Abstract
The aim of this report is to show that periodontitis and peri-implantitis with horizontal bone resorption in a 68-year-old male patient were successfully treated by non-surgical treatment. Scaling with an ultrasonic device was performed for moderate periodontitis around the mandibular left first premolar and moderate peri-implantitis around the maxillary right molar implants. Root planing with a metal curette was performed for the periodontal site, and debridement with a plastic curette was performed for the peri-implant site. A month after treatment, probing depth decreased from 5 to 2 mm at the periodontal site and 8 to 3 mm at the peri-implant site. The investigation of bacterial composition by sequencing the 16S rRNA gene amplicons showed that the composition similarly changed at both sites, 5 years after treatment; the change reflected the typical recovery of periodontitis. The clinical condition was maintained for 7 years after treatment at both sites. This was a successful case of non-surgical treatment for peri-implantitis with horizontal bone resorption, promoting recovery of the microbiota from dysbiotic shift.
Keywords: Peri-implantitis, periodontitis, non-surgical treatment, bacterial composition, periodontal pathogen
Introduction
Non-surgical treatment is a common management strategy for periodontitis. However, in cases of peri-implant inflammation, surgical management is a de facto standard because the implant surface is poorly debrided by non-surgical treatment. Non-surgical treatment fails to eliminate or reduce bacterial counts in peri-implantitis. 1 Thus, the application of non-surgical treatment is generally limited to peri-implant mucositis. A good prognosis of peri-implantitis after non-surgical treatment is considered rare. 2 However, the refractory cases of peri-implantitis still exist when surgical treatment is difficult to debride or apply because of multiple factors such as systemic diseases and advanced age. To consider non-surgical treatment for peri-implantitis as an alternative to surgical methods, bacterial composition at a peri-implant site would be comprehensively investigated before and after treatment. That being said, none of the previous case reports have addressed it except for investigating limited bacterial taxa. 1 High-throughput sequencing technology is one of the effective solutions for investigating composition of multiple bacterial taxa simultaneously, as an alternative to traditional ways such as bacterial culture and polymerase chain reaction. The aim of this case report is to present a successful case of non-surgical treatment for periodontitis and peri-implantitis sites with horizontal bone resorption in a patient, with the data of comprehensive investigation of bacterial composition using a high-throughput sequencer.
Case report
This report was approved by the Dental Research Ethics Committee of Tokyo Medical and Dental University (Tokyo, Japan; approval number D2015-535). A Japanese male aged 68 years presented with the discomfort in the maxillary right jaw area in June 2013. He was a nonsmoker, and his medical history was non-significant, although he had poor plaque control. Periodontitis was the primary reason for tooth loss and most of them had been restored by implants that were placed in other dental clinics. Written informed consent was obtained from the patient for this report.
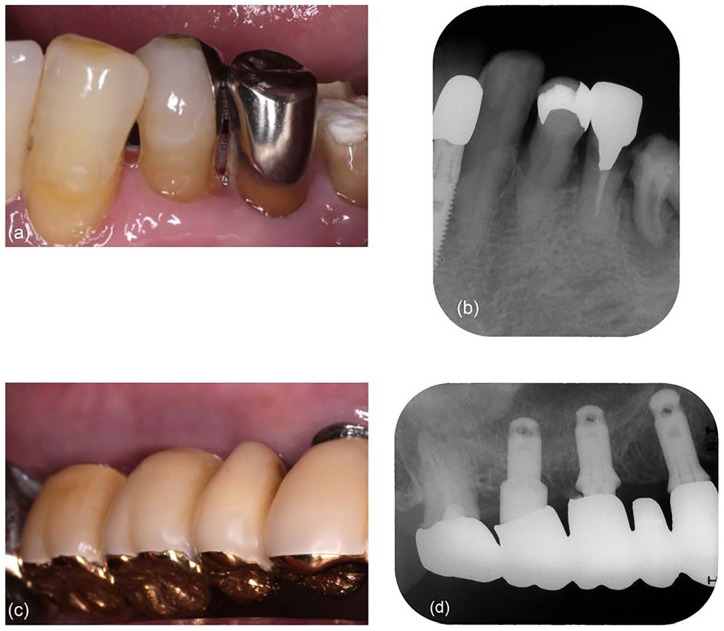
