Abstract
BACKGROUND & AIMS:
There is debate over the type of electrosurgical setting that should be used for polyp resection. Some endoscopists use a type of blended current (yellow), whereas others prefer coagulation (blue). We performed a single-blinded, randomized trial to determine whether type of electrosurgical setting affects risk of adverse events or recurrence.
METHODS:
Patients undergoing endoscopic mucosal resection of nonpedunculated colorectal polyps 20 mm or larger (n = 928) were randomly assigned, in a 2 × 2 design, to groups that received clip closure or no clip closure of the resection defect (primary intervention) and then to either a blended current (Endocut Q) or coagulation current (forced coagulation) (Erbe Inc) (secondary intervention and focus of the study). The study was performed at multiple centers, from April 2013 through October 2017. Patients were evaluated 30 days after the procedure (n = 919), and 675 patients underwent a surveillance colonoscopy at a median of 6 months after the procedure. The primary outcome was any severe adverse event in a per patient analysis. Secondary outcomes were complete resection and recurrence at first surveillance colonoscopy in a per polyp analysis.
RESULTS:
Serious adverse events occurred in 7.2% of patients in the Endocut group and 7.9% of patients in the forced coagulation group, with no significant differences in the occurrence of types of events. There were no significant differences between groups in proportions of polyps that were completely removed (96% in the Endocut group vs 95% in the forced coagulation group) or the proportion of polyps found to have recurred at surveillance colonoscopy (17% and 17%, respectively). Procedural characteristics were comparable, except that 17% of patients in the Endocut group had immediate bleeding that required an intervention, compared with 11% in the forced coagulation group (P = .006).
CONCLUSIONS:
In a randomized trial to compare 2 commonly used electrosurgical settings for the resection of large colorectal polyps (Endocut vs forced coagulation), we found no difference in risk of serious adverse events, complete resection rate, or polyp recurrence. Electrosurgical settings can therefore be selected based on endoscopist expertise and preference. Clinicaltrials.gov ID NCT01936948.
Keywords: colorectal cancer prevention, comparison, safety, surgery
Graphical Abstract
Electrosurgical snare resection represents the standard approach to resecting larger polyps since its introduction in 1971.1 The underlying mechanism is the conversion of electrical energy into heat, which enables transection of the polyp base while closing the snare.2 Electrosurgical snare resection, therefore, allows the removal of larger polyps in once piece. In addition, it may also ablate small residual polyp tissue at the resection margin and seal off small blood vessels during resection. However, there is little evidence to support these assumptions.3,4
Although electrosurgical application is a fundamental aspect of polypectomy, various currents and settings are clinically used, and there are no accepted standards of practice. As such, endoscopists typically perform polypectomy the way they were trained. A 2004 survey found that 46% of US endoscopists used coagulation current and 46% used a type of blended current.5
Several factors affect the ability of electrosurgical snare resection. These include voltage (at least 200 peak volt), current density, the percent duty cycle with which current is delivered, tissue impedance, and the snare surface area touching the tissue.6 Coagulation current delivers a higher voltage and interrupted current (low duty cycle) with a slow rise in heat in the tissue, resulting in dehydration and shrinkage (desiccation).2 This type of current may coagulate vessels and thereby prevent bleeding but may also cause deeper tissue heat injury and increase the risk for postpolypectomy syndrome. Cutting current uses lower voltage and continuous current (100% duty cycle), which leads to rapid heating of cells that then burst and vaporize, resulting in cleavage of tissue along the snare wire. Two retrospective studies found that coagulation current was associated with a greater risk of postprocedure bleeding and blended current with a greater risk of immediate bleeding.7,8
To date, not a single randomized trial has addressed the effectiveness or safety of different cautery approaches for polyp resection. We therefore aimed to compare 2 commonly applied electrosurgical settings: a pure coagulation current (forced coagulation) and a blended or alternating cut-coagulation current (Endocut Q) delivered by a modern microprocessor-controlled electrosurgical unit (Erbe Inc, Tübingen, Germany).
Methods
Study Design and Patient Selection
This multicenter, randomized, controlled, single-blinded study enrolled patients with a large colorectal polyp across 18 medical centers between April 2013 and October 2017. Patients were randomly assigned in a 2 × 2 factorial design to clip closure of the mucosal resection defect or no closure and to 1 of 2 electrosurgical settings for snare resection: a combination of blended and cutting current (Endocut Q) or pure coagulation current (forced coagulation) using the Erbe Vio 300D electrosurgical unit (Erbe USA Inc., Marietta, GA). The trial was designed to examine both the effect of clip closure on postprocedure bleeding and the effect of the electrosurgical setting on overall complications. It was powered based on the clip closure as the primary study intervention.9 The type of electrosurgical setting was the secondary intervention and is the subject of this report. Although a separate and explorative analysis of the effect of electrosurgical setting on safety and efficacy outcomes was planned a priori, only after completion of the trial was it possible to perform a test for interaction between the 2 interventions. This test showed no interaction (P = .957), and this lack of interaction allowed us then to pursue an analysis of the electrosurgical setting independent of the clip intervention.
The randomization list was computer generated, with patients assigned to 1 of 4 groups in blocks of 8, stratified by center. Assignments were kept in sequentially numbered and concealed envelopes. The randomization envelope was opened only after a potential study polyp was assessed during the colonoscopy and before starting endoscopic resection.
