Abstract
Natural gas has become the dominant source of electricity in the United States, and technologies capable of efficiently removing CO2 from natural gas-fired power plants emissions could reduce their emission intensity. However, given the low partial pressure of CO2 in the flue stream, separation of CO2 is particularly challenging. Taking inspiration from the crystal structures of diamine-appended metal–organic frameworks exhibiting two-step cooperative CO2 adsorption, we report a family of robust tetraamine-functionalized frameworks that retain cooperativity, leading to the potential for exceptional efficiency in capturing CO2 under the extreme conditions relevant to natural gas flue emissions. The ordered, multimetal coordination of the tetraamines impart the materials with extraordinary stability to adsorption-desorption cycling with simulated humid flue gas and enable regeneration using low-temperature steam in lieu of costly pressure or temperature swings.
One Sentence Summary:
Cooperative CO2 adsorption under challenging conditions, together with steam regeneration, is achieved using tetraamine-functionalized metal–organic frameworks enabling novel CO2 capture processes.
Carbon dioxide (CO2) emissions from fossil fuel combustion and industrial processes account for as much as 65% of anthropogenic greenhouse gas emissions (1–3). Carbon capture and sequestration (CCS), wherein CO2 is separated from the flue emissions of large point sources and permanently sequestered underground, is widely recognized as an essential component of strategies for meeting the ambitious climate targets established at the Paris Climate Conference (4). The development of capture technology has largely focused on coal flue emissions (5), but worldwide use of natural gas is projected to exceed that of coal by ~2032, necessitating the rapid development of CCS technology for natural gas emissions (6). The U.S. Department of Energy (DoE) has set an ambitious target of 90% capture of the CO2 from natural gas flue streams (7), which is particularly challenging given that the CO2 concentration in natural gas combined cycle (NGCC) emissions is typically only ~4%, compared to ~12 to 15% for coal emissions (7, 8). Additionally, NGCC emissions contain high concentrations of O2 (12.4%) and water (8.4%), and thus effective technologies must be stable and maintain CO2 capture performance in the presence of these species (7, 9).
Aqueous amine solutions, the most mature carbon capture technology to date (10), are susceptible to oxidative and thermal degradation and have low CO2 cycling capacities (11). Porous solid adsorbents such as zeolites, silicas, and metal–organic frameworks are emerging as promising CO2 capture materials because of their high surface areas, lower intrinsic regeneration energies, higher stabilities, and tunable surface chemistries (12, 13). Taking inspiration from amine-functionalized silicas (14), we and others have shown that amine-functionalized metal–organic frameworks can capture CO2 in the presence of water (15, 16). A major consideration for adsorptive CO2 capture from natural gas flue emissions is that the low partial pressure of CO2 requires materials with high adsorption enthalpies. In turn, higher temperatures or lower pressures are needed to desorb captured CO2, and these regeneration conditions are both costly and can substantially impact performance (12). The use of steam for CO2 recovery has been proposed as a cost-effective strategy for amine-functionalized adsorbents (17–22), particularly because low-grade steam is inexpensive and readily available in most industrial processes. Unfortunately, engineering adsorbents with stability in the presence of steam is an ongoing issue (19, 23–25).
We recently reported the promising CO2 capture properties of the diamine-appended framework mmen–Mg2(dobpdc) (mmen = N,N′-dimethylethylenediamine, dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) (Fig. 1A) (26). In this material, CO2 adsorption proceeds through a cooperative mechanism in which the gas inserts into the Mg–N bonds to form chains of oxygen–bound carbamate species charge-balanced by neighboring ammonium groups running along the pores (27). This mechanism gives rise to unusual step-shaped CO2 adsorption profiles and improved material CO2 cycling capacities that can be achieved with smaller pressure or temperature swings than are required for traditional adsorbents (27). The CO2 capture properties can further be tuned by varying the appended diamine, leading to adsorbents with remarkable potential for CO2 capture from coal flue gas emissions (Fig. 1B) (28–31) and even NGCC emissions (32).
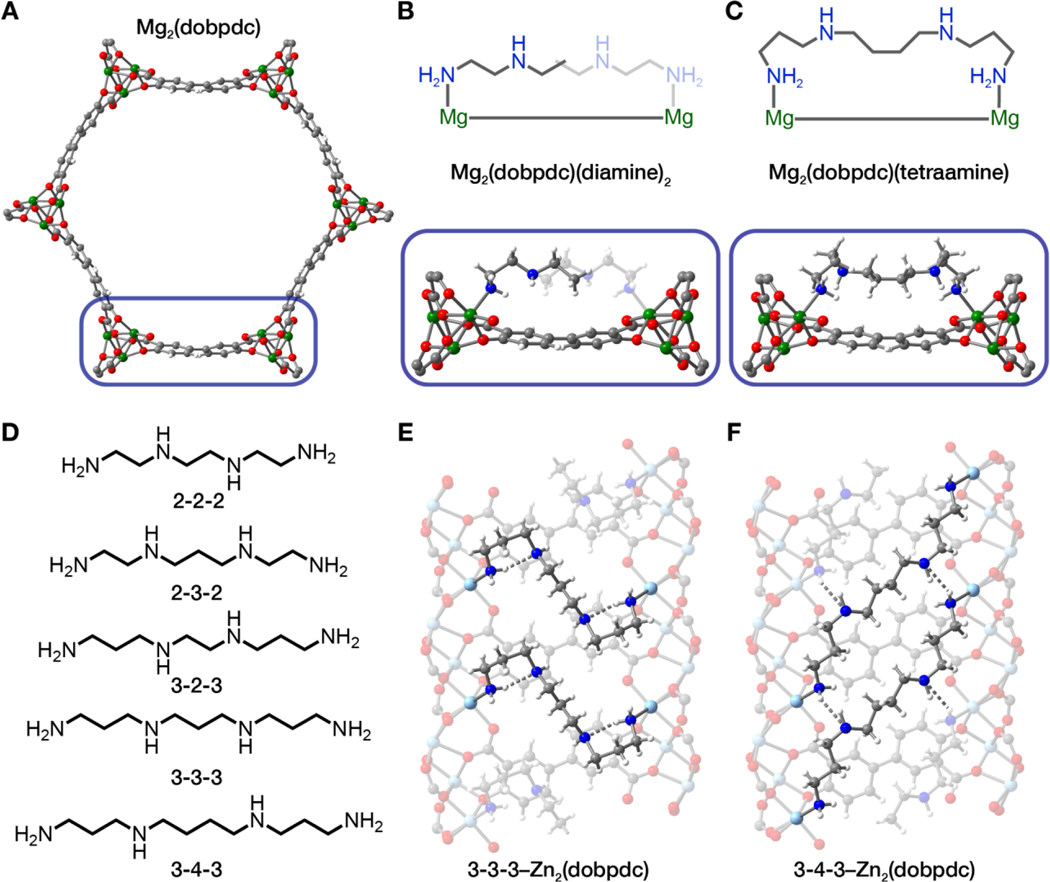
Fig. 1. Diamine versus tetraamine coordination in M2(dobpdc).