Clinical and radiographic examinations revealed moderate periodontitis around the mandibular left first premolar, 3 restored with a metal inlay and composite resin filling (Figure 1(a)). Probing depth (PD) for the tooth was 5 mm and bleeding on probing (BOP) was observed (Table 1). The radiograph showed horizontal bone resorption around the first premolar (Figure 1(b)). The patient was diagnosed with generalized periodontitis of stage IV with grade B 3 according to PD ⩾ 6 mm, furcation involvement class II, secondary occlusal trauma (tooth mobility degree ⩾ 2), 20 remaining teeth, and 0.76 bone loss/age. Teeth with severe periodontitis and/or furcation involvement were excluded from sampling because they were diagnosed for extraction. In addition, moderate peri-implantitis was observed around two implants in the maxillary right first and second molar region with radiographic bone level ⩾3 mm in combination with BOP and PDs ⩾ 6 mm using the criteria in the absence of previous radiographs, 4 having the metal-ceramic superstructure as part of the bridge (Figure 1(c)). The superstructure had an insufficient contour with overhanging margins below the gingival crest, and excess cement was found on the radiograph (Figure 1(d)). These two implants, the system of which was unknown, had been placed with sinus lift approximately 10 years back in another clinic. The PD for the second molar implant was 8 mm, and the BOP was observed (Table 1). Horizontal bone resorption with radiographic bone level ⩾ 3 mm was observed around the implants in the radiograph (Figure 1(d)). O’Leary plaque control record (PCR) was 54.8%.
Figure 1.
Baseline presentation: (a) intraoral view of the mandibular left molar area on the buccal side; (b) periapical radiograph of the mandibular left premolar area, showing horizontal bone resorption around the first premolar; (c) intraoral view of the maxillary right molar area on the buccal side. The margin of the superstructure is not visible because of being subgingival; and (d) periapical radiograph of the maxillary right molar area, showing the overhang of the superstructure, excess cement, and horizontal bone resorption.
Table 1.
Clinical assessment.
| Baseline |
5 Years |
7 Years |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buccal |
Palatal/lingual |
Buccal |
Palatal/lingual |
Buccal |
Palatal/lingual |
|||||||||||||
| Mesial | Mid | Distal | Mesial | Mid | Distal | Mesial | Mid | Distal | Mesial | Mid | Distal | Mesial | Mid | Distal | Mesial | Mid | Distal | |
| #17 (Implant) | ||||||||||||||||||
| PD (mm) | 4 | 7 | 8 | 5 | 7 | 4 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 2 | 4 | 3 | 2 | 3 |
| MR (mm) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 3 | 2 | 3 | 2 | 3 | 4 | 3 | 3 | 4 | 3 |
| BOP | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Mobility | 0 | 0 | 0 | |||||||||||||||
| #34 (Tooth) | ||||||||||||||||||
| PD (mm) | 4 | 5 | 4 | 4 | 4 | 5 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 |
| MR (mm) | 3 | 5 | 4 | 3 | 4 | 4 | 4 | 7 | 4 | 5 | 5 | 4 | 5 | 7 | 5 | 5 | 5 | 4 |
| BOP | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − |
| Mobility | 0 | 0 | 0 | |||||||||||||||
PD: probing depth; MR: mucosal recession; BOP: bleeding on probing.
Oral hygiene instructions were given to improve plaque control to the PCR level of less than 10%. Non-surgical treatment was then carried out for periodontitis around the mandibular left first premolar and peri-implantitis around the maxillary right molar implants. For periodontitis, scaling and root planing were performed without anesthesia. An ultrasonic device (Enac, Osada Electric Co. Ltd., Tokyo, Japan) with a stainless steel tip was used for scaling and a metal curette (Gracey curette, Hu-Friedy, Chicago, IL, USA) for root planing. For peri-implantitis, scaling and debridement were performed without anesthesia. The ultrasonic device and stainless steel tip similar to periodontitis were used for scaling. A plastic curette (Implacare, Hu-Friedy) was used for debridement. In these procedures, excess cement was removed.
