Abstract
Introduction:
Uterine fibroids (UFs) are the most common benign tumor arising from myometrium of reproductive age women, with significant financial burden estimated in hundreds of billions of dollars. Unfortunately, there are limitations in available long-term treatment options. Thus, there is a large unmet need in the UF space for noninvasive therapeutics.
Areas covered:
Authors reviewed the literature available for elagolix; an orally bioavailable, second-generation, non-peptide gonadotropin-releasing hormone (GnRH) antagonist recently approved by the US Food and Drug Administration (FDA) in combination with estradiol/norethindrone acetate for the management of heavy menstrual bleeding associated with UFs in premenopausal women.
Expert opinion:
The utility of new-generation oral GnRH-antagonists, such as elagolix, relugolix and linzagolix, is offering a new potential opportunity for the future therapy of UFs: elagolix has been the most studied drug of this class for treating benign gynecological diseases including endometriosis and UFs, for which it has been US FDA-approved in 2018 and 2020 respectively.
Keywords: Add-back Therapy, Elagolix, GnRH Antagonists, Heavy Menstrual Bleeding, Leiomyoma, Uterine Fibroids
1. Introduction:
Uterine Fibroids (UFs) are the most common benign tumor in reproductive age women worldwide worldwide, UFs are rare before puberty (menarche), their likelihood increases as women age during their reproductive years and reach its peak before menopause then UFs typically regress following menopause, with prevalence of 70% and 80% in Caucasian and African American women respectively by age of 50 [1–3]. Accordingly, it has a profound impact on health care costs worldwide. In the USA alone, UFs cost range estimated between 6 and 34 billion dollars annually [4]. The mechanism underlying UFs pathogenesis remains unclear, which in turn limits its effective treatment. Growing evidences support the theory that these monoclonal tumors originate from aberrant stem cells in the myometrium and involves numerous factors such as sex steroid hormones, estrogen and progesterone, various growth factors, and genetic factors [5].
1.1. Overview of the market:
If possible, many women with UFs would choose pharmacological treatment over a surgical treatment to secure their future family plans and avoid major surgeries [6, 7]. Several factors should be considered on individual basis when selecting appropriate UF treatment including patient’s age, symptoms severity, individual preferences and most importantly patient’s future family plans. Currently, there is limitation in available long-term medical treatment options. Thus, there is a large unmet need in the UF space for noninvasive therapeutics [8, 9]. Therefore, extensive studies exploring mechanistic insights behind UF pathogenesis are needed for sake of developing new UFs therapeutics. Estrogen and progesterone signaling are imperative for tumor growth. Therefore, current investigated treatment options aim to halt that hormonal effect including gonadotropin releasing hormone (GnRH) antagonists and selective progesterone receptor modulators (SPRMs) [10].
Till early 2020, the only agent FDA-approved medical treatments for UFs in the USA was leuprolide acetate (LA), gonadotropin-releasing hormone (GnRH) agonist, for improvement of anemia preoperatively only for short-term (3–6 months) [11]. This effect was due to suppression of ovarian estrogen and progesterone which shrink tumor volume and reduce surgical blood loss [12], Nevertheless, the resultant bone density loss along with the recurrence of UF symptoms following treatment cessation, discouraged the utility of this drug as long term therapy for UFs [13]. Later, GnRH Antagonists utility offered an advantage over GnRH agonists which is avoiding the initial flare effect resulted from receptor stimulation (up to 15 days), so they can exert faster effect of bleeding cessation [14, 15]. SPRMs are synthetic steroid ligands with a progesterone receptor-target and tissue-selective effects of mixed agonist and antagonist activities [16]. Ulipristal acetate (UPA) is commonly used SPRM and was approved against UFs in Europe and Canada with advantage of lacking the hypoestrogenic symptoms of GnRH analogs [16]. However, recent studies found that use of UPA is associated with the risk of liver failure that may require liver transplantation [17, 18]. Therefore, currently SPRM use is restricted. European medicine agency (EMA) recommends UPA can now only be used to treat uterine fibroids in premenopausal women for whom surgical procedures (including uterine fibroid embolization) are not appropriate or have not worked. Also, the medicines must not be used for controlling symptoms of uterine fibroids while awaiting surgical treatment [19]. Oral GnRH antagonist are extensively studied now for the treatment of heavy menstrual bleeding associated with UFs including elagolix, relugolix and linzagolix. Elagolix is the only FDA approved for the treatment of moderate to severe pain associated with endometriosis [20, 21] and elagolix supplemented with estradiol and norethindrone acetate was recently approved as first oral treatment for UFs associated heavy menstrual bleeding. In this drug profile we reviewed the literature of elagolix covering its chemistry, pharmacokinetics, pharmacodynamics, clinical efficacy and safety. In addition, where its stands among other therapies explored against UFs.
