Abstract
Patients who have undergone mastectomy, with or without reconstruction, are not universally screened with mammography or US. Therefore, clinical breast examination by the physician and patient-detected palpable abnormalities are crucial for detecting breast cancer or recurrence. Diagnostic US is the first-line modality for evaluation of postmastectomy palpable masses, with occasional adjunct use of diagnostic mammography for confirming certain benign masses. In the setting of a negative initial imaging evaluation with continued clinical concern, diagnostic MRI may aid in improving sensitivity. Knowledge of the typical multimodality imaging appearances and locations of malignant palpable abnormalities—such as invasive carcinoma recurrence, cancer in residual breast tissue, radiation-induced sarcoma, and metastatic disease—is crucial in diagnosis and treatment of these entities. In addition, familiarity with the range of benign palpable postmastectomy processes—including fat necrosis, fat graft, seroma, granuloma, neuroma, fibrosis, and infection—may help avoid unnecessary biopsies and reassure patients. The authors review common and rare benign and malignant palpable masses in mastectomy patients, describe multimodality diagnostic imaging evaluation of each entity, review radiologic and pathologic correlation, and acquaint the radiologist with management when these findings are encountered.
©RSNA, 2021
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ List the common benign and malignant entities that may manifest as a palpable lump in mastectomy patients.
■ Recognize the important clinical, pathologic, and imaging features of these abnormalities using a multimodality approach.
■ Describe the most common sites of cancer recurrence in mastectomy patients.
Introduction
Since the 2000s, there has been an increase in mastectomy rates for patients with breast cancer, including those with early-stage breast cancer. The recommendations for follow-up of breast cancer patients after mastectomy include clinical breast examination at 3–6-month intervals for 2 years and at 6–12-month intervals for years 3–5 after surgery (1). The role of imaging in routine surveillance is controversial, with limited evidence to support routine mammographic imaging and some literature supporting the role of screening breast US (1). Mixed literature has been provided on the utility of routine mammography in mastectomy patients with reconstruction, mostly owing to the technical difficulties of performing the mammography. Similarly, there is insufficient literature to support use of screening US in patients after mastectomy with or without reconstruction.
However, imaging does play an important role in management of clinical symptoms in mastectomy patients, especially those with palpable findings. The causes of palpable findings range from benign postoperative changes—including fat necrosis and fibrosis—to local-regional recurrence, which can occur in 2%–25% of patients, usually within 5 years (2,3). While US is the most common imaging modality performed in this diagnostic setting, there can be overlap in the imaging appearances of benign and malignant masses. Therefore, mammography and MRI can be helpful adjunct tools in differentiating benign palpable lumps from cancer recurrence.
Radiologists must be familiar with the normal imaging findings in patients with mastectomy and reconstruction—including the varying appearances after implant and autologous reconstruction—to recognize abnormal findings. In this article, we provide a comprehensive review of common benign and malignant masses that manifest as palpable lumps in mastectomy patients, including the multimodality imaging features, radiologic-pathologic correlation, and recommendations for clinical management.
Diagnostic Workup of Palpable Lumps
Given the wide availability of US, it has been routinely used as a first step in evaluating a palpable lump detected by the patient or clinician. In a study of 118 palpable lumps in mastectomy patients by Dashevsky et al (4), US showed a 97% negative predictive value and 27% positive predictive value, respectively. A study by Edeiken et al (5) that reviewed 39 recurrent cancers in autologous flaps found that US visualization of recurrent tumors seemed to be independent of tumor size, with the majority being 1 cm or smaller and 33% measuring 0.5 cm or smaller. Radiologists are highly encouraged to perform US in real time to be able to carefully make an accurate correlation with the exact point of concern.
Limited evidence is available to support the benefit of diagnostic mammography as the initial imaging modality in mastectomy patients with palpable lumps. In the studies mentioned earlier, it was found that diagnostic mammography yielded no additional cancers beyond those detected at US (4,5). However, mammography remains useful for problem solving in the event of an inconclusive US finding or an abnormality that is suggestive of a benign process, such as fat necrosis, oil cyst, or benign calcifications (4).
MRI is routinely performed after malignant tissue diagnosis of palpable recurrence to evaluate the extent of disease and the contralateral breast. MRI for evaluation of implant integrity in patients with implant reconstruction is sometimes requested as an initial step when the palpable lump is thought to be related to implant rupture. While some mastectomy patients with implant reconstruction may be imaged only with noncontrast MRI, it is important to carefully evaluate for any evidence of recurrence in the reconstructed breast or extramammary areas even in the absence of contrast material (eg, enlarged axillary or internal mammary lymph nodes and lung or liver lesions) (Fig 1). However, it is recommended that radiologists protocol MRI examinations for implant evaluation and parenchymal evaluation (ie, MRI with both the implant protocol and intravenous contrast material) in cases where implant rupture and recurrence are part of the differential diagnosis for a postmastectomy palpable lump.
Figure 1a.

Recurrent invasive ductal carcinoma (IDC) and lymphadenopathy detected incidentally at noncontrast MRI in a mastectomy patient with suspected implant rupture. Sagittal T2-weighted (a) and axial fat-saturated T1-weighted (b) images show a 1.5-cm irregular mass (arrow) superficial to the implant in the upper-outer reconstructed breast, with no evidence of silicone leak or intra- or extracapsular implant rupture. Incidentally noted is an enlarged internal mammary node (oval).
Figure 1b.
Recurrent invasive ductal carcinoma (IDC) and lymphadenopathy detected incidentally at noncontrast MRI in a mastectomy patient with suspected implant rupture. Sagittal T2-weighted (a) and axial fat-saturated T1-weighted (b) images show a 1.5-cm irregular mass (arrow) superficial to the implant in the upper-outer reconstructed breast, with no evidence of silicone leak or intra- or extracapsular implant rupture. Incidentally noted is an enlarged internal mammary node (oval).
Radiologists should be aware that a negative imaging study does not override a high level of clinical suspicion. Therefore, in the absence of an imaging correlate with a suspicious palpable lump, palpation-guided biopsy should be recommended (4).
Benign Abnormalities
An overview of the benign entities encountered in patients with mastectomy with or without reconstruction, their clinical manifestations, and their imaging features is presented in the Table.
Common Palpable Benign Abnormalities Encountered in Mastectomy Patients, Associated Symptoms, and Imaging Features
Fat Necrosis
Fat necrosis is a benign inflammatory change of adipose tissue that can often mimic breast cancer. It usually occurs after trauma, irradiation, or surgical intervention and can be commonly seen in mastectomy patients after revisions, breast reconstruction, implant removal, or infection (6). When fat necrosis is symptomatic, 97% of cases manifest as a palpable lump, with a minority of cases associated with pain, tenderness, or skin changes (7).
Pathologic Findings.—The pathologic process of fat necrosis takes place in many stages, one of which results in a mixed inflammatory response to the injured adipocytes and sometimes the surrounding extravasated blood. In many cases, foreign-body–type giant cells and lipid-laden histiocytes predominate. Resolution of fat necrosis results in both calcification and fibrosis. Calcifications vary in appearance and can sometimes raise suspicion for ductal carcinoma in situ. In addition, healing fibrosis can give lesions an irregular or infiltrative appearance radiologically, which may increase the suspicion for a malignant process (8).
Imaging Features.—Fat necrosis has a wide spectrum of imaging features that reflect the stage of evolution. Most commonly seen features at US include an ill-defined area of increased echogenicity with or without small cystic components, anechoic masses with posterior shadowing or enhancement, a completely solid hyper- or isoechoic mass, or anechoic masses with internal echoes or mural nodules (9) (Fig 2). Once fat necrosis is suspected and if technically feasible, mammography can be performed for confirmation. When fibrosis is mild, fat necrosis most often manifests as a radiolucent oil cyst. Other mammographic features commonly associated with palpable fat necrosis include dystrophic calcifications, focal asymmetries, or spiculated masses, making it difficult to differentiate from cancer.
Figure 2a.
Fat necrosis manifesting as a palpable lump in a patient with mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. (a) Gray-scale US image shows an avascular complex solid and cystic mass with an eccentric mural nodule (arrow). (b) Left craniocaudal mammogram with a BB marker placed over the area of concern (arrowhead) shows the correlate to be a circumscribed fat-containing mass with hyperdense borders (arrow), consistent with benign fat necrosis. No further evaluation was needed. (c) High-power photomicrograph of a specimen from another patient shows adipocytes (*) surrounded by inflammatory infiltrates, mainly histiocytes (arrow) and giant cells. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.)
Figure 2b.
![Fat necrosis manifesting as a palpable lump in a patient with mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. (a) Gray-scale US image shows an avascular complex solid and cystic mass with an eccentric mural nodule (arrow). (b) Left craniocaudal mammogram with a BB marker placed over the area of concern (arrowhead) shows the correlate to be a circumscribed fat-containing mass with hyperdense borders (arrow), consistent with benign fat necrosis. No further evaluation was needed. (c) High-power photomicrograph of a specimen from another patient shows adipocytes (*) surrounded by inflammatory infiltrates, mainly histiocytes (arrow) and giant cells. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48c5/8262178/0b7e9c07e0f7/rg.2021200161.fig2b.jpg)
Fat necrosis manifesting as a palpable lump in a patient with mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. (a) Gray-scale US image shows an avascular complex solid and cystic mass with an eccentric mural nodule (arrow). (b) Left craniocaudal mammogram with a BB marker placed over the area of concern (arrowhead) shows the correlate to be a circumscribed fat-containing mass with hyperdense borders (arrow), consistent with benign fat necrosis. No further evaluation was needed. (c) High-power photomicrograph of a specimen from another patient shows adipocytes (*) surrounded by inflammatory infiltrates, mainly histiocytes (arrow) and giant cells. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.)
Figure 2c.
Fat necrosis manifesting as a palpable lump in a patient with mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. (a) Gray-scale US image shows an avascular complex solid and cystic mass with an eccentric mural nodule (arrow). (b) Left craniocaudal mammogram with a BB marker placed over the area of concern (arrowhead) shows the correlate to be a circumscribed fat-containing mass with hyperdense borders (arrow), consistent with benign fat necrosis. No further evaluation was needed. (c) High-power photomicrograph of a specimen from another patient shows adipocytes (*) surrounded by inflammatory infiltrates, mainly histiocytes (arrow) and giant cells. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.)
MRI can be helpful in further characterizing palpable lumps with inconclusive findings at US and mammography, particularly when core biopsy is not technically feasible. MRI findings of fat necrosis are histologically based on the amount of inflammatory reaction, liquefied fat, and fibrosis present at the time of MRI (10). On noncontrast images, fat necrosis can manifest as a spiculated mass or area of architectural distortion, both secondary to fibrosis. Fibrosis can demonstrate hyper-, iso-, or hypointense T1-weighted signal intensity, depending on its stage. Although nonsaturated fat on T1-weighted images shows high signal intensity similar to that of surrounding fat, which significantly aids in diagnosis of fat necrosis (6), liquefied fat may demonstrate low signal intensity due to hemorrhage and inflammatory infiltrate. Large dystrophic calcifications may appear as signal void at MRI.
After administration of contrast material, enhancement may vary in morphology and kinetic pattern, ranging from slow and gradual to rapid (11). Evaluation of postcontrast fat-suppressed MR images must be performed in correlation with evaluation of precontrast T1-weighted non–fat-suppressed images (Fig 3). If enhancement is present and additional findings are inconclusive for fat necrosis, needle biopsy or surgical excision should be considered.
Figure 3a.
Fat necrosis in a patient with a history of right mastectomy, latissimus dorsi flap reconstruction, and subsequent fat grafting. (a) Gray-scale US image of the lump shows multiple echogenic circumscribed conglomerate masses (arrows) without associated vascularity (not shown). (b) On an axial precontrast non–fat-suppressed T1-weighted image, the masses (arrowheads) have internal high signal intensity similar to that of fat. (c) On an axial postcontrast fat-suppressed T1-weighted image, the masses (arrowheads) have rim enhancement with complete suppression of the internal fat. The findings are consistent with fat necrosis secondary to fat grafting, and no further workup was necessary.
Figure 3b.
