes derived from the trans-Golgi network and late endosomes, in a response orchestrated by RAB9 in mammals [87]. Although autophagosomes form in close proximity of the vacuole in yeast, no autophagosomal membrane supply has been reported for this organelle yet.
It is likely that these different membrane sources described in this section are not mutually exclusive and work either cooperatively or in a context-dependent manner, for instance in response to a bulk or selective stimulus or dependent on the kind of cargo.
3. Membrane modifications at the phagophore
3.1. The PI3P platform
There are two major lipid modifications serving as binding platforms for many autophagy effectors and driving autophagy, namely PI3P and PE-lipidated Atg8. While PI3P is not solely present at autophagosomal membranes, it fulfills many crucial signaling functions at autophagosomal membranes. In yeast, the percentage of the PI3P precursor lipid phosphatidylinositol (PI) at Atg8-containing membranes is with around 37% [80] much higher than the average abundance found at any other yeast organelle membrane with ~16% [88]. While PI3P can be generated via different pathways including dephosphorylation of phosphoinositides such as PI(3,4)P2 [89], the major route of PI3P production at autophagosomal membranes is believed to be via the PIK3C3-CI. In yeast and mammals, the core complex I is composed of Vps30/Atg6 (BECN1), Atg14 (ATG14L), Vps15 (VPS15/p150) and Vps34 (VPS34), whereas Vps34 is the catalytically active lipid kinase subunit and Atg14 is essential to link the PI3 kinase complex I to the autophagosome formation site [7,90,91] (Fig. 1). In addition, different regulators and subunits of the Vps34 kinase complexes are known, which influence the specificity and activity of the lipid kinase, e.g. during late steps in mammalian autophagy, ATG14L is replaced by UVRAG, forming the PIK3C3 complex II (PIK3C3-CII) [8,10,[92], [93], [94]].
During autophagy initiation, the mammalian PIK3C3-CI activity is enhanced by ULK1-dependent phosphorylation [95,96], which might partially be conserved in yeast [97]. Impairment of the PIK3C3-CI activity leads to autophagy defects both in yeast and in mammals [[7], [8], [9],90]. In yeast, the PIK3C3-CI seems to act primarily at the vacuole-phagophore contact site [64] and in mammals, PI3P is enriched at the omegasome [30]. At the nascent autophagosomal membrane PI3P signals to recruit a plethora of autophagy effector proteins, which can associate with PI3P directly through PI3P-binding domains [98]. Clustering of PI3P is found to induce small buds within membranes [99,100], which could enlarge the binding surface. A prominent example of PI3P-binding proteins important in autophagy is the PROPPIN family including yeast Atg18, Atg21 as well as mammalian WIPI1-4. While Atg21 only seems to have orthologues in yeast species [101], yeast Atg18 is closely related to WIPI1 and WIPI2, and mammalian WIPI3 and WIPI4 constitute a separate paralogue group [102]. In yeast, Atg18 or Atg21 ensure proper membrane elongation by mediating the recruitment of the lipid transfer protein Atg2 or of the Atg8 conjugation machinery, respectively. In mammals, similar functions have been described for WIPI4 and WIPI2 [[102], [103], [104], [105]]. Autophagosome formation is in addition supported by a positive feedback mechanism between ULK1 and PIK3C3-CI [106]. Also during late autophagy steps, PI3P plays a role by promoting the recruitment of autophagy factors, which regulate autophagosome fusion with the lytic compartment or are implicated in mammalian autophagosome trafficking [17,107]. Thus, due to the constant presence of PI3P at the autophagosomal membrane, PI3P signaling must be timely regulated. Next to alternative subunits and regulatory proteins acting on the PIK3C3-CI, this could be achieved through interdependent coordination between Rab GTPases and phosphoinositides [108]. In this line, VPS34/VPS15-containing PI3K complexes are effectors of RAB5 (yeast Vps21) [109]. RAB5 has been implicated in early autophagy steps (Fig. 2; Table 2) by influencing the recruitment of the Atg8 conjugation machinery via a stimulation of PI3P production by PIK3C3-CI [110,111]. RAB5 furthermore upregulates the activity of PI(4)- and PI(5)-phosphatases from PI(3,4,5)P3 and PI(3,4)P2, leading to an additional increase of cellular PI3P levels [112], which could enhance autophagy progression. During the maturation of autophagosomes, PI3P signaling is likely decreased [15,16] and in yeast, PI3P levels have been found to be reduced on mature autophagosomes [113]. This could be due to a reduction in Vps21/RAB5 activity during late steps of autophagosome formation as RAB5 influences PIK3C3-CI function on autophagic membranes. Similar to endosomal maturation, a Vps21/RAB5 and Ypt7/RAB7 conversion might take place, which could be mediated by Vps34 activity. Such a regulatory loop exists for endosomal RAB5, during which the GAP of RAB5 is recruited to the endosome in a VPS34-dependent manner [114]. The reduction of RAB5 activity would then lead to a decrease in PI3P abundance. In addition, RAB7 recruits the negative autophagy regulator Rubicon, which associates with PIK3C3-CII at the autophagosome and inhibits its activity [115]. Further studies focusing on the cooperation between Rab GTPases and the Vps34-kinase complexes will give important insight on how autophagosome formation is driven.
3.2. The Atg8 platform
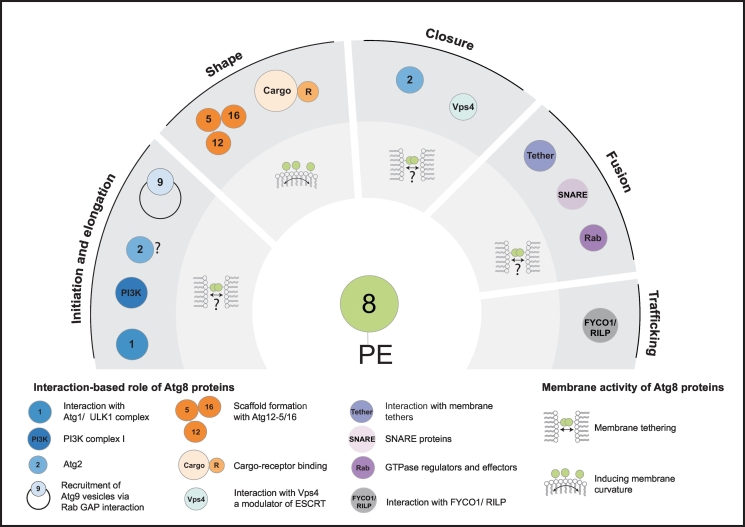
During autophagosome biogenesis, the elongation of the phagophore is promoted by the ubiquitin-like protein Atg8, which gets covalently but reversibly attached to phosphoethanolamine (PE) at the nascent autophagosomal membrane. Remarkably, Atg8 (including its homologues) is the only known cellular protein that has this unique lipid modification feature [116]. Although a few non-canonical roles of Atg8 members have been reported [117,118], Atg8 first and foremost regulates different aspects during autophagosome biogenesis and maturation (Fig. 4). Due to its trackable association with the autophagosomal membrane throughout different stages of autophagosome formation, Atg8 proteins are a widely used marker for autophagosome formation in both yeast and mammals.
Fig. 4.
Proposed roles of Atg8 proteins in autophagy. Atg8 proteins conjugated to PE serve as a binding platform for many autophagy effectors and regulate several steps during autophagy. In addition, lipidated Atg8 proteins have been shown to be membrane active, a feature allowing Atg8 proteins to facilitate distinct steps individually. See text for details.
While only one Atg8 protein is present in yeast, there are at least six homologues in mammals, which can be divided into the evolutionary distinct microtubule-associated proteins 1A/1B light chain 3 (LC3A, LC3B, LC3C) and gamma-aminobutyric acid receptor-associated protein (GABARAP, GABARAPL1, GABARAPL2/GATE-16) subfamily [119,120]. Common to all Atg8 family members is their ubiquitin-like core structure with two unique N-terminal helices and a conserved glycine within their C-terminus, which allows their lipidation [119,121,122]. Prior to the conjugation of Atg8 proteins to PE, their cytosolic precursors are constitutively processed by the cysteine protease Atg4 (ATG4A-D in mammals), thereby exposing the C-terminal glycine needed for lipidation [[122], [123], [124]]. Atg4 is also responsible to delipidate Atg8 and recycle Atg8 proteins from the outer autophagosomal membrane, which has been proposed to occur only once an autophagosome has fully matured [6,123,125,126].
Lipidation of Atg8 relies on a dual ubiquitin-like conjugation system with the E1-like Atg7, the E2-like Atg3 and the E3-like Atg12-Atg5/Atg16 complex. These proteins catalyze the formation of an amide bond between the C-terminal glycine of Atg8 and the amino head group of PE [127,128]. The second conjugation reaction in this dual system is the attachment of the ubiquitin-like modifier Atg12 to Atg5, which is mediated by Atg7 and Atg10 [127,129]. The Atg12-Atg5 conjugate then associates with Atg16, acting as the E3-like enzyme [130,131].
The availability of components of the conjugation machinery and the presence of PE at the autophagosomal membrane contribute to the spatial occurrence of Atg8 lipidation, which likely takes place at the growing edge of the phagophore [14,132,133]. This edge is characterized by its high curvature and allows the insertion of the curvature sensitive N-terminal amphipathic helix of the E2-like protein Atg3. Recruitment of Atg3 is promoted by the Atg12-Atg5/Atg16 complex and by Atg8 itself, hence, pointing to a positive feedback mechanism for Atg8 lipidation [[134], [135], [136]]. Moreover, in vitro Atg3 has been shown to mediate a local enrichment of PE [133], which further promotes Atg8 lipidation [47].
Efficient targeting of the Atg12-Atg5/Atg16 complex to the autophagosomal membrane relies on the interaction of Atg16 with the PI3P-binding protein Atg21 in yeast or the mammalian PROPPIN family member WIPI2 as well as mammalian RAB33B [104,134,[137], [138], [139]]. Atg21 and predictively also WIPI2 contain an amphipathic helix within their PROPPIN-domain [140,141], through which the E3-like complex could be recruited to the highly curved phagophore edge. Atg16 also harbors an amphipathic helix, which however is dispensable for the E3 complex recruitment to membranes but rather is required for the lipidation reaction itself [142]. As Atg12-Atg5/Atg16 are found to distribute along the growing phagophore [64], the complex might diffuse away from the edge into the phagophore leaflet together with Atg8. Other factors such as the Atg1/ULK1 kinase complex or direct PI3P-binding also contribute to Atg12-Atg5/Atg16 recruitment to autophagosomal membranes and enhance efficient Atg8 lipidation [[143], [144], [145], [146]]. Interestingly, the Atg12-Atg5/Atg16 complex remains localized on the outer surface of the expanding phagophore where it stabilizes the autophagosomal membrane in vitro [147], while lipidated Atg8 is found on both the convex and concave side [64,125,148].
During the initial steps in autophagosome formation, lipidation of Atg8 is the most downstream event at the newly formed phagophore. Atg8 conjugation is involved in key processes ranging from expanding and shaping the phagophore, cargo recognition, autophagosome closure to autophagosomal fusion with the lytic compartment [[12], [13], [14],22,98,103,[149], [150], [151]] (Fig. 4). In mammals, Atg8 proteins are further involved in autophagosome trafficking and might impact the degradation of the inner autophagosomal membrane after fusion [17,151,152]. In addition, positive feedback mechanisms exist during which the progression of Atg8 lipidation drives autophagy. Thus, the Atg8 interactome involves components of the Atg1/ULK1 kinase complex, the mammalian PIK3C3-CI and ATG2 [[153], [154], [155], [156]]. The interaction with ULK1 for example enhances ULK1 activation directly and indirectly via cargo-mediated receptors [157,158]. Phagophore formation and closure is also regulated by an interaction of mammalian ATG2 and GABARAPs [156].
Deletion of yeast Atg8 or proteins involved in its conjugation lead to the loss of autophagic activity and only a few autophagosomal structures are formed, which, however, are very small and incompetent to fuse with the vacuole [12,125,127,159]. In mammals, loss of all six known Atg8 homologues or of components of the conjugation machinery including ATG4 does not affect autophagosome quantities [160,161]. However, autophagosome formation occurs at decreased rates resulting in autophagosomes of reduced size [151,[160], [161], [162]]. Also, autophagosome-lysosome fusion is strongly defective in the absence of PE-conjugated Atg8 proteins [160,161,163], though it has been suggested that the formation of autolysosomes is only partially impaired, and instead, cargo acidification and degradation is blocked [151]. Studies focusing on individual mammalian Atg8 subfamilies highlight differences in the roles of LC3 or GABARAP proteins during autophagy. While GABARAP family members seem to play a dominant role during starvation-induced autophagy [158,160,164], LC3 proteins are important for selective autophagy [[165], [166], [167]]. However, neither family is limited to function in one type of autophagy exclusively, and overlapping as well as additive roles between the subfamilies exist [13,149,160]. In addition, GABARAP proteins regulate the final closure and autophagosome-lysosome fusion whereas the LC3 family members rather act in the efficient membrane elongation process during autophagosome biogenesis [13,160,163]. The different mechanisms, which determine the function of LC3 and GABARAP proteins are unknown, but likely involve distinct protein-protein interaction. ULK1, for instance, preferentially interacts with members of the GABARAP family [154], which enhance ULK1 activity and phagophore formation [158,168]. In contrast, LC3B and LC3C seem to negatively influence ULK1 activity, highlighting the distinct roles the two Atg8 protein families seem to have [158].
