Abstract
Aim:
This study examined the real-world effectiveness and safety outcomes of vedolizumab in ulcerative colitis (UC) patients who had failed anti-tumor necrosis factor (anti-TNF) therapy in Korea.
Methods:
A retrospective chart review study was conducted in adults with moderate to severely active UC who had failed anti-TNF agents and subsequently received vedolizumab. Clinical response and clinical remission at week 6 and 14 after vedolizumab initiation was evaluated. Safety outcomes were also reported. Outcome rates were compared with a matched sub-cohort derived from the open-label sub-cohort of the GEMINI 1 trial using the optimal matching method.
Results:
A total of 105 patients (mean age, 45.3 years; 63.8% male) were included. At week 6, 55.8% (n = 43/77) achieved a clinical response and 18.2% (n = 14/77) achieved clinical remission. At week 14, 73.2% (n = 52/71) achieved a clinical response and 39.4% (n = 28/71) achieved clinical remission. When non-response imputation was used, the clinical response rate at week 6 and week 14 were 40.1% (n = 43/105) and 49.5% (n = 52/105) respectively. Of the 105 patients, 16 (15.2%) experienced at least one adverse event. The matched analysis showed that the clinical response rate at week 6 was higher in the matched sub-cohort of this study (24/47, 51.1%) versus the matched sub-cohort from the GEMINI 1 open-label cohort (12/47, 34.3%, p = 0.019). The clinical remission rates at week 6 were similar (7/47, 14.9% versus 9/47, 19.1%, p = 0.785).
Conclusions:
In the real-world setting, vedolizumab is effective and well tolerated within the first 14 weeks of use in Korea. The proportion of patients experiencing clinical response and clinical remission at 6 and 14 weeks appeared to be largely consistent with that observed in real-world studies from other regions and populations.
Keywords: Korea, remission, response, ulcerative colitis, vedolizumab
Introduction
Anti-tumor necrosis factor (anti-TNF) agents, including infliximab, adalimumab, and golimumab, have transformed the therapeutic landscape of ulcerative colitis (UC), after their favorable efficacy data have been demonstrated in randomized controlled trials.1–3 However, up to one-third of the patients with UC have failed to respond to anti-TNF therapy.1,4,5 Following the failure to anti-TNF therapy, there is currently no clear guidelines on whether the next step should be to try other anti-TNF agents or to switch to drugs with different mechanism of action (e.g. vedolizumab). 6
Vedolizumab was recently made available for the treatment of patients with UC in Korea, and, since January 2020, is indicated for use in biologically naïve patients. Vedolizumab is a humanized monoclonal antibody that selectively antagonizes α4β7 integrin receptors, thereby inhibiting the migration of lymphocytes into the gastrointestinal tract. 7 Such migration is impaired without causing systemic immunosuppression, as opposed to other treatment options for UC such as anti-TNF agents and janus kinase (JAK) inhibitors. 8 As the mode of action of vedolizumab is different from that of anti-TNF, vedolizumab may represent an effective treatment option for patients with (or at higher risk of) either primary or secondary anti-TNF failure.
The landmark GEMINI 1 trial, which included 895 patients with UC across 34 countries, reported favorable results of vedolizumab induction and maintenance therapy. 9 The clinical trial found that clinical response rate at week 6 was almost double the placebo arm. At week 52, the proportion of patients who had experienced clinical remission was twice as high in the vedolizumab group (Q8W) versus the placebo group. Vedolizumab was also shown to be well tolerated with a favorable safety profile. 9
Following the GEMINI 1 trial, multiple real-world studies have since been conducted, albeit largely focused on Western populations.10–12 A recent systematic review and meta-analysis of these available real-world evidences suggested that the effectiveness and safety of vedolizumab were highly consistent with that reported by the pivotal GEMINI 1 study. 13 A recent controlled trial in Japan 14 has provided some evidence on a similar and favorable efficacy of vedolizumab for Japanese UC patients. Unfortunately, observation data on how much vedolizumab is effective and safe under the real-world clinical setting in Asia remain scarce. As a result, we conducted this study to evaluate the real-world effectiveness and safety of vedolizumab in patients with UC who failed anti-TNF therapy in Korea. We also sought to compare our data against a matched subset of the GEMINI 1 cohort.
Methods
A multicenter retrospective chart review of the electronic medical records was conducted across 13 centers in Korea, where data were collected using web-based case report forms. Adult patients with UC (⩾19 years of age) and the following characteristics were included: (i) diagnosed with definite UC, (ii) had failed anti-TNF therapy (primary or secondary) or discontinued due to other reasons such as patient preference and adverse event, and (iii) had received at least one dose of intravenous (IV) vedolizumab between August 2017 and December 2018. Vedolizumab were administered to the eligible patients via IV infusion at weeks 0, 2, and 6, then every 8 weeks thereafter.
The primary outcome was clinical response at week 6 after two infusions of vedolizumab (weeks 0 and 2). Clinical response was defined as a reduction in the partial Mayo score of at least 3 points and a decrease of at least 30% from the baseline score, with a decrease of at least 1 point on the rectal bleeding subscore, or an absolute rectal bleeding subscore of 0 or 1. 15 The secondary outcomes were (1) clinical responses at week 14, (2) clinical remission at week 6, (3) clinical remission at week 14, (4) endoscopic response at week 6, (5) endoscopic response at week 14, (6) endoscopic remission at week 6, (7) endoscopic remission at week 14, and (8) safety outcomes. Clinical remission was defined as a partial Mayo score of at most 2, without any subscore being greater than 1. 16 Endoscopic response was defined as a decrease of at least 50% from a baseline Mayo endoscopic subscore. Endoscopic remission was defined as a Mayo endoscopic subscore of 0–1; complete endoscopic remission was defined as a Mayo endoscopic subscore of 0.
