Abstract
Background:
We previously reported that Secreted frizzled-related protein-2 (SFRP2) is expressed in a variety of tumors, including sarcoma and breast carcinoma. SFRP2 stimulates angiogenesis and inhibits tumor apoptosis. Therefore, we hypothesized that a humanized SFRP2 monoclonal antibody (hSFRP2 mAb) would inhibit tumor growth.
Methods:
The lead humanized SFRP2 antibody was tested against a cohort of 22 healthy donors using a time course T-cell assay to determine the relative risk of immunogenicity. To determine hSFRP2 mAb efficacy, nude mice were subcutaneously injected with SVR angiosarcoma cells and treated with hSFRP2 mAb 4 mg/kg intravenously every 3 days for 3 weeks. Next, we injected Hs578T triple negative breast cells into the mammary fat pad of nude mice and treated for 40 days. Control mice received an IgG1 control. Resected SVR and Hs578T tumors were then stained using an apoptosis assay.
Results:
Immunogenicity testing of hSFRP2 mAb did not induce proliferative responses using SI≥2.0, p<0.05 threshold in any of the healthy donors. SVR angiosarcoma tumor growth was inhibited in vivo with significant tumor volume reduction in the hSFRP2 mAb-treated group, compared to controls (n=10, p<0.05). Likewise, Hs578T triple negative breast tumors were smaller in hSFRP2 mAb-treated group compared to controls (n=10, p<0.05). The hSFRP2 mAb treatment correlated with an increase in tumor cell apoptosis (n=11, p<0.05). Importantly, hSFRP2 mAb treatment was not associated with any weight loss or lethargy.
Conclusion:
We present a novel humanized hSFRP2 mAb with therapeutic potential in breast cancer and sarcoma that has no effect on immunogenicity.
Keywords: WNT, angiogenesis, NFAT, sarcoma
Introduction
Wnt ligands are secreted glycoproteins that activate downstream effectors through binding to cell surface frizzled-G-protein-coupled transmembrane receptors. Activation of Wnt signaling regulates normal embryonic development, but dysregulation of this pathway has been implicated in tumor progression for various cancers 1,2. Secreted frizzled-related proteins (SFRPs) were previously regarded as inhibitors of the canonical Wnt-beta (β)-catenin pathway1, suggesting a tumor suppressor function for SFRP2. However, several recent studies have shown that SFRP2 can act as a β-catenin agonist rather than as an antagonist 3–7, supporting a role in tumor promotion.
In endothelial cells, SFRP2 activates the non-canonical Wnt/Ca2+ pathway, rather than the canonical Wnt/β-catenin pathway, to stimulate angiogenesis 8,9 The Wnt/Ca2+ pathway is mediated through activated G-proteins and phospholipases. This leads to transient increases in cytoplasmic-free calcium and activation of the phosphatase and calcineurin that dephosphorylates the nuclear factor of activated T-cells (NFAT), after which NFAT translocates from the cytoplasm to the nucleus. SFRP2 stimulates endothelial tube formation, migration, and is anti-apoptotic. Antagonizing SFRP2 also directly inactivates NFAT in tumor cells and prevents tumor cell migration. 10
Substantial evidence now strongly supports the contribution of SFRP2 in promoting tumor growth in breast cancer10, angiosarcoma10, osteosarcoma 11, rhabdomyosarcoma 12, alveolar soft part sarcoma 13, malignant glioma 14, multiple myeloma 15, renal cell carcinoma 2, prostate cancer 16, lung cancer 17, and melanoma 18. Additionally, in vivo SFRP2 molecular imaging shows that SFRP2 expression increases proportionally with tumor size 19. A murine SFRP2 monoclonal antibody has been demonstrated to inhibit angiosarcoma and triple negative breast cancer growth in vivo 10. Moving toward clinical translation, we developed a humanized monoclonal antibody to SFRP2 (hSFRP2 mAb) that inhibits angiogenesis and tumor growth, while promoting tumor apoptosis.
Methods
Humanization of hSFRP2 monoclonal antibody
V region genes encoding the murine SFRP2 monoclonal antibody 80.8.6 10 were cloned and used to construct chimeric antibodies and then tested for purity and binding to SFRP2 (Supplementary method).
Immunogenicity testing
The lead fully-humanized anti-SFRP2 antibody and the reference chimeric anti-SFRP2 antibody were assessed for immunogenic potential (Supplementary method).
Determination of hSFRP2 mAb binding affinity, EC50, and Kd
A microplate solid phase protein binding (ELISA) assay was used to determine the half maximal effective concentration (EC50) for hSFRP2 mAb and its binding affinity for SFRP2, using the Cheng-Prusoff equation20 (Supplementary method).
