Abstract
Purpose:
To compare efficacy and safety of capecitabine and lapatinib with or without IMC-A12 (cituxumumab) in patients with HER2-positive metastatic breast cancer (MBC) previously treated with trastuzumab.
Patients and Methods:
Following an initial safety run-in cohort, patients were randomized 1:2 to Arm A (capecitabine and lapatinib) or to Arm B (capecitabine, lapatinib, and cituxumumab). Given the frequency of non-hematologic grade ≥3 adverse events in those receiving the three-drug combination in the safety cohort, lapatinib and capecitabine doses were reduced in Arm B only. The primary objective was to determine if the addition of cituxumumab to capecitabine and lapatinib improved progression-free survival (PFS) compared with capecitabine and lapatinib. Secondary objectives included a comparison between arms of other clinical endpoints, safety, change in overall quality of life (QOL) and self-assessed fatigue, rash, diarrhea, and hand-foot syndrome.
Results:
From July 2008 to March 2012, 68 patients (out of 142 planned) were enrolled and 63 were evaluable, including 8 for the safety run-in and 55 for the randomized cohort. Study enrollment was stopped early due to slow accrual. The addition of cituxumumab to capecitabine and lapatinib did not improve PFS (HR 0.93, 95% CI: 0.52-1.64). Furthermore, no difference in objective response rate or overall survival (OS) was observed. No difference between arms was observed in grade ≥3 adverse events, overall QOL change from baseline after 4 cycles of treatment.
Conclusion:
The addition of cituxumumab to lapatinib and capecitabine did not improve PFS or OS compared with lapatinib and capecitabine in patients with HER2-positive MBC.
Keywords: metastatic breast cancer, HER2-positive, insulin-like growth factor receptor, trastuzumab-resistance
Introduction
Metastatic breast cancer (MBC) is a major cause of mortality worldwide and claims the lives of approximately 40,000 women in the United States annually [1]. Approximately 15-20% of patients with MBC have HER2-positive disease, which is associated with more aggressive disease biology and historically, a poorer prognosis relative to other molecular subtypes [2]. In the last twenty years, eight HER2-directed agents have been approved by the U.S. Food and Drug Administration (FDA) for use for patients with primary HER2-positive breast cancer [3]. The advent of HER2-directed therapy has greatly improved patient outcomes; however, de novo and acquired resistance to HER2-directed therapies is common [4], and there are no FDA-approved therapies to overcome resistance. HER2-positive MBC remains incurable in the vast majority of patients [3], and better treatments, including those that can restore sensitivity to HER2-directed therapies, remain an unmet clinical need.
Insulin-like growth factor I receptor (IGF-1R) signaling has been implicated in de novo and acquired resistance to trastuzumab or EGFR-targeting agents in breast cancer models[5]. In trastuzumab-resistant subclones of HER-2 overexpressing cell lines, unique co-localization or heterodimerization of IGF-1R and HER2 has been identified [5,6]. Treatment of the resistant cells with anti-IGF-1R antibody or small molecule tyrosine kinase inhibitor (TKI) was shown to inhibit transactivation of HER2 and restore sensitivity to trastuzumab [5–7]. Similarly, addition of anti-IGF-1R agents to EGFR small molecule inhibitors or anti-EGFR antibodies have been shown to prevent, delay, or reverse resistance to these anti-EGFR agents [8,9].
The utility of small molecule inhibitors of the hetero- and homodimers of the IGF-1R and Insulin Receptor (IR) in breast and ovarian cancer have previously been examined [10]. Notably, inhibition of the IGF-1R/IR with BMS-554417 led to increased phosphorylation of HER2. In preclinical studies, combination regimens with IGF-1R targeted agents enhanced the anti-tumor activity of chemotherapy, radiotherapy, endocrine therapy [11], EGFR/HER2 inhibitors, and agents targeting the mammalian target of rapamycin (mTOR) pathway [12,13]. Cituxumumab (IMC-A12) is a fully human IgG1 monoclonal antibody that specifically targets the human IGF-1R. The toxicity profile observed in phase I studies was acceptable [14,15], and phase II studies were conducted in several tumor types [16–18]. In a meta-analysis of cituxumumab use, the most common adverse events (AEs) were hyponatremia (40%), fatigue (35%), and skin rash (35%) [16]. Other common AEs included hyperglycemia, anemia, nausea, and thrombocytopenia.
The combination of lapatinib, an EGFR/HER2 TKI, and capecitabine was considered as standard of care in mid-late 2000s for patients with HER2-positive MBC who had progressed on an anthracycline, taxane and trastuzumab [19]. Based on the aforementioned preclinical data, it was hypothesized that co-inhibition of IGF-1R and the HER family of receptors in combination with chemotherapy was a promising treatment strategy in trastuzumab-resistant breast cancer. Herein we report the findings of North Central Cancer Treatment Group (NCCTG) N0733, a patient safety study of a three-drug combination followed by a randomized phase II study with the aim of assessing progression-free survival (PFS) between two groups (capecitabine + lapatinib vs. capecitabine + lapatinib + cituxumumab). NCCTG is now part of the Alliance for Clinical Trials in Oncology.
Patients and Methods
Patient Eligibility
Women with histologically or cytologically confirmed locally advanced or metastatic HER2-positive breast cancer were eligible if they had disease progression after trastuzumab and an anthracycline and/or a taxane. Unlimited prior chemotherapy and endocrine therapy were allowed. Prior treatment with trastuzumab was required unless contraindicated. HER2-positive status was defined by 2007 ASCO/CAP guidelines, e.g., immunohistochemistry assay score of 3+ (uniform, intense staining of >30% of invasive tumor cells), or average HER2 gene copy number of > 6, or gene amplification HER2:D17Z1 ratio >2.20. Additional inclusion criteria included measurable disease according to RECIST criteria version 1.0 [20]; Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2; adequate cardiac function defined as a left ventricular ejection fraction ≥50%; central nervous system metastasis controlled by prior surgery and/or radiotherapy; and acceptable hematologic, renal and hepatic function.
Patients were excluded if within 5 years prior to registration they had been diagnosed with stage III or IV invasive cancer (other than breast cancer), or they were actively being treated for another malignancy, with the exception of non-melanoma skin cancer or carcinoma in situ of the cervix. Major surgery, chemotherapy, or immunotherapy ≤ 4 weeks prior to registration was not permitted. Other exclusion factors included New York Heart Association Class III or IV cardiovascular disease; active hepatic or biliary disease; leptomeningeal disease. Women of childbearing potential were required to use adequate contraception. The protocol was approved by the Mayo Clinic institutional review board, and patients provided written informed consent prior to screening and study registration.