The safety outcomes included adverse events of special interest (AESIs), serious adverse event (SAEs), and pregnancy-related outcomes documented during the treatment period. AEs that had occurred on or after the start of IV vedolizumab therapy were abstracted from the medical charts and coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 21.0. AESIs included serious infections, opportunistic infections, hepatitis viral infection, gastrointestinal infections, respiratory infections, other clinically significant infections, malignancies, infusion-related reactions, and hepatic injury. SAEs included UC exacerbation (e.g., new signs and symptoms of UC), cytomegalovirus infection, adenoiditis, mouth ulceration, and skin ulcers). SAE is referred to as any untoward medical occurrence that, at any dose, has resulted in death (or an immediate risk of death), persistent or significant disability or incapacity, and hospitalization (or prolongation of existing hospitalization). Safety analyses were performed for those who have received at least one dose of vedolizumab (i.e., full eligible study cohort).
Other covariates extracted included age, sex, body mass index (BMI), smoking history, family history of inflammatory bowel diseases, comorbidities, duration of UC, extent of UC, extraintestinal manifestations, and medication history (within 5 years prior to vedolizumab initiation) and concomitant medications use.
Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR) and categorical variables as number and percentage (%). Differences in continuous variables between subgroups were compared using the Mann–Whitney U test. Comparisons between categorical variables were analyzed using the Fisher’s exact test or Chi–squared test. Analysis of all study outcomes were performed on non-missing cases only (complete case analysis) as the primary analysis. Given the risk for attrition bias with real-world observational data and potential effect of right censoring, we also performed a non-response imputation (NRI) analysis for the clinical response at week 6 (the primary outcome) and at week 14. In the NRI, all cases with missing data at weeks 6 and 14 were imputed as non-responders, irrespective of their response status prior to these time points. Subgroup analyses were performed for the following factors: (1) duration of UC (<1 year, 1–<3 years, 3–<7 years, and ⩾7 years), disease extent (proctosigmoiditis, left-sided colitis, extensive colitis, pancolitis, and unknown), number of prior anti-TNF agents (one, two, and three), the type of failure (inadequate response, loss of response, intolerance, or others), and the type of concomitant medication exposure (corticosteroids, immunosuppressants, corticosteroids and immunosuppressants, and no corticosteroids or immunosuppressants).
In addition, outcome data were compared with a matched cohort (of vedolizumab-treated patients) derived from the open-label cohort of the GEMINI 1 pivotal trial. Only anti-TNF experienced subjects were included for matching. The two cohorts were matched by the following covariates: age, sex, BMI, smoking status, duration of UC, extent of UC, baseline Mayo score, number of prior hospitalization (12 months), and concomitant steroid use (yes/no). Matching was performed using the optimal matching method via the MatchIt package of the R statistical environment. 17 Optimal matching allows for the efficient finding of a matched comparator group that has the smallest average absolute distance by Mahalanobis measure across all the potential matched pairs within the two comparing cohorts. 18 Covariate balance was assessed using the t-test (for numerical variables) and the Chi–squared or the Fisher’s exact test (for categorical variables) after matching. All analyses were performed using the R version 3.3.1 and evaluated at a two-sided significance level of p < 0.05.
Ethical considerations
This study was approved by all the Independent Ethics Committees (IECs)/Institutional Review Boards (IRBs) of each participating center according to the local requirements (including Asan Medical Center IRB approval # 2018-0869). Reporting of this study is in accordance with the Strengthening the Reporting of Observational studies in Epidemiology guideline. 19
Results
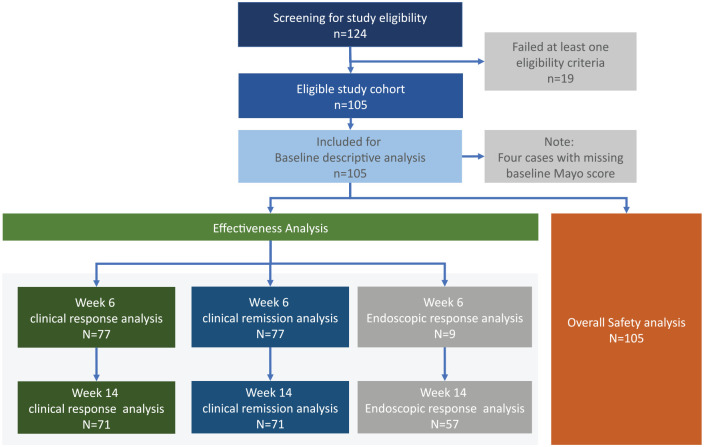
A total of 124 patients were identified and screened from the medical records, where 105 patients met the inclusion criteria. All 105 patients had adequate data for safety outcomes analysis (adverse events and pregnancy-related outcomes). Varying degree of patient attrition from baseline to weeks 6 and 14 were seen, resulting in the differing availability of outcome data for the various observation points. A flow chart is shown in Figure 1, which demonstrate the derivation and availability of data on the various outcome analyses (i.e., clinical and endoscopic response/remission at week 6 and 14).
Figure 1.
Flow diagram demonstrating the derivation of the analysis cohorts.
