Abstract
Loss of function of the lipid kinase diacylglycerol kinase ε (DGKε), encoded by the gene DGKE, causes a form of atypical hemolytic uremic syndrome that is not related to abnormalities of the alternative pathway of the complement, by mechanisms that are not understood. By generating a potentially novel endothelial specific Dgke-knockout mouse, we demonstrate that loss of Dgke in the endothelium results in impaired signaling downstream of VEGFR2 due to cellular shortage of phosphatidylinositol 4,5-biphosphate. Mechanistically, we found that, in the absence of DGKε in the endothelium, Akt fails to be activated upon VEGFR2 stimulation, resulting in defective induction of the enzyme cyclooxygenase 2 and production of prostaglandin E2 (PGE2). Treating the endothelial specific Dgke-knockout mice with a stable PGE2 analog was sufficient to reverse the clinical manifestations of thrombotic microangiopathy and proteinuria, possibly by suppressing the expression of matrix metalloproteinase 2 through PGE2-dependent upregulation of the chemokine receptor CXCR4. Our study reveals a complex array of autocrine signaling events downstream of VEGFR2 that are mediated by PGE2, that control endothelial activation and thrombogenic state, and that result in abnormalities of the glomerular filtration barrier.
Keywords: Nephrology
Keywords: Inositol phosphates, Microcirculation
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a form of thrombotic microangiopathy (TMA) characterized by hemolytic anemia, thrombocytopenia, and acute renal failure not associated with enteric infections caused by Shiga toxin–producing bacteria. TMA in aHUS affects invariably the microvasculature in the renal glomeruli, causing renal insufficiency. Mutations in complement and complement-regulating genes have been identified in about 50% of idiopathic aHUS cases, pointing at the central role of the complement cascade in this disease (1). Notwithstanding, we and others described a genetic form of aHUS that is caused by mutations in the gene DGKE that encodes the lipid kinase diacylglycerol kinase ε (DGKε), revealing that factors unrelated to the complement system can cause aHUS by mechanisms that are not understood (2, 3).
DGKε is a lipid kinase with high specificity for diacylglycerol (DAG) conjugated with arachidonic acid (AA) at the second carbonyl group of DAG (4–7). As an effect of the DGKε activity, cellular arachidonoyl poly-phosphatidyl-inositides (PtdIns) predominate over PtdIns acylated with other acyl moieties, and, as a consequence, in Dgke-knockout cells the total amount of PtdIns is 3-fold or lower than in WT cells (4–8). Under basal conditions phosphatidyl-inositol diphosphate [PtdIns(4,5) P2, henceforth PIP2] is the most abundant cellular PtdIns. Importantly, it is consumed upon activation of multiple receptors, including receptor tyrosine kinases such as VEGFR2, and it needs to be regenerated continuously through the phosphoinositide cycle (Figure 1A) (9–12).
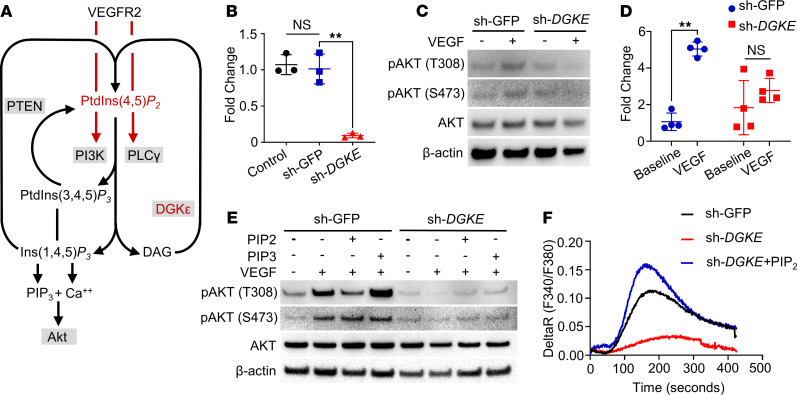
Figure 1. VEGFR2-dependent Akt activation is compromised in DGKE-knockdown human umbilical vein epithelial cells.
(A) Schematic representation of the phosphoinositide cycle and of some of the enzymes involved in the cycle (boxes). Red arrows point to the major enzymes (PI3K and phospholipase C γ, PLCγ) activated downstream of VEGFR2. Black arrows represent generation of PtdIns(1,4,5) P3 (PIP3) and Ca2+ downstream of PI3K and PLCγ, respectively, and activation of Akt. The common substrate of PI3K and PLCγ, PIP2, required for Akt activation is highlighted in red. DGKε is highlighted in red. (B) Efficiency of the sh-RNA knockdown in HUVECs measured by quantitative PCR (Q-PCR) compared with nontargeted control (sh-GFP) cells. Control: nontransfected cells. (C) Western blot showing impaired Akt activation (phosphorylation of threonine 308 and serine 473) in the shRNA-knockdown HUVECs upon VEGFA stimulation, compared with nontargeted control cells. (D) Expression of Cox2, measured by Q-PCR, is not induced in DGKE-knockdown HUVECs compared with nontargeted control cells stimulated with VEGFA. (E) Western blots showing that impaired Akt activation in the sh-RNA knockdown HUVECs upon VEGFA stimulation is partially reversed by PIP2 and PIP3 supplementation. (F) Changes in intracellular Ca2+ concentrations after VEGFA supplementation in DGKE-knockdown HUVECs, in DGKE-knockdown HUVECs after PIP2 supplementation, and in nontargeted HUVEC controls over time, measured by Fura-2 AM fluorescence. Data are from 3–4 independent experiments and are presented as mean ± SD. **: P < 0.01 by 1-way ANOVA in B and by Student’s t test in D. Each data point represents 1 experiment. PTEN, phosphatase and tensin homolog; pAKT, phosphorylated Akt; DeltaR: fluorescence ratio as 340 nm/380 nm.
Here, based on in vitro studies on primary endothelial cells and on the use of an endothelial specific Dgke-knockout mouse, we show that the signaling downstream of VEGFR2 is compromised in the absence of DGKε in the endothelium because of impaired activation of Akt and that the latter is restored by increasing cellular levels of PIP2. Mechanistically, we found that defective Akt activation in endothelial cells results in lack of expression of the gene prostaglandin-endoperoxide synthase 2, which encodes the enzyme cyclooxygenase 2 (Cox2) and induces the synthesis of its main product prostaglandin E2 (PGE2). Treating endothelial specific Dgke-knockout mice with a stable PGE2 analog reversed not only the aHUS, but also the proteinuria, possibly through CXCR4-mediated downregulation of matrix metalloproteinase 2 (MMP-2). Our data indicate that the endothelium is the tissue that is primarily affected by loss of Dgke and reveal multiple complex autocrine signaling events downstream of VEGFR2 that result in endothelial activation, thrombogenic state, and abnormalities of the glomerular filtration barrier. These signaling steps may be manipulated to modify the clinical course of the DGKE-related aHUS and conceivably of other diseases caused by endothelial activation or abnormal angiogenesis.
Results
VEGFR2-dependent Akt activation is compromised in DGKE-knockdown human umbilical vein endothelial cells and in human microvascular endothelial cells.
VEGFR2 is expressed in endothelial cells and exerts proproliferative, prosurvival, and promigratory functions, promoting angiogenesis upon stimulation by VEGFA (13–17). VEGFR2 signals downstream by autophosphorylating multiple tyrosine residues, where effector proteins are recruited (14). This results in, among other effects, activation of PLCγ, which then hydrolyzes PIP2 into inositol 1,4,5-triphosphate [Ins(1,4,5)P3, hereafter IP3] and DAG, and activation of phosphatidylinositol-3-kinase (PI3K), which leads to the generation of PIP3 from its precursor, PIP2, and ultimately to the activation of Akt (Figure 1A) (18–22). Since VEGF signaling is crucial to the maintenance of the glomerular microvasculature (23, 24), and because the disruption of VEGF signaling in humans and mice results in glomerular lesions that resemble those observed in humans with loss-of-function mutations in DGKE (2, 25), we hypothesized that the loss of DGKE may impair VEGF signaling in endothelial cells because of the shortage of PIP2 (Figure 1A). To test this hypothesis, we knocked down DGKE in human umbilical vein endothelial cells (HUVECs) by shRNA interference, using a nontargeting construct (sh-GFP) as a control (Figure 1B), and tested the activation of Akt by Western blot (phosphorylation of threonine 308 and serine 473 of Akt) upon VEGFA stimulation. Unlike untransfected and sh-GFP–transfected cells, DGKE-knockdown cells failed to activate Akt when exposed to VEGFA (Figure 1C and Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.146959DS1). Consistent with the impaired Akt activation, the expression of Cox2, which in endothelial cells is induced by VEGFA (26–29), was also compromised (Figure 1D). However, Akt activation was partially rescued when cells were exposed to PIP2 and, as expected, to PIP3 (Figure 1E and Supplemental Figure 1, C and D). Likewise, intracellular calcium levels that are mobilized from the endoplasmic reticulum by increased intracellular concentrations of IP3 did not rise after VEGFA stimulation, but they did increase after supplementing the culture medium with PIP2 (Figure 1F). We obtained overlapping results using human microvascular endothelial cells (HMECs) (Supplemental Figure 2, A and B). These results indicate that Akt activation downstream of VEGFR2 is defective in DGKE-knockdown endothelial cells because of decreased availability of PIP2.