At the time of pre-treatment examinations (pre-treatment) and 5 years after treatment (post-treatment), subgingival plaque was collected from the periodontal pocket around the mandibular left first premolar and from the peri-implant pocket around the maxillary right second molar implant. The plaque was collected from the deepest pocket of six sites by placing a sterile paper point for 30 s. Bacterial DNA was extracted from the plaque using a kit (Mora-extract AMR Inc., Tokyo, Japan), according to the manufacturer’s instructions. The 16S rRNA gene library was constructed by amplifying the V3-V4 region of the 16S rRNA gene, using the Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA, USA) and 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′ (forward) and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′ (reverse) primers. The library was sequenced using a MiSeq platform (Illumina, San Diego, CA, USA) to obtain 2 × 300 bp paired-end reads. The read data were submitted to the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/) under accession number DRA010158. The reads were processed with the Illinois Mayo Taxon Organization from the RNA Dataset Operations (IM-TORNADO) pipeline. 5 The preprocessed reads were clustered into operational taxonomic units (OTUs), taxonomically assigned at the genus level using the Human Oral Microbial Database v15.1 6 at 97% sequence identity.
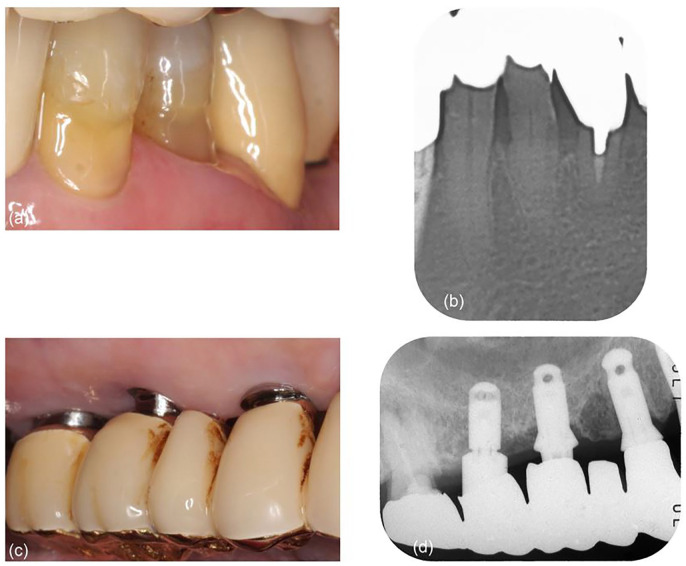
A month after treatment, the PD decreased to 2 mm for the periodontal and 3 mm for the peri-implant site. No BOP was observed at either site. These findings were maintained for 3 months and follow-up was performed thereafter (Table 1). At every visit during follow-up, professional mechanical cleaning was performed using a rubber cup (Merssage Cup No.1P, Shofu Inc, Kyoto, Japan) and/or air polishing (Quick Jet, Yoshida, Tokyo, Japan). Scaling was performed for the periodontal site using the ultrasonic device with a stainless steel tip and for the peri-implant site using the plastic curette. Irrigation with 0.2% benzethonium chloride solution (Neostelin Green, Japan Dental Pharmaceutical Manufacturing Co. Ltd., Yamaguchi, Japan) was performed for both sites. For a 5-year follow-up, periodontal and peri-implant health was maintained, and no significant problems occurred. The clinical outcomes were assessed 5 years after treatment, in May 2018. The PD was 2 mm for the periodontal site and 3 mm for the peri-implant site (Figure 2, Table 1). Peri-implant mucosal recession was observed at the peri-implant site (Figure 2). No BOP was observed for either site. In the radiograph, progression of bone resorption was not observed at both sites (Figure 2). Two years from the first visit, the mandibular left second premolar with a caries and periapical lesion was restored with a porcelain-fused-to-metal crown (Figure 2).
Figure 2.