Cell Culture
The origin and methods of culture for 2H11 murine endothelial, Hs578T human triple negative breast carcinoma, MDA-MB-231 human triple negative breast carcinoma and SVR murine angiosarcoma cell lines21 are described in supplementary methods. The SVR angiosarcoma tumor model was derived from the transfection of Ras into MS1 endothelial cells. MS1 cells were previously generated by immortalizing murine endothelial cells by expressing the temperature-sensitive large T antigen21. Upon implantation into mice, these cells form dormant hemangiomas21. MS1 cells were then transfected with Ras (SVR), and this cell line forms angiosarcomas when injected into nude mice21.
Endothelial tube formation assay
2H11 endothelial cells were analyzed for effects on tube formation in vitro (Supplementary method).
Proliferation Assay
Proliferation of Hs578T, MDA-MB-231 and SVR cells was assessed after treatment with the hSFRP2 mAb (Supplementary method).
Apoptosis/Necrosis
Effects of hSFRP2 mAb on apoptosis and necrosis were analyzed in Hs578T, MDA-MB-231 and SVR cells (Supplementary method).
Microplate Solid Phase Protein Binding (ELISA) Assay for Pharmacokinetics (PK) of hSFRP2 mAb
ELISA was performed and PK estimates were generated using non-compartmental analysis (NCA)22. See supplementary method for further details.
In vivo studies
Animal experiment protocols were consistent with NIH guidelines for the care and use of laboratory animals and approved by the Animal Care and Use Committee. The methods for PK, Maximum Tolerated Dose (MTD), angiosarcoma allografts and Hs578T triple negative breast carcinoma xenografts studies are described in supplementary method.
Immunohistochemistry
Formalin fixed, paraffin embedded SVR and Hs578T tumor sections were analyzed for proliferation by immunohistochemistry staining with Ki67 antibody (Supplementary method).
TUNEL assay
Formalin fixed, paraffin embedded sections from resected Hs578T and SVR tumors were stained for apoptotic cells following the manufacturer protocol for the Apoptag® Peroxidase In Situ Apoptosis Detection Kit (#S7100).
Statistics
For in vitro assays, statistical differences between IgG1 and hSFRP2 mAb treatments were calculated using a two-tailed student’s t-test, with p ≤0.05 considered significant. For in vivo tumor studies in angiosarcoma, a two-tailed student’s t-test was used. For Hs578T, two-sample t-test for each time point was used and compared the tumor volume between treated and control animals (Supplementary method).
Results
Humanization of SFRP2 mAb
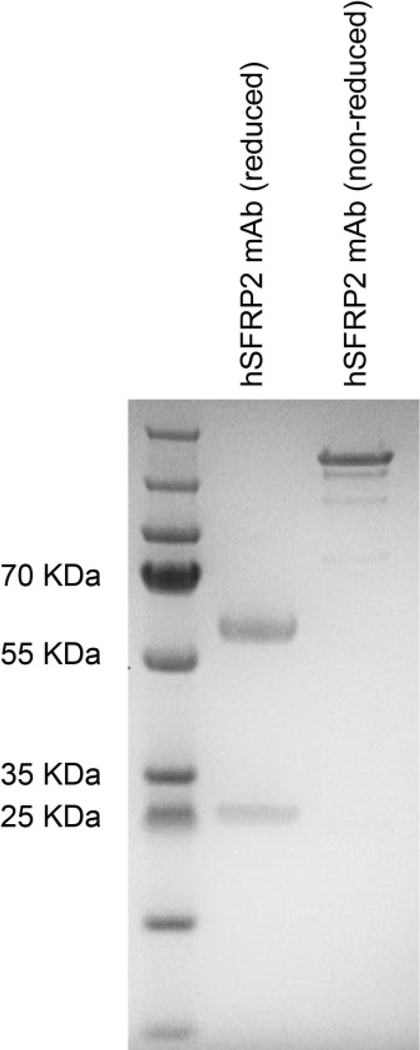
The chimeric antibodies and combinations of composite heavy and light chains (16 antibodies in total) were tested for binding to SFRP2 in a competition ELISA assay. This demonstrated that the binding efficiency of all composite antibodies to SFRP2 were broadly comparable to that of the chimeric antibodies, with all variants showing improvement when compared to the murine antibody (data not shown). The chimeric antibodies and composite variants of anti-SFRP2 were purified from cell culture supernatants on a Protein A sepharose column, buffer exchanged into PBS pH 7.4, and quantified by OD280nm, using an extinction coefficient (Ec (0.1%) = 1.76) based on the predicted amino acid sequence. Endotoxin testing of the lead hSFRP2 mAb showed endotoxin < 0.5EU/m. Western blot analyses of the lead hSFRP2 mAb indicated two bands corresponding to heavy and light chains (Figure 1).
Figure 1: Characterization of the purified candidate hSFRP2 mAb by SDS Page.
1 μg of purified lead hSFRP2 mAb was loaded on a 4–12% NuPAGE SDS gel. PageRuler Plus pre-stained ladder was loaded to allow sizing of bands. Lane 1 was reduced with β-mercaptoethanol; two bands were present for the sample corresponding to the heavy chain and light chain. Lane 2 was non-reduced.