Endothelial specific Dgke-knockout mice fully recapitulate the human disease.
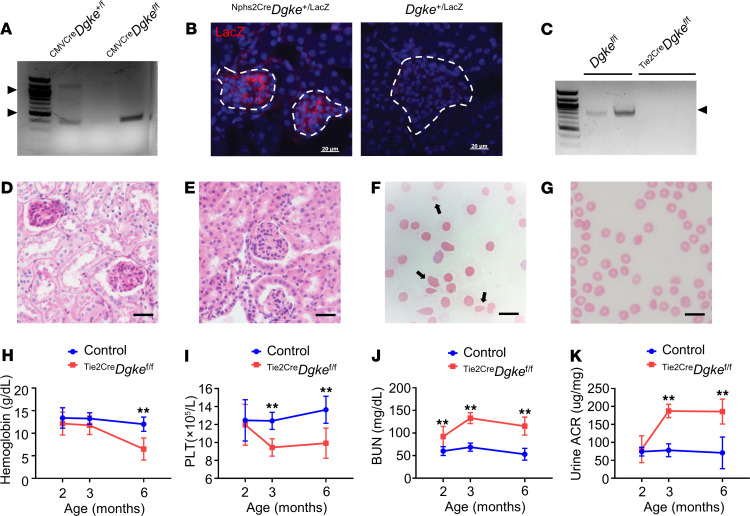
To test if VEGFA signaling in endothelial cells lacking Dgke is also compromised in vivo, we generated a conditional knockout mouse in which exon 2 of Dgke was deleted in a tissue-specific manner upon Cre recombination, using knockout first embryonic stem (ES) cells from the Knockout Mouse Project (KOMP) repository (30, 31). C57BL/6 Dgke-floxed mice are viable and fertile, and they produce progeny at the expected Mendelian ratio. We confirmed that exon 2 of Dgke is excised upon Cre recombination, by using the CMV-Cre mice to delete Dgke in all tissues (32) (Figure 2A). We also verified that the expression of the transgene is tissue specific, by generating Nphs2CreDgkeLacZ knockin mice and showing that the LacZ expression was present exclusively in the glomeruli (30, 33) (Figure 2B). To generate endothelial specific Dgke-knockout mice, Dgke-floxed mice were then crossed to mice in which the Cre recombinase is driven by the angiopoietin receptor Tie2 promoter in endothelial cells and in 2% to 7% of blood mononuclear cells (34). We assessed the knockout efficiency by real-time PCR (RT-PCR) on RNA extracted from Tie2CreDgkefl/fl lungs, which is one of the most vascularized tissues (Figure 2C), given that no antibody is available that reliably detects Dgke. We excluded the possibility that abnormal platelet function due to expression of Tie2 in megakaryocytes could contribute to the observed phenotype by performing in vitro studies of platelet aggregation (Supplemental Figure 3). Unlike the constitutive Dgke-knockout mice that do not have spontaneous phenotype, endothelial specific Tie2CreDgkefl/fl conditional knockout mice developed occlusion of the glomerular capillaries (Figure 2, D and E), schistocytosis (Figure 2, F and G, and Supplemental Figure 4A), hemolytic anemia (Figure 2H and Supplemental Figure 4B), thrombocytopenia (Figure 2I), and renal insufficiency as early as 2 months of age (Figure 2J). Remarkably, these mice also developed albuminuria (Figure 2K), which is observed in all patients affected by DGKE disease, by 3 months of age. The presence of albuminuria in endothelial specific Tie2CreDgkefl/fl knockout mice and its appearance at a later time, compared with the renal insufficiency, suggest that the endothelium is the cellular compartment responsible for the DGKE disease and that the impairment of the glomerular barrier may be a later and secondary event. Interestingly, we did not detect obvious signs of TMA in liver, small intestine, and spleen arterioles of Tie2CreDgkefl/fl knockout mice (Supplemental Figure 5), which may indicate a particular susceptibility of the glomerular microvasculature to this disease.
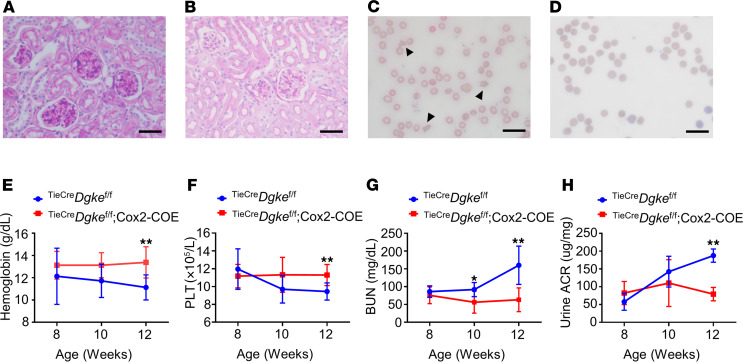
Figure 2. Endothelial specific Dgke-knockout mice recapitulate the full human phenotype.
(A) Agarose gel of the PCR products of the amplification of genomic DNA from ubiquitously Cre-expressing CMVCreDgkefl/fl mice compared with CMVCreDgke+/fl heterozygotes. Heterozygous mice show a long band of 1201 bp corresponding to the retained exon 2 and a shorter band of 365 bp corresponding to the excised exon 2 (arrowheads). Exon 2 is not retained in the homozygous status. (B) Immunofluorescence microscopy images of glomeruli (dashed lines) from Nphs2CreDgke+/LacZ knockin mice compared with Dgke+/LacZ controls, showing LacZ expression exclusively in glomeruli. Scale bars are 20 μm. (C) RT-PCR on RNA showing a band of 830 bp (arrowhead) in Dgkefl/fl mice and no product in Tie2CreDgkefl/fl mice. Primers were placed in the corresponding exon 1 and 5 in the cDNA of Dgke. (D) Representative bright-field microscopy images of glomeruli of Periodic acid–Schiff–stained (PAS-stained) and (E) H&E-stained sections of Tie2CreDgkefl/fl mouse kidneys showing near-complete occlusion of the capillary tuft. Scale bars are 50 μm. (F) Smears of blood from Tie2CreDgkefl/fl and (G) Dgkefl/fl controls at 6 months of age. Numerous schistocytes (arrows) are present in Tie2CreDgkefl/fl knockouts. Scale bars are 75 μm. (H–K) Serum hemoglobin, circulating platelets (PLT), blood urea nitrogen (BUN), and urine albumin to creatinine ratio (ACR) in Tie2CreDgkefl/fl compared with Dgkefl/fl controls at 2, 3, and 6 months of age. Data are presented as mean ± SD. **: P < 0.01 by Student’s t test. n = 6 mice per group.
A phosphatase and tensin homolog inhibitor rescues the phenotype of endothelial specific Dgke-knockout mice.