2. Elagolix
2.1. Chemistry
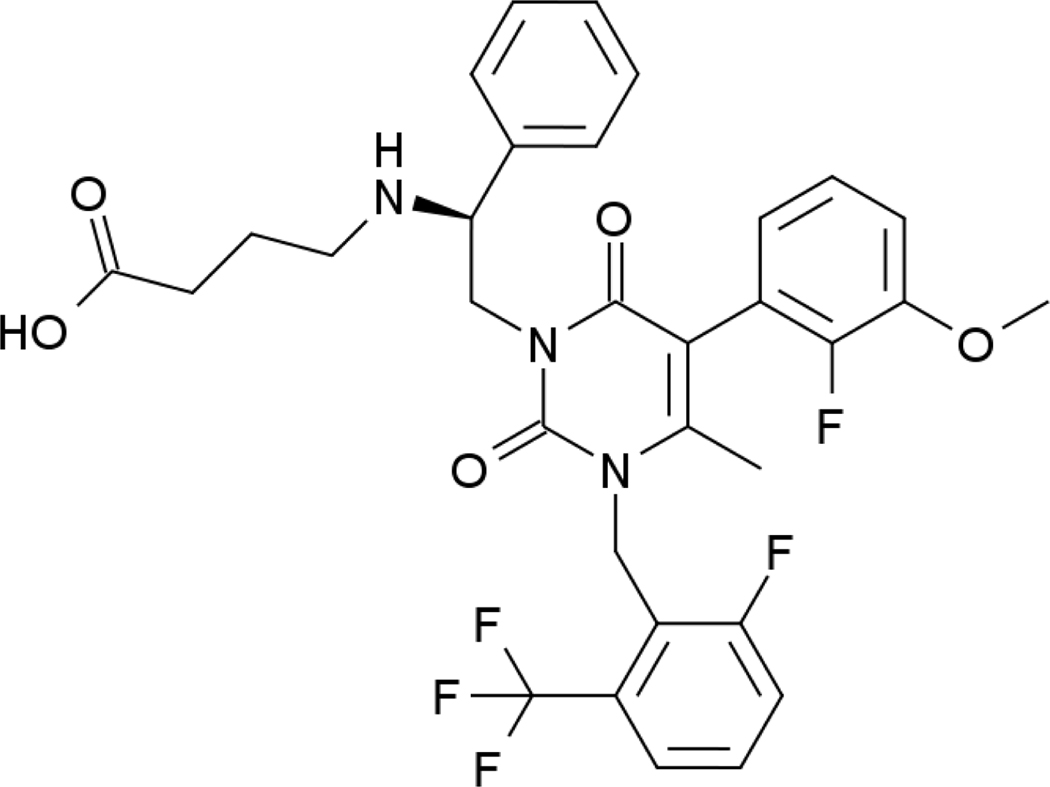
Elagolix has molecular formula of C32H30F5N3O5 and molecular weight of 631.6 g/mol. Elagolix sodium is a non-peptide, amorphous solid orally bioavailable small molecule, that is freely soluble in water. Elagolix at 150 or 200 mg dose, is highly soluble throughout the physiological pH range, and exhibits high aqueous solubility (approximately 1 mg/mL) as per the biopharmaceutics classification system (BCS) [22]. Chemical structure of elagolix is shown in figure 1.