Treatment Plan
For the safety run-in, patients were to receive lapatinib 1250 mg orally once daily continuously, capecitabine 1000 mg/m2 orally twice daily on days 1-14 of a 21-day cycle, in combination with IMC-A12 6 mg/kg intravenously on days 1, 8 and 15 of a 21-day cycle. Following the safety run-in, patients were randomized in a 1:2 ratio to the control arm, Arm A, comprised of capecitabine and lapatinib, or to Arm B, comprised of the three drug combination at the same dose and schedule as the safety run-in. No standard pre-medications or matching placebo infusion (Arm A only) were administered. Patients with moderate renal impairment (calculated creatinine clearance 30 - 50 mL/min) were started on capecitabine at a dose of 875 mg/m2 twice daily (Dose Level -1). If patients discontinued IMC-A12 due to AEs at least possibly attributed to the agent, lapatinib and capecitabine were continued if clinically appropriate. Patients continued treatment until unacceptable toxicity or disease progression. Due to toxicity observed in the safety run-in cohort, the doses of lapatinib and capecitabine were respectively reduced to 1000 mg orally once daily and 825 mg/m2 orally twice daily when given with IMC-A12 in Arm B, but in Arm A the doses of capecitabine and lapatinib were unchanged.
Safety and Efficacy Assessments
Patients were evaluated before each cycle of treatment. Toxicities were graded per the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 3.0. Disease status was assessed at baseline using CT or MRI and every six weeks thereafter using RECIST v1.0 [20]. In patients with known bone metastases, bone scans were performed at baseline, and then as clinically indicated. Cardiac surveillance by MUGA or echocardiogram was performed at baseline and prior to every fourth cycle of treatment. Fasting glucose was evaluated weekly for the first four cycles and lipid profiles were assessed every other cycle. For patients who completed more than 50 cycles of therapy, at the treating investigator’s discretion, patient visits and associated safety laboratory results could be extended to once every 3 cycles (9 weeks).
Quality of Life Assessments
In order to assess the qualitative impact of the addition of IMC-A12 to the combination of lapatinib and capecitabine, patients’ quality of life (QOL) was measured as a secondary outcome using the overall item of the Linear Analogue Self-Assessment (LASA6) supplemented by single-item measures of fatigue, rash, diarrhea, and hand-foot syndrome, four of the most common symptoms reported by patients receiving lapatinib and capecitabine [19]. The overall item of the LASA6 measures overall QOL using a numeric analogue scale of 0-10 [21]. This item has been validated in cancer patients and used in other clinical trials [22–24]. The single-item numeric analogue scales measure fatigue, rash, diarrhea, and hand-foot syndrome, and this approach have been previously validated [23,25,24]. These items were analyzed as separate individual constructs.
Statistical Design and Data Analysis Plan
A randomized phase II study design was selected to assess the addition of cituxumumab to capecitabine and lapatinib to PFS and safety outcomes. The primary endpoint, PFS, was defined as the time from randomization to the earliest date of documentation of disease progression or death. The sample size of 132 eligible patients (44 in Arm A, and 88 in Arm B) was calculated to yield 90% power to detect a 15% increase in a 6-month PFS from 60% to 75% (or a hazard ratio of 0.56) using a one-sided α = 0.1 log rank test, where the accrual time and follow-up time were assumed as 16.5 months and 9.4 months, respectively. However, due to poor accrual, the study was closed in 2012, and only patients in the randomized study cohort arms (n=55; 19 in Arm A and 36 in Arm B) were included in the analyses for PFS, overall survival (OS), time to treatment failure, tumor response rate, and QOL. Due to insufficient sample size, only hazard ratios (Arm A vs. Arm B) with 95% CI were reported. The safety analysis included 63 evaluable patients (8 patients for the safety run-in, and 55 patients evaluable for the efficacy analysis). Safety was evaluated by the severity and incidence rate of AEs. Kaplan-Meier survival curves [26] were plotted to assess the difference in PFS, OS, and time to treatment failure between the treatment groups. Log-rank tests [27] were used to compare the hazard rates between Arm A and Arm B, while Arm A is the reference in all the tests. OS was defined as the time from randomization to death due to any cause. Time to treatment failure was defined as the interval from the date of randomization to the date at which the patient discontinued study participation due to disease progression, AEs, or personal preference. The tumor response was classified as a partial response (PR) or complete response (CR) based on 2 consecutive evaluations at least 6 weeks apart among all patients who started treatment. The response rate, with 90% confidence interval, and duration of the response were reported. Overall QOL single-item linear analogue self-assessment (QOL-LASA) [28], including overall QOL, as well as the incidence and severity of fatigue, rash, diarrhea, and hand-foot syndrome, were evaluated by the change in each item between pretreatment and cycle 4, for each treatment arm, and the score change difference between Arm A and B was compared by 2-sided Wilcoxon rank sum test. [29]
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. All analyses were performed using SAS statistical software (version 9.4M6, SAS Institute, Cary, NC) on a dataset frozen on September 25, 2019. The Mayo Clinic Cancer Center (MCCC) Data Safety Monitoring Board (DSMB) reviewed safety data every 6 months based on reports provided by the MCCC Statistical Office.
Results
Patient Characteristics
From July 2008 to March 2012, 68 patients enrolled in the clinical trial. Within the safety run-in cohort, 1 of the 9 enrolled patients had documented disease progression prior to treatment and was excluded from the analysis. Within the randomized study cohort, 59 patients were enrolled and 4 were unevaluable for the final efficacy analysis due to: disease progression prior to treatment (n=1), major violation (n=1), declining study participation prior to the start date (n=1), and no measurable disease (n=1) [Figure 1]. Demographics and characteristics were generally similar in both the safety run-in and randomized study cohorts as summarized in Table 1. Among the 63 evaluable patients, 61.9% (n=39) were 50 years of age or older; 74.6% (n=47) were white, non-Hispanic; 6.4% (n=4) were white with unreported ethnicity; and 19.0% (n=12) were of racial/ethnic minority populations. More than half of patients, 55.6% (n=35) were asymptomatic of their disease with ECOG performance status 0 at study registration.
Fig.1.
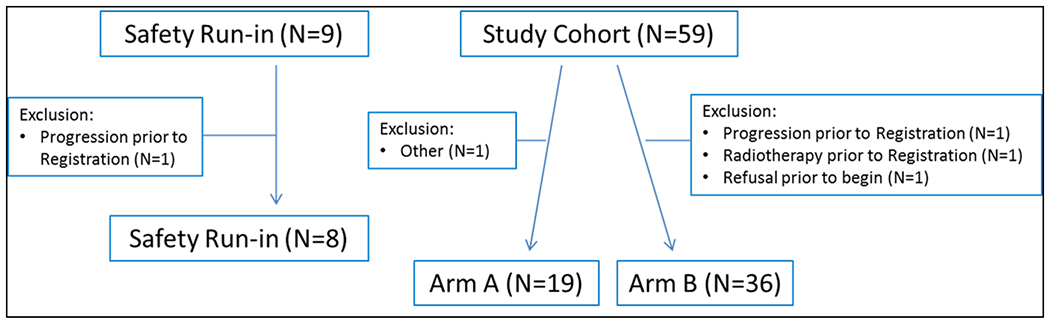
Consort Diagram
Note: Arm A is comprised of capecitabine and lapatinib; Arm B is the drug combination of capecitabine, lapatinib and cituxumumab.