Critical for Atg8 proteins to engage in the plethora of autophagosomal processes is their ability to bind a wide range of effector proteins via so-called LC3-interacting regions (LIR) (also known as Atg8-interaction motifs (AIM) in yeast) [[169], [170], [171], [172]]. Typically, LIR-motifs contain the core consensus sequence (W/F/Y)-X-X-(L/I/V) that is often preceded by negatively charged residues (E/D). The two hydrophobic residues of the LIR-motif present in the effector protein associate with two hydrophobic pockets within the Atg8 ubiquitin-like core [169,173]. The functional differences described above for LC3 and GABARAP family members can be attributed to variable affinities between the Atg8 proteins and distinct LIR motifs. Proteins with LIR-motifs of the type (W/F)-(V/I)-X-V prefer binding to members of the GABARAP subfamily, and are therefore also referred to as GIM (GABARAP interaction motif) [174]. A prominent example is PLEKHM1, which regulates autophagosome-lysosome fusion and has an 11-fold higher affinity towards GABARAP proteins, hence, offering an explanation for the apparent pronounced role of GABARAP proteins in autophagosome fusion [22,160,174]. In contrast, a number of proteins like FYCO1 favor the interaction with LC3 proteins [152,173]. A high-throughput study showed differences in the interactome between the LC3 and GABARAP subfamily with only a subset (~30%) of interaction partners shared among the two subfamilies [29]. The molecular basis of those differences is still under investigation but in addition to specified LIR-motifs like GIMs, residues outside the core consensus LIR sequence have been found to additionally define specificity towards LC3 or GABARAP binding [120,175]. Moreover, also non-canonical LIR motifs have been shown to promote the interaction with Atg8 proteins [166,173].
Besides its ability to form an important binding platform for many autophagy effectors, Atg8 proteins have been reported to tether membranes and to promote hemifusion (yeast Atg8) as well as complete fusion reactions (mammalian Atg8 proteins) in vitro [11,[176], [177], [178]]. While the membrane tethering properties of Atg8 proteins via homotypic transmembrane interactions have been confirmed in several studies [47,[176], [177], [178]], their fusogenic activity remains controversial. If and how mammalian LC3 or yeast Atg8 promote fusion under physiological conditions remains to be further analyzed [47,176,177]. In vitro, Atg8-mediated vesicle fusion is sensitive to the Atg8 concentration as well as the concentration of PE [11,47]. Accordingly, a local enrichment of PE and/or Atg8 proteins could be present at the autophagosomal membranes in vivo. Such an accumulation could be mediated by preferential Atg8-lipidation at the edge and/or Atg3-mediated concentration of PE [133].
LC3 and GABARAP proteins differ in their curvature sensitivity, with a preference for small vesicles with high curvature or for larger vesicles with lower curvature, respectively [177,178]. This could explain their observed pronounced function in either membrane elongation for LC3 members or autophagosome-lysosome fusion for the GABARAP family [13,160,163]. In summary, Atg8 proteins seem important in phagophore elongation, autophagosome closure as well as fusion between autophagosomes and the lytic compartment, however mechanistic details of their regulation and the precise mode of action need further investigation [11,177,179]. Such insight will not only advance the understanding of autophagy in general but is further of high importance as altered transcription and expression of Atg8 family members or impairment of the conjugation machinery have been associated with cancer and several pathological conditions such as neurodegenerative diseases [180,181].
While regulation of Atg8 function at the transcriptional and posttranslational level has been reported [[182], [183], [184], [185]], one important regulatory feature of Atg8 proteins is their reversible membrane conjugation. At least in yeast, deconjugation of Atg8 by Atg4 does not only ensure that erroneous lipidated Atg8 from non-autophagosomal membranes is removed to prevent mistargeting of interaction partners, but also maintains sufficient levels of free Atg8 [126,186,187]. Delipidation of Atg8 occurs also on autophagosomal structures, and mature yeast autophagosomes or autolysosomes contain only low levels of conjugated Atg8 on their outer membrane [122,123,125]. It has been speculated that the delipidation allows recycling of Atg8 for consecutive autophagy rounds [123,186,187], but also to regulate timely fusion of the autophagosome with the lytic compartment [126]. Thus, with the removal of Atg8 from the outer membrane, its interaction partners could be released [187], which otherwise might inhibit fusion. Such a mechanism would also prevent premature fusion reaction of unclosed autophagosomes with the lytic compartment. In the same line, PI3P-removal from mature autophagosomal membranes leading to the dissociation of Atg proteins has been described to be a prerequisite to allow fusion [15,16]. How the cell senses when an autophagosome has matured, and how it timely initiates removal of Atg8 and PI3P, is not well understood.
In yeast, controlled Atg8 deconjugation by Atg4 is also important during phagophore elongation. The expression of an Atg8 variant, which already exposes the C-terminal glycine needed for lipidation, is used to study the role of Atg4 beyond the initial priming step of Atg8. Expression of this Atg8 variant in cells lacking Atg4 resulted in defects already during phagophore expansion, leading to a reduction in autophagosome size and number [187,188]. This suggests that Atg4 is required throughout the autophagosome biogenesis process. Such an Atg4-dependency after initial priming of Atg8, however, has not been observed in higher eukaryotes [161], supporting previous notions that ATG8 conjugation is not a strict prerequisite for autophagosome formation in mammals [160,161]. Whereas the role of Atg4 in Atg8 priming during autophagy initiation and in removal of Atg8 from mature autophagosomes is well understood, its function during phagophore expansion requires further analysis.
As Atg8 proteins remain at the autophagosomal structures throughout autophagosome formation and premature release of Atg8 effectors needs to be avoided, a regulated mechanism to control Atg8-lipidation and delipidation must exist. It has been suggested that Atg8 interactors themselves could provide protection from premature Atg4 cleavage by shielding Atg8 and/or competing for Atg4 binding [147,189,190]. Then, upon autophagosome completion, release of effector proteins might allow Atg4 recruitment and subsequent deconjugation [190]. Release of Atg proteins from the maturing autophagosome has been described, for instance after PI3P removal [15]. Thus, PI3P cleavage might facilitate Atg4 targeting to closed autophagosomes. In addition to regulated protein interactions also reversible post-translational modifications control the release of Atg8 from mature autophagosomes. Atg8 proteins associate with the Atg1/ULK1 kinase [153,154], which phosphorylates Atg4, leading to an inhibition of Atg4 activity [191,192] and likely to a protection of Atg8 proteins from premature deconjugation. In mammals, dephosphorylation of ATG4 by protein phosphatase 2A (PP2A) restores ATG4 activity [192]. Further, direct phospho-regulation of mammalian LC3C and GABARAPL2 by the tank binding kinase 1 (TBK1) impedes their binding to Atg4 proteases, protecting them from delipidation [185]. Another regulatory mechanism reported is redox-dependent Atg4 activity regulation, observed both in yeast and mammals [193,194].
The understanding of how spatiotemporal Atg4 activity can be controlled throughout autophagosome formation will provide further insight into the fine-tuned autophagy process.
3.2.1. Atg8 proteins as possible integrators of Rab GTPase function
Rab GTPases as well as several of their regulators have been found to associate with different Atg proteins (Table 1, Table 2). Remarkably, a striking number of interactions with Rab GTPases or their regulators have been reported for Atg8 proteins in yeast and in mammals [28,29,195] (Table 1, Table 2) (Fig. 2). As Rab GTPases participate in various steps in autophagy, and Atg8 proteins serve as a central binding platform for many Atg factors at the autophagosomal membrane, it is tempting to speculate that Atg8 proteins are involved in mediating Rab GTPase function, as well as to connect different trafficking pathways to autophagy.
In yeast, Atg8 targets the GEF Mon1-Ccz1 to the autophagosomal membrane via evolutionary conserved LIR-motifs [196]. Mon1-Ccz1 recruits the Rab GTPase Ypt7 and stimulates its activity, promoting autophagosome-vacuole fusion [107,196]. RAB7, the mammalian homologue of Ypt7, localizes to autophagosomes during later steps of autophagosome biogenesis, predominantly during the fusion process to late endosomes/lysosomes [197]. RAB7 facilitates the fusion processes by mediating the recruitment of several membrane tethering proteins to the fusion site, thereby bringing the autophagosomal and lysosomal membranes into close proximity to allow SNARE bundling and subsequent fusion. Atg8 proteins interact with the RAB7-tethering effectors such as HOPS, PLEKHM1 or EPG5 at the autophagosome formation site [22,198,199]. In addition, it has been discussed that controlled autophagosomal transport in mammalian cells is a timely regulator of autophagosome-lysosomal fusion [200]. RAB7 is involved in intracellular autophagosomal trafficking via its association with the transport proteins FYCO1 [17] and RILP [201], which both can bind to autophagosomes in an LC3-dependent manner [17,152,202]. Other examples for a cross-talk between Atg8 proteins and RAB7 is via its regulators. Armus, a GAP for RAB7, associates with LC3 in a LIR-dependent manner and regulates autophagosome-lysosome fusion [203]. Overexpression of Armus results in the same phenotype as expression of inactive RAB7, namely the accumulation of enlarged autophagosomes [197,203]. The increased size of autophagosomes in cells lacking active RAB7 indicates a role of RAB7 in proper membrane elongation. In this line, another Atg8-binding GAP of RAB7, TBC1D15, regulates membrane expansion during mitophagy [204]. Moreover, ATG4B is an effector of the mammalian RAB7 paralogue RAB7B and similar to RAB7, RAB7B depletion results in the formation of abnormally large autophagosomes, however, in contrast to RAB7, without affecting autophagosome-lysosome fusion. Thus, the regulation of ATG4 proteases by RAB7B may play an important role in limiting autophagosomal size and coordinating the release of lipidated Atg8 [205]. If the RAB7 paralogues regulate autophagosomal size by similar or different mechanisms, is unknown.
While TBC1D15 is a RAB7 GAP found at mitochondria, the RAB7 GAP TBC1D5 localizes to the endosomal system and also binds Atg8 proteins [195]. TBC1D5 is involved in the regulation of RAB7 localization [57], and it has been suggested to facilitate the rerouting of Atg9 vesicles from retromer-coated endosomes to the autophagosomal site [56]. Next to TBC1D5, TBC1D9B is another GAP binding to Atg8 proteins [206], which might link Atg9-vesicle trafficking between the endosomal and autophagosomal pathway. TBC1D9B is a GAP for RAB11 [55] and RAB11-positive recycling endosomes have been identified as a crucial source for Atg9-derived vesicles during bulk autophagy [20,51,69,207]. These examples of Atg9 trafficking confirm the notion that GAP proteins bridge the endosomal and autophagic pathway through their interactions with endosomal factors and Atg8-proteins present at the autophagosome. In addition to a potential role of Atg8 together with GAPs in regulating Atg9 trafficking, further Atg8-GAPs interactions have been described. Gyp1, a GAP for Ypt1, binds to Atg8 and the interaction is suggested to stabilize the binding of cargo receptors to Atg8 during selective autophagy [208].
OATL1, a GAP for RAB33B [209], which mainly binds GABARAPs, has been implicated in autophagosome-lysosome fusion though the mechanism is unclear [209].
In summary, Atg8 proteins are one important binding platform for autophagy factors including Rab GTPases and their regulators. Since Atg8 proteins have key roles during autophagosome formation, their spatiotemporal regulation and the degree of functional conservation from yeast to mammal needs to be addressed further.
4. Autophagosome closure and maturation
After phagophore elongation is complete and the cargo is enwrapped, the nascent autophagosome remains with a small membrane opening at the place where the growing phagophore edge converges (Fig. 1). This pore needs to be closed prior to the final fusion of the autophagosome with the lytic compartment. Pore closure requires membrane scission, in which the initial single continuous phagophore membrane is separated into an outer and an inner autophagosomal membrane [210,211]. Considering membrane topology, this membrane scission event resembles the formation of multivesicular bodies (MVBs), which is achieved by the action of ESCRT proteins [212,213] (Fig. 1). The ESCRT machinery consists of the ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III subcomplexes, the modulating ATPase Vps4 and several accessory proteins [212,213]. While proteins of the ESCRT-III complex are sufficient to mediate scission of model membranes in vitro [214], the other factors mainly promote the recruitment and assembly of the ESCRT-III subcomplex at distinct membrane sites.
Recent genetic studies and high-resolution imaging approaches, which allow to distinguish between open and closed autophagosomal structures [215,216], revealed that only a subset of the ESCRT machinery promotes autophagosome closure in yeast and mammalian cells [215,217]. Depletion of these subunits results in a partial reduction of autophagic flux and accumulation of autophagosomal structures in the cytosol [215,217]. Predominantly, subunits of the ESCRT-III complex influence autophagosome closure, i.e. Snf7 and Vps20 in yeast and CHMP2A, CHMP3 and CHMP7 in mammals [215,217]. Although the mammalian core ESCRT-III subunit CHMP4B showed no prominent defect in the above-mentioned studies [215], it transiently co-localizes with nascent autophagosomes during late stages of autophagosome maturation [216]. The ATPase protein Vps4 shows transient localization to Atg8-positive structures in yeast and in mammals [217,218]. Vps4 is important to modulate ESCRT-III subunit assembly and similar to ESCRT mutants [214,219], lack of Vps4 function in both organisms leads to an increase in unclosed autophagosomal structures and a reduction in autophagic flux [[215], [216], [217]]. Besides ESCRT-III subunits, other ESCRT proteins show an impairment in scission, though less prominent. For example, the ESCRT-I protein Vps37A co-localizes with LC3 [218,220] and depletion of Vps37A or disturbance of ESCRT-I assembly causes an increase of unclosed autophagosomal structures [218,220].
The recruitment of the ESCRT machinery to the autophagosomal structures is regulated by the Rab GTPase Vps21 (mammalian RAB5) (Fig. 2). Vps21 deletion in yeast shows similar autophagy defects observed as upon loss of the ESCRT machinery proteins Snf7 and Vps4 [21,217,221]. In addition, deletion of Vps21, its GEF Vps9, or certain effector proteins, leads to a strong reduction in the transient co-localization of Snf7 with Atg8-positive structures. Snf7 also interacts with the scaffold proteins Atg11 and Atg17, and to some degree with Atg29 [217]. Artificial tethering of Snf7 to Atg17 can partially rescue the reduced autophagic flux observed in vps21Δ cells [217], suggesting that the ESCRT-III subunit Snf7 is recruited to the autophagosome by Vps21, where it is stabilized via its interaction with Atg17 to promote autophagosome closure. Targeting of the ESCRT machinery to the nascent autophagosome might further be dependent on the presence of PI3P, as members of the ESCRT machinery have PI3P binding sites [212,213]. The release of ESCRT subunits after scission could then be facilitated by the removal of PI3P, as this step is required to release several Atg proteins and occurs prior to the fusion of autophagosomes with the lytic compartment [15,16]. Transient localization of ESCRT components could also be controlled by the action of the ATPase Vps4, which promotes the remodeling of the ESCRT-III subcomplex assemblies leading to subunit release [[212], [213], [214],219].
