Abstract
Background/objectives
To estimate the risks of term-small for gestational age (SGA), preterm-appropriate for gestational age (AGA), and preterm SGA associated with maternal height and body mass index (BMI) and to calculate the population attributable fractions (PAF) of term SGA, preterm AGA, and preterm SGA associated with maternal short stature.
Subjects/methods
A population-based cohort of 13,230 women with pre-pregnancy height and weight followed from 2012 to 2016 in Sylhet, Bangladesh. We analyzed data of 2655 singleton live born infants. The babies born <37 weeks of gestation were considered preterm and weight <10th percentile of Intergrowth sex-specific gestational age were considered SGA. Risk factors for term SGA, preterm AGA, and preterm SGA were examined using multinomial logistic regression that estimated relative risk ratios (RRR) and 95% confidence intervals (CI).
Results
Maternal short stature <145 cm was significantly associated with term SGA (RRR 1.88, 95% CI 1.37, 2.58; p < 0.001), preterm AGA (RRR 1.45, 95% CI 1.02, 2.05; p < 0.05), and preterm SGA (RRR 14.40, 95% CI 1.82, 113.85; p < 0.05). Maternal underweight status (BMI < 18.5 kg/m2) was significant predictor of term SGA (RRR 1.32, 95% CI 1.10, 1.59; p < 0.01), and preterm AGA (RRR 1.39, 95% CI 1.12, 1.71; p < 0.01). PAF for maternal short stature were 23.2, 7.3, and 73.9% for term SGA, preterm AGA, and preterm SGA, respectively.
Conclusions
To address the problem of undernutrition, Bangladesh needs to strengthen implementation of its multi-sectoral nutrition program comprising nutrition specific and sensitive interventions. Implementation of the program with high coverage and quality would improve maternal nutrition and perinatal outcomes including preterm births and SGA.
Introduction
Each year, about 16% of all live born infants or an estimated 20 million infants worldwide are born low birthweight (LBW, <2500 gm), mostly because of prematurity or intrauterine growth restrictions (IUGR) [1, 2]. In 2010, an estimated 14.9 million babies or about 11% of all live birth babies worldwide were born preterm (<37 weeks gestation); more than 60% of preterm babies were born in South Asia and sub-Saharan Africa [2]. Small for gestational age (SGA) describes a baby who is less than tenth percentile of sex-specific population-based weight for gestational age [3]. In 2012, an estimated 23.3 million infants were born with SGA in low and middle income countries (LMIC); South Asia had the largest burden [4]. Both term and preterm babies can be appropriate for gestational age (AGA) or SGA. Babies born with SGA or preterm are at higher risks of morbidity, mortality, and long term disability including deficits in physical growth, neurocognitive development [2, 4, 5], and other chronic illness; preterm SGA babies experience the highest risk [5–8].
There are many established maternal risk factors that are associated with SGA, including primi-parity, maternal short stature and low body mass index (BMI), multiple births, Asian origin, smoking, and alcohol use [9–12]. Maternal pregnancy risk factors for SGA include vaginal bleeding [9], inadequate weight gain during pregnancy [10, 13] and maternal infectious diseases [11], hypertension, and renal disease [9, 14].
Maternal short stature (in particular, <150 cm) is considered to be an important risk factor for both SGA and preterm birth, particularly in a resource poor settings where SGA and preterm birth rates are high [6, 8, 15]. A meta-analysis using data from 12 population-based cohort studies from LMICs reported that ~6.5 million SGA and preterm births could be attributed in part to maternal short stature [6]. Maternal low BMI has been shown to be associated with spontaneous preterm birth [16].
The prevalence of maternal short stature and under-weight status are high in Bangladesh; 13% of ever-married women have a height of <145 cm and about a third of ever-married women are underweight [17]. Using population-based data from a large cohort of pregnant women in Sylhet district located in north-east Bangladesh, we quantified the relationship of maternal height and BMI with preterm birth and SGA.
Materials and methods
Study design and study population
This is a population-based study that uses data from the Maternal Infection Screening and Treatment (MIST) trial conducted in two rural sub-districts of Sylhet district in Bangladesh. The details of the study methods were previously published [18]. Briefly, a census of the study population allowed enumeration of married women of reproductive age (MWRA). Between January 2012 and March 2016 period, locally resident community health workers (CHWs) with a minimum of 10th grade education and 6 weeks training collected pre-pregnancy height and weight data of MWRA (n = 13,230) after obtaining informed consent. CHWs visited study women monthly and performed a urine pregnancy test if last menstrual period (LMP) was >4 weeks ago. Pregnant women were followed until the end of pregnancy to collect data on pregnancy care, pregnancy outcomes, and other vital events including births, deaths and migrations (in and out). CHWs collected weight of live born babies within 72 h of birth. They also collected data on women’s personal, household and community characteristics including age, education, husbands’ education, household size, and household socioeconomic data. The majority of the population in this agrarian community are poor, with low levels of education, and more than one third of the men and women have no formal schooling. The burden of preterm birth and perinatal mortality are high at 21% [19] and 65 per 1000 live births [20], respectively.
