Abstract
Objectives:
Inpatient alcohol withdrawal syndrome (AWS) is common and early treatment improves outcomes, but no prior study has used electronic health record (EHR) data, available at admission, to predict the probability of inpatient AWS. This study estimated the probability of inpatient AWS using prior-year EHR data, hypothesizing that documented alcohol use disorder (AUD) and AWS would be strongly associated with inpatient AWS while exploring associations with other patient characteristics.
Methods:
The study investigated patients hospitalized ≥24 hours on medical services in the Veterans Health Administration during 2013 using EHR data extracted from the Veterans Health Administration Corporate Data Warehouse. ICD-9-CM diagnosis code, demographic, and healthcare utilization data documented in the year before admission defined prior-year AUD, AWS, and other factors associated with inpatient AWS. The primary outcome, inpatient AWS, was defined by inpatient ICD-9-CM codes.
Results:
The unadjusted probability of AWS was 5.0% (95% CI 4.5%–5.4%) among 209,151 medical inpatients overall, 26.4% (95% CI 24.4%–28.4%) among those with prior-year AUD, and 62.5% (95% CI 35.2%–39.7%) among those with prior-year AWS. Of those with AWS, 86% had documented prior-year AUD and/or AWS. Other patient characteristics associated with increased probability of inpatient AWS (P < 0.001) were: male sex, single relationship status, homelessness, seizure, and cirrhosis.
Conclusions:
Although inpatient providers often use history to predict AWS, this is the first study in hospitalized patients to inform and validate this practice, showing that prior-year diagnosis of AUD and/or AWS in particular, can identify the majority of inpatients who should be monitored for AWS.
Keywords: alcohol withdrawal syndrome, electronic health record, general hospital, predictor, risk factor
Unhealthy alcohol use is the third-leading cause of preventable death in the United States and the number 1 killer of people aged 15 to 49 worldwide.1 The burden of alcohol use disorder (AUD) is especially concentrated in general hospital settings, where 15% to 20% of patients have an alcohol-related condition.2 Among hospitalized patients, one of the most common and potentially dangerous manifestations of AUD is alcohol withdrawal syndrome (AWS).3–6 AWS causes brain damage in patients through glutamate-mediated excitotoxicity,7 complicates and prolongs inpatient care,4,5,8 and can be fatal without proactive diagnosis and management.9 Experts, therefore, 1recommend early identification of patients at risk for AWS but there is little consensus on the optimal approach for risk-stratifying hospitalized patients.10
The only validated screening tool for identifying AWS in hospitalized patients includes 10 items and relies heavily on self-reported data from patients.11,12 Patients who are too sick or unable to communicate (mechanically ventilated, non-English-speaking, etc) cannot be evaluated reliably and the clinical workload associated with this approach may be a barrier to its use. Alternatively, historical data captured in the electronic health record (EHR) could potentially be used to identify patients at increased risk for AWS on admission, improving early identification of AWS while reducing the clinical burden placed on patients and providers. Patients with a documented history of AUD and/or AWS are generally considered high-risk for developing inpatient AWS,11 but the strength and magnitude of these associations have not been described in hospitalized patients. In addition, the value of other electronically documented, readily extractable demographic and clinical data for predicting the probability of inpatient AWS is unknown.
This study used data documented in the EHR in the year before hospital admission to estimate the probability of inpatient AWS. Specifically, the probability of inpatient AWS was estimated based on prior-year diagnosis and procedure codes indicating (1) AUD, (2) AWS, and (3) AUD and/or AWS. Changes in the predicted probability of inpatient AWS were explored across patient demographic and other health characteristics associated with AUD. The a priori hypothesis was that prior-year AUD and AWS diagnoses would each be strong independent predictors of inpatient AWS.
METHODS
Study Data and Sample
This retrospective cohort study used EHR data of veterans engaged in Veterans Health Administration (VHA) care and hospitalized during fiscal year 2013 (FY2013—October 1, 2012 to September 30, 2013).13 All patients admitted to medical services for at least 24 hours at facilities with acute and intensive care capabilities were included. Among patients with multiple eligible admissions, one hospitalization per patient was randomly selected (‘‘index’’ hospitalization hereafter), prioritizing admissions complicated by AWS (eFigure 1, http://links.lww.com/JAM/A234, Supplemental Digital Content showing patient selection flowchart). Demographic data, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes, dates of outpatient visits and hospitalizations, treating specialties, and facility data were obtained from the Veterans Affairs (VA) Corporate Data Warehouse, a national repository of clinical data generated by the VHA healthcare system. The study received approval and waivers of informed consent and HIPAA authorization from the VA Puget Sound Health Care System and University of Washington Institutional Review Boards.