(A) Illustration of a hexagonal channel of Mg2(dobpdc) viewed in the ab-plane, using single-crystal X-ray diffraction data for the isostructural framework Zn2(dobpdc). (B) The diamine-functionalized material features coordination of one diamine to each Mg2+ site (28) whereas (C) tetraamines can coordinate to two Mg2+ sites. (D) Tetraamines explored in this work and their abbreviations. (E,F) Single-crystal x-ray diffraction structures (100 K) of isostructural Zn2(dobpdc) functionalized with 3–3-3 and 3–4-3 tetraamines, respectively. The tetraamines span metal centers across the pore that are 10.4637(11) Å apart (3–3-3) and 16.8312(19) Å apart (3–4-3). Green, light blue, gray, red, blue, and white spheres represent Mg, Zn, C, O, N, and H, respectively.
Nonetheless, these materials are susceptible to diamine volatilization upon regeneration, which has limited their applicability in a practical capture processes (30). We now report that tetraamine-functionalized Mg2(dobpdc) materials (Fig. 1C) exhibited high thermal stability and cooperatively captured CO2 at concentrations as low as parts per million (ppm), enabled by a two-step ammonium carbamate chain-formation mechanism. The ordered, multimetal coordination mode achieved with the tetraamines dramatically increased the stability of these adsorbents and enables CO2 desorption with inexpensive steam for the first time in amine-functionalized Mg2(dobpdc).
Synthesis and structure of tetraamine-functionalized Mg2(dobpdc)
Our pursuit of tetraamine-functionalized Mg2(dobpdc) was motivated by structural analysis of diamine-appended analogs, which indicated that tetraamines with chain lengths on the order of two N-alkylethylenediamines could bridge nearest neighbor metals across the pore (Fig. 1, B and C) (28). Such multiple metal coordination could yield adsorbents exhibiting cooperative CO2 capture and greatly enhanced thermal and hydrolytic amine stability. Soaking Mg2(dobpdc) with various tetraamines (Fig. 1D) in toluene (see the Supplementary Methods) produced loadings of ~1 tetraamine per Mg2+ site, or twice the desired 1:2 tetraamine:metal ratio (Fig. 1C), as determined by 1H nuclear magnetic resonance (NMR) spectra of acid-digested samples. The desired loading could be achieved through subsequent thermal activation (see the Supplementary Methods and tables S1 and S2 and figs. S3 and S4). Thermogravimetric analysis indicated that the tetraamine-grafted Mg2(dobpdc) materials were resistant to further diamine loss up to ~250 to 290°C (figs. S3 and S4). Here, we refer to the tetraamines using shorthand based on the number of carbon atoms in the alkyl groups bridging the amine moieties, for example N,N′-bis(3-aminopropyl)-1,3-diaminopropane and N,N′-bis(3-aminopropyl)-1,4-diaminobutane are referred to as 3–3-3 and 3–4-3, respectively (Fig. 1D).
Single-crystal x-ray diffraction (XRD) data obtained for 3–3-3 and 3–4-3-appended variants of the isostructural framework Zn2(dobpdc) revealed that the tetraamines coordinate in a highly ordered fashion (Fig. 1, E and F). Tetraamine 3–3-3 bound two metal centers separated by a distance of 10.4637(11) Å (Fig. 1E) whereas 3–4-3 bridges metal sites were separated by 16.8312(19) Å (Fig. 1F). Longer tetraamines could ostensibly also be accommodated in the framework and bridge metal atoms at even greater distances. In 3–3-3-functionalized Zn2(dobpdc), there was extensive intramolecular hydrogen bonding, whereas 3–4-3–Zn2(dobpdc) primarily exhibited intermolecular hydrogen bonding between adjacent tetraamines along the pore direction. These experimental structures confirmed the tetraamine coordination mode in M2(dobpdc) and accounted for the extremely high thermal stability of these materials (see below).
Adsorption properties and optimization for natural gas combined cycle post-combustion capture
Remarkably, all of the Mg2(dobpdc)(tetraamine) variants exhibited sharp step-shaped CO2 adsorption profiles consistent with cooperative adsorption and ammonium carbamate chain formation (Fig. 2 and figs. S14 to S16) (27, 28, 30, 32). In each case, the CO2 adsorption capacity at 30°C approached the theoretical capacity of two CO2 molecules per tetraamine. Interestingly, with the exception of Mg2(dobpdc)(3–3-3), the frameworks exhibited two-step adsorption profiles, with each step corresponding to half of the theoretical capacity. Analogous two-step adsorption profiles were previously observed in bulky diamine-appended variants of Mg2(dobpdc) and was attributed to steric conflict between neighboring ammonium carbamate chains across the pore (30). In the case of tetraamine-appended Mg2(dobpdc), we hypothesize that initial chemisorption of CO2 occurred at one amine end, generating ammonium carbamates running along one vertex of the hexagonal pore. The formation of the second set of ammonium carbamate chains likely required reorientation of the unreacted, bound amines.