Although ESCRT proteins play a role in autophagosome closure, also other factors might mediate this step, as ESCRT mutants only show partial defects in autophagy flux [[215], [216], [217]]. Defects in the ESCRT machinery result in the accumulation of autophagic membranes and unsealed autophagosomes in the cytosol, increased Atg8 lipidation and the persistence of Atg proteins at the autophagosome. Various studies identified other factors with similar defects in autophagy. These factors include the casein kinase CK1delta/Hrr25 [222], sphingomyelin phosphodiesterase 1 (SMPD1) [223], the transmembrane protein VMP1 [224], [225], the RAB7 effector protein EPG5 [218], the TRAPPIII subunit TRAPPC11 [226], mammalian ATG2A/B [156,224], as well as Atg8 proteins and their conjugation machinery including Atg4 [151,156,162,225] and most have been discussed to play a role in autophagosome closure. Some of these factors such as yeast or mammalian TrappIII and SMPD1 regulate Atg9 trafficking to the autophagosome formation site [51,223,227]. Also, VMP1 seems to mediate ATG9-vesicle recruitment to the nascent autophagosome as in cells lacking VMP1 Atg9-positive vesicles are found away from autophagosomal structures [225]. VMP1 is involved during different steps in autophagy [31,228] and modulates the membrane contact site between the ER and the phagophore [229,230]. As Atg9 in yeast or Atg8 proteins in mammals were found to sequester Atg2 to the growing phagophore edge [63,156] and Atg2 transports lipids from the ER to the phagophore [39,61], which requires membrane-membrane contact sites, the above-mentioned factors might act cooperatively to promote autophagosome closure. It should be noted, however, that due to the function of Atg2 in lipid transfer, an observed accumulation of unclosed autophagosomes in the various mutant strains might be a result of decreased lipid transport to the edge of the forming autophagosome rather than an impairment in scission. Indeed, it has recently been reported that VMP1 acts as a scramblase and interacts with ATG2 in vitro, leading to a model wherein VMP1 at the ER (possibly in complex with another scramblase TMEM41B) and ATG9 at the growing phagophore re-equilibrate the lipid pools between the respective bilayers, which would be otherwise imbalanced due to the ATG2 mediated lipid transfer [79].
Atg8 proteins fulfill a broad functional spectrum in autophagy (Fig. 4). In addition to their role in promoting the elongation of the phagophore, they might also partake in autophagosome closure and autophagosome fusion with the lytic compartment. In mammals, loss of components involved in Atg8 lipidation such as ATG3, ATG5 and ATG4A/B lead to the presence of unclosed elongated autophagosomal structures in the cytosol [13,162,225,231]. Moreover, loss of ATG3 results in impaired scission [151], suggesting that either components of the conjugation machinery themselves and/or the presence of lipidated Atg8 proteins are important for autophagosome closure [210]. Members of the GABARAP subfamily seem to predominately control this late autophagy step [156], as loss of the GABARAP subfamily negatively affects autophagic flux and the release of the Atg machinery, which is less pronounced for the LC3 subfamily [13,163]. In addition, mutation of the GABARAP specific ATG4A results in the accumulation of unclosed autophagosomal membranes [13,122].
Many Atg8-mediated functions rely on their ability to assume a wide range of LIR-dependent interactions. Thus, Atg8 proteins might mediate autophagosome closure by recruiting relevant scission factors. Potato Vps4 interacts with an Atg8 paralogue in a LIR-dependent manner, supporting such a function [232]. In the same line, mammalian ATG2 binds GABARAP and GABARAP-L1 via a LIR-motif, and abrogation of this interaction causes autophagosome maturation defects [156]. Atg8 proteins might also support autophagosome closure by interacting with cargo receptors at the inner concave autophagosomal membrane. This is supported by the notion that the interaction between receptor proteins and Atg8 allows membrane bending around the cargo and its entire uptake [150,233]. In this scenario, tight engagement between cargo and the inner Atg8 coat of the growing autophagosome would leave only a small opening that needs to be closed. This could be achieved by the ESCRT machinery, which is proficient in sealing pores with a neck diameter of around 50–100 nm [234]. Alternatively, Atg8 proteins might promote autophagosome scission themselves [210]. The N-terminal helices of Atg8 proteins have been shown to confer membrane tethering and hemifusion activity [11,176]. Thus, if membranes are in sufficient proximity of around 10 nm or less, Atg8 proteins can promote membrane tethering by either trans-dimerization via the N-terminus of two opposing Atg8 proteins or by directly inserting their N-terminus into membranes [176,178] (Fig. 4). This tethering activity or membrane disturbance could induce spontaneous scission, resulting in the closure of the autophagosomal pore, as spontaneous membrane scission can occur when the pore diameter reaches a critical size of about 1–5 nm [234].
Autophagosome closure and fusion with the lytic compartment is tightly connected, as these processes occur successively in a timely ordered manner. Defects in autophagosomal membrane closure lead to impaired or delayed fusion [151,215,216]. Membrane fusion relies on the action of Rab GTPases, tethering complexes, and SNARE proteins. In this minimal setup, the active membrane-bound Rab GTPase enables tethering factors to bring the opposing membranes into close contact (Fig. 1). This allows SNARE proteins from opposing membranes to assemble into a four-helical bundle, which drives membrane fusion. In yeast, autophagosome-vacuole fusion relies on the Rab GTPase Ypt7, its GEF Mon1-Ccz1, the presence of PI3P, the tethering complex HOPS, the vacuole-anchored SNARES Vti1 and Vam3, the SNARE Vam7, and the autophagosomal SNARE Ykt6 [23,235]. In metazoan, autophagosome fusion with the lysosome appears more complex and depends on RAB7, RAB2, MON1, HOPS and further tethering factors, such as EGP5, BRUCE and PLEKHM1 [22,198,199,[236], [237], [238], [239]]. Two SNARE complexes are implicated in the fusion process, which are either formed by lysosomal VAMP7/8, the SNARE SNAP29, and autophagosomal STX17, or by lysosomal STX7, SNAP29, and autophagosomal YKT6 [240,241].
Since fusion must be timely regulated to avoid premature fusion with the lytic compartment, it had been suggested that STX17 is only recruited to matured and sealed autophagosomes [240]. In more recent studies, STX17 has been detected at the nascent autophagosome of scission-defective cells [151,215]. In these cells, aberrant fusion events with the lysosome take place, which lead to abnormal turnover of the inner autophagosomal membrane [151]. This indicates that the fusion machinery can be recruited before autophagosome sealing and must be tightly controlled. In yeast and mammals, the Atg1/ULK1 kinase complex is involved in the regulation of SNARE proteins (Fig. 1). In yeast the autophagosomal SNARE Ykt6 is targeted to autophagosomes already during early PAS assembly in an Atg1 kinase complex dependent manner [242]. In order to avoid premature fusion, Ykt6 is kept fusion-inactive by Atg1 phosphorylation at the nascent autophagosome, which impairs its ability to bundle with the vacuolar SNARE proteins [243]. These findings imply that controlled dephosphorylation is then required to relieve this inhibition once a mature and closed autophagosome has formed. A responsible phosphatase, however, has not been identified yet. Also, the vacuole-associated SNARE Vam7 interacts with the Atg1 complex component Atg17 already during early PAS formation, and disruption of this interaction leads to reduced fusion [244]. If Vam7 is also regulated by Atg1-phosphorylation remains unknown.
In higher eukaryotes two SNARE complexes promoting autophagosome-lysosome fusion exist, depending either on YKT6 or STX17. Although ULK1 consensus sites also exist on mammalian YKT6 [243], a possible regulation of YKT6 by ULK1 remains to be investigated. ULK1, however, controls SNARE function of STX17-decorated autophagosomes. ULK1 interacts directly with the SNARE STX17 and enhances SNARE bundling [245]. This seems to be independent of ULK1 kinase activity as no STX17 phosphorylation could be detected [245]. Instead, the action of ULK1 in this situation depends on its phosphorylation status at Ser423. A phospho-mimicking variant of ULK1 impairs STX17 recruitment and SNARE assembly without affecting ULK1 function during autophagy initiation [245]. The apparent difference in Atg1/ULK1 function could point to a regulatory mechanism between YKT6 and STX17-decorated autophagosomes, in which two distinct SNARE complexes mediate autophagosomal fusion in mammals [241], which might by differently regulated by ULK1.
Analogous to SNARE proteins, the recruitment of fusions factors such as HOPS must be coordinated and their function timely regulated (Fig. 1). The SNARE proteins Ykt6 and Vam7 directly bind the HOPS complex [246,247]. As both Ykt6 and Vam7 are recruited to the autophagosome at an early stage of autophagosome formation [242,244], HOPS association with the autophagosome likely needs to be regulated in a timely manner to avoid premature tethering and aberrant fusion. Similar to its role in preventing premature SNARE bundling, Atg1 phosphorylation of Ykt6 could inhibit untimely targeting of the HOPS complex to the autophagosome. Recruitment of yeast HOPS to the autophagosome and to the vacuole is also mediated by the Rab GTPase Ypt7 [23,235]. In the endosomal pathway, Ypt7 acts on late endosomes [26]. Similarly, Ypt7 is likely only active on mature autophagosomes. Hence, premature HOPS recruitment might be prevented by a cooperative binding of Ypt7 and the SNARE proteins, which takes place only at mature autophagosomes.
In mammals, the Ypt7 homologue RAB7 is present on autophagosomes and lysosomes [197,237]. In contrast to Ypt7, RAB7 does not directly interact with HOPS, but HOPS recruitment depends on RAB7 effectors as well as other Rab GTPases [22,201,238,248]. One example is the RAB7 effector PLEKHM1, which is required to target HOPS to the lysosome [22]. By binding to GABARAP, PLEKHM1 has been suggested to then bring the HOPS complex into close proximity of the autophagosome [22] (Fig. 4). With Atg8 proteins, the SNARE STX17, and RAB2, several proteins are present on the autophagosome, which are known to directly interact with HOPS [24,198,236,238,240]. As ULK1 also interacts with RAB2 and STX17 [24,245], it will be interesting to investigate if ULK1 controls HOPS recruitment in mammals.
PI3P is an additional factor affecting fusion. While dephosphorylation of PI3P is critical to release the Atg machinery for subsequent fusion [15,16], the presence of PI3P serves also as an important signal during fusion. PI3P aids in the recruitment of proteins relevant for fusion such as the HOPS complex or Mon1-Ccz1 [107,246], suggesting a spatiotemporal regulation of PI3P abundance. Atg8 proteins individually or in cooperation with PI3P also recruit several fusion factors, such as Mon1-Ccz1, HOPS, PLEKHM1, BRUCE, EPG5, as well as STX17 [22,163,196,198,199,239,249]. While many players in autophagosome closure and maturation have been identified, the transition between these steps and how they are controlled remains largely unclear. An interplay between Atg1/ULK1, PI3P and the PIK3C3-CI as well as Atg8 proteins could fine-tune the fusion process. In order to better understand the spatiotemporal regulation of the autophagosomal scission and fusion processes, the recruitment and function of the involved factors need to be better understood.
5. Membrane curvature
During autophagosome formation, a small membrane precursor elongates and bends into a cup-shaped phagophore with three different surfaces: a concave and a convex membrane side, and a highly curved edge. Depending on the curvature, the degree of the lipid packing varies and hydrophobic cavities can be exposed, providing different protein binding options without requiring protein-protein interactions [250]. Moreover, proteins can distinguish between distinct membranes with similar lipid composition but different curvature; or, as in the case of growing phagophores, distinguish between different regions of the same membranous structure that have different curvatures. In vitro, Atg1 and Atg13 preferentially bind highly curved vesicular membranes of about 30 nm diameter, which reflects the approximate size of Atg9 vesicles [6,44]. This preferential binding has been suggested to mediate the tethering of Atg9 vesicles to initiate phagophore formation [44]. During phagophore elongation, Atg1 then localizes all along the phagophore whereas Atg13 only localizes to the phagophore edge [64], suggesting a regulation of their membrane binding properties and a differential role of Atg13 and Atg1 at the elongated autophagosomal membrane [251].
Membrane shape does not only direct localization of proteins to the membrane surface but also influences protein function. The PIK3C3-CI is a prominent example of how membrane properties can affect protein function. Members of the PIK3C3-CI have membrane-binding properties, and the catalytic activity of the PIK3C3-CI is induced upon binding to curved membranes in vitro [252,253]. Specificity for autophagosomal membranes is controlled by Atg14/ATG14L [90,91,93]. Mammalian ATG14 contains a BATS-domain by which ATG14 binds to highly curved and loosely packed lipids with a preference for PI3P-containing membranes [254]. This preference indicates a positive feedback mechanism between PI3P production and PIK3C3-CI recruitment. Vps30/Atg6 and its mammalian homologue BECN1 contain a C-terminal evolutionary conserved domain (ECD) and β/α-repeated, autophagy-related (BARA) domain. The ECD-BARA domain allows efficient membrane binding and might also induce curvature itself [252,[255], [256], [257]].
The functionality of Rab GTPases themselves likely depends on membrane shape as well. For instance, the Rab GTPases RAB1 and RAB5 involved in early autophagy steps are targeted to curved membranes with lipid packing defects, which are present at the growing phagophore edge [258].
As discussed above, Atg2 acts as a lipid supplier for the growing phagophore [39,61], where it associates with the autophagosomal edge [28,64]. Atg2 possesses an amphipathic helix, which preferably associates with highly curved membranes and facilitates Atg18 recruitment to the autophagosome formation site [60,259]. Vice versa, Atg2 localization to the phagophore is mediated by yeast Atg18 or mammalian WIPI proteins [38,102,103,224], which associate through PI3P binding with the membrane [102,260]. Thus, the recruitment of the Atg2/Atg18 module occurs cooperatively. Atg9 affects proper Atg2 targeting in addition, as a loss in Atg9-Atg2 interaction results in an aberrant Atg2 localization away from the edge, leading to an autophagy defect [63]. These findings nicely exemplify how several factors such as protein-protein interaction, membrane modification or membrane curvature can influence membrane association of a protein in different manners, and that the localization of Atg proteins at the phagophore is defined and crucial for their proper function.