Eligible patients included all those who presented for endoscopic mucosal resection (EMR) of a large (≥20-mm) nonpedunculated colorectal polyp. Patients with inflammatory bowel disease, a known coagulopathy (international normalized ratio ≥1.5; 50,000 platelets per μL), severe comorbidities (American Society of Anesthesiologists class IV), or a poor bowel preparation quality10 were excluded. Eligible study polyps included those that were nonpedunculated and met the minimum size requirements as measured by an open snare before starting the EMR. Polyps were not eligible if they were not considered amenable to endoscopic mucosal resection (eg, high suspicion for invasive cancer) or if they were pedunculated (Paris Ip), subpedunculated (Paris Isp), or ulcerated (Paris III). The institutional review boards of each center approved the study, and all patients gave written informed consent to participate. The study was registered at clinicaltrials.gov (NCT01936948).
Procedures
Preparation for colonoscopy and procedural sedation followed the clinical standard at the participating center. Periprocedural antithrombotic medications followed professional society guidelines.11,12 Upon detection of a potential study polyp, the polyp was assessed for eligibility. If eligible, the patient was randomized, and the polyp was resected according to group assignment following the principles of the EMR technique.13 Before resection, the polyp was lifted from the muscularis propria by submucosal injection. The injection solution contained a solute (eg, normal saline or a viscous solution such as hydroxyethyl starch) and a contrast agent (eg, methylene blue or indigo carmine). After submucosal injection, the polyp was then removed by electrosurgical snare resection as assigned by randomization. The choice of injection solution and snare was at the discretion of the treating endoscopist. Cautery in both groups was applied following standardized settings in both groups (Endocut Q [referred to as Endocut]: effect 2, duration 1, interval 4; forced coagulation: effect 2, 25 W). The selection was based first on endoscopists’ clinical preference, as obtained in a prestudy survey. Different settings were then tested on chicken meat. The final selection represents frequently used Endocut and forced coagulation settings that best matched with respect to the observed thermal effect on tissue. Endoscopists were allowed to modify the setting if cutting was considered not sufficient. If participants had multiple polyps removed, the same cautery assignment was used for all polyps. All study polyp resection sites were marked with a tattoo unless the location was easily defined by anatomic landmarks as in the cecum or rectum. Postprocedure care was at the discretion of the treating endoscopists, who provided final guidance for timing of restarting antithrombotic medications and dietary restrictions.
Primary Outcome and Definitions
The primary outcome was the rate of any intraprocedure or postprocedure severe adverse events. Intraprocedure adverse events were defined as those occurring during the procedure. Postprocedure adverse events were defined as those occurring after the patient left the endoscopy unit and up to 30 days after the procedure. Severe adverse events were defined as clinically significant adverse events that were a threat of permanent disability or death that required hospitalization, blood transfusion, a colonoscopy, or surgery.14 In addition, any postpolypectomy syndrome was also considered a severe adverse event, even if treated as on an outpatient basis. Postprocedure adverse events were ascertained by phone call or during a clinic visit at least 30 days after the procedure and by review of medical records.
Secondary outcomes of interest were the proportion of polyps completely excised per visual inspection and the rate of recurrence at first surveillance colonoscopy at all resection sites that were identified. Recurrence was defined as biopsyproven recurrence of neoplasia at the prior resection site. Endoscopists were instructed to sequentially examine the resection site with white light and image-enhanced endoscopy (eg, NBI) and to obtain biopsy specimens. In some instances, biopsies were deferred because of the lack of any visible tissue that could represent polyp regrowth (ie, flat scar without identifiable tissue that could represent polyp tissue). This approach has been shown to have a sensitivity of 93%.15 We present recurrence of all EMR resection sites that were available for evaluation and, in addition, provide results of all resection sites that were identified.
We further assessed procedural characteristics that reflect the ease, safety, and efficiency of resection, including proportion of polyp removed en bloc, need for adjunctive therapy to remove residual polyp tissue, intraprocedural bleeding during resection of the polyp, and time of resection. Intraprocedure bleeding was defined as bleeding that occurred during resection of the polyp and that required treatment (eg, clipping, soft coagulation to ablate a blood vessel, epinephrine injection).
Analysis
The primary analysis was performed based on an intention to treat analysis. Primary and secondary outcomes are presented as absolute risks and comparison between groups as absolute risk difference with a 95% confidence interval (95% CI). We compared severe adverse events between groups, and all outcomes are presented as proportions using the chisquared test or the Fisher’s exact test where appropriate. Baseline characteristics are presented as means with standard deviation for normally distributed variables and as medians with interquartile range (IQR) for nonnormally distributed variables. Comparison of means was performed with the Student t test and of medians with the Kruskal-Wallis test. A 2-sided P value of <.05 was considered a significant difference.
We further performed a per protocol analysis among all patients who underwent the allocated intervention. Finally, we examined whether a possible effect of cautery setting was affected by periprocedural antithrombotic use, presence of multiple polyps, polyp location (proximal vs distal), polyp size, or clip closure using the Mantel-Haenszel test for interaction.
The sample size calculation was based on the primary intervention—the comparison of postprocedure bleeding events between the clip group and the no-clip group.9 The calculated required number of patients to be randomly assigned to the clip vs no-clip group was 920. For the secondary comparison of Endocut vs forced coagulation, this sample size of 920 patients would show a 5% absolute difference (eg, 10% vs. 5%) in the rate of severe adverse events as significant at a power of 0.79. For clarity of presentation, results were rounded in the text; tables and figures provide more precision.