Figure 1:
Chemical structure of elagolix
2.2. Pharmacokinetics
2.2.1. Absorption
Studies showed that elagolix is rapidly absorbed with a time to maximum concentration (Tmax) of approximately 1 hour, while the maximum concentration [Cmax] and area under the curve [AUC]) are dose proportional from 100 to 400 mg twice daily dose, and more than dose proportional with single doses of 600–1200 mg based on clinical pharmacokinetics studies in healthy subjects [23].
An absorption study was conducted in 6 healthy subjects to examine elagolix 100 mg pharmacokinetics (PK) administered to the stomach via oral solution, and to the jejunum, ileum, and colon via a radiolabeled remote drug delivery capsule. Briefly, both routes resulted in comparable overall systemic exposure, except for the colon administration which represented less than 10% that of the same dose deposited into the stomach/duodenum, jejunum, or ileum [24].
Data derived from 1624 subjects involved in five phase I and four phase III studies were employed in population PK analyses, with results showing that none of the tested covariates were significantly associated with Elagolix PK parameters, including age, body weight, race/ethnicity, hepatic or renal function, except for hepatic uptake transporter OATP1B1 genotype status which was significant (i.e. specifically for CL/F) [25].
2.2.2. Food Effect
The effect of food on elagolix plasma exposure was assessed via administration of a high-fat meal with result observed of a slight reduction relative to the fasted condition. Consequently, Elagolix was administrated at least 1 h before or 2 h after a meal in attempt to avoid a potential lower exposure in three of four endometriosis phase III pivotal clinical trials. Interestingly, elagolix was administered without regard to meals in the fourth endometriosis phase III extension trial and the clinical efficacy results were similar, regardless of drug administration instructions with respect to meals. Therefore, elagolix can be administered with or without food [24].
2.2.3. Distribution
About 80% of elagolix is bound to human plasma proteins, with blood-to-plasma ratios of 0.6 as drug found to preferentially partitioned into plasma rather than blood cellular components [26]. Population PK data showed that elagolix estimated apparent central volume of distribution (Vc/F) was 257 L[24]. In vitro studies in addition to pharmacogenetics analysis showed that elagolix is a substrate of hepatic uptake transporter OATP1B1. However, population PK analysis did not identify the OATP1B1 genotype as a significant covariate on elagolix Vc/F [25].
2.2.4. Metabolism
In vitro studies showed that elagolix is metabolized by multiple cytochrome P450 (CYP450) enzymes with major contribution from CYP3A4 (approximately 50%), and minor contributions from other CYP450s [24]. Following the administration of a single oral dose of 150 mg of radiolabeled [14C] elagolix to 6 healthy subjects, about 69% of elagolix was recovered in feces and urine as metabolites. These data suggest that unchanged elagolix is the major drug-derived material in human plasma and elagolix is extensively metabolized [24].
2.2.5. Elimination
After reaching Cmax, elagolix concentration–time profile exhibits biphasic characteristic, with an apparent terminal elimination half-life (t1/2) of approximately 4–6 hrs. Therefore, repeated dosing of elagolix once or twice per day does not result in significant drug accumulation in plasma [23, 25]. Elagolix dose adjustment is not required in women with any degree of renal impairment or end stage renal disease (including women receiving dialysis). While in women with severe hepatic impairment (Child–Pugh C), elagolix Cmax and AUC were increased by 520% and 570%, respectively. Therefore, elagolix is contraindicated in women with severe hepatic impairment (Child–Pugh C) [27]. Table 1 summarizes pharmacokinetics parameters for elagolix in healthy volunteers.
