Abstract
Purpose/Objective(s)
To evaluate feasibility and safety of prostate stereotactic body radiotherapy (SBRT) neoadjuvant to radical prostatectomy (RP) in a phase I trial. Primary endpoint was treatment completion rate without severe acute surgical complications. Secondary endpoints included patient-reported quality-of-life and physician-reported toxicities.
Materials/Methods
Patients with nonmetastatic high-risk or locally advanced prostate cancer received 24 Gy in 3 fractions to the prostate and seminal vesicles over five days, completed two weeks prior to RP. Patients with pN1 disease were treated following multi-disciplinary discussion and shared decision-making. Patient-reported quality-of-life (I-PSS and EPIC-26 questionnaires) and physician-reported toxicity (CTCAEv4.03) were assessed prior to SBRT, immediately before surgery, and at 3-month intervals for one year.
Results
12 patients enrolled, 11 completed treatment (one had advanced disease on PSMA PET after enrollment, before treatment). There were no significant surgical complications. After RP, two patients underwent additional RT to nodes with androgen suppression for pN1 disease. Median follow-up after completion of treatment was 20.1 months, with 9/11 patients having follow-up greater than 12 months. Two patients had biochemical recurrence (PSA >= 0.05) within the first 12 months, with an additional two patients found to having biochemical recurrence after the 12-month period. Highest CTCAEv4 genitourinary grade was 0/1/2/3 (n=1/4/4/2) and highest gastrointestinal grade was 0/1/2 (n=9/1/1). At 12 months, incontinence was the only grade ≥2 toxicity. One and two of nine patients had grade 2 or 3 incontinence, respectively. On EPIC-26, mean/median changes in scores from baseline to 12 months were −32.8/−31.1 for urinary incontinence, −1.6/−6.2 for urinary irritative/obstructive, −2.1/0 for bowel, −34.4/−37.5 for sexual function, and −10.6/−2.5 for hormonal. Mean/median change in I-PSS score from baseline to 12 months was 0.5/0.5.
Conclusions
RP following neoadjuvant SBRT appears to be feasible and safe at the dose tested. Severity of urinary incontinence may be higher than RP alone.
Introduction
Radiotherapy (RT) neoadjuvant to surgery is a standard-of-care for multiple cancers. Salvage radiotherapy is standard-of-care for biochemically recurrent prostate cancer after radical prostatectomy (RP). 1–3 RT neoadjuvant to RP has been evaluated in two prior trials. A Duke University Phase I dose escalation trial studied 12 men receiving intensity-modulated radiotherapy (39.6–54 Gy to prostate/SVs in 22–30 fractions with pelvic nodes treated up to a maximal dose of 45 Gy) followed by prostatectomy.4 No intraoperative morbidity was observed. Acute toxicity was limited to Grade 1 (GU and GI). Late toxicity, primarily GU, was within the range expected for adjuvant post-operative RT. Another phase I trial from University of Toronto studied 15 patients receiving preoperative RT to prostate (25 Gy in 5 consecutive daily fractions), utilizing conformal technique without image guidance.5 Patients in this study were found to have higher than expected urinary toxicity – including 2 patients (13.3%) with G2 toxicity and 6 patients (40%) with G3 toxicity.
Now a standard treatment option in NCCN guidelines for localized prostate cancer,6 ultrahypofractionation or stereotactic body radiotherapy (SBRT) utilizes image-guidance to deliver a higher dose per fraction than possible by conventional techniques. SBRT offers advantages including reduction in treatment duration, smaller irradiated volumes of normal tissues, and possibly a biological advantage given the low alpha/beta ratio for prostate cancer. Two recent trials of post-operative SBRT for prostate cancer have reported early toxicity results.7,8
Currently, three other registered trials are evaluating SBRT neoadjuvant to RP.9–11 The primary endpoint of this Phase I study was treatment completion rate without severe acute surgical complications, measured at 4 weeks post-surgery. Secondary endpoints were patient-reported quality-of-life based on International Prostate Symptom Score (I-PSS) and Expanded Prostate Cancer Index Composite-26 (EPIC-26) questionnaires, and physician-reported toxicity based on Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Materials and Methods
Men with biopsy-confirmed nonmetastatic unfavorable intermediate or higher prostate adenocarcinoma were eligible for enrollment in our study, although all patients that enrolled were found to be high-risk or locally advanced. Patients must have been medically fit, with Karnofsky performance status >= 70, and willing and able to undergo prostatectomy. To confirm absence of distant metastases, patients must have undergone CT scan or MRI of the abdomen/pelvis within 120 days prior to registration, and bone scan within 120 days prior to registration (if the bone scan was equivocal, a plain x-ray and/or MRI was obtained to rule out metastasis prior to registration). Androgen deprivation therapy (ADT) was allowed but only one patient was on ADT at time of RT and surgery.