Five-year follow-up: (a) gingiva is receded and tightened around the mandibular left first premolar; (b) section of panoramic radiograph shows the increase of bone density around the first premolar; (c) peri-implant mucosal recession is observed around the maxillary right molar implants. The margin of the superstructure is exposed to be supragingival; and (d) bone density is increased around the implants.
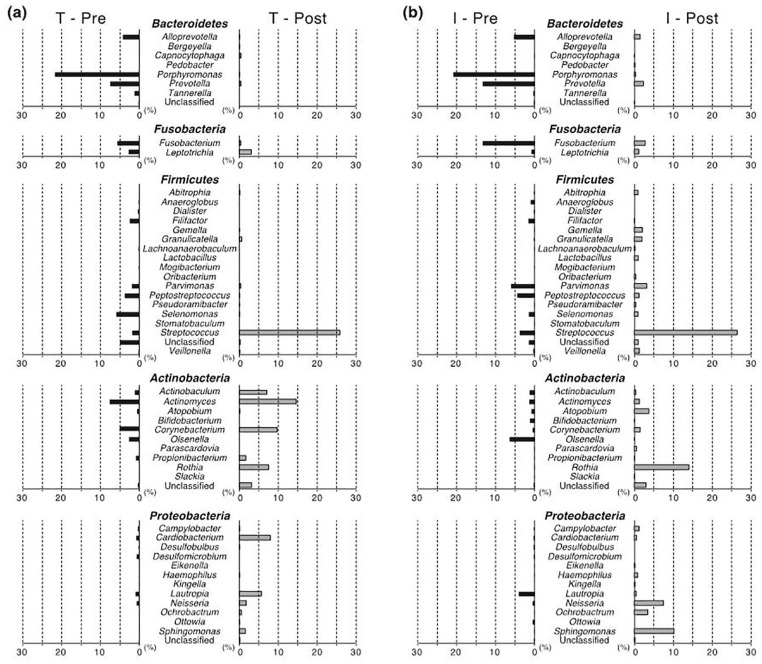
At both pre-treatment and post-treatment, bacterial composition was similar at the periodontal and peri-implant site (Figure 3). At the phylum level, Bacteroidetes was most predominant in its proportion in the pre-treatment plaque (35.2% at the periodontal and 40.0% at the peri-implant site), followed by the major phyla such as Firmicutes, Actinobacteria, Fusobacteria, and Proteobacteria. In the post-treatment plaque, Firmicutes, Actinobacteria, and Proteobacteria were predominant, followed by Bacteroidetes and Fusobacteria.
Figure 3.
Bacterial composition at the phylum level. The percentage abundance of each phylum is indicated in a bar chart (a) for the periodontal site and (b) for the peri-implant site. The data at the pre-treatment is shown at the left side, and the data at the post-treatment is shown at the right side. For example, “T—Pre” means the data for the periodontal site (i.e. “T” for the tooth and “I” for the implant) at the pre-treatment (i.e. “Pre” for the pre-treatment and “Post” for the post-treatment). The lowermost “Unclassified” is the taxon that is not classifiable at the phylum level but is classified in the domain of Bacteria.
From pre-treatment to post-treatment, the bacterial composition was remarkably changed at both sites. An increase was observed in Firmicutes, Actinobacteria, and Proteobacteria, whereas a decrease was observed in Bacteroidetes, Fusobacteria, and Synergistetes. The proportion of the phylum Synergistetes, consisting of only the genus Fretibacterium, decreased to zero. Similarly, the proportion of the phylum Spirochaetes, consisting of only the genus Treponema, decreased to zero at the periodontal site (Figure 3(a)), whereas there was a slight change at the peri-implant site (Figure 3(b)).
