Abstract
Limited data exist regarding systolic blood pressure (SBP) through mid- to late-life and late-life cardiac function and heart failure (HF) risk. Among 4,578 HF-free participants in the Atherosclerosis Risk in Communities study attending the 5th Visit (2011-2013; age 75±5 years), time-averaged cumulative SBP was calculated as the sum of averaged SBPs from adjacent consecutive visits (Visits 1-5) indexed to total observation time (24±1 years). Calculations were performed using measured SBPs and also incorporating antihypertensive medication specific effect constants (‘underlying’ SBP). Outcomes included comprehensive echocardiography at Visit 5 and post-Visit 5 incident HF, HF with preserved ejection fraction (HFpEF), and reduced ejection fraction (HFrEF). Higher cumulative SBP was associated with greater LV mass and worse diastolic measures (all p <0.001), associations that were stronger with underlying compared to cumulative SBP (all p<0.05). At 5.6±1.2 years follow-up post-Visit 5, higher cumulative measured and underlying SBP were associated with incident HF (HR per 10 mmHg for measured: 1.12 [1.01-1.24]; underlying: 1.19 [95%CI 1.10-1.30]) and HFpEF (measured: 1.15 [1.00-1.33]; underlying: 1.28 [1.14-1.45]), but not HFrEF (measured: 1.11 [0.94-1.32]; underlying: 1.11 [0.96-1.24]). Associations with HF and HFpEF were more robust with cumulative underlying compared to measured SBP (all p <0.05). Time-averaged cumulative SBP in mid- to late-life is associated with worse cardiac function and risk of incident HF, especially HFpEF, in late-life. These associations were stronger considering underlying as opposed to measured SBP, highlighting the importance of prevention and effective treatment of hypertension to prevent late-life cardiac dysfunction and HF.
Keywords: Time-averaged cumulative blood pressure, cardiac structure, cardiac function, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction
INTRODUCTION
Hypertension is a well-established and potent risk factor for cardiovascular diseases (CVD), including heart failure (HF)1. The risk of HF is greatest in late life, a time of life when the large majority of persons have been diagnosed with hypertension2,3. While therapeutic trials have clearly demonstrated reduced incidence of HF with effective treatment of hypertension in the elderly4, 5, it is likely that lifetime hypertension burden is more relevant to HF risk than blood pressure at a single time point in late life. Prior studies have demonstrated associations of higher blood pressure over time (cumulative blood pressure) with heightened risk of all-cause mortality,6 coronary heart disease (CHD),7,8,9 and heart failure (HF)7,9,10. Higher cumulative blood pressure through mid-life also associates with greater left ventricular mass index (LVMI) and wall thickness,11,12 while higher cumulative blood pressure in young adults associates with reduced systolic and diastolic function in mid-life.13 However, despite this wealth of data, important knowledge gaps persist, particularly in late life. Limited data exist regarding the relationship of cumulative blood pressure from mid- to late-life with late life cardiac function and HF risk, particularly subsequent risk of HF with preserved left ventricular ejection fraction (HFpEF). The extent to which cumulative blood pressure – as an expression of the burden of hypertension throughout mid-to late-life – is superior to blood pressure in late life alone in predicting late-life cardiac function and HF risk is not known. Finally, underlying hypertension severity – reflected in both the measured blood pressure and concomitant antihypertensive medication to achieve that measured blood pressure – may indicate more advanced underlying arterial dysfunction, longer exposure to untreated hypertension, or a genetic predisposition to hypertension not captured by measured blood pressure alone. However, it remains unclear whether measured blood pressure or underlying hypertension severity is most relevant for these endpoints in late life.
We determined the association of time-averaged cumulative systolic blood pressure (SBP) assessed over 25-years with late life cardiac structure and function and risk of incident HFpEF and HF with reduced LVEF (HFrEF) among 4,578 HF-free participants in the Atherosclerosis Risk in Communities (ARIC) study who attended the fifth study visit. We compared the magnitude and strength of associations of cumulative SBP with those observed for SBP at Visit 5 alone, and of cumulative SBP based on measured SBPs with that based on estimated underlying SBP calculated using medication specific effect constants.
METHODS
Study population
The ARIC study14 is an ongoing, prospective observational cohort study which recruited a total of 15,792 participants aged 45 to 64 years old between 1987 and 1989 (Visit 1) from four communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland. Subsequent visits were: Visit 2 (1990-92), Visit 3 (1993-95), Visit 4 (1996-98), Visit 5 (2011-13), Visit 6 (2016-2017), and Visit 7 (2018-2019). The ARIC study has been approved by Institutional Review Boards (IRB) of all participating institutions and all participants provided written informed consent. Data availability and detailed policies for requesting Atherosclerosis Risk in Communities (ARIC) data can be found at https://www2.cscc.unc.edu/aric/pubs-policies-and-forms-pg. ARIC data can also be obtained from the NHLBI BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/).
A total of 6,118 participants attended the fifth study visit and underwent echocardiography. For this analysis, those with missing BP measurements or antihypertensive medication information at any of the five study visits, those with an ejection fraction <50% on the Visit 5 echocardiogram, those with prevalent HF at Visit 5, and those with significant valvular heart disease (defined as more than moderate aortic or mitral valve disease) were excluded. Also, as medication specific effect constants to estimate underlying BP were available only for black and white race/ethnicity, participants reporting Asian and Native American race/ethnicity (total n=14) were excluded from the analysis. A total of 4,578 participants were included in this analysis.
Ascertainment of clinical covariates
Clinical covariates including age, sex, race, body mass index (BMI), and medication usage were identified at every visit. Antihypertensive medications were classified as angiotensin-converting-enzyme inhibitors (ACE inhibitors), alpha blocker, beta blocker, calcium channel blocker, and diuretics (thiazide, loop, or potassium sparing), with nitrates, angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRA) categorized as “other antihypertensive medications”. The presence of co-morbidities including diabetes, obesity (BMI ≥30 kg/m2), chronic kidney disease (CKD; eGFR <60 ml/min/1.73 m2), anemia (hemoglobin <12g/dL for female, <13g/dL for male), history of coronary heart disease, and atrial fibrillation were identified at the time of Visit 5 or based on diagnosis from prior visits. Prevalent HF at Visit 5 was ascertained from multiple sources either based on International Classification of Disease code for those hospitalized prior to 2005, physician-adjudicated HF hospitalization for those happened after 2005, any physician report of HF prior to the Visit 5 exam date, self-reported HF, or self-reported HF medication use.