Table 1.
Baseline patient and tumor characteristics
| Safety Run-in | Study Cohort | |||||
|---|---|---|---|---|---|---|
| (n=8) | Arm A (n=19) | Arm B (n=36) | ||||
| N | % | N | % | N | % | |
| Age at registration (years) | ||||||
| <50 | 2 | 25.0 | 6 | 31.6 | 16 | 44.4 |
| 50-60 | 4 | 50.0 | 4 | 21.0 | 11 | 30.6 |
| 60+ | 2 | 25.0 | 9 | 47.4 | 9 | 25.0 |
| Race | ||||||
| White | 8 | 100.0 | 15 | 78.9 | 30 | 83.3 |
| Black or African American | 3 | 15.8 | 4 | 11.1 | ||
| Other | 1 | 5.3 | 2 | 5.6 | ||
| Ethnicity | ||||||
| Hispanic or LatinX | 0 | 0 | 0 | 0 | 3 | 8.3 |
| Non-Hispanic | 6 | 75.0 | 18 | 94.7 | 32 | 88.9 |
| Not Reported | 2 | 25.0 | 1 | 5.3 | 1 | 2.8 |
| Performance Score | ||||||
| 0 | 4 | 50.0 | 10 | 52.6 | 21 | 58.3 |
| 1 | 3 | 37.5 | 8 | 42.1 | 14 | 38.9 |
| 2 | 1 | 12.5 | 1 | 5.3 | 1 | 2.8 |
| ER/PR status | ||||||
| ER+/PR+ | 3 | 15.8 | 13 | 36.1 | ||
| ER+/PR- | 1 | 12.5 | 7 | 36.8 | 3 | 8.3 |
| ER-/PR+ | 1 | 12.5 | ||||
| ER-/PR- | 6 | 75.0 | 9 | 47.4 | 20 | 55.6 |
| Common sites of metastases | ||||||
| Bone (Yes) | 1 | 12.5 | 6 | 31.6 | 15 | 41.7 |
| Liver (Yes) | 3 | 37.5 | 9 | 47.4 | 11 | 30.6 |
| Lung (Yes) | 3 | 37.5 | 10 | 52.6 | 16 | 44.4 |
| Nodal (Yes) | 4 | 50.0 | 11 | 57.9 | 24 | 66.7 |
| Skin (Yes) | 3 | 15.8 | 7 | 19.4 | ||
| Neoadjuvant/Adjuvant Chemotherapy (Yes) | 7 | 87.5 | 14 | 73.7 | 24 | 66.7 |
| Prior hormonal therapy for metastases (Yes) | 1 | 12.5 | 3 | 15.8 | 8 | 22.2 |
| Previous radiation therapy (Yes) | 6 | 75 | 14 | 73.7 | 23 | 63.9 |
Safety Analysis
The incidence and severity of AEs by treatment arm are presented in Table 2. In the safety run-in arm, grade ≥3 AEs were observed in at least one of the 8 patients for the following: anorexia, dehydration, diarrhea, fatigue, hand-foot syndrome, hypotension, hypocalcemia, mucositis, and thrombosis. The most common grade 3 non-hematologic AEs were diarrhea (n=3, 38%), mucositis (n=3, 38%), and fatigue (n=2, 25%). Grade ≥3 AEs were observed in 5 of 8 (62.5%) patients in the safety run-in cohort (data not shown).
Table 2.
Observed common toxicity (grade 2+ adverse events) in all study cohorts
| Common toxicity | Safety run-in (%, n=8) | Study Arm A (%, n=19) | Study Arm B (%, n=36) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gr 2 | Gr 3 | Gr 4 | Gr 2 | Gr 3 | Gr 4 | Gr 2 | Gr 3 | Gr 4 | |
| Alanine aminotransferase increased | 12.5 | 0 | 0 | 0 | 15.8 | 0 | 0 | 2.8 | 0 |
| Aspartate aminotransferase increased | 0 | 0 | 0 | 5.3 | 15.8 | 0 | 0 | 2.8 | 0 |
| Bilirubin increased | 0 | 0 | 0 | 10.5 | 0 | 0 | 5.6 | 0 | 0 |
| Hemoglobin decreased | 12.5 | 0 | 0 | 15.8 | 0 | 0 | 11.1 | 0 | 0 |
| Lipase increased | 0 | 0 | 0 | 0 | 0 | 5.3 | 0 | 2.8 | 0 |
| Anorexia | 12.5 | 12.5 | 0 | 0 | 0 | 0 | 11.1 | 0 | 0 |
| Dehydration | 12.5 | 12.5 | 0 | 0 | 5.3 | 0 | 0 | 5.6 | 0 |
| Diarrhea | 12.5 | 37.5 | 0 | 15.8 | 26.3 | 0 | 36.1 | 11.1 | 0 |
| Fatigue | 37.5 | 25.0 | 0 | 42.1 | 5.3 | 0 | 36.1 | 2.8 | 0 |
| Hand-and-foot syndrome/reaction | 50.0 | 12.5 | 0 | 42.1 | 5.3 | 0 | 19.4 | 25.0 | 0 |
| Hypotension | 0 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucositis oral | 37.5 | 37.5 | 0 | 10.5 | 5.3 | 0 | 13.9 | 0 | 0 |
| Nausea | 0 | 0 | 0 | 10.5 | 10.5 | 0 | 22.2 | 2.8 | 0 |
| Hypocalcemia | 0 | 12.5 | 0 | 0 | 0 | 0 | 2.8 | 0 | 0 |
| Thrombosis | 0 | 0 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 12.5 | 0 | 0 | 10.5 | 10.5 | 0 | 8.3 | 5.6 | 0 |
In the randomized cohort, the most common grade ≥3 AEs observed in Arm A (n=19) included: grade 3 increase in alanine aminotransferase (n=3, 16%), increase in aspartate aminotransferase (n=3, 16%), and diarrhea (n=5, 26%). Elevation in serum lipase was the only grade 4 event. One patient had grade 5 event in Arm A (due to disease progression while on treatment). In Arm B (n=36), the most common grade ≥3 AEs observed were diarrhea (n=4, 11%) and hand-foot syndrome (n=9, 25%). No grade 4 or 5 events were reported in Arm B. There were 11 out of 19 patients in Arm A (57.9%) and 22 out of 36 patients in Arm B (61.1%) who experienced a grade ≥3 AE (data not shown).
