Abstract
Abundant and well-preserved fossil microbenthos occurs in siliciclastic deposits of all Earth ages, from the early Archean to today. Studies in modern settings show how microbenthos responds to sediment dynamics by baffling and trapping, binding, biostabilization, and growth. Results of this microbial-sediment interaction are microbially induced sedimentary structures (MISS). Successful prospection for rich MISS occurrences in the terrestrial lithological record requires unraveling genesis and taphonomy of MISS, both of which are defined only by a narrow range of specific conditions. These conditions have to coincide with high detectability which is a function of outcrop quality, bedding character, and rock type. Assertions on biogenicity of MISS morphologies must be based on the presence of microbially induced sedimentary textures (MIST), which are MISS-internal textures comprising replacement minerals arranged into microscopic biological morphologies, ancient carbonaceous matter, trace fossils, and geochemical signals. MISS serve as possible templates for the decryption of ancient life-processes on Mars. This article closes with a perspective on selected deposits and ancient environments in Meridiani Planum, Gale Crater, and Jezero Crater, Mars, regarding their potential for MISS occurrences. The earlier hypothesis of structures on Mars as potentially being MISS is revised.
Key Words: MISS, Early life, Mars, Biosignature, MIST, Archean
1. Introduction
It is a common expectation that if life on Earth's neighbor planet ever existed, it must have been microbial. Historically, life exploration on other planets is rooted in the paleontological work on early microbial life chronicled in terrestrial Archean rocks. Here, pioneering studies revealed body fossils of microbial cells and filaments, stromatolites, and a wealth of chemical signals and biomarker molecules (reviews by Hickman-Lewis et al., 2018; Lepot, 2020). Naturally, proposed search strategies for extraterrestrial life are nurtured by the large data sets on these features, already tested from all angles of perspectives (Summons et al., 2011 [for MSL]; Westall et al., 2015; Vago and Westall, 2017; and Vago et al., 2017 [for ExoMars]; McMahon et al., 2018 [for Mars2020]). In contrast, Archean siliciclastic deposits have long been regarded as comparably poor in paleontological information and only more recently addressed in more detail (review by Noffke et al., 2021). In siliciclastic lithologies, microbially induced sedimentary structures (MISS) constitute one window into past life. Because clastic sediments and sedimentary rocks form a large volume of deposits on Mars, the aim of this paper is to shed light on the significance of MISS as potentially important, but until now little discussed, biosignatures. This contribution starts with a review of where Earth's exceptionally preserved MISS can be found. Then equivalent clastic deposits and paleoenvironments on Mars will be discussed with respect to their potential for hosting such valuable fossil sites.
2. Which Characteristics Are Typical for Fossil-rich Sites?
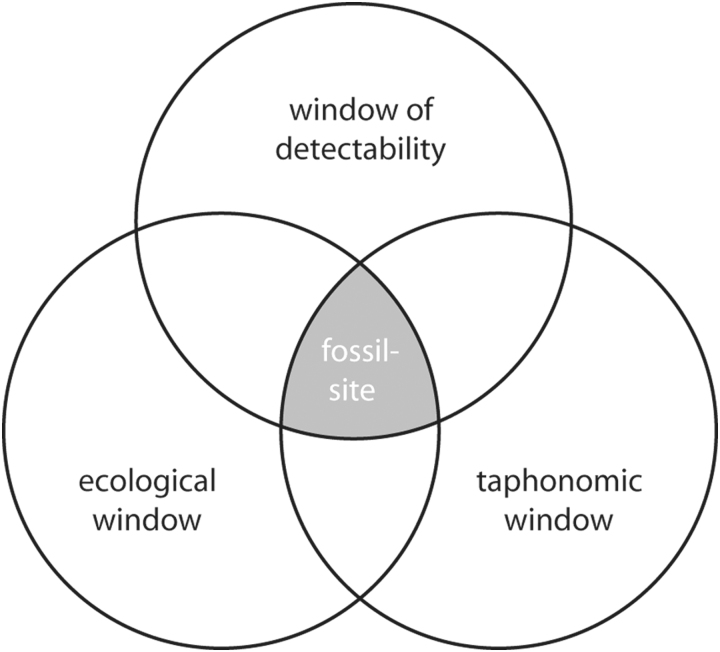
In order to find locales rich in well-preserved fossils, three aspects are of concern. First, it is necessary to understand the paleoenvironment intricately documented in rock successions (stacks of rock layers) and whether conditions for habitation have once been favorable enough to support high numbers of organisms. Second, of equal importance to habitability are the ancient conditions that eventually lead to preservation of organisms as fossils in the substrate. Ancient sedimentary conditions must have fostered transformation of organic matter to mineralic substances or the in situ recrystallization of organismic hard parts to highly resistant mineralogies. In rare circumstances, original organic material may have endured time and aggressive diagenetic alteration resulting in soft parts of ancient organisms preserved in detail. Third, the practical issue of detectability of a fossil-rich locale must be discussed. Fossils buried under a heap of debris or preserved in host rocks deep below Earth's surface may be plentiful and beautiful but—obviously—of not much use. The easiest way to explore a fossil site is to investigate in outcrop, where rock layers are widely exposed and easily visible. However, where intense chemical and physical weathering, modern or at some point in the past, has altered the rock, the quality of fossil presentation is diminished. Ideally, habitability of a paleoenvironment and a high preservation potential of the ancient sediments should overlap to produce abundant and complete fossils, and the fossil site should be easily detectable (Fig. 1; Noffke et al., 2002).
FIG. 1.
Three factors controlling the quality of fossil-rich sites. Where favorable habitability (the ecological window) and favorable preservation conditions (the taphonomic window) overlap, the potential for bountiful occurrences of fossils in a rock succession is high. Good detectability increases the value of such locales (the window of detectability).
3. Biofilms and Microbial Mats in Clastic Deposits
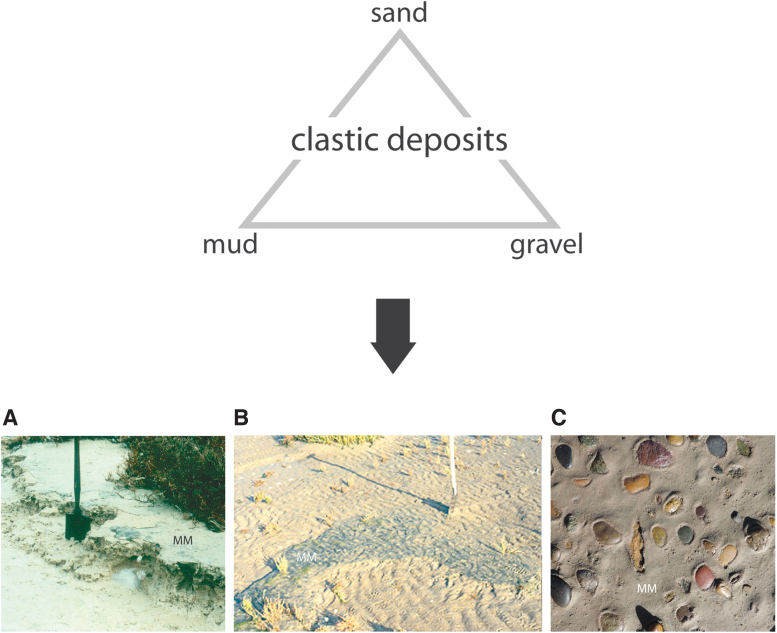
Clastic deposits are substrates composed of loose particles of mud-, silt-, sand-, and gravel-sizes (Fig. 2). Depending on climate conditions, evaporitic grains or cement may be present. On Earth, sediments also include a bulk of organisms, including microbenthos (Fig. 2). Like everywhere in natural settings, the benthic microorganisms commonly arrange into biofilms (Stoodley et al., 2002; Espinoza-Ortiz and Gerlach, 2021). These are layers of cells and the mucus, called extracellular polymeric substances (EPS; Decho and Gutierrez, 2017), that the cells secrete. At ecologically favorable locales, substantial centimeter-thick biofilms of meter-scale extensions may develop. Such biofilms are called microbial mats (Cohen and Rosenberg, 1989; Franks and Stolz, 2009). Best known examples are those mats predominantly constructed by cyanobacteria growing in coastal lagoons and on tidal flats and shelves (Hardie and Garrett, 1977; Horodyski and Bloeser, 1977; Ginsburg, 1991; Stal and Caumette, 1994; Pearl et al., 2000; Stolz, 2000; Visscher and Stolz, 2005; Noffke, 2010; Carmona et al., 2012). However, with water being the fundamental limiting requirement for life (Westall and Brack, 2018), microbial mats may develop at all sites, where this prerequisite is offered, plentiful or at least to bare minimum: playas and sabkhas, rivers and flood plains, in and around lakes, under ice, in interdune flats, and many other places (Hardie and Garrett, 1977; Horodyski and Bloeser, 1977; Pearl et al., 2000; Gallardo and Espinoza, 2007; Gerbersdorf et al., 2008). Such settings provide environmental conditions favored by microbenthos composed by cyanobacteria and numerous other microorganisms (Caumette et al., 1994).
FIG. 2.
Clastic deposits and microbenthos. Top: Clastic deposits are loose grains of mud, sand, or gravel sizes. After consolidation, they form mudstone (shale, if fissile), sandstone, and conglomerates. Bottom: Photos show microbial mats (MM) colonizing modern mud (A), sand (B), and gravel (C). Also, microbenthos, once expired, is subjected to fossilization and becomes part of the sedimentary rock.
It is readily apparent that microbenthos must compete with sediment dynamics to maintain a finely tuned and functional biofilm community. For many microbes, a muddy deposit (particles with diameters less than 0.004 mm) constitutes a difficult substrate for colonization, because accumulations of fine-grained particles (especially in the presence of clay minerals) are cohesive. Such a substrate is difficult to move through, for example by mobile cyanobacteria (Stal, 2003). More so, where fines remain suspended in the water column and block essential light from reaching the bottom, photoautotrophy as an energy-providing mechanism may fall short. That said, at sites of prolonged subaerial exposure and only low input of fine debris, cyanobacteria may form mats on top of a surface, even a muddy one. Intrasedimentary chemotrophic microbes relying on diffusion processes, however, may make use of the stability of coherent mud (Fig. 2A).
It appears that for photoautotrophic microbes, sand (particles with diameters ranging from 0.06 to 2 mm) offers the best substrate for microbial mat formation (Stal, 2003). This is the case especially where merely gentle currents and waves occur, too weak to erode sand-sized grains and to transport them as suspension load (Fig. 2B). Where in addition the sandy deposit is composed of translucent quartz grains, photosynthesis is unhampered, and growth of microbes may be supported by ample provision of nutrients through water circulating in abundant pore space and across the sedimentary surface.
Gravel (particles >2 mm) is also a difficult substrate for microbenthos: gravel accumulates where water currents are too strong for mud- or sand-sized particles to be deposited. Such turbulent water currents cause mechanical abrasion by moving pebbles, which would lead to rupture of any biofilms and mats and to dispersal of organic fragments. Only where a channel or a beach has migrated away from its original position, fine debris and clastic material may fill in the large pore space between the gravel components, providing a suitable substrate for biofilms and microbial mats to establish (Fig. 2C).
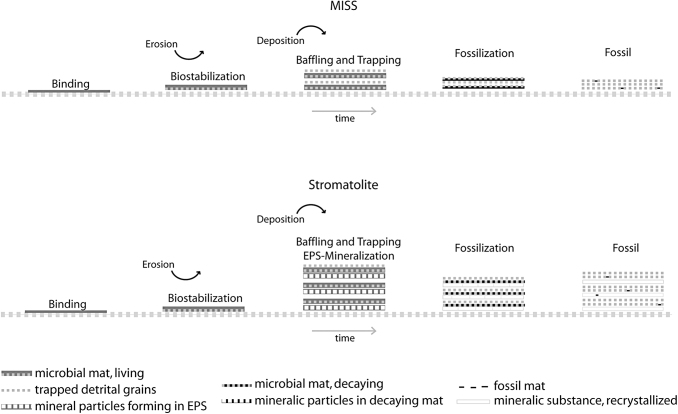
In marine settings dominated by carbonate precipitation (e.g., in the tropical climate zone), microbial mats construct massive, rigid buildups generally called microbialites, of which stromatolites are commonly more familiar (Grey and Awramik, 2020). Aside from microbial accumulation of loose grains by microbial baffling and trapping (Black, 1933), the main process leading to positive topographies of such domal microbialites is the in situ lithification of abundant EPS. In siliciclastic areas, where such EPS-lithification fades, microbial mats only construct sedimentary structures of more planar morphologies (Fig. 3).
FIG. 3.
Difference between carbonate stromatolites and clastic MISS. Top: MISS derive from binding (the formation of microbial mat fabrics), biostabilization (the fixation of sedimentary grains by the microbial mat), baffling and trapping (sediment accumulation by microbial mats), as well as subsequent diagenetic processes of lithification. Bottom: In stromatolites, the same processes occur. The one decisive difference in stromatolites is that a high amount of extracellular polymeric substances (EPS) rapidly mineralizes to carbonate, which contributes to their typical domal or columnar morphology. From: Noffke and Awramik, 2013.
Here it is mostly the mechanically complex interaction of microbes with the loose sediment that results in characteristic MISS (Noffke et al., 2001; Noffke, 2010), Figs. 4 and 5. Also, while due to the nature of their formation, the most conspicuous feature of stromatolites is their internal lamination (Grotzinger and Knoll, 1999), MISS—with few exceptions—lack thick stacks of laminae. As will be explained in more detail below, the formation of MISS can be monitored in modern aquatic settings.
FIG. 4.
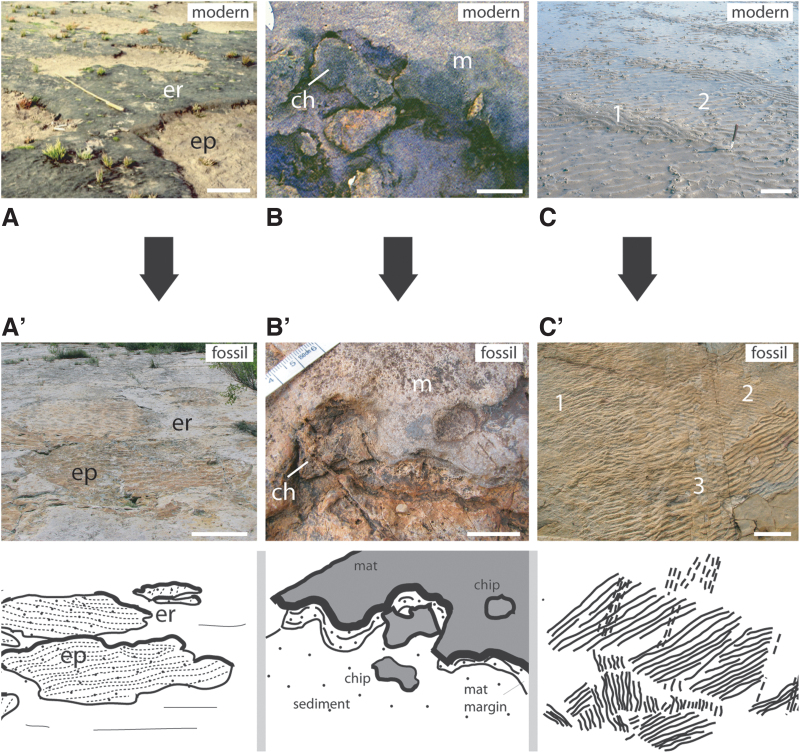
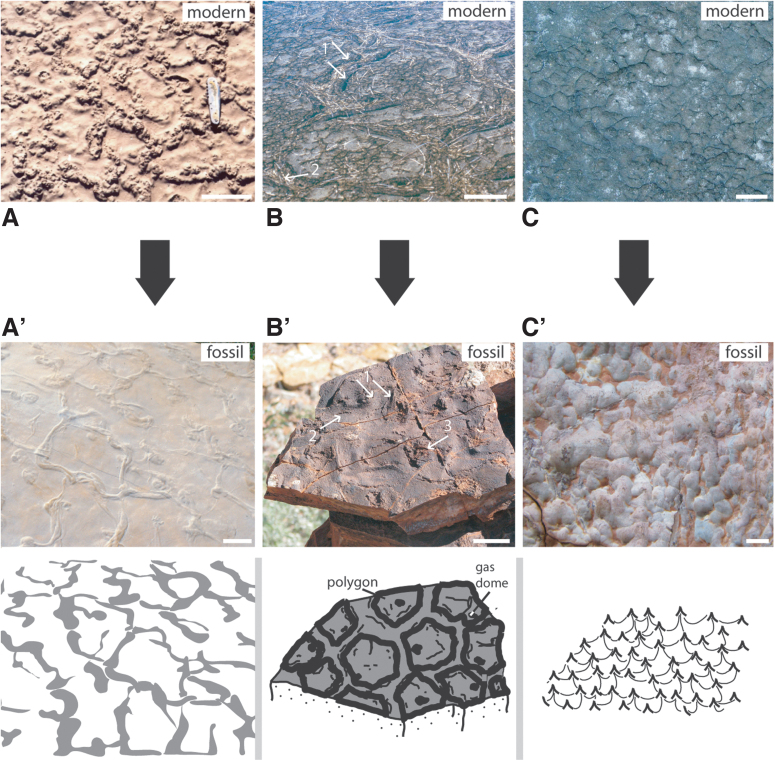
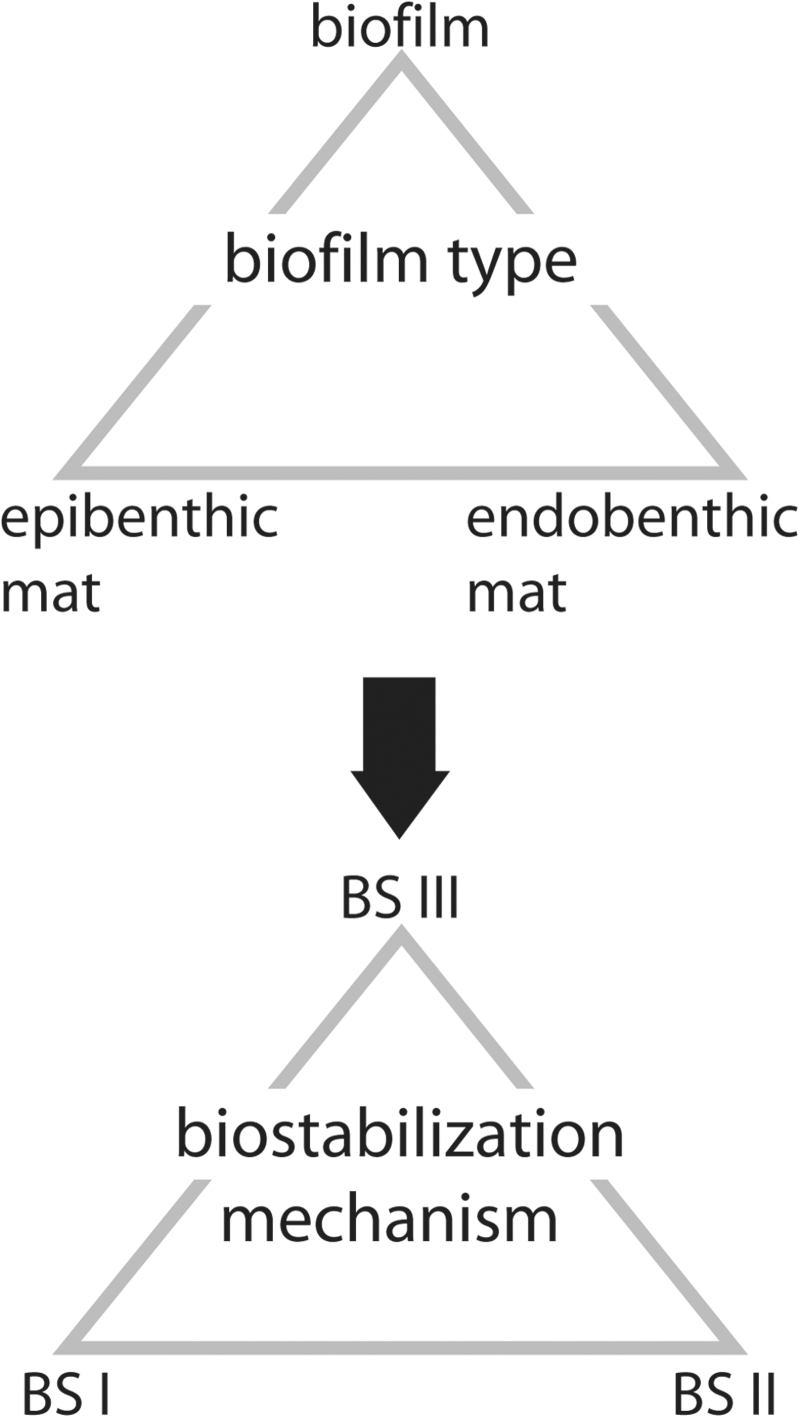
Examples for MISS formed in an environment with horizontally directed water flow (e.g., water currents crossing bottom sediments). The top row of photos (A–C) shows modern MISS; the bottom row of photos (A’–C’) shows fossil counterparts, sketched for clarity. (A/A’) Erosional remnants and pockets. The surface morphology is composed of elevated parts (erosional remnants = er; covered by sediment-stabilizing microbial mats) and of depressions (erosional pockets = ep; that show the barren substrate, often with ripple marks). Such a structure rises from partial erosion of a microbial mat–covered surface by tidal flood currents. Modern example from Mellum Island, Germany; fossil example from the Cretaceous Dakota Sandstone, Colorado, USA; scales ca. 50 cm. (B/B’) Fragments (chips = ch) were ripped off the margin (m) of a microbial mat by a strong current and immediately redeposited directly below the mat margin. Modern example from Mellum Island, Germany; fossil examples from the 3.48 Ga Dresser Formation, Pilbara, Western Australia; scales ca. 2.5 cm. (C/C’) Multidirectional ripple marks result from a succession of episodic storms causing strong currents to cross the sedimentary surface. The episodic currents interfere with continuing mat development. Such ripple mark patterns develop in course of the late summer and fall. Modern example from Mellum Island, Germany, with two ripple mark directions (1 and 2); fossil example from the 2.9 Ga Pongola Supergroup, South Africa, with three ripple mark directions (1 to 3); scales ca. 25 cm.