Measurements
Women’s pre-pregnancy weight was measured using a portable UNICEF Redline scale within nearest 100 gm and height was measured within nearest 0.1 cm using a locally constructed portable height stadiometer. Weight of live born infants was measured with a digital hand-held scale (Virtual Measurement and Control, Inc., Model VH372) with a precision of 20 gm. Infant weight was considered birth-weight if measured within 72 h of birth. All equipment was calibrated daily. Maternal height and body mass index are the main explanatory variables of this analysis. Women were classified into four categories based on height < 145 cm, 145–149.9 cm, 150–154.9 cm, and ≥155 cm. Body mass index (BMI) was defined as the ratio of weight in kilograms to height in meters squared (weight in kg/height in m2). Using BMI, women were classified as: normal (BMI 18.5–24.9 kg/m2), underweight (<18.5 kg/m2), and over-weight/obese ≥ 25.0 kg/m2).
The main outcome variables were preterm birth and SGA. Gestational age was determined by subtracting maternal reported date of last menstrual period (LMP) from date of birth. Date of LMP was collected using a prospectively maintained home menstrual calendar along with monthly pregnancy surveillance visits [18]. SGA status was defined as birth weight <10th percentile of INTER-GROWTH sex-specific weight for gestational age [21]. Infants who were ≥10th percentile weight for gestational age were considered as appropriate for gestational age (AGA). Using term or preterm and AGA or SGA status, we categorized infants into four groups: term AGA, term SGA, preterm AGA, and preterm SGA.
We created household wealth scores based on housing materials and household possessions using principle component analysis. The wealth scores were used to divide the households in to wealth quintiles. We created a variable ‘community level food availability’ as a proxy measure for community level food shortages dividing the calendar year into pre-harvest (July–December, presumed inadequate) and post-harvest (January–June, presumed adequate) seasons. We created a binary “smoking” variable based on women’s reported history of smoking by herself or any member of the household during pregnancy.
Data analysis
We conducted bivariate analysis to determine the association of four infant outcome categories (term AGA, term SGA, preterm AGA, and preterm SGA) with maternal height, maternal body mass index, and selected individual, household, and community level characteristics. In bivariate analysis, we have used chi-squared test to examine if the outcome categories were independent or associated with each of the explanatory variables. Variables with a p-value of <0.10 in the bivariate analyses were included in the multivariate analysis. We used multivariate multinomial logistic regression to identify factors predicting term SGA, preterm AGA, and preterm SGA. We calculated the population attributable fractions (PAF) of term SGA, preterm AGA, and preterm SGA to estimate the proportion of