Regarding radiation fractionation, 24 Gy in 3 fractions was delivered over five days, completed two weeks prior to robot-assisted laparoscopic radical prostatectomy (RALRP). This fractionation scheme was selected given its similar biologically effective dose (BED) to bladder and rectum (α/β=3–4) when compared with 54 Gy in 30 fractions that was safely delivered in the Duke University study,4 while also delivering a similar BED as conventionally fractionated post-operative radiotherapy α/β=1.5–2) to prostate tumor tissue. Clinical target volume (CTV) was prostate, SVs, and any visible/suspected extraprostatic extension. Planning treatment volume was CTV plus 5 mm. SBRT was delivered with volumetric-modulated arc therapy (VMAT) involving four half-arcs; kV imaging was performed prior to each half-arc with alignment to fiducials; cone-beam CT was performed to verify bladder and rectal filling prior to treatment initiation. Organ at risk (OAR) constraints were as previously described,12 scaled down to a prescription dose of 24 Gy.
Upon completion of surgery, patients with pN1 disease were treated following multi-disciplinary discussion and shared decision-making with the patient. Patients were evaluated pre-SBRT, immediately before surgery, and at 3-month intervals for one year. At each evaluation, patients were evaluated by physician for toxicity and asked to complete I-PSS and EPIC-26 questionnaires.
Results
Of 12 patients enrolled, one dropped out after PSMA PET/CT showed advanced disease. The remaining 11 patients completed treatment with preoperative SBRT, followed by RALRP (1/8/2 with no/unilateral/bilateral nerve-sparing, respectively) 14+/−3 days later. Although MRI of the prostate was not required per protocol, all had pre-treatment prostate MRI that was fused to CT simulation for planning.
Average estimated blood loss was 218 cc (range 100–400 cc). Average operative time was 3.5 hours (range 3.0–4.5 hours). Patients were discharged on average 1.2 days after their operative date (range 1.0–2.0 days). These parameters are similar to those reported for RALRP alone.13–15 No significant acute surgical complications occurred. Notably, there were no urethral anastomotic leaks or bowel injuries.
An overview of all treated patients – including preoperative stage, operative findings, and post-operative management – is shown in Table 1. One patient was on ADT during SBRT and surgery (1 month of leuprolide). After RALRP, two of the four patients with pN1 disease underwent RT+ADT to pelvic nodes; further treatment was deferred in one patient because PSA remained undetectable and in another because of patient preference.
Table 1.
Preoperative stage, operative findings, and post-operative management shown for each individual patient treated.
| Preoperative staging | Pathologic staging and adjuvant treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| ID | cStage | Gleason Grade | Cores positive | iPSA | pStage | Nerve-sparing | Margins | Adjuvant treatment (if applicable) |
| 1 | cT2bNoMo | 4+4=8 | 11/13 | 7.9 | pT3aNoMo | Right | Negative | |
| 2 | cT3aNoMo | 4+3=7 | 10/12 | 11 | pT3bNoMo | None Bilateral | Negative | |
| 3 | cTlcNoMo | 4+5=9 | 11/12 | 11.1 | pT3aNoMo | (partial) | Positive | |
| 4 | cT2bNoMo | 4+4=8 | 6/12 | 10.9 | pT3aNoMo | Right | Negative | |
| 5 | cT2aNoMo | 4+5=9 | 8/12 | 5.9 | pT3aNoMo | Right | Negative | |
| 6 | cTIcNIMo | 4+3=7 | 3/12 | 24.1 | pT3bNlMo | Left | Negative | RT+ADT to nodes |
| 7 | cT3aNlMo | 4+4=8 | 7/14 | 4.5 | pT3aNlMo | Right | Negative | RT+ADT to nodes |
| 8 | cT2aNoMo | 4+4=8 | 5/12 | 18.6 | pT2NlMo | Bilateral | Negative | Deferred (patient preference) |
| Deferred (concern re: urinary incontinence) | ||||||||
| 9 | cT2cN1Mo | 4+3=7 | 12/14 | 18.3 | pT3bNlMo | Right | Negative | |
| 10 | cTlcNoMo | 4+5=9 | 8/14 | 13.5 | pT2NoMo* | Right | Negative | |
| 11 | cT3aNoMo | 4+4=8 | 9/12 | 13.5 | pT2NoMo | Left | Negative | |
On ADT(1-month leuprolide) at time of RT and RP.