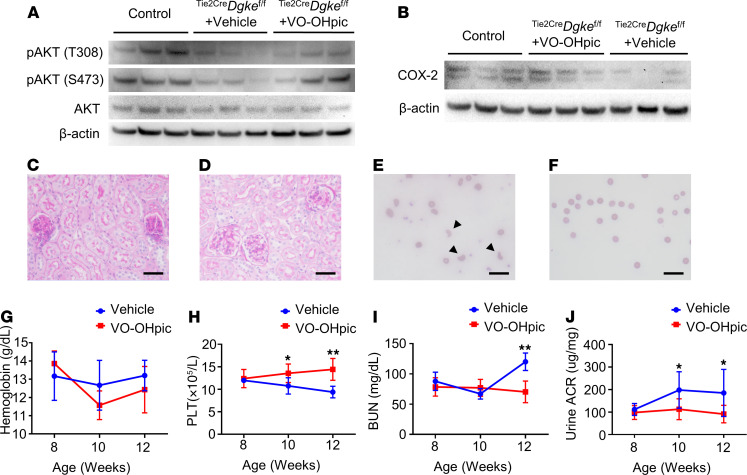
As observed in the DGKE-knockdown cells, Akt phosphorylation was lower in kidney cortexes of Tie2CreDgkefl/fl mice, compared with controls (Figure 3A, lanes 1 through 6; and Supplemental Figure 1, E and F). Hence, we tested whether increasing the endothelial levels of PIP3 would rescue the phenotype in vivo. To this end, we crossed Tie2CreDgkefl/fl mice to Pten-floxed mice (35), to obtain Tie2CreDgkefl/fl Pten+/fl in which only 1 of the 2 Pten alleles is present in endothelial cells; however, we were not able to obtain pups of the desired genotype, likely because of in utero mortality. For this reason, we used a potent small molecule inhibitor of PTEN (VO-OHpic) (36), and injected 8-week-old Tie2CreDgkefl/fl mice i.p. daily for 4 weeks. As expected, treating the Tie2CreDgkefl/fl mice with the PTEN inhibitor resulted in increased Akt activation in renal cortexes (Figure 3A, lanes 4 through 9; and Supplemental Figure 1, E and F). Consistent with Akt activation, the expression of Cox2 in kidney cortexes was increased in Tie2CreDgkefl/fl mice treated with PTEN inhibitor compared with vehicle-treated Tie2CreDgkefl/fl mice (Figure 3B and Supplemental Figure 1G). Remarkably, VO-OHpic–treated mice were protected from developing glomerular capillary obstruction (Figure 3, C and D), thrombocytopenia (Figure 3H), renal insufficiency, and proteinuria (Figure 3, I and J). Although schistocytosis was clearly evident in peripheral blood smears of vehicle-treated mice (Figure 3, E and F, and Supplemental Figure 4C), the serum hemoglobin concentrations did not significantly differ from those of VO-OHpic–treated mice (Figure 3G). These results indicate that Akt activation is impaired in kidneys of endothelial specific knockout mice and that increasing PIP3 cellular levels is sufficient to rescue the symptoms in Tie2CreDgkefl/fl knockout mice.
Figure 3. A PTEN inhibitor rescues the phenotype of endothelial-specific Dgke-knockout mice.
(A) Western blot showing impaired Akt activation (phosphorylation of threonine 308 and serine 473) in kidney cortex extracts of Tie2CreDgkefl/fl mice compared with controls. Akt activation was partially rescued in VO-OHpic–treated Tie2CreDgkefl/fl littermates. (B) Western blot showing increased protein levels of COX2 in kidney cortex extracts of VO-OHpic–treated Tie2CreDgkefl/fl mice compared with Tie2CreDgkefl/fl littermates. (C) Representative bright-field microscopy images of glomeruli of PAS-stained Tie2CreDgkefl/fl mouse kidneys and (D) Tie2CreDgkefl/fl littermates’ kidneys after 4 weeks of treatment with the PTEN inhibitor VO-OHpic. The occlusion of the glomerular capillaries is rescued after VO-OHpic treatment. Scale bars are 50 μm. (E) Smears of blood from Tie2CreDgkefl/fl mice and (F) Tie2CreDgkefl/fl littermates after VO-OHpic treatment at 3 months of age. Schistocytes are found in Tie2CreDgkefl/fl mice (arrowheads) but not in VO-OHpic–treated mice. Scale bars are 50 μm. (G–J) Serum hemoglobin, circulating PLT, BUN, and urine ACR in Tie2CreDgkefl/fl mice compared with VO-OHpic–treated Tie2CreDgkefl/fl littermates at 8, 10, and 12 weeks of age. Data are presented as mean ± SD. *: P < 0.05, **: P < 0.01 by Student’s t test. n = 6 mice per group.
Overexpression of Cox2 in endothelial cells rescues the phenotype ofTie2CreDgkefl/flmice. We previously reported that the expression of the gene prostaglandin-endoperoxide synthase 2, also known as cyclooxygenase 2 (Cox2), and the synthesis of its main product PGE2 were impaired in Dgke constitutive knockout mice (37). However, still undetermined is if impaired Cox2 induction is only associated with or determines the phenotype in Dgke-knockout mice. To answer this question, we crossed Tie2CreDgkefl/fl mice with B6.129S4-Tg(CAG-EGFP,-Ptgs2,-hrluc) (Cox2-COE) mice to obtain Tie2CreDgkefl/fl Cox2-COE double transgenics. Cox2-COE mice were engineered to carry a transgene that contains a CAG promoter that drives the expression of Cox2. The promoter is followed by a loxP-flanked sequence containing enhanced GFP and a transcriptional/translational STOP sequence that allows tissue-specific overexpression of Cox2 in the presence of Cre recombinase (38). As observed in Tie2CreDgkefl/fl mice treated with the PTEN inhibitor, Tie2CreDgkefl/fl Cox2-COE mice did not develop glomerular capillary occlusion (Figure 4, A and B), schistocytosis and hemolytic anemia (Figure 4, C–E, and Supplemental Figure 4D), thrombocytopenia (Figure 4F), and renal insufficiency and proteinuria (Figure 4, G and H). These results show that the lack of endothelial expression of Cox2 is causative of the Dgke phenotype. They also denote that the failed induction of Cox2 in endothelial cells and not the lack of its substrate AA causes aHUS and proteinuria in Tie2CreDgkefl/fl mice.
Figure 4. Overexpression of Cox2 in endothelial cells rescues the phenotype of endothelial specific Dgke-knockout mice.
(A) Representative bright-field microscopy images of glomeruli of PAS-stained Tie2CreDgkefl/fl mouse kidneys and (B) Tie2CreDgkefl/fl Cox2-COE mouse kidneys at 12 weeks of age. The occlusion of the glomerular capillaries is rescued in Tie2CreDgkefl/fl Cox2-COE mice. (C) Smears of blood from Tie2CreDgkefl/fl knockout mice and (D) Tie2CreDgkefl/fl Cox2-COE mice at 3 months of age. Schistocytes are visible in Tie2CreDgkefl/fl knockout mice (arrowheads) and are absent in Tie2CreDgkefl/fl Cox2-COE mice. Scale bars are 50 μm. (E–H) Serum hemoglobin, circulating PLT, BUN, and urine ACR in Tie2CreDgkefl/fl and Tie2CreDgkefl/fl Cox2-COE mice at 8, 10, and 12 weeks of age. Data are presented as mean ± SD. *: P < 0.05, **: P < 0.01 by Student’s t test. n = 6 mice per group.
A stable PGE2 analog rescues the phenotype of endothelial specific Dgke-knockout mice.
Cox2 is the rate-limiting enzyme for the generation of PGE2, a potent promoter of endothelial cell migration, survival, and angiogenesis (39, 40). VEGFA induces Cox2 and PGE2 in endothelial cells (26, 28, 29, 41), which suggests that its proangiogenic effect might be mediated at least partly by the production of PGE2 by mechanisms that are not completely understood (27, 42, 43). In a precedent work we showed that urinary concentration of PGE2, but not of the stable PGI2 metabolite 6-keto PGF1α, was reduced in the Dgke constitutive knockouts compared with control mice. Dgke-knockout mice showed defective vascularization of surgical sponges implanted subcutaneously, and this defect was rescued by injecting the sponges with PGE2 (44), which pointed to the existence of a defect of PGE2-mediated angiogenesis in these mice. To clarify the role of PGE2 in determining the clinical manifestations in Tie2CreDgkefl/fl mice, we tested if increasing circulating levels of PGE2 would rescue the hematologic and renal manifestations in our mouse model. To this end, we implanted subcutaneous osmotic pumps in 8-week-old Tie2CreDgkefl/fl mice to continuously deliver the stable PGE2 analog 16,16 dimethyl-PGE2 (dmPGE2) and looked for changes in the phenotype after 4 weeks. Infusion of dmPGE2 was sufficient to normalize all the clinical manifestations in Tie2CreDgkefl/fl mice (Figure 5, A–H, and Supplemental Figure 4E). These results indicate that impaired PGE2 production is a key factor in determining the presence of disease in the absence of Dgke and that the anemia observed in these mice is hemolytic and not caused by bone marrow failure.