Of the 105 patients, the median age was 47.0 (IQR = 23.0) years and 63.8% were males. The median full Mayo score at baseline was 9.0 (IQR = 2.0) where 46.7% had pancolitis. The majority of the patients had been previously treated with one or more aminosalicylates (93/105, 88.6%), corticosteroids (71/105, 67.6%), and immunosuppressants (56/105, 53.3%) prior to vedolizumab initiation. About 31.4% (33/105) of the study cohort had more than one prior anti-TNF therapy. The median duration of these prior anti-TNF therapy was 8.4 months (IQR = 16.7). The full demographic and clinical characteristics of the study cohort is shown in Table 1.
Table 1.
Demographic and clinical characteristics of the UC cohort at baseline.
| Demographics and clinical characteristics | Overall cohort |
|---|---|
| Age (year) | |
| Median (IQR) | 47.0 (23.0) |
| Sex, n (%) | |
| Male | 67 (63.8) |
| Female | 38 (36.2) |
| BMI (kg/m2) | |
| Median (IQR) | 21.4 (4.3) |
| Smoking status – n (%) | |
| Current smoker | 4 (3.8) |
| Former smoker | 31 (29.5) |
| Never smoked | 62 (59.0) |
| Unknown | 8 (7.6) |
| Family history, n (%) | |
| Ulcerative colitis | 3 (2.9) |
| Sibling(s) | 2 (1.9) |
| Parent(s) | 1 (1.0) |
| Comorbidities, n (%) | |
| Hypertension | 5 (4.8) |
| Diabetes mellitus | 2 (1.9) |
| Autoimmune hepatitis | 1 (1.0) |
| Rheumatoid arthritis | 1 (1.0) |
| Categorical duration of UC, n (%) | |
| <1 year | 3 (2.9) |
| ⩾1–<3 years | 22 (21.0) |
| ⩾3–<7 years | 30 (28.6) |
| ⩾7 years | 13 (12.4) |
| Unknown | 37 (35.2) |
| Disease extent, n (%) (up to a week before or at baseline) | |
| Proctosigmoiditis | 24 (22.9) |
| Left-sided colitis | 22 (21.0) |
| Extensive colitis | 2 (1.9) |
| Pancolitis | 49 (46.7) |
| Unknown | 8 (7.62) |
| Partial Mayo score at baseline | |
| Median (IQR) | 6.0 (2.0) |
| Unknown (%) | 4 (3.8) |
| Full Mayo score at baseline | |
| Median (IQR) | 9.0 (2.0) |
| Unknown (%) | 10 (9.5) |
| Extraintestinal manifestations, n (%) (up to 12 months before or at baseline) | |
| Yes | 6 (5.7) |
| No | 99 (94.3) |
| Prior medications n (%) | |
| Anti-TNFs | 105 (100.0) |
| Infliximab | 71 (67.6) ¥ |
| Adalimumab | 55 (52.4) ¥ |
| Golimumab | 20 (19.0) ¥ |
| Aminosalicylates | 93 (88.6) |
| Corticosteroids | 71 (67.6) |
| Immunosuppressants * | 56 (53.3) |
| Antibiotics | 21 (20.0) |
| Probiotics | 3 (2.9) |
| Opioids | 1 (1.0) |
| Duration of prior anti-TNF therapy (months) | |
| Median (IQR) | 8.4 (16.7) |
| Number of prior anti-TNF therapy, n (%) | |
| One (1) prior anti-TNF | 72 (68.6) |
| Two (2) prior anti-TNFs | 25 (23.8) |
| Three (3) prior anti-TNFs | 8 (7.6) |
| Type of prior anti-TNF failure + , n (%) | |
| Inadequate response | 53 (50.5) |
| Loss of response | 32 (30.5) |
| Intolerance | 10 (9.5) |
| Unknown | 10 (9.5) |
| Concomitant medications for UC, n (%) | |
| Corticosteroids only | 14 (13.3) |
| Immunosuppressants only | 6 (5.7) |
| Corticosteroids and immunosuppressants | 5 (4.8) |
| No corticosteroids or immunosuppressants | 80 (76.2) |
Sum to more than 100% due to more than one use.
Immunosuppressants considered are azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, mycophenolate mofetil, tacrolimus, and thalidomide.
Data captured as free-text initially and subsequently coded manually into the four categories. Entries such as ‘dampened responsiveness’, ‘primary non-response’, ‘no response’, and ‘no effect’ were coded as inadequate responses. Entries such as ‘secondary loss of response’, ‘recurrence of symptoms’, and ‘loss of response’ were coded as a loss of response. Entries such as ‘cytomegalovirus infection’, ‘hypotension’, and ‘intolerance’ were coded as intolerance. Other free-text entries such as ‘principal investigator’s judgement’ and ‘drug change’ etc. were considered as unknowns.
BMI, body mass index; IQR, interquartile range; SD, standard deviation; TNF, tumor necrosis factor; UC, ulcerative colitis.
A total of seventy-seven subjects provided sufficient data for the assessment of clinical response and clinical remission at week 6; 71 subjects did so for the same assessment at week 14 (Figure 1). Based on these cases, more than half of the available cases (43/77, 55.8%) achieved clinical responses at week 6, while 18.2% (14/77) achieved clinical remission. At week 14, almost three quarters of the cases with sufficient data (52/71, 73.2%) achieved clinical response and 39.4% (28/71) achieved clinical remission (Table 2). Changes of the partial Mayo score and subscores at week 6 and week 14 from baseline are presented in Table 3. Using NRI for the analysis of the primary outcome, the proportion achieving a clinical response at week 6 was 40.1% (n = 43/105). At week 14, NRI analysis indicated a clinical response rate of 49.5% (n = 52/105).
Table 2.