Peripheral BP measurements and derived SBP measurements
At Visits 1 through 4, brachial blood pressure was measured using a random-zero sphygmomanometer. At Visit 5, blood pressure was measured using an automatic sphygmomanometer (OMRON HEM-907XL). Measurements were made by trained technicians according to specific study protocols as detailed elsewhere15. A minimum of 5 minutes of resting time was required prior to the first BP measurements. For visits except the fourth, an average of the second and the third BP measurements were recorded and an average of two measurements were recorded as Visit 5 SBP.
Underlying SBP accounts for both measured SBP and the number and type of concomitant antihypertensive medications necessary to achieve the measured SBP, and may be more relevant to cardiac dysfunction and HF risk than measured SBP alone. To estimate underlying SBP among participants on antihypertensive medications, we employed antihypertensive medication specific effect constants (table S1) calculated as the weighted average of effect estimates for six major antihypertensive medications from 165 antihypertensive clinical trials,16 and previously validated in ARIC17. For participants who are not on antihypertensive medication, the underlying pressure was equivalent to the measure blood pressure.
Time-averaged cumulative SBP over the five study visits was defined as the sum of averaged underlying SBPs from adjacent consecutive visits, indexed to total exposure time between visits 1 and 5 (mean time 24±1 years) as follows (see also figure S1):
VnSBP: SBP at Visit n
TV[n,n+1]: Time between Visit n and n+1
TVtotal: Total time between Visit 1 and 5
Cumulative SBP was calculated using the measured SBP values (‘cumulative measured SBP’), and using the estimated underlying SBP values (‘cumulative underlying SBP’). Blood pressure measurements performed at Visit 6 and 7 were not included in this analysis.
Echocardiographic measures of cardiac structure and function
Comprehensive echocardiography was performed at Visit 5 according to a study-specific protocol and using uniform equipment by dedicated, trained, and certified study sonographers as previously described18. Quantitative measures of cardiac structure, systolic function, and diastolic function were performed by a central reading center blinded to clinical information, and according to recommendations from the American Society of Echocardiography (Supplemental materials for further details)19. Reproducibility metrics have been previously published18.
Assessment of clinical outcomes
Incident of HF after Visit 5 was ascertained based on physician adjudication aided by comprehensive abstraction of medical records from hospitalizations with an ICD code related to potential HF as previously described20. Incident HFpEF was defined as an adjudicated HF event with a documented LVEF ≥50% at the time of HF hospitalization, and incident HFrEF was defined as adjudicated HF with an LVEF <50% at the time of hospitalization. If LVEF was not available from the time of hospitalization, the closest available LVEF from a hospitalization within 6 months prior to the index hospitalization was used if there was no intercurrent adjudicated MI. Among 243 incident HF events over 5.6 ± 1.2 years of follow-up, 85 (35%) were HFrEF, 120 (49%) were HFpEF, and no LVEF was available in 38 (16%). Additional analyses were performed for the composite of each of HF event (overall HF, HFpEF, and HFrEF) with all-cause death post Visit 5. All-cause deaths were ascertained by ARIC surveillance or the National Death Index21.
Statistical Analysis
For descriptive analysis, studied participants were categorized into the following groups: (1) those whose SBP was <130 mmHg at each study visit without any antihypertensive use; (2) those whose SBP was <130 mmHg at each study visit with antihypertensive use at ≥1 visit; (3) tertiles of cumulative SBP for the remaining participants. Trends in clinical characteristics and echocardiographic measures at Visit 5 were assessed across these five categories, except trend in antihypertensive medication use at Visit 5 was assessed across tertiles of participants with SBP ≥130 mmHg with or without antihypertensive medication use at ≥1 visit. Trend p-values were adjusted for demographics including age, sex, race, field center, and BMI at Visit 5.
For association of SBP with echocardiographic measures and incident HF, we employed the following exposures: (1) measured SBP at Visit 5, (2) time-averaged cumulative measured SBP from Visits 1 through 5, (3) predicted underlying SBP at Visit 5, and (4) time-averaged cumulative predicted underlying SBP from Visits 1 through 5. The continuous associations between SBP and echocardiographic measures were initially assessed by restricted cubic spline analysis to assess for potential non-linear associations. Multivariable linear regression was used for linear relationships. Statistical comparison between time-averaged cumulative SBP and single time point Visit 5 SBP was performed by testing for the significance of the delta between the time-averaged cumulative SBP and Visit 5 SBP in a multivariable model with Visit 5 SBP, demographics (age, sex, race, field center, BMI), and co-morbidities (smoking status, diabetes, CKD, obesity [BMI ≥30 kg/m2], CHD, and atrial fibrillation at Visit 5) as model covariates. Similarly, the statistical differences in associations between time-averaged cumulative measured SBP and time-averaged cumulative underlying SBP were tested by assessing the significance of the delta between the standardized time-averaged cumulative underlying SBP and the standardized time-averaged cumulative measured SBP. Adjustment covariates were selected based on a priori knowledge.
For the association of SBP measures with incident HF, multivariable Cox proportional hazard regression was used with the primary endpoints of incident HF overall, incident HFrEF, and incident HFpEF. The modeling approach was similar to that employed for echocardiographic measures above. Secondary endpoints included the composite of each HF outcome with death. The proportional hazard assumptions were confirmed for all associations based on Schoenfeld residuals. Effect modification by cross-categories based on sex and race (i.e. white women, white men, black women, and black men) was assessed using multiplicative interaction terms in linear and Cox regression models.
To quantify the proportion of the association between time-averaged cumulative SBP and incident HF accounted for by the associated echocardiographic alterations, we determined the proportional reduction in the magnitude of the β-coefficient for time-averaged cumulative SBP in multivariable Cox proportional hazard models with versus without echocardiographic measures as adjustment covariates. Associated 95% confidence intervals were obtained from 2000 bootstrap replications using the percentile method.
All analyses were performed with STATA 15.0 (College Station, TX). A two-sided P-values of <0.05 was considered statistically significant.
RESULTS
The mean age at Visit 5 was 75±5 years, 41% were male, and 82% were white. At the time of Visit 5, 72% had a diagnosis of hypertension, and 71% were on antihypertensive medication (table S2). Measured SBP at Visit 5 was 130 ± 18 mmHg, underlying SBP was 143 ± 20 mmHg, time-averaged measured SBP was 124 ± 13 mmHg, and time-averaged underlying SBP was 131 ± 16 mmHg (Figure 1). Clinical characteristics of participants included in and those excluded from this analysis are presented in table S2. Included participants were modestly younger with fewer co-morbidities.