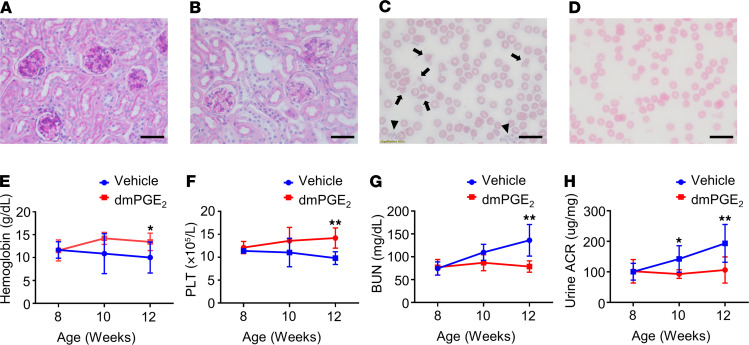
Figure 5. A stable PGE2-analog rescues the phenotype of Tie2CreDgkefl/fl mice.
(A) Representative bright-field microscopy images of glomeruli of PAS-stained Tie2CreDgkefl/fl mouse kidneys and (B) Tie2CreDgkefl/fl mouse kidneys after 4 weeks of subcutaneous infusion of the PGE2 stable analog dmPGE2. The occlusion of the glomerular capillaries is rescued after the dmPGE2 treatment. Scale bars are 50 μm. (C) Smears of blood from Tie2CreDgkefl/fl knockout mice and (D) Tie2CreDgkefl/fl mice after dmPGE2 treatment at 3 months of age. Tie2CreDgkefl/fl knockout mice show numerous schistocytes (arrows) and circulating reticulocytes (arrowheads). Scale bars are 50 μm. (E–H) Serum hemoglobin, circulating PLT, BUN, and urine ACR in Tie2CreDgkefl/fl compared with dmPGE2-treated Tie2CreDgkefl/fl mice at 8, 10, and 12 weeks of age. Data are presented as mean ± SD. *: P < 0.05, **: P < 0.01 by Student’s t test. n = 6 mice per group.
Treatment with a PGE2 stable analog normalizes endothelial cell activation and prothrombotic diathesis in Tie2CreDgkefl/fl mice.
DGKE-knockdown endothelial cells have been shown to express markers of activation and the initiator of coagulation tissue factor (TF) in vitro (45). We tested the expression of P selectin and TF in kidney cortexes of Tie2CreDgkefl/fl mice at 3 months of age and of plasma thrombin-antithrombin complexes (TAT) and found that all these parameters were significantly increased, compared with control mice (Figure 6, A–C), confirming in vivo in Tie2CreDgkefl/fl mice that lack Dgke have activation and prothrombotic state of the endothelium. In line with this observation, we also observed increased endothelial susceptibility to procoagulant inflammatory stimuli, by treating Tie2CreDgkefl/fl mice and controls at 2 months of age with the synthetic double-strand DNA polyinosinic:polycytidylic acid [poly(I:C)], a potent inducer of interferon-α (46) (Figure 6D). Of note, these results suggest that VEGFA signaling controls the activation and the thrombogenic state of the endothelium indirectly through the regulation of PGE2.
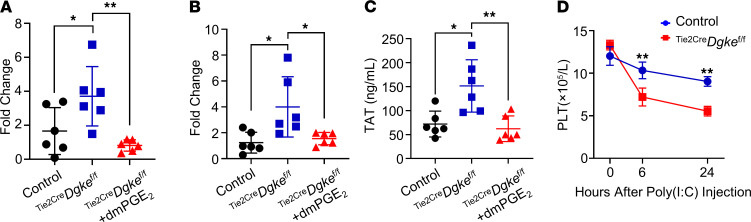
Figure 6. Treatment with a PGE2 stable analog normalizes endothelial cell activation and prothrombotic diathesis in Tie2CreDgkefl/fl mice.
Q-PCR results showing increased (A) P selectin and (B) TF expression in kidney cortexes of Tie2CreDgkefl/fl mice compared with controls that were within normal range in Tie2CreDgkefl/fl littermates after 4 weeks of subcutaneous infusion of dmPGE2. (C) Thrombin-antithrombin complex (TAT) levels in plasma showing increased TAT levels in Tie2CreDgkefl/fl mice, compared with control mice. TAT levels were normalized after dmPGE2 infusion. (D) Counts of circulating PLT of Tie2CreDgkefl/fl mice and controls at 0, 6, and 24 hours after poly(I:C) injection. Data are presented as mean ± SD. *: P < 0.05, **: P < 0.01 by 1-way ANOVA in A–C, where each data point represents 1 mouse, and by Student’s t test in D; n = 6 mice per group.
PGE2 controls the endothelial expression of CXCR4 in the kidney.
Stromal-derived factor 1 (SDF-1, also known as CXCL12) is a soluble chemokine expressed in the stromal component of several organs, and CXCR4 is its cognate receptor expressed on cells of the hematopoietic linage and on endothelial cells. In the kidney, like VEGFA, SDF-1 is expressed in podocytes (47–49). The activation of the SDF-1/CXCR4 signaling in endothelial cells exerts promigratory and proangiogenic effects, and it is required for the development of the renal vasculature (50). Hypoxia is a strong inducer of VEGFA, SDF-1, and CXCR4 (51, 52). In addition, high levels of SDF-1 are known to act in a feed-forward loop to further increase CXCR4 expression in endothelial cells (53). Because endothelial disfunction is expected to result in reduced tissue oxygenation, we tested the expression of hypoxia-induced genes in cortex extracts of Tie2CreDgkefl/fl and control mice and found that the expression of HIF-1 and HIF-2 was higher in Tie2CreDgkefl/fl kidney cortexes, compared with controls (Figure 7, A and B), as well as finding higher expression of HIF-1 and HIF-2 transcriptional targets SDF-1 and VEGFA (Figure 7, C and D). However, in spite of the hypoxic milieu of the Tie2CreDgkefl/fl kidney cortexes, the expression of CXCR4 was surprisingly low, compared with controls (Figure 7E). To confirm that the lower levels of CXCR4 in Tie2CreDgkefl/fl kidneys were caused by differences in CXCR4 expression in endothelial cells and not in other cell populations, we performed flow cytometry analysis of single-cell suspensions of kidney cortexes of Tie2-CreDgkefl/fl and control mice double-stained with antibodies against CXCR4 and against the endothelial cell marker VE-Cadherin. Consistent with other reports (54), VE-Cadherin+ endothelial cells represented about 3%–4% of the cells in our samples (Supplemental Figure 6B, Q2 + Q3). This analysis confirmed that, compared with controls, the proportion of CXCR4-expressing endothelial cells was reduced in Tie2-CreDgkefl/fl mice (Figure 7, F and G; and Supplemental Figure 6, A and C). Of note, because CXCR4 expression can be induced by PGE2 through the activation of adenylate cyclase (55–57), we tested the expression of CXCR4 in DGKE-knockdown HUVECs and HMECs by Q-PCR and found that CXCR4 expression was strongly downregulated in these cells, but it was rescued to normal levels after supplementation with PGE2 (Figure 7H and Supplemental Figure 2C). Remarkably, treatment with dmPGE2 increased the number of endothelial cells expressing CXCR4 in kidney cortexes of Tie2-CreDgkefl/fl mice (Figure 7, I and J). These results show that in the kidneys, PGE2 controls the endothelial expression of CXCR4 and thus the activity of the SDF-1/CXCR4 signaling axis.
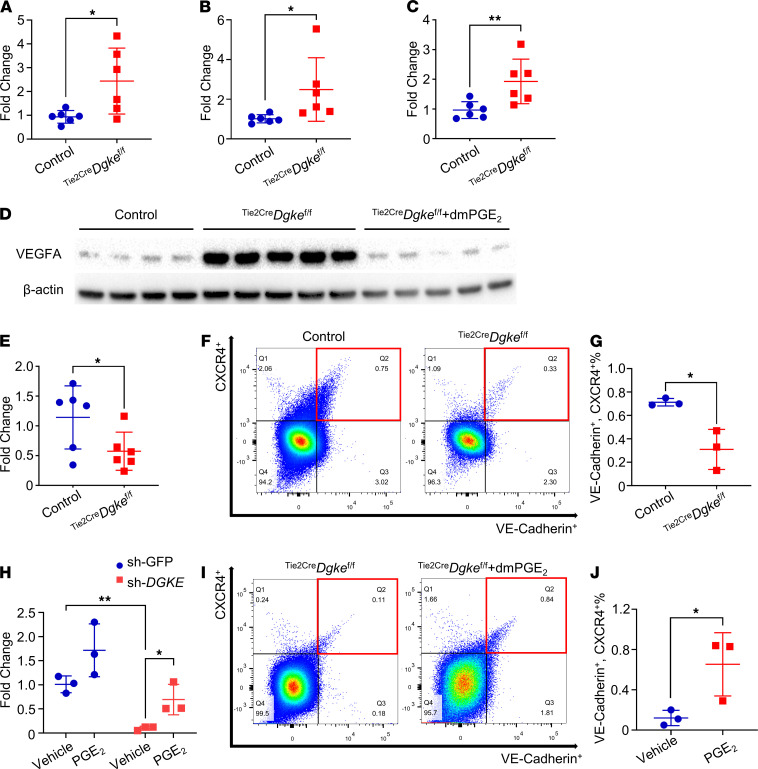
Figure 7. PGE2 controls the endothelial expression of CXCR4 in the kidney.