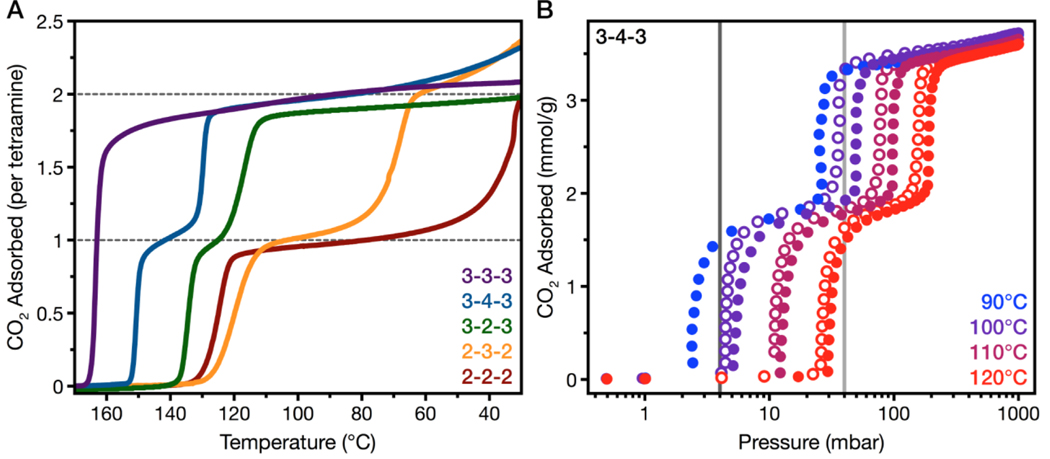
Fig. 2. CO2 uptake in tetraamine-appended Mg2(dobpdc).
(A) Adsorption isobars obtained through thermogravimetric analysis of Mg2(dobpdc)(tetraamine) under pure CO2 at atmospheric pressure. Dashed lines indicate the theoretical capacities for binding of one and two CO2 molecules per tetraamine. (B) Adsorption (filled circles) and desorption (open circles) isotherms for CO2 uptake in Mg2(dobpdc)(3–4-3) at 90°, 100°, 110°, and 120°C. The CO2 pressures in an untreated NGCC flue emission stream (40 mbar) and following 90% capture (4 mbar) are indicated by light gray and dark gray lines, respectively.
Consistent with this proposed mechanism, increasing tetraamine length coincides with a decrease in the material step separations up to 3–3-3, for which there is only a single adsorption step. Two-stepped adsorption returns in the case of slightly larger 3–4-3, which we attribute to this tetraamine likely bridging two metals at a longer distance than 3–3-3 and the other smaller tetraamines (Fig. 1E,F). Furthermore, when appended with triamines 3–3 (bis(3-aminopropyl)amine) and 3–4 (N-(3-aminopropyl)-1,4-diaminobutane), which can only form one set of ammonium carbamate chains, the Mg2(dobpdc) frameworks exhibited CO2 capacities that correspond to adsorption of one molecule of CO2 for every two Mg2+ sites (figs. S12 and S13). For practical applications, a single-step adsorption profile, or a two-step adsorption with closely spaced adsorption steps, is desirable to maximize the operating range over which the full material adsorption capacity can be accessed (32).
The temperature of the first adsorption step for Mg2(dobpdc)(3–3-3) and Mg2(dobpdc)(3–4-3) is 163° and 150°C, respectively. The temperatures are among the highest reported for any amine-appended variant of Mg2(dobpdc) (26, 28–30, 32). Traditional adsorbents with Langmuir-type adsorption profiles typically show optimal performance at the lowest possible adsorption temperature, where the adsorption capacity is maximized. The high adsorption step temperatures for the tetraamine-appended materials directly correlated with adsorption steps at low pressures sufficient to enable 90% CO2 capture from a NGCC flue stream (Fig. 2B and figs. S14 to S16) (7). For example, stepped CO2 adsorption isotherm for Mg2(dobpdc)(3–2-3) at 75°C indicated that the material should be able to reduce the CO2 content in a NGCC flue stream to 0.4% (fig. S16). Additionally, our preliminary results (figs. S28 and S39) indicate that these materials may also be suitable for direct capture of CO2 from air. While the majority of tetraamine-appended materials exhibit stable CO2 cycling capacities, Mg2(dobpdc)(3–3-3) shows a gradual decrease in CO2 cycling capacity over time (fig. S10), thus Mg(dobpdc)(3–4-3) was chosen for further study due to its practical step position and fundamentally interesting two-step adsorption behavior.
The adsorption data for Mg2(dobpdc)(3–4-3) suggested that in a post-combustion capture process from NGCC flue emissions, this framework could achieve a 90% CO2 capture rate, referring to the removal of CO2 to a residual concentration of 10% that of the feed (reduction from 40 mbar to 4 mbar at atmospheric pressure). Single-component isotherms collected between 90° and 120°C reveal that step-shaped CO2 capture at 4 mbar (corresponding to 90% capture) was retained up to 90°C under dry conditions (Fig. 2B). Using the Clausius-Clapeyron relationship, we calculated differential adsorption enthalpy (Δhads) and entropy (Δsads) values of 99 ± 3 kJ/mol and 223 ± 8 J/mol·K, respectively, at a loading of 1 mmol CO2/g (figs. S19 and S20). These values followed a correlation previously observed between Δhads and Δsads values determined for diamine-appended Mg2(dobpdc) (fig. S21) (28). The Δhads value for Mg2(dobpdc)(3–4-3) is among the highest reported for amine-functionalized materials. Such a high enthalpy of adsorption should enable CO2 capture at high temperatures and result in lower energy consumption in a temperature-swing adsorption process by minimizing the required temperature swing (33–37). Additionally, Mg2(dobpdc)(3–4-3) exhibited minimal adsorption-desorption hysteresis at atmospheric pressure (fig. S11).