Several Atg factors controlling membrane curvature have been identified to regulate curvature at autophagosomal structures. Atg9 homotrimers for instance can deform lipids layers and increase the curvature at the edge of the phagophore [76]. This high energetic state at the edge consequently results in bending of the phagophore [76]. In addition, Atg9 scramblase activity could generate membrane curvature by partitioning lipids with different head groups [261]. With the progression in elongation of the phagophore, positive and negative curvature is generated at the membrane and curvature regulators will be needed at the surfaces. The forces and factors involved in this process are poorly understood, but the fact that the main protein complexes controlling autophagy have at least one curvature sensing component points to the importance of the regulation [262]. The Atg8 lipidation machinery is one candidate to control membrane shape and stability during autophagosome formation (Fig. 4). The E2-like protein Atg3 contains an amphipathic alpha helix that mediates Atg3 recruitment preferentially to the highly curved autophagosomal edge [132]. Here, Atg3 might aid in the enrichment of PE [133], which has a cone shape and can induce membrane curvature [261]. Further, PE-conjugated Atg8 itself induces and stabilizes high membrane curvature [14]. Atg8 also fulfills further functions at other sites on the phagophore, such as cargo binding on the inner concave side and the recruitment of downstream factors on the convex side, likely requiring its diffusion throughout the phagophore membrane. Conjugated Atg8 can interact with the Atg12-Atg5/Atg16 complex at the convex surface of the isolation membrane [64,135,137], where oligomerized E3-complexes form a coat-like structure that has been suggested to aid in shaping and stabilizing the forming autophagosome [130,147]. On the concave side, Atg8 proteins bind to cargo receptors during selective autophagy. This association could force controlled bending of the phagophore around the cargo either mediated via multiple LIR-motifs within one receptor or via receptor oligomerization [150,233].
In addition, proteins containing a BAR domain can generate and stabilize membrane curvature [250]. Several proteins with a BAR-domain such as Atg20, Atg24, mammalian SNX4, SNX18 and BIF-1 have been identified to stimulate autophagy, mainly by coordinating Atg9 vesicle trafficking to the nucleating and growing phagophore [[263], [264], [265], [266], [267]]. Some factors further colocalize with the nascent autophagosome and hence could also be involved in the induction or stabilization of membrane curvature at the growing phagophore [263,268,269].
Further studies are needed to understand the function of membrane- and curvature-sensing domains in autophagy proteins and effectors, and their role in modulating the autophagy pathway.
6. Conclusion and perspective
Many Atg proteins control both early and late steps during autophagosome biogenesis and maturation. Their timely regulation is mediated by signaling molecules such as PI3P, but could additionally also be regulated by the association of different Rab GTPases and their effector proteins. While many Rab GTPases are involved at different steps during autophagosome formation, their specific role remains still largely elusive. Future experiments are required to dissect their function and regulation as well as the conversion between Rab modules, which could be coordinated by Atg8-mediated processes. The presented data further show that during autophagosome formation partially redundant as well as cooperative mechanisms exist which could facilitate rapid adaptation of the autophagy pathway under different cellular conditions.
Declaration of competing interest
None.
Acknowledgements
The authors would like to thank David Hollenstein and Mariya Licheva for critical reading of the manuscript. The Kraft laboratory has received funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation): SFB 1381 (Project-ID 403222702); SFB 1177 (Project-ID 259130777); GRK 2606 (Project ID 423813989) and under Germany's Excellence Strategy (CIBSS - EXC-2189- Project ID 390939984), from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under grant agreement No 769065, and from the European Union's Horizon 2020 research and innovation programme under grant agreement No 765912. This work reflects only the authors' view and the European Union's Horizon 2020 research and innovation programme is not responsible for any use that may be made of the information it contains.
References
- 1.Suzuki K., Kirisako T., Kamada Y., Mizushima N., Noda T., Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20(21):5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenstein D.M., Gómez-Sánchez R., Ciftci A., Kriegenburg F., Mari M., Torggler R., Licheva M., Reggiori F., Kraft C. Vac8 spatially confines autophagosome formation at the vacuole in S. Cerevisiae. J. Cell Sci. 2019;132(22) doi: 10.1242/jcs.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto H., Fujioka Y., Suzuki S.W., Noshiro D., Suzuki H., Kondo-Kakuta C., Kimura Y., Hirano H., Ando T., Noda N.N. The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes. Dev. Cell. 2016;38(1):86–99. doi: 10.1016/j.devcel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Torggler R., Papinski D., Brach T., Bas L., Schuschnig M., Pfaffenwimmer T., Rohringer S., Matzhold T., Schweida D., Brezovich A. Two independent pathways within selective autophagy converge to activate Atg1 kinase at the vacuole. Mol. Cell. 2016;64(2):221–235. doi: 10.1016/j.molcel.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka Y., Alam J.M., Noshiro D., Mouri K., Ando T., Okada Y., May A.I., Knorr R.L., Suzuki K., Ohsumi Y. Phase separation organizes the site of autophagosome formation. Nature. 2020;578(7794):301–305. doi: 10.1038/s41586-020-1977-6. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H., Kakuta S., Watanabe T.M., Kitamura A., Sekito T., Kondo-Kakuta C., Ichikawa R., Kinjo M., Ohsumi Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J. Cell Biol. 2012;198(2):219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kametaka S., Okano T., Ohsumi M., Ohsumi Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem. 1998;273(35):22284–22291. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- 8.Kihara A., Noda T., Ishihara N., Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J. Cell Biol. 2001;153(3):519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petiot A., Ogier-Denis E., Blommaart E.F.C., Meijer A.J., Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 2000;275(2):992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 10.Itakura E., Kishi C., Inoue K., Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell. 2008;19(12):5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatogawa H., Ichimura Y., Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation, mediates membrane tethering and hemifusion. Cell. 2007;130(1):165–178. doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Xie Z., Nair U., Klionsky D.J. Atg8 controls phagophore expansion during autophagosome formation. Mol. Biol. Cell. 2008;19(8):3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidberg H., Shvets E., Shpilka T., Shimron F., Shinder V., Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29(11):1792–1802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knorr R.L., Nakatogawa H., Ohsumi Y., Lipowsky R., Baumgart T., Dimova R. Membrane morphology is actively transformed by covalent binding of the protein Atg8 to PE-lipids. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebollero E., Van Der Vaart A., Zhao M., Rieter E., Klionsky D.J., Helms J.B., Reggiori F. Phosphatidylinositol-3-phosphate clearance plays a key role in autophagosome completion. Curr. Biol. 2012;22(17):1545–1553. doi: 10.1016/j.cub.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Cheng S., Zhao H., Zou W., Yoshina S., Mitani S., Zhang H., Wang X. PI3P phosphatase activity is required for autophagosome maturation and autolysosome formation. EMBO Rep. 2014;15(9):973–981. doi: 10.15252/embr.201438618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pankiv S., Alemu E.A., Brech A., Bruun J.A., Lamark T., Øvervatn A., Bjørkøy G., Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end - directed vesicle transport. J. Cell Biol. 2010;188(2):253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winslow A.R., Chen C.W., Corrochano S., Acevedo-Arozena A., Gordon D.E., Peden A.A., Lichtenberg M., Menzies F.M., Ravikumar B., Imarisio S. α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J. Cell Biol. 2010;190(6):1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakuta S., Yamamoto H., Negishi L., Kondo-Kakuta C., Hayashi N., Ohsumi Y. Atg9 vesicles recruit vesicle-tethering proteins Trs85 and Ypt1 to the autophagosome formation site. J. Biol. Chem. 2012;287(53):44261–44269. doi: 10.1074/jbc.M112.411454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longatti A., Lamb C.A., Razi M., Yoshimura S.I., Barr F.A., Tooze S.A. TBC1D14 regulates autophagosome formation via Rab11- and ULK1-positive recycling endosomes. J. Cell Biol. 2012;197(5):659–675. doi: 10.1083/jcb.201111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Zhou F., Zou S., Yu S., Li S., Li D., Song J., Li H., He Z., Hu B. A Vps21 endocytic module regulates autophagy. Mol. Biol. Cell. 2014;25(20):3166–3177. doi: 10.1091/mbc.E14-04-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwan D.G., Popovic D., Gubas A., Terawaki S., Suzuki H., Stadel D., Coxon F.P., de Stegmann D.M., Bhogaraju S., Maddi K. PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins. Mol. Cell. 2015;57(1):39–54. doi: 10.1016/j.molcel.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Bas L., Papinski D., Licheva M., Torggler R., Rohringer S., Schuschnig M., Kraft C. Reconstitution reveals Ykt6 as the autophagosomal SNARE in autophagosome–vacuole fusion. J. Cell Biol. 2018;217(10):3656–3669. doi: 10.1083/JCB.201804028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X., Jiang X., Tian R., Zhao P., Li L., Wang X., Chen S., Zhu Y., Mei M., Bao S. RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells. Autophagy. 2019;15(10):1774–1786. doi: 10.1080/15548627.2019.1596478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekbulat F., Schmitt D., Feldmann A., Huesmann H., Eimer S., Juretschke T., Beli P., Behl C., Kern A. RAB18 loss interferes with lipid droplet catabolism and provokes autophagy network adaptations. J. Mol. Biol. 2020;432(4):1216–1234. doi: 10.1016/j.jmb.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 27.Müller M.P., Goody R.S. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases. 2018;9, no. 1–2:5–21. doi: 10.1080/21541248.2016.1276999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graef M., Friedman J.R., Graham C., Babu M., Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell. 2013;24(18):2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behrends C., Sowa M.E., Gygi S.P., Harper J.W. Network organization of the human autophagy system. Nature. 2010;466(7302):68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axe E.L., Walker S.A., Manifava M., Chandra P., Roderick H.L., Habermann A., Griffiths G., Ktistakis N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182(4):685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itakura E., Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6(6):764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biazik J., Ylä-Anttila P., Vihinen H., Jokitalo E., Eskelinen E.L. Ultrastructural relationship of the phagophore with surrounding organelles. Autophagy. 2015;11(3):439–451. doi: 10.1080/15548627.2015.1017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee C., Song D., Mehra D., Mancebo A., Kim D.-H., Puchner E.M. Quantitative super-resolution microscopy reveals distinct ULK1 oligomeric states and nanoscopic morphologies during autophagy initiation. bioRxiv. 2020 doi: 10.1101/2020.07.03.187336. [DOI] [Google Scholar]
- 34.Lin M.G., Hurley J.H. Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell Biol. 2016;39:61–68. doi: 10.1016/j.ceb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas J.N.S., Wang C., Bunker E., Hao L., Maric D., Schiavo G., Randow F., Youle R.J. Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol. Cell. 2019;74(2) doi: 10.1016/j.molcel.2019.02.010. (pp. 347-362.e6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hailey D.W., Rambold A.S., Satpute-Krishnan P., Mitra K., Sougrat R., Kim P.K., Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141(4):656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamasaki M., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N., Oomori H., Noda T., Haraguchi T., Hiraoka Y. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495(7441):389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 38.Maeda S., Otomo C., Otomo T. The autophagic membrane tether ATG2A transfers lipids between membranes. Elife. 2019;8 doi: 10.7554/eLife.45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valverde D.P., Yu S., Boggavarapu V., Kumar N., Lees J.A., Walz T., Reinisch K.M., Melia T.J. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 2019;218(6):1787–1798. doi: 10.1083/JCB.201811139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mari M., Griffith J., Rieter E., Krishnappa L., Klionsky D.J., Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 2010;190(6):1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki S.W., Yamamoto H., Oikawa Y., Kondo-Kakuta C., Kimurac Y., Hirano H., Ohsumi Y. Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc. Natl. Acad. Sci. U. S. A. 2015;112(11):3350–3355. doi: 10.1073/pnas.1421092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekito T., Kawamata T., Ichikawa R., Suzuki K., Ohsumi Y. Atg17 recruits Atg9 to organize the pre-autophagosomal structure. Genes Cells. 2009;14(5):525–538. doi: 10.1111/j.1365-2443.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 43.He C., Song H., Yorimitsu T., Monastyrska I., Yen W.L., Legakis J.E., Klionsky D.J. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. J. Cell Biol. 2006;175(6):925–935. doi: 10.1083/jcb.200606084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao Y., Perna M.G., Hofmann B., Beier V., Wollert T. The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy. Nat. Commun. 2016;7 doi: 10.1038/ncomms10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahrami A.H., Lin M.G., Ren X., Hurley J.H., Hummer G. Scaffolding the cup-shaped double membrane in autophagy. PLoS Comput. Biol. 2017;13(10) doi: 10.1371/journal.pcbi.1005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agudo-Canalejo J., Knorr R.L. Formation of autophagosomes coincides with relaxation of membrane curvature. Methods Mol. Biol. 2019;1880:173–188. doi: 10.1007/978-1-4939-8873-0_10. [DOI] [PubMed] [Google Scholar]