Median follow-up after completion of treatment is 20.1 months, with nine patients having follow-up greater than 12 months. An overview of PSA follow-up and physician-reported toxicity is shown in Table 2. Two patients (#3 and #9) were found to have biochemical recurrence within the first 12 month of completing treatment. Patient #3 (pT3aN0, Gleason 4+5, positive margins) was found to have a progressively rising PSA (max 1.8 ng/ml upon completing treatment 22 months previously), with PSMA PET eventually revealing internal iliac nodal involvement, but no local tumor. The patient subsequently underwent salvage IMRT to pelvic LNs (plus boost to PSMA-avid disease) with ADT. Patient #9 (pT3bN1 whose adjuvant pelvic RT was deferred given concern about urinary incontinence) had a gradually rising PSA to max of 0.27 ng/ml; PSMA PET done at that time, however, was unrevealing. Two additional patients (both with pT3aN0, Gleason 4+4, negative margins) were found to have minor elevations in PSA after the 12-month follow-up period: patient #1 with PSA of 0.05 ng/ml 36 months post-treatment and patient #4 with PSA 0.17 as of 22 months post-treatment.
Table 2.
Long-term PSA and physician-graded toxicity follow-up for each individual patient treated.
| PSA trends and subsequent treatment | GU toxicities | Gl toxicities | |||||
|---|---|---|---|---|---|---|---|
| ID | PSA @ 12 months | Latest PSA | Salvage treatment | Anytime | 12 months | Anytime | 12 months |
| 1 | <0.05 | 0.05 | G3 (incontinence) | G3 (incontinence) | GO | GO | |
| 2 | <0.05 | <0.05 | G1 (frequency) | G1 (frequency) | GO | GO | |
| 3 | 0.47 | 1.8 | PSMA PET revealed internal iliac nodal involvement; s/p salvage IMRT to pelvic LNs (plus boost to PSMA-avid disease) and ADT | G1 (urgency/frequency) | GO | GO | GO |
| 4 | <0.05 | 0.17 | GO | GO | GO | GO | |
| 5 | <0.05 | <0.05 | G2 (incontinence) | GO | GO | GO | |
| 6 | <0.05 | <0.05 | G1 (incontinence) | G1 (incontinence) | Gl (diarrhea) | GO | |
| 7 | <0.05 | <0.05 | G3 (incontinence) | G3 (incontinence) | G2 (rectal bleeding) | GO | |
| 8 | <0.05 | <0.05 | G2 (incontinence) | G2 (incontinence) | GO | GO | |
| 9 | N/A | 0.27 | G2 (incontinence) | N/A | GO | N/A | |
| 10 | <0.05 | <0.05 | G1 (incontinence) | G1 (incontinence) | GO | GO | |
| 11 | N/A | <0.05 | G2 (incontinence) | N/A | GO | N/A | |
Highest CTCAEv4 genitourinary toxicity at any time was grade 0 (n=1), grade 1 (n=4), grade 2 (n=4), and grade 3 (n=2); highest gastrointestinal toxicity was grade 0 (n=9), grade 1 (n=1), and grade 2 (n=1). The patient with grade 2 gastrointestinal toxicity had rectal bleeding after pelvic radiation for pN1, which resolved with argon plasma coagulation. Of the nine patients evaluated at 12 months, one patient had Grade 2 incontinence and two patients had Grade 3 incontinence. In both Grade 3 patients, placement of artificial urinary sphincter [AUS] resolved incontinence. There were no other GU or GI Grade ≥2 toxicities at 12 months or later.