Cooperative adsorption mechanism
We used in situ infrared (IR) and solid-state NMR spectroscopies to characterize Mg2(dobpdc)(3–4-3) before and after CO2 adsorption to investigate the two-step cooperative adsorption mechanism. In situ diffuse reflectance IR spectroscopy (DRIFTS) characterization of the framework in the presence of CO2 revealed a characteristic carbamate C–N stretch at 1339 cm−1 and a C–O stretch at 1689 cm−1 (see isotopic difference spectrum, Fig. 3A) (27–30). In addition, spectra collected simultaneously with a volumetric, equilibrium CO2 isotherm at 120°C indicated that the ammonium carbamate species was responsible for cooperative adsorption. Distinct features corresponding to hydrogen bonding between neighboring ammonium carbamate chains were observed in the N–H regions of the difference spectrum (3300 to 3000 and 3500 to 3400 cm−1) and distinct hydrogen bonding environments were characterized for each of the two adsorption steps (fig. S26).
Fig. 3. Spectroscopic investigation of CO2 adsorption in Mg2(dobpdc)(3–4-3).
(A) Raw in situ DRIFTS spectra of activated 3-4-3-Mg2(dobpdc) (grey curve), 3-4-3-Mg2(dobpdc) dosed with 400 mbar of 12CO2 and 13CO2 at 120°C (dark blue and yellow curves, respectively), and the isotopic difference spectrum (light blue curve). The 13CO2 spectrum was used as a baseline, isolating vibrations caused by inserted CO2. Vibrations corresponding to diagnostic carbamate bands are labeled. (B) Room-temperature 13C solid-state magic angle spinning NMR (16.4 T) of Mg2(dobpdc)(3-4-3) dosed with 1038 mbar of 13CO2. The resonance at 162.6 ppm was assigned as carbamate. (C) 1H⟶13C heteronuclear correlation (contact time 100 μs) spectrum and correlation assignments. (D) Predicted structures of Mg2(dobpdc)(3-4-3), Mg2(dobpdc)(3-4-3)(CO2), and Mg2(dobpdc)(3-4-3)(CO2)2 obtained from structural relaxations with vdW-corrected DFT. Green, grey, red, blue, and white spheres represent Mg, C, O, N, and H atoms, respectively.
Corroborating evidence for ammonium carbamate formation was obtained from magic angle spinning solid-state NMR experiments. A single carbamate resonance was observed at 162.6 parts per million in the 13C NMR spectrum of Mg2(dobpdc)(3–4-3) dosed with 1.04 bar of 13CO2 (Fig. 3B), which coincides with resonances reported previously for ammonium carbamate formed upon 13CO2 adsorption in diamine-appended Mg2(dobpdc) (38). The presence of a single carbamate feature suggested that all of the chemisorbed CO2 was in the same chemical environment after adsorption at 1 bar. We also observed five resonances for the tetraamine alkyl carbons, supporting a symmetric reaction between the tetraamine and CO2 at full capacity (Fig. 3B). Two-dimensional heteronuclear correlation data further revealed that CO2 reacted with the primary amine and that a secondary ammonium group formed nearby the carbamate (Fig. 3C) (38), and solid-state 15N NMR data confirmed the symmetric formation of ammonium and carbamate species (fig. S27). The NMR and IR results confirmed that our tetraamine-functionalized Mg2(dobpdc) variants operated through the envisaged two-step cooperative adsorption mechanism.
The structures of Mg2(dobpdc)(3–4-3) after adsorption of CO2 to half and full capacity were further investigated using van der Waals (vdW)-corrected density functional theory (DFT) calculations (Fig. 3D). Lattice parameters obtained from high-resolution powder XRD diffraction experiments under vacuum and after exposure to 1 bar CO2 were used as starting points for structural relaxations (fig. S29 and table S4). The computed binding energies (ΔEads) for the structures are within ±10 kJ/mol of the corresponding experimental Δhads values (table S7) (38). At full CO2 capacity (i.e., two molecules of CO2 per tetraamine), our calculations support two-step cooperative insertion of CO2, and the calculated NMR chemical shifts for this structure are in good agreement with the experimental values (table S8) (38). The computed ΔEads values indicate that the half capacity structure was more stable than the full capacity structure, which further supports our proposed two-step cooperative adsorption mechanism. In addition, the tetraamines in our optimized structure of activated Mg2(dobpdc)(3–4-3) bridge the same set of metals spanning ~16.7 Å in the ab-plane of the framework, consistent with the single-crystal structure of Zn2(dobpdc)(3–4-3). Comparing the activated and full CO2 capacity structures to synchrotron PXRD patterns by Rietveld analysis show reasonable fits (figs. S32 and S33 and table S9). Taken together, our results from XRD, NMR, IR, and DFT data indicate that tetraamine-functionalized frameworks indeed enhance cooperative CO2 adsorption relative to diamine-appended materials.