Fat necrosis in a patient with a history of right mastectomy, latissimus dorsi flap reconstruction, and subsequent fat grafting. (a) Gray-scale US image of the lump shows multiple echogenic circumscribed conglomerate masses (arrows) without associated vascularity (not shown). (b) On an axial precontrast non–fat-suppressed T1-weighted image, the masses (arrowheads) have internal high signal intensity similar to that of fat. (c) On an axial postcontrast fat-suppressed T1-weighted image, the masses (arrowheads) have rim enhancement with complete suppression of the internal fat. The findings are consistent with fat necrosis secondary to fat grafting, and no further workup was necessary.
Figure 3c.
Fat necrosis in a patient with a history of right mastectomy, latissimus dorsi flap reconstruction, and subsequent fat grafting. (a) Gray-scale US image of the lump shows multiple echogenic circumscribed conglomerate masses (arrows) without associated vascularity (not shown). (b) On an axial precontrast non–fat-suppressed T1-weighted image, the masses (arrowheads) have internal high signal intensity similar to that of fat. (c) On an axial postcontrast fat-suppressed T1-weighted image, the masses (arrowheads) have rim enhancement with complete suppression of the internal fat. The findings are consistent with fat necrosis secondary to fat grafting, and no further workup was necessary.
Fat Graft
Autologous fat grafting is widely performed for breast reconstruction and augmentation. When used in conjunction with reconstruction after mastectomy, it is often performed to fill depressions and smooth contour irregularities along the margins of the flaps (12). Before reinjection of the lipoaspirate, which is commonly taken from the abdomen or upper thigh, it is centrifuged to separate the oil from the intact adipocyte. Revascularization of the transplanted adipocytes in the recipient bed is required for successful engraftment. Failure of this process can lead to graft resorption, scarring, fat necrosis, and calcifications, many of which, when palpable, can result in challenging imaging features difficult to differentiate from those of recurrence (13).
Pathologic Findings.—Histologic analysis of fat grafts depends on the age of the graft. In the early postoperative period, focal necrosis and macrophages with fine vacuolated cytoplasm are seen, which are indicative of lipid digestion. In later stages, normal mature fat tissue is usually seen as a predominant component of the graft, with sporadic fibrosis and few mature macrophages (14).
Imaging Features.—Fat necrosis and oil cysts are seen in most patients with fat grafts and are therefore considered the expected outcome. When palpable, a circumscribed avascular mass is typically seen at US at the site of fat injection and has 100% negative predictive value for malignancy (15). However, biopsy may be warranted to exclude recurrence when the mass demonstrates irregular margins or internal vascularity (15).
Mammography may be helpful to confirm benign features such as oil cysts, dystrophic calcifications, or calcifications with radiolucent centers. At MRI, areas of fat injection typically demonstrate circumscribed masses with enhancing rims, which represent inflammation and fibrosis. On T1-weighted non–fat-saturated and T2-weighted images, oil cysts have central hyperintense and hypointense signal, respectively, and may therefore be differentiated from malignancy (Fig 3).
Fibrosis or Scarring
Fibrosis or scarring is a benign sequela of surgical intervention or radiation therapy that frequently manifests as a palpable lump near the surgical scar in mastectomy patients. Permanent fibrotic change can also occur months to years after chest wall irradiation, particularly in patients with underlying collagen vascular disease (16). Differentiating between benign focal fibrosis or scarring and tumor recurrence can be a major clinical and radiologic challenge and therefore often requires further imaging workup or core needle biopsy for diagnosis (4).
Pathologic Findings.—Healing and scar formation are the primary processes responsible for fibrosis after lumpectomy or mastectomy. In the normal breast, benign fibrosis results from proliferation of fibrous tissue, leading to obliteration of mammary ducts and acini, mostly seen in premenopausal women. After mastectomy, focally fibrous stroma surrounding atrophied epithelium may develop secondary to surgical intervention (17).
Imaging Features.—The radiologic appearance of fibrosis may often overlap with that of malignant tumors. At US, it usually appears as an irregular hypoechoic mass with posterior acoustic shadowing, which is often associated with architectural distortion at mammography (Fig 4). If serial screening mammograms are available, stability or slow regression over the years usually indicates a benign process and helps rule out tumor recurrence (2).
Figure 4a.
Fibrosis manifesting as postmastectomy palpable lumps and skin dimpling. (a) Gray-scale (left) and color Doppler (right) US images of both areas of concern in the outer and inner right breast show suspicious hypoechoic masses with spiculated margins (arrows in left image). US-guided biopsy revealed dense fibrosis and elastosis at both sites. (b) High-power photomicrograph shows fibrous proliferation (yellow arrows) with surrounding elastic fibers (black arrows) without any evidence of adjacent breast parenchyma. (H-E stain; original magnification, ×40.)
Figure 4b.
Fibrosis manifesting as postmastectomy palpable lumps and skin dimpling. (a) Gray-scale (left) and color Doppler (right) US images of both areas of concern in the outer and inner right breast show suspicious hypoechoic masses with spiculated margins (arrows in left image). US-guided biopsy revealed dense fibrosis and elastosis at both sites. (b) High-power photomicrograph shows fibrous proliferation (yellow arrows) with surrounding elastic fibers (black arrows) without any evidence of adjacent breast parenchyma. (H-E stain; original magnification, ×40.)
Given the challenge of distinguishing focal fibrosis or scarring from malignancy, MRI for problem solving can be useful for evaluation. Fibrosis often demonstrates low T2-weighted signal intensity with no or faint enhancement on dynamic postcontrast images and a delayed enhancement pattern on the kinetic curve. While it can be differentiated from tumor recurrence, which typically demonstrates intermediate or slightly increased T2-weighted signal intensity compared with that of the breast parenchyma with washout kinetics, overlap between the two can certainly result in false-positive MRI interpretation. This is commonly seen in the first 6–12 months of the postoperative or postirradiation period, often related to enhancing granulation tissue (18).
Seroma or Hematoma
Fluid collections are well-expected sequelae of surgical intervention, specifically during the early postoperative phase. Seromas usually resorb over time, although in some patients they can persist for months or even years despite multiple aspirations. The more extensive the surgery, the more likely a seroma is to develop.
In mastectomy patients, seromas can be seen with flap reconstruction, revisions, or axillary lymph node dissection. Flaps that require less surgical dissection, such as the deep inferior epigastric perforator (DIEP) flap, are less likely to be associated with seromas or hematomas than the free or pedicled transverse rectus abdominis myocutaneous (TRAM) flap (12,19). Although rarely needed, percutaneous aspiration or surgical drainage may be performed for symptom relief.
Pathologic Findings.—Seromas arise from extensive surgical manipulation and dissection of blood vessels and lymphatics, leading to leakage and accumulation of benign inflammatory exudate, which typically consists of benign inflammatory cells such as neutrophils, lymphocytes, and macrophages (20).
Imaging Features.—US can be sufficient for evaluating palpable seromas or hematomas and useful for guiding fluid aspiration if desired. An oval or round circumscribed anechoic fluid collection in a surgical bed is usually consistent with a benign seroma (Fig 5a). If initial static US images are obtained by a technologist, it may be prudent for the radiologist to scan in real time for complete evaluation. Hematomas and complicated seromas can be noted immediately postoperatively as a collection with internal septa, loculi, or thick walls and usually resorb over time, replaced by fibrosis and scarring (Fig 5b).
Figure 5a.
Palpable postmastectomy seroma. (a) Gray-scale US image shows a typical circumscribed anechoic seroma at the site of axillary lymph node dissection. (b) Gray-scale US image in a male patient shows a complex seroma at the mastectomy scar containing a questionable solid lesion versus avascular debris (arrow). Aspiration was indicated and yielded benign seroma with blood and fibrinous material.
Figure 5b.
Palpable postmastectomy seroma. (a) Gray-scale US image shows a typical circumscribed anechoic seroma at the site of axillary lymph node dissection. (b) Gray-scale US image in a male patient shows a complex seroma at the mastectomy scar containing a questionable solid lesion versus avascular debris (arrow). Aspiration was indicated and yielded benign seroma with blood and fibrinous material.
At MRI, a circumscribed fluid collection with central T2 signal hyperintensity is typical of a seroma or hematoma. Hematomas can have variable T1-weighted and T2-weighted signal intensity, depending on evolution of the blood products. At postcontrast imaging, a uniformly smooth enhancing rim is often noted. If internal enhancement, focal thickening, or a solid nodule is noted, sampling should be performed to exclude malignancy (18).
Suture Granuloma
Foreign-body granulomas occur as the body’s reaction to immunologically inert materials, either exogenous such as nonabsorbable suture, silicone, gauze, sewing needles, or paraffin or endogenous such as fat necrosis (21). The clinical manifestation can vary from mild inflammation and erythema, swelling, or pain to finally rejection of the foreign-body material, depending on the inflammatory stage, resulting in a solid palpable lump that reflects a chronic inflammatory reaction with granuloma formation. However, this can form months to years after the surgical procedure. According to a retrospective study by Illman et al (22), 33% of breast granulomas were found to be silicone from prior implant rupture or free injections, 29% were fat necrosis, and 11% were suture granulomas, with the remainder found to be idiopathic granulomatous mastitis.
Pathologic Findings.—At the histologic level, both silicone and suture granulomas demonstrate an inflammatory mixed infiltrate, mainly multinucleated giant cells, histiocytes, and lymphocytes. In cases of suture granuloma, remnants of suture material are polarizable in most cases (23) (Fig 6d).
Figure 6d.
Suture granuloma. (a) Gray-scale US image shows a hypoechoic circumscribed mass at the area of concern containing linear echogenicity (arrow), which represents suture material. Biopsy results confirmed the diagnosis. (b) Postprocedure mammogram shows two isodense subareolar masses containing suture material (arrowheads) and a biopsy clip in the palpable mass (arrow). (c) PET/CT image in a patient with bilateral biopsy-proven suture granulomas shows increased fluorodeoxyglucose (FDG) uptake (arrows). (d) High-power photomicrograph shows central foreign material (arrowhead) surrounded by multinucleated giant cells (arrows) and reactive fibrosis. (H-E stain; original magnification, ×40.)
Figure 6b.

Suture granuloma. (a) Gray-scale US image shows a hypoechoic circumscribed mass at the area of concern containing linear echogenicity (arrow), which represents suture material. Biopsy results confirmed the diagnosis. (b) Postprocedure mammogram shows two isodense subareolar masses containing suture material (arrowheads) and a biopsy clip in the palpable mass (arrow). (c) PET/CT image in a patient with bilateral biopsy-proven suture granulomas shows increased fluorodeoxyglucose (FDG) uptake (arrows). (d) High-power photomicrograph shows central foreign material (arrowhead) surrounded by multinucleated giant cells (arrows) and reactive fibrosis. (H-E stain; original magnification, ×40.)
Imaging Features.—Suture granuloma can have a classic US appearance with high specificity, identified as a serpiginous tubular single or double echogenic line in a circumscribed hypoechoic mass (Fig 6a). This rail-like line usually indicates the suture material and can help in excluding recurrence (24). In a retrospective study by Rettenbacher at al (24), high-frequency US allowed identification of 91% of suture granulomas on the basis of this finding. Unilateral mammography can provide confirmation by demonstrating suture knots with or without calcifications.
Figure 6a.
Suture granuloma. (a) Gray-scale US image shows a hypoechoic circumscribed mass at the area of concern containing linear echogenicity (arrow), which represents suture material. Biopsy results confirmed the diagnosis. (b) Postprocedure mammogram shows two isodense subareolar masses containing suture material (arrowheads) and a biopsy clip in the palpable mass (arrow). (c) PET/CT image in a patient with bilateral biopsy-proven suture granulomas shows increased fluorodeoxyglucose (FDG) uptake (arrows). (d) High-power photomicrograph shows central foreign material (arrowhead) surrounded by multinucleated giant cells (arrows) and reactive fibrosis. (H-E stain; original magnification, ×40.)
At MRI, imaging findings of suture granulomas can vary on the basis of the clinical stage but often show no correlating signal intensity abnormality. Occasionally, if associated with calcifications, suture granuloma can appear as a mildly enhancing mass with central signal intensity drop, denoting the suture material. PET is found to be less helpful, as some of these granulomas can show some false-positive fluorodeoxyglucose (FDG) uptake (25) (Fig 6c).
Figure 6c.
Suture granuloma. (a) Gray-scale US image shows a hypoechoic circumscribed mass at the area of concern containing linear echogenicity (arrow), which represents suture material. Biopsy results confirmed the diagnosis. (b) Postprocedure mammogram shows two isodense subareolar masses containing suture material (arrowheads) and a biopsy clip in the palpable mass (arrow). (c) PET/CT image in a patient with bilateral biopsy-proven suture granulomas shows increased fluorodeoxyglucose (FDG) uptake (arrows). (d) High-power photomicrograph shows central foreign material (arrowhead) surrounded by multinucleated giant cells (arrows) and reactive fibrosis. (H-E stain; original magnification, ×40.)