Q-PCR showing increased (A) Hif1a, (B) Hif2a, and (C) Sdf-1 expression in kidney cortexes of Tie2CreDgkefl/fl mice compared with controls. (D) Western blot showing increased protein levels of VEGFA in kidney cortexes of Tie2CreDgkefl/fl mice compared with controls that were reversed in Tie2CreDgkefl/fl littermates after 4 weeks of subcutaneous infusion of dmPGE2. (E) Q-PCR showing impaired CXCR4 expression in kidney cortexes of Tie2CreDgkefl/fl mice compared with controls. (F) Representative flow cytometry plots and (G) statistical analysis of 3 independent flow cytometry experiments demonstrating decreased CXCR4+ endothelial cells (CXCR4+, VE-Cadherin+) in kidney cortexes of Tie2CreDgkefl/fl mice compared with controls. (H) mRNA expression of CXCR4 in sh-DGKE HUVECs and nontarget control cells (sh-GFP) with or without PGE2 supplementation showing that CXCR4 expression is rescued by dmPGE2. (I) Representative flow cytometry plots and (J) statistical analysis of 3 independent flow cytometry experiments showing decreased CXCR4+ endothelial cells (CXCR4+, VE-Cadherin+) in kidney cortexes from Tie2CreDgkefl/fl mice. CXCR4+, VE-Cadherin+ cells increased after 4 weeks’ infusion of dmPGE2 in Tie2CreDgkefl/fl littermates. Data are from 3 independent experiments and are presented as mean ± SD. **: P < 0.01, *: P < 0.05 by 1-way ANOVA in H and by Student’s t test in A–C, E, G, and J; n = 3–6 per group. Each data point represents 1 experiment or 1 mouse.
PGE2 suppresses the expression of MMP-2 in Tie2CreDgkefl/fl mouse kidneys.
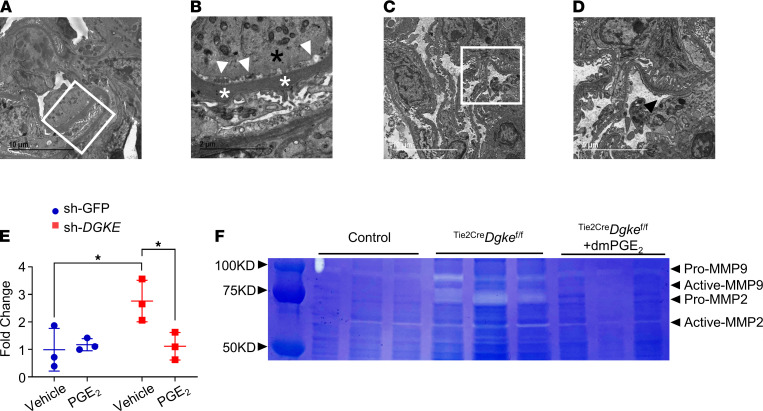
DGKE nephropathy is invariably associated with proteinuria (3, 25, 58). Proteinuria was also present in 100% of endothelial specific Tie2-CreDgkefl/fl knockout mice at a later time compared with the appearance of the hematologic signs, which suggests that the filtration barrier is secondarily affected by primary endothelial abnormalities. To gain insights into the pathogenesis of the proteinuria in the Tie2-CreDgkefl/fl knockout mice, we performed transmission electron microscopy (TEM) of renal cortexes of these mice at 3 months of age, when albuminuria is detectable. Tie2-CreDgkefl/fl knockout mouse glomeruli showed moderate podocyte foot process effacement, subendothelial widening, and, interestingly, segmental thickening of the glomerular basement membrane (GBM) (Figure 8, A and B), but all these abnormalities were not detectable in dmPGE2-treated Tie2-CreDgkefl/fl mice, in concordance with the normalized albuminuria (Figure 8, C and D, and Figure 5F). For this reason, we investigated if the proteinuria in Tie2-CreDgkefl/fl mice could result from abnormalities of the GBM induced by the activated endothelium (59). In the bone marrow niche, SDF-1 and CXCR4 are essential regulators of the homing of hematopoietic progenitor cells (HPCs) (60–63), and inhibition of CXCR4 is used to force HPCs to egress from their niche into the bloodstream, when large numbers of HPCs need to be harvested for bone marrow transplantation (64). The mobilizing effect of CXCR4 inhibitors and other hematopoietic cell mobilizers is mediated by the induction of several proteinases, including MMPs (65). Considering that the expression of CXCR4 in endothelial cells can be induced by PGE2 (55–57), we tested by Q-PCR if impaired endothelial induction of PGE2 in the absence of Dgke would be indirectly associated with increased expression of MMP-2 and MMP-9, the MMPs most abundantly expressed in the glomeruli (66), compared with controls. We found that the expression of MMP-2 was increased in DGKE-knockdown HUVECs and HMECs and normalized by supplementing the culture medium with PGE2 (Figure 8E and Supplemental Figure 2D). MMP-2, but not MMP-9, expression was also higher in cortex extracts of Tie2-CreDgkefl/fl mice in vivo, but not in kidneys of mice 28 days after they were injured by folic acid injection (67) (Supplemental Figure 7). We further confirmed by gel zymography analysis of cortex lysates that the total MMP-2 protein levels and its proteolytic activity were increased in Tie2-CreDgkefl/fl mice but not in Tie2-CreDgkefl/fl mice treated with dmPGE2 (Figure 8F). These results indicate that MMP-2 activity is high in endothelial specific Tie2-CreDgkefl/fl mouse kidneys and suggest that chronic impairment of the VEGF signaling in these mice may favor the remodeling of the GBM, maybe by controlling PGE2 and CXCR4-dependent MMP-2 expression. Further studies that are beyond the scope of this work will be needed to further explore this possibility.
Figure 8. PGE2 suppresses the expression of MMP-2 in Tie2CreDgkefl/fl mouse kidneys.
(A and B) Representative TEM images of a glomerulus of a 3-month-old Tie2CreDgkefl/fl mouse. (B) Higher magnification of the inset in A. White arrowheads point at subendothelial widening at the base of a swollen endothelial cell (black asterisk). White asterisks denote thickened GBM; black arrows, effaced foot processes. Scale bars: 10 μm (A), 2 μm (B). (C and D) Representative TEM image of a glomerulus of a 3-month-old Tie2CreDgkefl/fl mouse after 4 weeks’ subcutaneous infusion with dmPGE2. (D) Higher magnification of the inset in C. Black arrow points to normal foot processes; black arrowhead, healthy fenestrated endothelium and normal GBM. Scale bars: 10 μm (C), 5 μm (D). (E) Q-PCR showing MMP-2 expression in sh-DGKE–knockdown HUVECs and nontarget control cells (sh-GFP) before and after supplementation of the culture medium with PGE2. Data are from 3 independent experiments and are presented as mean ± SD. *: P < 0.05 by 1-way ANOVA, n = 3 per group. Each data point represents 1 experiment. (F) Gelatin-polyacrylamide gel showing the zymographic analysis of kidney cortex lysates of Tie2CreDgkefl/fl littermates of the same age after 4 weeks of dmPGE2 administration. Both pro–MMP-2 (72 kDa) and active MMP-2 (~60 kDa) are present in the Tie2CreDgkefl/fl mouse kidney, compared with controls, but disappear after treatment with dmPGE2.
