Abstract
BACKGROUND:
Meropenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa are the two most common nosocomial pathogens causing ventilator-associated pneumonia. To combat this resistance, different combinations of antibiotics have been evaluated for their efficacy in laboratories as well as in clinical situations.
AIM:
The aim of the study was to investigate the effect of combined colistin and meropenem against meropenem-resistant isolates of A. baumannii and P. aeruginosa by checkerboard method.
MATERIALS AND METHODS:
Fifty meropenem-resistant isolates of A. baumannii (n = 25) and P. aeruginosa (n = 25) from endotracheal aspirates were studied. The MIC of colistin and meropenem was found using the microbroth dilution method. The fractional inhibitory concentration was calculated for the combination of antibiotics by checkerboard assay and the antibiotic interactions were assessed. Fisher's exact test was carried out for statistical comparison of categorical variables.
RESULTS:
A synergistic effect between colistin and meropenem was observed in 18/25 (72%) and 6/25 (24%) isolates of Acinetobacter baumannnii and P. Aeruginosa, respectively, with fractional inhibitory concentration indices of ≤0.5. None of the tested isolates exhibited antagonism.
CONCLUSION:
Our results showed that combinations of colistin and meropenem are associated with improvement in minimum inhibitory concentration and may be a promising strategy in treating meropenem-resistant A. baumannii respiratory tract infections.
Keywords: Checkerboard assay, Clinical Laboratory Standards Institute, fractional inhibitory concentration indices, minimum inhibitory concentration, ventilator-associated pneumonia
Introduction
In recent times, testing for antimicrobial interactions has become very essential as there is an increase in the proportion of drug-resistant microorganisms and due to restricted choices for the management of the infections caused by those organisms.[1] This is particularly obvious when the infections are caused by nonfermenters.[2,3] Numerous combinations of antibiotics have been assessed to determine synergistic activity for various organisms. Favorable outcome was achieved for combination of different antibiotics with colistin such as meropenem, fluoroquinolones, and rifampicin.[3,4,5] However, a study conducted by Soudeiha et al. in 2017 showed that there was only additive effect and there was no synergism when evaluating combination of colistin and meropenem in Acinetobacter baumanni.[6] Although combination of antimicrobial therapy is known to broaden the spectrum and increase the bactericidal activity, its use still remains debatable in certain strains such as Pseudomonas aeruginosa.[7] A meta-analysis conducted by Paul et al. found that there was no change in the mortality rate when combination therapy was used over monotherapy.[8]
Of the different methods available for testing synergistic activity, time-kill assay (TKA) gives consistent and reliable results, however, as this method is too cumbersome, checkerboard assay (CB) and E-test has been extensively followed.[2] The most common organisms causing ventilator-associated pneumonia (VAP) in our center are A. baumannii and P. aeruginosa. Since the rate of meropenem resistance is increasing in these two organisms, we aimed to evaluate the effect of combined colistin and meropenem against meropenem-resistant isolates of A. baumannii and P.eruginosa by checkerboard method.
Materials and Methods
The approval for the study was obtained from the Institute Human ethics committee (JIP/IEC/2018/0142) and was carried out in Department of Microbiology. Study isolates comprised meropenem-resistant A. baumanni and P. aeruginosa (25 each) from endotracheal aspirates of patients with suspected VAP. Broth microdilution was used to determine the MIC for meropenem and colistin using pure powders of the drugs (Sigma-Aldrich, India). Serial doubling dilution was performed for both the antibiotics to cover 4–5 dilutions below and above the MIC. Four times the desired concentration of these antibiotics was prepared, as antibiotic gets diluted by four times in the wells of the microliter plate. Checkerboard arrays were created by dispensing 25 μl of these titrations in the appropriate wells of the microliter plate. Meropenem was dispersed in the columns (1–11) and colistin was dispensed in rows (A-G). The final row (row H) and final column (column 12) contained only meropenem and colistin, respectively.