Immnnogenicity testing of hSFRP2 mAb
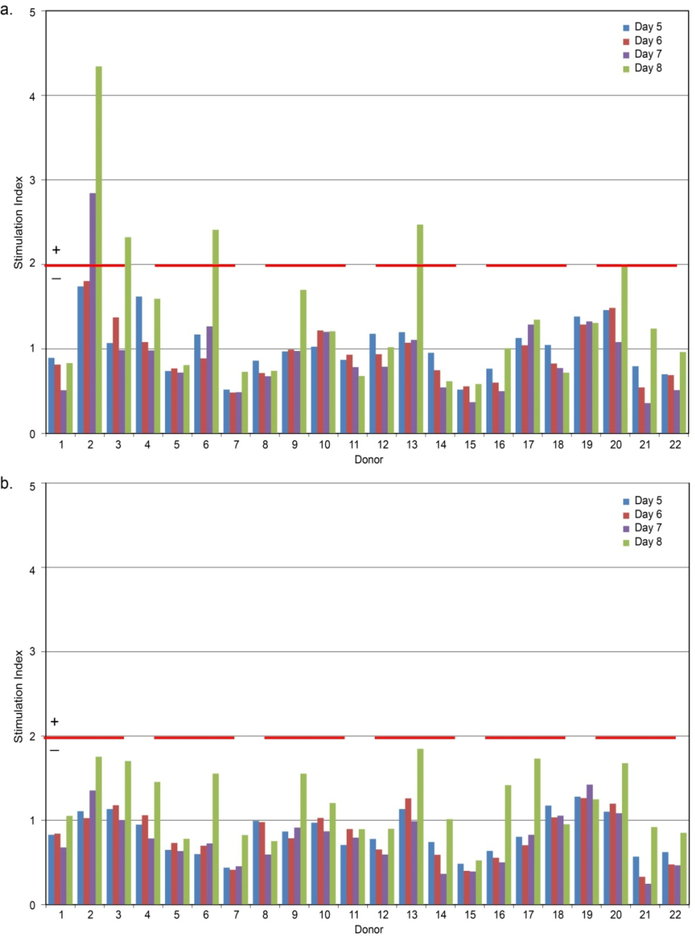
In order to determine the relative risk of immunogenicity, the lead, fully humanized, and chimeric anti-SFRP2 antibodies were tested against a cohort of 22 healthy donors using EpiScreen™ time course T-cell assay. Fully humanized anti-SFRP2 antibody induced no positive responses using SI ≥ 2.0, p < 0.05 threshold in any of the donors in the proliferation assay, whereas the chimeric anti-SFRP2 antibody induced positive T-cell proliferation responses in 23% of the donors. Results with the control antigen KLH show that there was a good correlation (< 10% inter-assay variability) between positive and negative results in repeat studies, indicating high reproducibility in the assay (Figure 2).
Figure 2: Healthy donor T-cell proliferation responses to test antibodies.
PBMCs from bulk cultures were sampled and assessed for proliferation on days 5, 6, 7 and 8 after incubation with the three test samples. Proliferation responses with an SI ≥ 2.0 (p < 0.05), indicated by red dotted line that were significant (p < 0.05) using an unpaired, two sample student’s t-test were considered positive. None of the test antibodies tested positive. (a) Chimeric antibody (b) Fully humanized antibody.
Humanized SFRP2 mAb binds SFRP2 with high affinity
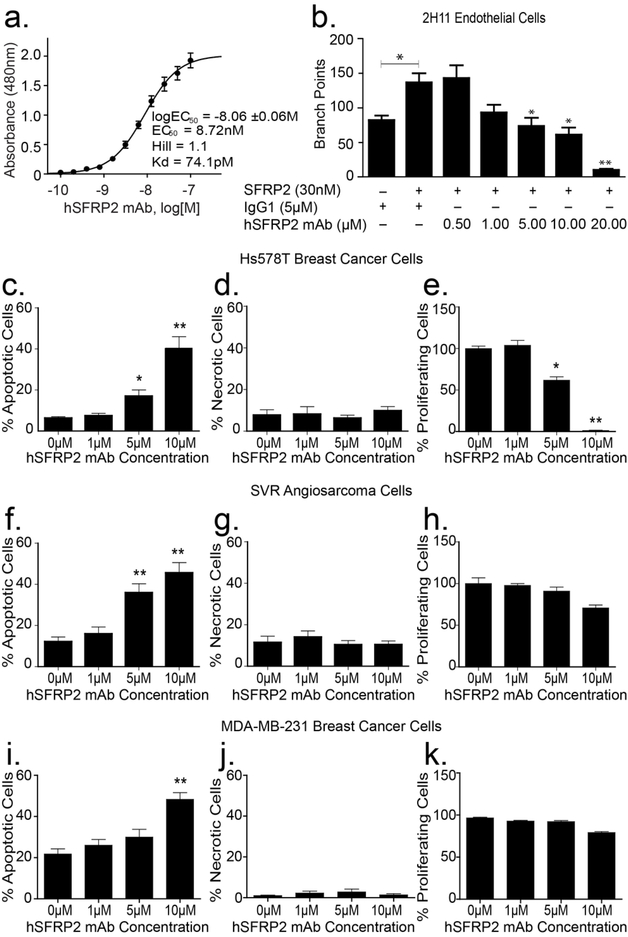
To determine binding affinity of the lead hSFRP2 mAb to SFRP2, SFRP2 (1μM) was incubated with increasing concentrations of hSFRP2 mAb in a microplate solid phase protein binding ELISA assay. The hSFRP2 mAb bound SFRP2 with an EC50 of 8.7 nM and a Kd of 74.1 pM (Figure 3a).