PFS, OS, and time to treatment failure
The median number of treatment cycles in Arm A was 6 (range: 1 - 16) and 5.5 (range: 0 - 89) in Arm B. The median duration of follow-up in Arm A was 27.4 months (range: 1.7 - 65.8) and Arm B was 19.1 months (range: 1.0-96.4). The median PFS in Arm A and B was 6.0 months (95% CI: 4.3-8.6) and 5.0 months (95% CI: 2.9-8.5) respectively. There was no PFS difference between the two arms (HR 0.93, 95% CI: 0.52-1.64) [Figure 2]. The median OS was 30.1 months (95% CI: 15.0-39.5) in Arm A and 21.9 months (95% CI: 12.0-45.4) in Arm B, and there was no OS difference between the two arms (HR 1.08, 95% CI: 0.55 - 2.12) [Figure 3]. Furthermore, the median time to treatment failure was 4.6 months (95% CI: 2.5-5.5) in Arm A and 4.2 months (95% CI: 2.9-5.7) in Arm B, and there was no difference in time to treatment failure between the two arms (HR 0.87, 95% CI: 0.48 - 1.59) [Figure 4].
Fig.2.
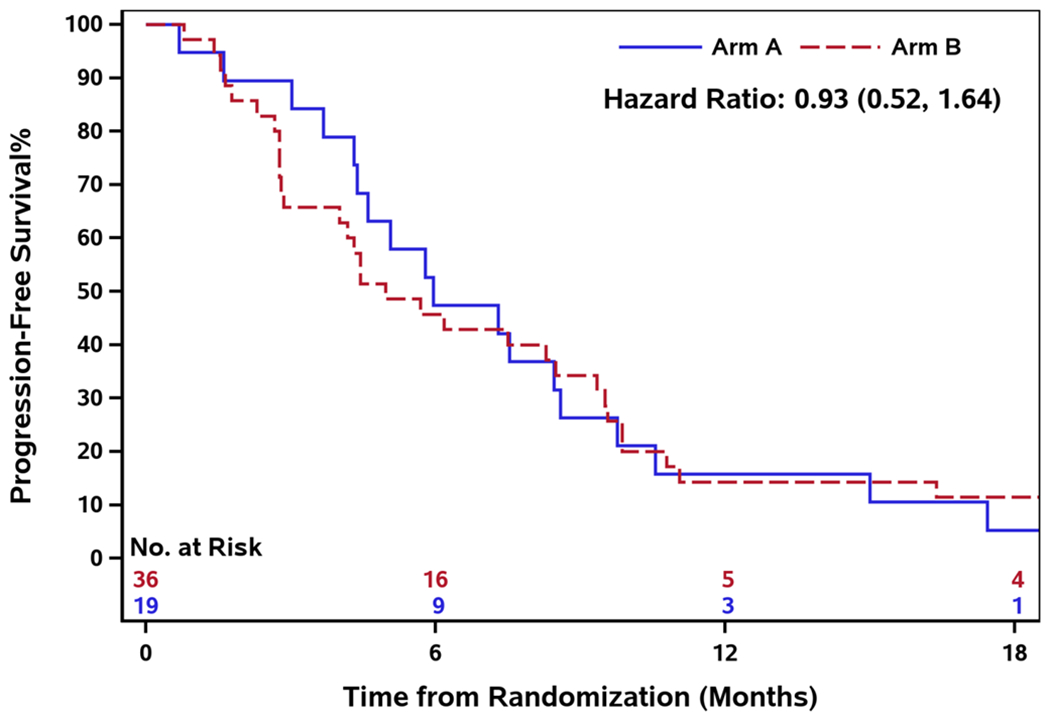
Progression-free survival by treatment arm
Fig.3.
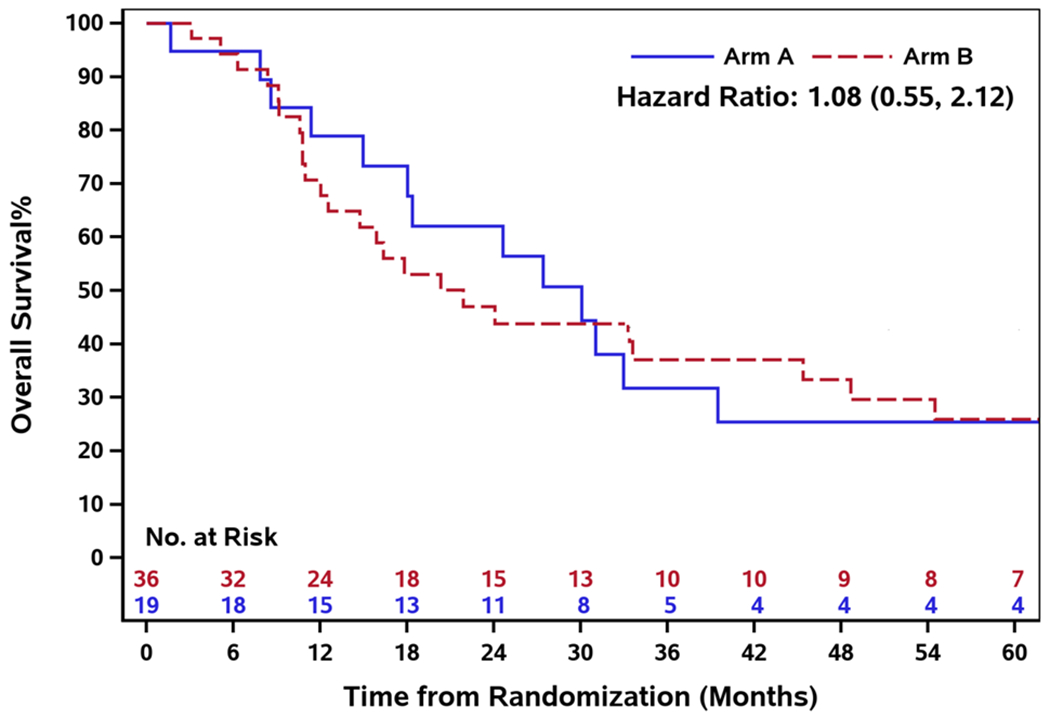
Overall survival by treatment arm
Fig.4.