these outcomes that would be averted at the population level if the height of Bangladeshi women reach to the level of the height of US women. For this calculation, we have used proportional distribution of height of US women (0.57% of women < 145 cm, 3.03% of women 145 to < 150 cm, 9.66% of women 150 to < 155 cm, and 86.74% of women ≥ 155 cm) from the US national health and nutrition examination survey 2011–2012 [22]. Stata version 14.0 (Stata Corp., College Station, TX, USA) was used for all analyses. We obtained ethical approval from the Johns Hopkins University Institutional Review Board and Ethical Review Committee of icddr,b.
Results
We had pre-pregnancy height and weight data of 13,230 women. Among this cohort, 4291 incident pregnancies were identified from 3549 women (Fig. 1). Pregnancy outcomes were ascertained for 4198 pregnancies of which 3300 were singleton livebirths. Birthweight was missing in 507 babies and 168 babies had improbable gestation age (Fig. 1). The final analytic sample comprised of 2655 singleton livebirth babies (Fig. 1).
Fig. 1.
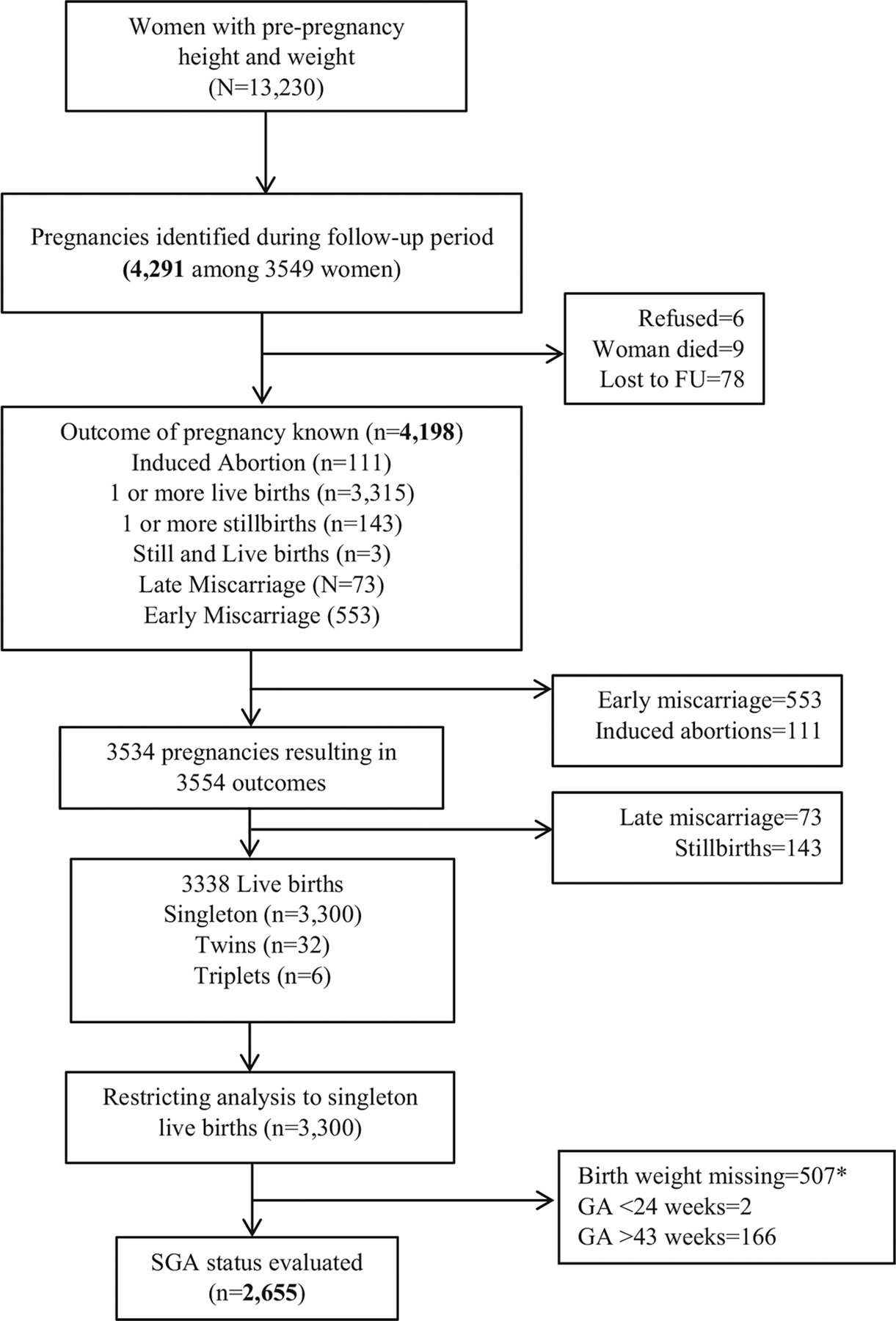
Study Flow Chart
*30 babies with gestational age >43 weeks also had birth weight missing
Distributions of pre-pregnancy height and BMI of women and rates of preterm births and SGA are presented in Table 1. About 16.3% of the women had short stature of <145 cm and another 32.7% had a height of 145 − < 150 cm. About two-fifths of the women were underweight with a BMI of <18.5 kg/m2. About 22.9% of the babies were preterm and 34.4% were SGA. Overall, 33.2% of the babies were term SGA, 21.7% were preterm AGA, and 1.2% were preterm SGA (Table 1).
Table 1.
Distribution of pre-pregnancy height and weight of women and adverse birth outcomes in rural Bangladesh
| Frequency (%) | 95% CI | |
|---|---|---|
| Maternal characteristics | ||
| Height (cm) | ||
| <145 | 434 (16.3) | 15.0, 17.8 |
| 145–149.9 | 868 (32.7) | 30.9, 34.5 |
| 150–154.9 | 899 (33.9) | 32.1, 35.7 |
| ≥155 | 454 (17.1) | 15.7, 18.6 |
| BMI (kg/m2) | ||
| <18.5 | 1,042 (39.3) | 37.4, 41.1 |
| 18.5–24.9 | 1,487 (56.0) | 54.1, 57.9 |
| ≥25 | 126 (4.7) | 3.9, 5.6 |
| Infant characteristics | ||
| Term and preterm (weeks) status | ||
| ≥37 | 2,047 (77.1) | 75.5, 78.7 |
| <37 | 608 (22.9) | 21.3, 24.5 |
| Small for gestational age | ||
| AGA | 1,742 (65.6) | 63.8, 67.4 |
| SGA < 10% | 913 (34.4) | 32.6, 36.2 |
| Preterm and SGA | ||
| Term AGA | 1,167 (43.9) | 42.1, 45.9 |
| Term SGA | 880 (33.2) | 31.4, 34.9 |
| Preterm AGA | 575 (21.7) | 20.1, 23.2 |
| Preterm SGA | 33 (1.2) | 0.09, 1.7 |
BMI body mass index, SGA small for gestational age, AGA appropriate for gestational age, CI confidence interval
In bivariate analysis, women’s height, BMI, age, parity, education, smoking during pregnancy, any qualified antenatal care (ANC), household wealth, type of latrine and water sources were associated with infants’ gestational age and size for gestational age (term AGA, term SGA, preterm AGA, preterm SGA) status (Table 2).
Table 2.