To prepare the inoculum, two to three isolated colonies from overnight incubated plates were taken and suspended in cation-adjusted Mueller–Hinton broth to match the turbidity of 0.5 McFarland (108 CFU/mL). This was followed by dilution to reach the final concentration of 5 × 105 CFU/mL. Fifty microliter of this inoculum was dispensed in all the wells except the drug control wells. Then, the microliter plates were incubated at 37°C for 18–24 h. Readings were taken following incubation and fractional inhibitory concentration (FIC) was calculated as follows:
FIC = MIC of colistin in combination /MIC of colistin alone + MIC of meropenem in combination / MIC of meropenem alone
An FIC ≤0.5 indicated synergy, 0.5 to ≤2 indicated additive effect, and combination was said to be antagonistic if FIC >4.[6]
Statistical analysis
Epidata v 3.01 software (Epidata association, Odense Denmark, 1999) was used for entering the data and the analysis was done using SPSS version 19.0( Armonk, NY:IBM Corp). Statistical analysis of categorical variables was carried out using Fisher's exact test and P < 0.05 was taken as significant.
Results
All isolates were resistant to meropenem as per the MIC results, while 6 (24%) isolates of A. baumannii and 5 (20%) isolates of P. aeruginosa were resistant to colistin. The FIC indexes were calculated and their interactions are depicted in Tables 1 and 2. Of the 25 A. baumannii isolates which were subjected to synergy testing, 18 (72%) isolates showed synergism, while 7 (28%) isolates demonstrated additive effect and out of the 25 P. aeruginosa isolates, only 6 (24%) isolates showed synergism, 4 (16%) showed indifference, and the rest 15 (60%) isolates showed an additive effect. Neither A. baumannii nor P. aeruginosa demonstrated antagonism with this combination [Tables 1,2 and Figure 1]. Synergy was demonstrated in all six colistin-resistant isolates of A. baumannii (100%), whereas it was demonstrated in only 3 (3/5) colistin-resistant strains of P. aeruginosa (60%). When synergism was compared between colistin resistant and colistin susceptible isolates of A. baumannii (P = 0.137) and P. aeruginosa (P = 0.115), it was observed that most of the resistant isolates showed synergism, however, this difference was not statistically significant. This could be due to the fact that our study was underpowered to pick a statistical difference between them (back calculated power = 41.76%) due to inadequate sample size.
Table 1.
Effect of combined colistin and meropenem against Acinetobacter baumannii isolates (n=25)
| Isolate number | MIC of colistin alone µg/ml | MIC of meropenem alone µg/ml | MIC of colistin with meropenem µg/ml | MIC of meropenem with colistin µg/ml | FIC | Interpretation |
|---|---|---|---|---|---|---|
| A1 | 1 | 32 | 0.25 | 4 | 0.37 | Synergy |
| A2 | 8 | 64 | 1 | 1 | 0.14 | Synergy |
| A3 | 8 | 128 | 0.5 | 1 | 0.067 | Synergy |
| A4 | 1 | 64 | 0.5 | 2 | 0.53 | Additive |
| A5 | 2 | 32 | 1 | 0.5 | 0.51 | Additive |
| A6 | 2 | 64 | 0.5 | 2 | 0.28 | Synergy |
| A7 | 1 | 32 | 0.25 | 2 | 0.31 | Synergy |
| A8 | 8 | 64 | 2 | 0.5 | 0.26 | Synergy |
| A9 | 2 | 16 | 1 | 1 | 0.56 | Additive |
| A10 | 8 | 64 | 1 | 0.5 | 0.13 | Synergy |
| A11 | 2 | 64 | 0.5 | 2 | 0.28 | Synergy |
| A12 | 2 | 128 | 0.25 | 0.5 | 0.13 | Synergy |
| A13 | 8 | 64 | 2 | 0.5 | 0.26 | Synergy |
| A14 | 2 | 64 | 1 | 64 | 1.5 | Additive |
| A15 | 16 | 64 | 2 | 1 | 0.14 | Synergy |
| A16 | 1 | 32 | 0.25 | 2 | 0.31 | Synergy |
| A17 | 1 | 64 | 0.25 | 8 | 0.37 | Synergy |
| A18 | 0.5 | 32 | 0.125 | 2 | 0.26 | Synergy |
| A19 | 0.5 | 128 | 0.125 | 16 | 0.75 | Synergy |
| A20 | 0.25 | 64 | 0.125 | 4 | 0.56 | Synergy |
| A21 | 1 | 64 | 0.25 | 2 | 0.28 | Synergy |
| A22 | 0.5 | 16 | 0.25 | 0.5 | 0.53 | Additive |
| A23 | 1 | 16 | 0.5 | 0.5 | 0.53 | Additive |
| A24 | 2 | 128 | 0.25 | 0.5 | 0.26 | Synergy |
| A25 | 0.125 | 256 | 0.025 | 128 | 1 | Additive |
MIC=Minimum inhibitory concentration, FIC=Fractional inhibitory concentration
Table 2.