Figure 3: Humanized SFRP2 mAb in vitro activity.
(a) Humanized SFRP2 mAb binds recombinant human SFPR2 protein (SFRP2) with high affinity. Concentration-response curve showing the 480 nm absorbance measured after binding increasing concentrations of hSFRP2 mAb to a preset concentration of 1 μM SFRP2 in an ELISA assay (n=16). EC50: half-maximal effective concentration; Kd: equilibrium dissociation constant; Hill: Hill coefficient. (b) Bar graph showing the effects of SFRP2 and hSFRP2 mAb on 2H11 endothelial tube formation. 2H11 cells were incubated and either treated with IgG1 control only (5 μM), or IgG1 (5μM) + SFRP2 protein (30nM), or a combination of SFRP2 (30 nM) and hSFRP2 mAb (from 0.5 to 10μM). n=4 *: p≤ 0.05; **: p≤ 0.001. (c-h) Bar graphs showing the effects of increasing concentrations of hSFRP2 mAb (0 to 10 μM) on apoptosis (c, f, i), necrosis (d, g, j), and proliferation (e, h, k), in Hs578T breast cancer cells (c-e), and SVR angiosarcoma cells (f-h), and MDA-MB-231 cells (i-k). *: p ≤ 0.05; **: p ≤ 0.001. Proliferation was measured using Cyquant®, while apoptosis and necrosis were measured using Annexin V and propidium iodide. Results are a compilation of 3 independent experiments containing 4 wells each (n=12) for Hs578T and SVR; and 2 experiments containing 4 repeats for MDA-MD-231 (n=8).
Humanized SFRP2 mAb inhibits endothelial tube formation and promotes tumor apoptosis
Consistent with previous reports8, SFRP2 induced an increase in the number of endothelial branch points in an endothelial tube formation assay, compared to control cells (n = 4, p ≤ 0.05) (Figure 3b). Conversely, increasing concentrations of hSFRP2 mAb significantly counteracted SFRP2 effects on tube formation (n = 4, p ≤ 0.05). The IC50 for hSFRP2 mAb inhibition of SFRP2-stimulated tube formation was calculated after Cyquant proliferation assay and found to be 4.9 ± 2 μM.
SFRP2 antagonism with murine SFRP2 mAb has been shown to increase apoptosis without affecting proliferation10. Therefore, we evaluated whether the hSFRP2 mAb affects tumor cell proliferation, apoptosis and necrosis in Hs578T triple negative breast carcinoma, MDA-MB-231 breast carcinoma, and SVR angiosarcoma cells, in vitro. Treatment with hSFRP2 mAb for 2 hours increased tumor apoptosis significantly in Hs578T cells (*p ≤ 0.05 for 5 μM, **p ≤ 0.001 for 10 μM hSFRP2 mAb compared to control, n=12, Figure 3c); SVR angiosarcoma cells (**p ≤ 0.001 for 5 μM and 10 μM hSFRP2 mAb compared to control, n=12, Figure 3f); and MDA-MB-231 cells (**p ≤0.001 for 10 μM compared to control, n=8, Figure 3i). There was no change in necrosis with hSFRP2 mAb treatment in any of the three cell lines (Figure 3d, g, j). Treatment with hSFRP2 mAb had no effect on SVR or MDA-MB-231 proliferation at 72 hours (Figure 3h, k), but significantly reduced tumor cell proliferation of Hs578T cells compared to control (5 μM *p ≤ 0.05, 10 μM **p ≤ 0.001, n=12, Figure 3e).