Inpatient AWS
Inpatient AWS was defined by primary or secondary ICD-9-CM diagnosis and/or procedure codes indicating recognition and/or treatment of AWS during the index hospitalization in FY2013 (eBox 1, http://links.lww.com/JAM/A234, Supplemental Digital Content showing specific ICD-9-CM codes).14
Prior-Year History of AUD and/or AWS
Prior-year AUD and AWS were each defined using inpatient and outpatient ICD-9-CM diagnosis and/or procedure codes documented in the 365 days before the date of index admission (eBox 1, http://links.lww.com/JAM/A234, Supplemental Digital Content showing specific ICD-9-CM codes).
Other Demographic and Health Characteristics Potentially Associated With AWS
Demographic, diagnostic, and healthcare utilization measures were obtained from the EHR in the 365 days before the index hospitalization. Prior-year medical, substance use, and mental health diagnoses associated with AUD were identified using ICD-9-CM diagnosis codes (available upon request). Health services utilization was determined using outpatient clinic codes and inpatient bed codes, grouped into categories of outpatient [emergency department (ED), primary care, specialty medical/surgical care, or mental health/substance use care] or inpatient (medical admission, surgical admission, or mental health/substance use admission) health services.
Analyses
Descriptive analyses characterized the study sample of medical inpatients admitted to VHA hospitals in FY2013. The predicted probability of inpatient AWS based on prior-year AUD, AWS, and AUD and/or AWS was estimated using logistic regression. The a priori hypothesis that prior-year AUD and AWS diagnoses would each be strong independent predictors of inpatient AWS was tested using both unadjusted and adjusted mixed-effects logistic regression models with hospital facility as a random effect to account for covariance at the hospital level. Unadjusted models are presented for their greater applicability to real-world clinical practice (in the absence of EHR algorithms that adjust for other patient characteristics). Adjusted models included patient demographic, diagnosis, and healthcare utilization covariates. In practice, AUD and AWS are part of the same pathological spectrum of disease; therefore, models also estimated probability of inpatient AWS based on a composite measure of prior-year AUD and/or AWS.
Secondary analyses evaluated the adjusted probability of inpatient AWS across demographic and other health characteristics, stratified by prior-year AUD and/or AWS. Two mixed-effects multivariable logistic regression models were used—one for patients with prior-year AUD and/or AWS and one for patients without. The variables in the 2 models were otherwise identical, including all demographic, diagnosis, and healthcare utilization covariates hypothesized to influence the risk of inpatient AWS for purposes of exploring independent associations.
For all analyses, odds ratios, predicted probabilities, and marginal effects were estimated using the ‘‘melogit’’ and ‘‘margins’’ commands in Stata, v16.0 (StataCorp LP, College Station, TX). Predicted probabilities and marginal effects were calculated as average adjusted predictions derived from the models using the observed values of covariates (as opposed to mean values).15 Due to the large sample size and exploratory analyses of multiple characteristics potentially associated with AWS, α was set to 0.001 a priori to reduce the probability of Type I error.
RESULTS
In this sample of 209,151 patients hospitalized on medical services in the VHA, 32,028 (15.3%) had a documented AUD and 6905 (3.3%) had documented inpatient or outpatient AWS in the year before admission (Table 1). The sample was on average, 68 years old, predominantly male (95.8%), majority White (67.2%), and many had pain-related conditions (73.1%), diabetes (41.1%), chronic obstructive pulmonary disease (30.3%), and depression (27.0%) in the year before admission. A majority (62.7%) received care in an ED and 39.3% were hospitalized on a medical service in the year before admission. Compared to patients without prior-year AUD and/or AWS, patients with prior-year AUD and/or AWS were on average, 9 years younger, had greater evidence of social isolation (ie, single and homeless), higher frequency of medical diagnoses associated with alcohol use (ie, seizures, gastrointestinal disorders, cirrhosis, and trauma), lower frequency of other medical diagnoses (ie, malignancy, diabetes, chronic kidney disease, heart failure, pneumonia, cerebrovascular disease, and dementia), greater burden of mental illness, and more frequent use of ED, mental health, and medical inpatient services (eTable1, http://links.lww.com/JAM/A234, Supplemental Digital Content showing characteristics of inpatients by prior-year history of AUD and/or AWS).
TABLE 1.