Carbon capture from flue gas with steam desorption
Because flue gas streams are saturated with water, any candidate carbon capture material must be able to adsorb CO2 under humid conditions. When exposed to CO2 streams with ~2.6% H2O (see Supplementary Methods) (32), Mg2(dobpdc)(3–4-3) again exhibited step-shaped isobars, with steps shifted to higher temperatures relative to those under dry CO2 (fig. S40) as has been reported previously for diamine-appended Mg2(dobpdc) (29, 30, 32). Notably, this temperature shift indicated that water enhanced CO2 binding in the material and should promote CO2 capture from a flue gas stream (29, 30, 32). Furthermore, essentially no changes in the CO2 adsorption profile and capacity were observed after the material was held under flowing air at 100°C for 12 h, indicating the stability of the framework to oxygen in the flue gas stream (fig. S43).
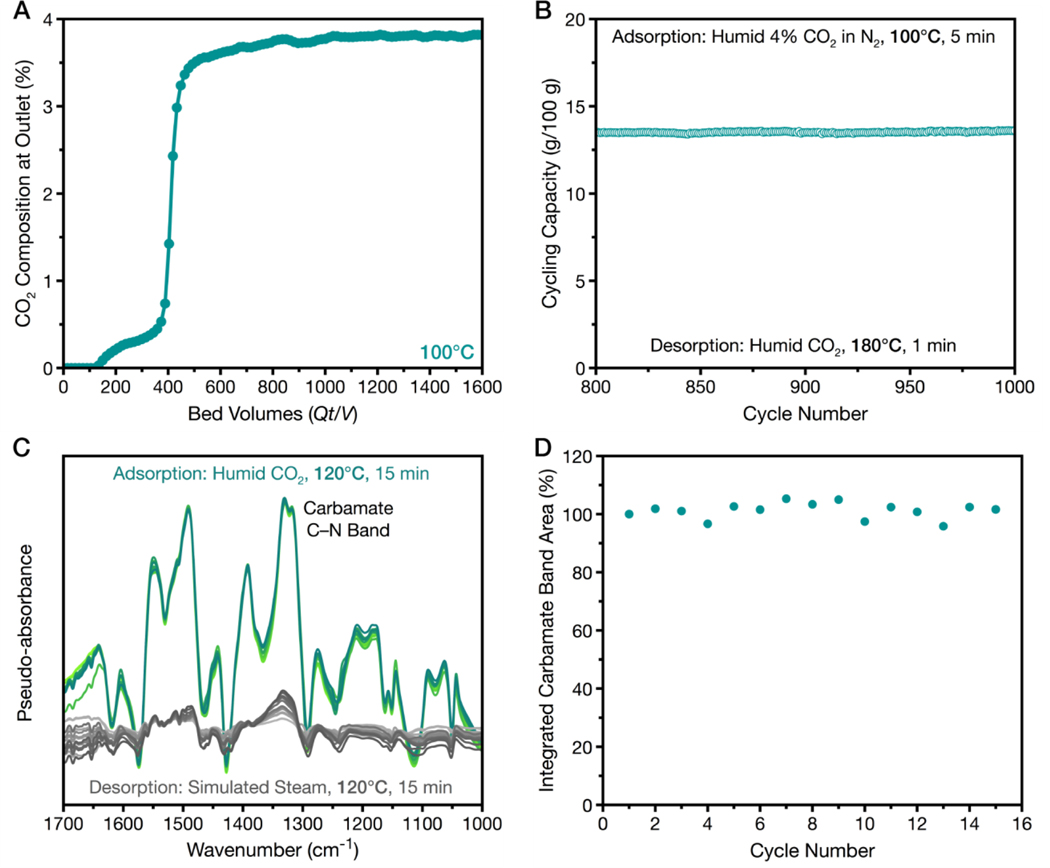
We also performed larger scale breakthrough experiments at 100°C under the same humid conditions to simulate a real fixed-bed adsorption process. Under these conditions, Mg2(dobpdc)(3–4-3) exhibited a high CO2 capture rate of 90% with a breakthrough capacity of 2.0±0.2 mmol/g (Fig. 4A and figs. S45 to S48). It also exhibited minimal tetraamine volatilization during the course of 1000 CO2 adsorption-desorption cycles performed under humid conditions using a thermogravimetric analyzer, and the material remains crystalline with a stable cycling capacity of 13.6 g/100 g (Fig. 4B and fig. S50 and S51). Thus, Mg2(dobpdc)(3–4-3) could cycle a large quantity of CO2 while meeting the U.S. DoE target for capturing 90% of the CO2 from natural gas flue emissions (7).
Fig. 4. CO2 adsorption in Mg2(dobpdc)(3-4-3) in the presence of water.
(A) CO2 breakthrough profile for Mg2(dobpdc)(3-4-3) under simulated humid (~2.6% H2O) natural gas flue gas (4% CO2 in N2) at 100°C and atmospheric pressure. (B) Extended temperature-swing cycling of Mg2(dobpdc)(3-4-3) carried out using a thermogravimetric analyzer at atmospheric pressure. Adsorption conditions: humid (~2.6% H2O) 4% CO2 in N2 for 5 min at 100°C; desorption conditions: humid (~2.6% H2O) CO2 stream at 180°C for 1 min. (C) Infrared spectra showing 15 cycles of adsorption under humid CO2 (bright green spectra) and desorption under simulated steam (light grey spectra, estimated 51 to 65% water content, balance N2). Increasingly darker colored curves indicate progressive cycles. Spectra of the activated framework under flowing steam/N2 were used as a baseline. (D) Plot of the integrated area for the carbamate band in C (~1290-1360 cm−1) versus adsorption cycle number, illustrating stable formation of the carbamate species across 15 cycles.