Effect of combined colistin and meropenem against Pseudomonas aeruginosa isolates (n=25)
| Isolate number | MIC of colistin alone µg/ml | MIC of meropenem alone µg/ml | MIC of colistin with meropenem µg/ml | MIC of meropenem with colistin µg/ml | FIC | Interpretation |
|---|---|---|---|---|---|---|
| P1 | 2 | 64 | 1 | 128 | 2.5 | Indifference |
| P2 | 4 | 64 | 1 | 2 | 0.28 | Synergy |
| P3 | 2 | 64 | 1 | 64 | 1.5 | Additive |
| P4 | 16 | 64 | 0.5 | 1 | 0.26 | Synergy |
| P5 | 0.5 | 4 | 8 | 0.25 | 2.5 | Indifference |
| P6 | 0.5 | 64 | 0.25 | 0.5 | 0.26 | Synergy |
| P7 | 0.5 | 64 | 1 | 64 | 3 | Indifference |
| P8 | 0.5 | 32 | 0.5 | 32 | 2 | Additive |
| P9 | 0.5 | 128 | 0.25 | 32 | 0.75 | Additive |
| P10 | 4 | 16 | 2 | 8 | 1 | Additive |
| P11 | 16 | 8 | 8 | 4 | 1 | Additive |
| P12 | 0.5 | 128 | 0.25 | 32 | 0.75 | Additive |
| P13 | 1 | 16 | 0.5 | 8 | 1 | Additive |
| P14 | 0.5 | 32 | 0.5 | 32 | 2 | Additive |
| P15 | 0.25 | 32 | 0.125 | 8 | 0.75 | Additive |
| P16 | 2 | 256 | 0.5 | 128 | 0.75 | Additive |
| P17 | 1 | 16 | 1 | 16 | 2 | Additive |
| P18 | 0.125 | 16 | 0.0625 | 16 | 3 | Indifference |
| P19 | 1 | 32 | 1 | 32 | 2 | Additive |
| P20 | 0.125 | 16 | 0.0625 | 8 | 1 | Additive |
| P21 | 2 | 32 | 0.5 | 16 | 0.75 | Additive |
| P22 | 1 | 64 | 0.5 | 2 | 0.53 | Additive |
| P23 | 1 | 4 | 0.25 | 0.5 | 0.37 | Synergy |
| P24 | 8 | 8 | 0.125 | 0.5 | 0.26 | Synergy |
| P25 | 0.5 | 64 | 0.125 | 4 | 0.31 | Synergy |
MIC=Minimum inhibitory concentration, FIC=Fractional inhibitory concentration
Figure 1.
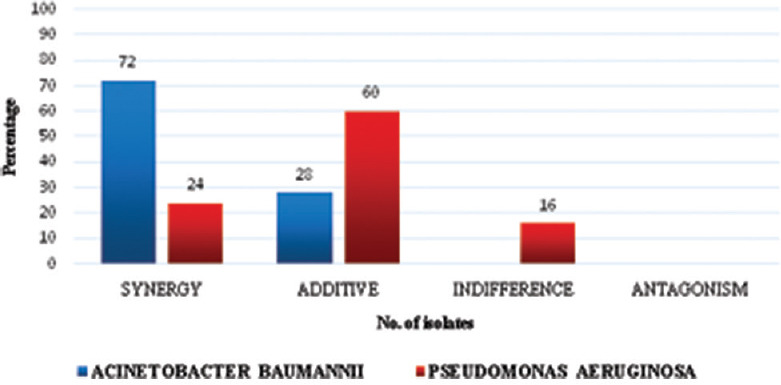
Results of in vitro synergy testing using checkerboard method for Acinetobacter baumannii and Pseudomonas aeruginosa
Discussion
A. baumannii and P. aeruginosa are the emerging nosocomial pathogens, which are now becoming resistant to commonly used antibiotics such as aminoglycosides, beta-lactams, beta-lactam + beta-lactamase inhibitors in combination and meropenem, so colistin is considered as the last line treatment option.[9,10] Of note, it has been known that biofilm formation accounts for approximately 80% of the human bacterial infection, and meropenem is said to have a poor anti-biofilm activity when administered alone.[11,12] Colistin, a polypeptide antibiotic, has a wide range of activity against Gram-negative organisms and acts mainly by disruption of lipopolysaccharide layer by interacting with the outer membrane of the Gram-negative bacteria. When this drug is given in combination with meropenem, synergism is thought to occur by favoring better access of meropenem to its target site, thereby leading to cell death. In addition, anti-biofilm activity of the combination may also contribute to synergism. A review and meta-analysis done by Jiang et al. showed that in time-kill assay (TKD), a lower synergy rate was shown by colistin (polymyxin E) when compared to polymyxin B, whereas an opposite finding was seen in the checkerboard synergy method.[13]