FIG. 5.
Examples for MISS formed in an environment with vertically oriented water flow (e.g., groundwater oscillating up and down). The top row of photos (A–C) shows modern MISS; the bottom row of photos (A’–C’) shows fossil counterparts, sketched for clarity. (A/A’) Petees developing in semiarid sabkha settings as a result of upward migrating groundwater evaporating at the sediment surface. The cauliflower-like appearance of the microbial mat is caused by evaporite crystals precipitating within the mat fabrics. Modern example from the sabkha El Bibane, Tunisia; fossil example is a cast from a surface of a Jurassic deposit in the French Alps, provided by Paul Bernier; scales ca. 10 cm. (B/B’) Polygonal oscillation cracks forming in a microbial mat in a sabkha. Modern example from the sabkha El Bibane, Tunisia; fossil example from the 3.48 Ga Dresser Formation, Pilbara, Western Australia. The cracks show two parallel rims (arrows 1). The rims are the margins of the microbial mat polygons that are defined by the cracks. The cracks themselves are overgrown by a thin microbial mat layer (arrows 2) establishing during humid weather conditions. In subsequent dry weather conditions, the polygons shrink, and the cracks open again. Repetition of growth and desiccation leads to such an oscillation of the polygons, and the bulged rims (arrows 1) form. During desiccation, the centers of the polygons may be pushed upward due to gases accumulating beneath the microbial mats. Eventually these gas domes open and collapse. Close examination of the fossil polygonal oscillation cracks in the Dresser Formation reveals a hole close to or in the centers of many polygons (arrow 3); scales ca. 10 cm. (C/C’) Reticulate pattern of ridges and tufts on a surface of a microbial mat. Modern example from Portsmouth Island, North Carolina, USA; fossil example from the 3.48 Ga Dresser Formation, Pilbara, Western Australia; scales ca. 2.5 cm.
Overburden and diagenetic alterations lead to consolidation of deposits, meaning sediment turns into sedimentary rock. Depending on the composition of a clastic parent-substrate, mudstone (called “shale” if fissile), siltstone, sandstone, or conglomerates form. Obviously, MISS including expired microbenthos are subject to lithification as well.
MISS occur in aquatic sediments and sedimentary rocks of all Earth ages including the early Archean (Schieber, 1986, 1999; Gerdes and Krumbein, 1987; contributions in Hagadorn et al., 1999; Eriksson et al., 2000; Gerdes et al., 2000; Noffke, 2000; Prave, 2002; Noffke et al., 2002, 2003, 2006a, 2006b, 2008, 2013; Pruss et al., 2006; Sarkar et al., 2006; contributions in Schieber et al., 2007; Gehling and Droser, 2009; Heubeck, 2009; contributions in Noffke, 2009; Javeaux et al, 2010; Carmona et al., 2012; Flannery and Walter, 2012; contributions in Noffke and Chafetz, 2012; Sheldon, 2012; Beraldi-Campesi, 2013; Wilmeth et al., 2014, 2019; Chu et al., 2015; Homann et al., 2015; Taher and Abdel-Motelib, 2015; Peterffy et al., 2016; Cuadrado and Pan, 2018; Homann, 2019; Maisano et al., 2019; Basilici et al., 2020; Noffke et al., 2021, and many more contributions). Despite the fact that in a geological field survey they are relatively difficult to detect (compared to stromatolites, for example), MISS appear to have a much higher abundance than such precipitated microbialites. Their ubiquitous occurrence throughout the geological record makes them promising targets for quests for ancient life in clastic successions, especially successions otherwise dreaded for their lithological monotony.
Understanding the formation of MISS provides information for assessment of the (ancient) habitability of an environment. Understanding the mode of preservation of MISS provides criteria to pinpoint occurrences of well-preserved specimens in vast outcrop. This will be explored in the following sections, starting with the formation of MISS.
4. Formation of MISS
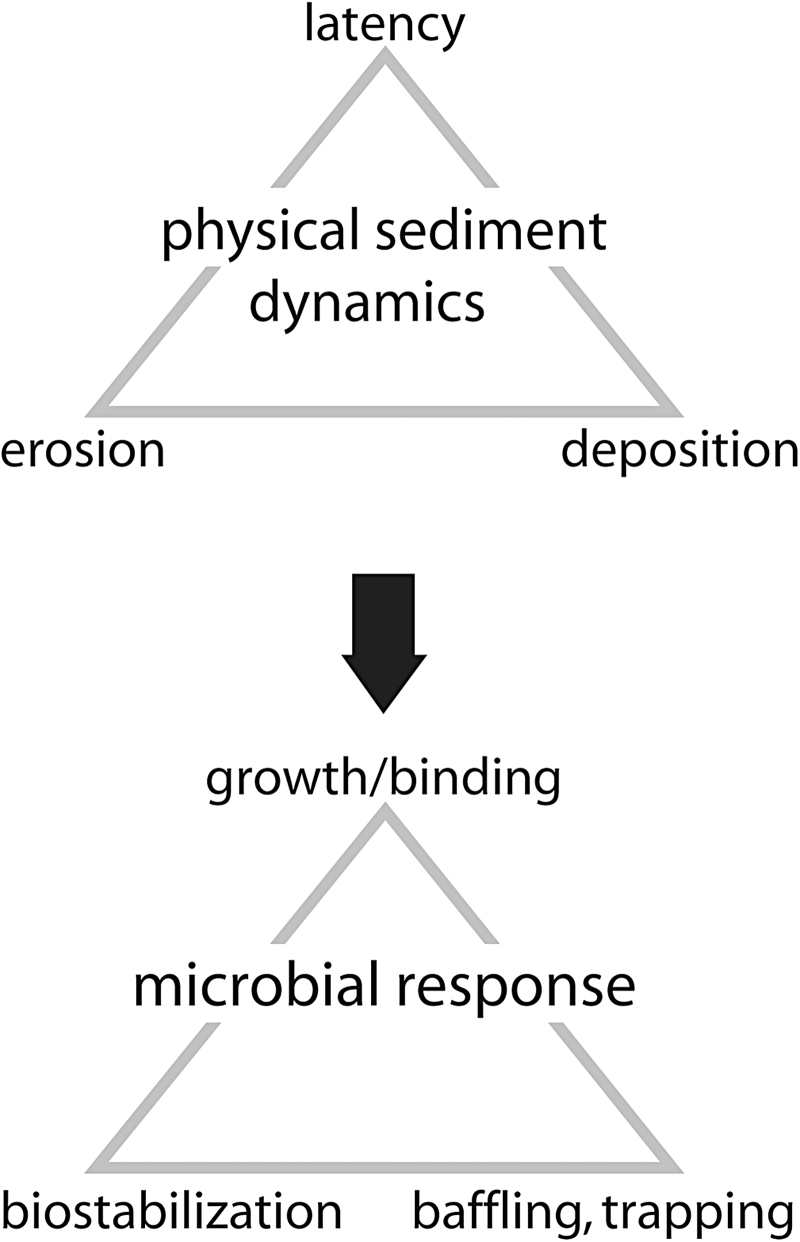
Clastic sedimentary deposits are affected by erosion and deposition (Fig. 6). Deposition is accumulation of sediment by moving water (waves and currents). In arid climates, vertically directed water movement (ascending/descending capillary groundwater) dominates and contributes to evaporite mineral precipitation at the sedimentary surface. In consequence of deposition or mineral precipitation, the sedimentary surface rises. Erosion, on the other hand, is the shear and uplift of sedimentary grains by horizontally moving water, or, in arid climates, dissolution of evaporite minerals. In consequence of erosion or dissolution, the sedimentary surface lowers over time. In between those events, there is a time period called “latency” of no effect on sediment. During latencies, the sedimentary surface is stable.
FIG. 6.
Physical sediment dynamics and microbial response. Top: Clastic deposits are governed by physical sediment dynamics. This dynamics includes erosion and deposition of sediment. Dynamic events are separated by periods of quiescence, called latencies. Bottom: The microbenthos must respond to these sediment dynamics in order to ensure survival. During latencies, biofilms and microbial mats establish by binding and growth. Biostabilization acts versus erosion. Baffling and trapping is triggered by deposition of sediment.
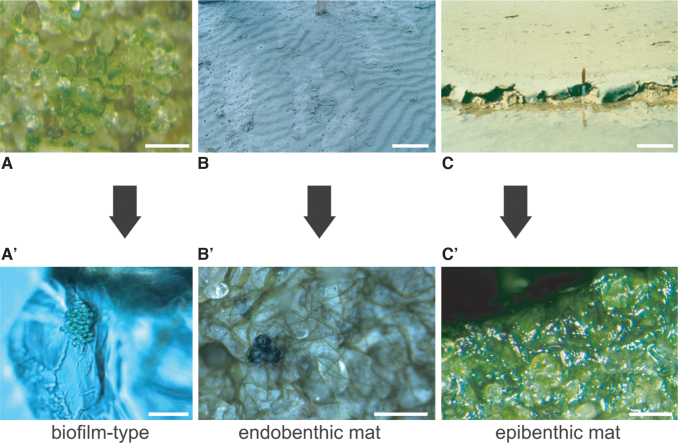
Microbial mats like those in tidal flats balance such sediment dynamics by modifying erosional and depositional effects. Indeed, they bioengineer suitable dynamic conditions (Noffke, 2010). Starting with microbial response to latencies, the time period of dynamic quiescence, a microbial mat develops by growth and/or binding. Binding is the organization of a functioning biofilm by microorganisms moving actively through the sediment and constructing a carpetlike network (Bebout and Garcia-Pichel, 1995; Shepard and Sumner, 2010). In contrast, growth includes cell replication and EPS production—the mat becomes thicker. Due to the hydrodynamic pattern being a function of geomorphology, different sites within an environmental setting have different latencies, and different types of microbial mats may develop. Independent from their community composition, the mats can be roughly divided into epibenthic (living on the substrate) and endobenthic (living in the sediment) mat types (Noffke, 2010). Biofilms, of course, are the initial stage for both (Fig. 7).
FIG. 7.
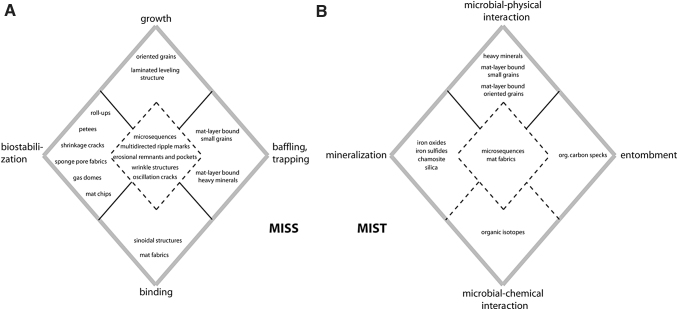
Different types of microbial colonization. Photos in the top row (A–C) show biofilm and microbial mats in macroscopic view; photos in the bottom row (A’–C’) are closeups under the microscope. (A/A’) Biofilm on quartz grains; scale in A = 1 cm, in A’ = 10 μm. (B/B’) Endobenthic microbial mat growing within the sedimentary surface—the ripple marks are well visible despite the microbial mat. Note in B’ how the filaments entangle sediment grains that due to only little EPS have grain-to-grain contact; scale in B = 15 cm, in B’ = 5 mm. (C/C’) Epibenthic microbial mat growing on top of a sedimentary surface, which due to the thickness of the mat appears planar. Grains in C’ have almost no grain-to-grain contact anymore and are embedded in thick EPS; scale in C = 20 cm, in C’ = 0.5 mm.
The microbial response to deposition of sediment is different. With increasing rate of particle fall-out, filaments orientate themselves perpendicularly to the mat surface and reach into the supernatant water. This baffling and trapping behavior reduces water velocity (Black, 1933; Noffke, 2010; Frantz et al., 2015; Noffke et al., 2021). The drop in hydrodynamic energy releases grains of smaller sizes or of heavier weights that otherwise—under the same dynamic conditions—would remain suspended in the water column. Indeed, in thin-section viewed under the microscope, many mat layers include populations of such small or heavy mineral grains.
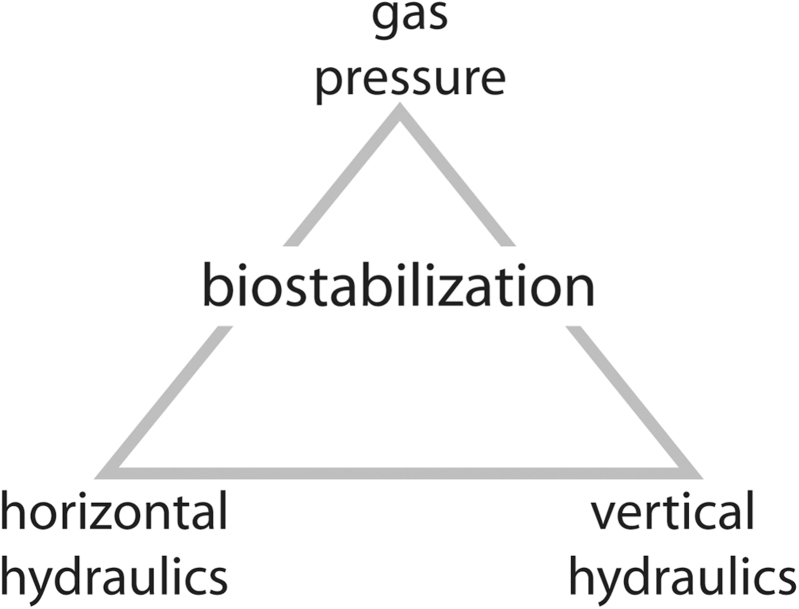
Microbes respond to erosion by biostabilization (Paterson, 1994; Amos et al., 2004; Gerbersdorf and Wieprecht, 2015). More generally, microbial biostabilization simply means sediment fixation by filaments and their adhesive mucilages (EPS). In more detail, there are three types of biostabilization, Fig. 8.