Distribution of infants’ gestational age and size for gestational age (term AGA, term SGA, preterm AGA, preterm –SGA) by maternal height, BMI and selected sociodemographic and community characteristics
| Term AGA N, % | Term SGA N, % | Preterm AGA N, % | Preterm SGA N, % | p value | |
|---|---|---|---|---|---|
| Individual | |||||
| Height (cm) | |||||
| <145 | 150 (34.6) | 166 (38.3) | 107 (24.7) | 11 (2.5) | <0.001 |
| 145–149.9 | 384 (44.2) | 298 (34.3) | 175 (20.2) | 11 (1.3) | |
| 150–154.9 | 408 (45.4) | 291 (32.4) | 190 (21.1) | 10 (1.1) | |
| ≥155 | 225 (49.6) | 125 (27.5) | 103 (22.7) | 1 (0.2) | |
| BMI (kg/m2) | |||||
| <18.5 | 399 (38.3) | 372 (35.7) | 259 (24.9) | 12 (1.2) | <0.001 |
| 18.5–24.9 | 691 (46.5) | 476 (32.0) | 299 (20.1) | 21 (1.4) | |
| ≥25 | 77 (61.1) | 32 (25.4) | 17 (13.5) | 0 (0.0) | |
| Age (years) | |||||
| <25 | 387 (45.7) | 311 (36.8) | 138 (16.3) | 10 (1.2) | <0.01 |
| 25–29 | 324 (43.4) | 234 (31.4) | 179 (24.0) | 9 (1.2) | |
| ≥30 | 456 (42.9) | 335 (31.5) | 258 (24.3) | 14 (1.3) | |
| Parity (number) | |||||
| <3 | 768 (45.1) | 587 (34.5) | 325 (19.1) | 22 (1.3) | <0.01 |
| 3–4 | 310 (43.4) | 217 (30.4) | 178 (24.9) | 9 (1.3) | |
| ≥5 | 89 (37.2) | 76 (31.8) | 72 (30.1) | 2 (0.8) | |
| Education (year of schooling) | |||||
| No education | 123 (36.0) | 110 (32.2) | 103 (30.1) | 6 (1.8) | <0.001 |
| 1–5 years | 525 (41.7) | 430 (34.2) | 285 (22.7) | 18 (1.4) | |
| ≥6 years | 519 (49.2) | 340 (32.2) | 187 (17.7) | 9 (0.9) | |
| Smoking during pregnancy | |||||
| No | 476 (48.6) | 301 (30.7) | 191 (19.5) | 12 (1.2) | <0.01 |
| Yes | 691 (41.3) | 579 (34.6) | 384 (22.9) | 21 (1.3) | |
| Any qualified ANC care | |||||
| No | 544 (40.7) | 436 (32.6) | 340 (25.4) | 18 (1.4) | <0.001 |
| Yes | 623 (47.3) | 443 (33.7) | 235 (17.9) | 15 (1.1) | |
| ANC visits 4+ | |||||
| No | 1050 (43.3) | 805 (33.2) | 539 (22.3) | 29 (1.2) | 0.06 |
| Yes | 117 (50.7) | 74 (32.0) | 36 (15.6) | 4 (1.7) | |
| Household | |||||
| Wealth quintile | |||||
| Lowest quintile | 279 (37.4) | 258 (34.5) | 198 (26.5) | 12 (1.6) | <0.001 |
| Second lowest quintile | 196 (42.3) | 155 (33.5) | 106 (22.9) | 6 (1.3) | |
| Middle quintile | 212 (44.2) | 166 (34.6) | 96 (20.0) | 6 (1.3) | |
| Second highest quintile | 248 (45.9) | 180 (33.3) | 106 (19.6) | 6 (1.1) | |
| Highest quintile | 232 (54.6) | 121 (28.5) | 69 (16.2) | 3 (0.7) | |
| Husband’s education | |||||
| No education | 123 (36.0) | 110 (32.2) | 103 (30.1) | 6 (1.8) | <0.001 |
| 1–5 years | 594 (42.2) | 488 (34.7) | 302 (21.5) | 23 (1.6) | |
| ≥6 years | 450 (49.7) | 282 (31.1) | 170 (18.8) | 4 (0.4) | |
| Family size | |||||
| 1–4 | 198 (42.0) | 150 (31.8) | 116 (24.6) | 8 (1.7) | 0.36 |
| 5–6 | 252 (42.1) | 197 (32.9) | 145 (24.2) | 5 (0.8) | |
| 7–8 | 232 (45.7) | 171 (33.7) | 98 (19.3) | 7 (1.4) | |
| ≥9 | 485 (45.1) | 362 (33.6) | 216 (20.1) | 13 (1.2) | |
| Type of latrine | |||||
| Improveda | 1009 (46.2) | 690 (31.6) | 458 (21.0) | 28 (1.3) | <0.001 |
| Not-improvedb | 158 (33.6) | 190 (40.4) | 117 (24.9) | 5 (1.1) | |
| Source of drinking water | |||||
| Improvedc | 586 (47.4) | 370 (29.9) | 267 (21.6) | 14 (1.1) | <0.01 |
| Not-improvedd | 581 (41.0) | 510 (36.0) | 308 (21.7) | 19 (1.3) | |
| Type of cooking fuel | |||||
| Improvede | 6 (60.0) | 3 (30.0) | 1 (10.0) | 0 (0.0) | 0.71 |
| Not-improvedf | 1161 (43.9) | 877 (33.2) | 574 (21.7) | 33 (1.3) | |
| Community | |||||
| Food availability | |||||
| Pre-harvestg | 489 (44.9) | 371 (34.0) | 217 (19.9) | 13 (1.2) | |
| Post-harvesth | 678 (43.3) | 509 (32.5) | 358 (22.9) | 20 (1.3) | |
| Time to Upazila HQ | |||||
| <45 min | 733 (43.4) | 549 (32.5) | 384 (22.7) | 23 (1.4) | 0.27 |
| ≥45 min | 434 (44.9) | 331 (34.3) | 191 (19.8) | 10 (1.0) | |
Improved latrine included all flushed and pit latrines with slab
Non-improved latrine included pit latrine without slab, hanging latrine, dry latrine, and no latrine/bush/field