Review of literature revealed that different studies have quoted different FIC interpretative results, for example, Liu et al. considered FIC indices (FICI) ≤0.5 as synergy, 0.5 < FICI ≤ 4 as additive and indifference, and FICI >4 as antagonism; van Belkum et al. considered FICI ≤0.5 to be synergistic, FICI 0.5 to ≤1.0 to be additive, FICI 1.0 to ≤2 as indifferent, and, finally FICI ≥3 as antagonistic, whereas Le Minh et al. considered as follows: synergistic when FICI ≤0.5, indifferent 0.5 < FICI <4, and antagonistic when FICI ≥4.[14,15,16] However, we followed the interpretation of Soudeiha et al. who had done a similar study in 2017 by using the same combination of antibiotics (colistin and meropenem) on the same organism (A. baumanni).[6]
In our study, we found that synergistic activity was not influenced by the MIC of meropenem (i.e., both low and high meropenem MIC showed similar activity), whereas a study conducted by Fan et al. in murine thigh infection model showed that strains with low meropenem MIC (≤32 mg/L) exhibited more synergistic activity than strains having higher MIC (≥64 mg/L).[17]
Out of the 25 A. baumannii isolates tested, 18 (72%) isolates showed synergy (FIC ≤0.5) and 7 (28%) showed additive effect (0.5 < FIC ≤2). Our findings were in agreement with those that Le Minh et al. who found that 68% of A. baumannii isolates showed synergistic activity between meropenem and colistin and they also noted that the synergistic activity was more in carbapenem-resistant A. baumannii isolates than in carbapenem-susceptible isolates.[14] Similar studies by Liu et al.[15] and Yavaş et al.[18] showed that there existed 100% synergy among A. baumannii isolates when combining colistin with meropenem. In contrast to our finding, a recent study by Kheshti et al. showed that there was only 10% synergy when combining colistin and meropenem among A. baumannii isolates.[19]
Our study results showed that for P. aeruginosa (n = 25) when combining colistin and meropenem using checkerboard method, there was a high rate of additive effect – 60% (15/25) and synergistic effect was seen in only 24% (6/25) and 16% (4/25) of isolates showed indifference. Our study supports the finding of Daoud et al. who observed that 63.6% (7/11) had additive effect and 27.2% (3/11) had synergy when combining colistin and meropenem using the checkerboard method for P. aeruginosa.[20] In contrast, Ramadan et al. found that 63.6% showed synergistic effect and 36.4% showed the additive effect.[21] No antagonistic effect was seen in the current study both for A. baumannii and P. aeruginosa.