- 47.Nair U., Jotwani A., Geng J., Gammoh N., Richerson D., Yen W.L., Griffith J., Nag S., Wang K., Moss T. SNARE proteins are required for macroautophagy. Cell. 2011;146(2):290–302. doi: 10.1016/j.cell.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipatova Z., Belogortseva N., Zhang X.Q., Kim J., Taussig D., Segev N. Regulation of selective autophagy onset by a Ypt/Rab GTPase module. Proc. Natl. Acad. Sci. U. S. A. 2012;109(18):6981–6986. doi: 10.1073/pnas.1121299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Menon S., Yamasaki A., Chou H.T., Walz T., Jiang Y., Ferro-Novick S. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc. Natl. Acad. Sci. U. S. A. 2013;110(24):9800–9805. doi: 10.1073/pnas.1302337110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webster C.P., Smith E.F., Bauer C.S., Moller A., Hautbergue G.M., Ferraiuolo L., Myszczynska M.A., Higginbottom A., Walsh M.J., Whitworth A.J. The C9orf72 protein interacts with Rab1a and the ULK 1 complex to regulate initiation of autophagy. EMBO J. 2016;35(15):1656–1676. doi: 10.15252/embj.201694401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamb C.A., Nühlen S., Judith D., Frith D., Snijders A.P., Behrends C., Tooze S.A. TBC1D14 regulates autophagy via the TRAPP complex and ATG 9 traffic. EMBO J. 2016;35(3):281–301. doi: 10.15252/embj.201592695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kakuta S., Yamaguchi J., Suzuki C., Sasaki M., Kazuno S., Uchiyama Y. Small GTPase Rab1B is associated with ATG9A vesicles and regulates autophagosome formation. FASEB J. 2017;31(9):3757–3773. doi: 10.1096/fj.201601052R. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura T., Tamura N., Kono N., Shimanaka Y., Arai H., Yamamoto H., Mizushima N. Autophagosome formation is initiated at phosphatidylinositol synthase-enriched ER subdomains. EMBO J. 2017;36(12):1719–1735. doi: 10.15252/embj.201695189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orsi A., Razi M., Dooley H.C., Robinson D., Weston A.E., Collinson L.M., Tooze S.A. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol. Biol. Cell. 2012;23(10):1860–1873. doi: 10.1091/mbc.E11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallo L.I., Liao Y., Ruiz W.G., Clayton D.R., Li M., Liu Y.J., Jiange Y., Fukuda M., Apodaca G., Yin X.M. TBC1D9B functions as a GTPase-activating protein for Rab11a in polarized MDCK cells. Mol. Biol. Cell. 2014;25(23):3779–3797. doi: 10.1091/mbc.E13-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popovic D., Dikic I. TBC1D5 and the AP2 complex regulate ATG9 trafficking and initiation of autophagy. EMBO Rep. 2014;15(4):392–401. doi: 10.1002/embr.201337995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jimenez-Orgaz A., Kvainickas A., Nägele H., Denner J., Eimer S., Dengjel J., Steinberg F. Control of RAB7 activity and localization through the retromer-TBC1D5 complex enables RAB7-dependent mitophagy. EMBO J. 2018;37(2):235–254. doi: 10.15252/embj.201797128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puri C., Vicinanza M., Ashkenazi A., Gratian M.J., Zhang Q., Bento C.F., Renna M., Menzies F.M., Rubinsztein D.C. The RAB11A-positive compartment is a primary platform for autophagosome assembly mediated by WIPI2 recognition of PI3P-RAB11A. Dev. Cell. 2018;45(1) doi: 10.1016/j.devcel.2018.03.008. (pp. 114-131.e8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawa-Makarska J., Baumann V., Coudevylle N., von Bülow S., Nogellova V., Abert C., Schuschnig M., Graef M., Hummer G., Martens S. Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation. Science. 2020;369(6508) doi: 10.1126/SCIENCE.AAZ7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotani T., Kirisako H., Koizumi M., Ohsumi Y., Nakatogawa H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl. Acad. Sci. U. S. A. 2018;115(41):10363–10368. doi: 10.1073/pnas.1806727115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osawa T., Kotani T., Kawaoka T., Hirata E., Suzuki K., Nakatogawa H., Ohsumi Y., Noda N.N. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat. Struct. Mol. Biol. 2019;26(4):281–288. doi: 10.1038/s41594-019-0203-4. [DOI] [PubMed] [Google Scholar]
- 62.Chowdhury S., Otomo C., Leitner A., Ohashi K., Aebersold R., Lander G.C., Otomo T. Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A-WIPI4 complex. Proc. Natl. Acad. Sci. U. S. A. 2018;115(42):E9792–E9801. doi: 10.1073/pnas.1811874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gómez-Sánchez R., Rose J., Guimarães R., Mari M., Papinski D., Rieter E., Geerts W.J., Hardenberg R., Kraft C., Ungermann C. Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J. Cell Biol. 2018;217(8):2743–2763. doi: 10.1083/jcb.201710116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki K., Akioka M., Kondo-Kakuta C., Yamamoto H., Ohsumi Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J. Cell Sci. 2013;126(11):2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- 65.Tang Z., Takahashi Y., He H., Hattori T., Chen C., Liang X., Chen H., Young M.M., Wang H.G. TOM40 targets Atg2 to mitochondria-associated ER membranes for phagophore expansion. Cell Rep. 2019;28(7) doi: 10.1016/j.celrep.2019.07.036. (pp. 1744-1757.e5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reggiori F., Tucker K.A., Stromhaug P.E., Klionsky D.J. The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Dev. Cell. 2004;6(1):79–90. doi: 10.1016/S1534-5807(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 67.Young A.R.J., Chan E.Y.W., Hu X.W., Köchl R., Crawshaw S.G., High S., Halley D.W., Lippincott-Schwartz J., Tooze S.A. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J. Cell Sci. 2006;119(18):3888–3900. doi: 10.1242/jcs.03172. [DOI] [PubMed] [Google Scholar]
- 68.Webber J.L., Tooze S.A. New insights into the function of Atg9. FEBS Lett. 2010;584(7):1319–1326. doi: 10.1016/j.febslet.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 69.Puri C., Renna M., Bento C.F., Moreau K., Rubinsztein D.C. Diverse autophagosome membrane sources coalesce in recycling endosomes. Cell. 2013;154(6):1285–1299. doi: 10.1016/j.cell.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lees J.A., Reinisch K.M. Inter-organelle lipid transfer: a channel model for Vps13 and chorein-N motif proteins. Curr. Opin. Cell Biol. 2020;65:66–71. doi: 10.1016/j.ceb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong L.H., Gatta A.T., Levine T.P. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat. Rev. Mol. Cell Biol. 2019;20(2):85–101. doi: 10.1038/s41580-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 72.Girardi J.P., Pereira L., Bakovic M. De novo synthesis of phospholipids is coupled with autophagosome formation. Med. Hypotheses. 2011;77(6):1083–1087. doi: 10.1016/j.mehy.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Fengsrud M., Erichsen E.S., Berg T.O., Raiborg C., Seglen P.O. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur. J. Cell Biol. 2000;79(12):871–882. doi: 10.1078/0171-9335-00125. [DOI] [PubMed] [Google Scholar]
- 74.Maeda S., Yamamoto H., Kinch L.N., Garza C.M., Takahashi S., Otomo C., Grishin N.V., Forli S., Mizushima N., Otomo T. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat. Struct. Mol. Biol. 2020 doi: 10.1038/s41594-020-00520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matoba K., Kotani T., Tsutsumi A., Tsuji T., Mori T., Noshiro D., Sugita Y., Nomura N., Iwata S., Ohsumi Y. Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat. Struct. Mol. Biol. 2020 doi: 10.1038/s41594-020-00518-w. [DOI] [PubMed] [Google Scholar]
- 76.Guardia C.M., Tan X.F., Lian T., Rana M.S., Zhou W., Christenson E.T., Lowry A.J., Faraldo-Gómez J.D., Bonifacino J.S., Jiang J. Structure of human ATG9A, the only transmembrane protein of the core autophagy machinery. Cell Rep. 2020;31(13) doi: 10.1016/j.celrep.2020.107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papinski D., Schuschnig M., Reiter W., Wilhelm L., Barnes C.A., Maiolica A., Hansmann I., Pfaffenwimmer T., Kijanska M., Stoffel I. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Mol. Cell. 2014;53(3):471–483. doi: 10.1016/j.molcel.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bushell S.R., Pike A.C.W., Falzone M.E., Rorsman N.J.G., Ta C.M., Corey R.A., Newport T.D., Christianson J.C., Scofano L.F., Shintre C.A. The structural basis of lipid scrambling and inactivation in the endoplasmic reticulum scramblase TMEM16K. Nat. Commun. 2019;10(1) doi: 10.1038/s41467-019-11753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghanbarpour A., Valverde D.P., Melia T.J., Reinisch K.M. A model for a partnership of lipid transfer proteins and scramblases in membrane expansion and organelle biogenesis. Proc. Natl. Acad. Sci. 2021;118(16) doi: 10.1073/pnas.2101562118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schütter M., Giavalisco P., Brodesser S., Graef M. Local fatty acid channeling into phospholipid synthesis drives phagophore expansion during autophagy. Cell. 2020;180(1) doi: 10.1016/j.cell.2019.12.005. (pp. 135-149.e14) [DOI] [PubMed] [Google Scholar]
- 81.Shima T., Kirisako H., Nakatogawa H. COPII vesicles contribute to autophagosomal membranes. J. Cell Biol. 2019;218(5):1503–1510. doi: 10.1083/jcb.201809032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ge L., Zhang M., Schekman R. Phosphatidylinositol 3-kinase and COPII generate LC3 lipidation vesicles from the ER-Golgi intermediate compartment. Elife. 2014;3(November):1–13. doi: 10.7554/eLife.04135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ge L., Zhang M., Kenny S.J., Liu D., Maeda M., Saito K., Mathur A., Xu K., Schekman R. Remodeling of ER -exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep. 2017;18(9):1586–1603. doi: 10.15252/embr.201744559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreau K., Ravikumar B., Renna M., Puri C., Rubinsztein D.C. Autophagosome precursor maturation requires homotypic fusion. Cell. 2011;146(2):303–317. doi: 10.1016/j.cell.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.English L., Chemali M., Duron J., Rondeau C., Laplante A., Gingras D., Alexander D., Leib D., Norbury C., Lippé R. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009;10(5):480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baba M., Tomonaga S., Suzuki M., Gen M., Takeda E., Matsuura A., Kamada Y., Baba N. A nuclear membrane-derived structure associated with Atg8 is involved in the sequestration of selective cargo, the Cvt complex, during autophagosome formation in yeast. Autophagy. 2019;15(3):423–437. doi: 10.1080/15548627.2018.1525475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nishida Y., Arakawa S., Fujitani K., Yamaguchi H., Mizuta T., Kanaseki T., Komatsu M., Otsu K., Tsujimoto Y., Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461(7264):654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- 88.Li L., Tong M., Fu Y., Chen F., Zhang S., Chen H., Ma X., Li D., Liu X., Zhong Q. Lipids and membrane-associated proteins in autophagy. Protein and Cell. 2020:1–25. doi: 10.1007/s13238-020-00793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Craene J.O., Bertazzi D.L., Bär S., Friant S. Phosphoinositides, major actors in membrane trafficking and lipid signaling pathways. Int. J. Mol. Sci. 2017;18(3):634. doi: 10.3390/ijms18030634. (MDPI AG) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Obara K., Sekito T., Ohsumi Y. Assortment of phosphatidylinositol 3-kinase complexes-Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Mol. Biol. Cell. 2006;17(4):1527–1539. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsunaga K., Morita E., Saitoh T., Akira S., Ktistakis N.T., Izumi T., Noda T., Yoshimori T. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J. Cell Biol. 2010;190(4):511–521. doi: 10.1083/jcb.200911141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhong Y., Wang Q.J., Li X., Yan Y., Backer J.M., Chait B.T., Heintz N., Yue Z. Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat. Cell Biol. 2009;11(4):468–476. doi: 10.1038/ncb1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma M., Liu J.J., Li Y., Huang Y., Ta N., Chen Y., Fu H., Ye M. Da, Ding Y., Huang W. Cryo-EM structure and biochemical analysis reveal the basis of the functional difference between human PI3KC3-C1 and -C2. Cell Res. 2017;27(8):989–1001. doi: 10.1038/cr.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Funderburk S.F., Wang Q.J., Yue Z. Trends Cell Biol. vol. 20, no. 6. NIH Public Access; 2010. The Beclin 1-VPS34 complex - at the crossroads of autophagy and beyond; pp. 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russell R.C., Tian Y., Yuan H., Park H.W., Chang Y.Y., Kim J., Kim H., Neufeld T.P., Dillin A., Guan K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15(7):741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wold M.S., Lim J., Lachance V., Deng Z., Yue Z. ULK1-mediated phosphorylation of ATG14 promotes autophagy and is impaired in Huntington’s disease models. Mol. Neurodegener. 2016;11(1):76. doi: 10.1186/s13024-016-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papinski D., Kraft C. Regulation of autophagy by signaling through the Atg1/ULK1 complex. J. Mol. Biol. 2016;428(9):1725–1741. doi: 10.1016/j.jmb.2016.03.030. (Academic Press) [DOI] [PubMed] [Google Scholar]
- 98.Lystad A.H., Simonsen A. Phosphoinositide-binding proteins in autophagy. FEBS Lett. 2016;590(15):2454–2468. doi: 10.1002/1873-3468.12286. (Wiley Blackwell) [DOI] [PubMed] [Google Scholar]