Figure 1 –

Histograms of time averaged cumulative measured SBP and time averaged cumulative measured SBP among study participants.
Higher time-averaged cumulative measured SBP was associated with older age and black race, and with higher prevalence of common cardiovascular co-morbidities including diabetes, obesity, coronary heart disease (CHD), atrial fibrillation, chronic kidney disease, and anemia even after adjusting for participant demographics (Table 1). Higher time-averaged cumulative measured SBP was also associated with higher pulse pressure, NT-proBNP, and high sensitivity troponin T concentrations. Notably, higher prevalence of co-morbidities and concentrations of cardiac biomarkers were also observed among participants with SBP <130 mmHg at all visits but who required antihypertensive medication compared to those who did not (Table 1). Similar results were noted for categories based on time-averaged cumulative underlying SBP (table S3).
Table 1 –
Clinical characteristics across participant categories based on measured SBP.
| Always SBP <130 mmHg (n=1,531) |
SBP ≧130 mmHg at ≥1 study visit (total n=3,047) |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Clinical characteristics | Without BP medication (n=647) | With BP medication (n=884) | Low cum BP (n=1016) [100-124 mmHg] | Borderline cum BP (n=1016) [125-133 mmHg] | High cum BP (n=1015) [134-183 mmHg] | Trend | Adj* |
| Time-ave cum SBP, mmHg | 109 ± 7 | 113 ± 6 | 119 ± 4 | 128 ± 2 | 142 ± 8 | < 0.001 | - |
| Time over V1-5, year | 23.6 ± 0.9 | 23.7 ± 1.0 | 23.7 ± 1.0 | 23.7 ± 1 | 23.7 ± 1 | 0.02 | 0.4 |
| Demographic at V5 | |||||||
| Age, year, mean | 74.1 ± 4.8 | 74.6 ± 4.9 | 75.0 ± 4.9 | 76 ± 5.1 | 76.8 ± 5.2 | < 0.001 | - |
| Male, % | 271 (42 %) | 372 (42 %) | 430 (42 %) | 421 (41 %) | 368 (36 %) | 0.014 | - |
| White, % | 596 (92 %) | 748 (85 %) | 869 (86 %) | 816 (80 %) | 711 (70 %) | < 0.001 | - |
| Visit center | 0.3 | - | |||||
| Forsyth County, % | 189 (29 %) | 191 (22 %) | 286 (28 %) | 215 (21 %) | 188 (19 %) | ||
| Jackson, % | 47 (7 %) | 125 (14 %) | 130 (13 %) | 185 (18 %) | 276 (27 %) | ||
| Minneapolis, % | 247 (38 %) | 288 (33 %) | 305 (30 %) | 314 (31 %) | 302 (30 %) | ||
| Washington Country, % | 164 (25 %) | 280 (32 %) | 295 (29 %) | 302 (30 %) | 249 (25 %) | ||
| Comorbidities at V5 | |||||||
| Hypertension, % | 10 (2 %) | 648 (74 %)† | 709 (70 %) | 913 (90 %) | 993 (98 %) | < 0.001 | < 0.001 |
| Obese (BMI ≥30), % | 111 (17 %) | 333 (38 %)† | 327 (32 %) | 418 (41 %) | 405 (40 %) | < 0.001 | < 0.001 |
| Diabetes, % | 115 (18 %) | 339 (38 %)† | 308 (30 %) | 381 (38 %) | 388 (38 %) | < 0.001 | - |
| CHD, % | 19 (3 %) | 117 (14 %)† | 107 (11 %) | 108 (11 %) | 98 (10 %) | 0.023 | < 0.001 |
| Afib, % | 14 (2 %) | 51 (6 %)† | 67 (7 %) | 59 (6 %) | 78 (8 %) | < 0.001 | 0.005 |
| COPD, % | 55 (9 %) | 78 (9 %) | 105 (10 %) | 82 (8 %) | 86 (8 %) | 0.67 | |
| CKD, % | 85 (13 %) | 215 (25 %)† | 236 (23 %) | 287 (28 %) | 337 (34 %) | < 0.001 | 0.57 |
| Anemi,a % | 71 (11%) | 144 (17%)† | 168 (17%) | 224 (23%) | 207 (21%) | < 0.001 | < 0.001 |
| Physical exam at V5 | |||||||
| SBP | 117 ± 9 | 116 ± 9† | 130 ± 14 | 134 ± 14 | 146 ± 17 | < 0.001 | < 0.001 |
| DBP | 63 ± 8 | 62 ± 9† | 67 ± 10 | 68 ± 10 | 71 ± 11 | < 0.001 | < 0.001 |
| Pulse BP | 54 ± 8 | 54 ± 9 | 62 ± 11 | 67 ± 12 | 75 ± 16 | < 0.001 | < 0.001 |
| Heart rate | 61 ± 9 | 62 ± 11 | 61 ± 10 | 63 ± 10 | 63 ± 11 | 0.005 | 0.19 |
| Laboratory values at V5 | |||||||
| BMI, kg/m2 | 26 ± 5 | 29 ± 5 | 28 ± 5 | 29 ± 5 | 29 ± 6 | < 0.001 | - |
| BSA, m2 | 2.5 ± 0.3 | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.6 ± 0.3 | 2.6 ± 0.3 | 0.029 | - |
| HbA1c, % | 5.6 ± 0.5 | 5.9 ± 0.8† | 5.9 ± 0.8 | 5.9 ± 0.8 | 6 ± 0.9 | < 0.001 | < 0.001 |
| Hs-Tn, ng/l | 0.8 [0.6, 1.2] | 1.0 [0.7, 1.4] † | 1.0 [0.7, 1.4] | 1.1 [0.7, 1.6] | 1.2 [0.8, 1.7] | < 0.001 | < 0.001 |
| NT-proBNP, pg/mL | 89 [52, 156] | 105 [57, 195] † | 118 [62, 221] | 125 [66, 240] | 155 [79, 298] | < 0.001 | < 0.001 |
| eGFR, ml/min/1.73m2 | 77 [66, 85] | 74 [60, 84] † | 73 [61, 84] | 71 [58, 83] | 68 [54, 82] | < 0.001 | < 0.001 |
| Hemoglobin mg/dL | 13.6 [12.8, 14.4] | 13.6 [12.7, 14.4] | 13.4 [12.5, 14.3] | 13.3 [12.3, 14.2] | 13.2 [12.3, 14.2] | < 0.001 | 0.033 |
| Medication at V5 | |||||||
| Any BP med use, % | - | 825 (93 %) | 670 (66 %) | 831 (82 %) | 920 (91 %) | < 0.001 | < 0.001 |
| Alpha blocker, % | - | 41 (5 %) | 47 (5 %) | 49 (5 %) | 86 (8 %) | < 0.001 | < 0.001 |
| Beta blocker, % | - | 311 (35 %) | 257 (25 %) | 314 (31 %) | 405 (40 %) | < 0.001 | < 0.001 |
| ACE-inhibitor, % | - | 247 (28 %) | 215 (21 %) | 252 (25 %) | 274 (27 %) | < 0.001 | < 0.001 |
| CC blocker, % | - | 172 (19 %) | 176 (17 %) | 324 (32 %) | 406 (40 %) | < 0.001 | < 0.001 |
| Diuretic, % | - | 394 (45 %) | 339 (33 %) | 425 (42 %) | 544 (54 %) | < 0.001 | < 0.001 |
| Others, % | - | 97 (11 %) | 114 (11 %) | 185 (18 %) | 203 (20 %) | < 0.001 | < 0.001 |
Trend P-value indicates comparison across all five groups.