Characteristics of Medical Inpatients in the Veterans Health Administration in Fiscal Year 2013
| Medical Inpatients (n = 209,151) | |
|---|---|
| Age yrs, mean (sd) | 67.9 (12.8) |
| Male, no. (%) | 200,369 (95.8) |
| Race, no. (%) | |
| White | 140,572 (67.2) |
| Black | 42,232 (20.2) |
| Hispanic/Latino | 10,874 (5.2) |
| Other Race/Ethnicity | 5699 (2.7) |
| Unknown | 9774 (4.7) |
| Single, no. (%) | 120,088 (57.4) |
| Homeless, no. (%) | 15,466 (7.4) |
| Prior-year medical diagnoses, no. (%) | |
| Pain-related | 152,966 (73.1) |
| Seizure | 10,713 (5.1) |
| Cerebrovascular disease | 32,608 (15.6) |
| Dementia | 21,241 (10.2) |
| Heart failure | 47,893 (22.9) |
| Chronic obstructive pulmonary disease | 63,429 (30.3) |
| Gastrointestinal disorder | 18,798 (9.0) |
| Cirrhosis | 11,348 (5.4) |
| Chronic kidney disease | 40,062 (19.2) |
| Acute kidney injury | 28,303 (13.5) |
| Diabetes | 85,953 (41.1) |
| Malignancy | 43,578 (20.8) |
| Trauma | 47,390 (22.7) |
| Pneumonia | 30,044 (14.4) |
| Sepsis/shock | 26,053 (12.5) |
| Prior-year AUD, no. (%) | 32,028 (15.3) |
| Prior-year AWS, no (%) | 6905 (3.3) |
| Prior-year other substance use disorder*, no. (%) | 20,292 (9.7) |
| Prior-year mental health diagnoses, no. (%) | |
| Anxiety | 27,054 (12.9) |
| Depression | 56,545 (27.0) |
| Post-traumatic stress disorder | 30,144 (14.4) |
| Bipolar disorder | 8392 (4.0) |
| Psychotic disorder | 13,872 (6.6) |
| Prior-year outpatient care, no. (%) | |
| Emergency Department care | 122,776 (62.7) |
| Primary care | 184,306 (93.0) |
| Specialty Medical/Surgical care | 175,938 (88.9) |
| Mental Health/Substance use care | 72,354 (37.5) |
| Prior-year inpatient care, no. (%) | |
| Medical admission | 82,128 (39.3) |
| Surgical admission | 16,491 (7.9) |
| Mental health/Substance use admission | 7780 (3.7) |
AUD indicates alcohol use disorder; AWS, alcohol withdrawal syndrome.
Includes only drug use disorders.
Table 2 shows the unadjusted and adjusted probabilities of inpatient AWS, overall and for those with prior-year AUD, AWS, and AUD and/or AWS. The overall probability of inpatient AWS was 5.0% (unadjusted 95% CI 4.5%–5.5%; adjusted 95% CI 4.5%–5.4%). In unadjusted analyses, the probability of AWS was 26.4% (95% CI 24.4%–28.4%) among patients with prior-year AUD, 62.5% (95% CI 35.2%–39.7%) among patients with prior-year AWS, and 26.7.% (95% CI 16.4%–19.3%) among patients with prior-year AUD and/or AWS. The adjusted probability estimates for inpatient AWS based on prior-year AUD, AWS, and AUD and/or AWS were more attenuated: 17.5% (95% CI 16.1%–18.8%), 37.5% (95% CI 35.2%–39.7%), and 17.9% (95% CI 16.4%–19.3%), respectively. Overall, 86% of patients (n = 8322 of 9727) with AWS during their index hospitalization had prior-year AUD and/or AWS, whereas 88% of patients (n = 175,420 of 199,424) without AWS did not have prior-year AUD and/or AWS.
TABLE 2.
Probability of AWS Among Medical Inpatients in the Veterans Health Administration in Fiscal Year 2013
| Unadjusted* |
Adjusted† |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR‡ | (95% CI) | Pred % | (95% CI) | OR‡ | (95% CI) | Pred % | (95% CI) | |
| All (n = 209,151) | — | — | 5.0 | (4.5 – 5.5) | — | — | 5.0 | (4.5 – 5.4) |
| Prior-year AUD (n = 32,028) | 39.5 | (37.3–41.8) | 26.4 | (24.4 – 28.4) | 24.6 | (23.1–26.3) | 17.5 | (16.1 – 18.8) |
| Prior-year AWS (n = 6,905) | 65.6 | (61.9–69.6) | 62.5 | (60.1 – 65.0) | 35.0 | (32.3–37.9) | 37.4 | (35.2 – 39.7) |
| Prior-year AUD/AWS (n = 32,326) | 44.6 | (42.1–47.3) | 26.7 | (24.7 – 28.6) | 28.3 | (26.4–30.3) | 17.9 | (16.4 –19.3) |
| No prior-year AUD or AWS (n = 176,825) | 0.02 | (0.02–0.02) | 0.9 | (0.8 – 1.0) | 0.04 | (0.03–0.04) | 1.0 | (0.9 – 1.1) |
AUD indicates alcohol use disorder; AWS, alcohol withdrawal syndrome; CI, confidence interval; OR, odds ratio; Pred %, predicted probability.