FIG. 8.
Biostabilization and the dynamic processes of the setting. First, where currents cross a mat surface (horizontal hydraulics), the microbenthos prohibits erosion of sedimentary particles. Second, where vertically oscillating capillary groundwater contributes to evaporite crystal formation in a subaerially exposed mat, the pressure caused by the growing crystals deforms the mat layer. A mat layer can also be deformed by desiccation. Third, where a mat is subaerially exposed, the EPS of the mat reduce gas exchange between sediment and atmosphere. The resulting gas pressure may deform the mat as well.
(i) A mat layer atop the sedimentary surface shelters the deposits against erosion by currents and waves. This biostabilization against erosion is three-fold (Fig. 9), in principle a function of the mat type (biofilm, endobenthic, epibenthic; Noffke, 2010). If erosion by horizontally moving water exceeds the biostabilization properties of a microbial mat, erosional remnants and pockets (Noffke, 1999), mat chips, as well as multidirectional ripple marks (Noffke, 1998) form (Fig. 4A to C). Microbial mats commonly are firmly attached to their substrates and hence in situ. Mat chips that may be ripped off can be transported over several hundred meters until they are accumulated behind current obstacles. Therefore, mat chips are the only allochthonous MISS (Figs. 4B and 13A). All other MISS are in situ. That said, mat chips may regrow onto their new substrate within hours (Fig. 13B).
FIG. 9.
Different types of microbenthos (epibenthic mat, biofilm, endobenthic mat; top diagram) respond to erosion by three different ways of biostabilization (BSI, BSII, and BSIII; bottom diagram). Epibenthic microbial mats protect their substrates by forming a smooth surface inducing non-eroding laminar flow (BSI). Endobenthic microbial mats reduce the surface roughness and with that the turbidity of the passing water current (BSII). In settings where the sediment is constantly reworked, only biofilms can form, not microbial mats. Here, biofilms attach onto grains forming biotic-mineral aggregates. In turbulent water, the aggregates stay in suspension as long as water motion continues (BSIII).
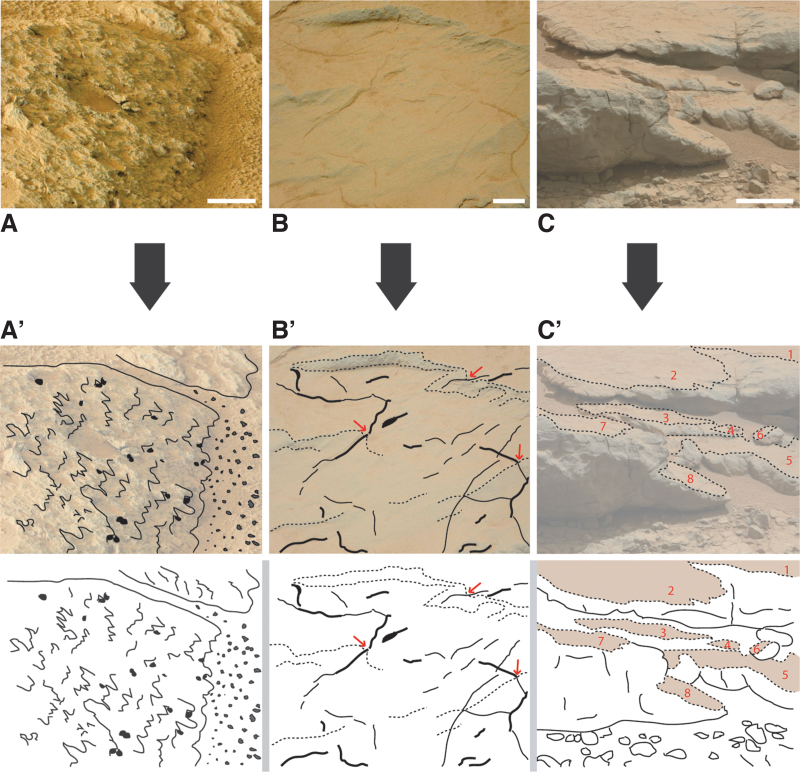
FIG. 13.
MISS of small scales. The top row of photos (A–C) shows modern MISS; the bottom row of photos (A’–C’) shows fossil counterparts, sketched for clarity. (A/A’) A microbial mat chip deposited on dry sand. Note the characteristic outline of the mat chip with lobes and embayments; a few small, round holes perforate the chip. Modern example from Portsmouth Island, North Carolina, USA; fossil example from the 3.48 Ga Dresser Formation, Pilbara, Western Australia; scales ca. 2 cm. (B/B’) Microbial mat chips growing back onto the moist surface of the parent mat. Modern example from Portsmouth Island, North Carolina, USA; fossil examples from the 3.48 Ga Dresser Formation, Pilbara, Western Australia; scales ca. 2 cm. (C/C’) Small-scale polygonal oscillation cracks. Modern example from a puddle close to Fryheid, South Africa; fossil example from the 3.2 Ga Moodies Group, South Africa; scales ca. 2 cm.
(ii) Because the otherwise loose grains of sand are fixed within the organic mat or biofilm layer, the sandy-organic substrate reacts to deformation in the same ductile manner as a cohesive mud would (Fig. 8). Desiccation of a microbial mat produces cracks defining mat polygons with upward-curled margins. In evaporitic settings of vertical groundwater oscillation, the pressure of evaporite crystals growing inside a mat causes folding of the mat and the formation of cauliflower-shaped petees, not to be confused with abiotic tepees (Fig. 5A).
(iii) Biostabilization also prohibits release of intrasedimentary gases into the atmosphere (Fig. 8). The increasing gas pressure produces high porosity in the substrate and may even locally lift the mat up (gas dome, Fig. 5B).
Important to note is that microbial mats show seasonality in their distribution. In consequence, the MISS they form may be seasonal phenomena. For example, multidirectional ripple marks (Fig. 4C) are a typical phenomenon in the moderate climate zone and can be observed at the end of the summer (Noffke, 1998). Microbial mat chips are released predominantly in the fall, when mats degrade. In a semiarid sabkha, evaporite mineral precipitation and gas dome upheaval take place during the hot and dry seasons, whereas subsequent humid conditions lead to evaporite mineral dissolution and dome collapse. Intimately connected to gas domes are polygonal oscillation cracks (Fig. 5B). Also tufts (Fig. 5C) are abundant in sabkhas and playas (Gerdes et al., 2000; Noffke, 2010; Taher, 2014; Aref and Taj, 2018).
Based on the microbial activities and their interaction, MISS were classified into five categories: structures formed by growth (category 1), biostabilization (category 2), baffling and trapping (category 3), binding (category 4), all microbial activities (category 5) (Noffke et al., 2001; Noffke, 2010; Fig. 10A).
FIG. 10.
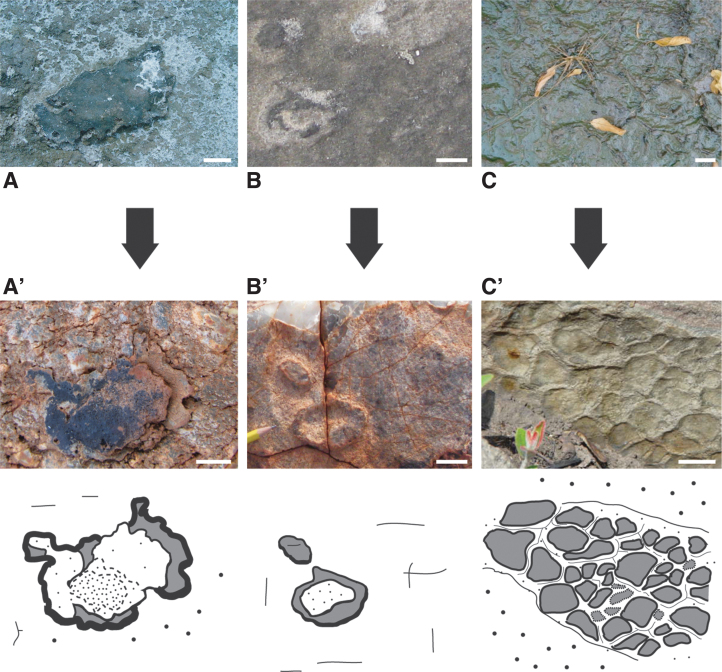
Classification of microbially induced sedimentary structures (MISS) and their daughter-group microbially induced sedimentary textures (MIST). (A) The classification of MISS. The individual structures are categorized according to their main modes of formation (growth, binding, biostabilization, baffling and trapping). The small rhombus in the center includes MISS that rise from the interaction of all four microbial activities. For practicality, the individual structures were given descriptive names. (B) The classification of MIST. In microscopic close-up view, MISS include body fossils preserved by mineral replacement, trace fossils caused by microbial interaction with sediment, as well as organic carbon preserved through rapid entombment. Organic carbon and replacement minerals are in clastic material commonly associated, but here listed separately due to their differing modes of formation and analytical exploration. Isotope signals are commonly present testifying microbial-chemical interaction. The small rhombus in the center includes MIST that include all four characteristics. The presence of MIST indicates biogenicity of MISS (Noffke, 2010). From: Noffke et al., 2021.
In the field, many MISS occur in association. For example, erosional remnants and pockets may co-occur with multidirectional ripple marks, individual gas domes, and heaps of mat chips. Also, changes in morphology across a field site are possible. One example would be erosional remnants and pockets that show a sharp-edged vertical projection in lower supratidal areas but turn to unassuming surface morphologies toward the lower intertidal zone (Noffke and Krumbein, 1999). Based on these data, occurrences and morphologies of MISS in a paleoenvironment are indeed predictable.
5. Preservation of MISS
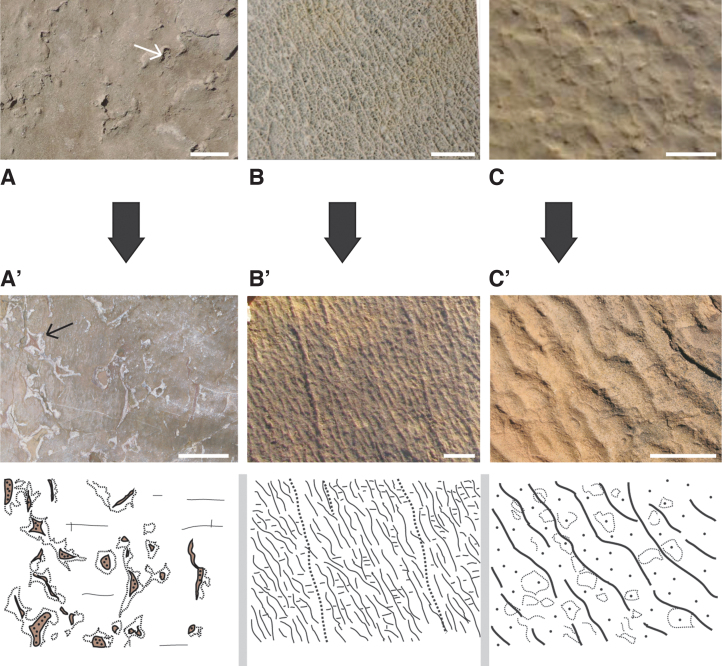
Understanding formation and distribution patterns, the next question is how MISS are preserved. Until now, MISS were described to be seemingly sole products of mechanical sediment-microbial interaction producing sedimentary structures—almost like the formation of traces in sand (trace fossils; Häntzschel, 1962). This perception of a trace fossil character, however, is not entirely correct. It is true that microscopic textures such as oriented grains (Fig. 11A), mat-layer-bound grain sizes, or accumulations of heavy minerals are traces, rising purely from sediment-organismic interaction. At microscopic scales, however, MISS include not only traces but also direct evidence of mineralized mat fabrics (Fig. 11B), and body fossils of microorganisms (Fig. 11C).
FIG. 11.
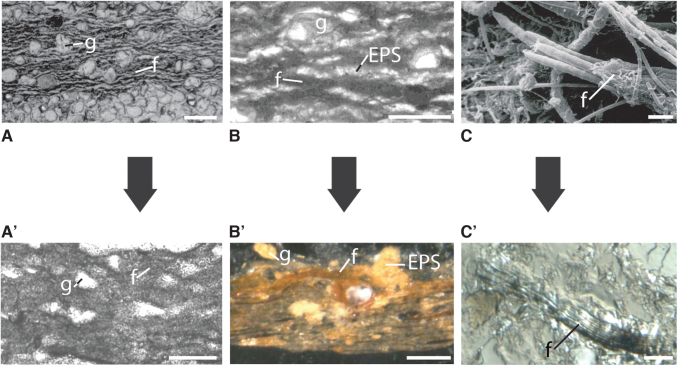
Examples for microscopic textures in Archean MISS as seen in vertical thin sections through mat layers. The top row of photos (A–C) shows modern textures; the bottom row of photos (A’–C’) shows fossil counterparts. f = filament, g = grain, EPS = extracellular polymeric substances. (A/A’) Oriented grains floating in the organic matrix of an epibenthic microbial mat. Note that the grains in the mat layer have no contact to each other; scales ca. 5 mm. (B/B’) Filaments and grains forming mat fabrics; in the fossil example (B’) EPS is replaced by chert (silica opal), filaments by goethite (weathered pyrite); scales ca. 5 mm. (C/C’) Close-ups of a filament bundle (C in scanning electron microscope; C’ in light microscope); scales ca. 20 μm.
All characteristics taken together, MISS include (i) a macroscopic sedimentary structure (already visible in the field, e.g., multidirectional ripple marks); (ii) microscopic traces (e.g., mat-layer-bound small grains); and (iii) microscopic, in situ preserved fossil biofilm. Due to this, MISS are said to have a ternary character (Noffke, 2010; Fig. 12).
FIG. 12.
The ternary character of MISS. MISS have the characteristics of macroscopic sedimentary structures, of trace (fossils) and of body fossils.
This is fundamentally different from stromatolites, where only conservatively estimated 1% of all described stromatolites show fossils of microbes (Grotzinger and Knoll, 1999). In MISS, such body fossils are preserved by various lithification processes transforming organic matter to stone. Commonly, sandy sedimentary rocks are notorious for oftentimes poor body fossil preservation. With respect to the taphonomy of MISS, however, the preservation potential of sandy sediments is improved by biofilms and microbial mats. For example, even in porous sandy substrates, the EPS produced ubiquitously by microbes reduce transfer of gases and water up to 10,000 times compared to sterile sand. In situ mineralization of organic mass of biofilms in clastic deposits is therefore common, even in modern, oxygenated settings, and delivers lithological products analytically accessible. These steps of mineralization of organic matter are well studied (Ferris et al., 1987; Schultze-Lam et al., 1996; Westall, 1999; Laflamme et al., 2011; Konhauser and Riding, 2012; Blumenberg et al., 2015; Newman et al., 2017; Gomes et al., 2020, and many more contributions) and elucidated briefly in the following. In vertical section, a microbial mat consists of a stack of layers, each layer containing specific microbes (Stal et al., 1985; Stal and Caumette, 1994; Franks and Stolz, 2009). The population of microbes of each layer interacts with the population of the layer above and below. Due to this metabolic interlocking, this stack of layers functions like a bioreactor: the top layer (usually cyanobacteria) harvests sunlight and transforms it into organic matter. Once the cyanobacteria are deceased, their organic matter is decomposed by chemoorganotrophic microbes beneath. Small biomolecules released from this process are further decomposed by chemolithoautotrophic microbes. Finally, the ions released as waste provide excellent docking sites for ions and water molecules derived from the surrounding medium. First, precipitates such as tenorite (FeS0) form. Over time, the crystallinity of the precipitates increases, and water molecules are pressed out. For example, tenorite would transform into pyrite (FeS2). In the end, the original organic matter is largely replaced by mineralic substance, the composition of which depends on the spectrum of ions provided by the surrounding medium. Also, the nature of the organic matrix, whether cell wall, EPS, or cell lumen, appears to dictate the type of minerals that are being formed (Kah and Knoll, 1996; Tice et al., 2011; Alleon et al., 2016; Hays et al., 2017; Manning-Berg and Kah, 2017; Hickman-Lewis et al., 2020). In clastic sedimentary rocks, pyrite, hematite, and chamosite may line ancient cell walls, whereas silica in the form of opal may have replaced EPS (Noffke, 2000). In sabkha or playa settings, microscopic calcite dumbbells and gypsum lenses contribute to multilayered biovarvites of decimeter thicknesses (Gerdes et al., 1985, 2000; Gerdes and Krumbein, 1987; Barbieri, et al., 2006; Kremer et al., 2008; Perri et al. 2017; Gomes et al., 2020). Gomes et al. (2020) describe that the mat architecture translates into a biased appearance of fossil mat textures that seemingly record for the presence of solely cyanobacteria (or cyanobacteria precursors). The reason is that sheaths of cyanobacteria are most resistant against degradation and remain intact while all other biological elements of a mat are erased. However, replacement of organic matter quite commonly is incomplete, and organic carbon may persist for a long time. Philippot et al. (2007), van Zuilen (2008), Chela-Flores (2019), Gomes et al. (2020), and many more discuss in detail carbon and sulfur isotope signals as relatively reliable lithological indicators echoing photoautotrophy and the metabolic activity of sulfur-reducing bacteria. In a similar vein, fossil MISS should include organic isotope signals (Noffke et al., 2003, 2006b, 2008, 2013).
Returning to the ternary character of MISS, the search for ancient life on Mars (and Earth, of course) is to a great extent both enabled and limited by the capabilities of analytical instrumentation. For practicability, the trace and body fossils within MISS are differentiated into the following subcategories: (i) the aforementioned trace fossils, (ii) mineralic congregations replacing organic material, (iii) original carbon of biofilm, and (iv) isotope signatures. Each subcategory is analytically accessible with common technologies also represented on remotely controlled rovers. To serve convenience, the four subcategories are summarized as “microbially induced sedimentary textures—MIST” (Fig. 10B; Noffke et al., 2021).