- 99.Stefan C.J., Trimble W.S., Grinstein S., Drin G., Reinisch K., De Camilli P., Cohen S., Valm A.M., Lippincott-Schwartz J., Levine T.P. Membrane dynamics and organelle biogenesis-lipid pipelines and vesicular carriers. BMC Biol. 2017;15(1):102. doi: 10.1186/s12915-017-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carlsson S.R., Simonsen A. Membrane dynamics in autophagosome biogenesis. J. Cell Sci. 2015;128(2):193–205. doi: 10.1242/jcs.141036. [DOI] [PubMed] [Google Scholar]
- 101.Meijer W.H., van der Klei I.J., Veenhuis M., Kiel J.A.K.W. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy. 2007;3(2):106–116. doi: 10.4161/auto.3595. [DOI] [PubMed] [Google Scholar]
- 102.Proikas-Cezanne T., Takacs Z., Dönnes P., Kohlbacher O. WIPI proteins: essential PtdIns3P effectors at the nascent autophagosome. J. Cell Sci. 2015;128(2):207–217. doi: 10.1242/jcs.146258. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki K., Kubota Y., Sekito T., Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12(2):209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 104.Juris L., Montino M., Rube P., Schlotterhose P., Thumm M., Krick R. PI3P binding by Atg21 organises Atg8 lipidation. EMBO J. 2015;34(7):955–973. doi: 10.15252/embj.201488957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Polson H.E.J., de Lartigue J., Rigden D.J., Reedijk M., Urbé S., Clague M.J., Tooze S.A. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6(4):506–522. doi: 10.4161/auto.6.4.11863. [DOI] [PubMed] [Google Scholar]
- 106.Karanasios E., Stapleton E., Manifava M., Kaizuka T., Mizushima N., Walker S.A., Ktistakis N.T. Dynamic association of the ULK1 complex with omegasomes during autophagy induction. J. Cell Sci. 2013;126(22):5224–5238. doi: 10.1242/jcs.132415. [DOI] [PubMed] [Google Scholar]
- 107.Cabrera M., Nordmann M., Perz A., Schmedt D., Gerondopoulos A., Barr F., Piehler J., Engelbrecht-Vandré S., Ungermann C. The Mon1-Ccz1 GEF activates the Rab7 GTPase Ypt7 via a longin-fold-rab interface and association with PI3P-positive membranes. J. Cell Sci. 2014;127(5):1043–1051. doi: 10.1242/jcs.140921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jean S., Kiger A.A. Coordination between RAB GTPase and phosphoinositide regulation and functions. Nat. Rev. Mol. Cell Biol. 2012;13(7):463–470. doi: 10.1038/nrm3379. [DOI] [PubMed] [Google Scholar]
- 109.Christoforidis S., Miaczynska M., Ashman K., Wilm M., Zhao L., Yip S.C., Waterfield M.D., Backer J.M., Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1999;1(4):249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- 110.Ravikumar B., Imarisio S., Sarkar S., O’Kane C.J., Rubinsztein D.C. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J. Cell Sci. 2008;121(10):1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dou Z., Pan J.A., Dbouk H.A., Ballou L.M., DeLeon J.L., Fan Y., Chen J.S., Liang Z., Li G., Backer J.M. Class IA PI3K p110β subunit promotes autophagy through Rab5 small GTPase in response to growth factor limitation. Mol. Cell. 2013;50(1):29–42. doi: 10.1016/j.molcel.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shin H.W., Hayashi M., Christoforidis S., Lacas-Gervais S., Hoepfner S., Wenk M.R., Modregger J., Uttenweiler-Joseph S., Wilm M., Nystuen A. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J. Cell Biol. 2005;170(4):607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng J., Fujita A., Yamamoto H., Tatematsu T., Kakuta S., Obara K., Ohsumi Y., Fujimoto T. Yeast and mammalian autophagosomes exhibit distinct phosphatidylinositol 3-phosphate asymmetries. Nat. Commun. 2014;5(1):1–12. doi: 10.1038/ncomms4207. [DOI] [PubMed] [Google Scholar]
- 114.Law F., Seo J.H., Wang Z., DeLeon J.L., Bolis Y., Brown A., Zong W.X., Du G., Rocheleau C.E. The VPS34 PI3K negatively regulates RAB-5 during endosome maturation. J. Cell Sci. 2017;130(12):2007–2017. doi: 10.1242/jcs.194746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tabata K., Matsunaga K., Sakane A., Sasaki T., Noda T., Yoshimori T. Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain. Mol. Biol. Cell. 2010;21(23):4162–4172. doi: 10.1091/mbc.E10-06-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ray A., Jatana N., Thukral L. Lipidated proteins: spotlight on protein-membrane binding interfaces. Prog. Biophys. Mol. Biol. 2017;128:74–84. doi: 10.1016/j.pbiomolbio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Heckmann B.L., Green D.R. LC3-associated phagocytosis at a glance. J. Cell Sci. 2019;132(5) doi: 10.1242/jcs.222984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu X.M., Yamasaki A., Du X.M., Coffman V.C., Ohsumi Y., Nakatogawa H., Wu J.Q., Noda N.N., Du L.L. Lipidation-independent vacuolar functions of Atg8 rely on its noncanonical interaction with a vacuole membrane protein. Elife. 2018;7 doi: 10.7554/eLife.41237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shpilka T., Weidberg H., Pietrokovski S., Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12(7):1–11. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jatana N., Ascher D.B., Pires D.E.V., Gokhale R.S., Thukral L. Human LC3 and GABARAP subfamily members achieve functional specificity via specific structural modulations. Autophagy. 2020;16(2):239–255. doi: 10.1080/15548627.2019.1606636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paz Y., Elazar Z., Fass D. Structure of GATE-16, membrane transport modulator and mammalian ortholog of autophagocytosis factor Aut7p. J. Biol. Chem. 2000;275(33):25445–25450. doi: 10.1074/jbc.C000307200. [DOI] [PubMed] [Google Scholar]
- 122.Kabeya Y., Mizushima N., Yamamoto A., Oshitani-Okamoto S., Ohsumi Y., Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004;117(13):2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 123.Kirisako T., Ichimura Y., Okada H., Kabeya Y., Mizushima N., Yoshimori T., Ohsumi M., Takao T., Noda T., Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000;151(2):263–275. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tanida I., Sou Y.S., Ezaki J., Minematsu-Ikeguchi N., Ueno T., Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J. Biol. Chem. 2004;279(35):36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 125.Kirisako T., Baba M., Ishihara N., Miyazawa K., Ohsumi M., Yoshimori T., Noda T., Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 1999;147(2):435–446. doi: 10.1083/jcb.147.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu Z.Q., Ni T., Hong B., Wang H.Y., Jiang F.J., Zou S., Chen Y., Zheng X.L., Klionsky D.J., Liang Y. Dual roles of Atg8-PE deconjugation by Atg4 in autophagy. Autophagy. 2012;8(6):883–892. doi: 10.4161/auto.19652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ichimura Y., Kirisako T., Takao T., Satomi Y., Shimonishi Y., Ishihara N., Mizushima N., Tanida I., Kominami E., Ohsumi M. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 128.Hanada T., Noda N.N., Satomi Y., Ichimura Y., Fujioka Y., Takao T., Inagaki F., Ohsumi Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007;282(52):37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 129.Mizushima N., Noda T., Yoshimori T., Tanaka Y., Ishii T., George M.D., Klionsky D.J., Ohsumi M., Ohsumi Y. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 130.Kuma A., Mizushima N., Ishihara N., Ohsumi Y. Formation of the ~350-kDa Apg12-Apg5·Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J. Biol. Chem. 2002;277(21):18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- 131.Mizushima N., Kuma A., Kobayashi Y., Yamamoto A., Matsubae M., Takao T., Natsume T., Ohsumi Y., Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J. Cell Sci. 2003;116(9):1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 132.Nath S., Dancourt J., Shteyn V., Puente G., Fong W.M., Nag S., Bewersdorf J., Yamamoto A., Antonny B., Melia T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat. Cell Biol. 2014;16(5):415–424. doi: 10.1038/ncb2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang S., Li Y., Ma C. Atg3 promotes Atg8 lipidation via altering lipid diffusion and rearrangement. Protein Sci. 2020;29(6):1511–1523. doi: 10.1002/pro.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell. 2008;19(5):2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Romanov J., Walczak M., Ibiricu I., Schüchner S., Ogris E., Kraft C., Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31(22):4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sakoh-Nakatogawa M., Kirisako H., Nakatogawa H., Ohsumi Y. Localization of Atg3 to autophagy-related membranes and its enhancement by the Atg8-family interacting motif to promote expansion of the membranes. FEBS Lett. 2015;589(6):744–749. doi: 10.1016/j.febslet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 137.Dooley H.C., Razi M., Polson H.E.J., Girardin S.E., Wilson M.I., Tooze S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell. 2014;55(2):238–252. doi: 10.1016/j.molcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pantoom S., Konstantinidis G., Voss S., Han H., Hofnagel O., Li Z., Wu Y.W. RAB33B recruits the ATG16L1 complex to the phagophore via a noncanonical RAB binding protein. Autophagy. 2020:1–15. doi: 10.1080/15548627.2020.1822629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Itoh T., Fujita N., Kanno E., Yamamoto A., Yoshimori T., Fukuda M. Golgi-resident small GTPase Rab33B interacts with Atg16L and modulates autophagosome formation. Mol. Biol. Cell. 2008;19(7):2916–2925. doi: 10.1091/mbc.E07-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Busse R.A., Scacioc A., Krick R., Pérez-Lara Á., Thumm M., Kühnel K. Characterization of PROPPIN-phosphoinositide binding and role of loop 6CD in PROPPIN-membrane binding. Biophys. J. 2015;108(9):2223–2234. doi: 10.1016/j.bpj.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gopaldass N., Fauvet B., Lashuel H., Roux A., Mayer A. Membrane scission driven by the PROPPIN Atg18. EMBO J. 2017;36(22):3274–3291. doi: 10.15252/embj.201796859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lystad A.H., Carlsson S.R., de la Ballina L.R., Kauffman K.J., Nag S., Yoshimori T., Melia T.J., Simonsen A. Distinct functions of ATG16L1 isoforms in membrane binding and LC3B lipidation in autophagy-related processes. Nat. Cell Biol. 2019;21(3):372–383. doi: 10.1038/s41556-019-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gammoh N., Florey O., Overholtzer M., Jiang X. Interaction between FIP200 and ATG16L1 distinguishes ULK1 complex-dependent and-independent autophagy. Nat. Struct. Mol. Biol. 2013;20(2):144–149. doi: 10.1038/nsmb.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nishimura T., Kaizuka T., Cadwell K., Sahani M.H., Saitoh T., Akira S., Virgin H.W., Mizushima N. FIP200 regulates targeting of Atg16L1 to the isolation membrane. EMBO Rep. 2013;14(3):284–291. doi: 10.1038/embor.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Harada K., Kotani T., Kirisako H., Sakoh-Nakatogawa M., Oikawa Y., Kimura Y., Hirano H., Yamamoto H., Ohsumi Y., Nakatogawa H. Two distinct mechanisms target the autophagy-related E3 complex to the pre- autophagosomal structure. Elife. 2019;8 doi: 10.7554/eLife.43088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dudley L.J., Cabodevilla A.G., Makar A.N., Sztacho M., Michelberger T., Marsh J.A., Houston D.R., Martens S., Jiang X., Gammoh N. Intrinsic lipid binding activity of ATG 16L1 supports efficient membrane anchoring and autophagy. EMBO J. 2019;38(9) doi: 10.15252/embj.2018100554. (p. e100554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kaufmann A., Beier V., Franquelim H.G., Wollert T. Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell. 2014;156(3):469–481. doi: 10.1016/j.cell.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 148.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lystad A.H., Ichimura Y., Takagi K., Yang Y., Pankiv S., Kanegae Y., Kageyama S., Suzuki M., Saito I., Mizushima T. Structural determinants in GABARAP required for the selective binding and recruitment of ALFY to LC3B-positive structures. EMBO Rep. 2014;15(5):557–565. doi: 10.1002/embr.201338003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sawa-Makarska J., Abert C., Romanov J., Zens B., Ibiricu I., Martens S. Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane-cargo apposition during selective autophagy. Nat. Cell Biol. 2014;16(5):425–433. doi: 10.1038/ncb2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsuboyama K., Koyama-Honda I., Sakamaki Y., Koike M., Morishita H., Mizushima N. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science. 2016;354(6315):1036–1041. doi: 10.1126/science.aaf6136. [DOI] [PubMed] [Google Scholar]