Table 1:
Pharmacokinetic Properties of elagolix in Healthy Subjects
| Absorption | |
| Tmax (h) | 1.5(1.0 – 4.0) |
| Effect of Food | |
| High-fat meal (826kcal, 52% fat, relative to fasting) | AUC: ↓25%, Cmax: ↓36% |
| Distribution | |
| % Bound to human plasma proteins | 80 |
| Blood-to-plasma ratio | 0.6 |
| Metabolism | CYP3A (major)Minor pathways include: CYP2D6, CYP2C8, and uridine glucuronosyl transferases (UGTs) |
| Elimination | |
| Major route of elimination | Hepatic metabolism |
| Terminal phase elimination half-life (t1/2) (h) | 5.9± 2.1 |
| % of dose excreted in urine | <3 |
| % of dose excreted in feces | 90 |
2.3. Pharmacodynamics
Elagolix has a dose-dependent suppression effect on the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) which subsequently decreased blood concentrations of the ovarian sex hormones estrogen (E2) and progesterone [20, 23, 28].
Elagolix induced a dose-dependent suppression of sex hormones in premenopausal females when consumed in regimens; 150 mg once daily, or 100, 200, 300, or 400 mg twice daily for 21 days as compared to placebo. Interestingly, it took only hours following first dose administration of the on day 1 to achieve that effect which continued through day 21. Notably, maximum E2 suppression was achieved with regimen starting from 200 mg elagolix twice daily or higher. Interestingly, LH suppression was more noticeable than FSH in all groups except for the 150 mg once daily group. Upon elagolix discontinuation, LH and FSH levels rose within 24 h after the last dose, and E2 levels began to rise 24 h after the last dose was administered [24, 29].
The effects elagolix in different doses and dosing regimens either alone or in combination with standard dose hormonal add-back therapy (E2/norethindrone acetate (NETA) 1 mg/0.5 mg) on ovulation, ovarian activity, and ovarian reserve were evaluated in a study in healthy adult premenopausal females for 3 month with similar finding of dose dependent hormonal suppression [27, 30]. Studies showed that the standard dose of E2/NETA as 1 mg/0.5 mg, when administered exogenously with elagolix, provided the optimal plasma levels of E2 which was similar among endometriosis patients as well as healthy subjects [24, 26, 31].
Following pivotal phase III ELARIS studies on premenopausal women moderate-to-severe pain associated with endometriosis, recent study explored the relationships between elagolix exposures and clinical efficacy response rates for dysmenorrhea and non-menstrual pelvic pain [32]. This study employed nonlinear mixed-effects discrete-time first order Markov modeling approach to characterize the relationships between elagolix average concentrations and efficacy responses. Results suggested no preference of one regimen over the other to treat endometriosis-associated pain for any patient subpopulation based on tested covariate groups.
2.4. Drug interactions
Several studies explored potential drug-drug interactions of elagolix with other drugs such as Ketoconazole, rosuvastatin, digoxin, midazolam, sertraline, fluconazole and rifampin in terms of change in elagolix Cmax and AUC with conclusion of concomitant use of strong inhibitors of OATP1B1 is contraindicated with elagolix [33–35]. To account quantitatively for the contribution of metabolizing enzymes, transporters, and their interplay in Elagolix PK, a whole-body physiologically based pharmacokinetic (PBPK) model of Elagolix was developed which adequately recovered the observed drug–drug interaction [34]. Importantly, results showed that combinations of elagolix with norethindrone or triphasic oral contraceptives (OCs) did not negatively impact the hormonal effects of each other, consequently no dose adjustment is required. [36].