Clinical response and remission rate at week 6 and week 14 post vedolizumab administration (complete case analysis and analysis with non-response imputation).
| Outcomes at week 6 | Complete case analysis (n = 77) (%) | Analysis with NRI * (n = 105) (%) |
|---|---|---|
| Clinical response rate, n (%) | 43 (55.8) | 43 (40.1) |
| Clinical remission rate, n (%) | 14 (18.2) | – |
| Outcomes at week 14 | Complete case analysis (n = 71) (%) | Analysis with NRI * (n = 105) (%) |
| Clinical response rate, n (%) | 52 (73.2) | 52 (49.5) |
| Clinical remission rate, n (%) | 28 (39.4) | – |
Clinical response rate at week 6 and week 14 where patients with missing partial Mayo score were assumed to be a non-responder to vedolizumab. “–” means analysis not performed.
NRI, non-response imputation.
Table 3.
Changes of the partial Mayo score and subscore at week 6 and week 14 post vedolizumab administration from baseline.
| Patients with valid partial Mayo score at | ||
|---|---|---|
| Week 6 (n = 77) | Week 14 (n = 71) | |
| Reduction in partial Mayo score | ||
| Median (IQR) | 3.0 (3.0) | 3.0 (3.0) |
| Reduction in stool frequency subscore | ||
| Median (IQR) | 0.0 (1.0) | 1.0 (2.0) |
| Reduction in rectal bleeding subscore | ||
| Median (IQR) | 1.0 (1.0) | 1.0 (2.0) |
| Reduction in physician’s global assessment subscore | ||
| Median (IQR) | 1.0 (1.0) | 1.0 (1.0) |
IQR, interquartile range.
Only nine patients had a valid Mayo endoscopic subscore assessment done at week 6. Of those, two patients (2/9, 22.2%) achieved an endoscopic response at week 6. At week 14, 57 patients had a valid Mayo endoscopic subscore, where close to half (28/57, 49.1%) achieved both an endoscopic response and an endoscopic remission. Endoscopic outcomes at week 6 and week 14 are presented in Table 4.
Table 4.
Endoscopic response and remission rates at week 6 and week 14 post vedolizumab administration.
| Post vedolizumab administration | ||
|---|---|---|
| Week 6 | Week 14 | |
| Number of patients with a valid Mayo endoscopic subscore among those with a baseline Mayo endoscopic subscore ⩾ 2 | 9 | 57 |
| Endoscopic response rate, n (%) | 2 (22.2) | 28 (49.1) |
| Endoscopic remission rate, n (%) | 2 (22.2) | 28 (49.1) |
| Complete endoscopic remission rate, n (%) | 0 (0) | 4 (7.0) |
Endoscopic response was defined as a decrease of at least 50% from baseline Mayo endoscopic subscore. Endoscopic remission was defined as Mayo endoscopic subscore of 0–1. Complete endoscopic remission was defined as Mayo endoscopic subscore of 0.
Overall, 15.2% (16/105) of the patients experienced at least one AE. The most common AEs were UC exacerbation (6/105, 5.7%), upper respiratory tract infections (5/105, 4.8%), and arthralgia (2/105, 1.9%). Five patients (4.8%) had at least one SAE, which included: UC exacerbation (3/105, 2.9%), cytomegalovirus infection (1/105, 1.0%), adenoiditis, and mouth/skin ulceration (1/105, 1.0%).
Data from 47 patients in this study were successfully matched (1:1) to 47 anti-TNF experienced subjects from the open-label cohort of the GEMINI 1 study. Table 5 shows the covariate balance for the two sub-cohorts before and after optimal matching. Compared with the matched (open label) sub-cohort of the GEMINI 1 trial, the week 6 clinical response rate was higher in the matched sub-cohort of this study (24/47, 51.1% versus 12/47, 25.5%, p = 0.019). No statistically significant difference was observed in terms of clinical remission rate [7/47, 14.9% (this study) versus 9/47, 19.1% (GEMINI 1 open label matched sub-cohort), p = 0.785] (Table 6).
Table 5.
Comparison of covariates balance pre- and post-optimal matching.
| Pre-matching | Post-matching | |||||
|---|---|---|---|---|---|---|
| Korea | GEMINI 1 | p-value | Korea | GEMINI 1 | p-value | |
| n = 105 | n = 222 | n = 47 | n = 47 | |||
| Age, years | ||||||
| Median (IQR) | 47.0 (23.0) | 39.7 (21.5) | <0.001 | 43.0 (24.5) | 38.2 (24.9) | 0.559 |
| Male sex, n (%) | 67 (63.8) | 122 (55.0) | 0.127 | 28 (59.6) | 27 (57.4) | 0.836 |
| BMI, kg/m2 | ||||||
| Median (IQR) | 21.4 (4.3) | 24.0 (6.1) | <0.001 | 21.3 (4.5) | 22.0 (5.7) | 0.407 |
| Smoking status, n (%) | ||||||
| Current smoker | 4 (3.8) | 15 (6.8) | 0.019 | 4 (8.5) | 1 (2.1) | 1.000 |
| Former smoker | 31 (29.5) | 76 (34.2) | 13 (27.7) | 19 (40.4) | ||
| Never smoked | 62 (59.0) | 131 (59.0) | 30 (63.8) | 27 (57.4) | ||
| Disease duration, years | ||||||
| Median (IQR) | 3.6 (4.0) | 5.7 (8.3) | <0.001 | 4.1 (3.3) | 3.9 (3.6) | 0.959 |
| Number of prior hospitalizations | ||||||
| Median (IQR) | 0 (0.0) | 0 (1.0) | 0.976 | 0 (1.0) | 0 (1.0) | 1.000 |
| Concomitant steroid use, n (%) | 19 (18.1) | 114 (51.4) | <0.001 | 10 (21.3) | 12 (25.5) | 0.631 |
| Disease extent, n (%) | ||||||
| Proctosigmoiditis | 24 (22.9) | 23 (10.4) | 0.044 | 13 (27.7) | 4 (8.5) | 0.550 |
| Left-sided colitis | 22 (21.0) | 76 (34.2) | 11 (23.4) | 19 (40.4) | ||
| Extensive colitis | 2 (1.9) | 24 (10.8) | 1 (2.1) | 5 (10.6) | ||
| Pancolitis | 49 (46.7) | 99 (44.6) | 22 (46.8) | 19 (40.4) | ||
| Baseline Mayo score | ||||||
| Median (IQR) | 9.0 (2.0) | 8.0 (3.0) | 0.022 | 9.0 (2.0) | 9.0 (2.0) | 0.947 |
BMI, body mass index; IQR, interquartile range.