The increase and decrease in phyla were attributed to particular genera (Figure 4). The genus Streptococcus remarkably increased in abundance in the phylum Firmicutes at both sites (1.87%–25.8% at the periodontal site and 3.80%–26.4% at the peri-implant site). In the phylum Actinobacteria, a decrease in the genus Olsenella and an increase in the genus Rothia were observed at both sites. However, a decrease was observed in the genera Porphyromonas, Prevotella, Alloprevotella, and Tannerella in the phylum Bacteroidetes, and in the genus Fusobacterium in the phylum Fusobacteria. Among them, the decrease in Porphyromonas was most remarkable at 21.7%–0.0426% at the periodontal site and 20.9%–0.236% at the peri-implant site. Although most of the increase and decrease at the genus level were similar at both sites, the genus Lautropia in the phylum increased at the periodontal site (Figure 4(a); 0.990%–5.68%) but decreased at the peri-implant site (Figure 4(b); 4.04%–0.408%). The ratio of Porphyromonas to Streptococcus (Porphyromonas/Streptococcus ratio) showed a remarkable decrease, as 11.6 to 0.00165 at the periodontal site and 5.49 to 0.00892 at the peri-implant site.
Figure 4.
Bacterial composition at the genus level: the percentage abundance of each genus is shown in the same manner as Figure 3: (a) for the periodontal site and (b) for the peri-implant site. Genera are listed according to their upper rank of phylum. The lowermost “Unclassified” in each phylum is the taxon that is not classifiable at the genus level but is classifiable at a rank higher than genus in the domain of Bacteria.
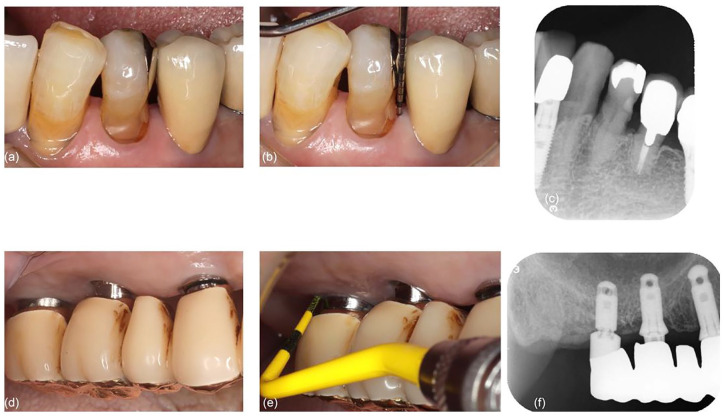
The clinical outcomes were assessed after 7 years of treatment, in March 2020. The PD was 3 mm for the periodontal site and 4 mm for the peri-implant site (Figure 5, Table 1). No BOP was observed at either site. Progression of bone resorption was not observed at both sites on the radiographs (Figure 5). The maxillary right third molar was extracted after 6 years of treatment because of caries and root fracture (Figure 5); the extraction was already recommended before treatment, but the patient did not agree until the discomfort became significant enough.
Figure 5.
Seven-year follow-up: (a) gingiva around the mandibular left first premolar is still tightened; (b) the BOP is not observed around the first premolar, with the PD of 3 mm; (c) bone density is maintained around the first premolar; (d) tightness of peri-implant mucosa is maintained around the maxillary right molar implants; (e) the BOP is not observed around the implants, with the PD of 4 mm; and (f) bone density is maintained around the implants.
Discussion
In this case, an excellent outcome was observed by non-surgical treatment for periodontitis and peri-implantitis sites with horizontal bone resorption in the same oral cavity. After 5 years of treatment, both sites showed similar changes in plaque bacterial composition to be typical of healthy sites. The change in bacterial composition through the treatment indicated a good prognosis as this patient maintained healthy periodontal and peri-implant sites. This case report is remarkable in longitudinally investigating alteration in bacterial composition through non-surgical treatment using a high-throughput sequencer comprehensively, although a few studies have been reported for cross-sectionally comparing bacterial composition between periodontitis and peri-implantitis within the same oral cavity.7–9 In addition, this case was followed up for 7 years, which was longer than the previous reports in which the duration of follow-up was from 3 to 12 months.1,10,11
First, the outcome of non-surgical treatment for the index case was expected to be poor because of risk factors such as a history of periodontitis, poor plaque control, and overhanging superstructure at the peri-implant site. 12 Although replacement of the superstructure was recommended, it could not be performed as the patient did not agree. However, the outcome was excellent, possibly due to improvement of plaque control and removal of excess cement. The peri-implant mucosal recession resulted in exposure of the subgingival margin of the implant superstructure to the supragingival area, which made plaque control for this site easier. Treatment of the adjacent tooth would also have resulted in the improvement of periodontal condition at the periodontal site. A previous study demonstrated excess cement in 81% of the cases of peri-implant disease, and the removal of excess cement resulted in disappearance of the disease signs in 74%. 13 Moreover, the horizontal bone resorption at the periodontal and peri-implant sites should be discussed. In this patient, significant vertical resorption of the alveolar bone was not observed at the periodontal and peri-implant sites. A previous study showed that the reduction of pocket depth and the gain of clinical attachment by root planing for the periodontal sites were significantly larger in horizontal bone resorption than in vertical. 14 In this case, horizontal bone resorption would have contributed to the good outcome because it was observed at both of the periodontal and peri-implantitis sites before treatment. In addition, in terms of debridement methods, the use of plastic curette for peri-implantitis after scaling using an ultrasonic device may also have resulted in the success of the treatment.