Silicone Granuloma and Implant Rupture
Silicone implant rupture should also be in the differential diagnosis when evaluating focal symptoms in mastectomy patients with silicone implant reconstruction. The prevalence of rupture increases with implant age through weakening of the elastomer shell. Most reported symptoms include palpable lumps, skin tightening, focal pain, and changes in the reconstructed breast size or shape.
These symptoms are found to correspond to silicone gel freely escaping the fibrous capsule and infiltrating into the parenchyma or aggregating into silicone granulomas. Small amounts of silicone can also travel through the lymphatic system and aggregate in the axillary or internal mammary lymph nodes (26). These silicone-laden lymph nodes can enlarge over time and become palpable by the patient.
Pathologic Findings.—Silicone granuloma is an organized group of macrophages with a variable number of lymphocytes, fibroblasts, and blood vessels. Silicone appears as refractile nonpolarizable material within empty spaces surrounded by inflammatory infiltrate (23).
Imaging Features.—Free silicone aggregates or granulomas in the breast and axilla may vary in their US appearance. Classic snowstorm and echogenic-noise artifacts are highly specific for silicone granuloma and free silicone, respectively, although anechoic cysts and isoechoic solid masses can occasionally be seen at the site of the palpable lump (Fig 7).Therefore, core needle biopsy may be necessary for diagnosis, especially if the finding is associated with posterior acoustic shadowing (27). Implant rupture may be suggested at mammography by an irregular implant contour or high-density circumscribed mass attached to or separate from the implant, specifically if underlying the palpable lump marker.
Figure 7a.
Postmastectomy silicone granuloma manifesting as a palpable left axillary lump in the setting of known previous silicone implant rupture and revision. (a) Gray-scale US image of the left axilla shows a hyperechoic round mass with a well-defined anterior margin (arrow) and poorly defined posterior margin with a snowstorm appearance (arrowhead). (b, c) Axial (b) and sagittal (c) water-saturated T2-weighted images show silicone in the left internal mammary nodes (arrows) and intact implants, consistent with the history of prior silicone implant rupture. (d) Medium-power photomicrograph shows silicone-induced giant cell foreign-body reaction: refractile foreign-body material (yellow arrows) surrounded by multinucleated giant cells (black arrows). (H-E stain; original magnification, ×20.)
Figure 7b.
Postmastectomy silicone granuloma manifesting as a palpable left axillary lump in the setting of known previous silicone implant rupture and revision. (a) Gray-scale US image of the left axilla shows a hyperechoic round mass with a well-defined anterior margin (arrow) and poorly defined posterior margin with a snowstorm appearance (arrowhead). (b, c) Axial (b) and sagittal (c) water-saturated T2-weighted images show silicone in the left internal mammary nodes (arrows) and intact implants, consistent with the history of prior silicone implant rupture. (d) Medium-power photomicrograph shows silicone-induced giant cell foreign-body reaction: refractile foreign-body material (yellow arrows) surrounded by multinucleated giant cells (black arrows). (H-E stain; original magnification, ×20.)
Figure 7c.

Postmastectomy silicone granuloma manifesting as a palpable left axillary lump in the setting of known previous silicone implant rupture and revision. (a) Gray-scale US image of the left axilla shows a hyperechoic round mass with a well-defined anterior margin (arrow) and poorly defined posterior margin with a snowstorm appearance (arrowhead). (b, c) Axial (b) and sagittal (c) water-saturated T2-weighted images show silicone in the left internal mammary nodes (arrows) and intact implants, consistent with the history of prior silicone implant rupture. (d) Medium-power photomicrograph shows silicone-induced giant cell foreign-body reaction: refractile foreign-body material (yellow arrows) surrounded by multinucleated giant cells (black arrows). (H-E stain; original magnification, ×20.)
Figure 7d.
Postmastectomy silicone granuloma manifesting as a palpable left axillary lump in the setting of known previous silicone implant rupture and revision. (a) Gray-scale US image of the left axilla shows a hyperechoic round mass with a well-defined anterior margin (arrow) and poorly defined posterior margin with a snowstorm appearance (arrowhead). (b, c) Axial (b) and sagittal (c) water-saturated T2-weighted images show silicone in the left internal mammary nodes (arrows) and intact implants, consistent with the history of prior silicone implant rupture. (d) Medium-power photomicrograph shows silicone-induced giant cell foreign-body reaction: refractile foreign-body material (yellow arrows) surrounded by multinucleated giant cells (black arrows). (H-E stain; original magnification, ×20.)
A silicone-specific MRI protocol is needed when implant rupture is suspected. Silicone granulomas and extruded free silicone typically follow silicone signal intensity, which includes low signal intensity on T1-weighted images with fat saturation and high signal intensity on water-suppressed T2-weighted images (Fig 8). As with suture granulomas, PET can demonstrate uptake by extracapsular silicone as well as granulomas in the axillary or internal mammary lymph nodes (23).
Figure 8a.
Intra- and extracapsular implant rupture in a mastectomy patient manifesting as a new palpable lump and deformity in the lower inner reconstructed breast. (a) Gray-scale US image shows a heterogeneous irregular mass (arrowhead) with an underlying fluid collection (*) contiguous to the adjacent implant. (b) Gray-scale US image shows stacked echogenic lines (stepladder sign) (arrows) corresponding to the collapsed shell of the silicone implant, consistent with intracapsular rupture. (c, d) Axial short inversion time inversion-recovery (STIR) (c) and postcontrast fat-suppressed T1-weighted (d) images show a T2-hyperintense mass with silicone signal intensity (arrows) at the lower inner breast without any suspicious enhancement and a collapsed implant with wavy appearance of the elastomer shell (linguine sign).
Figure 8b.
Intra- and extracapsular implant rupture in a mastectomy patient manifesting as a new palpable lump and deformity in the lower inner reconstructed breast. (a) Gray-scale US image shows a heterogeneous irregular mass (arrowhead) with an underlying fluid collection (*) contiguous to the adjacent implant. (b) Gray-scale US image shows stacked echogenic lines (stepladder sign) (arrows) corresponding to the collapsed shell of the silicone implant, consistent with intracapsular rupture. (c, d) Axial short inversion time inversion-recovery (STIR) (c) and postcontrast fat-suppressed T1-weighted (d) images show a T2-hyperintense mass with silicone signal intensity (arrows) at the lower inner breast without any suspicious enhancement and a collapsed implant with wavy appearance of the elastomer shell (linguine sign).
Figure 8c.
Intra- and extracapsular implant rupture in a mastectomy patient manifesting as a new palpable lump and deformity in the lower inner reconstructed breast. (a) Gray-scale US image shows a heterogeneous irregular mass (arrowhead) with an underlying fluid collection (*) contiguous to the adjacent implant. (b) Gray-scale US image shows stacked echogenic lines (stepladder sign) (arrows) corresponding to the collapsed shell of the silicone implant, consistent with intracapsular rupture. (c, d) Axial short inversion time inversion-recovery (STIR) (c) and postcontrast fat-suppressed T1-weighted (d) images show a T2-hyperintense mass with silicone signal intensity (arrows) at the lower inner breast without any suspicious enhancement and a collapsed implant with wavy appearance of the elastomer shell (linguine sign).
Figure 8d.
Intra- and extracapsular implant rupture in a mastectomy patient manifesting as a new palpable lump and deformity in the lower inner reconstructed breast. (a) Gray-scale US image shows a heterogeneous irregular mass (arrowhead) with an underlying fluid collection (*) contiguous to the adjacent implant. (b) Gray-scale US image shows stacked echogenic lines (stepladder sign) (arrows) corresponding to the collapsed shell of the silicone implant, consistent with intracapsular rupture. (c, d) Axial short inversion time inversion-recovery (STIR) (c) and postcontrast fat-suppressed T1-weighted (d) images show a T2-hyperintense mass with silicone signal intensity (arrows) at the lower inner breast without any suspicious enhancement and a collapsed implant with wavy appearance of the elastomer shell (linguine sign).
Peri-implant Fluid Collection or Effusion
A collection or effusion surrounding an implant is estimated to occur in approximately 5% of patients who have had breast reconstruction with implants (28). Patients can report feeling a focal bulge over an area of peri-implant fluid collection (27). Early peri-implant seromas occur during the 1st postoperative year and can manifest as pain, swelling, redness, or a focal palpable abnormality. These can be treated or drained if the patient is symptomatic to exclude infection and do not require further follow-up.
Late peri-implant effusions develop at least 1 year but typically 7–10 years after implant placement and warrant further workup to exclude breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) (29,30). Other causes of delayed effusion include infection, implant rupture, and mechanical shearing (20).
Pathologic Findings.—Early peri-implant effusion aspirate typically consists of serous fluid and can also contain benign inflammatory cells such as neutrophils, lymphocytes, and macrophages (20). In the absence of infection, this fluid is typically not sent for cytologic analysis. Late effusions should be aspirated (at least 50 mL) and sent for cytologic analysis to evaluate cell morphology, flow cytometry for T-cell evaluation, and immunohistochemistry to include CD30 and anaplastic lymphoma kinase (ALK) expression (29,30).
Imaging Features.—US is typically the first-line imaging modality for evaluating early and late peri-implant effusions. Early implant effusion appears as hypoechoic fluid surrounding the implant, either diffusely or loculated. The effusion fluid occupies the space between the elastomer shell and fibrous capsule (Fig 9). If MRI is performed, a simple effusion typically appears as T2-hyperintense fluid between the elastomer shell and fibrous capsule (31). In late effusion, dynamic postcontrast imaging is useful to evaluate for capsular enhancement, adenopathy, and capsular masses, especially if US results are indeterminate (30).
Figure 9.
Peri-implant fluid collection in a patient who underwent mastectomy and implant reconstruction 7 months before presenting with focal pain. Gray-scale US images show simple anechoic fluid in a radial fold between the implant’s elastomer shell (arrow) and fibrous capsule (arrowhead). No further workup was deemed necessary, given the early postoperative period and benign US appearance.
Implant Valve
Traditional saline implants are known to have fill valves or injection ports along the anterior elastomer shell of the implant, usually placed in a subareolar location (Fig 10). After skin-sparing mastectomy, in the absence of subcutaneous fat, fill valves can often be palpated and reported by some patients as an area of concern (27).
Figure 10a.
Implant valve correlating with a palpable lump in a mastectomy patient with saline implants. (a) Gray-scale US image directed to the palpable lump shows the implant valve (arrowheads) and normal overlying tissue. (b) Craniocaudal mammogram shows the radiolucent saline implant with its valve in a subareolar location (yellow line). (c) Axial non–fat-suppressed T2-weighted image shows bilateral hyperintense saline implants with low-signal-intensity valves (arrows) in the anterior wall of the implants. (d) Drawing shows a saline implant with a valve in its anterior wall, used to fill the implant with saline.
Figure 10d.
Implant valve correlating with a palpable lump in a mastectomy patient with saline implants. (a) Gray-scale US image directed to the palpable lump shows the implant valve (arrowheads) and normal overlying tissue. (b) Craniocaudal mammogram shows the radiolucent saline implant with its valve in a subareolar location (yellow line). (c) Axial non–fat-suppressed T2-weighted image shows bilateral hyperintense saline implants with low-signal-intensity valves (arrows) in the anterior wall of the implants. (d) Drawing shows a saline implant with a valve in its anterior wall, used to fill the implant with saline.
The valves are easily identified mammographically through a radiolucent saline implant and can also be visible at directed US (Fig 10a, 10b). Careful evaluation of the overlying tissue should be performed to exclude malignancy before documenting images of the implant valve as a definitive correlation. In the setting of bilateral saline implants, a symmetric palpable lump is usually felt in the contralateral breast, and US confirmation can be obtained if necessary.
Figure 10b.

Implant valve correlating with a palpable lump in a mastectomy patient with saline implants. (a) Gray-scale US image directed to the palpable lump shows the implant valve (arrowheads) and normal overlying tissue. (b) Craniocaudal mammogram shows the radiolucent saline implant with its valve in a subareolar location (yellow line). (c) Axial non–fat-suppressed T2-weighted image shows bilateral hyperintense saline implants with low-signal-intensity valves (arrows) in the anterior wall of the implants. (d) Drawing shows a saline implant with a valve in its anterior wall, used to fill the implant with saline.