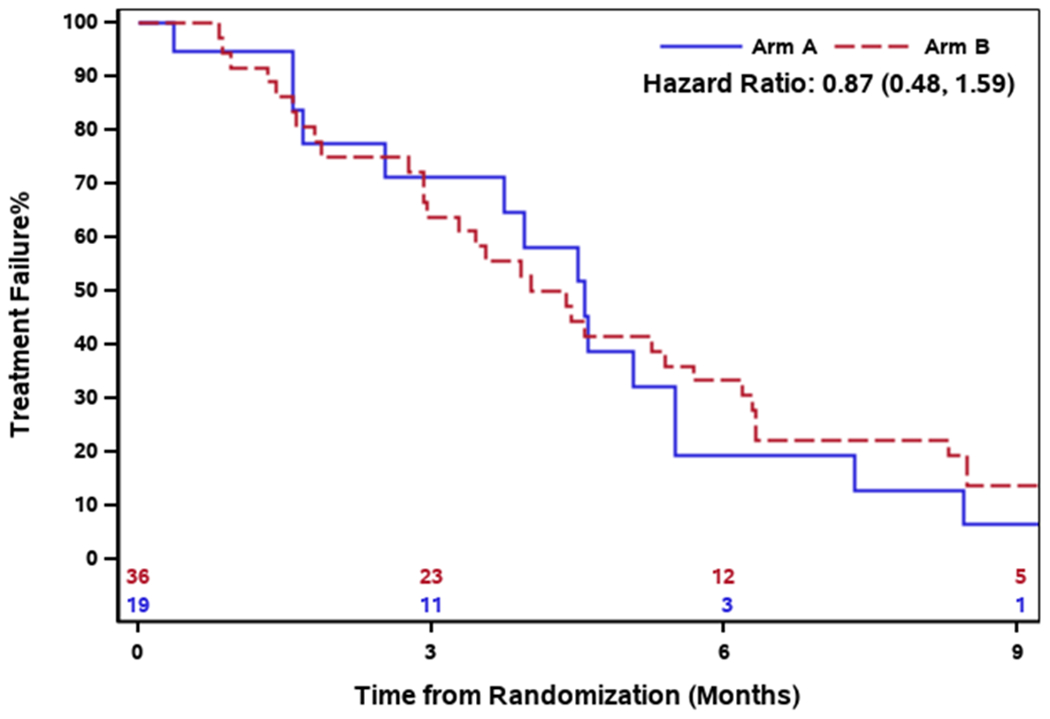
Time to treatment failure survival by treatment arm
The most common off-study reasons in Arm A were: disease progression (n=9, 47.4%), AE (n=4, 21.1%), and refusal of further treatment (n=3, 15.8%). In Arm B the most common off-study reasons were: disease progression (n=24, 66.7%), refusal of further treatment (n=8, 22.2%), and AE (n=3, 8.3%). The median of duration of follow-up in Arm A was 27.4 months (range: 1.7 - 65.8) and in Arm B was 19.1 months (range: 1.0 - 96.4).
Tumor response rate and duration of response
The overall response rate was 36.8% (90% CI: 18.8%-55.1%) in Arm A and 27.8% (90% CI: 15.5%-47.1%) in Arm B. The duration of response in Arm A ranged from 2.7 to 6.9 months, with a median of 3.2 months. The duration of response in Arm B ranged from 1.3 to 95.0 months with a median of 4.3 months.
Quality of Life
Eleven patients in Arm A (53.2%) and 25 patients in Arm B (69.4%) completed both baseline and cycle 4 overall QOL questionnaires. Paired single-item symptom assessments for fatigue, rash, diarrhea, and hand-foot syndrome were completed by all patients, except for 1 missing cycle 4 rash assessment in Arm A. The median and range of score change between baseline and cycle 4 in overall QOL, fatigue, rash, diarrhea, and hand-foot syndrome are reported in the Table 3. No significant differences in overall QOL or self-reported symptoms were observed between arms.
Table 3.
QOL point change at cycle 4 from baseline between Arm A and B
| QOL | Arm A | Arm B | p-value | ||
|---|---|---|---|---|---|
| n | median (range) | n | median (range) | ||
| QOL overall | 11 | 0 (−5, 1) | 25 | −1 (−6, 5) | 0.26 |
| Fatigue | 11 | 1 (−3, 7) | 25 | 0 (−4, 8) | 0.75 |
| Rash | 10 | 0 (−4, 2) | 25 | 0 (−5, 10) | 0.095 |
| Diarrhea | 11 | 2 (−10, 8) | 25 | 1 (−8, 8) | 0.99 |
| Hands and feet syndrome (skin or pain change) | 11 | 2 (−1, 8) | 25 | 4 (−7, 10) | 0.42 |
Note: The positive value of QOL overall score represents better QOL after 4 cycles of treatment; the positive value of fatigue, rash, diarrhea, and hands and feet syndrome score represents worse syndrome at cycle 4. N represents sample size.
Discussion
In this randomized phase II trial of capecitabine and lapatinib +/− IMC-A12 (cituxumumab), all patients had previously received trastuzumab and an anthracycline and/or a taxane. Thus they were appropriate candidates for the FDA-approved regimen of capecitabine and lapatinib [30]. The addition of cituxumumab to capecitabine and lapatinib, as evaluated in Arm B, did not increase median PFS (6.0 months in Arm A vs. 5.0 months in Arm B) or median OS (30.1 months in Arm A vs. 21.9 months in arm B) compared with lapatinib and capecitabine. Substantial toxicity was observed in the safety run-in cohort, as well as both randomized study arms, with similar percentage of patients experiencing a grade ≥3 AE in each cohort. No difference for patient-reported overall QOL change from baseline at cycle 4 was reported, which may have been due to a small sample size (<50% of patients completed both baseline and cycle 4 QOL questionnaires). Furthermore, there was no significant difference between study arms in patient-reported symptom assessments for fatigue, rash, diarrhea and hand-foot syndrome.
Data from phase II studies evaluating endocrine therapy or chemotherapy +/− cituxumumab in hormone receptor-positive (HR+), HER2-negative advanced breast cancer, in addition to other tumor types, have consistently demonstrated a similar lack of clinical efficacy, although the toxicity profile was generally more favorable [18,16,31–36]. Results from other studies of agents targeting the IGF-1R (i.e., figitumumab, ganitumab, dalotuzumab, others) in breast cancer and other solid tumors have also been disappointing [37–39,21,40–42]. For example, a phase II trial of figitumumab in combination with exemestane as first-line therapy in HR+, HER2-negative advanced breast cancer did not improve PFS compared with exemestane monotherapy [36,41]. In a phase II randomized trial of ganitumab with either exemestane or fulvestrant in postmenopausal women with advanced HR+ breast cancer, the addition of ganitumab to endocrine therapy did not improve PFS [38]. Similarly, there was no benefit for adding ganitumab and metformin to standard anthracycline and taxane-based neoadjuvant chemotherapy in patients with locally advanced, HER2-negative breast cancer as pathologic complete response rates were similar in the ganitumab-treated cohort compared with the control cohort in the I-SPY2 trial [42]. A notable exception for efficacy and tolerability of cituxumumab monotherapy was observed in patients with relapsed thymic epithelial malignancies [43].