- 152.Olsvik H.L., Lamark T., Takagi K., Larsen K.B., Evjen G., Øvervatn A., Mizushima T., Johansen X.T. FYCO1 contains a C-terminally extended, LC3A/B-preferring LC3-interacting region (LIR) motif required for efficient maturation of autophagosomes during basal autophagy. J. Biol. Chem. 2015;290(49):29361–29374. doi: 10.1074/jbc.M115.686915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kraft C., Kijanska M., Kalie E., Siergiejuk E., Lee S.S., Semplicio G., Stoffel I., Brezovich A., Verma M., Hansmann I. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 2012;31(18):3691–3703. doi: 10.1038/emboj.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alemu E.A., Lamark T., Torgersen K.M., Birgisdottir A.B., Larsen K.B., Jain A., Olsvik H., Øvervatn A., Kirkin V., Johansen T. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 2012;287(47):39275–39290. doi: 10.1074/jbc.M112.378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Birgisdottir Å.B., Mouilleron S., Bhujabal Z., Wirth M., Sjøttem E., Evjen G., Zhang W., Lee R., O’Reilly N., Tooze S.A. Members of the autophagy class III phosphatidylinositol 3-kinase complex I interact with GABARAP and GABARAPL1 via LIR motifs. Autophagy. 2019;15(8):1333–1355. doi: 10.1080/15548627.2019.1581009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bozic M., van den Bekerom L., Milne B.A., Goodman N., Roberston L., Prescott A.R., Macartney T.J., Dawe N., McEwan D.G. A conserved ATG2-GABARAP family interaction is critical for phagophore formation. EMBO Rep. 2020;21(3) doi: 10.15252/embr.201948412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Padman B.S., Nguyen T.N., Uoselis L., Skulsuppaisarn M., Nguyen L.K., Lazarou M. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 2019;10(1):1–13. doi: 10.1038/s41467-019-08335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grunwald D.S., Otto N.M., Park J.M., Song D., Kim D.H. GABARAPs and LC3s have opposite roles in regulating ULK1 for autophagy induction. Autophagy. 2020;16(4):600–614. doi: 10.1080/15548627.2019.1632620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abeliovich H., Dunn W.A., Kim J., Klionsky D.J. Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. J. Cell Biol. 2000;151(5):1025–1033. doi: 10.1083/jcb.151.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nguyen T.N., Padman B.S., Usher J., Oorschot V., Ramm G., Lazarou M. Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation. J. Cell Biol. 2016;215(6):857–874. doi: 10.1083/jcb.201607039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Agrotis A., Pengo N., Burden J.J., Ketteler R. Redundancy of human ATG4 protease isoforms in autophagy and LC3/GABARAP processing revealed in cells. Autophagy. 2019;15(6):976–997. doi: 10.1080/15548627.2019.1569925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujita N., Hayashi-Nishino M., Fukumoto H., Omori H., Yamamoto A., Noda T., Yoshimori T. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol. Biol. Cell. 2008;19(11):4651–4659. doi: 10.1091/mbc.E08-03-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vaites L.P., Paulo J.A., Huttlin E.L., Harper J.W. Systematic analysis of human cells lacking ATG8 proteins uncovers roles for GABARAPs and the CCZ1/MON1 regulator C18orf8/RMC1 in macroautophagic and selective autophagic flux. Mol. Cell. Biol. 2018;38(1) doi: 10.1128/mcb.00392-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Engedal N., Seglen P.O. Autophagy of cytoplasmic bulk cargo does not require LC3. Autophagy. 2016;12(2):439–441. doi: 10.1080/15548627.2015.1076606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shvets E., Abada A., Weidberg H., Elazar Z. Dissecting the involvement of LC3B and GATE-16 in p62 recruitment into autophagosomes. Autophagy. 2011;7(7):683–688. doi: 10.4161/auto.7.7.15279. [DOI] [PubMed] [Google Scholar]
- 166.von Muhlinen N., Akutsu M., Ravenhill B.J., Foeglein Á., Bloor S., Rutherford T.J., Freund S.M.V., Komander D., Randow F. LC3C, bound selectively by a noncanonical LIR motif in NDP52, is required for antibacterial autophagy. Mol. Cell. 2012;48(3):329–342. doi: 10.1016/j.molcel.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maruyama Y., Sou Y.S., Kageyama S., Takahashi T., Ueno T., Tanaka K., Komatsu M., Ichimura Y. LC3B is indispensable for selective autophagy of p62 but not basal autophagy. Biochem. Biophys. Res. Commun. 2014;446(1):309–315. doi: 10.1016/j.bbrc.2014.02.093. [DOI] [PubMed] [Google Scholar]
- 168.Joachim J., Jefferies H.B.J., Razi M., Frith D., Snijders A.P., Chakravarty P., Judith D., Tooze S.A. Activation of ULK kinase and autophagy by GABARAP trafficking from the centrosome is regulated by WAC and GM130. Mol. Cell. 2015;60(6):899–913. doi: 10.1016/j.molcel.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Noda N.N., Kumeta H., Nakatogawa H., Satoo K., Adachi W., Ishii J., Fujioka Y., Ohsumi Y., Inagaki F. Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cells. 2008;13(12):1211–1218. doi: 10.1111/j.1365-2443.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- 170.Ichimura Y., Kumanomidou T., Sou Y.S., Mizushima T., Ezaki J., Ueno T., Kominami E., Yamane T., Tanaka K., Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. J. Biol. Chem. 2008;283(33):22847–22857. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- 171.Pankiv S., Clausen T.H., Lamark T., Brech A., Bruun J.A., Outzen H., Øvervatn A., Bjørkøy G., Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 172.Noda N.N., Ohsumi Y., Inagaki F. Atg8-family interacting motif crucial for selective autophagy. FEBS Lett. 2010;584(7):1379–1385. doi: 10.1016/j.febslet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 173.Johansen T., Lamark T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J. Mol. Biol. 2020;432(1):80–103. doi: 10.1016/j.jmb.2019.07.016. [DOI] [PubMed] [Google Scholar]
- 174.Rogov V.V., Stolz A., Ravichandran A.C., Rios-Szwed D.O., Suzuki H., Kniss A., Löhr F., Wakatsuki S., Dötsch V., Dikic I. Structural and functional analysis of the GABARAP interaction motif (GIM) EMBO Rep. 2017;18(8):1382–1396. doi: 10.15252/embr.201643587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wirth M., Zhang W., Razi M., Nyoni L., Joshi D., O’Reilly N., Johansen T., Tooze S.A., Mouilleron S. Molecular determinants regulating selective binding of autophagy adapters and receptors to ATG8 proteins. Nat. Commun. 2019;10(1):1–18. doi: 10.1038/s41467-019-10059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Weidberg H., Shpilka T., Shvets E., Abada A., Shimron F., Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell. 2011;20(4):444–454. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 177.Landajuela A., Hervás J.H., Antón Z., Montes L.R., Gil D., Valle M., Rodriguez J.F., Goñi F.M., Alonso A. Lipid geometry and bilayer curvature modulate LC3/GABARAP-mediated model autophagosomal elongation. Biophys. J. 2016;110(2):411–422. doi: 10.1016/j.bpj.2015.11.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taniguchi S., Toyoshima M., Takamatsu T., Mima J. Curvature-sensitive trans-assembly of human Atg8-family proteins in autophagy-related membrane tethering. Protein Sci. 2020;29(6):1387–1400. doi: 10.1002/pro.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kriegenburg F., Ungermann C., Reggiori F. Coordination of autophagosome–lysosome fusion by Atg8 family members. Curr. Biol. 2018;28(8):R512–R518. doi: 10.1016/j.cub.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 180.Jacquet M., Guittaut M., Fraichard A., Despouy G. The functions of Atg8-family proteins in autophagy and cancer: linked or unrelated? Autophagy. 2021;17(3):599–611. doi: 10.1080/15548627.2020.1749367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lystad A.H., Simonsen A. Mechanisms and pathophysiological roles of the ATG8 conjugation machinery. Cells. 2019;8(9):973. doi: 10.3390/cells8090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cherra S.J., Kulich S.M., Uechi G., Balasubramani M., Mountzouris J., Day B.W., Chu C.T. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010;190(4):533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bartholomew C.R., Suzuki T., Du Z., Backues S.K., Jin M., Lynch-Day M.A., Umekawa M., Kamath A., Zhao M., Xie Z. Ume6 transcription factor is part of a signaling cascade that regulates autophagy. Proc. Natl. Acad. Sci. U. S. A. 2012;109(28):11206–11210. doi: 10.1073/pnas.1200313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang R., Xu Y., Wan W., Shou X., Qian J., You Z., Liu B., Chang C., Zhou T., Lippincott-Schwartz J. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell. 2015;57(3):456–466. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 185.Herhaus L., Bhaskara R.M., Lystad A.H., Gestal-Mato U., Covarrubias-Pinto A., Bonn F., Simonsen A., Hummer G., Dikic I. TBK1-mediated phosphorylation of LC3C and GABARAP-L2 controls autophagosome shedding by ATG4 protease. EMBO Rep. 2020;21(1) doi: 10.15252/embr.201948317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nakatogawa H., Ishii J., Asai E., Ohsumi Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy. 2012;8(2) doi: 10.4161/auto.8.2.18373. [DOI] [PubMed] [Google Scholar]
- 187.Nair U., Yen W.L., Mari M., Cao Y., Xie Z., Baba M., Reggiori F., Klionsky D.J. A role for Atg8-PE deconjugation in autophagosome biogenesis. Autophagy. 2012;8(5):780–793. doi: 10.4161/auto.19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hirata E., Ohya Y., Suzuki K. Atg4 plays an important role in efficient expansion of autophagic isolation membranes by cleaving lipidated Atg8 in Saccharomyces cerevisiae. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nair U., Cao Y., Xie Z., Klionsky D.J. Roles of the lipid-binding motifs of Atg18 and Atg21 in the cytoplasm to vacuole targeting pathway and autophagy. J. Biol. Chem. 2010;285(15):11476–11488. doi: 10.1074/jbc.M109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Abreu S., Kriegenburg F., Gómez-Sánchez R., Mari M., Sánchez-Wandelmer J., Skytte Rasmussen M., Soares Guimarães R., Zens B., Schuschnig M., Hardenberg R. Conserved Atg8 recognition sites mediate Atg4 association with autophagosomal membranes and Atg8 deconjugation. EMBO Rep. 2017;18(5):765–780. doi: 10.15252/embr.201643146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sánchez-Wandelmer J., Kriegenburg F., Rohringer S., Schuschnig M., Gómez-Sánchez R., Zens B., Abreu S., Hardenberg R., Hollenstein D., Gao J. Atg4 proteolytic activity can be inhibited by Atg1 phosphorylation. Nat. Commun. 2017;8(1):1–10. doi: 10.1038/s41467-017-00302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pengo N., Agrotis A., Prak K., Jones J., Ketteler R. A reversible phospho-switch mediated by ULK1 regulates the activity of autophagy protease ATG4B. Nat. Commun. 2017;8(1):1–10. doi: 10.1038/s41467-017-00303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Perez-Perez M.E., Zaffagnini M., Marchand C.H., Crespo J.L., Lemaire S.D. The yeast autophagy protease Atg4 is regulated by thioredoxin. Autophagy. 2014;10(11):1953–1964. doi: 10.4161/auto.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Popovic D., Akutsu M., Novak I., Harper J.W., Behrends C., Dikic I. Rab GTPase-activating proteins in autophagy: regulation of endocytic and autophagy pathways by direct binding to human ATG8 modifiers. Mol. Cell. Biol. 2012;32(9):1733–1744. doi: 10.1128/mcb.06717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gao J., Langemeyer L., Kümmel D., Reggiori F., Ungermann C. Molecular mechanism to target the endosomal Mon1-Ccz1 GEF complex to the pre-autophagosomal structure. Elife. 2018;7 doi: 10.7554/eLife.31145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gutierrez M.G., Munafó D.B., Berón W., Colombo M.I. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J. Cell Sci. 2004;117(13):2687–2697. doi: 10.1242/jcs.01114. [DOI] [PubMed] [Google Scholar]
- 198.Manil-Ségalen M., Lefebvre C., Jenzer C., Trichet M., Boulogne C., Satiat-Jeunemaitre B., Legouis R. The C.elegans LC3 acts downstream of GABARAP to degrade autophagosomes by interacting with the HOPS subunit VPS39. Dev. Cell. 2014;28(1):43–55. doi: 10.1016/j.devcel.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 199.Wang Z., Miao G., Xue X., Guo X., Yuan C., Wang Z., Zhang G., Chen Y., Feng D., Hu J. The Vici syndrome protein EPG5 is a Rab7 effector that determines the fusion specificity of Autophagosomes with late endosomes/lysosomes. Mol. Cell. 2016;63(5):781–795. doi: 10.1016/j.molcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 200.Nieto-Torres J., Shanahan S.-L., Chassefeyre R., Landeras-Bueno S., Encalada S., Hansen M. 2020. LC3B Phosphorylation Regulates FYCO1 Binding and Directional Transport of Autophagosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wijdeven R.H., Janssen H., Nahidiazar L., Janssen L., Jalink K., Berlin I., Neefjes J. Cholesterol and ORP1L-mediated ER contact sites control autophagosome transport and fusion with the endocytic pathway. Nat. Commun. 2016;7(1):1–14. doi: 10.1038/ncomms11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Khobrekar N.V., Quintremil S., Dantas T.J., Vallee R.B. The dynein adaptor RILP controls neuronal autophagosome biogenesis, transport, and clearance. Dev. Cell. 2020;53(2) doi: 10.1016/j.devcel.2020.03.011. (pp. 141-153.e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carroll B., Mohd-Naim N., Maximiano F., Frasa M.A., McCormack J., Finelli M., Thoresen S.B., Perdios L., Daigaku R., Francis R.E. The TBC/RabGAP Armus coordinates Rac1 and Rab7 functions during autophagy. Dev. Cell. 2013;25(1):15–28. doi: 10.1016/j.devcel.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yamano K., Fogel A.I., Wang C., van der Bliek A.M., Youle R.J. Mitochondrial Rab GAPs govern autophagosome biogenesis during mitophagy. Elife. 2014;2014(3) doi: 10.7554/eLife.01612.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kjos I., Borg Distefano M., Sætre F., Repnik U., Holland P., Jones A.T., Engedal N., Simonsen A., Bakke O., Progida C. Rab7b modulates autophagic flux by interacting with Atg4B. EMBO Rep. 2017;18(10):1727–1739. doi: 10.15252/embr.201744069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liao Y., Li M., Chen X., Jiang Y., Yin X.M. Interaction of TBC1D9B with mammalian ATG8 homologues regulates autophagic flux. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Takahashi Y., Tsotakos N., Liu Y., Young M.M., Serfass J., Tang Z., Abraham T., Wang H.G. The Bif-1-Dynamin 2 membrane fission machinery regulates Atg9-containing vesicle generation at the Rab11-positive reservoirs. Oncotarget. 2016;7(15):20855–20868. doi: 10.18632/oncotarget.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mitter A.L., Schlotterhose P., Krick R. Gyp1 has a dual function as Ypt1 GAP and interaction partner of Atg8 in selective autophagy. Autophagy. 2019;15(6):1031–1050. doi: 10.1080/15548627.2019.1569929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Itoh T., Kanno E., Uemura T., Waguri S., Fukuda M. OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J. Cell Biol. 2011;192(5):838–853. doi: 10.1083/jcb.201008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Noda T., Fujita N., Yoshimori T. The late stages of autophagy: how does the end begin? Cell Death Differ. 2009;16(7):984–990. doi: 10.1038/cdd.2009.54. [DOI] [PubMed] [Google Scholar]