Adj; P-value adjusted for age, sex, race, V5 BMI and visit center
P-value comparing those with SBP always <130 mmHg without versus with BP medication in models adjusting for age, sex, race V5 BMI and visit center
For medication types, p-values reflect test of trend across categories with SBP ≧130 mmHg at ≥1 study visit
Legend: SBP, systolic blood pressure; CHD, coronary heart disease; Afib, atrial fibrillation; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DBP, diastolic blood pressure; BMI, body mass index; BSA, body surface area; Hs-Tn, high sensitivity troponin; NT-proBNP, N-terminal pro b-type natriuretic peptide; eGFR, estimated glomerular filtration rate; ACE, angiotensin-converting enzyme; CC, calcium channel
Associations between time-averaged cumulative measured and underlying SBP and cardiac function
When compared to lower values, higher time-averaged cumulative measured SBP was associated with greater LV wall thickness, chamber dimension, and mass index, and with lower TDI e’, higher E/e’ ratio, higher LA volume index, and higher pulmonary artery systolic pressure indicative of worse diastolic function (Table 2). No association was observed with LVEF, although higher time-averaged cumulative measured SBP was associated with altered LV systolic deformation including lower longitudinal strain and higher circumferential strain. Similar associations were observed with time-averaged cumulative underlying SBP (table S4). The continuous associations of measures of LV structure and diastolic function with cumulative measured and underlying SBP are presented in Figure 2 (figure S2 for LV systolic function). Cumulative underlying SBP demonstrated greater magnitude of association with all echocardiographic measures except LVEF and global longitudinal strain when compared to cumulative measured SBP. SBP, both measured and underlying, assessed at a single time point at Visit 5 demonstrated similar associations with echocardiographic measures (figures S3–S6), but of significantly smaller magnitude (per mmHg) compared to cumulative SBP for LV structural and diastolic measures.
Table 2 –
Echocardiographic measures of cardiac structure and function across participant categories based on measured SBP.
| Always SBP <130 mmHg (n=1531) |
Either of SBP ≧130 mmHg (total n=3071) |
P-value | |||||
|---|---|---|---|---|---|---|---|
| Echo parameters | Without BP medication (n=647) | With BP medication (n=884) | Low cum BP (n=1016) [100-124 mmHg] | Borderline cum BP (n=1016) [125-132 mmHg] | High cum BP (n=1015) [133-183 mmHg] | Trend | Adj* |
| Cardiac structure | |||||||
| LVEDD, cm | 4.29 ± 0.47 | 4.38 ± 0.47† | 4.38 ± 0.48 | 4.37 ± 0.49 | 4.40 ± 0.48 | < 0.001 | < 0.001 |
| LVESD, cm | 2.54 ± 0.40 | 2.58 ± 0.42† | 2.59 ± 0.45 | 2.57 ± 0.44 | 2.59 ± 0.44 | 0.18 | 0.17 |
| MWT, cm | 0.92 ± 0.11 | 0.97 ± 0.13† | 0.98 ± 0.12 | 0.99 ± 0.13 | 1.01 ± 0.14 | < 0.001 | < 0.001 |
| LVMI, g/m2 | 71 ± 15 | 76 ± 17† | 77 ± 18 | 79 ± 18 | 82 ± 19 | < 0.001 | < 0.001 |
| LVH, n (%) | 49 (8 %) | 183 (21 %)† | 214 (21 %) | 261 (26%) | 310 (33%) | < 0.001 | < 0.001 |
| LV Systolic Function | |||||||
| LVEF, % | 66 ± 5 | 66 ± 5 | 66 ± 5 | 66 ± 5 | 66 ± 5 | 0.46 | 0.28 |
| Average LS, % | −18.5 ± 2.3 | −18.3 ± 2.3 | −18.2 ± 2.3 | −18.0 ± 2.5 | −17.8 ± 2.5 | < 0.001 | < 0.001 |
| Average CS, % | −27.8 ± 3.7 | −27.8 ± 3.6 | −28.1 ± 3.5 | −27.9 ± 3.7 | −28.4 ± 3.7 | 0.002 | < 0.001 |
| LV Diastolic Function | |||||||
| Septal e’, cm/sec | 6.2 ± 1.4 | 5.9 ± 1.4† | 5.8 ± 1.4 | 5.6 ± 1.4 | 5.5 ± 1.5 | < 0.001 | < 0.001 |
| E/e’ septal | 10.9 ± 3.2 | 11.5 ± 3.7† | 12.0 ± 3.8 | 12.4 ± 4.2 | 13.1 ± 4.4 | < 0.001 | < 0.001 |
| LAVI, ml/m2 | 22.9 ± 6.6 | 24.3 ± 7.8† | 25.1 ± 7.4 | 25.9 ± 9.6 | 27.4 ± 9.1 | < 0.001 | < 0.001 |
| PASP, mmHg | 26 ± 4 | 28 ± 5† | 27 ± 5 | 28 ± 5 | 29 ± 6 | < 0.001 | < 0.001 |
Trend P-value indicates comparison across all five groups.
Adj; P-value adjusted for age, sex, race, V5 BMI and visit center
P-value comparing those with SBP always <130 mmHg without versus with BP medication in models adjusting for age, sex, race V5 BMI and visit center
Legend: LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MWT, mean wall thickness; LVMI, left ventricular mass indexed; LVH, left ventricular hypertrophy (defined as LV mass / height2.7 >41.5 for women, >45.0 for men); LVEF, left ventricular ejection fraction; LAVI, left atrial volume indexed; PASP, pulmonary arterial systolic pressure; LS, longitudinal strain; CS, circumferential strain.