MRI should not be performed for evaluation of saline implant rupture, as this should be clinically evident. If MRI is performed for other purposes, the valve may be noted as a low-signal-intensity structure, usually along the anterior wall of the T2-hyperintense saline implant (Fig 10c).
Figure 10c.
Implant valve correlating with a palpable lump in a mastectomy patient with saline implants. (a) Gray-scale US image directed to the palpable lump shows the implant valve (arrowheads) and normal overlying tissue. (b) Craniocaudal mammogram shows the radiolucent saline implant with its valve in a subareolar location (yellow line). (c) Axial non–fat-suppressed T2-weighted image shows bilateral hyperintense saline implants with low-signal-intensity valves (arrows) in the anterior wall of the implants. (d) Drawing shows a saline implant with a valve in its anterior wall, used to fill the implant with saline.
Traumatic or Amputation Neuroma
Traumatic neuromas are benign lesions that are usually found after trauma or surgical intervention. Neuromas reflect a hyperplastic and proliferative process of the severed nerve end in an attempt to reinnervate the affected region and therefore are not considered true tumors (32).
Although most neuromas are found in the extremities, breast and chest wall traumatic neuromas have been described in the literature but are considered extremely rare, with estimated incidence of less than 0.09% (32,33). Breast neuromas commonly manifest as a localized area of severe tenderness or a palpable chest wall lump after surgical excision or mastectomy. A frequently observed location of a postmastectomy neuroma is near the surgical scar in the upper outer aspect of the chest, where branches of the long thoracic, thoracodorsal, pectoral, and intercostal nerves are susceptible to injury.
Conservative management is usually attempted first, with measures such as acupuncture, physical therapy, or directed injection of local anesthetics, steroids, or alcohol (32,34). In the case of refractory pain beyond 6 months, surgical excision of the neuroma is usually indicated (34).
Pathologic Findings.—Traumatic neuroma represents a dense, nonneoplastic, and disorganized proliferation of nerve bundles with low vascularity surrounded by an outer layer of contracting scar tissue and inflammation. This layer is continuous with the perineurium of the intact nerve sheath. Inside the neuroma matrix, inflammatory cells can be seen (35).
Imaging Features.—Given its rare incidence, the appearance of neuroma at mammography is not well described in the literature. The most common US features of neuromas identified in mastectomy patients include an oval or fusiform circumscribed hypoechoic mass with parallel orientation and increased vascularity at color Doppler imaging. According to previous reports, neuromas often demonstrate the tail sign: an area of focal thickening contiguous with the visualized mass (Fig 11) (34,36).
Figure 11a.
Postmastectomy traumatic (amputation) neuroma manifesting as localized “excruciating” pain in the upper outer reconstructed breast. (a) Targeted gray-scale (top) and color Doppler (bottom) US images show a predominantly circumscribed hypoechoic mass in the surgical scar with a focal protrusion (tail sign) (arrow in top image). (b) Low-power photomicrograph shows fibroadipose tissue with haphazard nerve bundles (arrows). (H-E stain; original magnification, ×10.)
Figure 11b.
Postmastectomy traumatic (amputation) neuroma manifesting as localized “excruciating” pain in the upper outer reconstructed breast. (a) Targeted gray-scale (top) and color Doppler (bottom) US images show a predominantly circumscribed hypoechoic mass in the surgical scar with a focal protrusion (tail sign) (arrow in top image). (b) Low-power photomicrograph shows fibroadipose tissue with haphazard nerve bundles (arrows). (H-E stain; original magnification, ×10.)
Breast traumatic neuromas have rarely been visualized at MRI and often demonstrate nonspecific features. Peripheral traumatic neuromas are described as isointense to skeletal muscle on T1-weighted images with high signal intensity on T2-weighted images (37). Postcontrast heterogeneous enhancement is common, with a benign type 1 enhancement curve (37).
Palpable Recurrent Malignancy
Local-regional recurrence is defined as ipsilateral breast, chest wall, skin, axillary, supraclavicular, or parasternal nodal recurrence of histologically proven carcinoma (38). The incidence of local-regional recurrence after modified radical mastectomy varies significantly and can range from 2% to 25%, with the majority of recurrences developing within 3–5 years after treatment (39,40). This rate was found to be overall similar between patients who underwent mastectomy alone versus patients who underwent mastectomy with reconstruction (41). Tumor size and biology, histologic features, age at time of diagnosis, and presence of positive margins in the mastectomy specimen have been described in the literature as risk factors for recurrence (42).
With any modified radical mastectomy, a small amount of residual breast tissue can remain, carrying an unknown risk of cancer recurrence (40). According to published study analyses, residual breast tissue can be found in up to 60% of patients who underwent nipple- or skin-sparing mastectomy. This is due to the variable amount of terminal ductal-lobular units (TDLUs) that can be found in the nipple-areolar complex region and in skin flaps thicker than 5 mm (43,44).
Recurrence in Mastectomy with Reconstruction
According to the American Cancer Society, 20%–40% of patients with breast cancer who undergo mastectomy choose to have breast reconstruction (45). The most commonly used reconstruction techniques include implant placement, use of autologous tissue, or a combination of both (46). Common autologous flap reconstruction techniques include use of the transverse rectus abdominis myocutaneous (TRAM) flap, deep inferior epigastric perforator (DIEP) flap, or latissimus dorsi flap (LDF).
The choice of autologous reconstruction type depends on the type of mastectomy performed, the extent of disease, the patient’s anatomy and body habitus, and personal preference (12). The cancer recurrence rate in early-stage patients after mastectomy with autologous myocutaneous flap reconstruction is 7%, with a mean interval of 5 years between mastectomy for the initial cancer and the time of recurrence diagnosis (5). The TRAM flap is the most commonly used flap, with a low rate of recurrence of 2%–4%.
Histologic Features of Recurrence
Recurrence of ductal carcinoma in situ (DCIS) after mastectomy is rare, occurring in an estimated 3.6% of patients, with a prevalence of high-grade disease in 62.5% of those patients (47). Nipple-sparing mastectomy (NSM) is more commonly associated with DCIS recurrence. Although NSM is performed for tumors located more than 1–1.5 cm from the nipple or prophylactically in high-risk women with genetic mutations, DCIS may still develop or recur within the nipple-areola complex (48).
The literature indicates that the risk of DCIS recurrence appears to be associated with the histologic growth pattern in addition to the presence of positive margins in the mastectomy specimen. DCIS with micropapillary, papillary, or solid architecture is more likely to recur and results in an unfavorable outcome (49). Palpable DCIS in a mastectomy patient is rare and usually indicates more aggressive tumor biology such as high grade or comedonecrosis. Other manifestations such as nipple discharge and skin changes have also been reported (50).
The recurrence rate of invasive carcinoma is variable, depending on the histologic grade and molecular subtype, which is defined by immunohistochemical staining for estrogen receptors (ERs) or progesterone receptors (PRs) and human epidermal growth factor receptor 2 (HER2) score. Basal-like (triple-negative) and HER2-positive tumors have a significant increased risk of local recurrence with decreased overall survival in patients who have undergone mastectomy compared with the other subtypes (51,52). Invasive lobular carcinoma (ILC) typically manifests at a later stage than invasive ductal carcinoma (IDC), requiring mastectomy at a higher rate; however, they have both been reported to show a similar overall local recurrence rate (53).
Local recurrence after mastectomy can be an indicator of distant metastasis; therefore, subsequent metastasis workup is necessary. Different treatment options can be offered, depending on the size and location of the tumor and the presence of distance metastasis. If possible, in the absence of metastasis and prior radiation therapy, local resection is usually performed, followed by radiation therapy to the involved area and regional lymphatics (54).
Common Sites of Recurrence and Imaging Features
Common locations of cancer recurrence in patients after mastectomy with or without reconstruction are shown in Figure 12.
Figure 12.
Common sites of cancer recurrence in mastectomy patients. A = chest wall and pectoralis muscle, B = mastectomy scar, C = skin, D = contact zone between the native tissue and an autologous flap, E = ipsilateral axillary lymph nodes, F = supraclavicular lymph nodes, G = infraclavicular lymph nodes, H = contralateral axillary lymph nodes.
Chest Wall.—The presence of chest wall recurrence is a poor prognostic sign, with increased risk of metastasis and poor overall survival (55). Recurrence at the chest wall after mastectomy occurs in 10%–20% of patients with operable breast carcinoma (56). Regardless of the cancer size, chest wall invasion upgrades the tumor stage to at least stage IIIB (57).
Clinical breast examination or self-examination allows detection of superficial recurrence by identifying palpable masses or skin changes such as thickening, retraction, or erythema. However, other benign causes may lead to a similar clinical manifestation, as discussed earlier, thus leading to high false-positive results (40,58).
Isolated recurrence to the pectoralis muscle is rare and clinically more difficult to detect in mastectomy patients with reconstruction than in mastectomy patients without reconstruction (59) (Fig 13). A case report by Pitcher et al (55) describes an isolated subpectoral recurrence after mastectomy with immediate implant reconstruction and adjuvant chemotherapy; the recurrence was discovered only at the time of secondary revision of the breast reconstruction.
Figure 13a.
Pectoralis muscle and chest wall recurrence manifesting as erythema and swelling, mimicking cellulitis or abscess. (a) US images show at least three complex solid and cystic masses (arrowhead) with internal hypervascularity in the left pectoralis muscle, suspicious for recurrence. (b) Axial contrast-enhanced CT image of the chest shows a corresponding expansile left pectoralis muscle (large arrows) with overlying fat stranding and skin thickening (small arrows). (c) Axial CT image from a follow-up examination 2 months later shows enlarging soft tissue in the anterior chest wall invading and destroying the sternum (arrowheads) with increasing skin thickening (arrows), consistent with metastatic disease progressing to involve the skin and chest wall.
Figure 13b.
Pectoralis muscle and chest wall recurrence manifesting as erythema and swelling, mimicking cellulitis or abscess. (a) US images show at least three complex solid and cystic masses (arrowhead) with internal hypervascularity in the left pectoralis muscle, suspicious for recurrence. (b) Axial contrast-enhanced CT image of the chest shows a corresponding expansile left pectoralis muscle (large arrows) with overlying fat stranding and skin thickening (small arrows). (c) Axial CT image from a follow-up examination 2 months later shows enlarging soft tissue in the anterior chest wall invading and destroying the sternum (arrowheads) with increasing skin thickening (arrows), consistent with metastatic disease progressing to involve the skin and chest wall.
Figure 13c.
Pectoralis muscle and chest wall recurrence manifesting as erythema and swelling, mimicking cellulitis or abscess. (a) US images show at least three complex solid and cystic masses (arrowhead) with internal hypervascularity in the left pectoralis muscle, suspicious for recurrence. (b) Axial contrast-enhanced CT image of the chest shows a corresponding expansile left pectoralis muscle (large arrows) with overlying fat stranding and skin thickening (small arrows). (c) Axial CT image from a follow-up examination 2 months later shows enlarging soft tissue in the anterior chest wall invading and destroying the sternum (arrowheads) with increasing skin thickening (arrows), consistent with metastatic disease progressing to involve the skin and chest wall.
US workup for chest wall recurrence has been reported to have high positive predictive value (PPV) for Breast Imaging Reporting and Data System (BIRADS) category 5 lesions (60). However, the PPV is much lower for less suspicious-appearing masses, and differentiation from benign lesions such as fat necrosis, granuloma, or focal fibrosis becomes challenging, especially when mammography is not feasible (60). US evaluation of chest wall recurrence can reveal irregular, noncircumscribed, antiparallel hypoechoic masses with vascularity, although probably-benign features have also been reported (1).
If enough tissue is present, at least a single mammographic view with marking of the palpable lump or area of pain should be attempted. In patients with autologous or implant reconstruction, it is important to ensure that the technologist includes the area of interest to accurately correlate with the US abnormality and avoid false-negative results. Direct communication between the radiologist and mammography technologist is essential before imaging the patient so that the technologist is aware of the patient’s surgical history and the exact area of concern. This will facilitate obtaining diagnostically useful images.
At MRI, heterogeneously enhancing irregular masses with spiculated margins are known to have the highest positive predictive value for recurrence (Fig 14). However, studies have emphasized that any new focal enhancement in the chest wall should be worked up to rule out recurrence (58). This is especially true when the mastectomy or any subsequent surgical intervention was performed years earlier.