6. General Approach for the Prospection for MISS on Mars
Starting with the more familiar situation on Earth, microorganisms depend on environmental parameters such as the presence of water, the amount of suspension in the water column, water temperature, nutrient content, and vertically or horizontally directed hydrodynamics. Importantly for MISS prospection, all these parameters affect the host sediment. As a result, the characteristics of the sediment (grain size, sedimentary structures, and mineral composition) may reflect the habitability of an environment with respect to benthic microbial life. This fact becomes significant as soon as the environment passes and is translated into the rock record. After all, what remains of a paleoenvironment is the sum of all its signatures left behind in the sedimentary rock. So it is the sedimentary rock that constitutes the only source of information for the assessment of habitability of past environments. Prospection of a clastic martian paleoenvironment (visible in open landscape or translated from outcrop) can only be guided by terrestrial paleoenvironments serving as models. This may be straightforward in principle but less so in practice.
The martian rock record begins before 4 Ga, a statement that is probable though undemonstrated, because the actual age of most martian rocks remains to this date undetermined. During the Noachian age, Mars may have had an atmosphere, a hydrosphere, and warm temperatures (Carr, 1981; Malin and Edgett, 2003). Late Noachian and Hesperian landscapes are characterized by wide networks of river valleys, channels, and canyons (Grotzinger et al., 2011, 2015; Schon et al., 2012; McSween et al., 2019). However, later, with the onset of the Hesperian, Mars fell dry, temperatures sank, and life (if it ever existed) must have faded. Eventually, in the younger Amazonian age, fluid water largely disappeared with relic landscape morphologies, and deposits affected by fluid water show up rarely and only locally (McSween et al., 2019).
While it appears that modern life does not exist on Mars, the possibility of fossils preserved in martian sedimentary rocks must be taken into consideration (Carr, 1996). However, hypotheses must be carefully put forward respecting the undeniable fact that Earth and Mars are two different planets with an early planetary development only relatively similar to each other. After all, the environmental trajectories of both planets diverged more than 3.7 billion years ago. In a closer look, the late Noachian and the 3.7 to 3.0 Ga Hesperian on Mars correspond in age to the Archean on Earth. On Archean Earth, however, life was already bustling and highly diverse, producing a treasure trove of carbonaceous microfossils, stromatolites, and MISS. These structures and fossils in concert with abundant chemical signals provide a large data set for the reconnaissance of potential ancient life on Mars (reviews by Hickman-Lewis et al., 2018; Homann, 2019; Grey and Awramik, 2020; Lepot, 2020). However, with respect to detectability, the old martian rocks are surprisingly superior to their terrestrial Archean counterparts. One main reason easily overlooked is that the Noachian and early Hesperian record of Mars is by magnitudes more complete than the Archean rock record. Why is that? To step back for a moment: in simple terms, time is manifested by rocks. The more complete a rock succession is, the more pieces of the puzzle compose a picture of ancient environments. Earth history, especially the early chapter, consists of gaps caused by erosion of sediments, plate tectonic recycling, and rock-mangling metamorphic overprint. The sparse lithological script from which Archean Earth must be arduously deciphered barely compares to the substantial archive offered by the tremendous martian stratigraphy waiting to be explored. The reason is that, in contrast to Earth, Mars did not or did only to a negligible degree experience tectonic activities (Breuer and Spohn, 2003). It is even more convenient that billion-years-old martian surface is still crisply visible, and sediments and sedimentary rocks, including the oldest ones, are relatively undisturbed. This high level of preservation is due to the lack of thorough postdepositional weathering of the martian surface—much different from the gigantic conversion machine of Earth (Knoll et al., 2005). Therefore, the older martian stratigraphy offers a more complete script of a past, which, on Earth, is only testified in small fragments. Indeed, this well-preserved martian account may perhaps one day be the portrait that assists reconstructing what is missing from the Archean narrative on Earth. With some perseverance, Mars indeed offers opportunities for the curious and spirited researcher.
Likewise important for gauging the martian potential for MISS occurrence is that water-sediment mechanics and the resulting sedimentary structures on Mars are comparable with those on Earth despite the martian gravity being lower (Squyres et al., 2004; Grotzinger et al., 2005). For example, ripple marks on both Earth and Mars allow the reconstruction of horizontal water flow. These data on the background noise of the physical sedimentology of Mars allow pinpointing local derivation in morphologies from the martian norm potentially caused by life. Here, the nature of MISS plays favorably out. The only two prerequisites for MISS, from the surveying geologist's perspective, are loose clastic sediment and fluid water. Because the physical interactions between both water and sediment are well understood, any biological influence will clearly cause a morphological deviation in the structural product—a MISS-similar abnormality. More so, effects of mechanical microbial behavior onto the sediment are quite independent from the taxonomic position of the microbial group itself. Biostabilization, for instance, is a simple matter of biomass mechanically protecting the underlying substrate from erosion. The taxonomy of the microorganisms does not play a significant role for the principle of microbial sediment fixation. Indisputably, MISS morphologies potentially resulting from martian microbenthos in sand can be expected to be similar to terrestrial ones, varying merely in detail. In early Archean deposits on Earth, MISS such as erosional remnants and pockets (Fig. 4A) have been caused by already complex microbial communities (Noffke et al., 2013). On Mars, however, paleoenvironments still providing water are older than even the oldest terrestrial sedimentary rock record. It would therefore be cautious to consider the possibility that activity of emerging and just unfolding life would have been unimpressive and may not have bothered the host sediment to much degree (Westall et al., 2015). In consequence, MISS of subtle appearance including perhaps small reticulate patterns of ridges and tufts, mat chips, and polygonal oscillation cracks may have resulted (Figs. 13 and 14).
FIG. 14.
MISS of small scales. The top row of photos (A–C) shows modern MISS; the bottom row of photos (A’–C’) shows fossil counterparts, sketched for clarity. (A/A’) Cracks in the microbial mat expose the substrate beneath. Some of the mat margins along the cracks are folded back onto the mat surface. In the fossil example, the underside of the overfolded mat margins includes calcite, so the underside is showing on the rock bed surface as white starlike patterns. Modern example from Paso Seca, Argentina; fossil example from Neoproterozoic Flinders Range, South Australia; scales ca. 10 cm. (B/B’) Netlike meshwork formed by binding on the surface of a microbial mat. Modern example from Paso Seca, Argentina; fossil example from Neoproterozoic Flinders Range, South Australia; scales ca. 5 cm. (C/C’) Reticulate pattern of ridges and tufts caused by a thin biofilm on a rippled sandy surface. Modern example from the tidal flats Paso Seca, Argentina; fossil example from Neoproterozoic Flinders Range, South Australia; scales ca. 8 cm. Photos from Paso Seca provided by Diana Cuadrado.
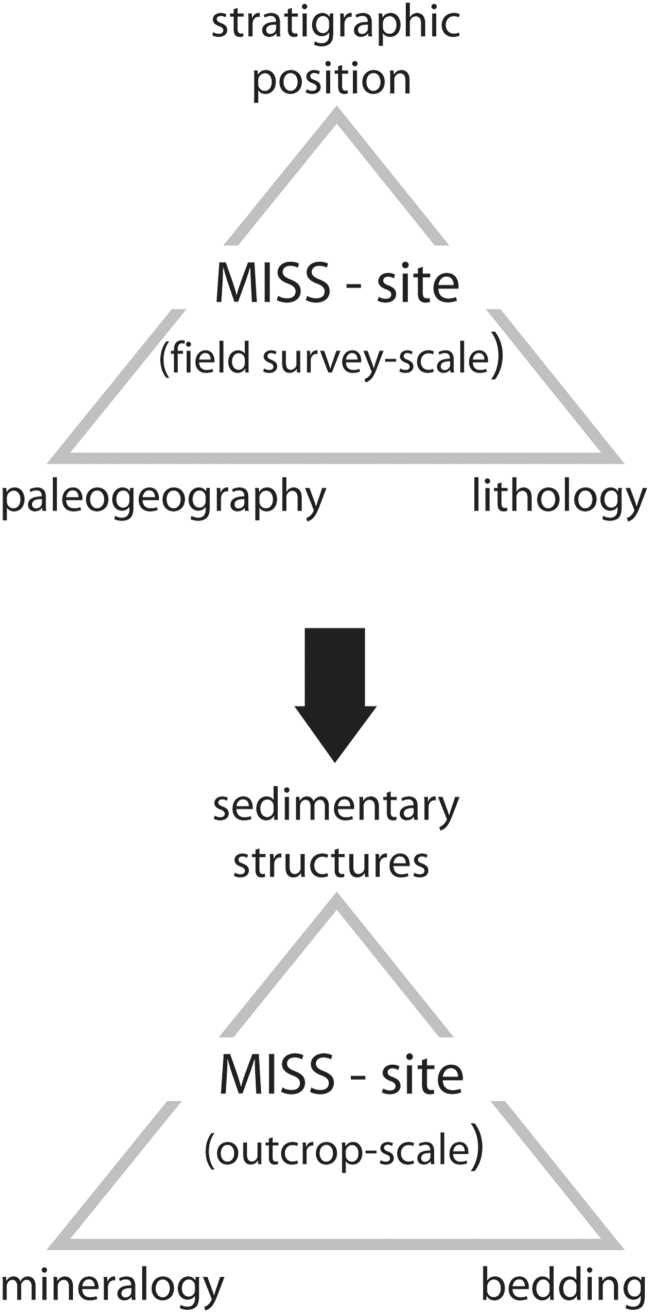
Turning to practical matters, with the endless martian landscapes spectacularly presented in rover imagery and abundant clastic rock successions readily available for sampling, the sobering question rises, where to start the search? How can the required qualities of habitability, preservation potential, and detectability of MISS occurrences be translated into a coordinated endeavor of prospection? Back on Earth, a geological field study conducted in the Neoproterozoic Nama Group, Namibia, specifically dealt with the prospection for MISS in landscape and outcrop situation (Noffke et al., 2002). The study showed that a targeted field survey should first be guided by the three factors: paleogeography, stratigraphic position, and lithology (Fig. 15).
FIG. 15. .
Geological prospection for MISS-bearing clastic deposits in the field. Top: the field survey–scale prospection for MISS considers the paleogeographic situation, the stratigraphic position, and the lithology. Bottom: in outcrop scale, a layer-by-layer analysis reveals the bedding character of rock layers (see Fig. 18), their mineralogical composition and grain size distribution, and any sedimentary structures.
This approach in explorative field survey allows converging at outcrop-scale locales of highest potential. First, a clastic paleogeography of suitable habitability includes all aquatic settings, such as oceans, lakes, rivers, and floodplains, either in landscape view or encrypted in stratigraphic rock profile. Seemingly inhospitable, dry settings in sabkhas, playas, or wind-driven dune fields may appear hopeless. However, here, capillary groundwater reliably oscillating up and down in substrates could have sustained life. The stratigraphic position of potentially MISS-harboring rock layers is the second important factor in gauging relevance. On Earth (and presumably all terrestrial planets where water bodies exist) the surface-water level in morphological basins (lakes, oceans) does not remain at a constant height. Rather, it rises and sinks slowly, back and forth, sometimes over hundreds of years' time, leaving rhythmic patterns of sediment layers behind. Receding water levels are called “regressions,” rising water levels leading to flooding “transgressions.” Fossil transgression and regressions are chronicled in outcrop by specific successions of rock beds. These rock successions include thinning-upward or thickening-upward sequences; that is, moving from base to top of the rock succession, the rock beds become either thinner or thicker (Fig. 16).
FIG. 16.
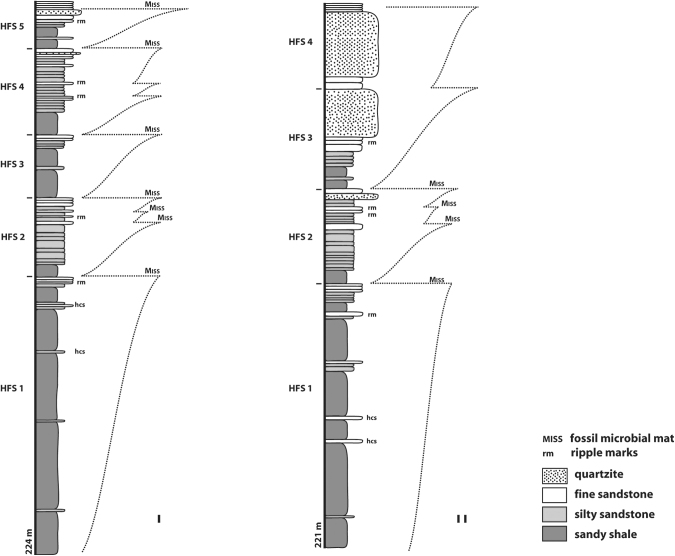
Transgression-regression cyclicity in two clastic rock successions ( = stratigraphic profiles) in the Brixton Formation, 2.9 Ga Witwatersrand Supergroup, South Africa. The lower parts of both successions are dominated by mudstone (shale). Such mudstone points toward a distal ( = far away from the shore) basin setting. Perhaps the water was also deeper. The profiles show how sandstone beds project more from the outcrop than the softer mudstone, less resistant to weathering. The sandstone beds record episodic or periodic input of coarser clastic sediment into the ancient basin. This could mean that a setting was closer to the shoreline (or closer to a local source of the coarser sediment, e.g. a delta or submarine fan). In general, MISS are absent in mudstone (shale) of a clastic succession. MISS are also largely absent in hummocky-cross-stratified sandstone beds (hcs) that chronicle a high-energy environment such as a submarine shoal bar. The two stratigraphic sections show, however, that MISS occur predominantly at transgressions. A gentle transgression causes wide-spaced shallow-water areas with only little disturbance of the proceeding substrates. Modified after Noffke et al., 2006a.
The Nama Group study and a study in the 2.9 Ga Witwatersrand Supergroup, South Africa, revealed a common concentration of MISS at transgressions (Noffke et al., 2002, 2006a). Because well-developed sequences are visible already from afar, orbital search for such rhythmic bedding and statistical analyses as described by Stack et al. (2013) may detect ancient transgressions in martian lithologies.
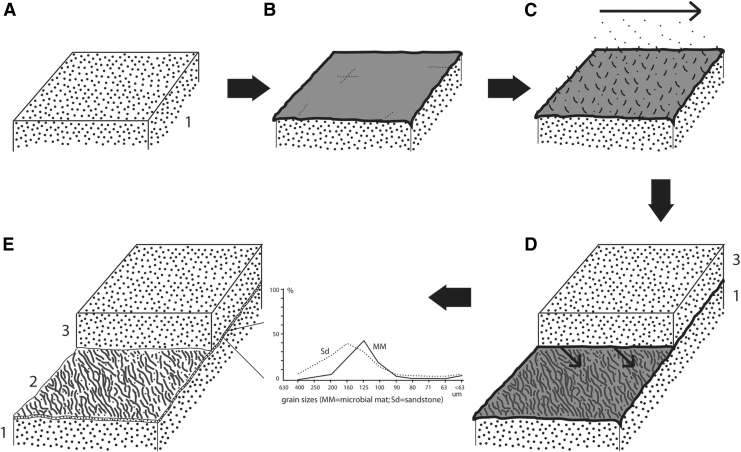
However, the Nama Group rock succession did not include MISS at each transgression branch. Further field analyses lead to define a third factor to be considered in the search for MISS: the lithological facies (Fig. 15). In outcrop scale, the lithofacies is a good indicator for hydrodynamic conditions and sedimentary properties, as outlined earlier. A lithofacies reflecting good habitability for MISS-producing microbenthos can be pinned down considering three characteristics: sedimentary structures, mineralogy of the substrate, and bedding character (Fig. 15). MISS are commonly restricted sandstone beds with medium-scale ripple marks characteristic for currents averaging between 2 and 20 cm/s velocities. These gentle currents prohibited fine-grained suspension to fall out and deposited as coherent drape on biofilms and microbial mats. On the other hand, such currents have been too weak to cause mechanical disruption of the microbenthos. In the Nama Group, translucent quartz grains of fine sand grain size have been preferred substrate for ancient photoautotrophic microbes. However, not all sandstone beds of this facies type indeed did display MISS. The reason is that while the synsedimentary conditions may have been supportive to mat development, they have not been automatically conducive to mat preservation. Indeed, while finding a paleoenvironment of higher habitability may be a good first step, it is more difficult to pinpoint potential sites of exceptional preservation. Here the bedding character (the way in which rock beds are forming a vertical succession in outcrop) plays in (Fig. 15). In the Nama Group, the layer-by-layer survey in the field revealed that MISS occur in the context of a typical succession including merely three rock beds (Fig. 17).
FIG. 17.
The taphonomic path of MISS. The taphonomic path is divided into five steps (A to E); not to scale. (A) Fine sand is deposited (layer 1). (B) A microbial mat develops. (C) Baffling and trapping accumulates silt-sized particles from suspension (layer 2 is forming). (D) The microbial mat is buried by a thick layer of sediment (layer 3). The mat itself (including its silt load) was so coherent that it was not mechanically disrupted during placement of layer 3. During deposition of layer 3, water inside the microbial mat is squeezed out, leaving irregular grooves in the microbial mat behind; layer 3 was so thick that microbes could not migrate upward anymore to recolonize the new sedimentary surface. (E) The graph on the right of the block diagram displays the sizes of baffled and trapped grains in microbial mats (MM) versus the host sediment (Sd). The fine-grained particles that were accumulated in the mat (MM) now form a miniscule layer of silt (layer 2) that separates sandstone bed 1 from sandstone bed 3. Heterotrophic microbes rapidly had replaced organic matter of biofilm by mineralic cement.