2.5. Clinical Efficacy
Phase I clinical studies regarding Elagolix showed that, 5 of 9 cohorts in phase I clinical study received a single dose of elagolix 25 mg, 50 mg, 100 mg, 200 mg, 400 mg, or placebo [29]. The first dose of elagolix was administered on day 7 + 1 after menstruation onset. Suppression of LH and FSH was achieved in all groups, whereas a dose dependent suppression of LH, FSH and E2 was noted [29]. In another phase I clinical study regarding to hormone response, safety, and PK, elagolix 150 mg once daily, as well as elagolix 100 mg, 200 mg, 300 mg, and 400 mg twice daily were compared to a placebo. Treatment intake was started two days after the onset of menstruation. The results were similar to previous studies: A dose-dependent suppression of E2, progesterone, LH, and FSH was observed, the suppression of FSH being the most efficient with elagolix 300 mg and 400 mg, while the clearest decrease in LH and E2 was found with elagolix 200 mg. The concentration of progesterone remained at anovulatory levels throughout the study period at the lowest dose of elagolix [23, 37]
2.5.1. Elagolix in endometriosis associated pain:
Starting from Phase II trials, elagolix was investigated in patients with endometriosis and UFs. The first stage of one Phase II study lasted 12 weeks and compared 3 groups with one another: a placebo control group; a group of patients receiving 150 mg elagolix once daily; and a third group receiving 250 mg once daily. Endometriosis-associated pain had decreased in all three groups by the end of the 12 weeks, as assessed by the Numeric Rating Scale. After 12 weeks, the patients of the placebo group were reallocated to one of the groups receiving elagolix. In this second stage, there was a further reduction of dysmenorrhea in both groups. Similar results were achieved for dyspareunia. Statistical significance was seen between elagolix 150 mg and placebo during weeks 8 to 12, as well as between elagolix 250 mg and placebo during weeks 4 to 8. Furthermore, a decrease in the use of prescription analgesics was seen in the elagolix groups, while the biggest improvement in quality of life was noted specifically in the elagolix 150mg group [38].
Similar results were described in another phase II clinical trial conducted showing the effect of 150 mg elagolix compared to placebo over an eight-week, double-blind, placebo- controlled treatment period, followed by a 16-week open-label treatment period [39].
Consequently, in May 2017, the results from two multicenter, double blind, randomized, placebo-controlled, phase III trials (Elaris EM-I and Elaris EM-II) were published. The patients enrolled in both studies were premenopausal women who had surgically diagnosed endometriosis and suffered from moderate to severe endometriosis-associated pain. 653 (74.9%) out of 871 women included in EM-I, completed the six-month study period. In the nearly identical EM-II,815 were women treated, with 632 (77.4%) completing the trial. The patients who completed the trials were enrolled in either a 6-month extension study (EM-III or EM-IV) or included in the 12-month follow-up period. The primary endpoints of the placebo-controlled EM-I and EM-II were efficacy, with regard to alleviation of dysmenorrhea and non-menstrual pelvic pain (NMPP), after three months of elagolix 150 mg once daily or Elagolix 200 mg twice daily, compared to placebo, measured in a reduced pain and reduced or equivalent intake of rescue analgesics. After three months of treatment in the EM-1, 46.4% of the women treated with elagolix 150 mg once daily,, and 75.8% of the patients treated with elagolix 200 mg twice daily, vs 19.6% of the women in the placebo group, were noted to be dysmenorrhea responders, which refers to participants who had a clinically meaningful reduction in the pain score and a decreased or stable use of rescue analgesic agents, as recorded in a daily electronic diary, while 50.4% of the women treated with elagolix 150 mg once daily and 54.5% of the women treated with 200 mg elagolix twice daily vs 36.5% of the women in the placebo group, were described as ‘non-menstrual pelvic pain responders.’ Similar results were found in the EM-II for the 150 mg once daily group, the 200 mg twice daily group, and the placebo group, with 43.4% and 72.4% vs 22.7%, respectively, of women who described a reduction of dysmenorrhea and 49.8% and 57.8% vs 36.5%, respectively, of women who described a reduction of non-menstrual pelvic pain, as well as a decrease or the same in intake of analgesics. After three and six months, the difference in intake of rescue analgesics between the elagolix 150 mg group and the placebo group was not found to be significant, while the contrary was noted in the group of women who received the higher dose compared to the placebo. Furthermore, a significant improvement in quality of life was noted in the elagolix-treated groups, as determined by 30-item Endometriosis Health Profile Scores [40].