Figure 2 -.
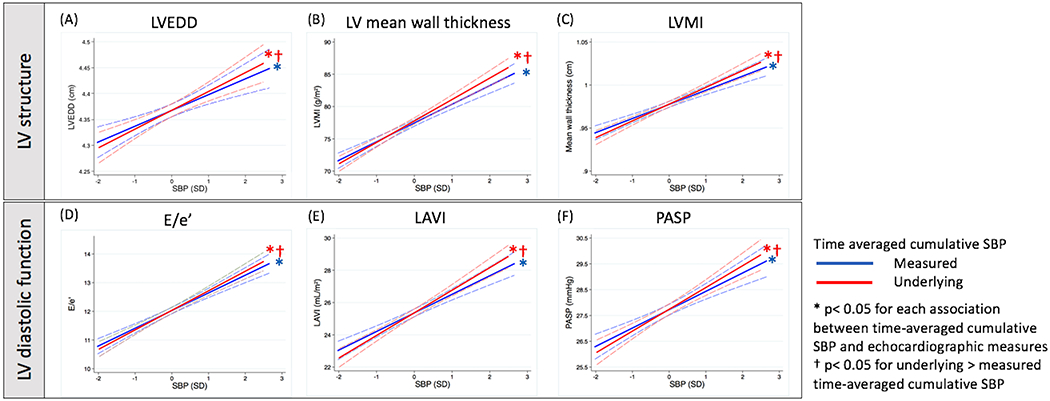
Relationship between time averaged cumulative SBP, both the measured and underlying, from Visits 1 to 5 and LV structure and diastolic function at Visit 5. Panels: (A) left ventricular end diastolic diameter, (B) left ventricular mean wall thickness, (C) left ventricular mass index, (D) E/e’, (E) left atrial volume index, (F) pulmonary arterial systolic pressure. Models are adjusted for age, sex race, V5 BMI, visit center, smoking status, DM, CKD, obesity, CHD, and atrial fibrillation at V5.
Relationship with HF outcomes
Incident HF occurred in 243 patients over a median follow-up of 5.6 [5.1-6.0] years: 85 incident HFrEF, 120 incident HFpEF, and 38 HF with unknown LVEF. Categories of higher time-averaged cumulative SBP, both measured and underlying, demonstrated higher risk of incident HF compared to participants with an SBP <130 mmHg at all visits (Table 3). Among participants with an SBP <130 mmHg at all visits, those requiring antihypertensive medication demonstrated a higher incidence of HF compared to those not on any antihypertensive medications.
Table 3 –
Association of categories based on time-averaged cumulative SBP with incident of HF post-V5.
| Time-average cumulative measured SBP |
Time-averaged cumulative underlying SBP |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for demographic | Adjusted for demographic + co-morbidities | Adjusted for demographic | Adjusted for demographic + co-morbidities | |||||||||||||
| BP categories | N | Event | Event rate per 100-person years [95%CI] | HR [95%CI] | P-value | HR [95%CI] | P-value | N | Event | Event rate per 100-person years [95%CI] | HR [95%CI] | P-value | HR [95%CI] | P-value | ||
| HF | ||||||||||||||||
| Always SBP <130mmHg |
Without BP meds | 647 | 10 | 0.28 [0.15-0.53] | - | - | - | - | 647 | 10 | 0.28 [0.15-0.53] | - | - | - | - | |
| With BP meds | 884 | 42 | 0.89 [0.66-1.20] | 2.66 [1.33-5.33] | 0.006 | 2.14 [1.02-4.49] | 0.044 | 286 | 18 | 1.18 [0.74-1.87] | 3.83 [1.77-8.32] | 0.001 | 2.97 [1.29-6.83] | 0.01 | ||
| Either of SBP ≧130mmHg | Low cum SBP | 1016 | 59 | 1.09 [0.85-1.41] | 3.37 [1.72-6.60] | < 0.001 | 2.96 [1.46-6.03] | 0.003 | 1215 | 52 | 0.79 [0.60-1.04] | 2.52 [1.28-4.96] | 0.008 | 2.27 [1.11-4.66] | 0.025 | |
| Borderline cum SBP | 1016 | 47 | 0.88 [0.66-1.17] | 2.47 [1.24-4.92] | 0.01 | 2.24 [1.09-4.62] | 0.029 | 1215 | 59 | 0.92 [0.72-1.19] | 2.61 [1.33-5.14] | 0.005 | 2.33 [1.14-4.75] | 0.021 | ||
| High cum SBP | 1015 | 85 | 1.64 [1.32-2.03] | 4.29 [2.20-8.36] | < 0.001 | 3.58 [1.76-7.26] | < 0.001 | 1215 | 104 | 1.67 [1.38-2.03] | 4.50 [2.32-8.72] | < 0.001 | 3.76 [1.86-7.59] | < 0.001 | ||
| Death | ||||||||||||||||
| Always SBP <130mmHg | Without BP meds | 647 | 59 | 1.67 [1.30-2.16] | - | - | - | - | 647 | 59 | 1.67 [1.30-2.16] | - | - | - | - | |
| With BP meds | 884 | 77 | 1.60 [1.28-2.00] | 0.95 [0.68-1.35] | 0.8 | 0.82 [0.57-1.18] | 0.3 | 286 | 24 | 2.54 [2.03-2.30] | 0.94 [0.58-1.51] | 0.794 | 0.81 [0.50-1.32] | 0.4 | ||
| Either of SBP ≧130mmHg | Low cum SBP | 1016 | 109 | 1.98 [1.64-2.39] | 1.13 [0.82-1.55] | 0.5 | 0.98 [0.70-1.34] | 0.9 | 1215 | 109 | 1.64 [1.36 [1.98] | 0.95 [0.69-1.30] | 0.73 | 0.89 [0.64-1.24] | 0.509 | |
| Borderline cum SBP | 1016 | 128 | 2.35 [1.98-2.80] | 1.21 [0.88-1.65] | 0.2 | 1.01 [0.73-1.40] | 0.9 | 1215 | 148 | 2.28 [1.94-2.68] | 1.23 [0.90-1.67] | 0.198 | 1.00 [0.72-1.38] | 0.998 | ||
| High cum SBP | 1015 | 161 | 3.02 [2.59-3.53] | 1.45 [1.06-1.98] | 0.019 | 1.21 [0.87-1.67] | 0.3 | 1215 | 194 | 3.03 [2.64-3.49] | 1.52 [1.12-2.06] | 0.007 | 1.23 [0.89-1.69] | 0.212 | ||
Demographic adjustment includes age, sex, race, V5 BMI, and visit center.