All coauthors had access to the study data and reviewed and approved the final manuscript.
Results
A total of 928 patients were randomly assigned equally to the Endocut and forced coagulation groups, 919 patients completed the 30-day follow-up, and 675 patients completed their first surveillance colonoscopy after a median of 6 months (Supplementary Figure 1). Patient baseline characteristics were similar between groups with the exception of a greater proportion of patients in the Endocut group with more than 1 study polyp (Table 1). Characteristics of study polyps were also similar between groups. The overall median size was 30 mm (IQR, 22–35), and two thirds of polyps were located at or proximal to the hepatic flexure (Table 2). In the Endocut group, slightly more polyps lifted completely with submucosal injection compared to the forced coagulation group (83% vs 78%; P = .060). In both groups, a similar proportion of resection defects were closed with clips.
Table 1.
Patient Baseline Characteristics
| Characteristics and outcomes | Endocut (n = 464) | Forced coagulation (n = 464) | P |
|---|---|---|---|
| Patients | |||
| Age, y, mean (SD) | 64.7 (9.7) | 65.4 (9.5) | .284 |
| Male sex, n (%) | 276 (59.5) | 276 (59.5) | 1.0 |
| Race or ethnic group, n (%) | .719 | ||
| Non-Hispanic white | 414 (89.2) | 407 (87.7) | |
| Non-Hispanic black | 27 (5.8) | 35 (7.5) | |
| Hispanic | 13 (2.8) | 14 (3.0) | |
| Asian | 3 (0.6) | 4 (0.9) | |
| Other/unknown | 7 (1.5) | 4 (0.9) | |
| Body mass index, kg/m2, mean (SD) | 29.3 (6.4) | 29.0 (5.6) | .521 |
| ASA class, n (%) | .718 | ||
| I | 42 (9.1) | 37 (8.0) | |
| II | 259 (55.8) | 254 (54.7) | |
| III | 163 (35.1) | 173 (37.3) | |
| Antithrombotic medications, n (%) | 128 (27.6) | 141 (30.4) | .347 |
| Antiplatelet agents | 115 (24.8) | 117 (25.2) | .879 |
| Anticoagulants | 18 (4.0) | 30 (6.6) | .083 |
| Procedure, n (%) | |||
| Sedation | .106 | ||
| No sedation | 1 (0.2) | 5 (1.1) | |
| Moderate sedation | 65 (14.0) | 51 (11.0) | |
| Monitored anesthesia care | 398 (85.8) | 408 (87.9) | |
| Quality of bowel preparation | .797 | ||
| Excellent | 126 (27.5) | 123 (26.2) | |
| Good | 256 (55.9) | 273 (58.1) | |
| Fair | 76 (16.6) | 74 (15.7) | |
| Any additional polyp (any size), n (%) | 201 (43.3) | 207 (44.6) | .692 |
| More than 1 ≥20 mm study polyp, n (%) | 40 (8.6) | 21 (4.5) | .012 |
ASA, American Society of Anesthesiologists.
Table 2.
Characteristics of Study Polyps and Polyp Resection
| Characteristics | Endocut (n = 513) | Forced Coagulation (n = 486) | P |
|---|---|---|---|
| Size, mm, median (IQR) | 30 (23–35) | 28 (22–35) | .809 |
| Location, n (%) | |||
| Proximal | 347 (67.6) | 319 (65.6) | .502 |
| Distal | 166 (32.4) | 167 (34.4) | |
| Morphology, n (%)a | .822 | ||
| Sessile | 220 (42.9) | 205 (42.2) | |
| Flat | 293 (57.1) | 281 (57.8) | |
| Histology, n (%) | .499 | ||
| Tubular adenoma | 231 (45.0) | 206 (42.4) | |
| Tubulovillous or villous adenoma | 116 (22.6) | 99 (20.4) | |
| Serrated lesionb | 104 (20.3) | 116 (23.9) | |
| High-grade dysplasia | 45 (8.8) | 47 (9.7) | |
| Cancer | 11 (2.1) | 15 (3.1) | |
| Other | 6 (1.2) | 3 (0.6) | |
| Prior resection attempts, n (%) | 72 (14.0) | 54 (11.1) | .164 |
| Difficulties with position during resection, n (%) | .480 | ||
| Minor | 342 (66.7) | 313 (64.4) | |
| Moderate/severe | 171 (33.3) | 173 (35.6) | |
| Submucosal lifting, n (%)c | .060 | ||
| Complete | 420 (82.7) | 378 (78.4) | |
| Partial | 85 (16.7) | 97 (20.1) | |
| Nonlifting | 3 (0.6) | 7 (1.4) | |
| Submucosal injection with epinephrine, n (%) | 207 (40.4) | 177 (36.4) | .202 |
| Ablation of resection margin, n (%) | 102 (19.9) | 101 (20.8) | .724 |
| Ablation of blood vessels to prevent bleeding, n (%) | 53 (10.3) | 42 (8.6) | .363 |
| Clip closure of the mucosal defect, n (%) | .458 | ||
| Complete | 186 (36.3) | 181 (37.2) | |
| Partial | 51 (9.9) | 60 (12.3) | |
| Not closed | 276 (53.8) | 245 (50.4) |
Sessile was defined as a polyp with a Paris Is component. Flat was defined as a polyp with Paris IIa, IIB, or IIc components.26
Includes 8 hyperplastic polyps, 198 sessile serrated adenomas/polyps, and 14 traditional serrated adenomas.