Including hospital as a random effect to account for covariance at the hospital level.
Adjusted for all patient demographics, diagnoses, and health services utilization covariates, with hospital facility as a random effect.
All P-values were significant to a level <0.001.
Prior-year AUD and/or AWS effectively stratified the sample into a ‘‘high AWS prevalence’’ group of 32,326 medical inpatients, 8322 (25.7%) with inpatient AWS, and a ‘‘low AWS prevalence’’ group of 176,825 medical inpatients, 1405 (0.8%) with inpatient AWS. Exploratory multivariable analyses of patient demographic, diagnosis, and utilization characteristics were thus stratified by presence/absence of prior-year AUD and/or AWS. Findings were generally similar in the two groups (eTable2, http://links.lww.com/JAM/A234, Supplemental Digital Content showing probability of AWS based on prior-year health characteristics stratified by prior-year AUD and/or AWS), though the associations between various health characteristics and inpatient AWS were more pronounced among patients with prior-year AUD and/or AWS. In both groups, factors associated with increased probability of inpatient AWS included: male sex, single relationship status, homelessness, and prior-year diagnosis of seizure and/or cirrhosis. Factors associated with decreased probability of inpatient AWS in both groups included: female sex, prior-year diagnosis of pain-related conditions, cerebrovascular disease, dementia, heart failure, chronic kidney disease, diabetes, malignancy, bipolar disorder, psychotic disorder, and/or receipt of outpatient care (ED, primary, and/or specialty care).
Unique associations with inpatient AWS in patients with and without prior-year AUD and/or AWS were also identified, suggesting possible differences between the 2 groups. Among patients with prior-year AUD and/or AWS, gastrointestinal disorders and trauma were associated with increased probability of inpatient AWS, while black relative to White race was associated with decreased probability. These relationships were not significant in patients without prior-year AUD and/or AWS. In contrast, prior-year other substance use disorder was strongly associated with inpatient AWS only in patients without prior-year AUD and/or AWS. Finally, prior-year inpatient medical and/or mental health care was associated with increased probability of AWS in patients with prior-year AUD and/or AWS and decreased probability of AWS in patients without prior-year AUD and/or AWS.
Figure 1 illustrates how each demographic and health characteristic changed the predicted probability of inpatient AWS, showing the marginal effects or difference in the adjusted probability between each category and the referent group. The magnitude of changes was very small for those without prior-year AUD and/or AWS (right panel)—from a 0.6% reduction in probability of AWS associated with female sex, chronic kidney disease, diabetes, and outpatient specialty care to a 0.7% increase in probability of AWS associated with cirrhosis. In contrast, the magnitude of changes was much larger for those with prior-year AUD and/or AWS (left panel)—from a 12.2% reduction in probability of AWS associated with malignancy to a 10.1% increase in probability of AWS associated with seizure.
FIGURE 1.
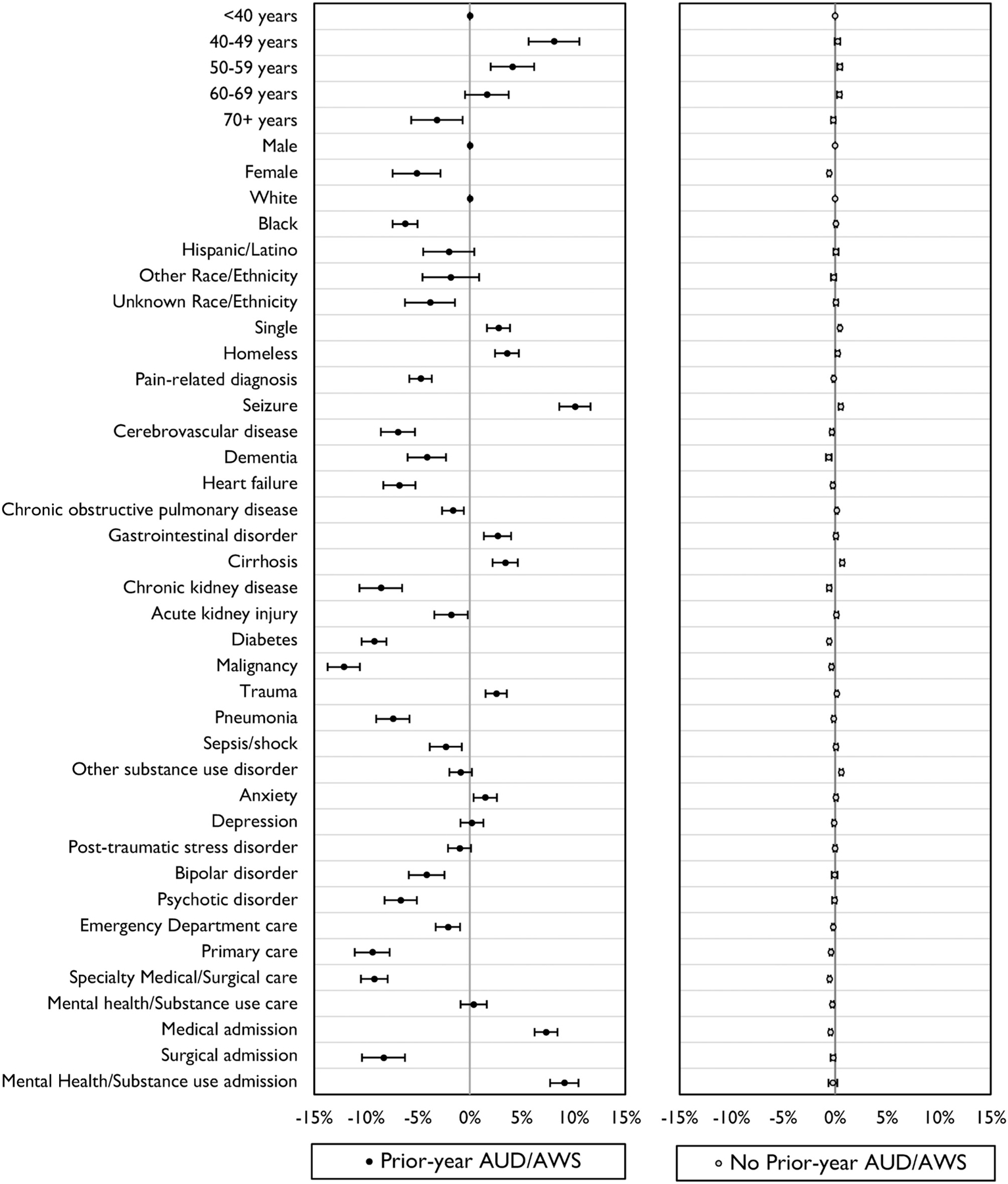