Improved sources of drinking water included water from pipe/tap, tube well and tank
Not-improved sources of drinking water included water from dug, spring, rain, and river/dam/lake/pond/stream/canal
Improved cooking fuel included cooking by electric, liquefied petroleum gas and kerosene
Not-improved cooking fuel included cooking by using wood, charcoal, straw/shrubs/grass, agricultural crop, and animal dung
Pre-harvest: period between July and December of a year
Post-harvest: period between January and June of a year
In the multivariable multinomial regression analyses, maternal height of <145 cm was a significant predictor of term SGA (RRR 1.88, 95% CI 1.37, 2.58), preterm AGA (RRR 1.45, 95% CI 1.02, 2.05), and preterm SGA (RRR 14.40. 95% CI 1.82, 113.85). Maternal height of 145–149.9 was also a significant predictor of term SGA (RRR 1.37, 95% CI 1.04, 1.79). Maternal underweight status (BMI < 18.5 kg/m2) was a significant predictor of term SGA (RRR 1.32, 95% CI 1.10, 1.59), and preterm AGA (RRR 1.39, 95% CI 1.12, 1.71) (Table 3). The risks of term SGA (RRR 1.37, 95% CI 1.00, 1.87) and preterm AGA (RRR 1.49, 95% CI 1.04, 2.15) were significantly higher among women belonging to household with the lowest wealth quintile (Table 3). Compared to women having a minimum of secondary level education, illiterate women had about 50% higher risk of preterm AGA (RRR 1.50, 95% CI 1.06, 2.14). The risk of preterm AGA was about 25% lower (RRR 0.75, 95% CI 0.58, 0.98) among women <25 years of age compared to women >30 years (Table 3). Compared to women who were not exposed to smoking during pregnancy, women who were exposed were at a significantly higher risk of term SGA (RRR 1.22, 95% CI 1.01, 1.47) (Table 3). Women who received any antenatal care had a lower risk of preterm AGA (RRR 0.72, 95% CI 0.58, 0.88) compared to women who did not receive antenatal care (Table 3). Use of antenatal care was not associated with the risk of term SGA or preterm SGA.
Table 3.
Multinomial logistic regression to determine the effect of maternal height and BMI on infants’ term/preterm status and AGA/SGA status (reference: term AGA)
| Term SGA RRR, 95% CI | Preterm AGA RRR, 95% CI | Preterm SGA RRR, 95% CI | |
|---|---|---|---|
| Height (cm) | |||
| <145 | 1.88 (1.37, 2.58) | 1.45 (1.02, 2.05) | 14.40 (1.82, 113.85) |
| 145–149.9 | 1.37 (1.04, 1.79) | 0.99 (0.73, 1.33) | 6.13 (0.78, 47.98) |
| 150–154.9 | 1.28 (0.98, 1.68) | 1.05 (0.78, 1.41) | 5.59 (0.71, 44.03) |
| ≥155 | ref | ref | ref |
| BMI (kg/m2) | |||
| <18.5 | 1.32 (1.10, 1.59) | 1.39 (1.12, 1.71) | 0.97 (0.47, 2.02) |
| 18.5–24.9 | ref | ref | ref |
| ≥25 | 0.69 (0.45, 1.07) | 0.62 (0.36, 1.08) | NA |
| Age (years) | |||
| <24 | 1.17 (0.94, 1.45) | 0.75 (0.58, 0.98) | 0.98 (0.41, 2.34) |
| 25–29 | 1.05 (0.84, 1.32) | 1.14 (0.89, 1.47) | 1.03 (0.43, 2.46) |
| ≥30 | ref | ref | |
| Education (years) | |||
| 0 | 1.14 (0.82, 1.58) | 1.50 (1.06, 2.14) | 1.80 (0.54, 5.96) |
| 1–5 | 1.10 (0.90, 1.35) | 1.17 (0.92, 1.48) | 1.47 (0.62, 3.51) |
| ≥6 | ref | ref | ref |
| Wealth quintile | |||
| Lowest quintile | 1.37 (1.00, 1.87) | 1.49 (1.04, 2.15) | 1.82 (0.46, 7.24) |
| Second lowest quintile | 1.23 (0.89, 1.70) | 1.28 (0.87, 1.87) | 1.46 (0.34, 6.23) |
| Middle quintile | 1.23 (0.90, 1.68) | 1.13 (0.77, 1.65) | 1.37 (0.32, 5.81) |
| Second highest quintile | 1.23 (0.92, 1.66) | 1.20 (0.84, 1.72) | 1.44 (0.35, 5.96) |
| Highest quintile | ref | ref | ref |
| Smoking during pregnancy | |||
| No | ref | ref | ref |
| Yes | 1.22 (1.01, 1.47) | 1.20 (0.96, 1.48) | 0.98 (0.47, 2.05) |
| Any qualified ANC care | |||
| No | ref | ref | ref |
| Yes | 0.98 (0.82, 1.18) | 0.72 (0.58, 0.88) | 0.91 (0.45, 1.87) |
If the height of Bangladeshi women would reach to the level of the height of US women, the proportion of term SGA, preterm AGA, and preterm SGA will be reduced by 23.2%, 7.3%, and 73.9%, respectively.