Table 6.
Clinical response and clinical remission * at week 6-comparison between the one-to-one matched cohorts from this study and the open-label sub-cohort of GEMINI 1 study.
| Outcomes | Comparative cohorts | Odds ratio (95% CI) and p-value | |
|---|---|---|---|
| Korean study; matched subjects (n = 47) | GEMINI 1 study; matched subjects from the open-label, anti-TNF failure cohort (n = 47) | ||
| Week 6 clinical response rate – n (%) | 24 (51.1) | 12 (25.5) | 3.01 (1.18–8.02); p = 0.019 |
| Week 6 clinical remission rate – n (%) | 7 (14.9) | 9 (19.1) | 0.74 (0.21–2.49); p = 0.785 |
Please see the Supplemental Table for a summary of definitions for clinical response and remission used in the GEMINI 1 study versus this observational study.
CI, confidence interval; TNF, tumor necrosis factor.
The overall AEs rate in this study was 15.2% (16/105). In the full anti-TNF experienced, vedolizumab-treated sub-cohort of GEMINI 1 trial [i.e., inclusive of both randomized cohort (a.k.a. cohort 1) and open-label cohort (a.k.a. cohort 2)], it was 53.9% (164/304) over the induction therapy period (up to 6 weeks). The SAE rates were similar for both studies (Table 7).
Table 7.
Adverse event rates between the full cohort of this study (up to week 14) and the full TNF-failure cohort from the GEMINI 1 study (induction phase only, up to week 6).
| Korean study; full cohort | GEMINI 1 study; full TNF-failure cohort | |
|---|---|---|
| n = 105 | n = 304 | |
| Safety outcomes | ||
| Any AE, n (%) | 16 (15.2) | 164 (53.9) |
| Any SAE, n (%) | 5 (4.8) | 12 (3.9) |
| Top 3 AEs in the current study | ||
| UC exacerbation (%) | 6 (5.7) | 11 (3.6) |
| Upper respiratory tract infection (%) | 5 (4.8) | 6 (2.0) |
| Arthralgia (%) | 2 (1.9) | 11 (3.6) |
AE, adverse event; SAE, serious adverse event; TNF, tumor necrosis factor; UC, ulcerative colitis.
Subgroup analyses found no significant difference between clinical responses across a range of subgroup factors at week 6 (Table 8). At week 14, the distribution of patients by the number of prior anti-TNF agents was different between the clinical responders and non-responders were more likely to have a lower number of prior anti-TNF therapy compared with non-responders (p < 0.001, unadjusted). Of the 47 patients previously exposed to one anti-TNF agent, 40 patients (85.1%) showed a clinical response, while none of the five patients previously exposed to three anti-TNF agents showed a clinical response at week 14 (Table 8).
Table 8.
Subgroup analyses of clinical response at week 6 and week 14.
| Clinical response stratified by | Week post-initiation of vedolizumab | |||||
|---|---|---|---|---|---|---|
| Week 6 | Week 14 | |||||
| No (n = 34) | Yes (n = 43) | p-value | No (n = 19) | Yes (n = 52) | p-value | |
| 1. Duration of UC | ||||||
| <1 year (%) | 0 (0.0) | 2 (7.7) | 0.201 | 0 (0.0) | 2 (5.4) | 0.539 |
| 1–<3 years (%) | 7 (26.9) | 8 (30.8) | 3 (23.1) | 12 (32.4) | ||
| 3–<7 years (%) | 11 (42.3) | 13 (50.0) | 6 (46.2) | 18 (48.6) | ||
| ⩾7 years (%) | 8 (30.8) | 3 (11.5) | 4 (30.8) | 5 (13.5) | ||
| 2. UC disease extent | ||||||
| Proctosigmoiditis (%) | 5 (14.7) | 14 (32.6) | 0.081 | 5 (26.3) | 13 (25.0) | 0.976 |
| Left-sided colitis (%) | 7 (20.6) | 9 (20.9) | 4 (21.1) | 14 (26.9) | ||
| Extensive colitis (%) | 1 (2.9) | 1 (2.3) | 0 (0.0) | 2 (3.8) | ||
| Pancolitis (%) | 17 (50.0) | 19 (44.2) | 10 (52.6) | 22 (42.3) | ||
| Unknown (%) | 4 (11.8) | 0 (0.0) | 0 (0.0) | 1 (1.9) | ||
| 3. Number of prior anti-TNF therapy | ||||||
| One (1) prior anti-TNF (%) | 19 (55.9) | 30 (69.8) | 0.478 | 7 (36.8) | 40 (76.9) | <0.001 |
| Two (2) prior anti-TNFs (%) | 12 (35.3) | 10 (23.3) | 7 (36.8) | 12 (23.1) | ||
| Three (3) prior anti-TNFs (%) | 3 (8.8) | 3 (7.0) | 5 (26.3) | 0 (0.0) | ||
| 4. Type of prior anti-TNF failure + | ||||||
| Inadequate response (%) | 20 (58.8) | 24 (55.8) | 0.766 | 8 (42.1) | 32 (61.5) | 0.452 |
| Loss of response (%) | 9 (26.5) | 10 (23.3) | 7 (36.8) | 13 (25.0) | ||
| Intolerance (%) | 1 (2.9) | 4 (9.3) | 2 (10.5) | 3 (5.8) | ||
| Unknown (%) | 4 (11.8) | 5 (11.6) | 2 (10.5) | 4 (7.7) | ||
| 5. Type of concomitant medication exposure | ||||||
| Corticosteroids only (%) | 3 (8.8) | 8 (18.6) | 0.640 | 2 (10.5) | 8 (15.4) | 0.691 |
| Immunosuppressants only (%) | 2 (5.9) | 3 (7.0) | 2 (10.5) | 2 (3.8) | ||
| Corticosteroids and immunosuppressants (%) | 1 (2.9) | 2 (4.7) | 0 (0.0) | 2 (3.8) | ||