In our study, 76% of the A. baumannni isolates and 80% of P. aeruginosa isolates were susceptible to colistin by microbroth dilution method. Even though monotherapy rather than in combination would decrease the development of adverse side effects caused by the use of additional antibiotics, it is not recommended with colistin. A study by Li et al., Lee et al., Cetin et al., and Bergen et al. showed that colistin when administrated alone leads to the development of heteroresistant Acinetobacter and Pseudomonas strains and can even cause colistin resistance among other isolates and can lead to proliferation of organisms which are innately resistant to colistin leading to secondary bacterial infection leaving the clinicians with no other treatment options.[22,23,24,25]
The rationale behind doing synergy testing is to find out whether the combination of antibiotics can improve the clinical outcomes in patients for whom in vitro synergy testing of antibiotics showed synergism to widen the empiric therapy and to delay the resistance development during antimicrobial treatment. A recent meta-analysis by Vardakas et al. showed that synergy-guided antibiotic combination therapy led to improved clinical outcome and lower mortality.[26] However, Nutman et al. found no significant improvement in outcomes in patients infected with organisms for which in vitro synergy testing showed synergism, they also found that patients infected with isolates that showed antagonism, in vitro synergy testing did not show worse outcome when a combination of antibiotics was tried.[27]
There are a few reasons for the discrepancy of experimental results with in vivo response. First, in vitro synergy testing does not consider the host immune system and the pathogen interaction. Next, the antimicrobial drug concentration achieved at the infection site varies from those needed for synergism in vitro, and although the desired synergistic concentration are achieved in vivo, the effect may not last.[28,29]
Although different methods are available for testing synergism, the checkerboard synergy method has the advantage of manipulating the antibiotic concentration used, unlike E-test strip where fixed concentrations of antibiotics are available. Time-kill test gives information on the rapidity of synergistic activity, yet it is unreasonable when testing many isolates, as it is tedious and consumes a lot of time. E-test is the least complex method, however, it is less standardized and constrained by fixed concentration of antibiotic on the strips.[2] Various studies have shown wide-ranging concordance of 33%–100% between CB and TKA, with lower rates of synergism by the checkerboard method.[28] A study by Ni et al. showed that increased rates of synergism are seen with TKD when compared to checkerboard method and E-test; therefore, standardization of the various existing methods for evaluating synergy has to be done to address these limitations.[30]
Conclusion
In the current study using the checkerboard method, when meropenem was combined with colistin, we had better synergistic activity against multidrug-resistant (MDR) A. baumannii isolates when compared to MDR P. aeruginosa isolates. However, more clinical trials are required to ascertain their efficacy and to explore its therapeutic potential, as in vivo settings cannot be entirely simulated in vitro.
Limitations
A single synergy testing method was used in our study (checkerboard method) and second, our sample size was small and the underlying mechanisms of resistance among the isolates were not determined.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge JIPMER for providing intramural research fund.
References
- 1.Eliopoulos GM, Eliopoulos CT. Antibiotic combinations: Should they be tested? Clin Microbiol Rev. 1988;1:139–56. doi: 10.1128/cmr.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laishram S, Pragasam AK, Bakthavatchalam YD, Veeraraghavan B. An update on technical, interpretative and clinical relevance of antimicrobial synergy testing methodologies. Indian J Med Microbiol. 2017;35:445–68. doi: 10.4103/ijmm.IJMM_17_189. [DOI] [PubMed] [Google Scholar]
- 3.Sopirala MM, Mangino JE, Gebreyes WA, Biller B, Bannerman T, Balada-Llasat JM, et al. Synergy testing by Etest, microdilution checkerboard, and time-kill methods for pan-drug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:4678–83. doi: 10.1128/AAC.00497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong JH, Clancy CJ, Cheng S, Shields RK, Chen L, Doi Y, et al. Characterization of porin expression in Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae identifies isolates most susceptible to the combination of colistin and carbapenems. Antimicrob Agents Chemother. 2013;57:2147–53. doi: 10.1128/AAC.02411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biancofiore G, Tascini C, Bisà M, Gemignani G, Bindi ML, Leonildi A, et al. Colistin, meropenem and rifampin in a combination therapy for multi-drug-resistant Acinetobacter baumannii multifocal infection. A case report. Minerva Anestesiol. 2007;73:181–5. [PubMed] [Google Scholar]