There are several potential reasons why compounds directed against the human IGF-1R have not succeeded in phase III clinical trials [44,45]. First, treatment with IGF-1R antibody therapy causes hyperglycemia and metabolic syndrome via its effects on IGF-1R homeostasis, which results in elevation of growth hormone and consequently, insulin resistance. In turn, insulin resistance elevates insulin and potentiates activation of the IR [46]. Secondly, IGF-1R and the IR are very similar with regard to their structure and function, and hybrids of both receptors can participate in cell signaling[47]. Thirdly, the monoclonal antibodies specifically inhibit IGF-1R and not the IR [48]. Although TKIs of IGF-1R effectively inhibited both the insulin and IGF-1R receptors, further clinical development of these agents was not pursued, likely due to the metabolic side-effects [21]. Taken together, it was concluded that targeting the entire IGF-1R and IR axis may result in a more complete inhibition of the biological functions of the IGF-1R/insulin receptor. Several phase I and II studies noted exceptional responses to anti-IGF-1R antibody monotherapy [44], the results of which were not borne out in phase III trials. Furthermore, unexpected toxicities not seen in earlier phase studies with IGF-1R inhibitors were reported. In one study, a post hoc analysis identified that approximately two-thirds of the study population had low IGF-1 serum levels, which was associated with an increased likelihood of treatment-related toxicity and an overall detrimental effect of IGF-1R inhibition [36]. Therefore, incorporation of predictive biomarkers in the design of future clinical trials to evaluate agents targeting the anti-IGF-1R network will be important.
IGF-1/2 neutralizing antibodies have been evaluated in early phase clinical trials. In breast cancer, two relevant compounds have been studied, MEDI-573 and BI836845 (xentuzumab) [44]. Notably, the breast cancer-specific trials have enrolled participants with HR+, HER2-negative disease as IGF-1R mediates therapeutic resistance in patients with estrogen-receptor positive or HER2-positive breast cancer. A phase I clinical trial of MEDI-573, an anti-IGF-1/2 monoclonal antibody, was reported in patients with advanced solid tumors; suppression of IGF-1 and IGF-2 was noted and a dose-limiting toxicity was not defined [37]. Subsequently, NCT01446159, a phaseI1b/2 randomized study of MEDI-573 in combination with an aromatase inhibitor (AI) versus an AI alone in women with MBC, was conducted; the combination therapy did not improve PFS, and further clinical evaluation of MEDI-573 in breast cancer is not planned. Xentuzumab is another IGF-1/2 neutralizing antibody which was noted in preclinical studies to inhibit ligand activation of IGF-1R/IR-A and decrease cellular proliferation, therefore evaluation in the clinical setting followed [49]. NCT02123823 was a phase Ib/II randomized study of xentuzumab in combination with exemestane and everolimus versus exemestane and everolimus alone in women with locally advanced or metastatic HR+, HER2-negative breast cancer. Results from the randomized phase II part of the study noted that PFS and toxicity outcomes were similar in the investigational and control arms; however, a prespecified subgroup analysis demonstrated that in the nonvisceral metastases subgroup, patients in the investigational arm had superior PFS outcomes compared with the everolimus and exemestane arm alone [50]. An ongoing phase II study (NCT03659136) is therefore evaluating xentuzumab, exemestane and everolimus in postmenopausal women with HR+, HER2-negative advanced breast cancer and nonvisceral disease. Further, NCT03099174 is a phase Ib study evaluating xentuzumab and the cyclin-dependent kinase (CDK) 4/6 inhibitor, abemaciclib, in advanced solid tumors and in combination with endocrine therapy in patients with advanced HR+, HER2-negative breast cancer. It remains to be seen whether further clinical development of IGF-1/2 neutralizing antibodies will be warranted in patients with MBC pending the results of these trials.
In summary, this phase II trial demonstrated that the addition of cituxumumab to lapatinib and capecitabine did not improve PFS or other clinical endpoints, while not impacting toxicity or self-reported QOL, when compared with lapatinib and capecitabine. However, as accrual goals were not met, this trial was under-powered. Cituxumumab and monoclonal antibodies that target the human IGF-1R are not being pursued for further clinical development in breast cancer. Ongoing studies will assess whether alternative approaches to target the IGF system demonstrate antitumor activity in breast cancer. In the meantime, many additional therapeutic options are FDA-approved with favorable side effect profiles in this patient population.
Acknowledgments:
The authors extend their gratitude to the patients who participated in this clinical trial. They further recognize and thank the clinical research staff at each of the participating study sites, as well as the breast cancer research committee leadership and centralized administrative support provided by the NCCTG and Alliance for Clinical Trials in Oncology.
Funding: Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA232760, U10CA180820 and UG1CA189859 (ECOG-ACRIN), U10CA180868 (NSABP/NRG Oncology), UG1CA189821 and U10CA180888 (SWOG), and https://acknowledgments.alliancefound.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest:
Jun He, Beiyun Chen, Donald Northfelt, Amylou C. Dueck, Karla V. Ballman, Kathleen S. Tenner, Hannah Linden, Joseph A. Sparano6, Judith O. Hopkins7, Chamath De Silva, Edith A. Perez. Ciara C. O’Sullivan declares research funding to institution (Mayo) from the following companies: Lilly, Seattle Genetics, Bavarian Nordic, Minnemarita Therapeutics, and Biovica. Matthew P. Goetz declares funding acknowledgement to named Professorship: Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D., and consulting fees to institution from Eagle Pharmaceuticals, Lilly, Biovica, Novartis, Sermonix, Context Pharm, Pfizer, and Biotheranostics, and grant funding to institution from Pfizer, Sermonix, and Lilly. Paul Haluska discloses he is a current employee and stockholder of Bristol Myers Squibb. Tufia C. Haddad declares grant funding to the Mayo Clinic from Takeda Oncology.
Footnotes
ClinicalTrials.gov Identifier: NCT00684983
Ethical Statement: The study was conducted in accordance with the Declaration of Helsinki, and this phase II therapeutic trial was monitored at least twice annually by the Data and Safety Monitoring Board, a standing committee composed of individuals from within and outside of the Alliance.
Consent to Participate: Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Consent to Publish: Patients signed informed consent regarding publishing their data.
Availability of data and material: Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center and is available upon request. All data generated or analyzed during this study are included in this published article.
Electronic Figure Submission: SAS statistical software (version 9.4M6, SAS Institute, Cary, NC) to generate the graphics.
