Abstract
Advancements in our understanding of polyamine molecular and cellular functions has led to increased interest in targeting polyamine metabolism for anticancer therapeutic benefits. The polyamines putrescine, spermidine, and spermine are polycationic alkylamines commonly found in all living cells and are essential for cellular growth and survival. This review summarizes the existing research on polyamine metabolism and function, specifically the role of polyamines in gastric immune cell and epithelial cell function. Polyamines have been implicated in a multitude of cancers, but in this review, we focus on the role of polyamine dysregulation in the context of Helicobacter pylori-induced gastritis and subsequent progression to gastric cancer. Due to the emerging implication of polyamines in cancer development, there is an increasing number of promising clinical trials using agents to target the polyamine metabolic pathway for potential chemoprevention and anticancer therapy.
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third most common cause of cancer-related death worldwide (1). The highest incidence of GC cases occurs in Latin America, and Eastern and Central Asia, while the lowest incidence rates are seen in North America, and North and East Africa. GC disproportionately affects older individuals, frequently men, and has a 5-year survival rate of 31% in the United States (1). There are two types of gastric carcinoma: 1) The intestinal type progresses through distinct histological steps of the “Correa Cascade” from non-atrophic gastritis (NAG), to multifocal atrophic gastritis (MAG), intestinal metaplasia (IM), dysplasia, and carcinoma (2–5). 2) The diffuse type of GC occurs when infiltrating neoplastic cells do not form glandular structures and although the precancerous pathway is less well-defined, it frequently arises in a background of IM (6).
Infection with Helicobacter pylori and genetic mutations are etiologic agents of GC. H. pylori is a Gram-negative bacterium classified as a type I carcinogen that infects approximately 50% of the world’s population (7). This pathogen specifically colonizes the mucosa of the human stomach and colonization typically correlates with development of chronic inflammation (8). Most colonized individuals do not exhibit symptoms, however long-term H. pylori carriers have increased risk of developing site-specific disease. Among those infected, approximately 10% develop peptic ulcer disease, less than 0.1% develop mucosa-associated lymphoid tissue lymphoma, and 1–3% develop gastric adenocarcinoma (8). Of importance, H. pylori infection is associated with both intestinal and diffuse GC (9). Several studies have shown that eradication of the bacterium results in attenuated progression to GC (9,10), but the treatment for H. pylori does not consistently reduce cancer risk, particularly once precancerous lesions are present (11,12). Moreover, since half of humans are infected (7), universal eradication is not practical and antibiotics are often ineffective, notably in underdeveloped regions (13,14). Lastly, H. pylori infection is indolent, mimicking a commensal (15), and therefore its presence cannot reliably predict who will develop disease progression. Stomach cancers in H. pylori-infected patients tend to develop slowly through pre-cancerous changes and rarely cause symptoms until late in the disease course, and thus often go undetected resulting in a low 5-year survival rate (8). Recently, two studies from our group have shown in a long-term cohort study in subjects in a high GC risk region of the Andean mountains of Colombia that H. pylori treatment is associated with reduced histological progression of precancerous lesions and development of GC, if subjects remained free of infection over a 16-year (16) and 20-year follow-up period (5). These findings indicate that detailed follow-up after H. pylori treatment may be helpful in determining long-term outcome.
Notably, while H. pylori is an important risk factor, a small percentage of diffuse GC arises from inherited cancer predisposition. Hereditary diffuse GC, a family GC syndrome, is commonly caused by variants of the CDH1 gene that encodes for cadherin-1 (17). Molecular consequences of CDH1 loss include: i) disruption of cell-cell adhesion, ii) induction of WNT/β-catenin oncogenic signaling, and iii) activation of EGFR signaling (18,19).
Environmental factors can increase the risk of developing GC. These include a diet high in salty or smoked food, a diet lacking fruits and vegetables, obesity, and smoking (20,21). In addition, host factors can directly, through their pro-oncogenic or anti-tumoral properties, or indirectly, by affecting the persistence of H. pylori and/or chronic gastritis, regulate H. pylori-induced GC (22). Polyamines are ubiquitous molecules found in microbes, plants, and animals (23,24). In mammals, they are produced by numerous different cells, including epithelial and immune cells, and play many important biological roles, from embryogenesis to aging (25). Due to their cationic nature, polyamines can interact with macromolecules, e.g., DNA, RNA, ATP, phospholipids, and proteins, and can thus contribute to gene regulation via epigenetic and chromatin structure modifications, translation, cell growth, proliferation, and differentiation (25,26). Therefore, it has been proposed that they have a major effect on cancer. In this context, we summarize herein the findings about the importance of polyamine metabolism in gastric carcinogenesis.
1. The metabolism of polyamines
1.1. Polyamine synthesis
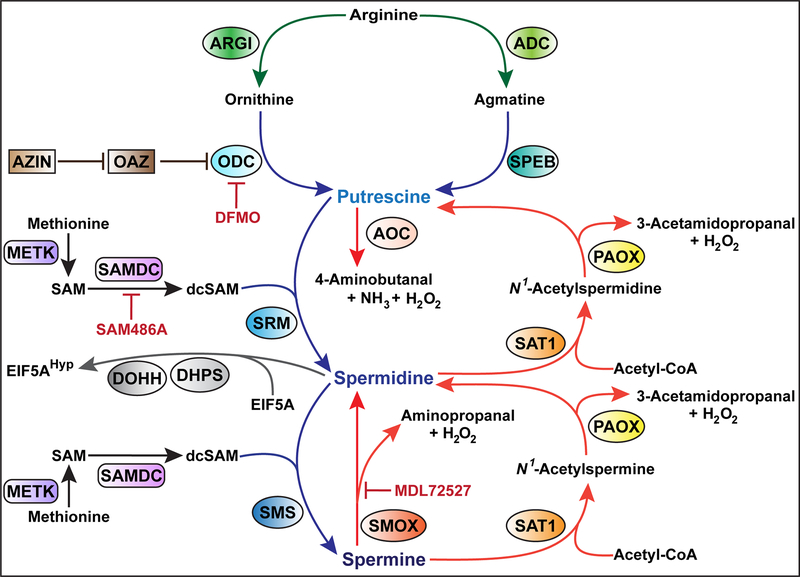
In mammals, there are three major polyamines– putrescine, spermidine, and spermine, which are sequentially synthesized from the catabolism of the amino acids L-arginine and L-methionine (23,24), as depicted in Fig. 1. First, arginase (ARGI), an enzyme involved in the urea cycle, catalyzes the hydrolysis of L-arginine to L-ornithine and urea (27). Two isoforms of ARGI exist: the cytosolic ARGI1, which is expressed predominantly in the liver and to a lesser extent in the bone marrow, and the mitochondrial ARGI2, which is present in a variety of peripheral mammalian tissues including the digestive and gastrointestinal tract, kidney, and prostate. While the two isoforms catalyze the same biochemical reaction, they differ in cellular expression, regulation, and localization (28). Second, L-ornithine is converted by the rate-limiting enzyme ornithine decarboxylase (ODC), encoded by the gene ODC1 (called ODC in this review to be consistent with the name of the gene in published studies), into the first polyamine putrescine (29,30). Alternatively, putrescine can also be generated from L-arginine by the arginine-agmatine-polyamine pathway (31). Arginine is metabolized to agmatine via arginine decarboxylase (ADC), followed by conversion of agmatine to putrescine by agmatinase (SPEB). Third, putrescine is sequentially converted into spermidine and spermine via spermidine synthase (SRM) and spermine synthase (SMS) respectively; both reactions require the donation of an aminopropyl group from decarboxylated S-adenosylmethionine (dcSAM) (23,25), which is synthesized by the sequential conversion of i) methionine to S-adenosylmethionine (SAM) by S-adenosylmethionine synthase (METK) and ii) SAM to dcSAM by the rate-limiting enzyme S-adenosylmethionine decarboxylase (SAMDC). The steady state levels of dcSAM are generally kept low to limit polyamine synthesis (25). It should be noted that the irreversible ODC inhibitor α-difluoromethylornithine (DFMO), which was synthesized over 40 years ago, is used to treat African trypanosomiasis, excessive hair growth on the face of women, and is under development as a chemotherapy for hyperproliferative diseases including cancer (32).