Figure 14a.
Palpable chest wall recurrence after mastectomy with implant reconstruction. (a) Targeted gray-scale US image shows a spiculated solid mass (arrowheads). Subsequent biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b) Axial postcontrast T1-weighted image shows corresponding abnormal enhancement (arrows) abutting and invading the chest wall and surrounding the sternum. (c) On a PET/CT image, the area of enhancement has abnormal FDG tracer uptake (arrows), with maximum standardized uptake value (SUV) of 18.1. Uptake is also noted in the hilar lymph nodes (arrowheads). (d) High-power photomicrograph shows malignant cells (arrows), singly and in groups, invading the stroma outside the ductal-tubular structure with no visible surrounding myoepithelial cells. (H-E stain; original magnification, ×40.)
Figure 14b.
Palpable chest wall recurrence after mastectomy with implant reconstruction. (a) Targeted gray-scale US image shows a spiculated solid mass (arrowheads). Subsequent biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b) Axial postcontrast T1-weighted image shows corresponding abnormal enhancement (arrows) abutting and invading the chest wall and surrounding the sternum. (c) On a PET/CT image, the area of enhancement has abnormal FDG tracer uptake (arrows), with maximum standardized uptake value (SUV) of 18.1. Uptake is also noted in the hilar lymph nodes (arrowheads). (d) High-power photomicrograph shows malignant cells (arrows), singly and in groups, invading the stroma outside the ductal-tubular structure with no visible surrounding myoepithelial cells. (H-E stain; original magnification, ×40.)
Figure 14c.
Palpable chest wall recurrence after mastectomy with implant reconstruction. (a) Targeted gray-scale US image shows a spiculated solid mass (arrowheads). Subsequent biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b) Axial postcontrast T1-weighted image shows corresponding abnormal enhancement (arrows) abutting and invading the chest wall and surrounding the sternum. (c) On a PET/CT image, the area of enhancement has abnormal FDG tracer uptake (arrows), with maximum standardized uptake value (SUV) of 18.1. Uptake is also noted in the hilar lymph nodes (arrowheads). (d) High-power photomicrograph shows malignant cells (arrows), singly and in groups, invading the stroma outside the ductal-tubular structure with no visible surrounding myoepithelial cells. (H-E stain; original magnification, ×40.)
Figure 14d.
Palpable chest wall recurrence after mastectomy with implant reconstruction. (a) Targeted gray-scale US image shows a spiculated solid mass (arrowheads). Subsequent biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b) Axial postcontrast T1-weighted image shows corresponding abnormal enhancement (arrows) abutting and invading the chest wall and surrounding the sternum. (c) On a PET/CT image, the area of enhancement has abnormal FDG tracer uptake (arrows), with maximum standardized uptake value (SUV) of 18.1. Uptake is also noted in the hilar lymph nodes (arrowheads). (d) High-power photomicrograph shows malignant cells (arrows), singly and in groups, invading the stroma outside the ductal-tubular structure with no visible surrounding myoepithelial cells. (H-E stain; original magnification, ×40.)
MRI is also helpful for evaluating the extent of disease and invasion of the pectoralis, serratus anterior, deep intercostal, or parasternal muscles or bone structures such as the sternum and ribs. As mentioned earlier, palpation-guided biopsy should be considered after negative imaging evaluation of a suspicious chest wall mass or even before MRI is performed. In patients with chest wall recurrence without distant metastasis, surgical resection of the chest wall may be effective for pain relief and avoiding local hemorrhage (61).
Mastectomy Scar.—Cancer recurrence in the mastectomy scar is reported in less than 1% of all mastectomies (62). Rarely, the mastectomy scar can contain metastasis, caused by spilling of tumor during curative surgery or a de novo tumor that may develop in residual glandular tissue after prophylactic mastectomy (62).
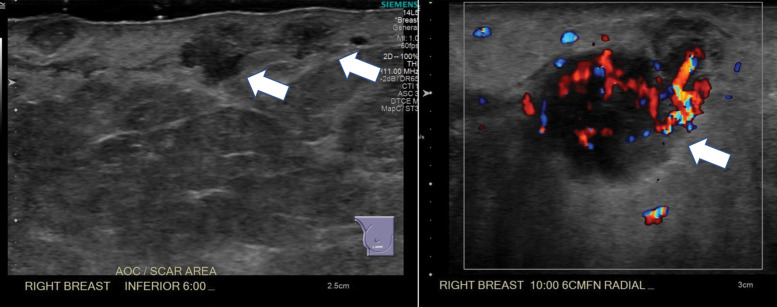
If a clinically indeterminate palpable lump is felt along the scar, imaging workup should be performed. As with chest wall masses, US should be performed first when a palpable mass is identified along the mastectomy scar. A small study by Rissanen et al (63) found that US had 91% sensitivity in evaluation of the mastectomy site. The addition of US has proved to be more accurate than mammography in the setting of a palpable lump, as recurrence tends to be close to the skin surface (40,63) (Fig 15). If enough tissue is present, mammographic views may be helpful in excluding benign causes such as fat necrosis before making decisions regarding biopsy.
Figure 15a.
Recurrence at the mastectomy scar. (a) Color Doppler (left) and gray-scale (right) US images at the areas of palpable concern show a subcutaneous, highly vascular, irregular mass at the central chest wall and an indistinct hyperechoic area inseparable from the skin at the lateral scar (arrowheads in right image). (b) Axial PET/CT images show metabolic activity in the soft-tissue mass (circle) and along the expected location of the mastectomy scar (small arrows), with a focal area of uptake laterally (large arrow). Surgical excision of the palpable mass and scar nodule yielded invasive ductal cancer involving both dermal and adipose tissue, with extensive permeation of dermal and subcutaneous lymphatics by tumor emboli.
Figure 15b.
Recurrence at the mastectomy scar. (a) Color Doppler (left) and gray-scale (right) US images at the areas of palpable concern show a subcutaneous, highly vascular, irregular mass at the central chest wall and an indistinct hyperechoic area inseparable from the skin at the lateral scar (arrowheads in right image). (b) Axial PET/CT images show metabolic activity in the soft-tissue mass (circle) and along the expected location of the mastectomy scar (small arrows), with a focal area of uptake laterally (large arrow). Surgical excision of the palpable mass and scar nodule yielded invasive ductal cancer involving both dermal and adipose tissue, with extensive permeation of dermal and subcutaneous lymphatics by tumor emboli.
Skin.—Local skin recurrence after mastectomy is reported in 5.2%–9.1% of patients (64,65). The literature considers skin recurrence an indicator of the presence of secondary metastasis. The clinical manifestation varies significantly from erythema and thickening to visible skin nodules and—in untreated cases—ulcers (Fig 16). Skin findings can often be mistaken for infection; therefore, skin punch biopsy should be performed to exclude recurrence.
Figure 16a.
Various manifestations of recurrent cancer in the skin in different mastectomy patients. (a–c) Photographs show discrete skin nodules (a), a sharply demarcated area of erythema (b), and extensive fungating skin lesions with ulcers (c). (d) High-power photomicrograph shows tumor cells (arrows) invading the epidermal layer (arrowhead). (H-E stain; original magnification, ×20.)
Figure 16b.

Various manifestations of recurrent cancer in the skin in different mastectomy patients. (a–c) Photographs show discrete skin nodules (a), a sharply demarcated area of erythema (b), and extensive fungating skin lesions with ulcers (c). (d) High-power photomicrograph shows tumor cells (arrows) invading the epidermal layer (arrowhead). (H-E stain; original magnification, ×20.)
Figure 16c.
Various manifestations of recurrent cancer in the skin in different mastectomy patients. (a–c) Photographs show discrete skin nodules (a), a sharply demarcated area of erythema (b), and extensive fungating skin lesions with ulcers (c). (d) High-power photomicrograph shows tumor cells (arrows) invading the epidermal layer (arrowhead). (H-E stain; original magnification, ×20.)
Figure 16d.
Various manifestations of recurrent cancer in the skin in different mastectomy patients. (a–c) Photographs show discrete skin nodules (a), a sharply demarcated area of erythema (b), and extensive fungating skin lesions with ulcers (c). (d) High-power photomicrograph shows tumor cells (arrows) invading the epidermal layer (arrowhead). (H-E stain; original magnification, ×20.)
Several factors may affect survival after isolated skin recurrence in mastectomy patients, such as number and size of skin nodules, presence of vascular emboli (inflammatory changes), site of recurrence, and disease-free interval after mastectomy (65). Once recurrence is confirmed via skin biopsy, diagnostic workup to evaluate the extent of disease, the presence of underlying concurrent recurrence, and distant metastasis becomes necessary (66). At US, skin recurrence can appear as skin thickening (usually >2 mm) or hypoechoic oval or indistinct masses with internal vascularity in the dermal layer (Fig 17), depending on the clinical manifestation. MRI can demonstrate patchy, nodular, or diffuse skin edema with heterogeneous enhancement correlating with the area of concern.
Figure 17a.

Skin recurrence in a patient with a history of skin-sparing mastectomy and deep inferior epigastric perforator (DIEP) flap reconstruction. (a) Photograph of the reconstructed right breast shows erythema and multiple palpable skin nodules at the 10-o’clock position and along the vertical surgical scar at the 6-o’clock position. (b) Gray-scale (left) and color Doppler (right) US images of the right breast show irregular hypoechoic masses (arrows in left image) in the dermal and subdermal layer with hypervascularity (arrow in right image). (c) Axial CT image shows multiple subcutaneous enhancing nodules (arrowheads), which correlate with the US findings.
Figure 17b.
Skin recurrence in a patient with a history of skin-sparing mastectomy and deep inferior epigastric perforator (DIEP) flap reconstruction. (a) Photograph of the reconstructed right breast shows erythema and multiple palpable skin nodules at the 10-o’clock position and along the vertical surgical scar at the 6-o’clock position. (b) Gray-scale (left) and color Doppler (right) US images of the right breast show irregular hypoechoic masses (arrows in left image) in the dermal and subdermal layer with hypervascularity (arrow in right image). (c) Axial CT image shows multiple subcutaneous enhancing nodules (arrowheads), which correlate with the US findings.
Figure 17c.
Skin recurrence in a patient with a history of skin-sparing mastectomy and deep inferior epigastric perforator (DIEP) flap reconstruction. (a) Photograph of the reconstructed right breast shows erythema and multiple palpable skin nodules at the 10-o’clock position and along the vertical surgical scar at the 6-o’clock position. (b) Gray-scale (left) and color Doppler (right) US images of the right breast show irregular hypoechoic masses (arrows in left image) in the dermal and subdermal layer with hypervascularity (arrow in right image). (c) Axial CT image shows multiple subcutaneous enhancing nodules (arrowheads), which correlate with the US findings.
Contact Zone.—In patients with skin- or nipple-sparing mastectomy and flap reconstruction, the most common area of cancer recurrence is the zone of attachment between the dermal layer of the native mastectomy flap and the skin or subcutaneous fat of the autologous flap, an area known as the contact zone (67,68) (Fig 18). The frequency of cancer recurrence in this location is likely secondary to the risk of residual tumor cells remaining in the native tissue after mastectomy (69). It is also believed that the centripetal nature of lymphatic drainage in the breast may transfer malignant cells to the dermal layer of the native mastectomy flap, leading to proliferation of residual tumor in the superficial dermal layer and the subcutaneous fat layer (67).
Figure 18a.
Contact zone of a transverse rectus abdominis myocutaneous (TRAM) flap. (a) Drawing shows the contact zone between the native mastectomy skin flap and the superficial subcutaneous layer of the autologous flap. (b) Sagittal non–fat-suppressed T1-weighted image of a reconstructed breast with a TRAM flap shows a low-signal-intensity line (arrows), which represents the dermal layer of the abdominal tissue (autologous flap) parallel to the native breast skin and an atrophied rectus abdominis muscle (*).
Figure 18b.

Contact zone of a transverse rectus abdominis myocutaneous (TRAM) flap. (a) Drawing shows the contact zone between the native mastectomy skin flap and the superficial subcutaneous layer of the autologous flap. (b) Sagittal non–fat-suppressed T1-weighted image of a reconstructed breast with a TRAM flap shows a low-signal-intensity line (arrows), which represents the dermal layer of the abdominal tissue (autologous flap) parallel to the native breast skin and an atrophied rectus abdominis muscle (*).