Co-morbidity adjustment includes smoking status, DM, CKD, obesity, CHD, and Afib.
Both measured and underlying cumulative SBP were independently associated with higher rates incident HF overall and HFpEF in fully adjusted models (Figure 3; Table 3). Both were associated with incident HFrEF in unadjusted analyses, but these associations become non-significant after adjusting for participant demographics (Figure 3). Associations with HF overall and HFpEF were greater in magnitude using cumulative underlying SBP compared to cumulative measured SBP (Figure 3; Table 3; figure S7). Higher single time point underlying – but not measured - SBP at Visit 5 was also independently associated with risk of incident HF and HFpEF, but the magnitude of association (per 10 mmHg) was significantly smaller than that of cumulative SBPs (figure S8). Similar results were found in analyses excluding events with intercurrent MI or revascularization (figure S9) Echocardiographic measures of LV structure (LVEDD, mean wall thickness, mass index) and diastolic function (E/e’ ratio, LA volume index) accounted for approximately 53% [95% CI: 32-88%] of the association of time-averaged cumulative underlying SBP with incident HF overall, and 42% [95% CI: 26-77%] of the association with incident HFpEF (table S5).
Figure 3 –

Relationship between time-averaged cumulative SBP, both the measured and underlying, and HF outcomes. The time averaged SBP based on measured SBP are in blue and those based on underlying SBP are in red. Hazard ratios are by per 10 mmHg change in SBPs. Demographic adjustment includes age, sex, race, Visit 5 BMI, and visit center. Co-morbidity adjustment includes smoking status, diabetes, chronic kidney disease, obesity, coronary heart disease, and atrial fibrillation at Visit 5.
Secondary analyses were performed for associations with the composite endpoints of death or incident HF (n=679), death or incident HFrEF (n=579), and death or incident HFpEF (n=609). Time averaged cumulative measured and underlying SBP were significantly associated with all composite outcomes in fully adjusted models (figure S10), while single time-point SBP at Visit 5 was not.
Interaction by sex-race group
No significant effect measure modification by sex and race group was observed for the associations of time-averaged cumulative SBP with either echocardiographic parameters or incident HF outcomes.
DISCUSSION
Clinical trials have repeatedly demonstrated reduction in HF risk with antihypertensive treatment generally,22,23,24 and in late life,25,26 but limited data is available regarding the long-term relationship of blood pressure through mid-to late-life with late life HF risk and subclinical cardiac dysfunction. We assessed the relationship of SBP in mid-to late-life with late life cardiac structure and function and HF risk, and compared the strength of these associations between cumulative versus single time point late-life SBP and between measured versus predicted underlying SBP. We report three novel findings. First, higher SBP in mid- to late-life is associated with worse late life LV structure and diastolic function and altered systolic deformation despite preserved LVEF. Second, higher SBP through mid-to-late life predicts incident HF – and incident HFpEF in particular – in late life independent of clinical co-morbidities. These associations with echocardiographic measures and HF risk were of greater magnitude with mid- to late-life SBP, calculated using time-averaged cumulative SBP compared to single time point late life SBP. Third, associations of time-averaged cumulative SBP with late-life LV structure, diastolic function, and risk of incident HF and HFpEF were of greater magnitude using underlying as opposed to measured SBP. Together, these findings highlight the importance of primordial prevention and effective long-term treatment of hypertension for the prevention of late-life HF generally, and HFpEF in particular.
Cumulative SBP from mid- to late-life and late-life cardiac structure and function
Chronic increase in LV afterload and arterial stiffness contributes to development of LV hypertrophy27. Among participants in the Framingham Heart Study free of CVD and not taking antihypertensive medication, higher thirty-year average SBP was a better predictor of greater LV mass and wall thickness than current BP at the time of echocardiography11. Other investigators have demonstrated similar associations of mid-life SBP with higher subsequent LV mass index13. In this analysis, time-averaged cumulative SBP from mid-to late-life was associated with greater late-life LV mass index, related to both larger LV cavity size and thicker LV walls, and also with worse diastolic measures, including lower e’, higher E/e’, and larger LA volume index. Consistent with prior studies, these structural and functional measures were more strongly associated with cumulative SBP as opposed to single time point concurrent SBP. Notably, cumulative SBP based on predicted underlying SBP – compared to measured SBP – was more strongly associated with LV structure and diastolic function. This finding suggests the importance of hypertension severity – beyond actual LV afterload – on LV structure and diastolic function.
Among ARIC participants with SBP <130 mmHg at every study visit, those who required antihypertensive medication demonstrated greater LV structural remodeling and worse diastolic function compared to those not requiring antihypertensive therapy. These findings extend upon those of Mancia et al., who previously reported alterations in cardiac structure among hypertensive patients with adequate BP control compared to normotensive patients in a cross-sectional study28. Therefore, while effective BP control may mitigate the impact of hypertension on cardiac structure and function, it does not completely prevent such alterations.
Concordant with data from CARDIA13, cumulative SBP was not associated with late life LVEF, although participants with frankly reduced LVEF (<50%) were excluded from this analysis. However, higher cumulative SBP was associated with lower absolute longitudinal strain but higher circumferential strain. These findings are consistent with prior cross-sectional analyses comparing patients with hypertensive heart disease to normotensive controls, and suggest hypertension-associated impairments in longitudinal strain with concomitant increase in circumferential strain, maintaining overall LVEF29,30,31,32,33.
Cumulative SBP and incident HF
Data from the Framingham Heart Study have demonstrated the prognostic importance of antecedent BP independent of current BP for risk of CVD generally,7 and incident HF specifically.10 We also found that time-averaged cumulative SBP was more robustly associated with risk of incident of HF than single time point SBP. These findings are concordant with a recent Mendelian randomization study suggesting the cardiovascular effects of SBP may depend on both magnitude and duration of exposure.34 We extend upon these results with two additional novel findings. First, we demonstrate that, while higher time-averaged cumulative SBP is associated with incident HFpEF and HFrEF, it is only independently predictive of incident HFpEF in late life. This finding aligns with data from randomized clinical trials demonstrating that effective BP control is an effective approach to prevent HF, and HFpEF in particular. For example, a sub-analysis of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) reported 50% reduction in the incidence of HFpEF with cholorthalidone35. Effective antihypertensive therapy can also regress LVH36,37 and improve diastolic function38,39, key risk factors for HFpEF development.