Fig. 1:
The polyamine metabolic pathway
Biosynthesis, interconversion, and degradation of putrescine, spermidine and spermine in mammals. Proteins abbreviations are in accordance with the guidelines from the Uniprot database (https://www.uniprot.org); note that the new abbreviation for ODC is DCOR.
Importantly, intracellular polyamine homeostasis is also maintained by the back conversion of spermine to spermidine, and spermidine to putrescine (Fig. 1). This occurs through two distinct pathways. The first pathway involves the enzymes diamine acetyltransferase 1 (SAT1) and peroxisomal N1-acetyl-spermine/spermidine oxidase (PAOX): SAT1 catalyzes the transfer of an acetyl group from acetyl-coenzyme A (acetyl-CoA) to either spermidine or spermine at the N1 position, then PAOX catalyzes the cleavage of acetylated polyamines, leading to the generation of spermidine or putrescine, 3-acetamidopropanal, and H2O2 (33,34). Note that only acetylated polyamines are exported outside of cells (35). The second pathway for polyamine back-conversion corresponds to the direct cleavage of spermine into spermidine, 3-aminopropanal, and H2O2 by spermine oxidase (SMOX) (36). SMOX is a major source of spermidine in tissues since it has been shown that deletion of the Smox gene in mice results in the reduction of spermidine content and accumulation of spermine (37). In addition, putrescine is also catabolized by the copper containing amiloride-sensitive amine oxidase (AOC1; Fig. 1), also known as diamine oxidase or amiloride binding protein, to produce 4-aminobutanal, NH3, and H2O2 (38).
1.2. Synthesis of hypusine
Spermidine serves an essential role as the substrate for the synthesis of hypusine [-(4-amino-2-hydroxybutyl) lysine], a unique amino acid found only in the protein eukaryotic translation initiation factor 5A (EIF5A) (39–41). Spermidine is cleaved by deoxyhypusine synthase (DHPS; Fig. 1), resulting in the transfer of the 4-aminobutyl moiety to the ϵ-amino group on a specific lysine (Lys50 in mice and humans) found on EIF5A (40,42). Then, deoxyhypusine hydroxylase (DOHH) hydroxylates this intermediate to form hypusine, thus activating EIF5A (43). This post-translational modification of EIF5A is called hypusination. Two isoforms of EIF5A have been characterized, EIF5A1, which is expressed in all cells and tissues, and EIF5A2, which is mainly present in the testes, brain, and tumor cells (44,45).
Hypusinated EIF5A (EIF5AHyp) can bind specific mRNAs that contain an AAAUGU consensus sequence (46–48). The EIF5AHyp/mRNA complex (Fig. 2) is translocated to the cytoplasm by the nuclear transporter exportin-1 (49), reaches ribosomes, and facilities translation (50). In addition, it has been shown that EIF5AHyp alleviates ribosome stalling at proline repeats during translation elongation of other non-polyproline motifs, such as peptides enriched in diglycyl motifs and in basic and acidic amino acids (51–53).
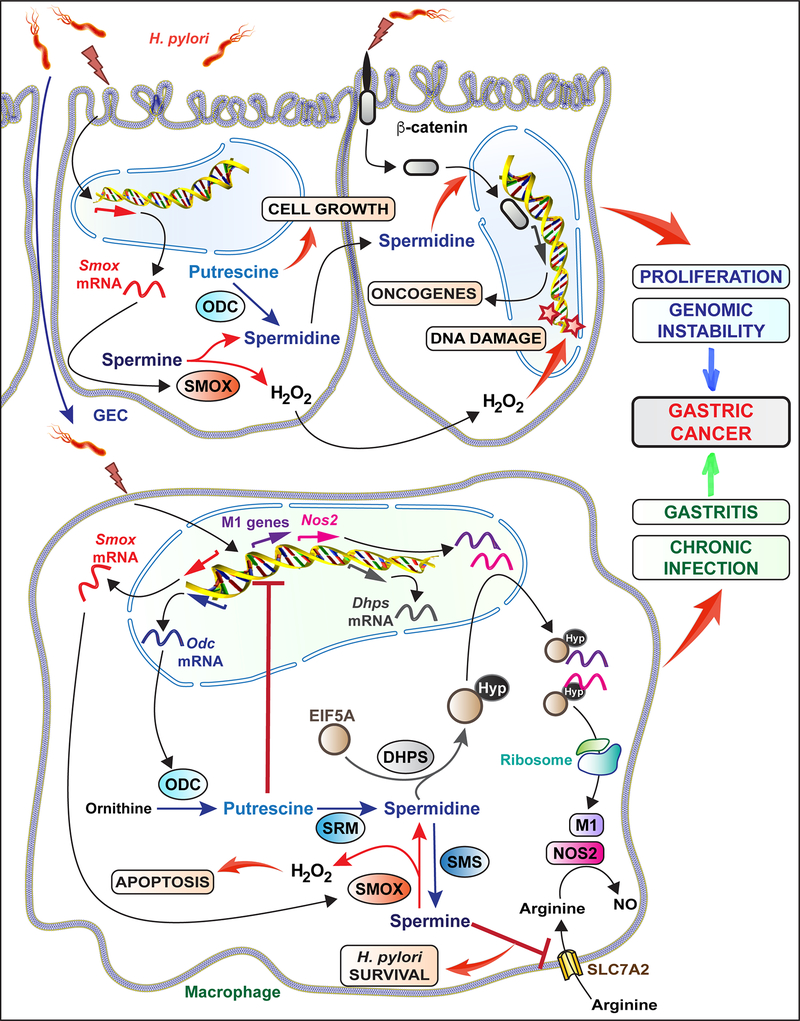
Fig. 2:
The role of polyamines on H. pylori-induced GC
The induction and the effect of polyamines in GECs and macrophages upon H. pylori infection are depicted.
Substitution of the critical lysine residue of EIF5A at the hypusination modification site with alanine, aspartic acid, or isoleucine inhibits yeast growth, revealing an essential role for EIF5A and its hypusine modification in yeast cell viability (54). Similarly, deletion of both EIF5A genes (55) or a single DHPS gene (56) causes loss of viability in yeast. Conversely, DOHH is not required for yeast growth (57). Moreover, knockout of Eif5a1, Dhps, or Dohh in mice leads to embryonic lethality, whereas heterozygous mice are viable and fertile (58,59). Interestingly however, homozygous Eif5a2 knockout mice are viable and fertile (60). These data clearly demonstrate the essential role for hypusination in embryogenesis, and cell growth and development. Furthermore, because EIF5AHyp is required for proliferation, it has also been proposed that the spermidine/hypusine pathway can play a major role in carcinogenesis. Thus, it has been shown that EIF5A, DHPS, and/or DOHH knockdown reduces tumor cell growth and proliferation ex vivo in glioblastoma, neuroblastoma, vulvar high grade intraepithelial neoplasia, hepatocellular carcinoma, and pancreatic ductal, cervical, prostate, and ovarian cancer, as well as GC (44).
Hypusination regulates the growth and proliferation of numerous cancerous cell lines (44). Coni et al. demonstrated that hypusination promotes colorectal cancer (CRC) cell growth through direct regulation of MYC biosynthesis (61). Silencing and inhibition of DHPS resulted in reduced proliferation and cell viability of CRC cell line HCT116 in vitro and in vivo in xenografts in nude mice (61). Similarly, treatment with the DHPS inhibitor, N1-guanyl-1,7-diaminoheptane (GC7) decreased EIF5AHyp levels, MYC protein levels, and polyp size in ApcMin/+ mice treated with azoxymethane (61). Altogether, these data suggest that hypusination may play a critical role in tumor growth.