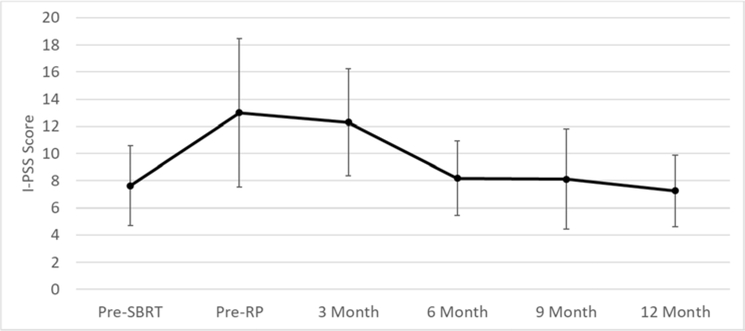
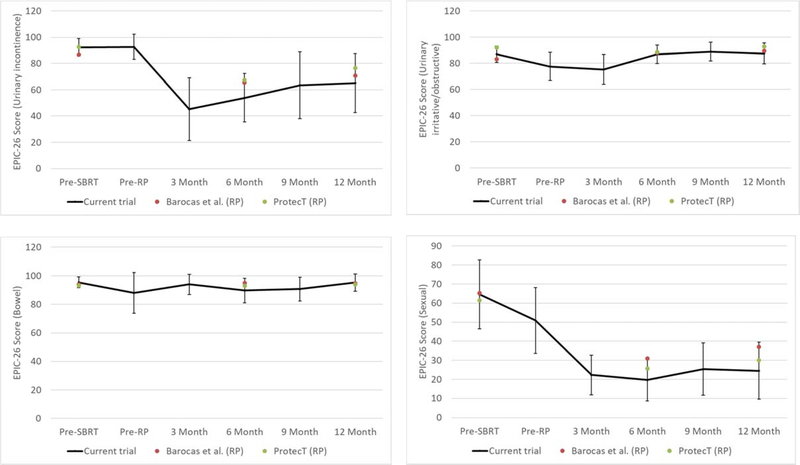
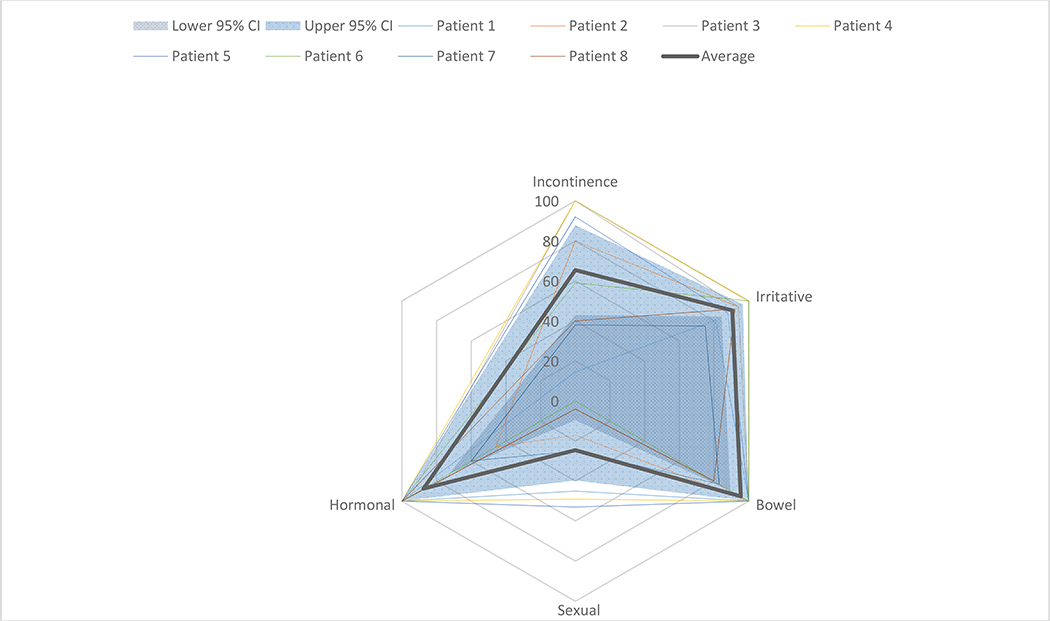
As shown in Figure 1, mean/median change in I-PSS score from baseline to 12 months was 0.5/0.5. Mean/median changes in EPIC-26 scores from baseline to 12 months were −32.8/− 31.1 for urinary incontinence, −1.6/−6.2 for urinary irritative/obstructive (UIO), −2.1/0 for bowel, −34.4/−37.5 for sexual function, and −10.6/−2.5 for hormonal (Figure 2A). EPIC scores of individual patients at 12 months (n=8) are shown in Figure 2B. Based on the midpoint of previously-defined minimally important difference (MID) ranges,16 at 12 months, clinically-relevant changes were found in 6/5/2/6/4 of 8 patients for incontinence, UIO, bowel, sexual, and hormonal toxicity, respectively.