This rock succession allowed the reconstruction of the taphonomic path of microbial mats (taphonomy is the sum of all processes of fossilization). The three-layered rock succession commonly includes at its base a fine sandstone bed, often with ripple marks caused by slower-moving water. Such a sandstone bed records ancient suitable conditions for MISS-forming microbes. The subsequent layer is a millimeter-thick siltstone bed composed of particles accumulated by baffling and trapping. Finally, the top of this rock succession is a sandstone bed, commonly quite thicker than the preceding one. This three-layered rock succession records a highly specific sequence of sedimentary events that had to take place to allow preservation of MISS. One important aspect should first be explained in more detail: most MISS are surface structures, and in consequence, in order to preserve, they must not be destroyed by syndepositional erosion. What does that mean? To understand this, it is necessary to visualize a rock bed as a single rock layer that, in lateral view, includes an upper and a lower bedding plane. All sediment manifested within this one rock layer was once accumulated by a constant rate of sedimentation of material of uniform composition. A sudden change of rate or composition, or both, induced a heterogeneity within the sediment texture. This heterogeneity in the sediment texture translated with lithification of the deposit into a bedding plane seen now in outcrop. The upper bedding plane of a rock bed commonly corresponds to an original sedimentary surface (e.g., an ancient sea floor) that once existed in a paleoenvironment. However, this is not always the case. There are actually two types of upper rock bed surfaces: (i) the already-mentioned “environmental surface” that is the original surface that once existed in the paleoenvironment (such as the ancient seafloor). It is fully preserved and includes all original sedimentary structures formed in the paleoenvironment, for example ripple marks once left behind by currents. (ii) A second type of upper rock bed surface, “erosive surfaces” are surfaces where part of the original environmental surface was eroded away before solidification and preservation of the surface could set in. That means the original surface was eroded before it could make it into the rock record. Causes for such syndepositional erosion could be strong currents or winds that eroded deeply into an existing surface (such as the seafloor), or it could be erosive effects caused by abrasion during subsequent placement of sediment onto the first, original surface. Returning for a moment to the taphonomic path of MISS, syndepositional erosion by placement of layer 3 must have been lower than the biostabilization by the buried microbial mat at the top of layer 2 (Fig. 17). In sedimentology, this process of eroding into a preceding sediment surface by freshly deposited material is called “amalgamation.” It results in “amalgamated rock beds.” Such erosive, upper bedding planes contain little to no information of the paleoenvironment except that there has been high-energy hydrodynamics. How environmental and erosive surfaces present in outcrop is demonstrated in Fig. 18.
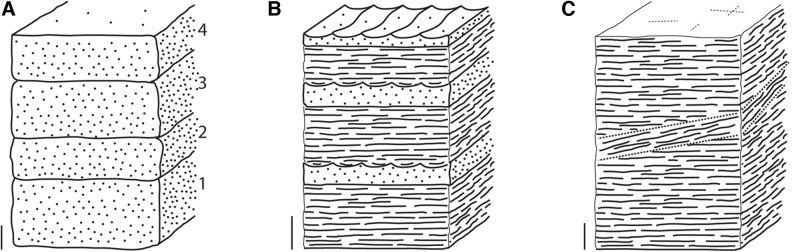
FIG. 18.
Difference between environmental surfaces and erosive surfaces of rock beds. (A) Thick sandstone bars may appear amalgamated. In formation, the original, environmental surface of the sediment was eroded away during placement of fresh sediment atop. In consequence, none of the environmental surfaces are preserved; only erosive upper sandstone bed surfaces of beds 1 to 4 exist. Such erosive surfaces do not contain any information on the ancient environment; scale ca. 25 cm. (B) Alternating bedding of mudstone units with sandstone beds. Because mud (formed in low hydraulic conditions) does not erode into a proceeding sandy surface during placement, the original environmental surface is well preserved, forming the upper bedding plane of the sandstone beds. Here, original ripple marks are still visible on each of the sandstone surfaces; scale ca. 5 cm. (C) A mudstone unit. The laminae composing the mudstone are bedding-parallel. However, tectonic effects or syndepositional settling may have overprint the original bedding planes, and fissility may be the result. Foliation may include a change in mineralogy, especially if a low-grade metamorphosis is in play. In any case, planes become visible in shale that represent tectonic shear or pressure, not the original environmental surfaces. Such secondarily derived planes are shown here for a tectonic shear zone (marked by the stippled lines); scale ca. 2 cm.
Mudstone (shale) successions, once produced in stable, low energy-settings with never-changing sediment source, may rise to monotonously laminated stacks of significant thicknesses (Fig. 18). While microbenthos may have occurred in abundance in the formerly muddy substrates, primary (environmental) surfaces are rare in the consolidated deposits. Mudstone tends to show fissility, meaning secondarily derived bed surfaces caused by settling of sediment and small-scale shear obscured or even erased the primary, environmental surfaces including potential biogenic print. Due to this reason, care must be taken when working in shale, not to confuse primary, environmental surfaces with those derived ones—a reason why the rationale for MISS prospection on Earth recommends focusing on tectonically and metamorphic largely unchallenged rock successions. In summary, stratigraphic sections of sandstone beds alternating with siltstone (or mudstone) units commonly provide best-preserved primary, environmental surfaces of type 1 (Fig. 18).
Returning one last time to the three-layered rock succession representing the taphonomic path of MISS, the sediment now forming the third layer was once so quickly deposited that microbes could not migrate upward to reach the new sedimentary surface.
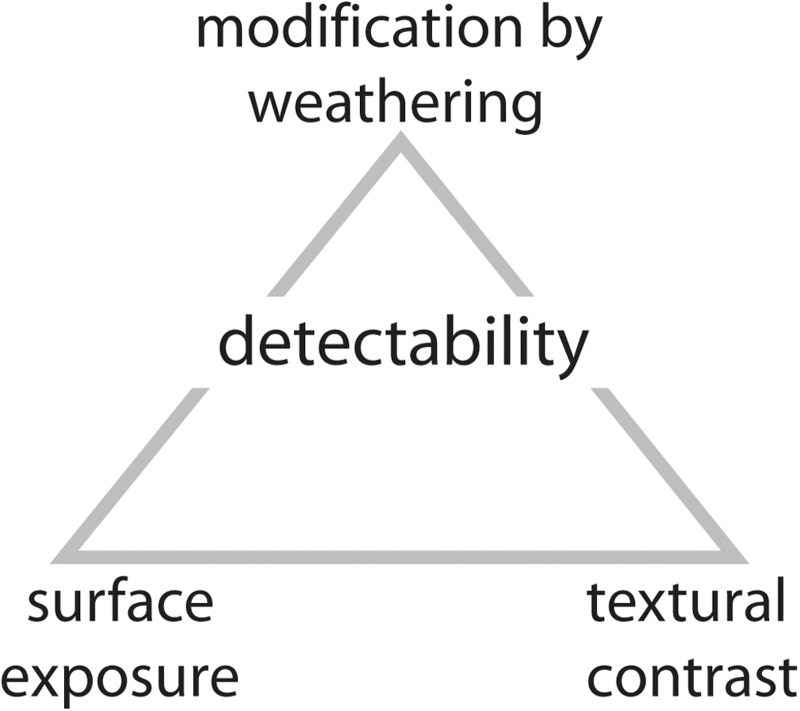
While independent from habitability and preservation potential, the aspect of detectability must be kept in mind when moving through an outcrop. Three criteria are important (Fig. 19): quality of exposure, modification by weathering, and textural contrast.
FIG. 19.
Detectability of MISS is a function of three main factors: the quality of surface exposure, the modification by weathering (destructive or exhuming), and the physical and/or chemical contrast between MISS and host deposit.
This will be elucidated in the following. (i) The quality of exposure of rock beds determines the direction the prospection takes during a visit to the locale. Be they terrestrial or extraterrestrial, outcrops occur where weathering has removed debris, such as along cliffs or channels. A firm cover of debris would frustrate visibility, let alone targeted sampling. In case of poor exposure of deposits, the problem can be circumvented (literally) by moving along the directions of strike-and-dip of candidate rock layers to seek better outcrops elsewhere. Logically, widely exposed and easily accessible rock bed surfaces facilitate field observations. (ii) Alteration of an in outcrop exposed rock surface by any type of weathering may cause dissenting interpretation. Weathering may destroy, modify, or cause morphologies on a rock bed surface, but it may also contribute to exhumation of ancient structures. Due to potential surface modification by weathering, careful judgment is needed in evaluating whether the morphology of a rock bed surface still represents the paleoenvironmentally designed one. One problem should be underscored: the widespread evidence for poor cementation of martian sandstones. Sand blasting may have obscured much of the original bedding surface morphology. Therefore, the search must focus on exposures not directly facing the brunt of main wind directions. Ideally, sedimentary structures such as ripple marks should be scouted out in order to determine with comfortable certainty the originality of a bedding surface in question (compare Fig. 18B). Illusionary similarity between ancient ripple marks in solid rock and more recent ripple marks in a loose dust cover requires additional testing. (iii) A definitive contrast between the backdrop provided by the textural qualities of the host deposit and the textural qualities of the MIST: the more MIST optically or chemically stand out from a monotonous backdrop, the better. This contrast is of significance for visibility in the consolidated rock. Biostabilization by microbenthos would not cause a well-defined texture in a muddy substrate already cohesive by nature. The difference between the cohesiveness of biofilm and the cohesiveness of the muddy substrate would not be significant enough to leave much of a signal. In consequence, the less coherent the original substrate, the more a texture once caused by biostabilization becomes visible. Due to the lower gravity on Mars, the same physical sedimentary processes affect deposits of slightly different grain sizes than on Earth (Herkenhoff et al., 2004; Grotzinger et al., 2005). For example, ripple formation is possible, even in comparison to Earth by a factor of 1.36 larger grain sizes (Grotzinger et al., 2005), allowing ripple marks to form in deposits of 1.2–1.3 mm grain sizes and finer (Lamb et al., 2012). Following the example of MISS formation on Earth, biostabilization, and for prospection necessary detectability, dictates a closer look at sedimentary rocks of such grain sizes. Textural contrast is provided especially well by a very mature substrate. Maturity of a sediment or sedimentary rock is reflected in grain size-spectrum, sphericity of the individual grains, and mineralogical composition. An example for very mature sediment would be modern beach sand. Many sandy beaches on Earth are dominantly composed of grains of fine to medium sand sizes. The sphericity of the grains is often rounded to subangular, and many beaches are composed mainly of quartz. Why is that? Originally, the grains now composing beach sand derived from high ground such as mountains and were transported toward the beach by rivers. The original rock in the mountains (to stay with this example) commonly would have a high mineralogical diversity. Granite would include micas, feldspars and quartz, gneiss, also abundant feldspar and pyroxenes. Erosion fractures this rock into boulders, and broken-off pieces are transported downslope by creeks and rivers. During transport, the grain size would decrease toward the shore, where sand sizes remain. This abrasion also causes angular grains to become more rounded. The reason that many sandy beaches are composed predominantly of quartz is that quartz is a very robust mineral that in comparison to other minerals such as feldspars or micas best withstands the abrasion during transport. More so, after deposition, beach sand is constantly reworked by waves generating the familiar, very homogeneous appearance. Due to genesis and appearance, it is said that beach sand is a “mature deposit.” Moving from inviting beach scenery back to Mars exploration, the exceptionally homogeneous composition of consolidated mature rock forms a great, monotonous backdrop against which any derivation such as a lamina of smaller grains or fossil biofilm fabrics clearly stands out. The same is true for geochemical signals that project sharply from a homogeneous background of low chemical diversity. In conclusion, the more homogeneous a substrate, the more prominent appears a textural abnormality. Such an abnormality is potentially pointing toward a biological origin if it cannot easily be explained by mere dynamic fluctuations or other abiotic processes. Biogenicity is especially suggested if there are abnormalities that are repetitive in nature and that may occur in higher numbers. Lastly, it should be remarked that core samples of sandstone, even less well cemented, are very stable, providing long-lasting encasing for far future analyses.
Returning to prospection in outcrop, thin-bedded, ripple-marked sandstones alternating with less than one millimeter-thick siltstone layers may be the most promising MISS-harboring lithologies. That said, in exception, robust biofilms definitively could have allowed the establishment of sandstone-sandstone bedding with the biofilms being the bed-separating medium (see discussion for microsequences; Noffke et al., 1997).
With those observations made on Earth, selected locales in Meridiani Planum, Gale Crater, and Jezero Crater are now discussed with respect to potential MISS occurrences.
7. Evaluation of Selected Rock Successions on Mars with Respect to Potential MISS Occurrence
Meridiani Planum is a wide plain close to the equator of Mars with a landscape morphology testifying episodic floods separated by longer-lasting subaerial exposure causing desiccation and sulfate evaporation (Herkenhoff et al., 2004; Squyres et al., 2004; Grotzinger et al., 2005; Squyres and Knoll, 2005; Hynek and Di Achille, 2017). The 22 km wide, Noachian age Endeavour Crater includes some of the older clastic sedimentary rocks and deposits of Meridiani Planum (Arvidson et al., 2014; Crumpler et al., 2015; Mittelfehldt et al., 2018). The less than 2 m thick, fine-grained and poorly bedded Matijevic Formation is a sedimentary rock of uncertain origin predating the impact that formed this crater. Upward through the stratigraphic section, the perhaps 3 m thick Shoemaker Formation is described by Mittelfehldt et al. (2018) as similar to the Bunte Brekzie (German for colorful breccia) of the Ries Crater in Germany. Accordingly, this coarse rock was probably formed during the impact that caused Endeavour Crater. The 1–2 m thick, poorly bedded Grasberg Formation may be the product of fall-out of atmospheric particles, though much more cannot be concluded (Crumpler et al., 2015). With respect to habitability, the lack of aquatic sedimentary structures such as ripple marks or cross-beds may point to absence of surface water; however, circulating intradepositional water is assumed to have been causative to the abundant sulfate-mineral filled veins (Kronyak et al., 2019a). While habitability of this lower stratigraphic section cannot be entirely excluded, the paleoenvironment and sedimentary processes may not have promoted development of a prime candidate rock for MISS search.
Toward the middle stratigraphic section, the 7 m thick, moderately rounded and well-sorted Burns Formation (early Hesperian time) becomes prevalent (Squyres et al., 2004; Grotzinger et al., 2005; Squyres and Knoll, 2005). The mafic clastic and evaporitic rocks are a “wetting-upward” sequence once formed in a cold climate (Grotzinger et al., 2005). Well displayed are the rocks for example at the ca. 150 m wide Endurance and the 20 m wide Eagle Craters. At Endurance Crater (Squyres et al., 2004; Grotzinger et al., 2005), the base of this fine- to medium-grained succession is formed by 1.50 m thick, eolian cross-beds documenting a dry dune paleoenvironment. The middle part of the succession changes into a finely laminated, well-sorted sandstone with some cross-bedding. Upward, the bedding becomes wavy with small cross-lamination—characteristic for an intermittently flooded interdune paleoenvironment that may have resembled a playa or sabkha. The interdune surface was episodically exposed subaerially and affected by the evaporite pump of ascending and descending capillary groundwater that induced tepees and salt ridges. Episodic, gentle water currents crossing the surface caused centimeter-scale ripple marks (Grotzinger et al., 2005; Knoll et al., 2005). Overall, McLennan et al. (2005) differ into four episodes of aquatic influence. On Earth, such interdune areas are preferred sites of microbial mat colonization since the Archean (Eriksson et al., 2000; Krumbein et al., 2004; Taher, 2014). In the presence of microbial mats, the formation of tepees may be modified toward more rounded, cauliflower-shaped petees. Where surface water periodically inundates a mat-overgrown area, polygonal oscillation cracks may establish, and episodic gas domes rise. Mat chips are released in high numbers during unfavorable seasons. But while ancient playa and sabkha settings on Earth would make prime candidates for MISS reconnaissance, the various sulfate minerals in the Burns Formation sandstones show that the ancient deposits here had once been saturated by toxic, acidic, and highly saline water—defining a quite hostile, though perhaps not completely life-excluding, setting (Squyres et al., 2004; Grotzinger et al., 2005; Squyres and Knoll, 2005). Grotzinger et al. (2005) discuss in detail a deflation surface in the Burns Formation. The deflation surface is in outcrop well visible; however, it merely records a time of strong abrasion of a subaerially exposed, environmental surface (Wellington Contact). Another paleosurface, the Whatanga Contact, would be quite a suitable starting point for biofilm exploration (if ancient water chemistry would have been conducive to life), because the pattern of ancient recrystallization points toward condensation of groundwater close to the surface. The observations of the two example surfaces demonstrate well how careful outcrop analyses of rock successions on Mars have to be conducted in order to pinpoint candidate layers for further analyses. Regarding the latter, freshly weathered rock beds that start to disintegrate into blocks, as it is the case in the upper unit of the Burns Formation, would offer a three-dimensional view on sedimentary structures.
Moving more upward in the Meridiani Planum stratigraphy, the 12 m studied rocks of Victoria Crater and the sedimentary section at the ca. 300 m wide Erebus Crater are considered as the younger stratigraphic continuation of the Eagle and Endurance Crater deposits. At Erebus Crater, interdune deposits including shrinkage cracks and rip-up clasts as well as small channel-fills are exposed (Grotzinger et al., 2006; Metz et al., 2009). In the interdune areas, repeated cycles of wetting and drying of well-laminated, mud-supported sandstone caused prismatic cracks to form—an ideal site for polygonal oscillation cracks to develop, would there have been biofilms. Rip-up clasts at Erebus Crater are centimeter-scale fragments broken off individual sandstone laminae. It appears that some clasts were still moist by the time of deposition and therefore could be deformed. The deposits at the ca. 750 m wide Victoria Crater seem to constitute a dry climate facies with only little aquatic influence except a possible groundwater infiltration (Squyres et al., 2009; Hayes et al., 2011). Given a choice and the ancient presence of nontoxic water, an Erebus Crater-situation would be more preferable for MISS prospection than a Victoria Crater-situation.