A randomized, double-blind, multicenter, placebo-controlled phase III trial showed that elagolix significantly reduces fatigue levels in women with moderate or severe endometriosis-associated pain [41]. Moreover, another study employed post hoc analysis of pooled data from the phase III ELARIS-I and ELARIS-II clinical trials to the relationship of clinically meaningful improvements in dyspareunia with health-related quality of life changes among women with endometriosis [42]. Several articles in the literature covered in details utility of elagolix for endometriosis associated pain over the past few years for further reading [43–66].
2.5.2. Elagolix in Uterine fibroids associated bleeding:
Two multicenter phase II studies evaluated elagolix in premenopausal women with UFs for its efficacy in controlling bleeding. In both trials, either elagolix monotherapy or in combination with hormonal add-back therapy (E2 and NETA) was evaluated and the primary endpoints in the Phase IIa study was mean change in MBL from baseline to the last complete menstrual cycle (last 28 days) during treatment and in the phase IIb study the primary endpoint was the percentage of women with MBL volume of less than 80 mL and of more than at least 50% reduction in MBL volume at the end of the study during last 28 days of treatment using accurate alkaline hematin method [30, 31]. In the phase IIa trial, seven dosing regimens of elagolix were explored including twice daily of 100 mg, 200 mg or 300 mg or once daily of 400 mg or 600 mg, or twice daily of 200 mg combined with continuous E2 0.5 mg/NETA 0.1 mg or twice daily 300 mg combined with both continuous E2 1 mg and cyclical progesterone 200 mg only for 12 days monthly, in attempt to find minimally efficacious elagolix doses with a lower frequency of administration. In the elagolix group, a significantly dose dependent reduction in MBL was observed (with highest reduction in patients receiving the drug at 300 mg BID) compared to the placebo group as well as significant decrease in largest UF volume among treated groups [30].
In the phase IIb trial, women were randomized into two cohorts, cohort 1 included 259 patients received 300 mg twice daily and cohort 2 included 308 women received 600 mg once daily. Each cohort was randomized into 4 groups including placebo, elagolix alone and elagolix with add-back therapy either low dose of E2 0.5 mg/NETA 0.1 mg or high dose of E2 1 mg/NETA 0.5 mg [31]. At the end of the study, primary endpoint criteria were met in 92% in elagolix alone groups of 300 mg BID and 90% in 600 mg QD, adding low dose add-back therapy reduced the percentage to 85% and 73% while high dose add-back therapy resulted in 79% and 82% in aforementioned groups, respectively (p < 0.001, each treatment group compared to placebo). Moreover, all treated groups showed significant UF tumor shrinkage at final visit from baseline, with the exception in 600 mg daily with 1.0 mg estradiol/0.5 mg norethindrone acetate. Finally, improvement in the symptom severity was observed in all patients in treatment groups [31].
Phase III trials, Elaris UF-I and Elaris UF-II were published early 2020 evaluating the safety, tolerability, and efficacy of 6-month Elagolix treatment in women with UFs. Tested dosage regimens were twice daily of 300 mg elagolix either alone or in combination with hormonal add-back therapy (E2 1 mg / NETA 0.5 mg). Elagolix-alone group was included to evaluate the add-back therapy effect on the hypoestrogenic effects of elagolix. Same as phase II trials, the primary endpoint was the percentage of women with MBL less than 80 mL at final month and at least 50% MBL reduction at final month from baseline [67]. Elagolix plus add-back therapy resulted in meeting criteria for the primary end point in 68.5% of 206 women in UF-1 and in 76.5% of 189 women in UF-2, while in placebo group only 8.7% of 102 women and 10% of 94 women only reported improvement (P<0.001). As expected, elagolix alone has better outcome in 84.1% of 104 women in UF-1 and in 77% of 95 women in UF-2. Nevertheless, add-back therapy attenuated elagolix hypoestrogenic effects especially BMD loss, while hot flushes (in both trials) and metrorrhagia (in UF-1) occurred significantly more commonly compared to placebo.