References
- 1. Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA: a cancer journal for clinicians 66 (1):7–30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2. Pinto AC, Ades F, de Azambuja E, Piccart-Gebhart M (2013) Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast (Edinburgh, Scotland) 22 Suppl 2:S152–155. doi: 10.1016/j.breast.2013.07.029 [DOI] [PubMed] [Google Scholar]
- 3. O’Sullivan CC, Smith KL (2014) Therapeutic Considerations in Treating HER2-Positive Metastatic Breast Cancer.Current breast cancer reports 6 (3):169–182. doi: 10.1007/s12609-014-0155-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zardavas D, Cameron D, Krop I, Piccart M (2013) Beyond trastuzumab and lapatinib: new options for HER2-positive breast cancer. American Society of Clinical Oncology educational book American Society of Clinical Oncology Meeting. doi: 10.1200/EdBook_AM.2013.33.e2 [DOI] [PubMed] [Google Scholar]
- 5. Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer research 65 (23):11118–11128. doi: 10.1158/0008-5472.can-04-3841 [DOI] [PubMed] [Google Scholar]
- 6. Jones HE, Gee JM, Taylor KM, Barrow D, Williams HD, Rubini M, Nicholson RI (2005) Development of strategies for the use of anti-growth factor treatments. Endocrine-related cancer 12 Suppl 1:S173–182. doi: 10.1677/erc.1.01004 [DOI] [PubMed] [Google Scholar]
- 7. Gee JM, Robertson JF, Gutteridge E, Ellis IO, Pinder SE, Rubini M, Nicholson RI (2005) Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocrine-related cancer 12 Suppl 1:S99–s111. doi: 10.1677/erc.1.01005 [DOI] [PubMed] [Google Scholar]
- 8. Camirand A, Zakikhani M, Young F, Pollak M (2005) Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast cancer research : BCR 7 (4):R570–579. doi: 10.1186/bcr1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakravarti A, Loeffler JS, Dyson NJ (2002) Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer research 62 (1):200–207 [PubMed] [Google Scholar]
- 10. Haluska P, Carboni JM, Loegering DA, Lee FY, Wittman M, Saulnier MG, Frennesson DB, Kalli KR, Conover CA, Attar RM, Kaufmann SH, Gottardis M, Erlichman C (2006) In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer research 66 (1):362–371. doi: 10.1158/0008-5472.can-05-1107 [DOI] [PubMed] [Google Scholar]
- 11. Frogne T, Jepsen JS, Larsen SS, Fog CK, Brockdorff BL, Lykkesfeldt AE (2005) Antiestrogen-resistant human breast cancer cells require activated protein kinase B/Akt for growth. Endocrine-related cancer 12 (3):599–614. doi: 10.1677/erc.1.00946 [DOI] [PubMed] [Google Scholar]
- 12. O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research 66 (3):1500–1508. doi: 10.1158/0008-5472.can-05-2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, Harkavy B, Shen N, Grohar P, Helman LJ (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26 (13):1932–1940. doi: 10.1038/sj.onc.1209990 [DOI] [PubMed] [Google Scholar]
- 14. Wilky BA, Rudek MA, Ahmed S, Laheru DA, Cosgrove D, Donehower RC, Nelkin B, Ball D, Doyle LA, Chen H, Ye X, Bigley G, Womack C, Azad NS (2015) A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours. British journal of cancer 112 (1):24–31. doi: 10.1038/bjc.2014.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higano CS, Berlin J, Gordon M, LoRusso P, Tang S, Dontabhaktuni A, Schwartz JD, Cosaert J, Mehnert JM (2015) Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors. Investigational new drugs 33 (2):450–462. doi: 10.1007/s10637-015-0217-7 [DOI] [PubMed] [Google Scholar]
- 16. Cao H, Cui L, Ma W, Zhu L, Wang K, Ni Y, Wang Y, Du J (2017) Adverse Events and Efficacy of Cixutumumab in Phase II Clinical Trials: A systematic Review and Meta-Analysis. Clinical drug investigation 37 (2):135–153. doi: 10.1007/s40261-016-0475-y [DOI] [PubMed] [Google Scholar]
- 17. Abou-Alfa GK, Capanu M, O’Reilly EM, Ma J, Chou JF, Gansukh B, Shia J, Kalin M, Katz S, Abad L, Reidy-Lagunes DL, Kelsen DP, Chen HX, Saltz LB (2014) A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. Journal of hepatology 60 (2):319–324. doi: 10.1016/j.jhep.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novello S, Scagliotti G, de Castro G Jr., Kiyik M, Kowalyszyn Rv, Deppermann KM, Arriola E, Bosquee L, Novosiadly RD, Nguyen TS, Forest A, Tang S, Kambhampati SR, Cosaert J, Reck M (2017) An Open-Label, Multicenter, Randomized, Phase II Study of Cisplatin and Pemetrexed With or Without Cixutumumab (IMC-A12) as a First-Line Therapy in Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 12 (2):383–389. doi: 10.1016/j.jtho.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 19. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. The New England journal of medicine 355 (26):2733–2743. doi: 10.1056/NEJMoa064320 [DOI] [PubMed] [Google Scholar]
- 20. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 92 (3):205–216 [DOI] [PubMed] [Google Scholar]
- 21. Sloan J, O’Fallon JR, Suman VJ, Sargent DJ (1998) Incorporating quality of life measurement into oncology clinical trials. Proc Am Stat Assoc:282–287 [Google Scholar]
- 22. Bretscher M, Rummans T, Sloan J, Kaur J, Bartlett A, Borkenhagen L, Loprinzi C (1999) Quality of life in hospice patients. A pilot study. Psychosomatics 40 (4):309–313. doi: 10.1016/S0033-3182(99)71224-7 [DOI] [PubMed] [Google Scholar]
- 23. Giorgi F, Cellerino R, Gramazio A, Tummarello D, Menichetti ET, Giordani P, Antognoli S, Carle F, Piga A (1996) Assessing quality of life in patients with cancer: a comparison of a visual-analogue and a categorical model. Am J Clin Oncol 19 (4):394–399 [DOI] [PubMed] [Google Scholar]
- 24. Hyland ME, Sodergren SC (1996) Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Qual Life Res 5 (5):469–480 [DOI] [PubMed] [Google Scholar]
- 25. Littman GS, Walker BR, Schneider BE (1985) Reassessment of verbal and visual analog ratings in analgesic studies. Clin Pharmacol Ther 38 (1):16–23 [DOI] [PubMed] [Google Scholar]
- 26. Kaplan EL, Meier P (1958) Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 53:457–481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 27. Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother 50 (3):163–170 [PubMed] [Google Scholar]
- 28. Locke DE, Decker PA, Sloan JA, Brown PD, Malec JF, Clark MM, Rummans TA, Ballman KV, Schaefer PL, Buckner JC (2007) Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. Journal of pain and symptom management 34 (6):628–638. doi: 10.1016/j.jpainsymman.2007.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilcoxon F (1945) Individual comparisons by ranking methods. Biometrics Bulletin 1 (6):80–83. doi: 10.2307/3001968 [DOI] [Google Scholar]