- 6.Soudeiha MA, Dahdouh EA, Azar E, Sarkis DK, Daoud Z. In vitro evaluation of the colistin-carbapenem combination in clinical isolates of A. baumannii using the checkerboard, Etest, and time-kill curve techniques. Front Cell Infect Microbiol. 2017;7:209. doi: 10.3389/fcimb.2017.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damas P, Garweg C, Monchi M, Nys M, Canivet JL, Ledoux D, et al. Combination therapy versus monotherapy: A randomised pilot study on the evolution of inflammatory parameters after ventilator associated pneumonia [ISRCTN31976779] Crit Care. 2006;10:R52. doi: 10.1186/cc4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: Systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett J. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 10.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of acinetobacter infections: A century of challenges. Clin Microbiol Rev. 2017;30:409–47. doi: 10.1128/CMR.00058-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geladari A, Simitsopoulou M, Antachopoulos C, Roilides E. Dose dependent synergistic interactions of colistin with rifampin, meropenem, and tigecycline against carbapenem resistant Klebsiella pneumoniae biofilms. Antimicrob Agents Chemother. 2019;63:e02357–18. doi: 10.1128/AAC.02357-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Römling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–61. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, He X, Li J. Synergy effect of meropenem-based combinations against Acinetobacter baumannii: A systematic review and meta-analysis. Infect Drug Resist. 2018;11:1083–95. doi: 10.2147/IDR.S172137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Minh V, Thi Khanh Nhu N, Vinh Phat V, Thompson C, Huong Lan NP, Thieu Nga TV, et al. In vitro activity of colistin in antimicrobial combination against carbapenem-resistant Acinetobacter baumannii isolated from patients with ventilator-associated pneumonia in Vietnam. J Med Microbiol. 2015;64:1162–9. doi: 10.1099/jmm.0.000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Zhao M, Chen Y, Bian X, Li Y, Shi J, et al. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents. 2016;48:559–63. doi: 10.1016/j.ijantimicag.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 16.van Belkum A, Halimi D, Bonetti EJ, Renzi G, Cherkaoui A, Sauvonnet V, et al. Meropenem/colistin synergy testing for multidrug-resistant Acinetobacter baumannii strains by a two-dimensional gradient technique applicable in routine microbiology. J Antimicrob Chemother. 2015;70:167–72. doi: 10.1093/jac/dku342. [DOI] [PubMed] [Google Scholar]
- 17.Fan B, Guan J, Wang X, Cong Y. Activity of colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PLoS One. 2016;11:e0157757. doi: 10.1371/journal.pone.0157757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yavaş S, Yetkin MA, Kayaaslan B, Baştuğ A, Aslaner H, But A, et al. Investigating the in vitro synergistic activities of several antibiotic combinationsagainst carbapenem-resistant Acinetobacter baumannii isolates. Turk J Med Sci. 2016;46:892–6. doi: 10.3906/sag-1408-14. [DOI] [PubMed] [Google Scholar]
- 19.Kheshti R, Pourabbas B, Mosayebi M, Vazin A. In vitro activity of colistin in combination with various antimicrobials against Acinetobacter baumannii species, a report from South Iran. Infect Drug Resist. 2019;12:129–35. doi: 10.2147/IDR.S182585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daoud Z, Mansour N, Masri K. Synergistic combination of carbapenems and colistin against P. aeruginosa and A. baumannii. Open J Med Microbiol. 2013:253–8. [Google Scholar]
- 21.Ramadan RA, Gebriel MG, Kadry HM, Mosallem A. Carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa: Characterization of carbapenemase genes and E-test evaluation of colistin-based combinations. Infect Drug Resist. 2018;11:1261–9. doi: 10.2147/IDR.S170233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cetin ES, Tekeli A, Ozseven AG, Us E, Aridogan BC. Determination of in vitro activities of polymyxin B and rifampin in combination with ampicillin/sulbactam or cefoperazone/sulbactam against multidrug resistant Acinetobacter baumannii by the E test and checkerboard methods. Jpn J Infect Dis. 2013;66:463–8. doi: 10.7883/yoken.66.463. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Bergen PJ, Bulitta JB, Tsuji B, Forrest A, Nation RL, et al. Synergistic activity of colistin and rifampin combination against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2013;57:3738–45. doi: 10.1128/AAC.00703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, et al. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50:2946–50. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, et al. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2011;55:5685–95. doi: 10.1128/AAC.05298-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardakas KZ, Athanassaki F, Pitiriga V, Falagas ME. Clinical relevance of in vitro synergistic activity of antibiotics for multidrug-resistant Gram-negative infections: A systematic review. J Glob Antimicrob Resist. 2019;17:250–9. doi: 10.1016/j.jgar.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Nutman A, Lellouche J, Temkin E, Daikos G, Skiada A, Durante-Mangoni E, et al. Colistin plus meropenem for carbapenem-resistant Gram-negative infections: In vitro synergism is not associated with better clinical outcomes. Clin Microbiol Infect. 2020;26:1185–91. doi: 10.1016/j.cmi.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, et al. Systematic review and meta-analysis 305 of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother. 2013;57:5104–11. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nutman A, Lellouche J, Temkin E, Daikos G, Skiada A, Durante-Mangoni E, et al. Colistin plus meropenem for carbapenem-resistant Gram-negative infections: In vitro synergism is not associated with better clinical outcomes. Clin Microbiol Infect. 2020;26:1185–91. doi: 10.1016/j.cmi.2020.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. In vitro synergy of polymyxins with other antibiotics for 372 Acinetobacter baumannii: A systematic review and meta-analysis. Int J Antimicrob Agents. 2015;45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]