Shifting the focus to hematite-rich deposits as possible hosts for biological textures, the conspicuous “blueberries” (Grotzinger et al., 2005), ancient diagenetic iron oxide concretions in Meridiani Planum deposits, certainly suggest the ancient existence of an iron mineral–related taphonomic path. Pleistocene deposits from Río Tinto on Earth represent a similar martian-type acidic fluvial paleoenvironment. Interestingly, the rocks include imprints of past prokaryotic cells (Fernandez-Remolar et al., 2005), which means that despite the unfavorable low pH range of this paleoenvironment, microbial life must have been plentiful. Goethite and flaming red hematite display an abundance of microbial fossils, preserved not as organic relics but as casts and molds. In a cast and mold, the organic matter is replicated by mineralic substance and encased by host rock. Also, gas bubbles and other macroscopic features became lithified. Despite the similarity to the Río Tinto sedimentary rocks, the more sulfate-rich Meridiani deposits show that they were formed under conditions of much stronger water limitation. A study therefore suggests a hypothetical sufficient but mostly low preservation potential in these martian rocks (Sumner, 2004).
Small sinter knobs displayed at Home Plate, a pyroclastic unit in the Noachian-age Gusev Crater, are compared with terrestrial stromatolites forming close to hot springs at El Tatio in Chile (Ruff and Farmer, 2016).
The approximately 155 km wide Gale Crater (volume by Grotzinger and Milliken, 2012; Grotzinger et al., 2014, 2015; McSween et al., 2019; Rampe et al., 2020) includes sedimentary rocks of late Noachian to early Hesperian age. The crater was formed in maximum between 3.6 to 3.8 billion years ago (Thomson et al., 2011). About 400 m stratigraphic profile of the sedimentary rocks in this crater, covering between several hundred thousand to a few million years of time, has so far been investigated (Grotzinger et al., 2015; Rampe et al., 2020). In the center of this crater is a 100 km long and 5 km high mound called Aeolis Mons (casually Mount Sharp). The ca. 3.6 to 3.2 billion-year-old deposits (Mount Sharp and Bradbury Groups) were sourced from the bold topography of the crater rim and have been transported by streams downward toward the crater center, which was occupied by a paleolake (Grotzinger et al., 2014, 2015; Rampe et al., 2020). The sediments released from the streams when entering the lake formed conglomeratic to sandy deltas proximal to the lake shore, whereas the deposition of decreasing grain sizes migrated toward the lake center. Pebble sizes suggest flow velocities of 0.20–0.75 m/s in some streams (Williams et al., 2013). While streams have been competent enough for gravel transportation, fluid water was not at constant supply. It appears from the bedding character that the water level in the lake was oscillating in the course of millions of years (Grotzinger et al., 2014). The climate was generally cold with episodic warm spells. Silicate minerals pointing toward water chemistry amicable to life occur predominantly in the older parts of the Mount Sharp stratigraphy, while in the younger parts sulfate minerals indicate less favorable life conditions (Milliken et al., 2010). In detail, the Mount Sharp Group includes the >300 m thick Murray Formation at its base, which comprises laminated mudstone with few intercalated sandstone units once deposited in the center of the lake. The Murray Formation is composed of seven named members, of which the four lower ones are discussed here: Pahrump Hills, Hartmann's Valley, Karasburg, and Sutton Island. The 13–25 m thick, with 2.2 mm finely laminated, lacustrine Pahrump Hills mudstones interfinger with the deltaic clastic sediments closer to the lake shore (Grotzinger et al., 2014, 2015; Stack et al., 2019). The lake included freshwater and appears to have been a paleoenvironment with high habitability potential. Individual, intercalated sandstone beds resulting from various different water levels in the lake exhibit excellently preserved sedimentary fabrics. Bearing in mind the need for bedding plane exposures in outcrop view, upper bedding planes largely unaffected by historic weathering may be focal points for MISS prospection. Mentioning finds here facies 3, cross-stratified sandstone such as the moderately well sorted Whale Rock comprising in average 0.6 mm grain sizes (if not caused by gravity flow). Also facies 4 with its mudstone-sandstone laminae comes to mind, where in outcrop individual, isolated clasts with their lobed and embayed outlines may morphologically resemble mat chips. Alas, careful survey showed a distribution related to a laminated source rock (Minitti et al., 2019). Petees could be located inside fractures, where evaporite mineral crystals have precipitated in several pulses (Minitti et al., 2019, their Fig. 12B). Conglomerates draped by mudstone laminae occur at the base of this section, suggesting a sudden change in hydraulic energy (Grotzinger et al., 2015). The 20–25 m Hartmann's Valley succession is characterized by steep, meter-scale cross-beds perhaps of eolian nature (Fedo et al., 2018) and could have been therefore less likely substrate for biofilm attachment. Habitability, preservation potential, as well as detectability are probably much higher in the 37–40 m Karasburg Member (Fedo et al., 2018; Stein et al., 2018; Sun et al., 2019), which is quite heterogeneous with mudstone units alternating with a few centimeter thick sandstone beds. Cross-bedding and small-scale polygons occur, that together with occasional gypsum laminae point toward a setting along a lake shore with episodic subaerial exposure. On Earth, such a setting would be a preferred colonization site of abundant MISS-forming microbial mats. The same lithologies are displayed in the Sutton Island Member. The 95–98 m thick mudstone includes intercalated sandstone beds with centimeter-scale ripple-cross and cross-bed stratification and desiccation cracks (Stein et al., 2018).
Another deposit of interest could be the siliciclastic Siccar Point Group (Kronyak et al., 2019b), where groundwater migration caused polygonal fracture patterns on depositional surfaces.
The Murray Formation interfingers with the Bradbury Formation (Grotzinger et al., 2015), the latter being cross-bedded delta sediments caused by energetic streams chronicled by commensurately high amounts of sand and conglomerate. Overall the composition is basaltic with one silica-rich, felsic horizon. Lacustrine Yellowknife Bay deposits form the base, and the clinoform sandstones of the Kimberley Formation are the middle part of this succession. Located in between are the deposits of the Cooperstown and Darwin Outcrops. The habitable Yellowknife Bay succession is a coarsening-upward sequence recording the change from a lacustrine (1.50 m Sheepbed Member) to fluvial sandy environment (2 m Gillespie Lake and 1.70 m Glenelg Members) (Grotzinger et al., 2014; Edgar et al., 2017). In general, the climate has been cold and mostly arid with negligible chemical weathering. Water must have been of moderate to neutral pH, and salinity was low, conditions that would allow chemoautotrophic microorganisms to flourish (Grotzinger et al., 2014). Especially synsedimentary geomorphological depressions may have been preferred colonization sites for biofilms. Medium-grained sandstone beds of the Gillespie Lake host structures morphologically similar to MISS for which abiotic or potential biotic origins were discussed in detail (Noffke, 2015).
The Glenelg Member was studied in multiple outcrops, where several facies have been distinguished. The ca. 70–80 cm thick Shaler outcrop (Edgar et al., 2017) is transcript of a fluvial-lacustrine setting with eolian overprint. In such settings, MISS-bearing facies could be (i) laminated sandstone characterized by desiccation cracks recording intermittent wetting-drying cyclicity; (ii) sandstone beds with small-scale ripple marks and a composition of well-sorted (mature) fine sand grains; (iii) fining-upward, fluvial-eolian rock beds that show no syndepositional erosive scour at their bases; and (iv) planar laminated lacustrine deposits that had experienced desiccation-wetting cyclicity. In general, care must be taken not to interpret ductile deformation features (convolute bedding) as biogenic. In doubt, further analyses must locate MIST.
The Cooperstown Outcrop (Le Deit et al., 2016) includes a basal, cross-bedded fine sandstone, which is overlain by the massive homogeneous sandstone of the Pine Plains unit. The top forms the 20 cm thick coarse sandstone of the Rensselaer Unit that also includes pebbles. The rock succession records a fluvial setting characterized by rapidly switching, shallow, braided streams of varying flow velocities. Clearly, where small, slightly elevated surface areas are avoided by stronger currents, MISS-forming biofilms may have been able to establish.
The Kimberly Formation is a fining-upward clastic sequence of a deltaic paleoenvironment. This habitat was characterized by a very shallow fluvial system with episodic subaerial exposure and eolian influence (Grotzinger et al., 2015; Rice et al. 2017). Small lakes, possibly interconnected by the same groundwater body, existed, each lasting about 100 to 10,000 years (Grotzinger et al., 2015). The maturity of the sands increases toward the top of this unit, together with the sphericity of the particles composing the sand (Grotzinger et al., 2015; Le Deit et al., 2016; Rice et al., 2017). The Kimberley succession includes the Point Coulomb, Liga, Square Top, Dillinger, and Mount Remarkable Members. Whereas the Point Coulomb Member is a conglomerate and a less likely (though still possible) substrate for ancient biofilms, the coarse-sandy, planar-bedded, and centimeter-thick beds of the Liga Member may be more suitable. That said, the on average ca. 4.7 mm, subangular to subrounded grains are poorly sorted; hence detectability of ancient biofilm fabrics may be unfavorable. Perhaps a better lithological setting is provided by the 1 m thick Square Top sandstone with subangular to rounded grain sizes of 1–1.4 mm, though its decimeter-thick bed-sets point toward a possibly too intense hydrodynamic situation. Of specific interest could be planar laminated thinner sandstone beds within this member, as long as they were formed in calmer water. The Dillinger Member overlies the Square Top Member, forming an unconformity (a surface that witnesses a time of cessation of sediment deposition). Its millimeter-scale laminated and occasionally cross-stratified deposits have grain sizes of 0.125–0.25 mm, on Earth a perfect substrate for MISS detection. The Mount Remarkable Member is merely a massive, coarse-grained sandstone probably of similar MISS preservation potential like the Point Coulomb Member. Also, Amazonian age fluvial geomorphologies and deposits are known from Gale Crater, opening possible new opportunities for life exploration (Grant and Wilson, 2019).
The ca. 45 km wide Jezero Crater has two inlet valleys and one outlet valley recording fluvial connection to a lake that possibly persisted for many millions of years in the crater center (Fassett and Head, 2005; Schon et al., 2012; Goudge et al., 2015; Stack et al. 2020). The fluvial-lacustrine system may have been active until about 3.8 Ga, leaving behind an exceptionally well preserved sedimentary sequence of high habitability and detectability (Schon et al., 2012). Clastic clay and carbonate minerals originally deriving from Noachian altered crust are widely abundant and most likely of detrital, not authogenic origin (Ehlmann et al., 2008; Goudge et al., 2015). The paleoclimate is assumed to have been quite dry (Schon et al., 2012). Deposits that are well-sorted and finer-grained appear to be a logical choice for MISS prospection. Stable paleo-substrates that have been affected only by low sediment input and low hydraulic reworking may be promising, as long as water availability was granted by occasional flooding or groundwater infiltration. Delta and flood plain settings including scroll bars, overbank and splay deposits, as well as not too fine grained prodelta settings may be a good bet, and so are paleolake shores. Shallow, meandering channels crossing a delta plain and the levees could be locales of interest. To insert an illustrative terrestrial example, thick microbial mats once draped the slopes of tidal channels in the Cretaceous Dakota Group, Colorado, USA (Noffke et al., 2019). Based on this observation on the younger Earth, one could assume that the channel sands of stratigraphically younger delta systems in the crater (Schon et al., 2012) may provide a considerable potential as well. Neretva Vallis (and layered units therein) and Nili Planum include potentially promising areas indicated by small-scale polygonal patterns. They probably represent a dry valley once shaped by fluvial activity. As outlined earlier, rock beds that have conformal contact may constitute environmental surfaces and should be more closely investigated. Generally good starting points in the field would be areas of quick facies transitions, for example, where fluvial and lacustrine deposits interfinger. Such outcrops offer a variety of lithologies in short distance from each other. Alluvial fans with their situation in steep relief topographies and their formation by episodic and strong streams may be less promising, especially where grain sizes and mineral compositions are of detection-challenging high diversity.
8. Are Conspicuous Structures in the Gillespie Lake Member Morphological Expression of Biotic or Abiotic Processes?
Outcrop views of the Gillespie Lake Member of the Yellowknife Bay succession display structures resembling in macroscopic morphology broadly that of MISS. Both abiotic and potentially biotic causes have been discussed (Noffke, 2015). The structures occur in medium-grained sandstone deposits once deposited in a playa lake. Both this lithology of the sandstone and the paleoenvironmental placement would be conducive to ancient microbial mat colonization and preservation. The structures show associations resembling those of MISS, and as a “play of thoughts,” temporal successions recording mat development and desiccation have been reconstructed from the various surface morphologies. The hypothesis was formulated that the structures in the Gillespie Lake Member may be of biological origin, which may be verified or falsified by further analyses (Noffke, 2015). This hypothesis is here revisited briefly.
Up to this section, biological activities and syndepositional processes modulating sediment morphologies have been explained. Now the focus shifts to the illustration of sedimentary processes affecting the surface any time after consolidation of the sedimentary rock. A consolidated sedimentary surface may become exposed to the elements for a long time, and many different physical and chemical weathering processes may modulate any preexisting surface morphology. Finally, such a heavily overprint sedimentary surface exposed in an outcrop chronicles a complex temporal succession of all such weathering processes. Identifying the individual steps of sculpturing is the key to arrive at a likely interpretation of the history witnessed by this surface.
On Mars, sand-blasting by wind is a widespread phenomenon leading to typical sedimentary features. For example, in Fig. 20A, a morphological slope of perhaps a decimeter height is deeply serrated by such aerial abrasion. Holes in this rock may have been locales from which concretions or pebbles may have fallen once their host rock was removed. In the image, the coarse grains accumulated in the trench along the slope could well be such pebbles or concretions. Many of the pebbles may also represent lithoclasts (rock fragments) originally derived from the sloping rock bed itself. Using ImageJ version 1.52a, a quick assessment of sphericity of selected grains in the trench shows a variety from subangular, subrounded, to rounded types, with most of the grains being subrounded. Along a transect perpendicular to the trench, the deepest part of the trench bottom, located close to the slope, includes the smallest grains, with average sizes of estimated 0.72 cm in diameter (standard deviation [sdev] = 0.31 cm). The area in the middle of the trench appears to be occupied by grains of average 0.97 cm estimated diameters (sdev = 0.30 cm). The highest part of the trench bottom is covered by the largest grains with sizes averaging estimated 1.31 cm (sdev = 0.54 cm). It appears from these values and from the asymmetry of the transect relief that the main abrasion takes place closer to the slope—perhaps an effect of scouring.
FIG. 20.
Examples of sedimentary structures caused by weathering, Mars; MSL Curiosity imagery (mars.jpl.nasa.gov/msl/multimedia/raw). The top row of photos (A–C) shows examples of such structures; the bottom row of photos (A’–C’) shows the same photos traced for clarity, and a sketch beneath. (A/A’) Slope affected by sand-blasting; note the serration caused by abrasion. Holes in the rock may have accommodated pebbles or concretions, some of which may now be accumulated in the trench on the right. The pebbles in the trench may also constitute abraded rock fragments that over time became rounded by turbulent reworking. Note that the pebbles closer to the slope, where the trench is deepest, show smaller grain sizes and higher sphericities than those more toward the right; scale ca. 10 cm; Sol 306. (B/B’) Eroded surface cutting into a rock bed at low angle; the surface is covered by cracks probably caused by insolation. Note that some cracks continue across ledges (stippled lines), making a syndepositional origin impossible; scale ca. 5 cm; Sol 155. (C/C’) Eroded surfaces (marked by stippled lines and numbered 1–8) in the Gillespie Lake Member sandstone. Note that each surface shows similar surface morphologies. The eroded surfaces do not correspond to the original environmental surface of the rock bed. In consequence, their surface morphologies were caused by weathering long after consolidation of the sandstone bed; scale ca. 20 cm; Sol 127.
Other sedimentary surface structures displayed in outcrop include centimeter-high flat-topped elevations of a meter or two extensions (Fig. 2 in Noffke, 2015). Such table-shaped elevations may represent parts of a rock bed that everywhere else was eroded away. Wind-transported dust is accumulated along the lee-sides of such mesas, leading to the impression of irregular edges of the mesa top layer. Such accumulations of dust also form arcuate, round-crested ripples on the mesa top giving rise to an impression of a formerly ductile surface.
Centimeter-deep pits in the outcrop are local blow-outs caused by wind erosion that now are filled in in part by dust. Selective dissolution may have assisted in erasing less resistant parts of the rock surface (Grotzinger et al., 2014). One larger pit (Fig. 3 in Noffke [2015]) may have been caused by pebble impact during a storm that also ejected material onto one side. This impact must have been more recent, because it covers a crack on the surface. On one bedding surface (Fig. 5 in Noffke [2015]), pit distribution appears to have been guided by a decimeter-scale polygonal pattern of cracks covering the sandstone surface. However, the pits may well have formed before such cracks established, so an apparent relation of pits to polygons may be a mere consequence of overlapping processes.
Gypsum (a mineral that is able to store water molecules) fills in many cracks on Mars (Chavdarian and Sumner, 2006). Irregularly bended cracks at the Gillespie Lake Member outcrop may be designed by recurrent mineral injection with the resulting crystallization generations now obscuring the crack symmetry. In outcrop, cracks may occur as negative relief forming a furrow in the sedimentary surface, or as positive relief forming two parallel ridges that project slightly from the bedding surface. The cracks may transition between such negative and positive relief (Fig. 6 in Noffke [2015]). At those areas, where the mineral filling of the cracks now projects from the surface, weathering may have eroded away the originally surrounding sediment.
In general sedimentology, weathering may be so intense that original surfaces in an outcrop are deconstructed completely and new surfaces are shaped. For example, in Fig. 20B, an exposed upper bedding plane appears to be oriented in a low angle to the rock bed itself. This is an important observation, because this arrangement of the bedding plane supports its interpretation as a secondarily eroded surface caused by weathering. Indeed, close examination of this surface reveals that cracks of linear to arcuate shapes cross any proceeding surface relief, suggesting that they formed only recently. They may be the result of insolation. Insolation is a weathering process caused by the much higher periodic temperature ranges in sun-exposed surface positions in comparison to shady settings (Thomas et al., 2005; Viles et al., 2010). The different ranges in temperatures in periodically sun-exposed areas lead to tension forces within a rock that eventually are released by sudden fracturing of the rock. Insolation may have lead also to shedding of centimeter-sized splinters now ubiquitously distributed at random across bedding surfaces on Mars.