1.3. Regulation of ODC by antizyme
Most cellular proteins are tagged for proteasomal degradation through ubiquitination. However, the non-covalent association of ODC with antizyme (OAZ; Fig. 1) enhances its interaction with the proteasome leading to increased ubiquitin-independent degradation of ODC (29). OAZ production is induced by polyamines, particularly spermidine and spermine, in a mechanism that involves frameshifting to stimulate translation of OAZ mRNA. The protein OAZ inhibitor (AZIN) binds OAZ with a higher affinity than ODC, resulting in the release of ODC from OAZ-ODC complexes (62). Thus, AZIN increases ODC activity and subsequent intracellular polyamine formation, promoting the development of cancerous cells (62).
2. Polyamine metabolism during H. pylori infection and gastric carcinogenesis
2.1. ODC expression
Several studies have reported an increase in ODC protein and mRNA expression in the gastric tissues and macrophages of H. pylori-infected patients. Patchett et al. used a [14C]ornithine bioassay to examine gastric mucosal ODC activity in 32 normal patients and 22 confirmed GC patients, which was compared to 47 patients with increased risk of upper gastrointestinal malignancy, specifically patients with partial gastric resection or those with FAP (63). Normal patients exhibited significantly lower ODC activity levels than those with gastric resection or confirmed GC. Importantly, ODC activity was significantly increased in the areas of gastric atrophy or IM regardless of the clinical group (63). Furthermore, in a cohort of patients with chronic superficial gastritis, chronic atrophic gastritis, gastric dysplasia, and GC, Miao et al. demonstrated by immunohistochemistry a positive correlation between ODC protein expression and degree of malignancy of the gastric mucosa (64), suggesting that ODC is a potential biomarker for precancerous lesions. These data were confirmed by Chaturvedi et al., who further demonstrated that ODC mRNA expression is increased in patients with H. pylori-induced gastritis and immunohistochemistry revealed that ODC was primarily expressed in mononuclear cells of the lamina propria in these infected patients (65). Furthermore, Hardbower et al. used immunofluorescence to reveal a significant increase in the number of CD68+ODC+ cells, indicative of expression in the gastric macrophages, in H. pylori-infected patients compared to uninfected individuals (66). These studies contrasted with the observation from Elitsur et al., who observed no significant change in ODC activity in the gastric mucosa of 6 H. pylori-infected versus 12 non-infected children along with no reported correlation between gastric inflammation and level of ODC (67). The 6 H. pylori-positive children displayed gastritis, specifically 3 cases of chronic mild gastritis and 3 cases of chronic active moderate gastritis, but no presence of gastric atrophy. Both the ODC activity assay and ODC immunohistochemistry analysis revealed no difference in gastric ODC activity between infected and uninfected children (67). This discrepancy between studies could be explained by the age of the patients and/or the lack of gastric atrophy seen in these infected children opposed to the adults assessed in the other studies (65,66).
To investigate the relationship between ODC induction and H. pylori presence, Konturek et al. administered H. pylori eradication therapy (omeprazole, amoxycillin, and clarithromycin) for seven days and vitamin C for three months to 20 patients with atrophic gastritis (68). A decrease in severity of gastritis in addition to a reduction in ODC mRNA levels was observed in gastric tissues three months after therapy. This echoed the result from the Sakai group, who had previously shown that eradication of H. pylori with triple therapy in atrophic gastritis and gastric ulcer patients decreased mucosal ODC activity and increased apoptosis in the gastric mucosa (69).
In vitro experiments have also been performed to study the induction of ODC by H. pylori. Asim et al. demonstrated that ODC mRNA is not upregulated in H. pylori-infected AGS or MKN28 human gastric carcinoma cells, and conditionally-immortalized mouse stomach cells (30). Further, it has been reported that H. pylori extracts inhibited ODC activity, but did not affect ODC mRNA expression in human gastric MKN45 cells (70). Although H. pylori extracts did not affect the viability of the gastric cells, extracts with proteins greater than 50 kDa significantly inhibited proliferation and ODC activity of the gastric cells. In contrast, ODC protein has been shown by Xu et al. to be induced through a phospho-CagA-mediated mechanism involving the Src/MEK/ERK/c-MYC pathway in the gastric epithelial cancer cell lines AGS and BGC-823 and the immortalized epithelial cell line GES-1 (71); it should be noted that the induction of ODC was surprisingly observed as soon as 2 hours post-infection, and ODC mRNA was not shown in this study. Overall, these studies suggest that the induction of ODC in transformed gastric epithelial cells (GECs) is not a major hallmark of the response to H. pylori.
However, Gobert et al. showed an increase in Odc mRNA levels in RAW 264.7 macrophages between 2 to 12 hours post co-culture with H. pylori SS1 and a significant increase in ODC enzymatic activity, accessed by the conversion of L-[14C]ornithine to [14C]CO2 after 24 hours with H. pylori SS1 (72). Furthermore, Cheng et al. demonstrated that infection of RAW 264.7 macrophages with H. pylori exhibited a time-dependent 12-fold increase in ODC promoter activity that peaked at 6 hours, which was similarly seen with ODC mRNA, protein, and enzyme activity (73). Additionally, to investigate the role of the macrophage-derived ODC seen in H. pylori-infected patients, Hardbower et al. generated mice with myeloid-specific deletion of Odc (OdcΔmye) by crossing C57BL/6 Odcfl/fl with a myeloid-specific LysMcre/cre driver (66). H. pylori-induced ODC protein was immunolocalized in CD68+ cells of C57BL/6 Odcfl/fl mice, but was not detected in gastric macrophages of OdcΔmye mice, demonstrating the specificity of the immunodetection.
Thus, H. pylori infection is associated with an increase of ODC expression, notably in macrophages (Fig. 2).
2.2. Induction of SMOX
SMOX is an essential enzyme in polyamine metabolism in the GI tract due to its efficient regulation of spermidine concentration (37). To examine the relationship between SMOX expression and H. pylori-associated GC risk, Chaturvedi et al. evaluated biopsies and H. pylori clinical isolates from Colombian patients living in either the Andean mountain region, in which the risk for developing GC is high, or in the Pacific coast region that is associated with a lower risk of GC in H. pylori-infected patients (74). Immunostaining of the gastric biopsies revealed that SMOX protein was more abundant in the epithelium of H. pylori-infected patients from the high-risk region compared to the low-risk population; this was observed for all stages of H. pylori-mediated disease, i.e., NAG, MAG, and IM, and SMOX levels increased progressively from NAG to MAG to IM (74). Furthermore, in vitro co-culture of the human gastric adenocarcinoma cell line AGS with clinical H. pylori isolates showed that SMOX mRNA expression was significantly more induced by high-risk strains compared to low-risk strains, establishing the essential role of H. pylori in SMOX induction (74). Additionally, Murray-Stewart et al. examined the epigenetic silencing of the tumor suppressor miR-124 from both the high-risk and low-risk gastric biopsies and its association with SMOX levels (75). H. pylori-infected AGS cells transfected with miR-124 maintained basal levels of SMOX mRNA, while the negative control and wild-type AGS cells exhibited a 6–8-fold increase in SMOX mRNA levels. Subsequent analysis of DNA methylation via pyrosequencing of gastric mucosa DNA from 90 patients in Columbia revealed a significantly elevated level of DNA methylation of three mir-124 gene loci in the high-risk population compared to the low-risk population, which was correlated with increased SMOX protein expression by immunohistochemistry (75).