Because of this superficial location, a palpable lump can often be detected by the patient or clinician; therefore, regular physical examination of the flap is crucial. Once an abnormality is identified, imaging with mammography (to exclude fat necrosis), US, and MRI should be performed. Small size or early recurrence can also be found incidentally at screening breast MRI of the contralateral breast (68). Localized recurrence at the contact zone can be surgically removed with preservation of the flap, as opposed to recurrence more posteriorly in the reconstructed breast, which commonly manifests at a later stage with distant metastasis, requiring systemic treatment.
One or two mammographic views can help evaluate the abnormality (and exclude fat necrosis) along the contact zone, depending on the size and location of the recurrence and the amount of scarring, with the contact zone appearing as a faint superficial line between the lucent flap and native fat. US usually demonstrates the palpable recurrent tumor as a solid indeterminate mass abutting the skin, with limited visualization of the interface due to its low resolution. The contact zone is best delineated at MRI and usually appears as a hypointense to intermediate-signal-intensity line on T1- and T2-weighted images, extending peripheral to the skin (70). Recurrence usually manifests as a solid focus, mass, or area of nonmass enhancement with suspicious characteristics anywhere along the contact zone (Fig 19).
Figure 19a.
Recurrence at the contact zone. (a) Gray-scale (top) and color Doppler (bottom) US images show an ill-defined heterogeneous area immediately under the skin containing oval anechoic masses. An echogenic line is noted (arrowheads in top image) separating the underlying subcutaneous tissue of the autologous flap and the native dermal flap, consistent with the contact zone. US-guided core biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b, c) Axial non–fat-saturated (b) and postcontrast (c) T1-weighted images show irregular masses (arrow) along the intermediate-signal-intensity line of the interface (arrowheads in b) between the autologous flap and the native skin flap, with subsequent enhancement after contrast material injection. (d) Low-power photomicrograph through the surgically excised specimen shows tumor cells (arrows) invading the dermal layer between the native flap and autologous flap. (H-E stain; original magnification, ×10.)
Figure 19b.
Recurrence at the contact zone. (a) Gray-scale (top) and color Doppler (bottom) US images show an ill-defined heterogeneous area immediately under the skin containing oval anechoic masses. An echogenic line is noted (arrowheads in top image) separating the underlying subcutaneous tissue of the autologous flap and the native dermal flap, consistent with the contact zone. US-guided core biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b, c) Axial non–fat-saturated (b) and postcontrast (c) T1-weighted images show irregular masses (arrow) along the intermediate-signal-intensity line of the interface (arrowheads in b) between the autologous flap and the native skin flap, with subsequent enhancement after contrast material injection. (d) Low-power photomicrograph through the surgically excised specimen shows tumor cells (arrows) invading the dermal layer between the native flap and autologous flap. (H-E stain; original magnification, ×10.)
Figure 19c.
Recurrence at the contact zone. (a) Gray-scale (top) and color Doppler (bottom) US images show an ill-defined heterogeneous area immediately under the skin containing oval anechoic masses. An echogenic line is noted (arrowheads in top image) separating the underlying subcutaneous tissue of the autologous flap and the native dermal flap, consistent with the contact zone. US-guided core biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b, c) Axial non–fat-saturated (b) and postcontrast (c) T1-weighted images show irregular masses (arrow) along the intermediate-signal-intensity line of the interface (arrowheads in b) between the autologous flap and the native skin flap, with subsequent enhancement after contrast material injection. (d) Low-power photomicrograph through the surgically excised specimen shows tumor cells (arrows) invading the dermal layer between the native flap and autologous flap. (H-E stain; original magnification, ×10.)
Figure 19d.
Recurrence at the contact zone. (a) Gray-scale (top) and color Doppler (bottom) US images show an ill-defined heterogeneous area immediately under the skin containing oval anechoic masses. An echogenic line is noted (arrowheads in top image) separating the underlying subcutaneous tissue of the autologous flap and the native dermal flap, consistent with the contact zone. US-guided core biopsy demonstrated recurrent invasive ductal carcinoma (IDC). (b, c) Axial non–fat-saturated (b) and postcontrast (c) T1-weighted images show irregular masses (arrow) along the intermediate-signal-intensity line of the interface (arrowheads in b) between the autologous flap and the native skin flap, with subsequent enhancement after contrast material injection. (d) Low-power photomicrograph through the surgically excised specimen shows tumor cells (arrows) invading the dermal layer between the native flap and autologous flap. (H-E stain; original magnification, ×10.)
Lymph Nodes.—The axillary lymph nodes receive over 97% of lymphatic drainage from the breast (71). Recurrence via lymphatic drainage from the breast most frequently occurs in the axillary, supraclavicular, or infraclavicular nodes (72). Regional lymph node recurrence, particularly supraclavicular, is not commonly seen but is associated with poor prognosis and can indicate concurrent or subsequent distant metastasis (73).
According to published reports, overall lymph node recurrence occurs in 1.7%–15.9% of patients with any stage of breast cancer (72). Lymph drainage outside the ipsilateral axillary lymph nodes occurs in 20%–57% of patients with breast cancer, mostly to the internal mammary chain and less commonly to the contralateral axilla (Fig 20). This rate increases after axillary lymph node dissection or radiation therapy and can be up to 70% (74).
Figure 20a.

Ipsilateral and contralateral lymph node recurrence in a patient with a history of mastectomy and lymph node dissection who presented with a palpable right axillary lymph node and left arm swelling. (a) Spot compression mediolateral oblique mammogram of the right axilla shows an underlying enlarged lymph node (arrow). (b) On a targeted US image, the mammographic finding corresponds to a morphologically abnormal lymph node (*). (c) Axial PET/CT image shows abnormal uptake in the right axillary lymph nodes (arrows) and intense metabolic activity in a conglomeration of left axillary lymph nodes (circle). (d) Low-power photomicrograph shows malignant glands (arrowheads) invading the parenchyma of a lymph node. (H-E stain; original magnification, ×10.)
Figure 20b.
Ipsilateral and contralateral lymph node recurrence in a patient with a history of mastectomy and lymph node dissection who presented with a palpable right axillary lymph node and left arm swelling. (a) Spot compression mediolateral oblique mammogram of the right axilla shows an underlying enlarged lymph node (arrow). (b) On a targeted US image, the mammographic finding corresponds to a morphologically abnormal lymph node (*). (c) Axial PET/CT image shows abnormal uptake in the right axillary lymph nodes (arrows) and intense metabolic activity in a conglomeration of left axillary lymph nodes (circle). (d) Low-power photomicrograph shows malignant glands (arrowheads) invading the parenchyma of a lymph node. (H-E stain; original magnification, ×10.)
Figure 20c.
Ipsilateral and contralateral lymph node recurrence in a patient with a history of mastectomy and lymph node dissection who presented with a palpable right axillary lymph node and left arm swelling. (a) Spot compression mediolateral oblique mammogram of the right axilla shows an underlying enlarged lymph node (arrow). (b) On a targeted US image, the mammographic finding corresponds to a morphologically abnormal lymph node (*). (c) Axial PET/CT image shows abnormal uptake in the right axillary lymph nodes (arrows) and intense metabolic activity in a conglomeration of left axillary lymph nodes (circle). (d) Low-power photomicrograph shows malignant glands (arrowheads) invading the parenchyma of a lymph node. (H-E stain; original magnification, ×10.)
Figure 20d.
Ipsilateral and contralateral lymph node recurrence in a patient with a history of mastectomy and lymph node dissection who presented with a palpable right axillary lymph node and left arm swelling. (a) Spot compression mediolateral oblique mammogram of the right axilla shows an underlying enlarged lymph node (arrow). (b) On a targeted US image, the mammographic finding corresponds to a morphologically abnormal lymph node (*). (c) Axial PET/CT image shows abnormal uptake in the right axillary lymph nodes (arrows) and intense metabolic activity in a conglomeration of left axillary lymph nodes (circle). (d) Low-power photomicrograph shows malignant glands (arrowheads) invading the parenchyma of a lymph node. (H-E stain; original magnification, ×10.)
While routine physical examination allows detection of palpable nodes in the axilla, the rate of false-negative findings remains as high as 39%, specifically with small size or early recurrence (73). This is secondary to multiple factors including the challenging anatomic location in the deep subcutaneous tissue within the axilla, extensive scarring from previous axillary lymph node dissection or radiation therapy, or overweight body habitus. Once recurrence is suspected at imaging, US-guided fine-needle aspiration or core biopsy can be performed to confirm the diagnosis.
De Novo Malignancy
Radiation-induced Sarcoma
Radiation therapy is not administered routinely after mastectomy owing to its unfavored side effects. According to the American Society of Clinical Oncology (ASCO), radiation therapy is recommended only for patients with tumors larger than 5 cm, metastasis to four or more lymph nodes, positive margins, or skin invasion (75). A known rare complication of radiation therapy is development of sarcomas, with an estimated incidence of 0.03%–0.08% (76,77). The interval between radiation exposure and development of sarcomas varies in different studies, although the estimated average is about 10–14 years. Certain criteria are required to categorize sarcomas as radiation induced, including history of radiation therapy, asymptomatic latency period of several years, tumor developing in a previously irradiated field, and histologic confirmation of sarcomatous nature (78).
Radiation-induced sarcomas are mostly soft-tissue aggressive neoplasms (usually of the chest wall), with the most common type being angiosarcoma, a malignant neoplasm arising from endothelial cells lining vascular channels after irradiation (76,79). Other types of radiation-induced sarcoma include leiomyosarcoma, liposarcoma, fibrosarcoma, and rarely undifferentiated sarcoma (76).
Angiosarcoma most commonly develops in the dermis of the irradiated skin, the chest wall, or breast parenchyma. It has been reported mostly in older patients, with a median age of 70 years (79,80). The clinical manifestation includes rapidly growing painful masses mimicking hematomas or focal areas of ecchymosis, skin thickening, or erythema mimicking bruising or inflammation; therefore, diagnosis can be challenging and is often delayed (78) (Fig 21). Skin changes should specifically raise concern when they develop years after treatment as a new finding, prompting diagnostic imaging workup and often punch biopsy. If the sarcoma is operable, long-term survival can be achieved with radical resection with wide negative margins (77,81).
Figure 21a.
High-grade angiosarcoma manifesting as chest wall skin erythema and severe swelling mimicking cellulitis; however, the finding significantly worsened over a course of 12 months. (a) Axial CT image of the lower chest shows a large infiltrative soft-issue mass (arrows) invading the entire width of the anterior chest wall. (b) PET/CT image shows significantly intense FDG uptake in the anterior chest wall (arrowheads) superimposed on the sternum and anterior ribs. (c) Whole-body maximum intensity projection (MIP) PET reconstruction shows the overall extent of the mass. Surgical débridement demonstrated spindle cell neoplasm, consistent with mixed high- and low-grade angiosarcoma.
Pathologic Findings.—Radiation-induced sarcoma is identified with immunohistochemical staining for the erythroblast transformation-specific (ETS)–related gene (ERG) (a transcription factor that confirms the vascular lining of the tumor), CD31 (platelet endothelial cell adhesion molecule), CD34 (human hematopoietic progenitor cell antigen), and factor VIII–related antigen (79). Microscopically, it generally shows a complex anastomosing network of vessels with infiltrative borders. Angiosarcoma demonstrates abnormal vessels lined by atypical endothelial cells. Higher-grade lesions may show solid sheets of atypical spindle cells or epithelioid cells without a network of vessels (82) (Fig 22).
Figure 22a.
Pathologic features of angiosarcoma. (a) Low-power photomicrograph shows an infiltrative lesion with solid areas of malignant cells and vessels (arrow). Arrowhead = normal lobular unit in the breast. (H-E stain; original magnification, ×10.) (b) Low-power photomicrograph obtained with CD34 immunohistochemical stains shows tumor reactivity to the stains (arrow), confirming the vascular origin. (Original magnification, ×10.)
Figure 22b.
Pathologic features of angiosarcoma. (a) Low-power photomicrograph shows an infiltrative lesion with solid areas of malignant cells and vessels (arrow). Arrowhead = normal lobular unit in the breast. (H-E stain; original magnification, ×10.) (b) Low-power photomicrograph obtained with CD34 immunohistochemical stains shows tumor reactivity to the stains (arrow), confirming the vascular origin. (Original magnification, ×10.)