In Fig. 20C, eight surfaces are visible in outcrop. Surface 1 potentially could represent an upper bedding plane displaying an original environmental surface of the top rock bed. In contrast, surface 2 is in a low angle to the rock bed and therefore should be interpreted as weathering surface. Surfaces 3, 4, and 5 either occupy the tops of two dislocated blocks that appear to have sheared off the main rock bed above, or that represent broken-off pieces from a rock bed located stratigraphically beneath the top bed. Surface 6 belongs to an individual, dislodged bolder. The large block in the foreground including surfaces 7 and 8 may be a sheared-off block that may have once belonged to the top rock bed. In any case, it appears to be allochthonous. Of importance here is that all surfaces 1 to 8 include similar surface morphologies independently from their angles of exposure. With that, the formation of the surface relief of each of these surfaces postdates the sediment formation itself.
Closing the discussion on the nature of the Gillespie Lake Member structures, these observations support that the macroscopic surface design can be easily attributed to widespread weathering processes. As discussed earlier, environmental bedding planes must be identified as such by the presence of confirmed syndepositional structures such as ripple marks. Biogenicity of MISS is supported by presence of MIST requiring core sampling or in situ analyses (Noffke, 2010).
9. Conclusions
Returning to the motivating question how to prospect for MISS occurrences in face of the vast martian clastic landscapes and lithologies, the main points are here reiterated.
Rich occurrences of MISS are found where aquatic paleoenvironments allowed abundant population by microbenthos and their excellent preservation. Such sites must be well detectable by remotely controlled technologies.
The structures are formed by biofilms and microbial mats. The benthic communities interact with physical sediment dynamics by biostabilization, growth, binding and baffling and trapping. Such processes demand moderate sediment dynamics that is manifested in sedimentary structures visible in outcrop analyses.
In outcrop, the search focuses on a typical rock bedding character representing dynamic conditions once permitting conservation of environmental paleosurfaces. A short succession of three rock beds consisting of centimeter-thick fine sandstone, millimeter-thick siltstone, and decimeter-thick sandstone testifies a complete taphonomic path leading potentially to exquisite MISS preservation. On Earth, regression-transgression branches in stratigraphy appear to be especially promising starting points for exploration. Clearly, the detailed sedimentological survey constitutes an important part of prospection.
MISS show characteristic macroscopic morphologies that differ significantly from that of precipitated microbialites. Optical documentation in field view from many angles allows collecting morphometric data that can be stored for future use. Similarities in surface morphologies of upper bedding surfaces displayed in outcrop in differing strikes and dips should raise suspicion of weathering being the causal factor for the surface morphology.
In microscale analyses of thin sections, MISS include a wealth of trace and body fossils, replacement minerals, organic matter, and isotope signals. Such daughter features are summarized as MIST. They must be present to confirm biogenicity. MIST pose a multifaceted opportunity for a great variety of standard analyses during missions as well as on returned sample cores. They serve as indicators for ancient biological processes, especially within a backdrop of mature clastic deposits.
Acknowledgments
The author is grateful for the insight and comments provided by the two reviewers and the editor Lewis Dartnell. Linda Kah, Francis Westall, and Andrew H. Knoll are thanked for discussing processes on Mars, and Katrina Shotorban and Caldwell Buntin for technical assistance.
Abbreviations Used
- EPS
extracellular polymeric substances
- MISS
microbially induced sedimentary structures
- MIST
microbially induced sedimentary textures
Author Disclosure Statement
No competing financial interests exist.
Funding Statement
No funding to declare.
References
- Alleon J, Bernard S, Le Guillou C, et al. (2016) Early entombment within silica minimizes the molecular degradation of microorganisms during advanced diagenesis. Chem Geol 437:98–108 [Google Scholar]
- Amos CL, Bergamasco A, Umgiesser G, et al. (2004) The stability of tidal flats in Venice Lagoon—the results of in situ measurements using two benthic, annular flumes. J Mar Syst 51:211–241 [Google Scholar]
- Aref MA and Taj RJ (2018) Recent evaporite deposition associated with microbial mats, Al-Kharrar supratidal–intertidal sabkha, Rabigh area, Red Sea coastal plain of Saudi Arabia. Facies 64:1–28 [Google Scholar]
- Arvidson RE, Squyres SW, Bell JF, et al. (2014) Ancient aqueous environments at Endeavour Crater, Mars. Science 343, doi: 10.1126/science.1248097 [DOI] [PubMed] [Google Scholar]
- Barbieri R, Stivaletta N, Marinangeli L, et al. (2006) Microbial signatures in sabkha evaporite deposits of Chott el Gharsa (Tunisia) and their astrobiological implications. Planet Space Sci 54:726–736 [Google Scholar]
- Basilici G, Soares MVT, Mountney NP, et al. (2020) Microbial influence on the accumulation of Precambrian aeolian deposits (Neoproterozoic, Venkatpur Sandstone Formation, Southern India). Precambrian Res 347, doi: 10.1016/j.precamres.2020.105854 [DOI] [Google Scholar]
- Bebout BM and Garcia-Pichel F (1995) UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl Environ Microbiol 61:4215-4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraldi-Campesi H (2013) Early life on land and the first terrestrial ecosystems. Ecol Process 2:1–17 [Google Scholar]
- Black M (1993) The precipitation of calcium carbonate on the Great Bahama Bank. Geological Magazine 70:455–466 [Google Scholar]
- Blumenberg M, Thiel V, and Reitner J (2015) Organic matter preservation in the carbonate matrix of a recent microbial mat—is there a ‘mat seal effect’? Org Geochem 87:25–34 [Google Scholar]
- Breuer D and Spohn T (2003) Early plate tectonics versus single-plate tectonics on Mars: evidence from magnetic field history and crust evolution. J Geophys Res Planets 108:5072–5085 [Google Scholar]
- Carmona NB, Ponce JJ, Wetzel A, et al. (2012) Microbially induced sedimentary structures in Neogene tidal flats from Argentina: paleoenvironmental, stratigraphic and taphonomic implications. Palaeogeogr Palaeoclimatol Palaeoecol 9:353–355 [Google Scholar]
- Carr M (1981) The Surface of Mars. Yale University Press, New Haven, CT [Google Scholar]
- Carr M (1996) Water on Mars. Oxford University Press, New York [Google Scholar]
- Caumette P, Matheron R, Raymond N, et al. (1994) Microbial mats in the hypersaline ponds of Mediterranean salterns (Salins-de-Giraud, France). FEMS Microbiol Ecol 13:273–286 [Google Scholar]
- Chavdarian GV and Sumner D (2006) Cracks and fins in sulfate sand: evidence for recent mineral-atmospheric water cycling in Meridiani Planum outcrops? Geology 34:229–232 [Google Scholar]
- Chela-Flores J (2019) Testing S isotopes as biomarkers for Mars. Int J Astrobiol 18:436–439 [Google Scholar]
- Chu D, Tong J, Song H, et al. (2015) Early Triassic wrinkle structures on land: stressed environments and oases for life. Sci Rep 5:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y and Rosenberg E, eds. (1989) Microbial mats: physiological ecology of benthic microbial communities. American Society for Microbiology, Washington, DC [Google Scholar]
- Crumpler LS, Arvidson RE, Bell J, et al. (2015) Context of ancient aqueous environments on Mars from in situ geologic mapping at Endeavour Crater. J Geophys Res Planets 120:538–569 [Google Scholar]
- Cuadrado DG and Pan J (2018) Field observations on the evolution of reticulate patterns in microbial mats in a modern siliciclastic coastal environment. J Sediment Res 88:24–37 [Google Scholar]
- Decho AW and Gutierrez T (2017) microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol 8:1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar LA, Gupta S, Rubin DM, et al. (2017) Shaler: in situ analysis of a fluvial sedimentary deposit on Mars. Sedimentology 65:96–122 [Google Scholar]
- Ehlmann BL, Mustard JF, Fassett CI, et al. (2008) Clay minerals in delta deposits and organic preservation potential on Mars. Nat Geosci 1:355–358 [Google Scholar]
- Eriksson PG, Simpson EL, Eriksson KA, et al. (2000) Muddy roll-up structures in siliciclastic interdune beds of the c. 1.8 Ga Waterberg Group, South Africa. Palaios 15:177–183 [Google Scholar]
- Espinoza-Ortiz EJ and Gerlach R (2021) Biofilms. In Treatise of Invertebrate Paleontology. edited by N Noffke (in press) [Google Scholar]
- Fassett CI and Head JW III (2005) Fluvial sedimentary deposits on Mars: Ancient deltas in a crater lake in the Nili Fossae region. Geophys Res Lett 32, doi: 10.1029/2005GL023456 [DOI] [Google Scholar]
- Fedo C, Grotzinger J, Gupta S, et al. (2018) Sedimentology and stratigraphy of the Murray Formation, Gale crater, Mars [abstract 2078]. In 49th Lunar and Planetary Science Conference Abstracts, Lunar and Planetary Institute Houston [Google Scholar]
- Fernandez-Remolar DC, Morris RV, Gruener JE, et al. (2005) The Río Tinto Basin, Spain: mineralogy, sedimentary geobiology, and implications for interpretation of outcrop rocks at Meridiani Planum, Mars. Earth Planet Sci Lett 240:149–167 [Google Scholar]
- Ferris FG, Fyfe WS, and Beveridge TJ (1987) Bacteria as nucleation sites for authigenic minerals in a metal-contaminated lake sediment. Chem Geol 63:225–232 [Google Scholar]
- Flannery DT and Walter MR (2012) Archean tufted microbial mats and the Great Oxidation Event: new insights into an ancient problem. Australian Journal of Earth Sciences 59:1–11 [Google Scholar]
- Franks J and Stolz JF (2009) Flat laminated microbial mat communities. Earth-Science Reviews 96:163–172 [Google Scholar]
- Frantz CM, Petryshyn VA, and Corsetti FA (2015) Grain trapping by filamentous cyanobacterial and algal mats: implications for stromatolite microfabrics through time. Geobiology 13:409–423 [DOI] [PubMed] [Google Scholar]
- Gallardo VA and Espinoza C (2007) New communities of large filamentous sulfur bacteria in the eastern South Pacific. Int Microbiol 10:97–102 [PubMed] [Google Scholar]
- Gehling JG and Droser ML (2009) Textured organic surfaces associated with the Ediacara biota in South Australia. Earth-Science Reviews 96:196–206 [Google Scholar]
- Gerbersdorf SU and Wieprecht S (2015) Biostabilization of cohesive sediments: revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture. Geobiology 13:68–97 [DOI] [PubMed] [Google Scholar]
- Gerbersdorf SU, Jancke T, Westrich B, et al. (2008) Microbial stabilization of riverine sediments by extracellular polymeric substances. Geobiology 6:57–69 [DOI] [PubMed] [Google Scholar]
- Gerdes G and Krumbein WE (1987) Biolaminated Deposits. Springer, Heidelberg [Google Scholar]
- Gerdes G, Krumbein WE, and Reineck HE (1985) The depositional record of sandy, versicolored tidal flats (Mellum Island, southern North Sea). Journal of Sedimentary Research 55:265–278 [Google Scholar]
- Gerdes G, Klenke T, and Noffke N (2000) Microbial signatures in peritidal siliciclastic sediments: a catalogue. Sedimentology 47:279–308 [Google Scholar]
- Ginsburg RN (1991) Controversies about stromatolites: vices and virtues. In Controversies in Modern Geology, edited by DW Müller, JA McKenzie and H Weisserts, Academic Press, London, pp 25–36 [Google Scholar]
- Gomes ML, Riedman LA, O'Reilly S, et al. (2020) Taphonomy of biosignatures in microbial mats on Little Ambergris Cay, Turks and Caicos Islands. Front Earth Sci 8, doi: 10.3389/feart.2020.576712 [DOI] [Google Scholar]
- Goudge TA, Mustard JF, Head JW, et al. (2015) Assessing the mineralogy of the watershed and fan deposits of the Jezero crater paleolake system, Mars. J Geophys Res Planets 120:775–808 [Google Scholar]
- Grant JA and Wilson SA (2019) Evidence for late alluvial activity in Gale crater, Mars. Geophys Res Lett 46:7287–7294 [Google Scholar]
- Grey K and Awramik S (2020) Handbook for the Study and Description Of Microbialites. GSWA Bulletin 147, Geological Survey of Western Australia, East Perth, Australia [Google Scholar]
- Grotzinger JP and Knoll AH (1999) Stromatolites in Precambrian carbonates: evolutionary mileposts or environmental dipsticks? Annu Rev Earth Planet Sci 27:313–358 [DOI] [PubMed] [Google Scholar]
- Grotzinger JP and Milliken RE, eds. (2012) Sedimentary Geology of Mars. SEPM Special Publication 102, Society for Sedimentary Geology, Tulsa, OK [Google Scholar]
- Grotzinger JP, Arvidson RE, Bell JF, et al. (2005) Stratigraphy and sedimentology of a dry to wet eolian depositional system, Burns Formation, Meridiani Planum, Mars. Earth Planet Sci Lett 240:11–72 [Google Scholar]
- Grotzinger J, Bell J III, Herkenhoff K, et al. (2006) Sedimentary textures formed by aqueous processes, Erebus crater, Meridiani Planum, Mars. Geology 34:1085–1088 [Google Scholar]
- Grotzinger J, Beaty D, Dromart G, et al. (2011) Mars sedimentary geology: key concepts and outstanding questions. Astrobiology 11:77–87 [DOI] [PubMed] [Google Scholar]
- Grotzinger JP, Sumner DY, Kah LC, MSL Science Team (2014) A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1242777 [DOI] [PubMed] [Google Scholar]
- Grotzinger J, Gupta S, Malin M, et al. (2015) Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science 350:78–103 [DOI] [PubMed] [Google Scholar]
- Hagadorn JW, Pflüger F, Bottjer DJ, eds. (1999) Unexplored microbial worlds [theme issue]. Palaios vol. 14 [Google Scholar]
- Häntzschel W (1962) Trace fossils and problematica. In Treatise on Invertebrate Paleontology, Part W, Miscellanea, edited by RC Moore, RA Robison, DL Clark, et al., Geological Society of America, Boulder, CO, and University of Kansas Press, Lawrence, KS, pp 177–249 [Google Scholar]
- Hardie L and Garrett P (1977) Sedimentation on the modern carbonate tidal flats of northwest Andros Island, Bahamas. Johns Hopkins University Press, Baltimore, MD, pp 1–2 [Google Scholar]
- Hayes A, Grotzinger J, Edgar L, et al. (2011) Reconstruction of eolian bed forms and paleocurrents from cross-bedded strata at Victoria Crater, Meridiani Planum, Mars. J Geophys Res Planets 116:1–17 [Google Scholar]
- Hays LE, Graham HV, Des Marais DJ, et al. (2017) Biosignature preservation and detection in Mars analog environments. Astrobiology 17:363–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenhoff KE, Squyres S, Arvidson R, et al. (2004) Evidence from Opportunity's microscopic imager for water on Meridiani Planum. Science 306:1727–1730 [DOI] [PubMed] [Google Scholar]
- Heubeck C (2009) An early ecosystem of Archean tidal microbial mats (Moodies Group, South Africa, ca. 3.2 Ga). Geology 37:931–934 [Google Scholar]
- Hickman-Lewis K, Cavalazzi B, Foucher F, et al. (2018) Most ancient evidence for life in the Barberton greenstone belt: microbial mats and biofabrics of the ∼3.47 Ga Middle Marker horizon. Precambrian Res 312:45–67 [Google Scholar]
- Hickman-Lewis K, Cavalazzi B, Sorieul S, et al. (2020) Metallomics in deep time and the influence of ocean chemistry on the metabolic landscapes of Earth's earliest ecosystems. Sci Rep 10, doi: 10.1038/s41598-020-61774-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M (2019) Earliest life on Earth: evidence from the Barberton Greenstone Belt, South Africa. Earth-Science Reviews 196, doi: 10.1016/j.earscirev.2019.102888 [DOI] [Google Scholar]
- Homann M, Heubeck C, Airo A, et al. (2015) Morphological adaptations of 3.22 Ga-old tufted microbial mats to Archean coastal habitats (Moodies Group, Barberton Greenstone Belt, South Africa). Precambrian Res 266:47–64 [Google Scholar]
- Horodyski RJ and Bloeser B (1977) Laminated algal mats from a coastal lagoon, Laguna Mormona, Baja California, Mexico. Journal of Sedimentary Research 47:680–696 [Google Scholar]
- Hynek BM and Di Achille G (2017) Geologic map of Meridiani Planum, Mars. Scientific Investigations Map 3356, U.S. Geological Survey, Reston, VA, doi:10.3133/sim; 3356 [Google Scholar]
- Javeaux E, Marshall CP, and Bekker A (2010) Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 463:72–83 [DOI] [PubMed] [Google Scholar]
- Kah LC and Knoll AH (1996) Microbenthic distribution of Proterozoic tidal flats: environmental and taphonomic considerations. Geology 24:79–82 [DOI] [PubMed] [Google Scholar]
- Knoll AH, Carr M, Clark B, et al. (2005) An astrobiological perspective on Meridiani Planum. Earth Planet Sci Lett 240:179–189 [Google Scholar]
- Konhauser K and Riding R (2012) Bacterial biomineralization. In Fundamentals of Geobiology, edited by AH Knoll, DE Canfield, and KO Konhauser, Wiley-Blackwell, Chichester, UK, pp 105–130 [Google Scholar]
- Kremer B, Kazmierczak J, and Stal L (2008) Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North Sea. Geobiology 6:46–56 [DOI] [PubMed] [Google Scholar]