Missing: n = 9.
Six polyps in the Endocut group (1.2%) were removed by forced coagulation, and 8 polyps in the forced coagulation (1.6%) group were removed by Endocut. For another 14 polyps in the forced coagulation group, the setting was switched to Endocut during the resection (2.9%). Reasons for changing the setting varied and included personal preference for the given polyp or, in the majority of cases, difficulty in removing the polyp with the initial setting.
Primary Outcome: Severe Adverse Events
The rates of severe adverse events in the Endocut and forced coagulation groups were 7.2% and 7.9%, respectively (P = .762) (Figure 1). There was no significant difference in the occurrence of intraprocedure or postprocedure events or in the types of severe adverse events (Table 3). For instance, postprocedure bleeding was observed in 5.0% in the Endocut group and 5.7% in the forced coagulation group, occurring at a median of 2 days (IQR, 1–8) and 2.5 days (IQR, 1–7) after the procedure, respectively. More patients had a perforation in the Endocut group than in the forced coagulation group (6 vs 3 patients), but this difference was not significant (P = .320). The proportion of patients requiring a repeat colonoscopy (for control of bleeding), blood transfusion, or surgery did not differ between groups.
Figure 1.
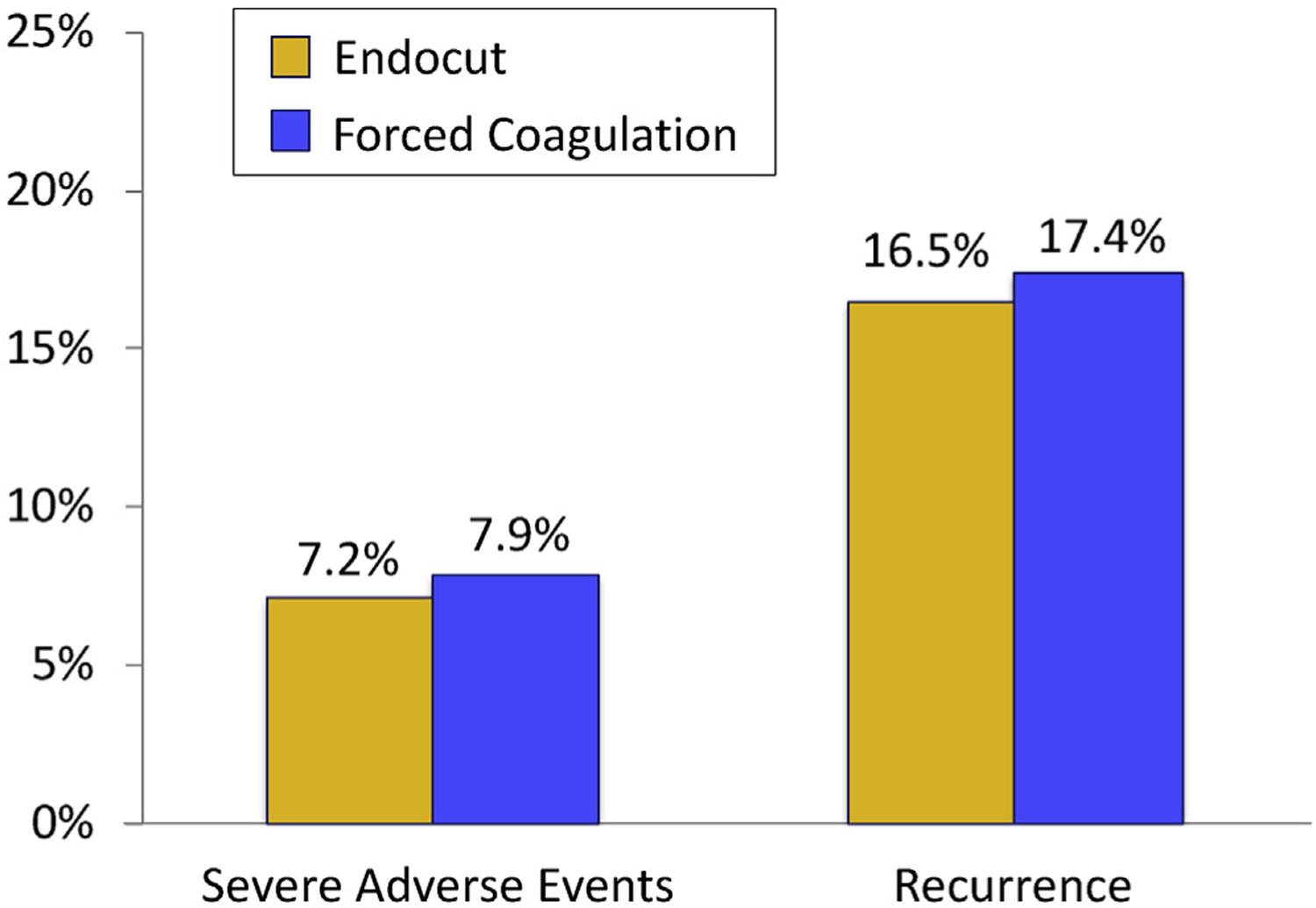
Rate of immediate and postprocedure severe adverse events (per patient analysis) and polyp recurrence at first surveillance colonoscopy (per polyp analysis).