Pharmacokinetics
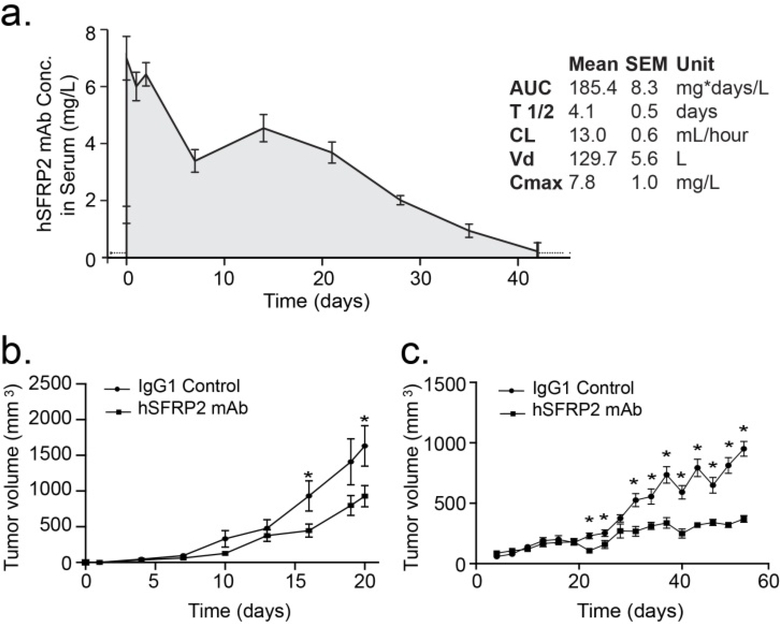
To study the pharmacokinetic properties of the antibody, a single dose of hSFRP2 mAb of 4 mg/kg was injected via the tail vein into nude mice and blood samples were collected at different time points (Figure 4a). The half-life of the antibody in the serum of the animals was 4.1 ± 0.5 days with a maximum serum concentration (Cmax) of 7.8 ± 1.0 mg/L and a clearance (CL) of 13.0 ± 0.6 mL/hour.
Figure 4: Humanized SFRP2 mAb inhibits tumor growth in angiosarcoma and breast cancer.
(a) Pharmacokinetic plot showing the decrease in concentration of hSFRP2 mAb in the serum of mice over time after a single i.v. injection of 4 mg/kg. AUC: Area Under Curve; T 1/2: Half-life; CL: clearance; Vd: volume of distribution; Cmax: maximum serum concentration. Each data point represents the mean ± SEM of the measurements of at least 3 independent samples (n=3 per time point). (b) Nude mice with SVR angiosarcoma allografts were treated with hSFRP2 mAb or IgG1 control. There was a 43% reduction in tumor growth in the hSFRP2 mAb-treated mice (n=10, *p<0.05). (c) Effects of IgG1 treatment vs hSFRP2 mAb on Hs578T tumor volume in vivo over time. Nude mice with Hs578T xenografts were treated with hSFRP2 mAb or IgG1 control for a total of 52 days. Day is counted from day of first treatment, which was 30 days from tumor inoculation. There was a 61% reduction in tumor volume in the hSFRP2 mAb treated mice, n=11, *p<0.05).
Determination of toxicity of hSFRP2 mAb in vivo
Mice injected with SVR angiosarcoma cells were treated with hSFRP2 mAb at dosages 2, 4, 10 and 20 mg/kg i.v. every three days; or IgG1 control, for 21 days. There was no weight loss or lethargy in any of the antibody-treated mice. H&E sections of kidney and liver from all mice at 20 mg/kg dose were reviewed by a board-certified pathologist (LS) and there were no pathologic changes in the liver or kidney between control and hSFRP2 mAb-treated mice (Supplementary Figure 1). At the end of the experiment, body weights remained similar among the groups (32.2 ± 1.4g for controls; 31.3 ± 1.1g for 2 mg/kg; 32.1 ± 0.5g for 4 mg/kg; 31.8 ± 0.9g for 10 mg/kg and 32.7 ± 1.0g for 20 mg/kg. The dose with the maximum effect was 4 mg/kg, where there was a 69% reduction in tumor volume (n = 5 per group, p = 0.05) (data not shown).
Determination of efficacy of hSFRP2 mAb in vivo
To confirm the efficacy of the 4 mg/kg dose identified in the maximum tolerated dose experiment, we repeated the experiment with the SVR angiosarcoma cells on a larger number of animals (n = 10 animals/group), treating them with 4 mg/kg hSFRP2 mAb. As described in Figure 4b, after 3 weeks, tumors treated with hSFRP2 mAb were 43% smaller than tumors treated with the IgG1 control (1,631.3 ± 283 mm3 for control, 928.5 ± 148 mm3 for hSFRP2 mAb; p ≤ 0.05).
Next, we asked whether hSFRP2 mAb could affect the growth of other tumor types. Mice with Hs578T triple negative breast carcinoma cells were treated with hSFRP2 mAb or IGg1 control. Treatment with IgG1 or hSFRP2 mAb began at day 30 when the Hs578T xenografts mice had palpable tumors approximately 100 mm3. Comparison between control and each treated group at each time point shows that treatment days 22, 25, and all time points from day 31 until the last day of treatment were statistically significant (Figure 4c, p < 0.05). At the end of the experiment, which was day 58 from tumor inoculation, after 28 days of treatment with hSFRP2 mAb, the Hs578T tumor volume was reduced by 61%, (*p < 0.05) (Figure 4c).