- 211.Knorr R.L., Lipowsky R., Dimova R. Autophagosome closure requires membrane scission. Autophagy. 2015;11(11):2134–2137. doi: 10.1080/15548627.2015.1091552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Christ L., Raiborg C., Wenzel E.M., Campsteijn C., Stenmark H. Cellular functions and molecular mechanisms of the ESCRT membrane-scission machinery. Trends Biochem. Sci. 2017;42(1):42–56. doi: 10.1016/j.tibs.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 213.Vietri M., Radulovic M., Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020;21(1):25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 214.Wollert T., Wunder C., Lippincott-Schwartz J., Hurley J.H. Membrane scission by the ESCRT-III complex. Nature. 2009;458(7235):172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Takahashi Y., He H., Tang Z., Hattori T., Liu Y., Young M.M., Serfass J.M., Chen L., Gebru M., Chen C. An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Nat. Commun. 2018;9(1):1–13. doi: 10.1038/s41467-018-05254-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhen Y., Spangenberg H., Munson M.J., Brech A., Schink K.O., Tan K.W., Sørensen V., Wenzel E.M., Radulovic M., Engedal N. ESCRT-mediated phagophore sealing during mitophagy. Autophagy. 2020;16(5):826–841. doi: 10.1080/15548627.2019.1639301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhou F., Wu Z., Zhao M., Murtazina R., Cai J., Zhang A., Li R., Sun D., Li W., Zhao L. Rab5-dependent autophagosome closure by ESCRT. J. Cell Biol. 2019;218(6):1908–1927. doi: 10.1083/JCB.201811173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Takahashi Y., Liang X., Hattori T., Tang Z., He H., Chen H., Liu X., Abraham T., Imamura-Kawasawa Y., Buchkovich N.J. VPS37A directs ESCRT recruitment for phagophore closure. J. Cell Biol. 2019;218(10):3336–3354. doi: 10.1083/JCB.201902170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mierzwa B.E., Chiaruttini N., Redondo-Morata L., Moser Von Filseck J., König J., Larios J., Poser I., Müller-Reichert T., Scheuring S., Roux A. Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat. Cell Biol. 2017;19(7):787–798. doi: 10.1038/ncb3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Flower T.G., Takahashi Y., Hudait A., Rose K., Tjahjono N., Pak A.J., Yokom A.L., Liang X., Wang H.G., Bouamr F. A helical assembly of human ESCRT-I scaffolds reverse-topology membrane scission. Nat. Struct. Mol. Biol. 2020;27(6):570–580. doi: 10.1038/s41594-020-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhou F., Zou S., Chen Y., Lipatova Z., Sun D., Zhu X., Li R., Wu Z., You W., Cong X. A Rab5 GTPase module is important for autophagosome. PLoS Genet. 2017;13(9) doi: 10.1371/journal.pgen.1007020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li Y., Chen X., Xiong Q., Chen Y., Zhao H., Tahir M., Song J., Zhou B., Wang J. Casein kinase 1 family member CK1δ/Hrr25 is required for autophagosome completion. Front. Cell Dev. Biol. 2020;8:460. doi: 10.3389/fcell.2020.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Corcelle-Termeau E., Vindeløv S.D., Hämälistö S., Mograbi B., Keldsbo A., Bräsen J.H., Favaro E., Adam D., Szyniarowski P., Hofman P. Excess sphingomyelin disturbs ATG9A trafficking and autophagosome closure. Autophagy. 2016;12(5):833–849. doi: 10.1080/15548627.2016.1159378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Velikkakath A.K.G., Nishimura T., Oita E., Ishihara N., Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol. Biol. Cell. 2012;23(5):896–909. doi: 10.1091/mbc.E11-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kishi-Itakura C., Koyama-Honda I., Itakura E., Mizushima N. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 2014;127(18):4089–4102. doi: 10.1242/jcs.156034. [DOI] [PubMed] [Google Scholar]
- 226.Stanga D., Zhao Q., Milev M.P., Saint-Dic D., Jimenez-Mallebrera C., Sacher M. TRAPPC11 functions in autophagy by recruiting ATG2B-WIPI4/WDR45 to preautophagosomal membranes. Traffic. 2019;20(5):325–345. doi: 10.1111/tra.12640. [DOI] [PubMed] [Google Scholar]
- 227.Shirahama-Noda K., Kira S., Yoshimori T., Noda T. TRAPP is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy. J. Cell Sci. 2013;126(21):4963–4973. doi: 10.1242/jcs.131318. [DOI] [PubMed] [Google Scholar]
- 228.Molejon M.I., Ropolo A., Re A. Lo, Boggio V., Vaccaro M.I. The VMP1-Beclin 1 interaction regulates autophagy induction. Sci. Rep. 2013;3 doi: 10.1038/srep01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tabara L.C., Escalante R. VMP1 establishes ER-microdomains that regulate membrane contact sites and autophagy. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhao Y.G., Chen Y., Miao G., Zhao H., Qu W., Li D., Wang Z., Liu N., Li L., Chen S. The ER-localized transmembrane protein EPG-3/VMP1 regulates SERCA activity to control ER-isolation membrane contacts for autophagosome formation. Mol. Cell. 2017;67(6) doi: 10.1016/j.molcel.2017.08.005. (pp. 974-989.e6) [DOI] [PubMed] [Google Scholar]
- 231.Sou Y.S., Waguri S., Iwata J.I., Ueno T., Fujimura T., Hara T., Sawada N., Yamada A., Mizushima N., Uchiyama Y. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol. Biol. Cell. 2008;19(11):4762–4775. doi: 10.1091/mbc.E08-03-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zess E.K., Jensen C., Cruz-Mireles N., De la Concepcion J.C., Sklenar J., Stephani M., Imre R., Roitinger E., Hughes R., Belhaj K. N-terminal β-strand underpins biochemical specialization of an ATG8 isoform. PLoS Biol. 2019;17(7) doi: 10.1371/journal.pbio.3000373. (p. e3000373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wurzer B., Zaffagnini G., Fracchiolla D., Turco E., Abert C., Romanov J., Martens S. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife. 2015;4(September) doi: 10.7554/eLife.08941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Rossman J.S., Lamb R.A. Viral membrane scission. Annu. Rev. Cell Dev. Biol. 2013;29:551–569. doi: 10.1146/annurev-cellbio-101011-155838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gao J., Reggiori F., Ungermann C. A novel in vitro assay reveals SNARE topology and the role of Ykt6 in autophagosome fusion with vacuoles. J. Cell Biol. 2018;217(10):3670–3682. doi: 10.1083/JCB.201804039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jiang P., Nishimura T., Sakamaki Y., Itakura E., Hatta T., Natsume T., Mizushima N. The HOPS complex mediates autophagosome-lysosome fusion through interaction with syntaxin 17. Mol. Biol. Cell. 2014;25(8):1327–1337. doi: 10.1091/mbc.E13-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hegedűs K., Takáts S., Boda A., Jipa A., Nagy P., Varga K., Kovács A.L., Juhász G. The Ccz1-Mon1-Rab7 module and Rab5 control distinct steps of autophagy. Mol. Biol. Cell. 2016;27(20):3132–3142. doi: 10.1091/mbc.E16-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lőrincz P., Tóth S., Benkő P., Lakatos Z., Boda A., Glatz G., Zobel M., Bisi S., Hegedűs K., Takáts S., Scita G., Juhász G. Rab2 promotes autophagic and endocytic lysosomal degradation. J. Cell Biol. 2017;216(7):1937–1947. doi: 10.1083/jcb.201611027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ebner P., Poetsch I., Deszcz L., Hoffmann T., Zuber J., Ikeda F. The IAP family member BRUCE regulates autophagosome–lysosome fusion. Nat. Commun. 2018;9(1):1030. doi: 10.1038/s41467-018-02823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Itakura E., Kishi-Itakura C., Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151(6):1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 241.Matsui T., Jiang P., Nakano S., Sakamaki Y., Yamamoto H., Mizushima N. Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin 17. J. Cell Biol. 2018;217(8):2633–2645. doi: 10.1083/jcb.201712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gao J., Kurre R., Rose J., Walter S., Fröhlich F., Piehler J., Reggiori F., Ungermann C. Function of the SNARE Ykt6 on autophagosomes requires the Dsl1 complex and the Atg1 kinase complex. EMBO Rep. 2020;21(12) doi: 10.15252/embr.202050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Barz S., Kriegenburg F., Henning A., Bhattacharya A., Mancilla H., Sánchez-Martín P., Kraft C. Atg1 kinase regulates autophagosome-vacuole fusion by controlling SNARE bundling. EMBO Rep. 2020;21(12) doi: 10.15252/embr.202051869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liu X., Mao K., Yu A.Y.H., Omairi-Nasser A., Austin J., Glick B.S., Yip C.K., Klionsky D.J. The Atg17-Atg31-Atg29 complex coordinates with Atg11 to recruit the Vam7 SNARE and mediate autophagosome-vacuole fusion. Curr. Biol. 2016;26(2):150–160. doi: 10.1016/j.cub.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang C., Wang H., Zhang D., Luo W., Liu R., Xu D., Diao L., Liao L., Liu Z. Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat. Commun. 2018;9(1):1–15. doi: 10.1038/s41467-018-05449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Stroupe C., Collins K.M., Fratti R.A., Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25(8):1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lürick A., Kuhlee A., Bröcker C., Kümmel D., Raunser S., Ungermann C. The Habc domain of the SNARE Vam3 interacts with the HOPS tethering complex to facilitate vacuole fusion. J. Biol. Chem. 2015;290(9):5405–5413. doi: 10.1074/jbc.M114.631465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marwaha R., Arya S.B., Jagga D., Kaur H., Tuli A., Sharma M. The Rab7 effector PLEKHM1 binds Arl8b to promote cargo traffic to lysosomes. J. Cell Biol. 2017;216(4):1051–1070. doi: 10.1083/jcb.201607085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kumar S., Jain A., Farzam F., Jia J., Gu Y., Choi S.W., Mudd M.H., Claude-Taupin A., Wester M.J., Lidke K.A. Mechanism of Stx17 recruitment to autophagosomes via IRGM and mammalian Atg8 proteins. J. Cell Biol. 2018;217(3):997–1013. doi: 10.1083/jcb.201708039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bigay J., Antonny B. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev. Cell. 2012;23(5):886–895. doi: 10.1016/j.devcel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 251.Lin M.G., Schöneberg J., Davies C.W., Ren X., Hurley J.H. The dynamic Atg13-free conformation of the Atg1 EAT domain is required for phagophore expansion. Mol. Biol. Cell. 2018;29(10):1228–1237. doi: 10.1091/mbc.E17-04-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Rostislavleva K., Soler N., Ohashi Y., Zhang L., Pardon E., Burke J.E., Masson G.R., Johnson C., Steyaert J., Ktistakis N.T. Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350(6257) doi: 10.1126/science.aac7365. (pp. aac7365–aac7365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ohashi Y., Tremel S., Masson G.R., McGinney L., Boulanger J., Rostislavleva K., Johnson C.M., Niewczas I., Clark J., Williams R.L. Membrane characteristics tune activities of endosomal and autophagic human VPS34 complexes. Elife. 2020;9:1–28. doi: 10.7554/eLife.58281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fan W., Nassiri A., Zhong Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L) Proc. Natl. Acad. Sci. U. S. A. 2011;108(19):7769–7774. doi: 10.1073/pnas.1016472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Fogel A.I., Dlouhy B.J., Wang C., Ryu S.-W., Neutzner A., Hasson S.A., Sideris D.P., Abeliovich H., Youle R.J. Role of membrane association and Atg14-dependent phosphorylation in Beclin-1-Mediated autophagy. Mol. Cell. Biol. 2013;33(18):3675–3688. doi: 10.1128/mcb.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huang W., Choi W., Hu W., Mi N., Guo Q., Ma M., Liu M., Tian Y., Lu P., Wang F.L. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012;22(3):473–489. doi: 10.1038/cr.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Noda N.N., Kobayashi T., Adachi W., Fujioka Y., Ohsumi Y., Inagaki F. Structure of the novel C-terminal domain of vacuolar protein sorting 30/autophagy-related protein 6 and its specific role in autophagy. J. Biol. Chem. 2012;287(20):16256–16266. doi: 10.1074/jbc.M112.348250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kulakowski G., Bousquet H., Manneville J.-B., Bassereau P., Goud B., Oesterlin L.K. Lipid packing defects and membrane charge control RAB GTPase recruitment. Traffic. 2018;19(7):536–545. doi: 10.1111/tra.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tamura N., Nishimura T., Sakamaki Y., Koyama-Honda I., Yamamoto H., Mizushima N. Differential requirement for ATG2A domains for localization to autophagic membranes and lipid droplets. FEBS Lett. 2017;591(23):3819–3830. doi: 10.1002/1873-3468.12901. [DOI] [PubMed] [Google Scholar]
- 260.Obara K., Sekito T., Niimi K., Ohsumi Y. The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J. Biol. Chem. 2008;283(35):23972–23980. doi: 10.1074/jbc.M803180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.McMahon H.T., Boucrot E. Membrane curvature at a glance. J. Cell Sci. 2015;128(6):1065–1070. doi: 10.1242/jcs.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Nguyen N., Shteyn V., Melia T.J. Sensing membrane curvature in macroautophagy. Journal of Molecular Biology. 2017;429(4):457–472. doi: 10.1016/j.jmb.2017.01.006. (Academic Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nice D.C., Sato T.K., Stromhaug P.E., Emr S.D., Klionsky D.J. Cooperative binding of the cytoplasm to vacuole targeting pathway proteins, Cvt13 and Cvt20, to phosphatidylinositol 3-phosphate at the pre-autophagosomal structure is required for selective autophagy. J. Biol. Chem. 2002;277(33):30198–30207. doi: 10.1074/jbc.M204736200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Takahashi Y., Meyerkord C.L., Hori T., Runkle K., Fox T.E., Kester M., Loughran T.P., Wang H.G. Bif-1 regulates Atg9 trafficking by mediating the fission of Golgi membranes during autophagy. Autophagy. 2011;7(1):61–73. doi: 10.4161/auto.7.1.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Popelka H., Damasio A., Hinshaw J.E., Klionsky D.J., Ragusa M.J. Structure and function of yeast Atg20, a sorting nexin that facilitates autophagy induction. Proc. Natl. Acad. Sci. U. S. A. 2017;114(47):E10112–E10121. doi: 10.1073/pnas.1708367114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Antón Z., Betin V.M.S., Simonetti B., Traer C.J., Attar N., Cullen P.J., Lane J.D. A heterodimeric SNX4–SNX7 SNX-BAR autophagy complex coordinates ATG9A trafficking for efficient autophagosome assembly. J. Cell Sci. 2020;133(14) doi: 10.1242/jcs.246306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Søreng K., Munson M.J., Lamb C.A., Bjørndal G.T., Pankiv S., Carlsson S.R., Tooze S.A., Simonsen A. SNX 18 regulates ATG 9A trafficking from recycling endosomes by recruiting Dynamin-2. EMBO Rep. 2018;19(4) doi: 10.15252/embr.201744837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhao D., Liu X.M., Yu Z.Q., Sun L.L., Xiong X., Dong M.Q., Du L.L. Atg20- and Atg24-family proteins promote organelle autophagy in fission yeast. J. Cell Sci. 2016;129(22):4289–4304. doi: 10.1242/jcs.194373. [DOI] [PubMed] [Google Scholar]
- 269.Takahashi Y., Meyerkord C.L., Wang H.G. Bif-1/Endophilin B1: a candidate for crescent driving force in autophagy. Cell Death Differ. 2009;16(7):947–955. doi: 10.1038/cdd.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]