Later, ELARIS UF EXTEND study was published as phase III multicenter, randomized, double-blind, extension study [68]. Where an additional 6 months (up to 12 months total) of elagolix 300 mg twice daily with hormonal add-back therapy (E2 1 mg and NETA 0.5 mg once daily) in women who completed an initial 6 months of the same treatment in one of two preceding phase 3 ELARIS UF-1 and UF-2 studies to evaluate the long-term efficacy, tolerability, and safety and, in particular, to characterize the impact of add-back on the hypoestrogenic effects of elagolix especially BMD changes and following the same endpoints of the original study. Results showed that total 12 months of combination treatment still sustained MBL reduction while no new or unexpected safety concerns were associated with an additional 6 months of treatment, also using add-back therapy attenuated the hypoestrogenic effects of elagolix alone [68]. Similarly, increasing number of articles discussing the beneficial role of elagolix in UF bleeding and increasing afflicted women quality of life are being published in literature [69–72]. Table 2 summarizing clinical trials conducted to explore efficacy of elagolix in UF associated bleeding.
Table 2:
Clinical trials exploring elagolix efficacy in uterine fibroids associated bleeding
| Trial | Type | Treatment groups | Enrolled patients | Treatment period | Primary Outcome Measures | Reference |
|---|---|---|---|---|---|---|
| NCT01441635 | Phase IIa Placebo-controlled, dose-ranging, multiple cohort (completed) | Elagolix 300 mg twice daily ± add-back Elagolix 600 mg once daily Elagolix 200 mg twice daily ± add-back Elagolix 400 mg once daily Elagolix 100 mg twice daily Placebo | Elagolix alone, 160 Elagolix + add-back, 61 Placebo, 50 Total, 271 | 3 months | Mean Change from Baseline to the Last 28 Days of Treatment in Menstrual Blood Loss (MBL) using the alkaline hematin method | [30] |
| NCT01817530 | Phase IIb Double-blind, placebo-controlled, parallel group (completed) | Elagolix 300 mg twice daily ± add-back Elagolix 600 mg once daily ± add-back Placebo | Elagolix alone, 142 Elagolix + add-back, 282 Placebo, 143 Total, 567 | 6 months | Percentage of women who had less than 80 mL menstrual blood loss and 50% or greater reduction in menstrual blood loss from baseline to the last 28 days of treatment. Safety assessments included changes in bone mineral density. | [31] |
| NCT02654054 (ELARIS UF-I) | Phase III Double-blind, placebo-controlled (completed) | Elagolix 300 mg twice daily Elagolix 300 mg twice daily ± add-back Placebo | Total 412 | 6 months | Percentage of responders, defined as participants who met the following conditions: - Menstrual blood loss (MBL) volume < 80 mL during the Final Month (the last 28 days prior to and including the last dose date), and - ≥ 50% reduction in MBL volume from Baseline to the Final Month. | [67] |
| NCT02691494 (ELARIS UF-II) | Phase III Double-blind, placebo-controlled (completed) | Elagolix 300 mg twice daily Elagolix 300 mg twice daily ± add-back Placebo | Total 378 | 6 months | Percentage of responders, defined as participants who met the following conditions: - Menstrual blood loss (MBL) volume < 80 mL during the Final Month (the last 28 days prior to and including the last dose date), and - ≥ 50% reduction in MBL volume from Baseline to the Final Month. | [67] |
| NCT02925494 (ELARIS UF-EXTEND) | Phase III Double-blind, placebo-controlled (completed) | Elagolix 300 mg twice daily ± add-back | Elagolix alone, 98 Elagolix + add-back, 218 Total 433 | Additional 6 months after completion of 6 months in ELARIS UF-I or II | Percentage of responders, defined as participants who met the following conditions: - Menstrual blood loss (MBL) volume < 80 mL during the Final Month (the last 28 days prior to and including the last dose date), and - ≥ 50% reduction in MBL volume from Baseline to the Final Month. Safety evaluations included adverse events and bone mineral density changes. Safety evaluations included adverse events and bone mineral density changes. | [68] |
| NCT03271489 | Phase IIIb, placebo-controlled, double-blinded in the first year and an open-label for the next three years (ongoing) | Elagolix + add-back Placebo | 500 participants estimated | 12 months | Change in Bone Mineral Density (BMD) | |