| No corticosteroids or immunosuppressants (%) | 28 (82.4) | 30 (69.8) | 15 (78.9) | 40 (76.9) | ||
Data captured as free-text initially and subsequently coded manually into the four categories. Entries such as ‘dampened responsiveness’, ‘primary non-response’, ‘no response’, and ‘no effect’ were coded as inadequate responses. Entries such as ‘secondary loss of response’, ‘recurrence of symptoms’ and ‘loss of response’ were coded as a loss of response. Entries such as ‘cytomegalovirus infection’, ‘hypotension’, and ‘intolerance’ were coded as intolerance. Other free-text entries such as ‘principal investigator’s judgement’ and ‘drug change’ etc. were considered as unknowns.
TNF, tumor necrosis factor; UC, ulcerative colitis.
Discussion
Limited Asian specific real-world studies concerning UC patients using vedolizumab have been reported or presented previously.20–23 This study adds to this body of evidence, showing favorable effectiveness at week 6 after administration of vedolizumab. More than half of the observed patients achieved a clinical response and close to 20% of them achieved clinical remission. At week 14, the effectiveness improved further; almost three quarters of the patients achieved a clinical response and close to 40% of them achieved clinical remission. Vedolizumab also showed a favorable safety profile, with a small number of reported AEs.
The results of the real-world effectiveness of vedolizumab shown in this study are comparable, and largely consistent with the open-label sub-cohort from GEMINI 1 study that was matched one-to-one to those in this study. In addition, the crude week 6 clinical response rate was close to the higher end of the 95% CI of the average reported in a systematic review of real-world evidence on vedolizumab (55.8% in this study versus 49% in the review), while the week 6 crude clinical remission rate (18.2%) was within the 95% CI range of the review (13–41%). In terms of safety outcomes, our data shows a lower AE rate, despite the longer observation period. Due to the different study design (i.e., retrospective observation via chart review versus prospective clinical trial) and the unmatched and unadjusted nature of the comparison, it is important that interpreting these two sets of AE rates crudely should be done with caution (further discussed on study limitation). However, based on other real-world studies reported so far, it is suggestive that the safety profiles of vedolizumab approximate the understanding generated from the key clinical trials data, particularly the GEMINI studies.9,13,15,22,24
When compared with several real-world studies that focus specifically on those who had failed anti-TNF therapy (similar to this study), the outcome rates appeared to be largely consistent.15,25,26 In a French study 15 and a US study with a multicenter cohort, 26 the clinical response/remission at week 6 were 41%/32% and 45.0%/15.0%, respectively, which were not quite different with the results of this study (55.8%/18.2%). Data from another anti-TNF failures-focused study which enrolled 29 patients with UC from 37 Belgian centers 25 has also indicated a similar trend, that is, the reported week 10-response rate was 59% and the remission rate was 10%. Evidence suggests that early response to biologic treatment in UC may be predictive of favorable outcomes over the longer term.27,28 As a result, our observation provides some support that patients treated with vedolizumab will likely have better, longer-term benefit given the positive outcomes observed at the early treatment stage. This study filled an important knowledge gap by focusing on the real-world effectiveness of vedolizumab in the subgroup of UC patients who have failed anti-TNF therapy which appear to be lacking in our current literature base.6,13 Our data provided the needed evidence on this aspect in a group of Eastern Asian who lack response to anti-TNF therapy. More recently, vedolizumab use for anti-TNF-naïve patients with UC has been approved in Korea. Real-world evidence from the West has suggested that early initiation of vedolizumab may offer additional outcomes benefits.29,30 Unfortunately, local data in Asia for this category of patients remain limited. Further effort should be invested to examine the potential benefits of vedolizumab among anti-TNF-naïve patients in Korea and Asia.