Humanized SFRP2 mAb induces apoptosis, with no change in proliferation in tumors in vivo
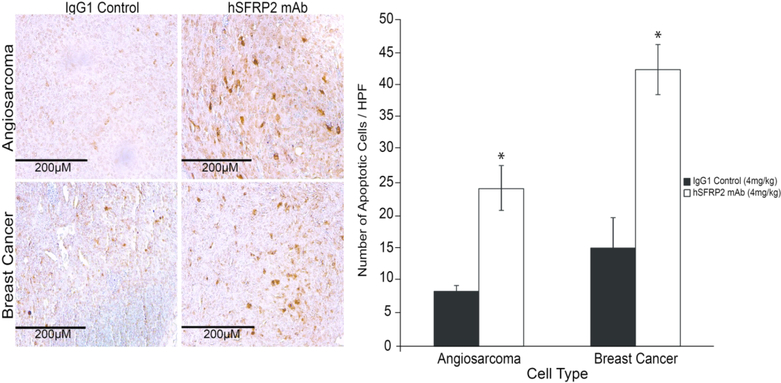
Since hSFRP2 mAb induces apoptosis in vitro, and inhibits proliferation in breast cancer cells, we investigated if these phenotypes were retained in vivo. While the proportion of proliferative (Ki67-positive) cells was not affected by hSFRP2 mAb treatment compared to IgG1 control tumors (23 ± 1.6% vs. 29 ± 4.2% for SVR tumors; 18 ± 2.7% vs. 18 ± 2.8% for Hs578T tumors, p = NS), the proportion of apoptotic cells increased by 188% in SVR tumors (8.4 ± 0.9 in IgG1 control, 24.2 ± 3.5 in hSFRP2 mAb treated; n = 10, p ≤ 0.05) and by 181% in Hs578T tumors (15.1 ± 4.9 in IgG1 control, 42.4 ± 3.9 in hSFRP2 mAb tumors; n = 10, p ≤ 0.05) (Figure 5).
Figure 5. Humanized SFRP2 mAb treatment promotes apoptosis in tumors.
Left: Paraffin embedded Hs578T breast and SVR angiosarcoma tumors were sectioned and processed for TUNEL staining. For each tumor, a total of 5 fields were photographed, the number of apoptotic cells (brown) was counted in each field, and averaged for each tumor. A total of 10 SVR tumors per treatment (n=10) and 11 Hs578T tumors (n=11) were used for the analysis. Right: Bar graph showing the increase in the number of apoptotic cells in tumors treated with SFRP2 mAb (white bars) compared to IgG1 control treated tumors (black bars). *:p≤ 0.05.
Discussion
We report the development of a humanized SFRP2 mAb that is not immunogenic and binds to recombinant human SFRP2 protein with high affinity. Our in vitro and studies show a significant increase in apoptosis in both Hs578T human triple negative breast carcinoma cells, MDA-MB-231 human triple negative breast carcinoma cells, and murine SVR angiosarcoma tumor cells. Hs578T and MDA-MB-231 cells were chosen because they are triple negative breast cancer cell lines, for which there is a great need for novel therapies. The SVR angiosarcoma cell line21 was chosen because angiosarcomas have been shown to represent the signaling abnormalities of pathologic angiogenesis23. SVR cells produce SFRP2 protein8 and silencing SFRP2 inhibited angiosarcoma tube formation in a Matrigel tube formation assay8. 2H11 endothelial cells were chosen because they were demonstrated to be the best murine endothelial cell line to model tumor endothelium for studying the antiangiogenic activity of therapeutic compounds in vitro24.
Most importantly, the hSFRP2 mAb significantly reduced tumor growth in both Hs578T triple negative breast and SVR angiosarcoma tumors in vivo with no signs of toxicity. These anti-tumorigenic effects are particularly important in the setting of sarcoma and triple negative breast, which are aggressive malignancies with poor responses to known chemotherapeutic regimens.
Sarcomas are a heterogeneous group of malignancies that includes >50 different subtypes, each with unique clinical and pathological characteristics. In general, there is a 50% mortality rate, and cures are only achieved with complete surgical resection. The efficacy of chemotherapeutic agents for unresectable or metastatic disease have been disappointing, with minimal long-term benefit, and a 5 year survival for patients with metastatic disease of only 15%25. Additionally, patients with late or metastatic disease rely on chemotherapy as the primary treatment. Unfortunately, doxorubicin has response rates of 10 to 25% in clinic 27
Angiosarcoma is a vascular sarcoma arising from soft tissues in various anatomic locations that has a particularly poor prognosis with an average 5-year survival of 35% and an aggressive course with as many as 44% of patients presenting with metastatic disease. This malignancy has poor response to chemotherapeutic options with the tendency to develop chemo-resistance 26,28. The SVR angiosarcoma line in this study is a transformed endothelial cell line that is a model of aggressive angiosarcoma in nude mice21. Thus, inhibition of tumor growth in this mouse model supports its potential efficacy in human angiosarcoma.