Marginal effects of prior-year health characteristics on the probability of AWS among medical inpatients with and without prior-year alcohol use disorder and/or AWS in the Veterans Health Administration in fiscal year 2013. AWS indicates alcohol withdrawal syndrome.
DISCUSSION
This is the first study to explore the associations between EHR data available at the time of hospital admission and the probability of inpatient AWS. Although the probability of AWS in this national sample of medical inpatients was 5% overall, it rose to 26% among patients with AUD and 63% among patients with AWS documented in the year before admission; after adjustment for patient demographic and other health characteristics, these associations remained significant. Findings from this study are important because they indicate EHR data could be used to identify hospitalized patients who should be monitored and potentially treated for inpatient AWS: 86% of medical inpatients with AWS had documented prior-year AUD and/or AWS (sensitivity) and 88% of medical inpatients without AWS did not have documented prior-year AUD and/or AWS (specificity). Other patient factors also influenced the probability of inpatient AWS but the magnitude of these differences was only clinically-meaningful in patients with previous AUD and/or AWS—in whom the probability of AWS ranged from 17% to 36% across demographic and other health characteristics. Based on clinical experience, inpatient providers may already use prior diagnoses to estimate the risk of AWS in their patients, but this is the first study to validate and inform this practice.
Previous studies of factors associated with AWS in hospitalized patients have evaluated the predictive value of alcohol screening questionnaires16,17 and a composite measure,11 which rely on patient self-report. The remainder of the literature on risk factors for AWS has been conducted in select samples (eg, detoxification centers and addiction care units) with unclear generalizability to hospitalized medical patients, and/or focused on predicting severe AWS (ie, delirium tremens) among samples with AWS.18–29 The present study substantiates and quantifies previously identified associations between history of AUD and/or AWS,11,23,25,28,29 male sex,17,23,28,30 prior seizure18,23,28,29 and subsequent development of AWS. Several significant associations were also identified that are rarely reported in the prior literature—single relationship status, homelessness, and cirrhosis were found to increase the probability of inpatient AWS, and other substance use disorder may be an important risk factor among patients with no documented history of AUD and/or AWS.
This study did not observe a previously described higher risk of AWS in patients with psychiatric disorders.12,31 Instead, serious mental health diagnoses were associated with lower probability of inpatient AWS. This may relate to the strong emphasis on diagnosis and treatment of mental health disorders in the VHA. Of patients with a documented mental health disorder in this study, 73% were seen by an outpatient mental health specialist in the year before admission. Other medical diagnoses with high morbidity and mortality (malignancy, pain-related conditions, cerebrovascular disease, dementia, heart failure, chronic kidney disease, and diabetes) were also associated with lower probability of inpatient AWS. These conditions may be associated with greater healthcare engagement and in turn lower risk of inpatient AWS, though further study of these novel associations is needed.