The high breakthrough capacity and exceptional stability of Mg2(dobpdc)(3–4-3) motivated us to explore the use of steam in place of heating at 180°C to desorb CO2 from the material. Low-grade steam could offer advantages over both temperature swing and pressure swing adsorption processes for post-combustion CO2 capture (17–22). The viability of using steam for CO2 desorption was tested in a custom-built flow-through cell for in situ DRIFTS equipped to cycle between flowing humid CO2 and simulated steam (estimated 51 to 65% water content, balance N2, see table S10) at 120°C and ~1 atm (Fig. 4C,D). The IR spectra collected for Mg2(dobpdc)(3–4-3) over the course of 15 cycles showed repeated growth and disappearance of both the ammonium (2800–1800 cm−1) and C–N (1339 cm−1) bands associated with carbamate chain formation, indicating the stability of the cooperative insertion mechanism to steam regeneration conditions. Postcycling isobaric analysis of the material revealed that step-shaped adsorption was retained to a high capacity (~16.6 g/100, see figs. S52 to S55 and table S11), and 1H NMR analysis revealed that the tetraamine loading remained unchanged (table S12). In stark contrast, the representative diamine-appended material Mg2(dobpdc)(e-2)2 (e-2 = N-ethylethylenediamine) (28, 30) exhibited substantial amine volatilization after only a single steam desorption cycle (table S13 and fig. S56). The impressive performance of Mg2(dobpdc)(3–4-3) underscores the stabilization afforded by the multimetal coordination modes.
Outlook
We have developed a class of tetraamine-functionalized metal–organic frameworks that exhibit cooperative CO2 adsorption and greatly enhanced stability compared to previous diamine-functionalized materials as a result of multiple, ordered metal–amine interactions. Critically, the nature of amine coordination in these materials gives rise to two-step adsorption of CO2 and large adsorption enthalpies suitable for CO2 capture from simulated natural gas flue streams. The top-performing material, Mg2(dobpdc)(3–4-3), achieved a large CO2 adsorption capacity of 2.0 ± 0.2 mmol/g in the presence of water while meeting the DoE target for 90% CO2 capture from natural gas flue emissions. Most strikingly, however, the enhanced stability of these tetraamine-functionalized frameworks enables regeneration using direct steam contact, a pathway that could afford significant energy savings compared to traditional processes. Overall, the molecular-level design strategy implemented here represents a step-change in the industrial viability of adsorption-based CO2 capture from natural gas flue emissions, a separation of growing importance in the global energy landscape.
Supplementary Material
Acknowledgements:
We thank the Philomathia Foundation and Berkeley Energy and Climate Institute for support of A.C.F. through a postdoctoral fellowship. J.-H.L.’s work was supported by the KIST Institutional Program (Project No. 2E29910). We thank the Miller Institute for Basic Research in Science for postdoctoral fellowship support of J.D.M. We thank the National Institute of General Medical Science of the National Institutes of Health for a postdoctoral fellowship for P.J.M. (F32GM120799). We thank Halle N. Redfearn for carrying out initial NMR measurements on tetraamine-functionalized metal–organic frameworks, Dr. Hiroyasu Furukawa and Dr. Julia Oktawiec for helpful discussions, and Dr. Katie R. Meihaus for editorial assistance.
Funding: We acknowledge ExxonMobil Research and Engineering Company for financial support of this work. The crystallographic studies of Zn2(dobpdc) were carried out at the Advanced Light Source at Lawrence Berkeley National Laboratory, a user facility supported by the Director, Office of Science, Office of Basic Energy Sciences, of the DoE under Contract No. DE-AC02-05CH11231. Synchrotron powder X-ray diffraction data were collected on the 17-BM-B Beamline at the Advanced Photon Source, a U.S. Department of Energy Office of Science User Facility operated by Argonne National Laboratory. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract DE-AC02-05CH11231. Additional computational resources were provided by the Department of Energy (NERSC). This research also used the Savio computational cluster resource provided by the Berkeley Research Computing program at the University of California, Berkeley.
Competing interests: The authors declare the following competing financial interest(s): J.R.L. has a financial interest in and serves on the board of directors of Mosaic Materials, Inc., a start-up company working to commercialize metal–organic frameworks for gas separations. S.C.W., J.M.F., J.R.L., E.J.K., R.L.S., J.D.M., and P.J.M. are inventors on patent application US16/175,708 (International: PCT/US2018/058287) held/submitted by the University of California, Berkeley and ExxonMobil Research and Engineering Co. that covers polyamine-appended metal–organic frameworks for carbon dioxide separations.
Footnotes
Disclaimers: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data and materials availability: The supplementary materials contain complete experimental and spectral details for all new compounds reported herein. Crystallographic data will be made available free of charge from the Cambridge Crystallographic Data Centre under reference numbers CCDC 1996313 and 1996314.