Imaging Features.—Obtaining adequate mammographic views can be challenging—depending on the clinical manifestation, presence of reconstruction, and degree of scarring—and they may demonstrate only nonspecific findings. US features can be variable and may include skin thickening or an irregular or oval mass with increased vascular flow at Doppler imaging. At MRI, skin or chest wall sarcoma usually demonstrates heterogeneous low T1 signal intensity and high T2 signal intensity. High-grade sarcoma usually demonstrates rapid enhancement and washout kinetics on dynamic postcontrast T1-weighted images.
Evaluation for involvement of the pectoralis and intercostal muscles and ribs is important to guide surgical planning. In addition to the findings listed earlier, feeding vessels in the periphery of the tumor are usually characteristic of angiosarcoma (83–85). Regardless of the location (cutaneous, chest wall, or breast parenchyma), angiosarcoma usually has intense FDG uptake at PET/CT, which also helps in evaluating disease extent (Fig 21b, 21c).
Figure 21b.
High-grade angiosarcoma manifesting as chest wall skin erythema and severe swelling mimicking cellulitis; however, the finding significantly worsened over a course of 12 months. (a) Axial CT image of the lower chest shows a large infiltrative soft-issue mass (arrows) invading the entire width of the anterior chest wall. (b) PET/CT image shows significantly intense FDG uptake in the anterior chest wall (arrowheads) superimposed on the sternum and anterior ribs. (c) Whole-body maximum intensity projection (MIP) PET reconstruction shows the overall extent of the mass. Surgical débridement demonstrated spindle cell neoplasm, consistent with mixed high- and low-grade angiosarcoma.
Figure 21c.

High-grade angiosarcoma manifesting as chest wall skin erythema and severe swelling mimicking cellulitis; however, the finding significantly worsened over a course of 12 months. (a) Axial CT image of the lower chest shows a large infiltrative soft-issue mass (arrows) invading the entire width of the anterior chest wall. (b) PET/CT image shows significantly intense FDG uptake in the anterior chest wall (arrowheads) superimposed on the sternum and anterior ribs. (c) Whole-body maximum intensity projection (MIP) PET reconstruction shows the overall extent of the mass. Surgical débridement demonstrated spindle cell neoplasm, consistent with mixed high- and low-grade angiosarcoma.
Conclusion
There is a spectrum of benign and malignant entities that often manifest as a palpable mass in mastectomy patients and may present a clinical and diagnostic challenge. Comprehensive diagnostic evaluation includes targeted US with mammography, when indicated, as it is often helpful in further delineating the underlying disease. In the absence of a discrete US or mammographic correlate, imaging evaluation may also include breast MRI. These common and uncommon entities should be kept in mind by the radiologist when evaluating a palpable abnormality in the mastectomy patient.
Presented as an education exhibit at the 2019 RSNA Annual Meeting.
For this journal-based SA-CME activity, the author V.L.M. has provided disclosures (see end of article); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
V.L.M. supported by Cancer Center Support Grant P30 CA008748 from the National Cancer Institute.
Disclosures of Conflicts of Interest.— : V.L.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: financial compensation from Koios Medical for time spent as a reader in a study. Other activities: disclosed no relevant relationships.
Abbreviations:
- FDG
- fluorodeoxyglucose
- H-E
- hematoxylin-eosin
References
- 1.Lee JH, Kim EK, Oh JY, et al. US screening for detection of nonpalpable locoregional recurrence after mastectomy. Eur J Radiol 2013;82(3):485–489. [DOI] [PubMed] [Google Scholar]
- 2.Kim SM, Park JM. Normal and abnormal US findings at the mastectomy site. RadioGraphics 2004;24(2):357–365. [DOI] [PubMed] [Google Scholar]
- 3.Fajardo LL, Roberts CC, Hunt KR. Mammographic surveillance of breast cancer patients: should the mastectomy site be imaged?. AJR Am J Roentgenol 1993;161(5):953–955. [DOI] [PubMed] [Google Scholar]
- 4.Dashevsky BZ, Hayward JH, Woodard GA, Joe BN, Lee AY. Utility and outcomes of imaging evaluation for palpable lumps in the postmastectomy patient. AJR Am J Roentgenol 2019;213(2):464–472. [DOI] [PubMed] [Google Scholar]
- 5.Edeiken BS, Fornage BD, Bedi DG, Sneige N, Parulekar SG, Pleasure J. Recurrence in autogenous myocutaneous flap reconstruction after mastectomy for primary breast cancer: US diagnosis. Radiology 2003;227(2):542–548. [DOI] [PubMed] [Google Scholar]
- 6.Kerridge WD, Kryvenko ON, Thompson A, Shah BA. Fat Necrosis of the Breast: A Pictorial Review of the Mammographic, Ultrasound, CT, and MRI Findings with Histopathologic Correlation. Radiol Res Pract 2015;2015:613139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taboada JL, Stephens TW, Krishnamurthy S, Brandt KR, Whitman GJ. The many faces of fat necrosis in the breast. AJR Am J Roentgenol 2009;192(3):815–825. [DOI] [PubMed] [Google Scholar]
- 8.D’Alfonso TM, Ginter PS, Shin SJ. A review of inflammatory processes of the breast with a focus on diagnosis in core biopsy samples. J Pathol Transl Med 2015;49(4):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilgen IG, Ustun EE, Memis A. Fat necrosis of the breast: clinical, mammographic and sonographic features. Eur J Radiol 2001;39(2):92–99. [DOI] [PubMed] [Google Scholar]
- 10.Chala LF, de Barros N, de Camargo Moraes P, et al. Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol 2004;33(3):106–126. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita T, Yashiro N, Yoshigi J, Ihara N, Narita M. Fat necrosis of breast: a potential pitfall in breast MRI. Clin Imaging 2002;26(4):250–253. [DOI] [PubMed] [Google Scholar]
- 12.Margolis NE, Morley C, Lotfi P, et al. Update on imaging of the postsurgical breast. RadioGraphics 2014;34(3):642–660. [DOI] [PubMed] [Google Scholar]
- 13.Pinell-White XA, Etra J, Newell M, Tuscano D, Shin K, Losken A. Radiographic implications of fat grafting to the reconstructed breast. Breast J 2015;21(5):520–525. [DOI] [PubMed] [Google Scholar]
- 14.Herly M, Ørholt M, Glovinski PV, et al. Quantifying long-term retention of excised fat grafts: a longitudinal, retrospective cohort study of 108 patients followed for up to 8.4 years. Plast Reconstr Surg 2017;139(5):1223–1232. [DOI] [PubMed] [Google Scholar]
- 15.Parikh RP, Doren EL, Mooney B, Sun WV, Laronga C, Smith PD. Differentiating fat necrosis from recurrent malignancy in fat-grafted breasts: an imaging classification system to guide management. Plast Reconstr Surg 2012;130(4):761–772. [DOI] [PubMed] [Google Scholar]
- 16.Yi A, Kim HH, Shin HJ, Huh MO, Ahn SD, Seo BK. Radiation-induced complications after breast cancer radiation therapy: a pictorial review of multimodality imaging findings. Korean J Radiol 2009;10(5):496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassar L, Baassiri A, Salah F, et al. Stromal Fibrosis of the Breast: A Spectrum of Benign to Malignant Imaging Appearances. Radiol Res Pract 2019;2019:5045908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devon RK, Rosen MA, Mies C, Orel SG. Breast reconstruction with a transverse rectus abdominis myocutaneous flap: spectrum of normal and abnormal MR imaging findings. RadioGraphics 2004;24(5):1287–1299. [DOI] [PubMed] [Google Scholar]
- 19.Hedegard W, Niell B, Specht M, Winograd J, Rafferty E. Breast reconstruction with a deep inferior epigastric perforator flap: imaging appearances of the normal flap and common complications. AJR Am J Roentgenol 2013;200(1):W75–W84. [DOI] [PubMed] [Google Scholar]
- 20.Di Napoli A, Pepe G, Giarnieri E, et al. Cytological diagnostic features of late breast implant seromas: from reactive to anaplastic large cell lymphoma. PLoS One 2017;12(7):e0181097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huda F, Kumar V, Kumar N, Kishore S. Suture granuloma mimicking axillary recurrence in bilateral breast carcinoma: a case report. Int Surg J 2017;4(12):4105. [Google Scholar]
- 22.Illman JE, Terra SB, Clapp AJ, et al. Granulomatous diseases of the breast and axilla: radiological findings with pathological correlation. Insights Imaging 2018;9(1):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Faria Castro Fleury E, D’Alessandro GS, Lordelo Wludarski SC. Silicone-induced granuloma of breast implant capsule (SIGBIC): histopathology and radiological correlation. J Immunol Res 2018;2018:6784971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rettenbacher T, Macheiner P, Hollerweger A, Gritzmann N, Weismann C, Todoroff B. Suture granulomas: sonography enables a correct preoperative diagnosis. Ultrasound Med Biol 2001;27(3):343–350. [DOI] [PubMed] [Google Scholar]
- 25.Takeshita N, Tohma T, Miyauchi H, et al. Suture Granuloma With False-Positive Findings on FDG-PET/CT Resected via Laparoscopic Surgery. Int Surg 2015;100(4):604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caskey CI, Berg WA, Hamper UM, Sheth S, Chang BW, Anderson ND. Imaging spectrum of extracapsular silicone: correlation of US, MR imaging, mammographic, and histopathologic findings. RadioGraphics 1999;19(Spec Issue):S39–S51; quiz S261–S262. [DOI] [PubMed] [Google Scholar]
- 27.Seiler SJ, Sharma PB, Hayes JC, et al. Multimodality Imaging–based Evaluation of Single-Lumen Silicone Breast Implants for Rupture. RadioGraphics 2017;37(2):366–382. [DOI] [PubMed] [Google Scholar]
- 28.Jordan SW, Khavanin N, Kim JYS. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg 2016;137(4):1104–1116. [DOI] [PubMed] [Google Scholar]
- 29.Clemens MW, Jacobsen ED, Horwitz SM. 2019 NCCN Consensus Guidelines on the Diagnosis and Treatment of Breast Implant–associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Aesthet Surg J 2019;39(suppl_1):S3–S13. [DOI] [PubMed] [Google Scholar]
- 30.Sharma B, Jurgensen-Rauch A, Pace E, et al. Breast implant–associated anaplastic large cell lymphoma: review and multiparametric imaging paradigms. RadioGraphics 2020;40(3):609–628. [DOI] [PubMed] [Google Scholar]
- 31.Roller R, Chetlen A, Kasales C. Imaging of Breast Implants and Their Associated Complications. J Am Osteopath Coll Radiol 2014;3(1):2–9. https://www.jaocr.org/articles/imaging-of-breast-implants-and-their-associated-complications.25328855 [Google Scholar]
- 32.Salemis NS. Traumatic neuroma as a rare cause of intractable neuropathic breast pain following cancer surgery: management and review of the literature. Intractable Rare Dis Res 2018;7(3):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Gao EL, Yang YL, Hu HY, Hu XQ. Traumatic neuroma in a patient with breast cancer after mastectomy: a case report and review of the literature. World J Surg Oncol 2012;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AlSharif S, Ferré R, Omeroglu A, El Khoury M, Mesurolle B. Imaging features associated with posttraumatic breast neuromas. AJR Am J Roentgenol 2016;206(3):660–665. [DOI] [PubMed] [Google Scholar]
- 35.Foltán R, Klíma K, Spacková J, Sedý J. Mechanism of traumatic neuroma development. Med Hypotheses 2008;71(4):572–576. [DOI] [PubMed] [Google Scholar]
- 36.Sung HS, Kim YS. Ultrasonographic features of traumatic neuromas in breast cancer patients after mastectomy. Ultrasonography 2017;36(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahlawat S, Belzberg AJ, Montgomery EA, Fayad LM. MRI features of peripheral traumatic neuromas. Eur Radiol 2016;26(4):1204–1212. [DOI] [PubMed] [Google Scholar]