Although contributing to the clinical epidemiology of inpatient AWS, the present study also has implications for clinical practice. A recent review evaluated diverse methods for identifying hospitalized patients at risk for AWS and recommended the Prediction of Alcohol Withdrawal Severity Scale (PAWSS) as the most promising approach.32 The PAWSS is the only AWS prediction tool that has been developed and validated in hospitalized patients and demonstrated excellent testing characteristics; however, it is potentially burdensome, requiring inpatient providers to gather a wide array of clinical and interview data.11 Further, 60% of inpatients were excluded from the validation study for commonly encountered reasons: transfer from another hospital, inability to communicate (or communicate in English), seizures, severe AWS on presentation, or being ‘‘too sick’’ to participate.12 These issues ultimately complicate the use of any assessment strategy relying on patient self-report in real-world hospital practice. By comparison, a valid EHR algorithm that efficiently identifies patients at increased risk for AWS on admission could be applied more broadly to patients and streamline the clinical workflow.
Limitations
Several limitations might impact the external and internal validity of this study. The study dataset included mostly male, older age, White patients engaged in VHA care and hospitalized in FY2013; additional research is required to assess whether results generalize to veterans hospitalized more recently or patients admitted to non-VHA hospitals. An existing secondary dataset was used to conduct this study; prospective and patient-reported data were unavailable. A validated measure of AWS severity does not yet exist and therefore, was not included in these analyses. Although the Clinical Instrument Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) is used to assess AWS severity in some hospital settings,33 it reflects both AWS severity and response to AWS treatment. The study was not large enough to support both a derivation and validation dataset. Prevalence of inpatient AWS in this study (~5%) was higher than what some studies using billing data have reported,21,22 but similar to single-center prospective cohorts.2,11,12 In the VHA, screening, and treatment for addictions are high priorities,13,34,35 and providers have access to a single EHR providing relatively comprehensive outpatient and inpatient data. In health systems with less care continuity, the presence or absence of prior-year AUD and/or AWS may be less reliable and results of this study will be less applicable.
Bias in EHR data may lead to biased predictions. For example, the finding that black patients with prior-year AUD and/or AWS were less likely than White patients to experience subsequent inpatient AWS could indicate that providers are more likely to document mild AUD and/or AWS (stigmatized diagnoses) in Black patients; prior VHA research lends credence to this hypothesis.36 The extent of such biases in EHR data could impact the validity of EHR algorithms for risk-stratifying patients, thus future research is needed to understand this finding. Internal validity might also be improved by combining multiple characteristics into a risk score for predicting inpatient AWS or considering other factors beyond the scope of this initial study—vital signs, laboratory tests, alcohol screening scores, and historical data predating the previous year.
Future studies should be designed to address the following issues: stratification by severity of AWS and/or severity of AUD, inclusion of gold standard measures for formal assessment of AWS, evaluation of whether EHR data predating the previous year improves prediction of AWS, and validation of this study’s findings in a secondary dataset. Evaluating the predictors of outcomes such as hospital length of stay, need for intensive care, and intubation among hospitalized patients with AWS would lend additional important insights. Subsequent research could then assess whether implementation of an EHR-based prediction model can improve proactive diagnosis, treatment, and clinical outcomes of hospitalized patients with AWS. In the meantime, this study’s novel findings provide an initial evidence-base for a practical approach to identifying hospitalized patients who warrant close monitoring for a common and morbid inpatient condition.3,6,8
Notwithstanding its limitations, this study has important strengths. With 209,151 hospitalized patients and 9727 patients with AWS, the study represents the largest cohort of inpatient AWS in the literature. By comparison, all 14 high-quality studies referenced in a recent review of AWS—together—included only 1355 patients with AWS, and only 27 patients with AWS from an unselected general hospital sample.12,32 The present study used a geographically diverse national sample of patients admitted to 100 different facilities across the U.S. and widely accessible EHR data (demographics, ICD codes, and inpatient/outpatient encounters) to identify factors associated with AWS. The magnitude of the findings in patients with previously documented AUD and/or AWS is large enough to be clinically important and prior-year AUD and/or AWS diagnoses can be extracted easily from the EHR by admitting clinicians or incorporated into clinical decision support tools.
CONCLUSIONS
In the VHA, AWS complicates 5% of medical hospitalizations. Inpatient AWS is associated with significant morbidity and mortality—outcomes that could be improved by early identification. This study suggests that 2 elements of the documented medical history, prior-year AUD and/or AWS, easily extracted from the EHR and available on admission, can identify the vast majority of medical inpatients who will develop AWS during their hospitalization.