References and Notes:
- 1.IPCC, “Climate Change 2013 - The physical science basis” (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013). [Google Scholar]
- 2.IPCC, “Climate change 2014: mitigation of climate change” (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2014). [Google Scholar]
- 3.International Energy Agency, “CO2 emissions from fuel combustion highlights” (2016), pp. 1–166. [Google Scholar]
- 4.Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Brown S, Fennel PS, Fuss S, Galindo A, Hackett LA, Hallett JP, Herzog HJ, Jackson G, Kemper J, Krevor S, Maitland GC, Matuszewski M, Metcalf IS, Petit C, Puxty G, Reimer J, Reiner DM, Rubin ES, Scott SA, Shah N, Smit B, Trusler JPM, Webley P, Wilcox J, Dowell NM, Carbon capture and storage (CCS): the way forward. Energy Environ. Sci. 11, 1062–1176 (2018). [Google Scholar]
- 5.Siegelman RL, Milner PJ, Kim E, Weston SC, Long JR, Challenges and opportunities for adsorption-based CO2 capture from natural gas combined cycle emissions. Energy Environ. Sci. 26, 2161–2173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Energy Information Administration, “International energy outlook 2019” (2019), pp. 1–85. [Google Scholar]
- 7.U.S. Department of Energy, National Energy Technology Laboratory, “Cost and performance baseline for fossil energy plants. Volume 1a: bituminous coal (PC) and natural gas to electricity. Revision 3” (2015). [Google Scholar]
- 8.Rubin ES, Zhai H, The cost of carbon capture and storage for natural gas combined cycle power plants. Environ. Sci. Technol. 46, 3076–3084 (2012). [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Energy, “Carbon capture opportunities for natural gas fired power systems” (2017), pp. 1–5. [Google Scholar]
- 10.Rochelle GT, Amine scrubbing for CO2 capture. Science. 325, 1652–1654 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Mazari SA, Si Ali B, Jan BM, Saeed IM, Nizamuddin S, An overview of solvent management and emissions of amine-based CO2 capture technology. Int J Greenh Gas Con. 34, 129–140 (2015). [Google Scholar]
- 12.Drage TC, Snape CE, Stevens LA, Wood J, Wang J, Cooper AI, Dawson R, Guo X, Satterley C, Irons R, Materials challenges for the development of solid sorbents for post-combustion carbon capture. J. Mater. Chem. 22, 2815–2823 (2012). [Google Scholar]
- 13.Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM, The chemistry and applications of metal–organic frameworks. Science. 341, 1230444 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Bollini P, Didas SA, Jones CW, Amine-oxide hybrid materials for acid gas separations. J. Mater. Chem. 21, 15100–15120 (2011). [Google Scholar]
- 15.Mason JA, McDonald TM, Bae T-H, Bachman JE, Sumida K, Dutton JJ, Kaye SS, Long JR, Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 137, 4787–4803 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Kong C, Chen L, Amine-functionalized metal–organic frameworks: structure, synthesis and applications. RSC Adv. 6, 32598–32614 (2016). [Google Scholar]
- 17.Chaikittisilp W, Kim H-J, Jones CW, Mesoporous alumina-supported amines as potential steam-stable adsorbents for capturing CO2 from simulated flue gas and ambient air. Energy Fuels. 25, 5528–5537 (2011). [Google Scholar]
- 18.Numaguchi R, Fujiki J, Yamada H, Chowdhury FA, Kida K, Goto K, Okumura T, Yoshizawa K, Yogo K, Development of post-combustion CO2 capture system using amine-impregnated solid sorbent. Energy Procedia. 114, 2304–2312 (2017). [Google Scholar]
- 19.Li W, Choi S, Drese JH, Hornbostel M, Krishnan G, Eisenberger PM, Jones CW, Steam-stripping for regeneration of supported amine-based CO2 adsorbents. ChemSusChem. 3, 899–903 (2010). [DOI] [PubMed] [Google Scholar]
- 20.Sanz-Pérez ES, Murdock CR, Didas SA, Jones CW, Direct capture of CO2 from ambient air. Chem. Rev. 116, 11840–11876 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni AR, Sholl DS, Analysis of equilibrium-based TSA processes for direct capture of CO2 from air. Ind. Eng. Chem. Res. 51, 8631–8645 (2012). [Google Scholar]
- 22.Kim C, Cho HS, Chang S, Cho SJ, Choi M, An ethylenediamine-grafted Y zeolite: a highly regenerable carbon dioxide adsorbent via temperature swing adsorption without urea formation. Energy Environ. Sci, 9, 1803–1811 (2015). [Google Scholar]
- 23.Jahandar Lashaki M, Khiavi S, Sayari A, Stability of amine-functionalized CO2 adsorbents: a multifaceted puzzle. Chem. Soc. Rev. 119, 3962–86 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Andirova D, Lei Y, Zhao X, Choi S, Functionalization of metal–organic frameworks for enhanced stability under humid carbon dioxide capture conditions. ChemSusChem. 8, 3405–3409 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Burtch NC, Jasuja H, Walton KS, Water stability and adsorption in metal–organic frameworks. Chem. Rev. 114, 10575–10612 (2014). [DOI] [PubMed] [Google Scholar]
- 26.McDonald TM, Lee WR, Mason JA, Wiers BM, Hong CS, Long JR, Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal–organic framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 134, 7056–7065 (2012). [DOI] [PubMed] [Google Scholar]
- 27.McDonald TM, Mason JA, Kong X, Bloch ED, Gygi D, Dani A, Crocellà V, Giordanino F, Odoh SO, Drisdell WS, Vlaisavljevich B, Dzubak AL, Poloni R, Schnell SK, Planas N, Lee K, Pascal T, Wan LF, Prendergast D, Neaton JB, Smit B, Kortright JB, Gagliardi L, Bordiga S, Reimer JA, Long JR, Cooperative insertion of CO2 in diamine-appended metal–organic frameworks. Nature. 519, 303–308 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Siegelman RL, McDonald TN, Gonzalez MI, Martell JD, Milner PJ, Mason JA, Berger AH, Bhown AS, Long JR, Controlling cooperative CO2 adsorption in diamine-appended Mg2(dobpdc) metal–organic frameworks. J. Am. Chem. Soc. 139, 10526–10538 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milner PJ, Siegelman RL, Forse AC, Gonzalez MI, Runčevski T, Martell JD, Reimer JA, Long JR, A diaminopropane-appended metal–organic framework enabling efficient CO2 capture from coal flue gas via a mixed adsorption mechanism. J. Am. Chem. Soc. 139, 13541–13553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner PJ, Martell JD, Siegelman RL, Gygi D, Weston SC, Long JR, Overcoming double-step CO2 adsorption and minimizing water co-adsorption in bulky diamine-appended variants of Mg2(dobpdc). Chem. Sci. 9, 160–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choe JH, Kang DW, Kang M, Kim H, Park JR, Kim DW, Hong CS, Revealing an unusual temperature-dependent CO2 adsorption trend and selective CO2 uptake over water vapors in a polyamine-appended metal–organic framework. Mater. Chem. Front. 48, 2783 (2019). [Google Scholar]