Discussion
We documented high prevalence of maternal stunting; 16.3% of the women were <145 cm and another 32.7% were between 145– < 150 cm. The prevalence of maternal pre-pregnancy underweight status was high at 39.3%. The burden of preterm birth (22.9 %) and SGA (34.4%) were also high. Short maternal stature was a significant predictor for all three outcomes (term SGA, preterm AGA, and preterm SGA). Compared to women of a height of ≥155 cm, women with a height of <145 cm had about twofold higher risk of term SGA and fourteen fold higher risk of preterm SGA. Compared to women with normal BMI (BMI 18.5–24.9 kg/m2), underweight (BMI < 18.5 kg/m2) women had about 32.0% and 39.0% higher risk of term SGA and preterm SGA, respectively (Table 3).
Our findings are consistent with earlier observations that short stature of women increases the risk of both preterm birth and SGA [6, 23]. A meta-analysis using data from LMIC countries that used INTERGROWTH-21st reference to define SGA reported that all women with short stature had statistically significantly higher risk of term SGA, preterm AGA, and preterm SGA births. Women < 145 cm had the highest adjusted risk ratios (aRRs) (term SGA: aRR 2.03, 95% CI: 1.76, 2.35; preterm AGA: aRR 1.45, 95% CI: 1.26, 1.66; preterm SGA: aRR 2.13, 95% CI: 1.42, 3.21). Another study reported that maternal height of <150 cm was associated with 1.60, 1.34, and 5.92 folds higher risk of term SGA, preterm AGA, and preterm SGA, respectively [23]. Millions of SGA and preterm births are attributed to short maternal stature [6]. We estimated that in our population about 23% of the term SGA, 7% of preterm AGA and three-fourth of the preterm SGA were attributable to short maternal stature. Our estimated PAF for term SGA and preterm AGA were almost identical to estimates of Kozuki et al. for the South Asia region [6]. Our calculation of PAFs assumes that the outcomes are either directly linked to maternal short stature or operating through underlying factors that are highly linked to short stature.
The association of maternal height and SGA may have a biomechanical plausibility and a biological plausibility [24]. Shorter women are more likely to possess low uterine cavity, which may lead to intrauterine growth restriction, and may contribute to spontaneous preterm birth because of earlier filling of the pelvis [24, 25]. They may have long term poor undernutrition leading to inadequate supply of nutrients to the fetus leading to IUGR [24]. Furthermore, they may also be more susceptible to infections during pregnancy increasing the risk of adverse pregnancy outcomes [26]. The increased risk may also be transgenerational due to genetic predisposition or placental epigenetic modifications which might contribute to intrauterine growth [27] and might play a role to determine adulthood height of later generations [28].
We have demonstrated that maternal short stature, a measure of chronic undernutrition and under-weight status, which is a measure of acute malnutrition, were independent risk factors for both SGA and preterm birth after controlling for maternal age, education and household wealth. Both prematurity and SGA are multifactorial, there are different biological processes involved and preterm babies are more vulnerable than SGA babies. In a pooled analysis of 20 identified cohorts providing data for 2,015,019 livebirths from Asia, Africa, and Latin America, the RRs for preterm babies were 6.82 (95% CI 3.56–13.07) for neonatal mortality and 2.50 (1.48–4.22) for post-neonatal mortality and for SGA were 1.83 (95% CI 1.34–2.50) for neonatal mortality and 1.90 (1.32–2.73) for post-neonatal mortality [5]. The neonatal mortality risk of babies who were both preterm and SGA was higher than that of babies with either characteristic alone (15.42; 9.11–26.12). Thus, differentiation of the burden and risk of babies born preterm and/or SGA rather than with low birthweight would guide prevention and management strategies to speed progress towards the reduction of child mortality.
The study has several limitations. Birth weight (BW) was missing in 507 (15.3%) newborns. Missingness of BW was not significantly associated with women’s height or BMI but was higher among younger, more educated women and women of wealthy households (data not shown). Neonatal death was ninefold higher among those who had a missing BW than those with the BW (56/507 vs 34/2793). Three-fourth of the neonatal deaths among missing BW was in the first 2 days compared to a third among those who had the BW suggesting that missing BW was partially due to survival bias. Birth weight measurements were often delayed up to 72 h. Since newborns usually lose 5–10% of their initial weight during the first 4 days of life [29], the delayed measurement might have contributed to an over-estimation of SGA rate. However, we did not observe any particular pattern. In multivariate multinomial logistic regression analysis only preterm SGA rate was higher among those who were measured between 24 and 47 h of birth but none of the adverse outcomes was different for those who were measured between 48 and 71 h compared to those who were measured within 24 h of birth (data not shown). We were not able to examine several risk factors, for example, weight gain during pregnancy and preceding birth interval, which could have been important covariates for this analysis. We did not include parity in the multivariate analysis because it was significantly correlated with women’s age. Also, the study did not include data on hypertension, infection, anemia, and management of illness in study women, which might be important for adverse perinatal outcomes. We used LMP to calculate gestational age, which might have led to misclassification of term and preterm status. However, the error in estimation of gestational age may be minimal because we conducted monthly pregnancy surveillance aided by a LMP calendar to prospectively record LMP and conducted pregnancy test in all women with a missed period of >4 weeks. Accuracy of classifying preterm birth identification in another study in Bangladesh was high based on this method with sensitivity of 86% and specificity of 96% compared to ultrasound based gestational age classification [30].