Table 3.
Adverse Events
| Characteristics and outcomes | Endocut (n = 461) | Forced coagulation (n = 458) | P |
|---|---|---|---|
| Severe adverse events | 33 (7.2) | 36 (7.9) | .686 |
| Immediate, n (%) | 5 (1.1) | 4 (0.9) | .743 |
| Bleeding | 0 | 1 (0.2) | |
| Perforation | 4 (0.9) | 2 (0.4) | |
| Abdominal pain | 1 (0.2) | 1 (0.2) | |
| Postprocedure | 28 (6.1) | 32 (7.0) | .844 |
| Bleeding, n (%) | 23 (5.0) | 26 (5.7) | |
| Time to bleeding, d, median (IQR) | 2 (1–8) | 2.5 (1–7) | .984 |
| Perforation, n (%) | 2 (0.4) | 1 (0.2) | |
| Postpolypectomy syndrome, n (%) | 2 (0.4) | 3 (0.7) | |
| Abdominal pain, n (%) | 0 | 1 (0.2) | |
| Other, n (%) | 1 (0.2)a | 1 (0.2)c | |
| Interventions, n (%) | |||
| Colonoscopy | 12 (2.6) | 16 (3.5) | .432 |
| Blood transfusion | 5 (1.1) | 5 (1.1) | .992 |
| Surgery | 1 (0.2) | 3 (0.7) | .313 |
| Outcome, n (%) | .134 | ||
| Recovered | 31 (93.9) | 36 (100) | |
| Dieda,b | 2 (6.1) | 0 |
One patient was found dead 18 days after the colonoscopy from an unclear cause.
One patient on anticoagulation medications died after severe postprocedure bleeding. The resection site was closed with clips.
Patient admitted with fever, with no source of infection identified, responded to antibiotics.
There were 2 deaths; both occurred in the Endocut group. One patient underwent clip closure and developed postprocedure bleeding after restarting anticoagulation. The patient then developed a myocardial infarction and subsequently died of multiorgan failure. The second patient was found dead at his home 18 days after the colonoscopy, without a clear cause of death.
Complete Resection and Polyp Recurrence
Visibly complete polyp removal was achieved for 96% of polyps in the Endocut group and 95% in the forced coagulation group. Among all 857 (92%) patients who were eligible for surveillance (Supplementary Figure 1), a similar proportion in each group underwent a first surveillance colonoscopy (79%). Endoscopists documented identification of resection sites in 91%. Overall polyp recurrence was observed in 17% at all previous EMR resection sites in either group (Figure 1). When restricting recurrence to identified sites, the rates were 18% and 19% in the respective groups (Table 4). Although most recurrences were macroscopically visible, histologic recurrence without visible polyp tissue was found slightly more frequently in the Endocut group than in the forced coagulation group (6.0% vs 3.1%; P = .07).
Table 4.
Findings at First Surveillance Colonoscopy (SC)
| Outcomes | Endocut | Forced coagulation | P |
|---|---|---|---|
| Patients eligible for first SC, n | 431 | 426 | |
| Patients with first SC, n (%) | 339 (78.7) | 336 (78.9) | .832 |
| Time to first SC, mo, median (IQR) | 6 (4–7) | 6 (4–8) | .006 |
| Polyp resection sites available for evaluation, n | 369 | 351 | .256 |
| Polyp resection sites identified, n (%) | 333 (90.2) | 322 (91.7) | .484 |
| Sites with biopsy or histology, n (%)a | 280 (75.9) | 256 (72.9) | .365 |
| Recurrence, n (%)a | 61 (16.5) | 61 (17.4) | .762 |
| Visible | 39 (10.6) | 50 (14.2) | .134 |
| Not visible | 22 (6.0) | 11 (3.1) | .070 |
Among all polyp sites; if restricted to those that were identified: 18.3% vs 18.9%, respectively.
Performance Characteristics
Overall performance characteristics were similar between both groups (Table 5). Most lesions were removed piecemeal, 90% with Endocut and 87% with forced coagulation. Small residual tissue islands after initial snare resection were slightly less frequent with Endocut than with forced coagulation (35% vs 41%; P = .041), yet adjunctive resection techniques to remove any residual tissue did not differ between the groups. For instance, any ablative method was used for 24% of polyps in either group.
Table 5.