Before treatment, the periodontal site showed typical bacterial composition with highly abundant periodontal pathogens; the microbiota was considered to be imbalanced (i.e. dysbiosis). 15 The genera Porphyromonas, Tannerella, and Treponema are generally found with high frequency in periodontal pockets, and the particular species in these genera are called the red complex. In addition, other genera also frequently found in periodontal pockets, such as Prevotella and Fusobacterium, 16 were higher at pre-treatment than post-treatment. Interestingly, the composition at the peri-implant site was similar to that at the periodontal site, suggesting the transmission of periodontal pathogens from periodontitis lesions to peri-implant sites. 17 In contrast, the genera Streptococcus and Rothia, reported to be predominant at healthy periodontal sites, 18 increased in prevalence from pre- to post-treatment. Although minor differences in the proportional change existed among the genera, the overall change in bacterial composition from inflamed to healthy microbiota strongly supported the success of non-surgical treatment for this patient. On the contrary, the genus Lautropia showed exceptional behavior, as it increased at the periodontal site and decreased at the peri-implant site. The species Lautropia mirabilis has been shown to be more abundant at nonrefractory periodontitis sites than refractory; 19 however, its role in the etiology of periodontitis and peri-implantitis is currently unknown. Such differences in the overall similar change of bacterial composition at both sites would provide a clue to disease-specific treatment, customized for peri-implantitis.
Moreover, this report clearly demonstrated the obvious decrease of periodontal pathogens, in contrast to a previous study showing the non-significance of the effect of non-surgical treatment on the change of subgingival bacterial composition at the peri-implant sites. 20 The periodontal pathogen Porphyromonas gingivalis disturbs the host immune system in periodontitis lesions. A decrease in periodontal pathogens, including such bacterial species, would be a sign of treatment success. The Porphyromonas/Streptococcus ratio, calculated from the two genera drastically changed in amount through the treatment, would be a good indicator for predicting the therapeutic effect and prognosis for peri-implantitis and periodontitis. This case report was, however, limited in the number of subjects and in dissecting alteration of bacterial composition, which was difficult to diagnose inflammation and to characterize particular bacterial taxa as causative agents of inflammation. Therefore, further studies are needed to investigate the alteration of this ratio and changes in multiple cases, including those of surgical treatment along with the assessment of the change in actual bacterial counts.
Conclusion
In this case, non-surgical treatment was effective for peri-implantitis with horizontal bone resorption and presence of excess cement. By non-surgical treatment, the periodontal and the peri-implant sites showed similar changes in the bacterial composition from the predominance of periodontal pathogens to the typical composition of healthy sites.
Acknowledgments
Supercomputing resource was provided by the Human Genome Center at the Institute of Medical Science (the University of Tokyo, Tokyo, Japan; http://sc.hgc.jp/shirokane.html). We thank Hugo Song, from HUGO LS (https://www.hugols.com), for editing the draft of this manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from the Dental Research Ethics Committee of Tokyo Medical and Dental University (Tokyo, Japan; approval number D2015-535).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by KAKENHI from the Japan Society for the Promotion of Science (Tokyo, Japan; grant numbers JP19K19016, JP19K10164, and JP20K09934).