Second, we observed a greater magnitude of association of time-averaged cumulative SBP with incident HF, and HFpEF specifically, when calculated using underlying compared to measured SBP. This finding is consistent with the stronger associations of underlying compared to measured cumulative SBP with LV structure and diastolic function. Together, these findings suggest that the severity of hypertension, reflected both in the measured SBP and the intensity of antihypertensive therapy over time from mid- to late-life, more fully reflect HF risk than measured SBP alone. These findings highlight the importance of interventions to prevent the development of hypertension for maintaining cardiac health into late life. Furthermore, concordant with previous studies of the adverse impact of masked hypertension40,41,42 and the benefits of early hypertension control,43,44,45 our findings also argue for the early detection and early, effective, and sustained control of hypertension to minimize cardiac end-organ impairments and HF risk in late life.
Alterations in cardiac structure and function accounted for approximately 40-50% of the association of cumulative underlying SBP with incident HF and HFpEF, suggesting that hypertension-related alterations in other end-organs (e.g. arterial dysfunction, renal injury) may also contribute appreciably to this risk. Furthermore, recent translational data suggest an important role for systemic inflammation, possibly related to hypertension in addition to other common cardiovascular comorbidities, in the development of HFpEF in particular46,47,48. Hypertension may therefore not only contribute to development of LV hypertrophy, diastolic dysfunction, and HFpEF risk through excessive increase in LV afterload, but also through an associated pro-inflammatory state. However, further studies are necessary to evaluate this hypothesis.
Study limitations
Several limitations of this study should be recognized. Survivor bias may lead to underestimation of the association of SBP measures with the echocardiographic and HF outcomes. Attendance bias at the fifth study visit may limit the generalizability of our findings, as this was not random. While the method we employed for estimating underlying SBP for participants taking antihypertensive medication have been previously used and validated in ARIC, information on adherence and medication dosage was not available and we assumed homogenous effect of antihypertensive medications. Death was more common than incident HF in this age group, and may represent an important competing risk. Importantly, similar findings were observed in sensitivity analysis with the composite of death or HF as outcomes. The relatively short follow-up time may have limited power, particularly for analyses of incident HFpEF or HFrEF. However, risk at 5 years is clinically relevant for persons in this age group. Missing data, particularly for BP measurements and medication usage, may have not been missing at random but was relatively modest in extent. Finally, this observational study cannot establish causality of the observed associations.
PERSPECTIVES
Among 4,578 community-based adults, higher cumulative time-averaged SBP over 25 years from mid- to late-life was independently associated with greater LV mass, worse LV diastolic function, altered LV systolic deformation, and higher risk of incident HF – and HFpEF in particular – in late-life. These associations were more robust using cumulative time-averaged underlying SBP, which takes into account measured SBP and antihypertensive medication use, compared to cumulative time-averaged measured SBP. Alterations in cardiac structure and function accounted for approximately 40-50% of the association of cumulative underlying SBP with incident HF and HFpEF, suggesting that hypertension-related alterations in other end-organs also contribute appreciably to HF risk. Furthermore, among participants with SBP <130 mmHg at all study visits, those requiring antihypertensive medication to achieve this goal demonstrated worse late-life cardiac structure and function and higher HF risk compared to those not requiring antihypertensive medication. Together, our findings highlight the importance of preventing hypertension, of early hypertension detection, and of early, effective, and sustained SBP control in prevalent hypertension to promote cardiac health, minimize end-organ damage, and prevent HF in late life.
CONCLUSION
Higher time-averaged cumulative SBP through mid- to late-life is associated with worse LV structure, worse diastolic function, and altered systolic deformation in late life, and with heightened risk of incident HF, particularly incident HFpEF. These associations were stronger with time-averaged cumulative SBP compared to single time point late life SBP, and with predicted underlying SBP accounting for antihypertensive medication use compared to measured SBP alone. These findings highlight the importance of primordial prevention of hypertension, and effective SBP control among those with hypertension, through mid- to late-life for maintenance of cardiac health into late-life.
Supplementary Material
Novelty and Significance.
What is new?
Higher time-averaged cumulative SBP over mid- to late-life was associated with alterations in cardiac structure and function, and with greater risk of incident heart failure – and HFpEF in particular – in late-life. These associations were more robust using the time-averaged cumulative SBP using an estimated underlying SBP based on measured SBP and accounting for antihypertensive medication usage, compared to time-averaged cumulative measured SBP.
What is Relevant?
These findings support to importance of preventing hypertension, early hypertension detection, and early, effective, and sustained SBP control in prevalent hypertension from mid- through late-life to promote cardiac health and prevent HF in late life.
Summary
Higher time-averaged cumulative SBP from mid- to late-life is independently associated with worse cardiac structure and function, and higher risk of HF and HFpEF, in late-life.
ACKNOWLEDGEMENT
The authors thank the staff and participants of the ARIC study for their important contribution.
SOURCE OF FUNDING
The Atherosclerosis Risk in Communities (ARIC) study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The authors thank the staff and participants of the ARIC study for their important contributions. The work for this manuscript was also supported by NHLBI grants R01HL135008, R01HL143224, R01HL150342, and K24HL152008 (A.M.S.), and a Watkins Discovery Award from the Brigham and Women’s Heart and Vascular Center (A.M.S.).
DISCLOSURES
Dr. Matsushita reports receiving research support from Kyowa Hakko Kirin, Fukuda Denshi and consulting fees from Kyowa Hakko Kirin, Apex, Healthy.io, and Akebia.
Dr. Claggett reports receiving consulting fees from Myokardia, Gilead, AOBiome, Boehringer Ingelheim.
Dr. John reports receiving research support from NIH T32 Grant.
Dr. Skali reports receiving ownership interest from OptimizeRx.
Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, Theracos, and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau.
Dr. Cheng reports receiving research support from NIH and consulting fee from Zogenix.
Dr. Shah reports receiving research support from Novartis through the Brigham and Women’s Hospital and consulting fees from Bellerophon and Philips Ultrasound.
The remaining authors have nothing to disclose.