The high burden of undernutrition among women and SGA and preterm births among babies that we have documented are important public health problems in Bangladesh and in many other LMICs. Our data suggest that maternal undernutrition, both chronic and acute, along with no or low maternal education, and low household wealth are the key factors that demand attention to reduce the burden of SGA and preterm birth. The Government of Bangladesh launched a multi-sectoral National Plan of Action for Nutrition (NPAN) in 1997. It comprises nutrition specific and nutrition-sensitive policies and interventions in an array of sectors including health, education, agriculture, fisheries and livestock, environment, social protection, women empowerment, and disaster management. Bangladesh has made considerable progress in nutrition in recent years [31]. The proportion of under 5-year-old children who are moderate to severely stunted reduced from 55% in 1997 to 41% in 2011 and 36% in 2014 [17, 32, 33]. Much of the improvements is explained by nutrition-sensitive drivers including improved access to education for girls; strong family planning program leading to smaller family sizes and increased birth intervals; delayed first pregnancy; more widespread use of safe water sources and better sanitation; improved infrastructure and electrification; pro-poor economic growth, improved agricultural production and diversification; a vibrant NGO sector supporting income generation and delivering basic services; expansion of non-farm business and manufacturing sectors creating employment opportunities; and remittances from labor migration [34]. Despite gains in recent years, one in three children in Bangladesh are stunted because the program is underfunded, under-staffed and the quality of implementation is inadequate [35]. As one of the lowest tax-to-gross domestic product (GDP) ratios country of the world, the Government of Bangladesh is constrained in what it can spend on health, population and nutrition programs. Currently, Bangladesh spends only about 3.4% of gross domestic product (GDP) on health, population and nutrition program, which is lower than the average spending of low-income countries (5.4%), and far below the average spending of the world (8.5%) [36]. Bangladesh needs to increase spending on health and nutrition programs to ensure adequate human and other resources for optimal implementation of the NPAN to achieve its long term nutritional goal.
The Government of Bangladesh is implementing a core set of nutrition specific interventions recommended in the Lancet maternal and child nutrition series for women during pregnancy [37]. These interventions include provision of iron-folate along with targeted protein energy supplementation during antenatal care services. However, the coverage of antenatal care is low; about 64 percent pregnant women receive just one antenatal visit and 31 percent receive the recommended four visits [17]. Our earlier findings from this population suggest that community-based approaches offer unique opportunity to reach poor and hard to reach women through communications and outreach strategies [38]. Community strategies would also allow integration of nutrition interventions with maternal, new-born and child health services.
Acknowledgements
We acknowledge the contribution of the study women and the dedication of Projahnmo field team. Projahnmo is a research partnership of Johns Hopkins University, the Bangladesh Ministry of Health and Family Welfare and other Bangladeshi institutions including ICDDR,B, and Shimantik.
Funding
The study was funded by grants from the NICHD (R01 HD066156-02).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.UNICEF, WHO. Low Birth Weight: Country. New York: Regional and Global Estimates; 2004. http://www.unicef.org/publications/index_24840. [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- 3.Schlaudecker EP, Munoz FM, Bardaji A, Boghossian NS, Khalil A, Mousa H, et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine. 2017;35:6518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ. 2017;358:j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozuki N, Katz J, Lee AC, Vogel JP, Silveira MF, Sania A, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low-and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. 2015;145:2542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozuki N, Lee AC, Silveira MF, Sania A, Vogel JP, Adair L, et al. The associations of parity and maternal age with small-forgestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13(Suppl 3):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozuki N, Katz J, LeClerq SC, Khatry SK, West KP Jr., Christian P. Risk factors and neonatal/infant mortality risk of small-forgestational-age and preterm birth in rural Nepal. J Matern Fetal Neonatal Med. 2015;28:1019–25. [DOI] [PubMed] [Google Scholar]
- 9.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–93. [DOI] [PubMed] [Google Scholar]
- 10.Simas TA, Waring ME, Liao X, Garrison A, Sullivan GM, Howard AE, et al. Prepregnancy weight, gestational weight gain, and risk of growth affected neonates. J Women Health. 2012;21:410–7. [DOI] [PubMed] [Google Scholar]
- 11.Leng J, Hay J, Liu G, Zhang J, Wang J, Liu H, et al. Small-forgestational age and its association with maternal blood glucose, body mass index and stature: a perinatal cohort study among Chinese women. BMJ Open. 2016;6:e010984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Sundquist K, Sundquist J. Risks of small-for-gestational-age births in immigrants: a nationwide epidemiological study in Sweden. Scand J Public Health. 2012;40:634–40. [DOI] [PubMed] [Google Scholar]