Polyp Resection Performance Characteristics of Endocut and Forced Coagulation
| Outcomes: EMR at index colonoscopy | Endocut, n (%) (n = 513) | Forced coagulation, n (%) (n = 486) | P |
|---|---|---|---|
| Complete resection | 494 (96.3) | 461 (94.9) | .267 |
| Piecemeal resection | 460 (89.7) | 425 (87.4) | .270 |
| Residual tissue after EMR snare resection | |||
| >5 mm residual islands | 52 (10.1) | 54 (11.1) | .617 |
| ≤5 mm residual islands | 180 (35.1) | 201 (41.4) | .041 |
| Adjunctive treatment of residual polyp that could not be removed with a snare | 159 (31.0) | 158 (32.5) | .607 |
| Cold forceps avulsion | 74 (14.4) | 87 (17.9) | .135 |
| Any ablative treatment | 125 (24.4) | 117 (24.1) | .914 |
| Hot forceps avulsion | 65 (12.7) | 60 (12.3) | .877 |
| Soft coagulation | 14 (2.7) | 27 (5.6) | .024 |
| APC | 54 (10.5) | 41 (8.4) | .260 |
| Othera | 6 (1.2) | 2 (0.4) | .289 |
| Intraprocedural bleeding, n (%) | 89 (17.4) | 55 (11.3) | .006 |
| Treatment of intraprocedural bleedingb | .023 | ||
| Cauterizing methodc only | 72 (14.0) | 43 (8.8) | |
| Epinephrine, no clips used | 3 (0.6) | 2 (0.4) | |
| Clip | 15 (2.9) | 10 (2.1) | |
| Time of resection, min, median (IQR)d | 17 (10–28) | 18 (10–33) | .058 |
NOTE. P values in bold represent significant differences. APC, argon plasma coagulation.
EndoCut: unknown (n = 1), radiofrequency ablation (n = 1), cap/suction (n = 1), endoscopic submucosal dissection (ESD) (n = 3), forced coagulation: ESD (n = 2).
Mutually exclusive. Epinephrine may have included cautery methods, and clip treatment may also have included cautery methods or epinephrine injection.
Includes soft coagulation, coagulation grasper, hot forceps, and APC.
Resection time from start of submucosal injection to completion of the resection, which includes adjunctive treatment but not clip closure of the defect.
Intraprocedural bleeding that required treatment occurred more frequently during resection with Endocut than with forced coagulation (17% vs 11%; P = .006). In the majority, bleeding was treated by a cauterizing method, and clips were placed in only 2.9% and 2.1% in the Endocut and forced coagulation groups, respectively. The median resection time was slightly shorter in the Endocut group compared to the forced coagulation group (17 vs 18 minutes; P = .058).
Per Protocol and Subgroup Analyses
The allocated intervention was applied in 454 patients (97.8%) with 505 study polyps in the Endocut group and in 432 patients (93.1%) with 461 study polyps in the forced coagulation group. The per protocol analysis did not show any difference with respect to severe adverse events, complete resection, or rate of recurrence (Supplementary Table 1).
In additional subgroup analyses, we examined whether cautery outcomes were different in selected subgroups of patients or polyps. Periprocedural antithrombotic medications, presence of more than 1 large polyp, polyp size (<40 mm vs ≥40 mm), proximal polyp location, clipping of the mucosal defect, or prior resection attempts did not significantly affect the effect of electrosurgical setting on the occurrence of severe adverse events (Supplementary Table 2), complete resection, or recurrence (test for interaction not significant, data not shown). There was a nonsignificant tendency of a greater risk of recurrence for polyps with prior resection attempts that were removed with forced coagulation (8/39 polyps; 20.5%) compared to those removed with Endocut (7/51; 13.7%).
Discussion
This randomized trial compared 2 commonly used electrosurgical settings for polyp resection, a combination of a blended and cutting current (Endocut Q) and a pure coagulation current (forced coagulation). The study found no difference in the rate of severe adverse events, complete resection, or risk of recurrence. There were a few differences in performance characteristics with either method. Most notably, Endocut more frequently caused intraprocedural bleeding that required treatment than forced coagulation (17% vs 11%). In contrast, small residual tissue islands were more frequently described in the forced coagulation group than in the Endocut group. Furthermore, there was a slight difference in the resection time favoring Endocut. However, these procedural differences did not affect overall safety and efficacy.
In our study, Endocut more frequently resulted in intraprocedural bleeding that required treatment, in most cases with a cauterizing method, yet neither of the 2 settings showed a convincing benefit in complete polyp removal, complications, or recurrence. The greater intraprocedural bleeding risk with Endocut suggests that the blended phase between the cutting pulses was not sufficient to seal bleeding vessels. It is possible that a different mode for this phase may result in less frequent bleeding, and future investigation into different settings may be worthwhile.
The greater risk of intraprocedural bleeding with Endocut may affect the field of view during resection and raise concerns about a greater risk of recurrence, as suggested by a previous study.16 Although we did not find a difference in recurrence between the 2 groups, our study cannot completely exclude this possibility. Other factors that we did not examine may play a modifying role and conceal a potential impact of bleeding during resection and recurrence. A more detailed examination of factors associated with recurrence would be valuable.
Of note, the imbalance in baseline characteristics with respect to the presence of multiple large polyps had no effect on the main outcomes. Furthermore, in none of the subgroup analyses on antithrombotics, polyp size, proximal location, multiplicity of polyps, prior resection attempts, or clipping of the mucosal defect did we find differences in main safety or efficacy outcomes between electrosurgical settings. There was a tendency of a greater risk of recurrence for polyps with prior resection attempts that were removed with forced coagulation compared to Endocut; however, the numbers are small, and other polyp and procedure factors may confound this observation.
We observed more perforations in the Endocut group than in the forced coagulation group (6 vs 3 perforations). Although the study was underpowered to show such difference as significant, an increased risk of perforation with Endocut may be real (type 2 error). To provide proof, a far larger study would be needed, which is unlikely to happen. Endoscopists using Endocut should therefore be aware of this potential risk and ensure that no muscularis propria is entrapped in the snare before electrosurgery is applied.