Additionally, we have shown that Mongolian gerbils infected for 12 weeks with the PZ5056 strain of H. pylori isolated from a subject from the high-risk region of Colombia exhibited more dysplasia and SMOX staining localized to the dysplastic glands than animals infected with a low-risk clinical isolate (74). In contrast, our group did not observe a significant increase in Smox mRNA expression nor an increase of spermidine concentration in the gastric tissue of C57BL/6 mice infected with H. pylori PMSS1 for 4 weeks compared to control animals; there was no Smox expression and a dramatic reduction of spermidine concentration in mice with Smox deletion, demonstrating that basal SMOX activity affects polyamine concentrations in stomach tissues (76). Additionally, in vitro experiments on RAW 264.7 macrophages infected with H. pylori showed a significant increase in Smox mRNA expression and subsequent spermine catabolism (77,78). These data demonstrate that SMOX is induced in GECs and macrophages in H. pylori gastritis (Fig. 2).
2.3. Other enzymes
AOC1 mRNA and protein expression is significantly increased in human stomach adenocarcinoma tissues compared to normal tissues (79), suggesting a role for this enzyme in the progression of human GC. In addition, the gene encoding for AZIN1, which supports ODC activity, has been identified as one of the 18 genes differentially expressed in human gastric tumors (80), and this was confirmed in a targeted analysis performed on 140 Japanese patients (81).
2.4. Polyamine concentrations in humans during H. pylori infection and GC
Because polyamines support proliferation, it could be considered that their levels may be a marker of tumorigenesis. Thus, Lundell & Rosengren analyzed gastric neoplastic tissue from patients undergoing a total or subtotal gastrectomy (82). The putrescine and spermidine concentrations were significantly increased compared to the normal gastric mucosa from the same individuals in all 16 patients; however, the spermine levels did not differ between the two groups. Subsequent analysis of ODC activity in 14 patients revealed an increase, although not statistically significant, in ODC activity in the tumor tissue compared to the normal gastric mucosa (82). In a clinical trial examining the influence of H. pylori infection on polyamine levels, Linsalata et al. evaluated the polyamine levels via high performance liquid chromatography in the gastric antral and body biopsies from 20 H. pylori-positive and 6 H. pylori-negative patients undergoing gastroscopy (83). The H. pylori-positive patients exhibited higher polyamine levels in the antrum as opposed to the body, but both H. pylori-positive biopsies contained higher polyamine levels compared to H. pylori-negative patients (83).
These data sustain the concept that enzymes involved in putrescine and spermidine synthesis –i.e. ODC and SMOX– are upregulated in H. pylori-infected patients and in GC.
3. The role of polyamines in gastric immune cell function
Upon infection, H. pylori primarily localizes to the gastric mucous layer and the gastric epithelial surface, but has also been shown to penetrate into the lamina propria (84). Colonization is followed by myeloid and lymphocyte infiltration into the gastric mucosa. These immune cells are activated by bacterial virulence factors, resulting in the release of inflammatory mediators that contribute to cellular damage and progression to cancer. The evasion of H. pylori from the innate and adaptive immune response leads to chronic persistence of infection and this may contribute to the progression to gastric adenocarcinoma (84). Several studies have implicated polyamines in the regulation of macrophage function, thus regulating H. pylori persistence and the potential risk for GC. The effect of polyamines on macrophage function is shown in Fig. 2.
Monocyte-derived phagocytic macrophages are vital to gastric mucosal homeostasis. Resident macrophages and infiltrating monocytes/macrophages present in the mucosa monitor and respond to invading pathogens. Stimulation by pathogen-associated molecular markers, damage-associated molecular markers, and the T helper (Th) 1 cytokine IFN-γ, polarize macrophages to the pro-inflammatory M1 phenotype (85,86). Th2 cytokines including IL-4 and IL-10, conversely, can stimulate alternatively activated M2 macrophages (87). Classically activated M1 macrophages produce high levels of antimicrobial effectors, such as nitric oxide (NO) or reactive oxygen intermediates, and pro-inflammatory cytokines TNF-α, IL-1β, IL-8, and IL-12 to recruit other immune cells to the site of infection; these cells thus exhibit antitumoral, antimicrobial and pro-inflammatory properties (85,86). In contrast, alternatively activated M2 macrophages have increased ARGI activity and express cytokines that dampen the immune response; these cells are associated with tissue repair and carcinogenesis (85). To assess the effect of polyamine regulation on macrophage activation, Hardbower et al. infected OdcΔmye macrophages in vitro with H. pylori SS1 and found increased M1 macrophage activation with Odc deletion and no effect on M2 markers (66). Subsequent addition of putrescine to H. pylori-infected OdcΔmye bone marrow-derived macrophages reversed this enhanced M1 macrophage activation (66), suggesting a role for ODC and putrescine as macrophage function regulators. OdcΔmye mice exhibited alterations in chromatin structure, specifically an increase in histone 3, lysine 4 monomethylation and histone 3, lysine 9 (H3K9) acetylation, along with decreased H3K9 di/trimethylation in in vivo and ex vivo primary macrophages, resulting in an upregulation of gene transcription, especially M1 gene expression (66). Together, these findings suggest that ODC reduces macrophage M1 response to bacterial infections through chromatin structure alterations, resulting in bacterial persistence and pathogenesis.
The polyamine spermidine is also required to support translation through hypusination. Recently, we demonstrated that H. pylori induces DHPS expression in murine macrophages (Fig. 2), resulting in enhanced hypusination of EIF5A (88). Humans and mice infected with this bacterium also exhibited increased levels of EIF5AHyp in CD68+ cells. To understand the role of hypusination in macrophage function, mice were generated with specific deletion of Dhps in myeloid cells (DhpsΔmye). Innate immune proteins induced by H. pylori and regulated by hypusination were identified from DhpsΔmye bone marrow-derived macrophages and GC7-treated macrophages. The proteins included major antimicrobial effectors, such as NOS2, IRG1, and proteins involved in autophagy (IRGM1 and SQSTM1). Accordingly, H. pylori survival was increased in macrophages with Dhps deletion or DHPS inhibition in vitro (88). These data indicate that translational regulation by hypusination is a hallmark of eukaryotic host defense against pathogenic bacteria. Thus, although blockade of DHPS results in inhibition of cancerous cell growth (61), it could be questioned whether this strategy might be efficient for GC since it could support the persistence of H. pylori in the stomach. In this context, further studies about the role of DHPS in animal models of H. pylori-mediated carcinogenesis are needed and are underway in our laboratory.
Besides the general effect of hypusination on translation, polyamines have been shown to specifically regulate the translation of inducible NO synthase (NOS2). It was reported that addition of spermine, and to a lower extent spermidine, to H. pylori-stimulated macrophages does not affect Nos2 mRNA expression, but reduces NOS2 translation and consequently NO synthesis, thus inhibiting NO-dependent H. pylori killing; this was similarly observed with Odc knockdown using siRNA (89). Similarly, Smox knockdown using shRNA in RAW 264.7 murine macrophages, which was associated with increased spermine concentration, resulted in a marked decrease in NOS2 protein induction, NO production, and H. pylori killing (80). Further, Chaturvedi et al. demonstrated that addition of spermine to H. pylori-stimulated RAW 264.7 macrophages in vitro blocked L-arginine uptake by the inducible L-arginine transporter solute carrier family 7 member 2 (SLC7A2, previously known as CAT2), whereas knockdown of Odc had the opposite effect (65). These findings demonstrate that the polyamine spermine can also affect the post-translational regulation of immune effectors during H. pylori infection.
It should be also noted that spermidine and spermine have been shown to stimulate apoptosis in H. pylori-stimulated macrophages (77,90) and that DFMO inhibited this cell death (72). Consistent with these in vitro data, Lewis et al. found that mice infected with H. pylori for 48 hours exhibited gastric macrophage apoptosis, which was eliminated in Arg2–/– mice (91). As a mechanism, Chaturvedi et al. proposed that H2O2 release during spermine catabolism by SMOX, downstream of ARGI2 and ODC, was responsible for mitochondrial membrane depolarization and subsequent apoptosis (77).
Collectively, these data indicate an important role for polyamines in macrophage function and subsequent persistence of H. pylori infection, implicating polyamines as potential players in progression of chronic gastritis to GC.