- 13.Xie C, Epstein LH, Eiden RD, Shenassa ED, Li X, Liao Y, et al. Stunting at 5 Years Among SGA Newborns. Pediatrics. 2016;137: e20152636. [DOI] [PubMed] [Google Scholar]
- 14.Hayward I, Malcoe LH, Cleathero LA, Janssen PA, Lanphear BP, Hayes MV, et al. Investigating maternal risk factors as potential targets of intervention to reduce socioeconomic inequality in small for gestational age: a population-based study. BMC Public Health. 2012;12:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derraik JG, Lundgren M, Cutfield WS, Ahlsson F. Maternal height and preterm birth: a study on 192,432 Swedish women. PLoS ONE. 2016;11:e0154304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinturache A, McKeating A, Daly N, Sheehan S. M T. Maternal body mass index and the prevalence of spontaneous and elective preterm deliveries in an Irish obstetric population: a retrospective cohort study. BMJ Open. 2017;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Population Research and Training (NIPORT), Ministry of Health and Family Welfare, Mitra and Associates (MA) Bangladesh demographic and health survey 2014. Rock-Ville, Maryland: The DHS program, ICF International; 2016.. [Google Scholar]
- 18.Lee ACC, Quaiyum MA, Mullany LC, Mitra DK, Labrique A, Ahmed P, et al. Screening and treatment of maternal genitourinary tract infections in early pregnancy to prevent preterm birth in rural Sylhet, Bangladesh: a cluster randomized trial. BMC Pregnancy and Childbirth. 2015;15:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baqui AH, Rosen HE, Lee AC, Applegate JA, El Arifeen S, Rahman SM, et al. Preterm birth and neonatal mortality in a rural Bangladeshi cohort: implications for health programs. J Perinatol. 2013;33:977–81. [DOI] [PubMed] [Google Scholar]
- 20.Khanam Rasheda, Baqui Abdullah H, Syed MamunIbneMoin, Harrison Meagan, Begum Nazma, Quaiyum Abdul, et al. Can facility delivery reduce the risk of intrapartum complications related perinatal mortality: findings from a cohort study. J Glob Health. 2017;8:010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–68. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control. NHANES 2011–2012 Examination data 2014. http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Examination&CycleBeginYear=2011 (2014) Accessed on 31 January 2018.
- 23.Muhihi A, Sudfeld CR, Smith ER, Noor RA, Mshamu S, Briegleb C, et al. Risk factors for small-for-gestational-age and preterm births among 19,269 Tanzanian newborns. BMC Pregnancy Childbirth. 2016;16:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozaltin E, Hill K, Subramanian SV. Association of maternal stature with offspring mortality, underweight, and stunting in low-to middle-income countries. JAMA. 2010;303:1507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer MS, McLean FH, Eason EL, Usher RH. Maternal nutrition and spontaneous preterm birth. Am J Epidemiol. 1992;136:574–83. [DOI] [PubMed] [Google Scholar]
- 26.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20: 439–50. [DOI] [PubMed] [Google Scholar]
- 28.Timasheva Y, Putku M, Kivi R, Kozich V, Mannik J, Laan M. Developmental programming of growth: genetic variant in GH2 gene encoding placental growth hormone contributes to adult height determination. Placenta. 2013;34:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez G, Ventura P, Samper MP, Moreno L, Sarria A, Perez-Gonzalez JM. Changes in body composition during the initial hours of life in breast-fed healthy term newborns. Biol Neonate. 2000;77:12–6. [DOI] [PubMed] [Google Scholar]
- 30.Gernand AD, Paul RR, Ullah B, Taher MA, Witter FR, Wu L, et al. A home calendar and recall method of last menstrual period for estimating gestational age in rural Bangladesh: a validation study. J Health Popul Nutr. 2016;35:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osmani SR, Ahmed A, Ahmed T, Hossain N, Huq S, A. S. Strategic Review of Food Security and Nutrition in Bangladesh. Executive Summary. Rome, Italy: World Food Programme; 2016. [Google Scholar]
- 32.Mitra SN, Ahmed A1-Sabir, Anne R, Cross, Jamil K Bangladesh Demographic and Health Survey, 1996–1997. Dhaka and Calverton, Maryland: National Institute of Population Research and Training (NIPORT), Mitra and Associates, and Macro International Inc.; 1997. [Google Scholar]
- 33.National Institute of Population Research and Training (NIPORT), Mitra and Associates (MA), ICF International Bangladesh Demographic and Health Survey 2011. Dhaka, Bangladesh and Calverton, Maryland, USA: NIPORT, Mitra and Associates, and ICF International.; 2013. [Google Scholar]
- 34.Nisbett N, Davis P, Yosef S, Akhtar N. Bangladesh’s story of change in nutrition: Strong improvements in basic and underlying determinants with an unfinished agenda for direct community level support. Glob Food Secur. 2017;13:21–9. [Google Scholar]
- 35.Unicef. Learning from nutrition programme evaluations: a thematic evaluation synthesis report. https://www.unicef.org/evaluation/files/Final_learning_from_nutrition_17_07_2014.pdf (2014) Accessed on 11 May 2018.
- 36.Majumder MAA. World health statistics 2011: how does Bangladesh compare with other south-east Asian countries. South East Asia. J Public Health. 2013;1:4–11. [Google Scholar]
- 37.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382:452–77. [DOI] [PubMed] [Google Scholar]
- 38.Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371:1936–44. [DOI] [PubMed] [Google Scholar]