Figure 1.
Patient-reported quality-of-life (I-PSS) before/after treatment. Error bars are 95% confidence intervals.
Figure 2A.
Patient-reported quality-of-life (EPIC-26) before/after treatment: urinary incontinence (upper-left), urinary irritative/obstructive (upper-right), bowel (bottom-left), and sexual (bottom-right). Error bars are 95% confidence intervals. Also plotted are EPIC-26 scores from patients undergoing radical prostatectomy (RP) in Barocas et al.20 (red) and EPIC scores from patients undergoing RP in ProtecT17 (green).
Figure 2B.
Radar chart plotting individual patient-reported quality-of-life (EPIC-26) scores (n=8) at 12 months after completion of treatment. Also shown is the average score (thick green line) and 95% confidence interval (area between dark blue and light blue shaded areas).
Discussion
Long-term efficacy and safety profile of neoadjuvant SBRT prior to RP requires longer follow-up and a larger sample size. Short-term toxicity included expected reduced sexual function and incontinence that may be more severe than RP alone given that two patients underwent placement of AUS (one of whom, however, was morbidly obese - a known risk factor for incontinence). Notably, 3 of 9 patients reported Grade 2 or higher incontinence at 12 months, similar to that found in the large prospective ProtecT trial where 26% of patients who underwent RP had Grade 2 or higher incontinence at 12 months, although numbers in the present trial are too low to draw definitive conclusions.17 While the rate of incontinence may be higher than expected for surgery alone,18 the very high-risk population studied in this trial is also more likely to require aggressive resection (only 2/11 patients receiving bilateral nerve sparing surgery which has been shown to improve incontinence rates).19
When reported, results from the other ongoing neoadjuvant SBRT trials will enable further assessments of this approach, although differences in dose may account for different outcomes. Pending these results, future studies may explore combination neoadjuvant ADT/SBRT strategies, or incorporation of PSMA PET imaging for treatment planning. In this realm, postoperative SBRT to the prostate fossa is another area of active exploration, with two Phase I dose-escalation trials recently published. A dose-escalation trial from USC studied 24 patients (12 of whom received the highest dose, 7.1 Gy x 5 fractions), and with at least 6 months of follow-up (median 14.1 months), 7/12 patients receiving the highest dose were found to have Grade 2 GI toxicity, although no patient was found to have grade >=3 GI/GU toxicity.7 A dose-escalation trial from City of Hope studied escalating doses of five-fraction SBRT to the prostate bed: 35 Gy (n=3), 40 Gy (n=8), and 45 Gy (n=15). In the group receiving 45 Gy, grade 3 GU toxicity was found in 2/15 patients, grade 2 GU toxicity was found in 4/15 patients, and grade 2 GI toxicity was found in 1/15 patient.8
Patients in the present neoadjuvant trial appeared to tolerate treatment more favorably compared to patients from the University of Toronto Phase I study where 6/15 patients had grade 3 toxicity and 2/14 patients had grade 2 GU toxicity.5 Although follow-up was considerably longer in this prior study (median follow-up 12.2 years), peak incidence of GU toxicity was seen within 18 months. Other possible explanations for the difference in toxicity between our study and the aforementioned trial include: RT technique (VMAT vs. 6-field conformal technique), every other day fractionation instead of daily fractionation, and surgical approach (RALRP vs. open retropubic RP).5 Notably, both the prior Toronto trial and this present trial are small.
Changes in EPIC-26 scores in this study were similar to those found in the EPIC scores in RP patients from ProtecT17 and EPIC-26 scores from Barocas et al.20 All domains (incontinence/irritative/bowel/sexual) reported in those studies were within the 95% confidence intervals of the present study (Figure 2A).