- 30. Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko CW, Sridhara R, Justice R, Pazdur R (2008) FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. The oncologist 13 (10):1114–1119. doi: 10.1634/theoncologist.2008-0816 [DOI] [PubMed] [Google Scholar]
- 31. Belani CP, Dahlberg SE, Rudin CM, Fleisher M, Chen HX, Takebe N, Velasco MR Jr., Tester WJ, Sturtz K, Hann CL, Shanks JC, Monga M, Ramalingam SS, Schiller JH (2016) Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508). Cancer 122 (15):2371–2378. doi: 10.1002/cncr.30062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gradishar WJ, Yardley DA, Layman R, Sparano JA, Chuang E, Northfelt DW, Schwartz GN, Youssoufian H, Tang S, Novosiadly R, Forest A, Nguyen TS, Cosaert J, Grebennik D, Haluska P (2016) Clinical and Translational Results of a Phase II, Randomized Trial of an Anti-IGF-1R (Cixutumumab) in Women with Breast Cancer That Progressed on Endocrine Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 22 (2):301–309. doi: 10.1158/1078-0432.ccr-15-0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussain M, Rathkopf D, Liu G, Armstrong A, Kelly WK, Ferrari A, Hainsworth J, Joshi A, Hozak RR, Yang L, Schwartz JD, Higano CS (2015) A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer. European journal of cancer (Oxford, England : 1990) 51 (13):1714–1724. doi: 10.1016/j.ejca.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu EY, Li H, Higano CS, Agarwal N, Pal SK, Alva A, Heath EI, Lam ET, Gupta S, Lilly MB, Inoue Y, Chi KN, Vogelzang NJ, Quinn DI, Cheng HH, Plymate SR, Hussain M, Tangen CM, Thompson IM Jr. (2015) SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol 33 (14):1601–1608. doi: 10.1200/jco.2014.59.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wagner LM, Fouladi M, Ahmed A, Krailo MD, Weigel B, DuBois SG, Doyle LA, Chen H, Blaney SM (2015) Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children’s Oncology Group. Pediatric blood & cancer 62 (3):440–444. doi: 10.1002/pbc.25334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langer CJ, Novello S, Park K, Krzakowski M, Karp DD, Mok T, Benner RJ, Scranton JR, Olszanski AJ, Jassem J (2014) Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol 32 (19):2059–2066. doi: 10.1200/jco.2013.54.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haluska P, Menefee M, Plimack ER, Rosenberg J, Northfelt D, LaVallee T, Shi L, Yu XQ, Burke P, Huang J, Viner J, McDevitt J, LoRusso P (2014) Phase I dose-escalation study of MEDI-573, a bispecific, antiligand monoclonal antibody against IGFI and IGFII, in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 20 (18):4747–4757. doi: 10.1158/1078-0432.ccr-14-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robertson JF, Ferrero JM, Bourgeois H, Kennecke H, de Boer RH, Jacot W, McGreivy J, Suzuki S, Zhu M, McCaffery I, Loh E, Gansert JL, Kaufman PA (2013) Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. The Lancet Oncology 14 (3):228–235. doi: 10.1016/s1470-2045(13)70026-3 [DOI] [PubMed] [Google Scholar]
- 39. Higano CS, Yu EY, Whiting SH, Gordon MS, LoRusso P, Fox F, Katz TL, Roecker JM, Schwartz JD (2007) A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. Journal of Clinical Oncology 25 (18_suppl):3505–3505. doi: 10.1200/jco.2007.25.18_suppl.3505 [DOI] [Google Scholar]
- 40. Baselga J, Morales SM, Awada A, Blum JL, Tan AR, Ewertz M, Cortes J, Moy B, Ruddy KJ, Haddad T, Ciruelos EM, Vuylsteke P, Ebbinghaus S, Im E, Eaton L, Pathiraja K, Gause C, Mauro D, Jones MB, Rugo HS (2017) A phase II study of combined ridaforolimus and dalotuzumab compared with exemestane in patients with estrogen receptor-positive breast cancer. Breast Cancer Res Treat 163 (3):535–544. doi: 10.1007/s10549-017-4199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryan PD, Neven P, Blackwell KL, Dirix LY, Barrios CH, Miller WH Jr, Fein LD, Fenton D, Benner RJ, Meech SJ, et al. 2011. Figitumumab plus exemestane versus exemestane as first-line treatment of postmenopausal hormone receptor-positive advanced breast cancer: a randomized, open-label phase II trial. Cancer Research 71 (24 Suppl):Abstract nr P1-17-01. 10.1158/0008-5472. SABCS11-P1-17-01 Accessed 28 January 2021. [DOI] [Google Scholar]
- 42. Yee et al. Abstract P6–11-04: The evaluation of ganitumab/metformin plus standard neoadjuvant therapy in high-risk breast cancer: Results from the I-SPY 2 trial. Cancer Res February 15 2017. (77) (4 Supplement) P6-11-04; DOI: 10.1158/1538-7445.SABCS16-P6-11-04. [DOI] [Google Scholar]
- 43. Rajan A, Carter CA, Berman A, Cao L, Kelly RJ, Thomas A, Khozin S, Chavez AL, Bergagnini I, Scepura B, Szabo E, Lee MJ, Trepel JB, Browne SK, Rosen LB, Yu Y, Steinberg SM, Chen HX, Riely GJ, Giaccone G (2014) Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. The Lancet Oncology 15 (2):191–200. doi: 10.1016/s1470-2045(13)70596-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ekyalongo RC, Yee D. Revisiting the IGF-1R as a breast cancer target. Precision Oncology (2017) 1:14 ; doi: 10.1038/s41698-017-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yee D (2018) Anti-insulin-like growth factor therapy in breast cancer. J Mol Endocrinol 61 (1):T61–t68. doi: 10.1530/jme-17-0261 [DOI] [PubMed] [Google Scholar]
- 46. Wilcox G (2005) Insulin and insulin resistance. Clin Biochem Rev 26 (2):19–39 [PMC free article] [PubMed] [Google Scholar]
- 47. De Meyts P, Sajid W, Palsgaard J, et al. Insulin and IGF-I Receptor Structure and Binding Mechanism. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6192/. Accessed 28 January 2021. [Google Scholar]
- 48. Feng Y, Dimitrov DS (2012) Antibody-based therapeutics against components of the IGF system. Oncoimmunology 1 (8):1390–1391. doi: 10.4161/onci.20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friedbichler K, Hofmann MH, Kroez M, Ostermann E, Lamche HR, Koessl C, Borges E, Pollak MN, Adolf G, Adam PJ (2014) Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Molecular cancer therapeutics 13 (2):399–409. doi: 10.1158/1535-7163.mct-13-0598 [DOI] [PubMed] [Google Scholar]
- 50. Crown J, Sablin MP, Cortés J, Bergh J, Im S, Lu YS, et al. Xentuzumab (BI 836845), an insulin-like growth factor-neutralizing antibody, combined with exemestane and everolimus in hormone receptor-positive locally advanced/metastatic breast cancer: randomized Phase 2 results. Presented at the San Antonio Breast Cancer Symposium, December 4–8, 2018. P6-21-01. [Google Scholar]