Both the Endocut and forced coagulation currents were delivered using the Erbe generator. We did not consider it feasible to use more than 1 generator in the study. Erbe delivers microprocessor-controlled current that adjusts for tissue resistance. In this regard, our study does not simulate older uncontrolled comparisons of blended and forced coagulation currents in which the actual currents delivered reflect the power settings dialed into the generator by the clinician.7,8 Thus, in older generators, a power setting of 20 W will deliver 20 W regardless of tissue resistance. Given this, we cannot say based on this study that current delivered by the Erbe generator and current delivered by a fixed power generator have equal safety. In fact, in one study, the use of microprocessor-controlled electrosurgical units decreased the risk of postprocedure bleeding complications, when compared to older units,17 supporting the use of microprocessor-controlled processors for polyp resection.
In addition, the amount of thermal injury delivered to the tissue is a function of the speed of snare transection, that is, the faster the transection, the lesser the thermal injury. Speed of transection can be controlled by factors other than current type or setting. For example, closing the snare tightly before applying current will increase the current density in the snare and result in a more rapid transection and less thermal injury compared to a loose grip on the tissue with a slow mechanical closure of the snare. This aspect of thermal injury was not controlled for but could have been used by study investigators to control thermal injury with each individual polyp resection.
Although polyp resection overall is moving toward cold snare resection without using any cautery, it will likely continue to be needed for larger polyps.18–23 Although we have not included polyps <20 mm in size, it seems unlikely that the results would differ because we have not found a difference when stratified by polyp size. We also did not include pedunculated polyps. Because these polyps have a greater risk of immediate bleeding, we may infer from our study that it may be safer to apply a coagulation current with a lower risk of immediate bleeding to these polyps.
Several limitations should be noted. It is possible that a different Endocut or forced coagulation setting would yield different results. The selected settings (Endocut: effect 2, duration 1, interval 4; forced coagulation: effect 2 at 25 W) were based first on our endoscopists’ clinical practice. The survey of our study endoscopists showed that the fraction of endoscopists using the 2.1.4 setting before the study was the same as the fraction using the setting of 3.1.6. According to a recent review issued by the manufacturer, both settings are within the recommended range.24 In our in vitro testing, the thermal effects of the 2.1.4 setting and forced coagulation current seemed similar, although in clinical practice, anecdotal experience shows that Endocut at a variety of settings provides faster tissue transection generally as well as better cutting through fibrotic tissue than forced coagulation current. The observation that, in 5% of polyps randomly assigned to the forced coagulation group, the physician used Endocut for a portion of the resection is consistent with the more effective cutting properties of Endocut. We did not enforce how the Endocut current was used. When the yellow pedal is depressed, cutting current is delivered first, and some clinicians tap the yellow pedal repeatedly so that the current does not cycle into the coagulation phase. Although different current settings for Endocut or different methods of application might produce a different result, the study design did compare a current with a significant cutting component to pure forced coagulation current. A further limitation is that the results are representative of endoscopists with sufficient experience to be considered local experts, because patients were recruited through referral from other colleagues. Although all endoscopists were instructed to be the primary endoscopist to perform the resections, trainees might have participated in polyp resection, which could have affected the outcome. We also did not have data on first surveillance colonoscopy in approximately one fifth of patients, yet this proportion was similar to previous studies.16,25 Finally, the study was underpowered to draw strong conclusions on low event outcomes such as perforation or postpolypectomy syndrome.
In summary, to our knowledge, this is the first randomized study that compared 2 commonly used electrosurgical settings for the resection of large (≥20-mm) nonpedunculated colorectal polyps. Overall, polyp resection with Endocut or forced coagulation did not differ with respect to severe adverse events, complete resection rate, or polyp recurrence. This study therefore supports an individual approach based on endoscopist preference.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND AND CONTEXT
There is debate over the type of electrosurgical setting that should be used for polyp resection. Some endoscopists use a type of blended current (yellow) whereas others prefer coagulation (blue).
NEW FINDINGS
In a randomized trial to compare 2 commonly used electrosurgical settings for the resection of large colorectal polyps (Endocut vs forced coagulation), we found no difference in risk of serious adverse events, complete resection rate, or poly recurrence.
LIMITATIONS
Larger prospective studies are needed to confirm these findings.
IMPACT
Electrosurgical settings can therefore be selected based on endoscopist expertise and preference
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Funding
The study was supported by a research grant from Boston Scientific. Boston Scientific was not involved in the design of the study, its conduct, analysis or interpretation of study results. The study was further supported by a research grant from the American College of Gastroenterology.
Abbreviations used in this paper:
- CI
confidence interval
- EMR
endoscopic mucosal resection
- IQR
interquartile range
Footnotes
Supplementary Material
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.03.014.