Despite the value of the data, there are some limitations to consider. Firstly, the potential effect of selection bias. Without a randomization mechanism to assigning treatment, clinicians are likely to initiate the interested treatment to those patients who feature a certain (set of) characteristics. 31 It is challenging to anticipate the exact direction of this source of bias. However, it is highly reasonable to believe that patients who are more likely to benefit by the treatment will have a higher probability of receiving it. As a result, the observed outcomes are more likely to be in favor to them in the real-world versus the clinical trial. Secondly, in reviewing the medical chart, investigators are more likely to ‘look’ for recorded information in the medical chart to fulfil their ‘expectation’. If the investigator expects that patients receiving vedolizumab should have a more favorable outcome, it is likely that they will ‘look harder’ for this information in order to ‘confirm’ their belief (hence confirmation bias). As a result, the data may be concluded that there is a higher effectiveness observed from those who received the treatment. In addition, due to the different study design [i.e., a prospective clinical trial (of GEMINI 1) versus a retrospective chart review], the way AEs captured and reported were accordingly different. In a prospective clinical trial, AE reporting is explicitly solicited from the trial participants by the investigator. In an observational study of retrospective nature (especially via chart review), AEs are typically noted/documented according to their significance to the patients and relevance to the clinicians. Patients were unlikely to report (and clinicians were unlikely to document) the mild and non-specific events. Events that result in significant morbidity and has a known specificity to their treatment are more likely to be escalated to the clinician (and hence being documented in the medical records). As a result, one would expect that AEs of greater significance reported in a real-world observational study will closer resemble those reported in the clinical trial. The mild and non-specific AEs will likely be under-reported. This expectation coincides well with our findings. We believe that the safety findings are meaningful to clinicians and researchers who would like to establish an understanding of the safety profile of vedolizumab under the real-world clinical setting and in the Korean population. The GEMINI safety data presented along will serve as the benchmark to facilitate their qualitative interpretation.
The issue of missing data is inherent in any real-world study, particularly in retrospective studies by chart review. For instance, there is a ~25% missing data on clinical response and remission at week 6 in this study. The reported outcome rates above were primarily performed based on a complete case approach, that is, cases with missing outcome data were excluded. In addition, we also considered a sensitivity analysis in which patients with missing a Mayo score at 6 weeks and 14 weeks were assumed to be non-responders to vedolizumab (a.k.a. NRI). The resultant clinical response rates based on this highly conservative assumption, remain highly consistent with the results observed from other real-world studies and randomized clinical trials, that is, clinical response rate of 43% (95% CI 38–49%) at week 6, and 56% (95% CI 50–62%) at week 14. This indicates that, even if we make the most conservative assumption on the pattern of (non-random) missing data in this analysis, the conclusion based on this real-world study will still be considered highly meaningful and significant.
Conclusion
In conclusion, this study has illustrated that, in the real-world setting, vedolizumab is effective and well tolerated within the first 14 weeks of use in patients with UC in Korea. The proportion of patients experiencing clinical response and clinical remission at weeks 6 and 14 appear to be largely consistent with that observed from real-world studies in other regions and populations.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_17562848211024769 for The real-world outcomes of vedolizumab in patients with ulcerative colitis in Korea: a multicenter retrospective study by Byong Duk Ye, Jae Hee Cheon, Ki Hwan Song, Joo Sung Kim, Young-Ho Kim, Hyuk Yoon, Kang-Moon Lee, Sang-Bum Kang, Byung Ik Jang, Jae Jun Park, Tae Oh Kim, Dae-Wook Lee, Chee Yoong Foo, Jeong Eun Shin and Dong Il Park in Therapeutic Advances in Gastroenterology
Acknowledgments
All co-authors have participated in the conception and design of the study; all clinician investigators collected the data with the support from IQVIA and Takeda; CYF analyzed the study data, and all co-authors interpreted the results. CYF wrote the first draft of this manuscript; all authors critically reviewed the content and approved the final version.
Footnotes
Conflict of interest statement: BDY reported receiving research grants from Celltrion, Inc. and Pfizer Korea, and lecture and/or consulting fees from Abbvie Korea, Daewoong Pharma., Ferring Korea, Janssen Korea, Kangstem Biotech, LG Chem., Shire Korea, Takeda Korea, IQVIA, Takeda, Celltrion, Inc., Chong Kun Dang Pharm., Medtronic Korea, and Pfizer Korea, outside of the submitted work. KHS reported receiving lecture fees from Eisai Korea, Celltrion, Inc., and Janssen Korea, outside the submitted work. YHK reported receiving personal fees from Takeda outside the submitted work. KML reported receiving lecture and/or consulting fees from Jassen, Takeda, and Celtrion, outside the submitted work. DWL is an employee of Takeda Korea at the time of this manuscript writing. CYF is an employee of IQVIA Real World Insights at the time of this manuscript writing. IQVIA has supported Takeda and all the clinician investigators in the design, execution, data management and the development of this manuscript. All other co-authors have nothing to disclose.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Takeda Pharmaceuticals Korea (Takeda Korea).
Ethical approval: The Ethics Committee of all the participating centers approved the conduct of this study and waived the need to obtain consent for the collection, analysis and publication of this retrospectively obtained and anonymized non-interventional study data. (Asan Medical Center IRB approval # 2018-0869)
Data sharing: Data used for the analyses are available on request.
Transparency: The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
ORCID iDs: Byong Duk Ye  https://orcid.org/0000-0001-6647-6325
https://orcid.org/0000-0001-6647-6325
Kang-Moon Lee  https://orcid.org/0000-0003-2850-4553
https://orcid.org/0000-0003-2850-4553
Chee Yoong Foo  https://orcid.org/0000-0002-1833-4290
https://orcid.org/0000-0002-1833-4290
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Byong Duk Ye, Department of Gastroenterology and Inflammatory Bowel Disease Center, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Korea.
Jae Hee Cheon, Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
Ki Hwan Song, Department of Surgery, KOO Hospital, Daegu, Korea.
Joo Sung Kim, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Young-Ho Kim, Division of Gastroenterology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Hyuk Yoon, Division of Gastroenterology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
Kang-Moon Lee, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea.