- Kronyak RE, Kah LC, Edgett KS, et al. (2019a) Mineral-filled fractures as indicators of multigenerational fluid flow in the Pahrump Hills Member of the Murray Formation, Gale Crater, Mars. Earth Space Sci 6:238–265 [Google Scholar]
- Kronyak R, Kah L, Miklusicak N, et al. (2019b) Extensive polygonal fracture network in Siccar Point group strata: fracture mechanisms and implications for fluid circulation in Gale Crater, Mars. J Geophys Res Planets 124:2613–2634 [Google Scholar]
- Krumbein EW, Gorbushina AA, and Holtkamp-Tacken E (2004) Hypersaline microbial systems of sabkhas: examples of life's survival in “extreme” conditions. Astrobiology 4:450–459 [DOI] [PubMed] [Google Scholar]
- Laflamme M, Schiffbauer JD, Narbonne GM, et al. (2011) Involvement of microbial mats in early fossilization by decay delay and formation of impressions and replicas of vertebrates and invertebrates. Lethaia 44:203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MP, Grotzinger JP, Southard JB, et al. (2012) Were aqueous ripples on Mars formed by flowing brines? In Sedimentary Geology of Mars, edited by JP Grotzinger and RE Milliken, SEPM Special Publication 102, Society for Sedimentary Geology, Tulsa, OK, pp 139–150 [Google Scholar]
- Le Deit L, Mangold N, Forni O, et al. (2016) The potassic sedimentary rocks in Gale Crater, Mars, as seen by ChemCam on board Curiosity. J Geophys Res Planets 121:784–804 [Google Scholar]
- Lepot K (2020) Signatures of early microbial life from the Archean (4 to 2.5 Ga) eon. Earth-Science Reviews 209, doi: 10.1016/j.earscirev.2020.103296 [DOI] [Google Scholar]
- Maisano L, Cuadrado DG, and Gomez EA (2019) processes of MISS-formation in a modern siliciclastic tidal flat, Patagonia (Argentinia). Sedimentary Geology 381:1–12 [Google Scholar]
- Malin MC and Edgett KS (2003) Evidence for persistent flow and aqueous sedimentation on early Mars. Science 302:1931–1934 [DOI] [PubMed] [Google Scholar]
- Manning-Berg AR and Kah L (2017) Proterozoic microbial mats and their constraints on environments of silicification. Geobiology 15:469–483 [DOI] [PubMed] [Google Scholar]
- McLennan MS, Bell JF III, Calvin WM, et al. (2005) Provenance and diagenesis of the evaporite-bearing Burns Formation, Meridiani Planum, Mars. Earth Planet Sci Lett 240:95–121 [Google Scholar]
- McMahon S, Bosak T, Grotzinger JP, et al. (2018) A field guide to finding fossils on Mars, J Geophys Res Planets 123:1012–1040 [DOI] [PMC free article] [PubMed]
- McSween HY Jr, Moersch JE, Burr DM, et al. (2019) Planetary Geoscience. Cambridge University Press, Cambridge, UK, pp 178–184 [Google Scholar]
- Metz JM, Grotzinger JP, Rubin DM, et al. (2009) Sulfate-rich eolian and wet interdune deposits, Erebus Crater, Meridiani Planum, Mars. Journal of Sedimentary Research 79:247–264 [Google Scholar]
- Milliken RE, Grotzinger JP, and Thomson BJ (2010) Paleoclimate of Mars as captured by the stratigraphic record in Gale Crater. Geophys Res Lett 37, doi: 10.1029/2009GL041870 [DOI] [Google Scholar]
- Minitti ME, Malin MC, Van Beek JK, et al. (2019) Distribution of primary and secondary features in the Pahrump Hills outcrop (Gale Crater, Mars) as seen in a Mars Descent Imager (MARDI) “sidewalk” mosaic. Icarus 328:194–209 [Google Scholar]
- Mittelfehldt DW, Gellert R, Ming DW, et al. (2018) Diverse lithologies and alteration events on the rim of Noachian-aged Endeavour Crater, Meridiani Planum, Mars: in situ compositional evidence. J Geophys Res Planets 123:1255–1306 [Google Scholar]
- Newman SA, Klepac-Ceraj V, Mariotti G, et al. (2017) Experimental fossilization of mat-forming cyanobacteria in coarse-grained siliciclastic sediments. Geobiology 15:484–498 [DOI] [PubMed] [Google Scholar]
- Noffke N (1998) Multidirectional ripple marks rising from biological and sedimentological processes in modern lower supratidal deposits (Mellum Island, southern North Sea). Geology 26:879–882 [Google Scholar]
- Noffke N (1999) Erosional remnants and pockets evolving from biotic–physical interactions in a Recent lower supratidal environment. Sedimentary Geology 123:175–181 [Google Scholar]
- Noffke N (2000) Extensive microbial mats and their influences on the erosional and depositional dynamics of a siliciclastic cold water environment (Lower Arenigian, Montagne Noire, France). Sedimentary Geology 136:207–215 [Google Scholar]
- Noffke N, ed. (2009) Microbial mats in Earth's fossil record of life: geobiology [theme issue]. Earth Science Reviews vol. 96, issue 3 [Google Scholar]
- Noffke N (2010) Microbial Mats in Sandy Deposits from the Archean Era to Today. Springer Science, Berlin [Google Scholar]
- Noffke N (2015) Ancient sedimentary structures in the <3.7 Ga Gillespie Lake Member, Mars, that resemble macroscopic morphology, spatial associations, and temporal succession in terrestrial microbialites. Astrobiology 15:169–192 [DOI] [PubMed] [Google Scholar]
- Noffke N and Awramik S (2013) Stromatolites and MISS—differences between relatives. GSA Today 23:4–9 [Google Scholar]
- Noffke N and Chafetz HS, eds. (2012) Microbial Mats in Siliciclastic Depositional Systems Through Time. SEPM Special Publication 101, Society for Sedimentary Geology, Tulsa, OK [Google Scholar]
- Noffke N and Krumbein WE (1999) A quantitative approach to sedimentary surface structures contoured by the interplay of microbial colonization and physical dynamics. Sedimentology 46:417–426 [Google Scholar]
- Noffke N, Gerdes G, Klenke T, et al. (1997) A microscopic sedimentary succession of graded sand and microbial mats in modern siliciclastic tidal flats. Sedimentary Geology 110:1–6 [Google Scholar]
- Noffke N, Gerdes G, Klenke T, et al. (2001) Microbially induced sedimentary structures: a new category within the classification of primary sedimentary structures. Journal of Sedimentary Research 71:649–656 [Google Scholar]
- Noffke N, Knoll AH, and Grotzinger JP (2002) Sedimentary controls on the formation and preservation of microbial mats in siliciclastic deposits: a case study from the Upper Neoproterozoic Nama Group, Namibia. Palaios 17:533–544 [Google Scholar]
- Noffke N, Hazen RM, and Nhleko N (2003) Earth's earliest microbial mats in a siliciclastic marine environment (2.9 Ga Mozaan Group, South Africa). Geology 31:673–676 [Google Scholar]
- Noffke N, Beukes N, Gutzmer J, et al. (2006a) Spatial and temporal distribution of microbially induced sedimentary structures: a case study from siliciclastic storm deposits of the 2.9 Ga Witwatersrand Supergroup, South Africa. Precambrian Res 146:35–44 [Google Scholar]
- Noffke N, Eriksson KA, Hazen RM, et al. (2006b) A new window into Early Archean life: microbial mats in Earth's oldest siliciclastic tidal deposits (3.2 Ga Moodies Group, South Africa). Geology 34:253–256 [Google Scholar]
- Noffke N, Beukes N, Hazen RM, et al. (2008) Exceptionally preserved microbial mats of Meso-Archean age: the Sinqueni Formation, Pongola Supergroup, South Africa. Geobiology 6:5–20 [DOI] [PubMed] [Google Scholar]
- Noffke N, Christian D, Wacey D, et al. (2013) Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old Dresser Formation, Pilbara, Western Australia. Astrobiology 13:1103–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noffke N, Hagadorn J, and Bartlett S (2019) Microbial structures and dinosaur trackways from a Cretaceous coastal environment (Dakota Group, Colorado, USA). Journal of Sedimentary Research 89:1096–1108 [Google Scholar]
- Noffke, N, Beraldi-Campesi H, Callefo F, et al. (2021). Microbially induced sedimentary structures. In Treatise of Invertebrate Paleontology, Prokaryota, Vol. 1 (in press) [Google Scholar]
- Paterson D (1994) Biological mediation of sediment erodibility: ecology and physical dynamics. In Cohesive Sediments, edited by N Burt, R Parker, and J Watts, John Wiley and Sons, London, pp 215–229 [Google Scholar]
- Pearl HW, Pinkney J, and Steppe TF (2000) Cyanobacterial-bactreial mat consortia: examining the functional unit of microbial survival and growth in extreme environments. Environ Microbiol 2:11–26 [DOI] [PubMed] [Google Scholar]
- Perri E, Tucker ME, Słowakiewicz M, et al. (2017) Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 65:1213–1245 [Google Scholar]
- Peterffy O, Calner M, and Vajda V (2016) Early Jurassic microbial mats—a potential response to reduced biotic activity in the aftermath of the end-Triassic mass extinction event. Palaeogeogr Palaeoclimatol Palaeoecol 464:76–85 [Google Scholar]
- Philippot P, van Zuilen M, Lepot K, et al. (2007) Early Archaen mivroorganisms preferred elemental sulfur, not sulfate. Science 317:1534–1537 [DOI] [PubMed] [Google Scholar]
- Prave AR (2002) Life on land in the Proterozoic: evidence from the Torridonian rocks of northwest Scotland. Geology 30:811–814 [Google Scholar]
- Pruss SB, Bottjer DJ, Corsetti FA, et al. (2006) A global marine sedimentary response to the end-Permian mass extinction: examples from southern Turkey and the western United States. Earth-Science Reviews 78:193–206 [Google Scholar]
- Rampe EB, Blake DF, Bristow TF, MSL Science Team (2020) Mineralogy and geochemistry of sedimentary rocks and eolian sediments in Gale Crater, Mars: a review after six Earth years of exploration with Curiosity. Geochemistry 80, doi: 10.1016/j.chemer.2020.125605 [DOI] [Google Scholar]
- Rice MS, Gupta S, Treiman AH, et al. (2017) Geologic overview of the Mars Science Laboratory rover mission at the Kimberley, Gale Crater, Mars. J Geophys Res Planets 122:2–20 [Google Scholar]
- Ruff SW and Farmer JD (2016) Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nat Commun 7, doi: 10.1038/ncomms13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Banerjee S, Samanta P, et al. (2006) Microbial mat-induced sedimentary structures in siliciclastic sediments: examples from the 1.6 Ga Chorhat Sandstone, Vindhyan Supergroup, MP, India. J Earth Syst Sci 115:49–60 [Google Scholar]
- Schieber J (1986) The possible role of benthic microbial mats during the formation of carbonaceous shales in shallow Mid-Proterozoic basins. Sedimentology 33:521–536 [Google Scholar]
- Schieber J (1999) Microbial mats in terrigenous clastics; the challenge of identification in the rock record. Palaios 14:3–12 [Google Scholar]
- Schieber J, Bose PK, Eriksson P, et al. (2007) Atlas of Microbial Mat Features Preserved within the Siliciclastic Rock Record. Elsevier, Amsterdam [Google Scholar]
- Schon SC, Head JW, and Fassett CI (2012) An overfilled lacustrine system and progradational delta in Jezero Crater, Mars: implications for Noachian climate. Planet Space Sci 67:28–45 [Google Scholar]
- Schultze-Lam S, Fortin D, Davis BS, et al. (1996) Mineralization of bacterial surfaces. Chem Geol 132:171–181 [Google Scholar]
- Sheldon ND (2012) Microbially induced sedimentary structures in the ca. 1100. Ma terrestrial midcontinent rift of North America. In Microbial Mats in Siliciclastic Depositional Systems Through Time. SEPM Special Publication 101, edited by N Noffke and H Chafetzs, Society for Sedimentary Geology, Tulsa, OK pp 153–162 [Google Scholar]
- Shepard RN and Sumner DY (2010) Undirected motility of filamentous cyanobacteria produces reticulate mats. Geobiology 8:179–190 [DOI] [PubMed] [Google Scholar]
- Squyres SW and Knoll AH (2005) Sedimentary rocks at Meridiani Planum: origin, diagenesis, and implications for life on Mars. Earth Planet Sci Lett 240:1–10 [Google Scholar]
- Squyres SW, Grotzinger JP, Arvidson RE, et al. (2004) In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306:1709–1714 [DOI] [PubMed] [Google Scholar]
- Squyres SW, Knoll AH, Arvidson RE, et al. (2009) Exploration of Victoria Crater by the Mars rover Opportunity. Science 324:1058–1061 [DOI] [PubMed] [Google Scholar]
- Stack KM, Grotzinger JP, and Milliken RE (2013) Bed thickness distributions on Mars: an orbital perspective. J Geophys Res Planets 118:1323–1349 [Google Scholar]
- Stack KM, Grotzinger JP, Lamb MP, et al. (2019) Evidence for plunging river plume deposits in the Pahrump Hills member of the Murray Formation, Gale Crater, Mars. Sedimentology 66:1768–1802 [Google Scholar]
- Stack KM, Williams NR, Calef F III, et al. (2020) Photogeologic map of the Perserverance rover field site in Jezero Crater constructed by the Mars 2020 Science Team. Space Sci Rev 216, doi: 10.1007/s11214-020-00739-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stal LJ (2003) Microphytobenthos, their extracellular polymeric substances, and the morphogenesis of intertidal sediments. Geomicrobiol J 20:463–478 [Google Scholar]
- Stal LJ and Caumette P, eds. (1994) Microbial Mats: Structure, Development and Environmental Significance. NATO ASI Series G, Ecological Sciences, 35, Springer, Berlin [Google Scholar]
- Stal LJ, Gemerden H, and Krumbein WE (1985) Structure and development of a benthic marine microbial mat. FEMS Microbiol Lett 31:111–125 [Google Scholar]
- Stein N, Grotzinger J, Schieber J, et al. (2018) Desiccation cracks provide evidence of lake drying on Mars, Sutton Island member, Murray Formation, Gale Crater. Geology 46:515–518 [Google Scholar]
- Stolz JF (2000) Structure of microbial mats and biofilms. In Microbial Sediments, edited by RE Riding and SM Awramik, Springer, Berlin [Google Scholar]
- Stoodley P, Sauer K, Davies DG, et al. (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209 [DOI] [PubMed] [Google Scholar]
- Summons RE, Amend JP, Bish D, et al. (2011) Preservation of martian organic and environmental records: final report of the Mars Biosignature Working Group, Astrobiology 11:157–181 [DOI] [PubMed] [Google Scholar]
- Sumner DY (2004) Poor preservation potential of organics in Meridiani Planum hematite-bearing sedimentary rocks. J Geophys Res Planets 109, doi: 10.1029/2004JE002321 [DOI] [Google Scholar]
- Sun VZ, Stack KM, Kah LC, et al. (2019) Late-stage diagenetic concretions in the Murray Formation, Gale Crater, Mars. Icarus 321:866–890 [Google Scholar]
- Taher AG (2014) Microbially induced sedimentary structures in evaporite–siliciclastic sediments of Ras Gemsa sabkha, Red Sea Coast, Egypt. J Adv Res 5:577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taher AG and Abdel-Motelib A (2015) New insights into microbially induced sedimentary structures in alkaline hypersaline El Beida Lake, Wadi El Natrun, Egypt. Geo-Marine Letters 35:341–353 [Google Scholar]
- Thomas M, Clarke JDE, and Pain CF (2005) Weathering, erosion and landscape processes on Mars identified from recent rover imagery, and possible Earth analogues. Australian Journal of Earth Sciences 52:365–378 [Google Scholar]
- Thomson BJ, Bridges NT, Milliken R, et al. (2011) Constraints on the origin and evolution of the layered mound in Gale Crater, Mars using Mars Reconnaissance Orbiter data. Icarus 214:413–432 [Google Scholar]
- Tice MM, Thornton DCO, Pope MC, et al. (2011) Archean microbial mat communities. Annu Rev Earth Planet Sci 39:297–319 [Google Scholar]
- Vago JL and Westall F (2017) Habitability on early Mars and the search for biosignatures with the ExoMars rover. Astrobiology 17:471–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago JL, Westall F, Pasteur Instrument Teams, Landing Site Selection Working Group, and Other Contributors (2017) Habitability on early Mars and the search for biosignatures with the ExoMars rover. Astrobiology 17:471–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuilen M (2008) Stable isotope ratios as a biomarker on Mars. Space Sci Rev 135:221–232 [Google Scholar]
- Viles H, Ehlmann B, Wilson CF, et al. (2010) Simulating weathering of basalt on Mars and Earth by thermal cycling. Geophys Res Lett 37:1–5 [Google Scholar]
- Visscher PT and Stolz JF (2005) Microbial mats as bioreactors: populations, processes, and products. In Geobiology: Objectives, Concepts, Perspectives, edited by N. Noffke, Elsevier, Amsterdam, pp 87–100 [Google Scholar]
- Westall F (1999) The nature of fossil bacteria: a guide to the search for extraterrestrial life. J Geophys Res 104:16437–16451 [Google Scholar]
- Westall F and Brack A (2018) The importance of water for life. Space Sci Rev 214, doi: 10.1007/s11214-018-0476-7 [DOI] [Google Scholar]
- Westall F, Foucher F, Bost N, et al. (2015) Biosignatures on Mars: what, where, and how? implications for the search for martian life. Astrobiology 15:998–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RME, Grotzinger JP, Dietrich WE, MSL Science Team (2013) Martian fluvial conglomerates at Gale Crater. Science 340:1068–1072 [DOI] [PubMed] [Google Scholar]
- Wilmeth DT, Dornbos SQ, Isbell JI, et al. (2014) Putative domal microbial structures in fluvial siliciclastic facies of the Mesoproterozoic (1.09 Ga) Copper Harbor Conglomerate, Upper Peninsula Michigan, USA. Geobiology 12:99–108 [DOI] [PubMed] [Google Scholar]
- Wilmeth DT, Corsetti F, Beukes NJ, et al. (2019) Neoarchean (2.7 Ga) lacustrine stromatolitic deposits in the Hartbeesfontain Basin, Ventersdorp Supergroup, South Africa: implications for oxygen oases. Precambrian Res 320:291–302 [Google Scholar]