- 38.Sagara Y, Barry WT, Mallory MA, et al. Surgical Options and Locoregional Recurrence in Patients Diagnosed with Invasive Lobular Carcinoma of the Breast. Ann Surg Oncol 2015;22(13):4280–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YS, Chen JLY, Huang CS, et al. High mammographic breast density predicts locoregional recurrence after modified radical mastectomy for invasive breast cancer: a case-control study. Breast Cancer Res 2016;18(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Destounis S, Morgan R, Arieno A, Seifert P, Somerville P, Murphy P. A review of breast imaging following mastectomy with or without reconstruction in an outpatient community center. Breast Cancer 2011;18(4):259–267. [DOI] [PubMed] [Google Scholar]
- 41.Reddy S, Colakoglu S, Curtis MS, et al. Breast cancer recurrence following postmastectomy reconstruction compared to mastectomy with no reconstruction. Ann Plast Surg 2011;66(5):466–471. [DOI] [PubMed] [Google Scholar]
- 42.Al-Himdani S, Timbrell S, Tan KT, Morris J, Bundred NJ. Prediction of margin involvement and local recurrence after skin-sparing and simple mastectomy. Eur J Surg Oncol 2016;42(7):935–941. [DOI] [PubMed] [Google Scholar]
- 43.Giannotti DG, Hanna SA, Cerri GG, Barbosa Bevilacqua JL. Analysis of Skin Flap Thickness and Residual Breast Tissue After Mastectomy. Int J Radiat Oncol Biol Phys 2018;102(1):82–91. [DOI] [PubMed] [Google Scholar]
- 44.Torresan RZ, dos Santos CC, Okamura H, Alvarenga M. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol 2005;12(12):1037–1044. [DOI] [PubMed] [Google Scholar]
- 45.American Cancer Society. Breast Cancer Facts & Figures 2019–2020. Atlanta, Ga: American Cancer Society, 2019. [Google Scholar]
- 46.Adrada BE, Whitman GJ, Crosby MA, Carkaci S, Dryden MJ, Dogan BE. Multimodality Imaging of the Reconstructed Breast. Curr Probl Diagn Radiol 2015;44(6):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bannani S, Rouquette S, Bendavid-Athias C, Tas P, Levêque J. The locoregional recurrence post-mastectomy for ductal carcinoma in situ: incidence and risk factors. Breast 2015;24(5):608–612. [DOI] [PubMed] [Google Scholar]
- 48.Leclère FM, Panet-Spallina J, Kolb F, et al. Nipple-sparing mastectomy and immediate reconstruction in ductal carcinoma in situ: a critical assessment with 41 patients. Aesthetic Plast Surg 2014;38(2):338–343. [DOI] [PubMed] [Google Scholar]
- 49.Hanna WM, Parra-Herran C, Lu FI, Slodkowska E, Rakovitch E, Nofech-Mozes S. Ductal carcinoma in situ of the breast: an update for the pathologist in the era of individualized risk assessment and tailored therapies. Mod Pathol 2019;32(7):896–915. [DOI] [PubMed] [Google Scholar]
- 50.Sundara Rajan S, Verma R, Shaaban AM, Sharma N, Dall B, Lansdown M. Palpable ductal carcinoma in situ: analysis of radiological and histological features of a large series with 5-year follow-up. Clin Breast Cancer 2013;13(6):486–491. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 2008;26(14):2373–2378. [DOI] [PubMed] [Google Scholar]
- 52.Meyers MO, Klauber-Demore N, Ollila DW, et al. Impact of breast cancer molecular subtypes on locoregional recurrence in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol 2011;18(10):2851–2857. [DOI] [PubMed] [Google Scholar]
- 53.García-Fernández A, Lain JM, Chabrera C, et al. Comparative Long-term Study of a Large Series of Patients with Invasive Ductal Carcinoma and Invasive Lobular Carcinoma: Loco-Regional Recurrence, Metastasis, and Survival. Breast J 2015;21(5):533–537. [DOI] [PubMed] [Google Scholar]
- 54.Halyard MY, Wasif N, Harris EER, et al. ACR Appropriateness Criteria® local-regional recurrence (LR) and salvage surgery: breast cancer. Am J Clin Oncol 2012;35(2):178–182. [DOI] [PubMed] [Google Scholar]
- 55.Pitcher AA, Chao JW, Varma S, Swistel AJ, Otterburn DM. Recurrent breast cancer in the subpectoral space after implant reconstruction. J Surg Oncol 2014;109(5):431–433. [DOI] [PubMed] [Google Scholar]
- 56.Haffty BG, Hauser A, Choi DH, et al. Molecular markers for prognosis after isolated postmastectomy chest wall recurrence. Cancer 2004;100(2):252–263. [DOI] [PubMed] [Google Scholar]
- 57.Lee SC, Jain PA, Jethwa SC, Tripathy D, Yamashita MW. Radiologist’s role in breast cancer staging: providing key information for clinicians. RadioGraphics 2014;34(2):330–342. [DOI] [PubMed] [Google Scholar]
- 58.Yilmaz MH, Esen G, Ayarcan Y, et al. The role of US and MR imaging in detecting local chest wall tumor recurrence after mastectomy. Diagn Interv Radiol 2007;13(1):13–18. [PubMed] [Google Scholar]
- 59.Scanlon EF. Local recurrence in the pectoralis muscles following modified radical mastectomy for carcinoma. J Surg Oncol 1985;30(3):149–151. [DOI] [PubMed] [Google Scholar]
- 60.Gweon HM, Son EJ, Youk JH, Kim JA, Chung J. Value of the US BI-RADS final assessment following mastectomy: BI-RADS 4 and 5 lesions. Acta Radiol 2012;53(3):255–260. [DOI] [PubMed] [Google Scholar]
- 61.Hanagiri T, Nozoe T, Yoshimatsu T, et al. Surgical treatment for chest wall invasion due to the local recurrence of breast cancer. Breast Cancer 2008;15(4):298–302. [DOI] [PubMed] [Google Scholar]
- 62.Woerdeman LAE, Kortmann JBJ, Hage JJ. Routine histologic examination of 728 mastectomy scars: did it benefit our patients?. Plast Reconstr Surg 2006;118(6):1288–1292. [DOI] [PubMed] [Google Scholar]
- 63.Rissanen TJ, Mäkäräinen HP, Mattila SI, Lindholm EL, Heikkinen MI, Kiviniemi HO. Breast cancer recurrence after mastectomy: diagnosis with mammography and US. Radiology 1993;188(2):463–467. [DOI] [PubMed] [Google Scholar]
- 64.Fentiman IS, Matthews PN, Davison OW, Millis RR, Hayward JL. Survival following local skin recurrence after mastectomy. Br J Surg 1985;72(1):14–16. [DOI] [PubMed] [Google Scholar]
- 65.Raimbault M, Lavoué V, Morcel K, et al. Isolated skin recurrence following salvage mastectomy for intramammary recurrence (after initial breast conservation therapy): is it a fatal event?. Breast 2011;20(4):380–384. [DOI] [PubMed] [Google Scholar]
- 66.Kim SJ, Kim JY. An unusual cutaneous recurrence of carcinoma in the mastectomy bed and its imaging features: a case report. Am J Case Rep 2019;20(800):805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoo H, Kim BH, Kim HH, Cha JH, Shin HJ, Lee TJ. Local recurrence of breast cancer in reconstructed breasts using TRAM flap after skin-sparing mastectomy: clinical and imaging features. Eur Radiol 2014;24(9):2220–2226. [DOI] [PubMed] [Google Scholar]
- 68.Al-Khalili R, Wynn RT, Ha R. The Contact Zone: A Common Site of Tumor Recurrence in a Patient Who Underwent Skin-sparing Mastectomy and Myocutaneous Flap Reconstruction. Curr Probl Diagn Radiol 2016;45(3):233–234. [DOI] [PubMed] [Google Scholar]
- 69.Huiskes JVM, Keemers-Gels ME, Fabré J, Strobbe LJA. DIEAP Flap Breast Reconstruction Followed by Local Recurrence of Breast Cancer. Case Rep Oncol 2018;11(2):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng C, Chang CB, Tso HH, Flowers CI, Hylton NM, Joe BN. MRI appearance of tumor recurrence in myocutaneous flap reconstruction after mastectomy. AJR Am J Roentgenol 2011;196(4):W471–W475. [DOI] [PubMed] [Google Scholar]
- 71.Konkin DE, Tyldesley S, Kennecke H, Speers CH, Olivotto IA, Davis N. Management and outcomes of isolated axillary node recurrence in breast cancer. Arch Surg 2006;141(9):867–872; discussion 872–874. [DOI] [PubMed] [Google Scholar]
- 72.He ZY, Wu SG, Zhou J, et al. Benefit of post-mastectomy radiotherapy of the supra-/infraclavicular lymphatic drainage area in breast cancer patients. Asian Pac J Cancer Prev 2014;15(14):5557–5563. [DOI] [PubMed] [Google Scholar]
- 73.Moon HJ, Kim MJ, Kim E-K, et al. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology 2009;252(3):673–681. [DOI] [PubMed] [Google Scholar]
- 74.Moossdorff M, Vugts G, Maaskant-Braat AJG, et al. Contralateral lymph node recurrence in breast cancer: regional event rather than distant metastatic disease—a systematic review of the literature. Eur J Surg Oncol 2015;41(9):1128–1136. [DOI] [PubMed] [Google Scholar]
- 75.Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol 2016;34(36):4431–4442. [DOI] [PubMed] [Google Scholar]
- 76.Kong J, Shahait AD, Kim S, Choi L. Radiation-induced undifferentiated pleomorphic sarcoma of the breast. BMJ Case Rep 2020;13(2):e232616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kara HV, Gandolfi BM, Williams JB, D’Amico TA, Zenn MR. Multidisciplinary approach to treatment of radiation-induced chest wall sarcoma. Gen Thorac Cardiovasc Surg 2016;64(8):492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng XY, Wu SK, Song ST, Wang T, Zhang SH, Jiang ZF. Clinical manifestations and radiological features may contribute to the early diagnosis of radiation-induced sarcoma after breast cancer. Clin Radiol 2014;69(12):1228–1234. [DOI] [PubMed] [Google Scholar]
- 79.Chesebro AL, Chikarmane SA, Gombos EC, Giardino AA. Radiation-associated angiosarcoma of the breast: what the radiologist needs to know. AJR Am J Roentgenol 2016;207(1):217–225. [DOI] [PubMed] [Google Scholar]
- 80.D’Angelo SP, Antonescu CR, Kuk D, et al. High-risk features in radiation-associated breast angiosarcomas. Br J Cancer 2013;109(9):2340–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapelier AR, Bacha EA, de Montpreville VT, et al. Radical resection of radiation-induced sarcoma of the chest wall: report of 15 cases. Ann Thorac Surg 1997;63(1):214–219. [DOI] [PubMed] [Google Scholar]
- 82.Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol 1994;102(6):757–763. [DOI] [PubMed] [Google Scholar]
- 83.Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: radiologic findings and pathologic correlation. II. malignant tumors. RadioGraphics 2003;23(6):1491–1508. [DOI] [PubMed] [Google Scholar]
- 84.Duncan MA, Lautner MA. Sarcomas of the breast. Surg Clin North Am 2018;98(4):869–876. [DOI] [PubMed] [Google Scholar]
- 85.Varghese B, Deshpande P, Dixit S, Koppiker CB, Jalnapurkar N. Primary angiosarcoma of the breast: a case report. J Radiol Case Rep 2019;13(2):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]






![Fat necrosis manifesting as a palpable lump in a patient with mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. (a) Gray-scale US image shows an avascular complex solid and cystic mass with an eccentric mural nodule (arrow). (b) Left craniocaudal mammogram with a BB marker placed over the area of concern (arrowhead) shows the correlate to be a circumscribed fat-containing mass with hyperdense borders (arrow), consistent with benign fat necrosis. No further evaluation was needed. (c) High-power photomicrograph of a specimen from another patient shows adipocytes (*) surrounded by inflammatory infiltrates, mainly histiocytes (arrow) and giant cells. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48c5/8262178/a30dc8437c28/rg.2021200161.fig2a.jpg)
![Fat necrosis manifesting as a palpable lump in a patient with mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap reconstruction. (a) Gray-scale US image shows an avascular complex solid and cystic mass with an eccentric mural nodule (arrow). (b) Left craniocaudal mammogram with a BB marker placed over the area of concern (arrowhead) shows the correlate to be a circumscribed fat-containing mass with hyperdense borders (arrow), consistent with benign fat necrosis. No further evaluation was needed. (c) High-power photomicrograph of a specimen from another patient shows adipocytes (*) surrounded by inflammatory infiltrates, mainly histiocytes (arrow) and giant cells. (Hematoxylin-eosin [H-E] stain; original magnification, ×40.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/48c5/8262178/cf563e13d840/rg.2021200161.fig2c.jpg)