Supplementary Material
Acknowledgments
Supported by VA Puget Sound Health Care System Research & Development Associate Chief of Staff (ACOS) Pilot Grant Program, the Center of Excellence for Substance Abuse Treatment & Education (CESATE), and K24AA022128.
Footnotes
The authors report no conflicts of interest.
All authors had access to the data supporting this study and a role in writing the manuscript.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.journaladdictionmedicine.com).
Contributor Information
Tessa L. Steel, Department of Seattle-Denver Center of Innovation (COIN), University of Washington, Division of Pulmonary, Critical Care, & Sleep Medicine, VA Puget Sound Health Care System, Seattle Division, 1660 South Columbian Way S-152, Seattle, WA.
Carol A. Malte, Center of Excellence in Substance Addiction Treatment and Education VA Puget Sound Health Care System, Seattle Division, Seattle, WA.
Katharine A. Bradley, Kaiser Permanente Washington Health Research Institute, Seattle, WA.
Eric J. Hawkins, Center of Excellence in Substance Addiction Treatment and Education Seattle-Denver Center of Innovation (COIN), VA Puget Sound Health Care System, Seattle Division, University of Washington, Department of Psychiatry and Behavioral Sciences, Seattle, WA.
REFERENCES
- 1.Griswold MG, Fullman N, Hawley C, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152): 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foy A, Kay J. The incidence of alcohol-related problems and the risk of alcohol withdrawal in a general hospital population. Drug Alcohol Rev. 1995;14(1):49–54. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien JM, Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35(2):345–350. [DOI] [PubMed] [Google Scholar]
- 4.Spies CD, Neuner B, Neumann T, et al. Intercurrent complications in chronic alcoholic men admitted to the intensive care unit following trauma. Intensive Care Med. 1996;22(4):286–293. [DOI] [PubMed] [Google Scholar]
- 5.Spies CD, Nordmann A, Brummer G, et al. Intensive care unit stay is prolonged in chronic alcoholic men following tumor resection of the upper digestive tract. Acta Anaesth Scand. 1996;40(6):649–656. [DOI] [PubMed] [Google Scholar]
- 6.Steel TL, Malte CA, Bradley KA, Lokhandwala S, Hough CL, Hawkins EJ. Prevalence and variation of clinically recognized inpatient alcohol withdrawal syndrome in the Veterans Health Administration. J Addict Med. 2020;14(4):300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witte PD, Pinto E, Ansseau M, Verbanck P. Alcohol and withdrawal: from animal research to clinical issues. Neurosci Biobehav Rev. 2003;27(3):189–197. [DOI] [PubMed] [Google Scholar]
- 8.Gupta NM, Lindenauer PK, Yu P-C, et al. Association between alcohol use disorders and outcomes of patients hospitalized with community-acquired pneumonia. JAMA Netw Open. 2019;2(6):e195172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monte R, Rabunal R, Casariego E, Lopez-Agreda H, Mateos A, Pertega S. Analysis of the factors determining survival of alcoholic withdrawal syndrome patients in a general hospital. Alcohol Alcohol. 2010;45(2): 151–158. [DOI] [PubMed] [Google Scholar]
- 10.Mayo-Smith MF, Beecher LH, Fischer TL, et al. Management of alcohol withdrawal delirium: An evidence-based practice guideline. Arch Intern Med. 2004;164(13):1405–1412. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado JR, Sher Y, Ashouri JF, et al. The prediction of alcohol withdrawal severity scale’’ (PAWSS): Systematic literature review and pilot study of a new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol. 2014;48(4):375–390. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado JR, Sher Y, Das S, et al. Prospective validation study of the prediction of alcohol withdrawal severity scale (PAWSS) in medically ill inpatients: A new scale for the prediction of complicated alcohol withdrawal syndrome. Alcohol Alcohol. 2015;50(5):509–518. [DOI] [PubMed] [Google Scholar]
- 13.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 14.Timko C, Bonn-Miller MO, McKellar J, Ilgen M. Detoxification history and 2-year outcomes of substance use disorder treatment and mutual-help group participation. J Drug Issues. 2014;44(1):4–21. [Google Scholar]
- 15.Williams R Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308–331. [Google Scholar]