Discussion
In this work we have investigated the molecular mechanisms by which biallelic loss-of-function mutations in DGKE cause a form of TMA and aHUS that it is not associated with defects of the alternative complement pathway (2, 3). By generating a potentially novel conditional knockout mouse for the gene Dgke, we demonstrated that endothelial specific deletion of Dgke is sufficient to phenocopy in full the human disease, which is remarkably similar to the disease reported in subjects treated with bevacizumab (2), highlighting the centrality of the endothelium in the DGKE disorder and suggesting that podocytes, which also express DGKE, are only secondarily affected. Mechanistically, we found that the shortage of PIP2, the intracellular substrate of PI3K, is the primary biochemical defect that causes the DGKE disease resulting in defective PIP3-dependent Akt activation in endothelial cells downstream of VEGFR2, as demonstrated in vitro and in vivo by the experiments in which a PTEN inhibitor was administered toTie2-CreDgkefl/fl mice. Thus, our data point at a central role of a defect of the Akt signaling in the endothelium of Tie2-CreDgkefl/fl mice and speak against a potential role of increased PKC activity in determining the DGKE phenotype, although this possibility cannot be completely excluded. Consistent with our in vivo data is also the conclusion, recently reported in an in vitro study, that by causing shortage of PIP2 (the common cellular substrate of both PI3K and PLC) loss of Dgke does not affect DAG signaling (8).
A somewhat unexpected connection that emerges from our studies is the relation between endothelial VEGF and Cox2/PGE2 signaling in endothelial cells. Although we previously reported the defect of Cox2 expression and PGE2 production in the constitutive Dgke-knockout mice, it was not clear how these abnormalities would be connected with the observed phenotype and if their correction would have been able to rescue the disease in Dgke-defective mice. The in vitro and in vivo experiments that we have reported here show that Dgke is required in endothelial cells to induce the expression of Cox2 and the production of PGE2 downstream of VEGFR2 and to promote the maintenance of the kidney microvasculature. This conclusion is supported by the ability of the endothelial overexpression of Cox2 and of the subcutaneous infusion of dmPGE2 to rescue the hematologic and renal manifestations observed in Tie2-CreDgkefl/fl mice. To this point, it is worthwhile to notice that treatment with dmPGE2 both rescued capillary occlusion and renal function, which could be explained by a vasodilator effect of PGE2 on the glomerular microvasculature, and avoided the activation of the endothelial cells and the expression of prothrombotic molecules, suggesting that the antiactivating effect of VEGFA on endothelial cells is exerted through the production of PGE2.
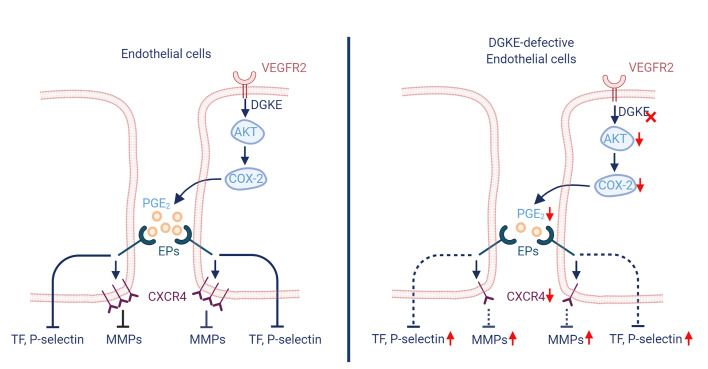
Finally, the studies that we have presented here imply an unanticipated interaction between VEGF and SDF-1/CXCR4 signaling in the kidney. A hypothetical general model that could be drawn from our observations would suggest the existence of a balance between proangiogenic VEGF-PGE2–mediated signaling that would cause, among other responses, the GBM remodeling by activating MMPs and negative feedback sustained by the counteracting SDF-1/CXCR4 signaling triggered by PGE2. According to such a model, the lack of Dgke and PGE2 would impair CXCR4-dependent suppression of MMP-2 in endothelial cells, and it would eventually result in glomerular filtration barrier damage (Graphical abstract). Although further experimental evidence has to be produced to better define the interplay between PGE2 and CXCR4 expression in vivo, our results reveal the existence of complex autocrine signaling events downstream of VEGFR2 that could conceivably be manipulated to modify the clinical course of aHUS and of other diseases characterized by endothelial activation or abnormal angiogenesis.
Methods
Animal studies.
All mice were on a C57BL/6J background. The Cox2-COE mice were provided by Harvey R. Herschman, University of California, Los Angeles (Los Angeles, California, USA), and were previously described (38).
Generation of Dgke conditional knockout mice.
These mice were generated by injecting ES cells with a knockout first construct, from the KOMP (https://www.komp.org) (30). The knockout first allele is initially a nonexpressive form, but it can be converted to a conditional allele, which carries 2 LoxP sites flanking exon 2 of Dgke, via Flp recombination. We confirmed by genomic sequencing that this exon was deleted after Cre recombination. Dgke-floxed mice are viable and fertile, and they produce progeny at the expected Mendelian ratio. Tie2-Cre mice were purchased from The Jackson Laboratory (stock 008863) and were previously described (68). CMV-Cre mice were purchased from The Jackson Laboratory (stock 006054). WT mice bred from the colony were used as controls, unless otherwise specified.
Endothelial cell culture.
HUVECs and human dermal microvascular endothelial cells (HMEC-1) were obtained from the American Type Culture Collection. Monolayers were cultured in medium 199 (Gibco, Thermo Fisher Scientific) supplemented with 20% heat-inactivated FBS (Gibco, Thermo Fisher Scientific), endothelial cell growth supplement (0.03 mg/mL), heparin (0.1 mg/mL), penicillin (100 IU/mL), and streptomycin (100 μg/mL). HMEC-1 monolayers were cultured in MCDB131 (Gibco, Thermo Fisher Scientific) supplemented with 15% heat-inactivated FBS (Gibco, Thermo Fisher Scientific), endothelial cell growth supplement (20 μg/mL), hydrocortisone (1 μg/mL), and glutamine (10 mM) in a humidified incubator at 37°C and 5% CO2/95% air.
Lentiviral infection and shRNA-mediated gene silencing.
DGKE and GFP-targeting shRNA lentiviral constructs were purchased from Thermo Fisher Scientific. The experiments were performed as previously described (69).
In vitro stimulation of HUVECs and HMECs with VEGF, with and without phosphoinositides.
Human VEGF recombinant protein was purchased from Gibco (Thermo Fisher Scientific). For VEGF treatment experiments, endothelial cells were first starved for 6 hours, then incubated with 80 ng/mL VEGF or vehicle control (PBS) for 15 minutes before being used for Western blot or intracellular calcium measurements. For phosphoinositide rescue experiments, cells were first starved for 6 hours. PIP2 or PIP3 (Echelon) was mixed with the carrier histone H1 (Echelon) at a 1:1 ratio. After a brief water bath sonication for 15 seconds, the mixture was incubated at room temperature for 15 minutes. Then it was added to the cell culture for 1 hour at the final concentration of 2.5 μM. Cells were then supplemented with VEGF or vehicle (PBS) as described above.
Western blots.
Tissues were lysed in lysis buffer (150 mM NaCl, 10 mM EDTA, 10 mM Tris pH 7.4, 1% Triton X-100, DTT, and 25 mM N-Ethylmaleimide) with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Roche). Protein concentration was calculated by NanoDrop (Thermo Fisher Scientific). Proteins were separated with NuPAGE 4%–12% Bis-Tris Gel (Invitrogen, Thermo Fisher Scientific) at 160 V, then transferred to a nitrocellulose membrane for 90 minutes at 100 V at 4°C. The membrane was blocked for 120 minutes in TBS-Tween with 5% BSA at room temperature and incubated with antibodies against anti–p-Akt (threonine 308) (Cell Signaling Technologies, 4056), anti–p-Akt (serine 473) (Cell Signaling Technologies, 9271), anti-Akt (Cell Signaling Technologies, 9272), anti-COX2 (Cell Signaling Technologies, 12282), and anti–β-actin (Cell Signaling Technologies, 8457) overnight at a dilution of 1:1000. Secondary rabbit antibody (Cell Signaling Technologies, 7074) and secondary mouse antibody (Cell Signaling Technologies, 7076) were incubated for 80 minutes at a concentration of 1:10,000 at room temperature. For exposure, Western ECL Substrate (Bio-Rad) was used. Imaging was done with ChemiDoc XRS+ System with Image Lab Software (Bio-Rad). Western blot densitometry was performed using ImageJ Version 1.53g, 2020 (NIH).
Measurement of intracellular calcium.