4. The role of polyamines in GECs
Since ODC is not markedly upregulated in GECs in response to H. pylori, the role of ODC in gastric epithelial cell function has not been deeply investigated. Nonetheless, transfection of GC cells SGC7901 and MGC803 with antisense RNA to ODC led to significantly suppressed tumor cell proliferation, G1 cell cycle arrest, and reduced invasiveness (90). Furthermore, Xu et al. 2012 demonstrated that knockdown of ODC protein production in the human GC cell line BGC-823 led to a decrease in cell proliferation, with or without H. pylori infection (71), indicating that ODC is essential for expansion of gastric cancerous cells, as observed with tumor cells derived from neuroblastomas (92), prostate cancer (93), or colorectal adenomas (94). Supporting this concept, 3% DFMO in the drinking water has been shown to inhibit the growth of human gastric tumor xenografts in athymic BALB/c mice (95); concomitantly, putrescine and spermidine levels were significantly decreased in the tumors, whereas spermine was not affected.
In contrast, the enzyme SMOX has been shown to be induced in GECs by H. pylori (96). SMOX inhibition with MDL72527 or transfection with siRNA reduces H2O2 synthesis and oxidative DNA damage, assessed by measurement of levels of 8-oxoguanosine, in gastric epithelial AGS cells. Moreover, H. pylori-induced DNA damage is attenuated by catalase, further evidencing the critical role of the SMOX/H2O2 pathway in DNA alteration. In parallel, inhibition of SMOX reduces mitochondrial membrane depolarization, cytochrome c release, and activation of caspase-3 (96). These results indicate that the induction of SMOX is involved in the development of GECs with DNA damage and increased apoptosis. These findings were recently recapitulated in vitro in primary GECs as Sierra et al. showed that gastric organoids from Smox-deficient mice exhibit reduced levels of phosphoserine 139 of H2FA histone family member X (pH2AFX), a reliable marker of DNA damage, compared to gastric organoid monolayers from wild-type mice (76). Importantly, β-catenin was less activated in H. pylori-infected gastric organoids from Smox deficient mice compared to WT gastroids, and this was recapitulated in human gastric organoids treated with the SMOX inhibitor SLH150–54 (76). The effect of Smox deletion or SMOX inhibition was reversed by adding back spermidine (76); however, the effects of SMOX silencing and spermidine supplementation were not observed in uninfected cells (76), demonstrating that spermidine facilitates β-catenin activation in H. pylori-infected cells, but does not initiate the process. Elucidation of the underlying molecular mechanisms deserves further investigation.
Of note, the knockdown of AOC1 via siRNA results in the inhibition of proliferation, cell invasion/migration, AKT signaling, and EMT transition with co-current promotion of apoptosis in the human GC cell lines AGS and MKN45 (79). This suggests an important role for AOC1 not only in polyamine homeostasis, but also in GC tumor progression.
Thus, although ODC is not induced in GECs during H. pylori infection, its activity plays a major role in GC progression, notably by supporting cell growth and proliferation; polyamine catabolism, notably by SMOX activity, in GECs is implicated in gastric carcinogenesis and GC progression (Fig. 2).
5. Polyamines as a target in GC
Polyamines are critical regulators of cell growth and differentiation in the GI tract and play essential roles in repairing gastric mucosal damage (97). However, uncontrolled production of polyamines can lead to aberrant differentiation and proliferation, which are hallmarks of carcinogenesis and cancer progression.
5.1. Effect of polyamines on gastritis and colonization
Studies focused on inhibiting ODC with a chemical inhibitor or through genetic knockout in mice were employed to elucidate its role in carcinogenesis progression and immune response. Administration of DFMO to C57BL/6 mice during a four-month infection with H. pylori SS1 revealed a two-log-order reduction in bacterial levels and a significant decrease in gastric inflammation; in addition, the two-fold increase in polyamine levels found in the gastric tissue of infected compared to control animals were reverted back to basal levels with DFMO treatment (65). Furthermore, isolated gastric macrophages from infected mice treated with DFMO exhibited an increase in L-arginine uptake, NOS2 protein, and NO (65). These data suggest that inhibition of ODC dampens gastric macrophage activation, and reduces H. pylori burden and development of gastric inflammation. Our data in OdcΔmye mice with the same H. pylori strain and infection length again showed decreased H. pylori colonization, but increased gastritis (66). There was a significant increase in the number of CD68+ODC+ cells in the H. pylori-infected versus control patients, which demonstrates an association between upregulation of ODC and bacterial infection in human gastric macrophages. Luminex multiple array analysis of the OdcΔmye mouse gastric tissues revealed that chemokines were significantly increased in OdcΔmye gastric tissues. This enhanced chemokine production suggests that ODC deletion in macrophages correlates with enhanced immune cell infiltration into the stomach, contributing to the enhanced inflammation observed (66). Additionally, the cytokines interleukin (IL)-17, a hallmark of the Th17 response (98), specifically in H. pylori infection (99), and tumor necrosis factor (TNF-α), were detected in significantly higher levels in the OdcΔmye gastric tissues (66). This emphasizes that ODC expression in myeloid cells reduces inflammation and thus the innate responses and antimicrobial activity against H. pylori. It should be also noted that DFMO exhibits a direct effect on H. pylori growth and virulence (100,101), which may explain the discrepancy in gastritis observed between the results observed with Odc knockdown and DFMO treatment; note that the effect of DFMO on H. pylori occurs independently of an effect on polyamines, but by causing direct oxidative damage (101). The infection of mice with specific deletion of Odc in GECs is warranted to understand the role of epithelial ODC on gastritis and bacterial persistence and is underway in our laboratory.
Smox-deficient mice exhibit a significant reduction in gastric spermidine levels and inflammation, but increased colonization (76), indicating that spermidine in the stomach supports gastritis and the antimicrobial effect. Interestingly, we also observed that the specific deletion of DHPS, which uses spermidine as a substrate to generate EIF5AHyp, in myeloid cells resulted in a significant increase of stomach colonization by H. pylori and gastritis (88), demonstrating that spermidine metabolism by the SMOX/DHPS metabolic pathway is critical for the antimicrobial response in vivo. It is now important to determine why SMOX and DHPS favor and dampen inflammation of the stomach, respectively.
5.2. Polyamines regulate gastric carcinogenesis
Investigation into the role of a carbonate supplemented diet on Wistar rats induced with spontaneous GC through gastric resection by Ehrnstrӧm et al. revealed that animals fed the carbonate diet exhibit an increase in ODC protein expression, which is correlated with enhanced cell proliferation and augmented incidence of GC (102). In addition, in the models of GC induced by N-methyl-N’-nitro-N-nitrosoguanidine (MNN) plus sodium chloride or by MNN plus tyrosine methyl ester, the ODC inhibitor 1,3-diaminopropane significantly reduces putrescine and spermidine production, and the number of cancers (103,104).
Further, the number of GECs with oxidative DNA damage and development of dysplasia and carcinoma were also significantly reduced by DFMO in Mongolian gerbils infected with the cancer-inducing clinical strain PZ5056G (74). In this study, the SMOX inhibitor MDL72527 has a similar effect as DFMO and that the combination of both compounds had a modest additive effect (74).
Thus, although the effect of polyamines on gastritis and colonization is complex and cell-specific, it seems that overall, polyamines are essential for gastric carcinogenesis and that inhibition of ODC and/or SMOX might represent a potent chemopreventive strategy in H. pylori-infected patients.