The Hs578T cell line is a triple negative human cell line that is categorized as a basal cancer meaning it is in the basal layer of the epithelium where progenitor cells reside29. It is further classified as Basal B type which has expression similar to stem cells with overlapping features with the triple negative tumor type30,31. This cell line has been found to express epidermal growth factor receptor (EGFR) and hypermethylate E-cadherin gene, which is a mutation that has been implicated in change in response to chemotherapeutics32. A microRNA study of multiple breast cancer cell lines categorized Hs578T into a mesenchymal-like cell line with epithelial to mesenchymal transition (EMT) features, and is similar to carcinosarcoma cell lines (metaplastic) which are frequently ER/PR and HER-2 negative33,34. Growth inhibition of this tumor in vivo is encouraging and could potentially lead to studies investigating utilization of hSFRP2 mAb on metaplastic breast cancers. Additionally, we previously showed that 85% of human triple negative breast cancer have SFRP2 present35, and a murine SFRP2 mAb inhibits human MDA-MB-231 in mice10. We also confirmed that the hSFRP2 mAb induces apoptosis in vitro on MDA-MB-231. Therefore, the hSFRP2 mAb may also be a treatment in general for triple negative breast cancer.
Targeted immunotherapies are a rapidly growing class of cancer treatments that have shown significant response and improvement in overall survival in various cancers. Recent investigation into combination therapies with other immunotherapies or in conjunction with traditional chemotherapeutic agents has also demonstrated promising results and is continuing to expand36. In particular, several clinical trials investigating immunotherapy in sarcomas are ongoing, however only a few drugs are approved for use with metastatic disease37,38. While there is some anti-tumor activity of targeted agents in sarcoma, improved therapeutic agents, and novel combinations of therapeutics, are essential to improve response and outcome. Here, we present a novel humanized monoclonal antibody to SFRP2 with great potential applications in clinic as it inhibits tumor growth and increases apoptosis in both aggressive sarcoma and triple negative breast cancer models in vivo.
Supplementary Material
Synopsis:
We report the humanization of a monoclonal antibody to secreted frizzled-related protein-2 that inhibits angiogenesis, induces tumor apoptosis, and prevents tumor growth in vivo.
Acknowledgements
Funding: This work was supported by the Department of Defense (W81XWH-18-1-0007) to NKD and AMB; the Sarcoma Foundation of American (NKD); R21CA137725, R01CA138930 to SM; The Cancer Prevention and Research Institute of Texas (CPRIT), WWWW Foundation, Inc. (QuadW), The St. Baldrick’s Foundation to JTY and NIH T32 Grant CA193201. Support from Hollings Cancer Center and Biostatistics Shared Resources (partly supported by P30 CA138313) at MUSC is also acknowledged.
Footnotes
Disclosure:
Novel Targets for Regulation of Angiogenesis, U.S. Patent No. 8,734,789. Inventors: Nancy Klauber-DeMore. MD.
Combination therapy of a humanized antibody to secreted frizzled related 2 with PD-1 inhibition for the treatment of cancer. Inventor: Nancy Klauber-DeMore, MD, U.S. Provisional App. No. 62/737,155
Dr. Klauber-DeMore is co-founder, shareholder, Chief Scientific Officer, and Board Member of Enci Therapeutics, Inc.
References:
- 1.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Pt 13):2627–2634. [DOI] [PubMed] [Google Scholar]
- 2.Yamamura S, Kawakami K, Hirata H, et al. Oncogenic functions of secreted Frizzled-related protein 2 in human renal cancer. MolCancer Ther. 2010;9(6):1680–1687. [DOI] [PubMed] [Google Scholar]
- 3.Esteve P, Sandonis A, Ibanez C, Shimono A, Guerrero I, Bovolenta P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development. 2011;138(19):4179–4184. [DOI] [PubMed] [Google Scholar]
- 4.Gehmert S, Sadat S, Song YH, Yan Y, Alt E. The anti-apoptotic effect of IGF-1 on tissue resident stem cells is mediated via PI3-kinase dependent secreted frizzled related protein 2 (Sfrp2) release. BiochemBiophysRes Commun. 2008;371(4):752–755. [DOI] [PubMed] [Google Scholar]
- 5.Lee JL, Chang CJ, Wu SY, Sargan DR, Lin CT. Secreted frizzled-related protein 2 (SFRP2) is highly expressed in canine mammary gland tumors but not in normal mammary glands. Breast Cancer Res Treat. 2004;84(2):139–149. [DOI] [PubMed] [Google Scholar]