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iDs: Takahiko Shiba  https://orcid.org/0000-0002-8388-6868
https://orcid.org/0000-0002-8388-6868
Takayasu Watanabe  https://orcid.org/0000-0001-5487-8191
https://orcid.org/0000-0001-5487-8191
References
- 1. Persson GR, Samuelsson E, Lindahl C, et al. Mechanical non-surgical treatment of peri-implantitis: a single-blinded randomized longitudinal clinical study. J Clin Periodontol 2010; 37(6): 563–573. [DOI] [PubMed] [Google Scholar]
- 2. Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 2008; 35(8 Suppl.): 305–315. [DOI] [PubMed] [Google Scholar]
- 3. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol 2018; 89(Suppl. 1): S159–S172. [DOI] [PubMed] [Google Scholar]
- 4. Renvert S, Persson GR, Pirih FQ, et al. Peri-implant health, peri-implant mucositis, and peri-implantitis: case definitions and diagnostic considerations. J Periodontol 2018; 89(Suppl. 1): S304–S312. [DOI] [PubMed] [Google Scholar]
- 5. Jeraldo P, Kalari K, Chen X, et al. IM-TORNADO: a tool for comparison of 16S reads from paired-end libraries. PLoS ONE 2014; 9(12): e114804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen T, Yu WH, Izard J, et al. The human oral microbiome database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database 2010; 2010: baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Komatsu K, Shiba T, Takeuchi Y, et al. Discriminating microbial community structure between peri-implantitis and periodontitis with integrated metagenomic, metatranscriptomic, and network analysis. Front Cell Infect Microbiol 2020; 10: 596490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiba T, Watanabe T, Kachi H, et al. Distinct interacting core taxa in co-occurrence networks enable discrimination of polymicrobial oral diseases with similar symptoms. Sci Rep 2016; 6: 30997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu XL, Chan Y, Zhuang L, et al. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin Oral Implants Res 2019; 30(8): 760–776. [DOI] [PubMed] [Google Scholar]
- 10. Persson GR, Salvi GE, Heitz-Mayfield LJ, et al. Antimicrobial therapy using a local drug delivery system (Arestin) in the treatment of peri-implantitis. Clin Oral Implants Res 2006; 17(4): 386–393. [DOI] [PubMed] [Google Scholar]
- 11. Maximo MB, de Mendonca AC, Renata Santos V, et al. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res 2009; 20(1): 99–108. [DOI] [PubMed] [Google Scholar]
- 12. Schwarz F, Derks J, Monje A, et al. Peri-implantitis. J Clin Periodontol 2018; 45(Suppl. 20): S246–S266. [DOI] [PubMed] [Google Scholar]
- 13. Wilson TG., Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol 2009; 80(9): 1388–1392. [DOI] [PubMed] [Google Scholar]
- 14. Choi YM, Lee JY, Choi J, et al. Effect of root planing on the reduction of probing depth and the gain of clinical attachment depending on the mode of interproximal bone resorption. J Periodontal Implant Sci 2015; 45(5): 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng ZL, Szafranski SP, Jarek M, et al. Dysbiosis in chronic periodontitis: key microbial players and interactions with the human host. Sci Rep 2017; 7: 3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998; 25(2): 134–144. [DOI] [PubMed] [Google Scholar]
- 17. Van Assche N, Van Essche M, Pauwels M, et al. Do periodontopathogens disappear after full-mouth tooth extraction. J Clin Periodontol 2009; 36(12): 1043–1047. [DOI] [PubMed] [Google Scholar]
- 18. Naginyte M, Do T, Meade J, et al. Enrichment of periodontal pathogens from the biofilms of healthy adults. Sci Rep 2019; 9: 5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colombo AP, Bennet S, Cotton SL, et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J Periodontol 2012; 83(10): 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nie J, Zhang Q, Zheng H, et al. Pyrosequencing of the subgingival microbiome in peri-implantitis after non-surgical mechanical debridement therapy. J Periodontal Res 2020; 55(2): 238–246. [DOI] [PubMed] [Google Scholar]