5.3. Human clinical trials
There is increased interest in targeting polyamine synthesis and metabolism for cancer prevention and therapy (Table 1). DFMO was one of the first drugs developed in the 1970s to target cancer cell growth via ODC activity inhibition (105). Initial in vitro studies with rat hepatoma and mouse leukemia cells revealed that DFMO administration decreased putrescine and spermidine concentrations, but barely affected spermine levels (106). However, DFMO was less effective in vivo in advanced small cell lung cancer and metastatic colon cancer clinical trials both alone and in combination with other agents (107). The ineffectiveness of DFMO was likely due to compensatory mechanisms that were activated upon polyamine depletion. Despite its mixed success, DFMO remains a possible anticancer agent due to its low toxicity, with only reversible ototoxicity reported (107). Other polyamine biosynthesis blockers, methylglyoxal bis (guanylhydrazone) and 4-amidinoindan-1-one-2’-amidinhydrazone (SAM486A), were investigated to target SAMDC. However, guanylhydrazone administration to hamsters resulted in undesired mitochondrial toxicity (108) and SAM486A displayed mixed success as a single agent in a phase II trial for relapsed or refractory non-Hodgkin’s lymphoma (109) and in combination with other anti-proliferative agents, including 5-fluorouracil and leucovorin, in a phase I metastatic colorectal cancer trial (110). SAM486A was also tested in a phase II clinical trial for patients with metastatic melanoma, however many patients experienced severe toxicity with no confirmed partial response (111).
Table 1.
Summary of clinical trial outcomes discussed in this review.
| Cancer Type | Target | Drug | Combination Therapy | Clinical Trial | Patients Enrolled | Outcome | Toxicity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Carcinoma (breast, prostate, stomach, female genital organ) or metastatic carcinoma of unknown origin | ODC | DFMO | Conventional chemotherapy | Phase I-II | 38 | No difference in disease progression in patients receiving DFMO + conventional chemotherapy vs. conventional chemotherapy only | Ototoxicity resulted in discontinuation of therapy for 6 patients | 107 |
| Relapsed or refractory non-Hodgkin’s lymphoma | SAMDC | SAM486A | None | Phase II | 41 | Overall response rate (complete plus partial response): 18.9% for the 7 evaluable patients | Hematological: anemia, febrile neutropenia, thrombocytopenia Nonhematological: nausea, vomiting, diarrhea, asthenia, abdominal pain, flushing |
109 |
| Metastatic colorectal cancer | SAMDC | SAM486A | 5-fluorouracil/leucovorin | Phase I | 27 | 8/26 patients received a partial response & 9/26 had stable disease | Fatigue, dizziness, facial paresthesia, somnolence, skin toxicity | 110 |
| Metastatic melanoma | SAMDC | SAM486A | None | Phase II | 15 | No significant therapeutic potential & no reduction in tumor metabolism | Fatigue/lethargy, myalgia, neutropenia | 111 |
| Neuroblastoma | ODC | DFMO | None | Phase II | 140 | - Maintenance therapy to prevent relapse after standard therapy: 2-year survival: 84% ± 4%; overall survival: 87 ± 2%. - Maintenance therapy after salvage therapy for relapsed/refractory disease: 2-year survival: 54 ± 8%; overall survival: 84 ± 6% |
67% of patients reported no treatment-related adverse events; the most common reported toxicity was Grade 2–3 liver injury | 112 |
| Sporadic colorectal adenoma | ODC | DFMO | Sulindac | Randomized placebo-control, double blind trial | 375 | Reduced recurrence of all adenomas (70%), advanced adenomas (92%), and recurrence of more than 1 adenoma (95%) | No differences in toxicity between groups | 113 |
Recently in a phase II trial to evaluate DFMO as a potential maintenance therapy for children with high-risk neuroblastoma, Sholler et al. demonstrated that twice daily oral administration of DFMO for two years was well tolerated and was associated with high event free survival and overall survival (112). In a randomized placebo-control, double blind trial for sporadic colorectal adenoma prevention, Meyskens et al. reported a reduction in recurrent adenomatous polyps, with few side effects, after oral administration of DFMO and sulindac, a known nonsteroidal anti-inflammatory drug, once daily for 36 months (113). The clinical success of DFMO in these two cancers has spurred interest for clinical trials in other polyamine-implicated cancers. To this end, based on our data showing reduced GC in H. pylori-infected gerbils (74) and other work presented in this review, our group has been conducting a clinical trial of DFMO in patients with precancerous gastric lesions in Latin America (ClinicalTrials.gov Identifier: NCT02794428); the study will be completed in late 2021.
Conclusions
Polyamine research has progressed considerably over the past few decades, elucidating the cellular and molecular functions of polyamines. Polyamines are essential for a variety of critical cellular functions including cell growth, proliferation, differentiation, and survival. Overall, in the context of H. pylori-induced GC development, polyamines are critical for carcinogenesis and for progression. Nonetheless, each polyamine can have differential effects in GECs and immune cells, and further deeper investigations are required to decipher their roles in the global oncogenic picture of the stomach. Regardless, recent advances have prompted clinical trials targeting key enzymes in the polyamine metabolic pathway including ODC.
Acknowledgements:
This work was funded by NIH grants R01CA190612 (K.T.W.), P01CA116087 (K.T.W.), P01CA028842 (K.T.W.), and R21AI142042 (K.T.W.); Veterans Affairs Merit Review grants I01BX001453 and I01CX002171 (K.T.W.); Department of Defense grant W81XWH-18-1-0301 (K.T.W.); Crohn’s & Colitis Foundation Senior Research Award 703003 (K.T.W.); the Thomas F. Frist Sr. Endowment (K.T.W.); and the Vanderbilt Center for Mucosal Inflammation and Cancer (K.T.W.). K.T.W. also receives support from the Vanderbilt Digestive Disease Research Center (NIH grant P30DK058404). K.M.M. was supported by NIH grant T32CA009592.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 3.Correa P, Piazuelo BM, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa P, Shiao Y. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s–3s. [PubMed] [Google Scholar]
- 5.Piazuelo MB, Bravo LE, Mera RM, Camargo MC, Bravo JC, Delgado AG, et al. The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology. 2021;160:1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge S, Xia X, Ding C, Zhen B, Zhou Q, Feng J, et al. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–9. [DOI] [PubMed] [Google Scholar]
- 8.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J-L, Zhang L, Brown LM, Li J-Y, Shen L, Pan K-F, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You W, Brown LM, Zhang L, Li J, Jin M, Chang Y, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83. [DOI] [PubMed] [Google Scholar]
- 13.Mera R, Fontham ETH, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camargo MC, Piazuelo MB, Mera RM, Fontham ETH, Delgado AG, Yepez MC, et al. Effect of smoking on failure of H. pylori therapy and gastric histology in a high gastric cancer risk area of Colombia. Acta Gastroenterol Latinoam. 2007;37:238–45. [PMC free article] [PubMed] [Google Scholar]
- 15.Blaser MJ. Ecology of Helicobacter pylori in the human stomach. J Clin Invest. 1997;100:759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018;67:1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–70. [DOI] [PubMed] [Google Scholar]
- 18.Kountouras J, Zavos C, Chatzopoulos D. New concepts of molecular biology on gastric carcinogenesis. Hepatogastroenterology. 2005;52:1305–12. [PubMed] [Google Scholar]
- 19.Li D, Lo W, Rudloff U. Merging perspectives: genotype-directed molecular therapy for hereditary diffuse gastric cancer (HDGC) and E-cadherin-EGFR crosstalk. Clin Transl Med. 2018;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etemadi A, Safiri S, Sepanlou SG, Ikuta K, Bisignano C, Shakeri R, et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardbower DM, Peek RM, Wilson KT. At the bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. J Leukoc Biol. 2014;96:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobert AP, Wilson KT. Human and Helicobacter pylori interactions determine the outcome of gastric diseases. Curr Top Microbiol Immunol. 2017;400:27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol Cell Physiol. 1982;243:C212–21. [DOI] [PubMed] [Google Scholar]