- 6.Melkonyan HS, Chang WC, Shapiro JP, et al. SARPs: a family of secreted apoptosis-related proteins. Proc NatlAcadSciUSA. 1997;94(25):13636–13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirotsou M, Zhang Z, Deb A, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc NatlAcadSciUSA. 2007;104(5):1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courtwright A, Siamakpour-Reihani S, Arbiser JL, et al. Secreted Frizzle-Related Protein 2 Stimulates Angiogenesis via a Calcineurin/NFAT Signaling Pathway. Cancer Res. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siamakpour-Reihani S, Caster J, Bandhu ND, et al. The Role of Calcineurin/NFAT in SFRP2 Induced Angiogenesis-A Rationale for Breast Cancer Treatment with the Calcineurin Inhibitor Tacrolimus. PLoSOne. 2011;6(6):e20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontenot E, Rossi E, Mumper R, et al. A novel monoclonal antibody to secreted frizzled-related protein 2 inhibits tumor growth. MolCancer Ther. 2013;12(5):685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Techavichit P, Gao Y, Kurenbekova L, Shuck R, Donehower LA, Yustein JT. Secreted Frizzled-Related Protein 2 (sFRP2) promotes osteosarcoma invasion and metastatic potential. BMC Cancer. 2016;16(1):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Vinson C, Gurley CM, et al. Impaired Ŵnt signaling in embryonal rhabdomyosarcoma cells from p53/c-fos double mutant mice. Am J Pathol. 2010;177(4):2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M, Homme M, Yamazaki Y, Shimizu R, Takazawa Y, Nakamura T. Modeling Alveolar Soft Part Sarcoma Unveils Novel Mechanisms of Metastasis. Cancer Res. 2017;77(4):897–907. [DOI] [PubMed] [Google Scholar]
- 14.Roth W, Wild-Bode C, Platten M, et al. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19(37):4210–4220. [DOI] [PubMed] [Google Scholar]
- 15.Oshima T, Abe M, Asano J, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106(9):3160–3165. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Zhu D, Chen F, et al. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene. 2016;35(33):4321–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, Xiao Y, Wen R, et al. Promoting roles of the secreted frizzled-related protein 2 as a Wnt agonist in lung cancer cells. Oncol Rep. 2015;34(5):2259–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur A, Webster MR, Marchbank K, et al. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature. 2016;532(7598):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuruta JK, Klauber-DeMore N, Streeter J, et al. Ultrasound molecular imaging of secreted frizzled related protein-2 expression in murine angiosarcoma. PloS one. 2014;9(1):e86642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng HC. The power issue: determination of KB or Ki from IC50. A closer look at the Cheng-Prusoff equation, the Schild plot and related power equations. J Pharmacol Toxicol Methods. 2001;46(2):61–71. [DOI] [PubMed] [Google Scholar]
- 21.Arbiser JL, Moses MA, Fernandez CA, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94(3):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. [DOI] [PubMed] [Google Scholar]
- 23.Arbiser JL, Bonner MY, Berrios RL. Hemangiomas, angiosarcomas, and vascular malformations represent the signaling abnormalities of pathogenic angiogenesis. Curr Mol Med. 2009;9(8):929–934. [DOI] [PubMed] [Google Scholar]
- 24.Walter-Yohrling J, Morgenbesser S, Rouleau C, et al. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res. 2004;10(6):2179–2189. [DOI] [PubMed] [Google Scholar]
- 25.Schoffski P, Cornillie J, Wozniak A, Li H, Hompes D. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37(6):355–362. [DOI] [PubMed] [Google Scholar]
- 26.Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11(10):983–991. [DOI] [PubMed] [Google Scholar]
- 27.Lorigan P, Verweij J, Papai Z, et al. Phase III trial of two investigational schedules of ifosfamide compared with standard-dose doxorubicin in advanced or metastatic soft tissue sarcoma: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 2007;25(21):3144–3150. [DOI] [PubMed] [Google Scholar]
- 28.Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11(3):241–247. [DOI] [PubMed] [Google Scholar]
- 29.Charafe-Jauffret E, Ginestier C, Monville F, et al. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25(15):2273–2284. [DOI] [PubMed] [Google Scholar]
- 30.Chung CH, Bernard PS, Perou CM. Molecular portraits and the family tree of cancer. Nat Genet. 2002;32 Suppl:533–540. [DOI] [PubMed] [Google Scholar]
- 31.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riaz M, van Jaarsveld MT, Hollestelle A, et al. miRNA expression profiling of 51 human breast cancer cell lines reveals subtype and driver mutation-specific miRNAs. Breast Cancer Res. 2013;15(2):R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai X, Cheng H, Bai Z, Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017;8(16):3131–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esses KM, Hagmaier RM, Blanchard SA, Lazarchick JJ, Riker AI. Carcinosarcoma of the breast: two case reports and review of the literature. Cases J. 2009;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhati R, Patterson C, Livasy CA, et al. Molecular characterization of human breast tumor vascular cells. Am J Pathol. 2008;172(5):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosn M, El Rassy E, Kourie HR. Immunotherapies in sarcoma: Updates and future perspectives. World JClin Oncol. 2017;8(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cancer Research Institute. How is Immunotherapy Changing the Outlook for Patients with Sarcoma? 2016, May; https://www.cancerresearch.org/immunotherapy/cancer-types/sarcoma.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.