Sang-Bum Kang, Division of Gastroenterology, Departments of Internal Medicine, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
Byung Ik Jang, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
Jae Jun Park, Division of Gastroenterology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
Tae Oh Kim, Division of Gastroenterology, Department of Internal Medicine, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
Dae-Wook Lee, Takeda Pharmaceutical Ltd, Medical Affairs, Asia-Pacific Region, Singapore.
Chee Yoong Foo, IQVIA, Real-world Insights, Asia-Pacific, Singapore.
Jeong Eun Shin, Division of Gastroenterology, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
Dong Il Park, Division of Gastroenterology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
References
- 1. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142: 257–265.e253. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95. [DOI] [PubMed] [Google Scholar]
- 4. Papamichael K, Rivals-Lerebours O, Billiet T, et al. Long-term outcome of patients with ulcerative colitis and primary non-response to infliximab. J Crohns Colitis 2016; 10: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Bosch O, Gisbert JP, Canas-Ventura A, et al. Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. J Crohns Colitis 2013; 7: 717–722. [DOI] [PubMed] [Google Scholar]
- 6. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res 2017; 15: 7–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park SC, Jeen YT. Anti-integrin therapy for inflammatory bowel disease. World J Gastroenterol 2018; 24: 1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis 2016; 10: 1437–1444. [DOI] [PubMed] [Google Scholar]
- 9. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 10. Kopylov U, Ron Y, Avni-Biron I, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm Bowel Dis 2017; 23: 404–408. [DOI] [PubMed] [Google Scholar]
- 11. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol 2017; 8: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stallmach A, Langbein C, Atreya R, et al. Vedolizumab provides clinical benefit over 1 year in patients with active inflammatory bowel disease – a prospective multicenter observational study. Aliment Pharmacol Ther 2016; 44: 1199–1212. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber S, Dignass A, Peyrin-Biroulet L, et al. Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018; 53: 1048–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One 2019; 14: e0212989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2016; 14: 1593–1601 e1592. [DOI] [PubMed] [Google Scholar]
- 16. Probert CS, Sebastian S, Gaya DR, et al. Golimumab induction and maintenance for moderate to severe ulcerative colitis: results from GO-COLITIS (golimumab: a phase 4, UK, open label, single arm study on its utilization and impact in ulcerative colitis). BMJ Open Gastroenterol 2018; 5: e000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho D, Imai K, King G, et al. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42: 24073. [Google Scholar]
- 18. Ho DE, Imai K, King G, et al. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2017; 15: 199–236. [Google Scholar]
- 19. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 20. Chiu Y-C, Chen C-C, Ko C-W, et al. Real-world efficacy and safety of vedolizumab among patients with inflammatory bowel disease: a single tertiary medical center experience in Central Taiwan. Adv Dig Med 2021; 8: 40–46. [Google Scholar]
- 21. Gan AT-M, Chan WP-W, Ling KL, et al. P634 real-world data on the efficacy and safety of vedolizumab therapy in patients with inflammatory bowel disease: a retrospective nation-wide cohort study in Singapore. J Crohns Colitis 2019; 13: S434–S435. [Google Scholar]
- 22. Kim J, Ham NS, Oh EH, et al. P277 Real life effectiveness and safety of vedolizumab induction and maintenance therapy for Korean IBD patients in whom anti-TNF treatment failed: a prospective cohort study. J Crohns Colitis 2019; 13: S237–S237. [Google Scholar]
- 23. Tai W-C, Tu C-H, Chou J-W, et al. PP 0463 Treatment persistence and clinical outcomes of vedolizumab in inflammatory bowel disease patients in Taiwan: real-world evidence from the TSIBD registry. E-Poster presented at the Asian Pacific Digestive Week 2019, 12–15 December 2019, Kolkata. [Google Scholar]
- 24. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014; 147: 618–627e613. [DOI] [PubMed] [Google Scholar]
- 25. De Vos M, Dhooghe B, Vermeire S, et al. Efficacy of vedolizumab for induction of clinical response and remission in patients with moderate to severe inflammatory bowel disease who failed at least two TNF antagonists. United European Gastroenterol J 2018; 6: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis 2015; 21: 2879–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol 2013; 28: 1829–1833. [DOI] [PubMed] [Google Scholar]
- 28. Barre A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther 2018; 47: 896–905. [DOI] [PubMed] [Google Scholar]
- 29. Bressler B, Yarur A, Silverberg MS, et al. Vedolizumab and anti-TNFα real-world outcomes in biologic-naïve inflammatory bowel disease patients: results from the EVOLVE study. J Crohns Colitis. Epub ahead of print 31 March 2021. DOI: 10.1093/ecco-jcc/jjab058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kopylov U, Verstockt B, Biedermann L, et al. Effectiveness and safety of vedolizumab in anti-TNF-naive patients with inflammatory bowel disease-a multicenter retrospective European study. Inflamm Bowel Dis 2018; 24: 2442–2451. [DOI] [PubMed] [Google Scholar]
- 31. Haneuse S. Distinguishing selection bias and confounding bias in comparative effectiveness research. Med Care 2016; 54: e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_17562848211024769 for The real-world outcomes of vedolizumab in patients with ulcerative colitis in Korea: a multicenter retrospective study by Byong Duk Ye, Jae Hee Cheon, Ki Hwan Song, Joo Sung Kim, Young-Ho Kim, Hyuk Yoon, Kang-Moon Lee, Sang-Bum Kang, Byung Ik Jang, Jae Jun Park, Tae Oh Kim, Dae-Wook Lee, Chee Yoong Foo, Jeong Eun Shin and Dong Il Park in Therapeutic Advances in Gastroenterology