- 24.Michael AJ. Polyamines in eukaryotes, bacteria, and archaea. J Biol Chem. 2016;291:14896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei G, Hobbs CA, Defeo K, Hayes CS, Gilmour SK. Polyamine-mediated regulation of protein acetylation in murine skin and tumors. Mol Carcinog. 2007;46:611–7. [DOI] [PubMed] [Google Scholar]
- 27.Munder M Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–32. [DOI] [PubMed] [Google Scholar]
- 29.Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–32. [DOI] [PubMed] [Google Scholar]
- 30.Asim M, Chaturvedi R, Hoge S, Lewis ND, Singh K, Barry DP, et al. Helicobacter pylori induces ERK-dependent formation of a phospho-c-Fos·c-Jun activator protein-1 complex that causes apoptosis in macrophages. J Biol Chem. 2010;285:20343–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, et al. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod. 2014;90:84. [DOI] [PubMed] [Google Scholar]
- 32.LoGiudice N, Le L, Abuan I, Leizorek Y, Roberts SC. Alpha-difluoromethylornithine, an irreversible inhibitor of polyamine biosynthesis, as a therapeutic strategy against hyperproliferative and infectious diseases. Med Sci (Basel). 2018;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsui I, Wiegand L, Pegg AE. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981;256:2454–9. [PubMed] [Google Scholar]
- 34.Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer DL, Diegelman P, Jell J, Vujcic S, Merali S, Porter CW. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem. 2008;283:4241–51. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, et al. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–11. [DOI] [PubMed] [Google Scholar]
- 37.Gobert AP, Al-Greene NT, Singh K, Coburn LA, Sierra JC, Verriere TG, et al. Distinct immunomodulatory effects of spermine oxidase in colitis induced by epithelial injury or infection. Front Immunol. 2018;9:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vakal S, Jalkanen S, Dahlström KM, Salminen TA. Human copper-containing amine oxidases in drug design and development. Molecules. 2020;25:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981;78:2869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park MH, Lee YB, Joe YA. Hypusine is essential for eukaryotic cell proliferation. Biol Signals. 1997;6:115–23. [DOI] [PubMed] [Google Scholar]
- 41.Chen K, Liu A. Biochemistry and function of hypusine formation on eukaryotic initiation factor 5A. Biol Signals. 1997;6:105–9. [DOI] [PubMed] [Google Scholar]
- 42.Murphey RJ, Gerner EW. Hypusine formation in protein by a two-step process in cell lysates. J Biol Chem. 1987;262:15033–6. [PubMed] [Google Scholar]
- 43.Abbruzzese A, Park MH, Folk JE. Deoxyhypusine hydroxylase from rat testis. Partial purification and characterization. J Biol Chem. 1986;261:3085–9. [PubMed] [Google Scholar]
- 44.Nakanishi S, Cleveland JL. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids. 2016;48:2353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins ZA, Hååg PG, Johansson HE. Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics. 2001;71:101–9. [DOI] [PubMed] [Google Scholar]
- 46.Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, et al. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu A, Jao DL-E, Chen KY. Identification of mRNA that binds to eukaryotic initiation factor 5A by affinity co-purification and differential display. Biochem J. 2004;384:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu A, Chen KY. Hypusine is required for a sequence-specific interaction of eukaryotic initiation factor 5A with postsystematic evolution of ligands by exponential enrichment RNA. J Biol Chem. 2001;276:2555–61. [DOI] [PubMed] [Google Scholar]
- 49.Aksu M, Trakhanov S, Görlich D. Structure of the exportin Xpo4 in complex with RanGTP and the hypusine-containing translation factor eIF5A. Nat Commun. 2016;7:11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park MH, Wolff EC. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J Biol Chem. 2018;293:18710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelechano V, Alepuz P. eIF5A facilitates translation termination globally and promotes the elongation of many non-polyproline-specific tripeptide sequences. Nucleic Acids Res. 2017;45:7326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuller AP, Wu CC-C, Dever TE, Buskirk AR, Green R. eIF5A functions globally in translation elongation and termination. Mol Cell. 2017;66:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutierrez E, Shin B-S, Woolstenhulme CJ, Kim J-R, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cano VSP, Jeon GA, Johansson HE, Henderson CA, Park J-H, Valentini SR, et al. Mutational analysis of human eIF5A-1: identification of amino acid residues critical for hypusine modification and eIF5A activity. FEBS J. 2008;275:44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki K, Abid MR, Miyazaki M. Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett. 1996;384:151–4. [DOI] [PubMed] [Google Scholar]
- 57.Park J-H, Aravind L, Wolff EC, Kaevel J, Kim YS, Park MH. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci U S A. 2006;103:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimura K, Lee SB, Park JH, Park MH. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 2012;42:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henning S, Pällmann N, Miller KK, Hermans-Borgmeyer I, Venz S, Sendoel A, et al. A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis Model Mech. 2014;7:963–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pällmann N, Braig M, Sievert H, Preukschas M, Hermans-Borgmeyer I, Schweizer M, et al. Biological relevance and therapeutic potential of the hypusine modification system. J Biol Chem. 2015;290:18343–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coni S, Serrao SM, Yurtsever ZN, Di Magno L, Bordone R, Bertani C, et al. Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis. 2020;11:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu S, Liu J, Xing F. Antizyme inhibitor 1: a potential carcinogenic molecule. Cancer Sci. 2017;108:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patchett SE, Alstead EM, Butruk L, Przytulski K, Farthing MJ. Ornithine decarboxylase as a marker for premalignancy in the stomach. Gut. 1995;37:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miao X-P, Li J-S, Li H-Y, Zeng S-P, Zhao Y, Zeng J-Z. Expression of ornithine decarboxylase in precancerous and cancerous gastric lesions. World J Gastroenterol. 2007;13:2867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, et al. Polyamines impair immunity to Helicobacter pylori by inhibiting L-arginine uptake required for nitric oxide production. Gastroenterology. 2010;139:1686–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A. 2017;114:E751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elitsur Y, Majumdar AP, Tureaud J, Dosescu J, Neace C, Velusamy L, et al. Tyrosine kinase and ornithine decarboxylase activation in children with Helicobacter pylori gastritis. Life Sci. 1999;65:1373–80. [DOI] [PubMed] [Google Scholar]
- 68.Konturek PC, Rembiasz K, Konturek SJ, Stachura J, Bielanski W, Galuschka K, et al. Gene expression of ornithine decarboxylase, cyclooxygenase-2, and gastrin in atrophic gastric mucosa infected with Helicobacter pylori before and after eradication therapy. Dig Dis Sci. 2003;48:36–46. [DOI] [PubMed] [Google Scholar]
- 69.Hirasawa R, Tatsuta M, Iishi H, Yano H, Baba M, Uedo N, et al. Increase in apoptosis and decrease in ornithine decarboxylase activity of the gastric mucosa in patients with atrophic gastritis and gastric ulcer after successful eradication of Helicobacter pylori. Am J Gastroenterol. 1999;94:2398–402. [DOI] [PubMed] [Google Scholar]
- 70.Takashima T, Fujiwara Y, Watanabe T, Tominaga K, Oshitani N, Higuchi K, et al. High molecular protein of Helicobacter pylori responsible for inhibition of ornithine decarboxylase activity of human gastric cultured cells. Aliment Pharmacol Ther. 2002;16:167–73. [DOI] [PubMed] [Google Scholar]