- 16.Dolman JM, Hawkes ND. Combining the AUDIT questionnaire and biochemical markers to assess alcohol use and risk of alcohol withdrawal in medical inpatients. Alcohol Alcohol. 2005;40(6):515–519. [DOI] [PubMed] [Google Scholar]
- 17.Pecoraro A, Ewen E, Horton T, et al. Using the AUDIT-PC to predict alcohol withdrawal in hospitalized patients. J Gen Intern Med. 2014; 29(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuckit MA, Tipp JE, Reich T, Hesselbrock VM, Bucholz KK. The histories of withdrawal convulsions and delirium tremens in 1648 alcohol dependent subjects. Addiction (Abingdon England). 1995;90(10): 1335–1347. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson JA, Suelzer CJ, Eckert GJ, Zhou X-H, Diffus RS. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11(7): 410–414. [DOI] [PubMed] [Google Scholar]
- 20.Wetterling T, Kanitz R-D, Besters B, et al. A new rating scale for the assessment of the alcohol-withdrawal syndrome (AWS Scale). Alcohol Alcohol. 1997;32(6):753–760. [DOI] [PubMed] [Google Scholar]
- 21.Shaw GK, Waller S, Latham CJ, Dunn G, Thomson AD. The detoxification experience of alcoholic in-patients and predictors of outcome. Alcohol Alcohol. 1998;33(3):291–303. [DOI] [PubMed] [Google Scholar]
- 22.Reoux JP, Malte CA, Kivlahan DR, Saxon AJ. The alcohol use disorders identification test (AUDIT) predicts alcohol withdrawal symptoms during inpatient detoxification. J Addict Dis. 2002;21(4):81–91. [DOI] [PubMed] [Google Scholar]
- 23.Fiellin DA, O’Connor PG, Holmboe ES, Horwitz RI. Risk for delirium tremens in patients with alcohol withdrawal syndrome 1. Subst Abus. 2002;23(2):83–94. [DOI] [PubMed] [Google Scholar]
- 24.Kraemer KL, Mayo-Smith MF, Calkins DR. Independent clinical correlates of severe alcohol withdrawal. Subst Abus. 2003;24(4):197–209. [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Jang MK, Lee JY, et al. Clinical predictors for delirium tremens in alcohol dependence. J Gastroen Hepatol. 2005;20(12):1833–1837. [DOI] [PubMed] [Google Scholar]
- 26.Wetterling T, Weber B, Depfenhart M, Schneider B, Junghanns K. Development of a rating scale to predict the severity of alcohol withdrawal syndrome. Alcohol Alcohol. 2006;41(6):611–615. [DOI] [PubMed] [Google Scholar]
- 27.Wright T, Myrick H, Henderson S, Peters H, Malcolm R. Risk factors for delirium tremens: A retrospective chart review. Am J Addict. 2006;15(3):213–219. [DOI] [PubMed] [Google Scholar]
- 28.Monte R, Rabunal R, Casariego E, Bal M, Pertega S. Risk factors for delirium tremens in patients with alcohol withdrawal syndrome in a hospital setting. Eur J Intern Med. 2009;20(7):690–694. [DOI] [PubMed] [Google Scholar]
- 29.Berggren U, Fahlke C, Berglund KJ, Blennow K, Zetterberg H, Balldin J. Thrombocytopenia in early alcohol withdrawal is associated with development of delirium tremens or seizures. Alcohol Alcohol. 2009;44(4): 382–386. [DOI] [PubMed] [Google Scholar]
- 30.Salottolo K, McGuire E, Mains CW. Occurrence, predictors, and prognosis of alcohol withdrawal syndrome and delirium tremens following traumatic injury. Crit Care Med. 2017;45(5):867–874. [DOI] [PubMed] [Google Scholar]
- 31.Moore DT, Fuehrlein BS, Rosenheck RA. Delirium tremens and alcohol withdrawal nationally in the Veterans Health Administration. Am J Addict. 2017;26(7):722–730. [DOI] [PubMed] [Google Scholar]
- 32.Wood E, Albarqouni L, Tkachuk S, et al. Will this hospitalized patient develop severe alcohol withdrawal syndrome?: The rational clinical examination systematic review. JAMA. 2018;320(8):825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Brit J Addict. 1989;84(11):1353–1357. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins EJ, Lapham GT, Kivlahan DR, Bradley KA. Recognition and management of alcohol misuse in OEF/OIF and other veterans in the VA: A cross-sectional study. Drug Alcohol Depen. 2010;109(1–3):147–153. [DOI] [PubMed] [Google Scholar]
- 35.Grossbard JR, Hawkins EJ, Lapham GT, et al. Follow-up care for alcohol misuse among OEF/OIF veterans with and without alcohol use disorders and posttraumatic stress disorder. J Subst Abuse Treat. 2013;45(5):409–415. [DOI] [PubMed] [Google Scholar]
- 36.Williams EC, Rubinsky AD, Lapham GT, et al. Prevalence of clinically recognized alcohol and other substance use disorders among VA outpatients with unhealthy alcohol use identified by routine alcohol screening. Drug Alcohol Depen. 2014;135:95–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