Conflicts of interest
These authors disclose the following: Heiko Pohl has received research funding from Boston Scientific, Cosmo, and US Endoscopy. Ian Grimm is a consultant for Boston Scientific. Matthew T. Moyer is a consultant for Boston Scientific. Douglas Pleskow is a consultant for Olympus, Boston Scientific, and Medtronic. Mouen Khashab is a consultant and on the medical advisory board for Boston Scientific and Olympus and a consultant for Medtronic. Abraham Mathew is a consultant for Boston Scientific. Daniel von Renteln is supported by a Fonds de Recherche du Québec Santé career development award, has received research funding from Erbe and Pentax and is a consultant for Boston Scientific. Seth Crockett is supported in part by a grant from the National Institutes of Health (KL2TR001109) and has received research funding from Exact Sciences and ColoWrap. Amit Rastogi is a consultant for Boston Scientific and Cook Endoscopy and has received a research grant from Olympus. Maria Pellise is a consultant for Norgine Iberia. Douglas K. Rex has been a consultant for Olympus Corp, Boston Scientific, Lumendi, Endokey, GI Supply, Braintree, and Salix and has received research support from Boston Scientific, EndoChoice, EndoAid, Medtronic, and Colonary Solutions. The remaining authors disclose no conflicts.
References
- 1.Deyhle P, Seuberth K, Jenny S, et al. Endoscopic polypectomy in the proximal colon. Endoscopy 1971;103–105.
- 2.ASGE Technology Committee, Tokar JL, Barth BA, et al. Electrosurgical generators. Gastrointest Endosc 2013; 78:197–208. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy—results of the complete adenoma resection (CARE) study. Gastroenterology 2013;144:74–80. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi A, Nakayama Y, Kajiyama M, et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc 2014; 79:417–423. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Harrison M, Rex DK. A survey of colonoscopic polypectomy practices among clinical gastroenterologists. Gastrointest Endosc 2004;60:414–418. [DOI] [PubMed] [Google Scholar]
- 6.Morris ML, Tucker RD, Baron TH, et al. Electrosurgery in gastrointestinal endoscopy: principles to practice. Am J Gastroenterol 2009;104:1563–1574. [DOI] [PubMed] [Google Scholar]
- 7.Van Gossum A, Cozzoli A, Adler M, et al. Colonoscopic snare polypectomy: analysis of 1485 resections comparing two types of current. Gastrointest Endosc 1992;38:472–475. [DOI] [PubMed] [Google Scholar]
- 8.Parra-Blanco A, Kaminaga N, Kojima T, et al. Colonoscopic polypectomy with cutting current: is it safe? Gastrointest Endosc 2000;51:676–681. [DOI] [PubMed] [Google Scholar]
- 9.Pohl H, Grimm IS, Moyer MT, et al. Clip closure prevents bleeding after endoscopic resection of large colon polyps in a randomized trial. Gastroenterology 2019; 157:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronchick CA, Lipshutz WH, Wright SH, et al. Validation of an instrument to assess colon cleansing. Am J Gastroenterol 1999;94:2667. [Google Scholar]
- 11.ASGE Standards of Practice Committee, Anderson MA, Ben-Menachem T, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc 2009;70:1060–1070. [DOI] [PubMed] [Google Scholar]
- 12.ASGE Standards of Practice Committee, Acosta RD, Abraham NS, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3–16. [DOI] [PubMed] [Google Scholar]
- 13.Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol 2012; 10:969–979. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. What is a serious adverse event? 2016. Available at: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event. Accessed October 6, 2019.
- 15.Desomer L, Tutticci N, Tate DJ, et al. A standardized imaging protocol is accurate in detecting recurrence after EMR. Gastrointest Endosc 2017;85:518–526. [DOI] [PubMed] [Google Scholar]
- 16.Tate DJ, Desomer L, Klein A, et al. Adenoma recurrence after piecemeal colonic EMR is predictable: the Sydney EMR recurrence tool. Gastrointest Endosc 2017;85:647–656. [DOI] [PubMed] [Google Scholar]
- 17.Burgess NG, Metz AJ, Williams SJ, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014; 12:651–661. [DOI] [PubMed] [Google Scholar]
- 18.Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270–297. [DOI] [PubMed] [Google Scholar]
- 19.Maruoka D, Arai M, Akizue N, et al. Residual adenoma after cold snare polypectomy for small colorectal adenomas: a prospective clinical study. Endoscopy 2018; 50:693–700. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura N, Takeuchi Y, Yamashina T, et al. Incomplete resection rate of cold snare polypectomy: a prospective single-arm observational study. Endoscopy 2017; 49:251–257. [DOI] [PubMed] [Google Scholar]
- 21.Papastergiou V, Paraskeva KD, Fragaki M, et al. Cold versus hot endoscopic mucosal resection for nonpedunculated colorectal polyps sized 6–10 mm: a randomized trial. Endoscopy 2018;50:403–411. [DOI] [PubMed] [Google Scholar]
- 22.Tate DJ, Awadie H, Bahin FF, et al. Wide-field piecemeal cold snare polypectomy of large sessile serrated polyps without a submucosal injection is safe. Endoscopy 2018; 50:248–252. [DOI] [PubMed] [Google Scholar]
- 23.Thoguluva Chandrasekar V, Spadaccini M, Aziz M, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc 2019;89:929–936. [DOI] [PubMed] [Google Scholar]
- 24.Eickhoff A, Repici A, Manner H, et al. Electrosurgical pocket guide for GI interventions; 2018;2020. Available at: https://www.erbe-med.com/erbe/media/Marketingmaterialien/85800-135_ERBE_EN_Pocket_Guide_Electrosurgery_D092444.pdf. Accessed July 2020.
- 25.Knabe M, Pohl J, Gerges C, et al. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol 2014;109:183–189. [DOI] [PubMed] [Google Scholar]
- 26.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58(6 Suppl):S3–S43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