Conclusion
SBRT two weeks neoadjuvant to RP appears to be feasible and safe at the dose tested. Acute surgical complications were not observed but the severity of urinary incontinence may be higher than that for RP alone based on rates of AUS placement. We look forward to comparisons with the results of the other ongoing neoadjuvant trials that use different dose fractionations.
Acknowledgments
Funding: UCLA Prostate Cancer SPORE, Prostate Cancer Foundation, UCLA JCCC
Footnotes
Conflict of interest:
- Dr. Parikh reports prior employment with McKinsey & Company.
- Dr. Kishan reports honoraria and consulting fees from Varian, and honoraria from Viewray, outside the submitted work.
- Dr. Kupelian reports employment with Varian.
- Dr. Rettig reports grants from Novartis, grants and personal fees from Johnson & Johnson, grants from Merck, grants from Astellas, grants from Medivation, grants from Progenics, personal fees from Bayer, other from Constellation Pharmaceuticals, outside the submitted work.
- Dr. Steinberg reports personal fees from ViewRay, personal fees from VisionRT, outside the submitted work.
- Dr. Nickols reports grants from Varian, grants from Janssen, grants from Progenics, grants from Bayer, outside the submitted work.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological t3n0m0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol 2009;181:956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalbasi A, Swisher-McClure S, Mitra N, et al. Low rates of adjuvant radiation in patients with nonmetastatic prostate cancer with high-risk pathologic features. Cancer 2014;120:3089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson ES, Margolis DJ, Mesko S, et al. Multiparametric mri identifies and stratifies prostate cancer lesions: Implications for targeting intraprostatic targets. Brachytherapy 2014;13:292–8. [DOI] [PubMed] [Google Scholar]
- 4.Koontz BF, Quaranta BP, Pura JA, et al. Phase 1 trial of neoadjuvant radiation therapy before prostatectomy for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2013;87:88–93. [DOI] [PubMed] [Google Scholar]
- 5.Glicksman R, Sanmamed N, Thoms J, et al. A phase 1 pilot study of preoperative radiation therapy for prostate cancer: Long-term toxicity and oncologic outcomes. Int J Radiat Oncol Biol Phys 2019;104:61–66. [DOI] [PubMed] [Google Scholar]
- 6.Network NCC. Prostate cancer (version 1.2020). In: Editor, editorêditors. Book Prostate cancer (version 1.2020).
- 7.Ballas LK, Luo C, Chung E, et al. Phase 1 trial of sbrt to the prostate fossa after prostatectomy. Int J Radiat Oncol Biol Phys 2019;104:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampath S, Frankel P, Vecchio BD, et al. Stereotactic body radiation therapy to the prostate bed: Results of a phase 1 dose-escalation trial. Int J Radiat Oncol Biol Phys 2020;106:537–545. [DOI] [PubMed] [Google Scholar]
- 9.Center HLMC, Research I. Dose escalation study of preoperative sbrt for high risk prostate cancer. https://ClinicalTrialsgov/show/NCT02572284.
- 10.University of Michigan Rogel Cancer C. Neoadjuvant stereotactic body radiotherapy prior to radical prostatectomy for high risk prostate cancer. https://ClinicalTrialsgov/show/NCT02946008.
- 11.Weill Medical College of Cornell U. Pre-prostatectomy mri-guided stereotactic body radiotherapy for high-risk prostate cancer trial (prepare sbrt). https://ClinicalTrialsgov/show/NCT03663218.
- 12.King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877–82. [DOI] [PubMed] [Google Scholar]
- 13.Farnham SB, Webster TM, Herrell SD, et al. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology 2006;67:360–3. [DOI] [PubMed] [Google Scholar]
- 14.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA 2009;302:1557–64. [DOI] [PubMed] [Google Scholar]
- 15.Simon RM, Howard LE, Moreira DM, et al. Predictors of operative time during radical retropubic prostatectomy and robot-assisted laparoscopic prostatectomy. Int J Urol 2017;24:618–623. [DOI] [PubMed] [Google Scholar]
- 16.Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology 2015;85:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhu V, Sivarajan G, Taksler GB, et al. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. Eur Urol 2014;65:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michl U, Tennstedt P, Feldmeier L, et al. Nerve-sparing surgery technique, not the preservation of the neurovascular bundles, leads to improved long-term continence rates after radical prostatectomy. Eur Urol 2016;69:584–589. [DOI] [PubMed] [Google Scholar]
- 20.Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA 2017;317:1126–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]