- 32.Siegelman RL, Milner PJ, Forse AC, Lee J-H, Colwell KA, Neaton JB, Reimer JA, Weston SC, Long JR, Water enables efficient CO2 capture from natural gas flue emissions in an oxidation-resistant diamine-appended metal–organic framework. J. Am. Chem. Soc. 141, 13171–13186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joss L, Gazzani M, Hefti M, Marx D, Mazzotti M, Temperature swing adsorption for the recovery of the heavy component: an equilibrium-based shortcut model. Ind. Eng. Chem. Res. 54, 3027–3038 (2015). [Google Scholar]
- 34.Joos L, Lejaeghere K, Huck JM, Van Speybroeck V, Smit B, Carbon capture turned upside down: high-temperature adsorption & low-temperature desorption (HALD). Energy Environ. Sci. 8, 2480–2491 (2015). [Google Scholar]
- 35.Yang M-W, Chen N-C, Huang C-H, Shen Y-T, Yang H-S, Chou C-T, Temperature swing adsorption process for CO2 capture using polyaniline solid sorbent. Energy Procedia. 63, 2351–2358 (2014). [Google Scholar]
- 36.Lee A, Xiao G, Xiao P, Joshi K, Singh R, Webley PA, High temperature adsorption materials and their performance for pre-combustion capture of carbon dioxide. Energy Procedia. 4, 1199–1206 (2011). [Google Scholar]
- 37.Wang XP, Yu JJ, Cheng J, Hao ZP, Xu ZP, High-temperature adsorption of carbon dioxide on mixed oxides derived from hydrotalcite-like compounds. Environ. Sci. Technol. 42, 614–618 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Forse AC, Milner PJ, Lee J-H, Redfearn HN, Oktawiec J, Siegelman RL, Martell JD, Dinakar B, Porter-Zasada LB, Gonzalez MI, Neaton JB, Long JR, Reimer JA, Elucidating CO2 Chemisorption in diamine-appended metal–organic Frameworks. J. Am. Chem. Soc. 140, 18016–18031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruker Analytical X-ray Systems Inc., SAINT, APEX2, and APEX3 Software for CCD Diffractometers. [Google Scholar]
- 40.Sheldrick GM, SADABS; University of Göttingen: Göttingen, Germany: (1996). [Google Scholar]
- 41.Sheldrick GM, SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. A. 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheldrick GM, Crystal structure refinement with SHELXL. Acta Cryst. C. 71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H, OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009). [Google Scholar]
- 44.Øien-Ødegaard S, Shearer GC, Wragg DS, Lillerud KP, Pitfalls in metal–organic framework crystallography: towards more accurate crystal structures. Chem. Soc. Rev. 50, 4867–4876 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Bürgi H-B, Alshmimri SA, Yaghi OM, Impact of disordered guest–framework interactions on the crystallography of metal–organic frameworks. J. Am. Chem. Soc. 140, 8958–8964 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Coelho A, Topas Academic v4.1 (2007). [Google Scholar]
- 47.March A. Mathematische Theorie Der Regelung Nach Der Korngestah Bei Affiner Deformation. Z. Kristallogr. Cryst. Mater. 81, 285–297 (1932). [Google Scholar]
- 48.Dollase WA. Correction of Intensities for Preferred Orientation in Powder Diffractometry: Application of the March Model. J. Appl. Crystallogr. 19, 267–272 (1986). [Google Scholar]
- 49.Blöchl PE, Projector augmented-wave method. Phys. Rev. B. 50, 17953 (1994). [DOI] [PubMed] [Google Scholar]
- 50.Kresse G, Joubert D, From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 59, 1758–1775 (1999). [Google Scholar]
- 51.Kresse G, Furthmüller J, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 52.Kresse G, Furthmüller J, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Kresse G, Hafner J, Ab initio molecular-dynamics simulation of the liquid-metal--amorphous-semiconductor transition in germanium. Phys. Rev. B. 49, 14251–14269 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Lee K, Murray ÉD, Kong L, Lundqvist BI, Langreth DC, Higher-accuracy van der Waals density functional. Phys. Rev. B. 82, 081101 (2010). [Google Scholar]
- 55.Elsässer C, Fähnle M, Chan CT, Ho KM, Density-functional energies and forces with Gaussian-broadened fractional occupations. Phys. Rev. B. 49, 13975–13978 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Campbell CT, Sellers JRV, Enthalpies and entropies of adsorption on well-defined oxide surfaces: experimental measurements. Chem. Rev. 113, 4106–4135 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Helfferich FG, Carr PW, Non-Linear Waves in Chromatography. J. Chromatogr. A. 629, 97–122 (1993). [Google Scholar]
- 58.Zhang W, Shan Y, Seidel-Morgenstern A, Breakthrough Curves and Elution Profiles of Single Solutes in Case of Adsorption Isotherms with Two Inflection Points. J. Chromatogr. A. 1107, 216–225 (2006). [DOI] [PubMed] [Google Scholar]
- 59.Mazzotti M, Rajendran A, Equilibrium Theory-Based Analysis of Nonlinear Waves in Separation Processes. Annu. Rev. Chem. Biomol. Eng. 4, 119–141 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Hefti M, Joss L, Bjelobrk Z, Mazzotti M, On the Potential of Phase-Change Adsorbents for CO2 Capture by Temperature Swing Adsorption. Faraday Discuss. 192, 153–179 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Krug RR, Hunter WG, Grieger RA, Statistical interpretation of enthalpy–entropy compensation. Nature. 261, 566–567 (1976). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.