REFERENCES
- 1.Kannel WB. Blood pressure as cardiovascular risk factor: prevention and treatment. JAMA. 1996; 275(20):1571–1576. [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. [DOI] [PubMed] [Google Scholar]
- 5.Willamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315(24):2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future CVD mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol. 2011;58(23):2396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasan RS, Massaro JM, Wilson PW, et al. Framingham Heart Study. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105(1):48–53. [DOI] [PubMed] [Google Scholar]
- 8.Allen N, Berry JD, Ning H, Van Horn L, Dyer A, Lloyd-Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: the cardiovascular lifetime risk pooling project. Circulation. 2012;125(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifonte A, Ayer T, Veledar E, Clark A, Wilson PW. Antecedent blood pressure as a predictor of cardiovascular disease. J Am Soc Hypertens. 2015;9(9):690–696.e1. [DOI] [PubMed] [Google Scholar]
- 10.Lee DS, Massaro JM, Wang TJ, et al. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50(5):869–76. [DOI] [PubMed] [Google Scholar]
- 11.Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1991;18(5):1287–94. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh AK, Hardy RJ, Francis DP, et al. Medical Research Council National Survey of Health and Development (NHSD) Scientific and Data Collection Team. Midlife blood pressure change and left ventricular mass and remodelling in older age in the 1946 British Birth Cohort Study. Eur Heart J. 2014;35(46):3287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age: The CARDIA Study. J Am Coll Cardiol. 2015;65(25):2679–87. [DOI] [PubMed] [Google Scholar]
- 14.The atherosclerosis risk in communities (aric) study: Design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.The National Heart, Lung, and Blood Institute of the National Institutes of Health. ARIC Manual 11 Sitting Blood Pressure - Collaborative Studies Coordinating Center. Available at: http://www.cscc.unc.edu/aric/visit/Sitting_Blood_Pressure_and_Postural_Changes_in_Blood_Pressure_and_Heart_Rate.1_11.pdf
- 16.Wu J, Kraja AT, Oberman A, et al. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18(7):935–42. [DOI] [PubMed] [Google Scholar]
- 17.Balakrishnan P, Beaty T, Young JH, Colantuoni E, Matsushita K. Methods to estimate underlying blood pressure: The Atherosclerosis Risk in Communities (ARIC) Study. PLoS One. 2017;12(7):e0179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012. March 1;5(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the atherosclerosis risk in communities (aric) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 22.Kostis JB1, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278(3):212–6. [PubMed] [Google Scholar]
- 23.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981–97. [DOI] [PubMed] [Google Scholar]
- 24.SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–98. [DOI] [PubMed] [Google Scholar]
- 26.Briasoulis A, Agarwal V, Tousoulis D, Stefanadis C. Effects of antihypertensive treatment in patients over 65 years of age: a meta-analysis of randomised controlled studies. Heart. 2014;100(4):317–23. [DOI] [PubMed] [Google Scholar]
- 27.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancia G, Carugo S, Grassi G, et al. Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) Study. Prevalence of left ventricular hypertrophy in hypertensive patients without and with blood pressure control: data from the PAMELA population. Pressioni Arteriose Monitorate E Loro Associazioni. Hypertension. 2002;39(3):744–9. [DOI] [PubMed] [Google Scholar]
- 29.Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengupta PP, Krishnamoorthy VK, Korinek J, et al. Left Ventricular form and function revisited: applied translational science to cardiovascular ultrasound imaging. J Am Soc Echocardiogr. 2007;20(5):539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr. 2008:1138–44. [DOI] [PubMed] [Google Scholar]
- 32.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond). 2004;106:53–60. [DOI] [PubMed] [Google Scholar]
- 33.Carasso S, Cohen O, Mutlak D, et al. Differential effects of afterload on left ventricular long- and short-axis function: insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am Heart J. 2009;158:540–5. [DOI] [PubMed] [Google Scholar]
- 34.Ference BA, Bhatt DL, Catapano AL, et al. Association of Genetic Variants Related to Combined Exposure to Lower Low-Density Lipoproteins and Lower Systolic Blood Pressure With Lifetime Risk of Cardiovascular Disease. AMA. 2019. September 2. doi: 10.1001/jama.2019.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis BR, Kostis JB, Simpson LM, et al. ALLHAT Collaborative Research Group. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118(22):2259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devereux RB, Dahlof B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110(11):1456–62. [DOI] [PubMed] [Google Scholar]
- 37.Schneider A, Schwab J, Karg MV, et al. Low-dose eplerenone decreases left ventricular mass in treatment-resistant hypertension. J Hypertens. 2017;35(5):1086–1092. [DOI] [PubMed] [Google Scholar]
- 38.Solomon SD, Janardhanan R, Verma A, et al. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–87. [DOI] [PubMed] [Google Scholar]
- 39.Solomon SD, Verma A, Desai A, et al. Effect of intensive versus standard blood pressure lowering on diastolic function in patients with uncontrolled hypertension and diastolic dysfunction. Hypertension. 2010;55(2):241–8. [DOI] [PubMed] [Google Scholar]
- 40.Tientcheu D, Ayers C, Das SR, McGuire DK, de Lemos JA, Khera A, Kaplan N, Victor R, Vongpatanasin W. Target Organ Complications and Cardiovascular Events Associated With Masked Hypertension and White-Coat Hypertension: Analysis From the Dallas Heart Study. J Am Coll Cardiol. 2015. November 17;66(20):2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redmond N, Booth JN 3rd, Tanner RM, Diaz KM, Abdalla M, Sims M, Muntner P, Shimbo D. Prevalence of Masked Hypertension and Its Association With Subclinical Cardiovascular Disease in African Americans: Results From the Jackson Heart Study. Am Heart Assoc. 2016. March 29;5(3):e002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pierdomenico SD, Pierdomenico AM, Coccina F, Clement DL, De Buyzere ML, De Bacquer DA, Ben-Dov IZ, Vongpatanasin W, Banegas JR, Ruilope LM, Thijs L, Staessen JA. Prognostic Value of Masked Uncontrolled Hypertension. Hypertension. 2018. October;72(4):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan BM, Bandyopadhyay D, Shaftman SR, Wagner CS, Zhao Y, Yu-Isenberg KS. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012. June;59(6):1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor PJ, Vazquez-Benitez G, Schmittdiel JA, Parker ED, Trower NK, Desai JR, Margolis KL, Magid DJ. Benefits of early hypertension control on cardiovascular outcomes in patients with diabetes. Diabetes Care. 2013. February;36(2):322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension: importance of early and sustained implementation of effective treatment strategies. Diabetes Care. 2011. May;34 Suppl 2:S297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 47.Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation. 2016;134(1):73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiattarella GG, Altamirano F, Tong D, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature. 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