VEGF-stimulated intracellular calcium release was measured in HUVECs. Briefly, cells were washed in PBS and subsequently loaded with 4 μM Fura-2 AM (Thermo Fisher Scientific) in Tyrode’s solution (140 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, 0.33 mM NaH2PO4, 5.5 mM glucose, 10 mM HEPES at pH 7.4 with NaOH) for 45 minutes at 37°C. The cells were then washed in PBS, seeded onto poly-l-lysine–coated glass coverslips, and incubated in complete medium for 1 hour at 37°C. After incubation, coverslips were transferred to Ca2+-free Tyrode’s solution, and the fluorescence ratio of the cells was measured at 340 nm and 380 nm at 37°C using MetaFluor Imaging Software (Molecular Devices), after 40 seconds of baseline recording (ΔR: fluorescence ratio as 340 nm/380 nm).
Quantitative RT-PCR.
Total RNA was extracted using the RNeasy Mini Kit (QIAGEN). RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). RT-PCR was performed using the CFX Connect Real-Time PCR Detection System (Bio-Rad) and iTaq Universal SYBR Green Supermix (Bio-Rad). All RT-PCR experiments were performed in triplicate. The sequences of PCR primers (IDT) are provided in Supplemental Table 1.
Measurement of BUN, plasma TAT, serum lactate dehydrogenase activity, urine albumin, and urine creatinine.
Blood samples were obtained from the retro-orbital plexus. BUN and plasma TAT were measured by ELISA (Abcam), according to the manufacturer’s instructions. Twenty-four-hour urine was collected using metabolic cages. Serum lactate dehydrogenase (LDH) activity was tested by LDH assay kit (MilliporeSigma). Urinary albumin concentration was determined using the Albuwell microalbumin ELISA (Exocell). Urinary creatinine concentration was determined using a P/ACE MDQ Capillary Electrophoresis System and photodiode detector (Beckman Coulter) at the Physiology Core of the O’Brien Center for Kidney Diseases in Dallas (70). ACR was calculated by dividing albumin concentration in milligrams by creatinine concentration in grams.
Measurement of circulating platelets and serum hemoglobin.
Mouse blood samples were collected from the retro-orbital plexus using heparinized capillary tubes. After acquisition, samples were immediately processed for platelet counts and hemoglobin measurements on an Advia 120 Hematology system (Siemens Medical Solutions USA Inc.) to avoid clot formation.
Mouse platelet isolation and in vitro platelet aggregation studies.
Mouse platelets were prepared as previously described (71, 72). Blood from anesthetized mice was drawn from the retro-orbital plexus and collected in 1.5 mL polypropylene tubes containing 300 μL of enoxaparin (0.3 mg/mL; Sanofi-Aventis). The blood was centrifuged at 100g for 5 minutes, and the platelet-rich plasma (PRP) was collected in a fresh tube. PRP (platelet count; 2 × 108/mL) from WT and endothelial specific knockout DGKE was stirred (178 RCF) at 37°C for 2 minutes in a whole blood/optical lumi-aggregometer (Chrono-log, model 700–2) before the addition of agonists (collagen or ADP). Aggregation was measured as percentage change in light transmission, where 100% refers to transmittance through the blank sample (platelet-poor plasma).
PGE2 infusion with subcutaneous osmotic minipumps.
Mice were treated with dmPGE2 (Cayman Chemical) and vehicle control through a subcutaneously implanted ALZET 1004 mini–osmotic pump (DURECT). Minipumps were loaded with 100 μL of dmPGE2 solution in sterile PBS. A release rate of 0.11 μL/h administered a total amount of 30 μg/kg dmPGE2 daily, a dose that resulted in elevated serum PGE2 without obvious adverse side effects, during the 28 days of the experimental setup.
PTEN inhibitor experiments.
Littermate Tie2Dgkefl/fl mice were treated with daily i.p. injection of the PTEN inhibitor VO-OHpic and vehicle control. VO-OHpic (BioVision, 1801-5) was used at 10 mg/kg/d, administered in a 10% DMSO solution.
Poly(I:C) injections.
Mice were treated with i.p. injections (1 μg/μL, 200 μg/mouse) of a solution of poly(I:C) (MilliporeSigma, P1530). Blood and urine samples were collected at 6 hours, 24 hours, and 7 days after. Platelets, BUN, and ACR were measured as described above.
Flow cytometry.
Fresh mouse kidney cortexes were digested in 2 mL of protease solution — 5 mM CaCl2, 10 mg/mL Bacillus licheniformis protease (Creative Enzymes, NATE0633), and 125 U/mL DNase I (Roche) in Dulbecco’s PBS — on ice for 30 minutes. The lysates were filtered through 70 μm cell strainers. Single-cell suspension was collected by rinsing with FACS buffer. Suspensions were lysed with red blood cell lysis buffer (Quality Biological) to remove red blood cells. After washing with 1× PBS, 1 × 106 cells were resuspended in 100 μL of FACS buffer and preincubated with anti–mouse CD16/32 antibody (BioLegend, 101302) for blocking prior to labeling with CD144-APC (BioLegend, 138012) and CD184-BV421 (BD Biosciences, 562738) antibodies for 30 minutes at room temperature. Cells were then washed with FACS buffer and analyzed on BD LSR II Violet at the University of Iowa Flow Cytometry Facility. All flow cytometry results were analyzed using FlowJo software (Version 9).
Zymography.
Kidney cortical tissues were lysed in lysis buffer (20 mM Tris-HCl, 125 mM NaCl, and 1% Triton X-100, pH 8.5) with protease inhibitor cocktail (Roche). Protein concentration was calculated by NanoDrop (Thermo Fisher Scientific). Samples were separated on Tris-glycine gels with 0.1% gelatin (Invitrogen, Thermo Fisher Scientific). After electrophoresis, the gels were incubated in 1× renaturing buffer (Thermo Fisher Scientific) for 2 hours at room temperature, then in 1× developing buffer (Thermo Fisher Scientific) for 48 hours. Gels were stained with Coomassie Blue R-250 (Bio-Rad) and destained in 20% ethanol and 10% acetic acid. MMP-2 activity was detected as clear bands on the blue background.
Statistics.
All quantitative data are presented as mean ± SD. Statistical significance (P ≤ 0.05 was considered significant) was calculated using the 2-tailed unpaired Student’s t test for 2 groups and 1-way ANOVA for multiple-group comparison by Prism 7 (GraphPad Software).
Study approval.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Iowa (protocol 7021976).
Author contributions
DL performed experiments, analyzed data, prepared figures, and contributed to writing the manuscript. QD, MP, BP, CL, MP, DFD, and MKN performed experiments and analyzed data. HJ, AKC, CS, and CLH provided reagents. MA designed experiments, analyzed data, prepared figures, and contributed to writing of the manuscript.
Supplementary Material
Acknowledgments
The flow cytometry data were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. This facility is funded through user fees and the financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veterans Administration Medical Center. Research reported in this publication was supported by the National Center for Research Resources of the NIH under award number 1 S10 OD016199-01A1. The AKC lab is supported by grants from the NIH (R35HL139926, R01NS109910, U01NS113388) and by the Established Investigator Award 18EIA33900009 from the American Heart Association. We want to thank Harvey R. Herschman, for providing us the Cox2-COE mice.
Version 1. 05/10/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, Liu et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2021;6(9):e146959.https://doi.org/10.1172/jci.insight.146959.
Contributor Information
Dingxiao Liu, Email: dingxiao-liu@uiowa.edu.
Qiong Ding, Email: qiong-ding@uiowa.edu.
Dao-Fu Dai, Email: dao-fu-dai@uiowa.edu.
Biswajit Padhy, Email: biswajit-padhy@uiowa.edu.
Manasa K. Nayak, Email: manasa-nayak@uiowa.edu.
Can Li, Email: lican811@gmail.com.
Heng Jin, Email: hengjin@tmu.edu.cn.
Chang Shu, Email: shuchang@csu.edu.cn.
Chou-Long Huang, Email: chou-long-huang@uiowa.edu.
