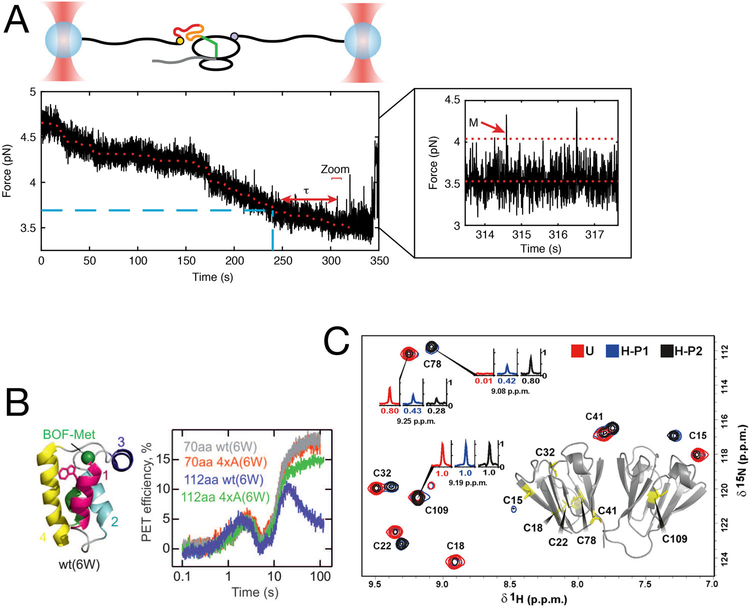
FIGURE 4.
Coupling of nascent chain elongation and folding gives rise to non-equilibrium effects. (A) Single-molecule experiments with optical tweezers allow direct observation of non-equilibrium effects on nascent protein folding. Partially synthesized calerythrin nascent chains populate a misfolded state (M, zoom inset). Formation of the misfolded state is significantly delayed (τ) even after the amino acids constituting the misfolded state have been synthesized, owing to non-equilibrium effects of translation and folding. From ref.[67] (B) Characterization of nascent chain dynamics by photon-induced electron transfer (PET) during active elongation shows that the nascent chain interconverts between different compact and elongated structures. As the nascent chain traverses through the exit tunnel, it adopts different conformations as reported by PET efficiencies. When 70 aa of the protein HemK are synthesized (70 aa construct), it adopts a compact structure before rearranging to final native state upon further elongation (112 aa construct). Disrupting the hydrophobic core of the domain (4×A mutants) does not interfere with compaction, but prevents the final rearrangement. From ref.[77], with permission. (C) Variations in the rate of translation elongation alters the final structure of gamma-B crystallin. The protein was heterologously expressed in E. coli, using either “unharmonized” (U) or “harmonized” (H) coding sequences. Even though both coding sequences are synonymous, NMR measurements reveal the presence of two sub-populations of the protein with distinct structures and patterns of disulfide bridges. From ref.[73]