- 71.Xu X, Liu Z, Fang M, Yu H, Liang X, Li X, et al. Helicobacter pylori CagA induces ornithine decarboxylase upregulation via Src/MEK/ERK/c-Myc pathway: implication for progression of gastric diseases. Exp Biol Med (Maywood). 2012;237:435–41. [DOI] [PubMed] [Google Scholar]
- 72.Gobert AP, Cheng Y, Wang J-Y, Boucher J-L, Iyer RK, Cederbaum SD, et al. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692–700. [DOI] [PubMed] [Google Scholar]
- 73.Cheng Y, Chaturvedi R, Asim M, Bussière FI, Scholz A, Xu H, et al. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J Biol Chem. 2005;280:22492–6. [DOI] [PubMed] [Google Scholar]
- 74.Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene. 2015;34:3429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray-Stewart T, Sierra JC, Piazuelo MB, Mera RM, Chaturvedi R, Bravo LE, et al. Epigenetic silencing of miR-124 prevents spermine oxidase regulation: implications for Helicobacter pylori-induced gastric cancer. Oncogene. 2016;35:5480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sierra JC, Piazuelo MB, Luis PB, Barry DP, Allaman MM, Asim M, et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene. 2020;39:4465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaturvedi R, Cheng Y, Asim M, Bussière FI, Xu H, Gobert AP, et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–73. [DOI] [PubMed] [Google Scholar]
- 78.Chaturvedi R, Asim M, Barry DP, Frye JW, Casero RA, Wilson KT. Spermine oxidase is a regulator of macrophage host response to Helicobacter pylori: enhancement of antimicrobial nitric oxide generation by depletion of spermine. Amino Acids. 2014;46:531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu F, Xu Y, Xiong J-H, Zhang J-H, Wu J, Luo J, et al. AOC1 contributes to tumor progression by promoting the AKT and EMT pathways in gastric cancer. Cancer Manag Res. 2020;12:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung MH, Kim SC, Jeon GA, Kim SH, Kim Y, Choi KS, et al. Identification of differentially expressed genes in normal and tumor human gastric tissue. Genomics. 2000;69:281–6. [DOI] [PubMed] [Google Scholar]
- 81.Okugawa Y, Toiyama Y, Shigeyasu K, Yamamoto A, Shigemori T, Yin C, et al. Enhanced AZIN1 RNA editing and overexpression of its regulatory enzyme ADAR1 are important prognostic biomarkers in gastric cancer. J Transl Med. 2018;16:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lundell L, Rosengren E. Polyamine levels in human gastric carcinoma. Scand J Gastroenterol. 1986;21:829–32. [DOI] [PubMed] [Google Scholar]
- 83.Linsalata M, Russo F, Notarnicola M, Berloco P, Di Leo A. Polyamine profile in human gastric mucosa infected by Helicobacter pylori. Ital J Gastroenterol Hepatol. 1998;30:484–9. [PubMed] [Google Scholar]
- 84.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. [DOI] [PubMed] [Google Scholar]
- 85.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–6. [PubMed] [Google Scholar]
- 88.Gobert AP, Finley JL, Latour YL, Asim M, Smith TM, Verriere TG, et al. Hypusination orchestrates the antimicrobial response of macrophages. Cell Rep. 2020;33:108510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bussière FI, Chaturvedi R, Cheng Y, Gobert AP, Asim M, Blumberg DR, et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem. 2005;280:2409–12. [DOI] [PubMed] [Google Scholar]
- 90.Xu C, Yan Y, Yang Y, Liu B, Xin J, Chen S, et al. Downregulation of ornithine decarboxylase by pcDNA-ODCr inhibits gastric cancer cell growth in vitro. Mol Biol Rep. 2011;38:949–55. [DOI] [PubMed] [Google Scholar]
- 91.Lewis ND, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB, et al. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol. 2011;186:3632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gamble LD, Purgato S, Murray J, Xiao L, Yu DMT, Hanssen KM, et al. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci Transl Med. 2019;11:eaau1099. [DOI] [PubMed] [Google Scholar]
- 93.Devens BH, Weeks RS, Burns MR, Carlson CL, Brawer MK. Polyamine depletion therapy in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:275–9. [DOI] [PubMed] [Google Scholar]
- 94.Milovic V, Turchanowa L. Polyamines and colon cancer. Biochem Soc Trans. 2003;31:381–3. [DOI] [PubMed] [Google Scholar]
- 95.Saydjari R, Alexander RW, Upp JR, Poston GJ, Barranco SC, Townsend CM, et al. The effect of tumor burden on ornithine decarboxylase activity in mice. Cancer Invest. 1991;9:415–9. [DOI] [PubMed] [Google Scholar]
- 96.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA Damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–5. [DOI] [PubMed] [Google Scholar]
- 97.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol. 1990;259:G584–592. [DOI] [PubMed] [Google Scholar]
- 98.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. [DOI] [PubMed] [Google Scholar]
- 99.Hardbower DM, Singh K, Asim M, Verriere TG, Olivares-Villagómez D, Barry DP, et al. EGFR regulates macrophage activation and function in bacterial infection. J Clin Invest. 2016;126:3296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barry DP, Asim M, Leiman DA, de Sablet T, Singh K, Casero RA, et al. Difluoromethylornithine is a novel inhibitor of Helicobacter pylori growth, CagA translocation, and interleukin-8 induction. PloS One. 2011;6:e17510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sierra JC, Suarez G, Piazuelo MB, Luis PB, Baker DR, Romero-Gallo J, et al. α-Difluoromethylornithine reduces gastric carcinogenesis by causing mutations in Helicobacter pylori cagY. Proc Natl Acad Sci U S A. 2019;116:5077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ehrnström RA, Veress B, Arvidsson S, Sternby NH, Andersson T, Lindström CG. Dietary supplementation of carbonate promotes spontaneous tumorigenesis in a rat gastric stump model. Scand J Gastroenterol. 2006;41:12–20. [DOI] [PubMed] [Google Scholar]
- 103.Iishi H, Tatsuta M, Baba M, Yano H, Sakai N, Uehara H, et al. Ornithine decarboxylase inhibitor lessens the rat gastric carcinogenesis enhancement caused by tyrosine methyl ester. Int J Cancer. 1997;73:113–6. [DOI] [PubMed] [Google Scholar]
- 104.Tatsuta M, Iishi H, Baba M, Yano H, Uehara H, Nakaizumi A. Ornithine decarboxylase inhibitor attenuates NaCl enhancement of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine. Carcinogenesis. 1995;16:2107–10. [DOI] [PubMed] [Google Scholar]
- 105.Prakash NJ, Schechter PJ, Grove J, Koch-Weser J. Effect of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on L1210 leukemia in mice. Cancer Res. 1978;38:3059–62. [PubMed] [Google Scholar]
- 106.Mamont PS, Duchesne M-C, Grove J, Bey P. Anti-proliferative properties of DL-α-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978;81:58–66. [DOI] [PubMed] [Google Scholar]
- 107.Horn Y, Schechter PJ, Marton LJ. Phase I-II clinical trial with alpha-difluoromethylornithine--an inhibitor of polyamine biosynthesis. Eur J Cancer Clin Oncol. 1987;23:1103–7. [DOI] [PubMed] [Google Scholar]
- 108.Nass MM. Analysis of methylglyoxal bis(guanylhydrazone)-induced alterations of hamster tumor mitochondria by correlated studies of selective rhodamine binding, ultrastructural damage, DNA replication, and reversibility. Cancer Res. 1984;44:2677–88. [PubMed] [Google Scholar]
- 109.Pless M, Belhadj K, Menssen HD, Kern W, Coiffier B, Wolf J, et al. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin’s lymphoma: results from a phase II multicenter study. Clin Cancer Res. 2004;10:1299–305. [DOI] [PubMed] [Google Scholar]
- 110.Zuylen L van, Bridgewater J, Sparreboom A, Eskens FALM, Bruijn P de, Sklenar I, et al. Phase I and pharmacokinetic study of the polyamine synthesis inhibitor SAM486A in combination with 5-fluorouracil/ leucovorin in metastatic colorectal cancer. Clin Cancer Res. 2004;10:1949–55. [DOI] [PubMed] [Google Scholar]
- 111.Millward MJ, Joshua A, Kefford R, Aamdal S, Thomson D, Hersey P, et al. Multi-centre phase II trial of the polyamine synthesis inhibitor SAM486A (CGP48664) in patients with metastatic melanoma. Invest New Drugs. 2005;23:253–6. [DOI] [PubMed] [Google Scholar]
- 112.Sholler GLS, Ferguson W, Bergendahl G, Bond JP, Neville K, Eslin D, et al. Maintenance DFMO increases survival in high-risk neuroblastoma. Sci Rep. 2018;8:14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa). 2008;1:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]