References
- 1.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(17):1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 2.Ozaltin F, et al. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J Am Soc Nephrol. 2013;24(3):377–384. doi: 10.1681/ASN.2012090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemaire M, et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet. 2013;45(5):531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang W, et al. Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Biol Chem. 1996;271(17):10237–10241. doi: 10.1074/jbc.271.17.10237. [DOI] [PubMed] [Google Scholar]
- 5.Milne SB, et al. Dramatic differences in the roles in lipid metabolism of two isoforms of diacylglycerol kinase. Biochemistry. 2008;47(36):9372–9379. doi: 10.1021/bi800492c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez de Turco EB, et al. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl- inositol lipid signaling. Proc Natl Acad Sci U S A. 2001;98(8):4740–4745. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shulga YV, et al. Molecular species of phosphatidylinositol-cycle intermediates in the endoplasmic reticulum and plasma membrane. Biochemistry. 2010;49(2):312–317. doi: 10.1021/bi901551e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. doi: 10.1101/633867. So V, et al. Phosphatidylinositol cycle disruption is central to atypical hemolytic-uremic syndrome caused by diacylglycerol kinase epsilon deficiency [preprint]. Published on bioRxiv May 27, 2019. [DOI]
- 9.Xu C, et al. Kinetic analysis of receptor-activated phosphoinositide turnover. J Cell Biol. 2003;161(4):779–791. doi: 10.1083/jcb.200301070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willars GB, et al. Differential regulation of muscarinic acetylcholine receptor-sensitive polyphosphoinositide pools and consequences for signaling in human neuroblastoma cells. J Biol Chem. 1998;273(9):5037–5046. doi: 10.1074/jbc.273.9.5037. [DOI] [PubMed] [Google Scholar]
- 11.Creba JA, et al. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike LJ, Casey L. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem. 1996;271(43):26453–26456. doi: 10.1074/jbc.271.43.26453. [DOI] [PubMed] [Google Scholar]
- 13.Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2(7):a006502. doi: 10.1101/cshperspect.a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch S, et al. Signal transduction by vascular endothelial growth factor receptors. Biochem. 2011;437(2):169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 15.Olsson AK, et al. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 16.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280(6):C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett CS, et al. Vascular growth factors and glomerular disease. Annu Rev Physiol. 2016;78:437–461. doi: 10.1146/annurev-physiol-021115-105412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, et al. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20(11):2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 20.Gerber HP, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 21.Graupera M, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453(7195):662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 22.Dayanir V, et al. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001;276(21):17686–17692. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- 23.Eremina V, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111(5):707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sison K, et al. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol. 2010;21(10):1691–1701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eremina V, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akarasereenont PC, et al. The expression of COX-2 in VEGF-treated endothelial cells is mediated through protein tyrosine kinase. Mediators Inflamm. 2002;11(1):17–22. doi: 10.1080/09629350210311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura M, et al. Vascular endothelial growth factor up-regulates cyclooxygenase-2 expression in human endothelial cells. J Clin Endocrinol Metab. 2002;87(7):3504–3507. doi: 10.1210/jcem.87.7.8796. [DOI] [PubMed] [Google Scholar]
- 28.Wheeler-Jones C, et al. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997;420(1):28–32. doi: 10.1016/S0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- 29.Kage K, et al. Basic fibroblast growth factor induces cyclooxygenase-2 expression in endothelial cells derived from bone. Biochem Biophys Res Commun. 1999;254(1):259–263. doi: 10.1006/bbrc.1998.9875. [DOI] [PubMed] [Google Scholar]
- 30.Skarnes WC, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474(7351):337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osterwalder M, et al. Dual RMCE for efficient re-engineering of mouse mutant alleles. Nat Methods. 2010;7(11):893–895. doi: 10.1038/nmeth.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwenk F, et al. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23(24):5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moeller MJ, et al. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35(1):39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 34.Venneri MA, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109(12):5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 35.Groszer M, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294(5549):2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 36.Mak LH, et al. Characterisation of the PTEN inhibitor VO-OHpic. J Chem Biol. 2010;3(4):157–163. doi: 10.1007/s12154-010-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, et al. Loss of diacylglycerol kinase epsilon in mice causes endothelial distress and impairs glomerular Cox-2 and PGE2 production. Am J Physiol Renal Physiol. 2016;310(9):F895–F908. doi: 10.1152/ajprenal.00431.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamei K, et al. Transgenic mouse for conditional, tissue-specific Cox-2 overexpression. Genesis. 2006;44(4):177–182. doi: 10.1002/dvg.20199. [DOI] [PubMed] [Google Scholar]
- 39.Rao R, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem. 2007;282(23):16959–16968. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- 40.Aoudjit L, et al. Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol Renal Physiol. 2006;290(6):F1534–F1542. doi: 10.1152/ajprenal.00267.2005. [DOI] [PubMed] [Google Scholar]
- 41.Gliki G, et al. Vascular endothelial growth factor-induced prostacyclin production is mediated by a protein kinase C (PKC)-dependent activation of extracellular signal-regulated protein kinases 1 and 2 involving PKC-delta and by mobilization of intracellular Ca2+ Biochem J. 2001;353(pt 3):503–512. doi: 10.1042/0264-6021:3530503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy JF, Fitzgerald DJ. Vascular endothelial growth factor induces cyclooxygenase-dependent proliferation of endothelial cells via the VEGF-2 receptor. FASEB J. 2001;15(9):1667–1669. doi: 10.1096/fj.00-0757fje. [DOI] [PubMed] [Google Scholar]
- 43.Chang SH, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101(2):591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, et al. Loss of diacylglycerol kinase epsilon in mice causes endothelial distress and impairs glomerular Cox-2 and PGE(2) production. Am J Physiol Renal Physiol. 2016;310(9):F895–F908. doi: 10.1152/ajprenal.00431.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruneau S, et al. Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood. 2015;125(6):1038–1046. doi: 10.1182/blood-2014-06-579953. [DOI] [PubMed] [Google Scholar]
- 46.Devendra D, Eisenbarth GS. Interferon alpha--a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin Immunol. 2004;111(3):225–233. doi: 10.1016/j.clim.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Sayyed SG, et al. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52(11):2445–2454. doi: 10.1007/s00125-009-1493-6. [DOI] [PubMed] [Google Scholar]
- 48.Romoli S, et al. CXCL12 blockade preferentially regenerates lost podocytes in cortical nephrons by targeting an intrinsic podocyte-progenitor feedback mechanism. Kidney Int. 2018;94(6):1111–1126. doi: 10.1016/j.kint.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding M, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12(9):1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- 50.Takabatake Y, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20(8):1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zagzag D, et al. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173(2):545–560. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zagzag D, et al. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65(14):6178–6188. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 53.Salcedo R, et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: in vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154(4):1125–1135. doi: 10.1016/S0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park J, et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360(6390):758–763. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoggatt J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoggatt J, et al. Differential stem- and progenitor-cell trafficking by prostaglandin E2. Nature. 2013;495(7441):365–369. doi: 10.1038/nature11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salcedo R, et al. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood. 2003;102(6):1966–1977. doi: 10.1182/blood-2002-11-3400. [DOI] [PubMed] [Google Scholar]
- 58.Brocklebank V, et al. Long-term outcomes and response to treatment in diacylglycerol kinase epsilon nephropathy. Kidney Int. 2020;97(6):1260–1274. doi: 10.1016/j.kint.2020.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jarad G, et al. Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116(8):2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 61.Petit I, et al. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12(5):557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.[No authors listed] Plerixafor:AMD 3100, AMD3100, JM 3100, SDZ SID 791. Drugs R D. 2007;8(2):113–119. doi: 10.2165/00126839-200708020-00006. [DOI] [PubMed] [Google Scholar]
- 65.Jujo K, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107(24):11008–11013. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sekiuchi M, et al. Expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of matrix metalloproteinases 2 and 1 in the glomeruli of human glomerular diseases: the results of studies using immunofluorescence, in situ hybridization, and immunoelectron microscopy. Clin Exp Nephrol. 2012;16(6):863–874. doi: 10.1007/s10157-012-0633-3. [DOI] [PubMed] [Google Scholar]
- 67.Jin H, et al. Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight. 2019;4(2):125490. doi: 10.1172/jci.insight.125490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kisanuki YY, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230(2):230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 69.Li B, et al. Increased hedgehog signaling in postnatal kidney results in aberrant activation of nephron developmental programs. Hum Mol Genet. 2011;20(21):4155–4166. doi: 10.1093/hmg/ddr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zinellu A, et al. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem. 2005;342(2):186–193. doi: 10.1016/j.ab.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 71.Nayak MK, et al. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018;2(15):2029–2038. doi: 10.1182/bloodadvances.2018022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nayak MK, et al. Metabolic enzyme pyruvate kinase M2 regulates platelet function and arterial thrombosis. Blood. 2021;137(12):1658–1668. doi: 10.1182/blood.2020007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.