| NCT03886220 | Phase III Double-blind, placebo-controlled (ongoing) | Elagolix Placebo | 48 participants estimated | 6 months | Percentage of Participants with Menstrual Blood Loss (MBL) volume < 80 mL at Final Month and >= 50% Reduction in MBL Volume from Baseline to the Final Month |
2.6. Safety
Similar adverse events were described throughout all clinical trials phases. Mild to moderate nausea and hot flashes were reported as the most common adverse events with the highest incidence of hot flashes occurred in women receiving an elagolix dose of at least 200mg twice daily. Furthermore, spotting was reported by seven patients included in this phase 1 study. However, these adverse events didn’t lead to a discontinuation of study participation. Additionally, no changes or differences from the placebo group were found with regard to electrocardiograms, blood tests, or vital signs [23, 73]. Importantly, a phase 2 conducted study in endometriosis patients showed a significant decrease in bone-mineral density (BMD) was noted in both the elagolix 150mg and elagolix 250mg groups [38] where a significant dose-dependent decrease of lumbar BMD was noted after six months of treatment in both groups. Laboratory results from a phase 3 study showed an elagolix-associated increase in low-density and high-density lipoprotein cholesterol, as well as triglycerides [73]. A phase 1 study was conducted in healthy premenopausal females to evaluate whether elagolix has potential QTc interval prolongation with conclusion of no clinical relevance [24, 74].
Data from four phase III studies were used in Exposure–BMD modeling where simulations of 36 months of continuous treatment suggested a mean percentage of 2% and 5% change in lumbar spine BMD from baseline for Elagolix 150 mg once daily or 200 mg twice daily respectively. This data is consistent with the observed 1% BMD change after 12 months treatment of 150 mg once-daily dosing. Collectively, these studies supported the indicated use of elagolix 150 mg once daily for 24 months or 200 mg twice daily for 6 months [33, 40, 75–78].
In Elaris UF-1 and UF-2, adverse reactions caused discontinuation rate of 10% among women treated with elagolix supplemented with add-back therapy and 7% among placebo-treated women. The most common reported adverse events leading to study drug discontinuation in the elagolix plus add-back group were nausea, headache, alopecia, metrorrhagia, menorrhagia, and hot flush (1% each) [75]. Furthermore, subset analysis of the Phase III studies was performed to evaluate the safety and efficacy of elagolix (300 mg twice a day) with add-back therapy (1 mg E2/0.5 NETA once a day) in reducing HMB associated with UFs in various subgroups of women over 6 months of treatment where subgroups analyzed included age, body mass index, race, ethnicity, baseline MBL, fibroid location, and uterine and primary fibroid volume (largest fibroid identified by ultrasound). Study concluded efficacy of elagolix with hormonal add-back therapy in reducing HMB associated with UFs independent of any of these factors [79].
2.7. Regulatory affairs:
Pharmacoeconomic study was conducted to assess the cost–effectiveness of Elagolix versus GnRH agonist LA in women with moderate to severe endometriosis pain using a Markov model with a positive net monetary benefit (NMB) in favor of Elagolix over LA over 1- and 2-year time horizons [80].
Elagolix was developed by AbbVie and Neurocrine Biosciences for management of hormone-dependent reproductive disorders in women. Subsequently, In July 2018, the US FDA approved elagolix tablets (ORILISSA™) for management of moderate to severe pain associated with endometriosis. In May 2020, FDA approved ORIAHNN™ (Elagolix, estradiol, and norethindrone acetate capsules; Elagolix capsules) as the first oral medication for the management of heavy menstrual bleeding due to UFs in pre-menopausal women as One capsule (elagolix 300 mg, E2 1 mg, NETA 0.5 mg) in the morning and one capsule (elagolix 300 mg) in the evening for up to 24 months [33, 75].
3. Conclusion:
Uterine Fibroids are the most common benign tumor in reproductive age women worldwide, with financial burden of hundreds of billions of dollars annually including direct cost of hospital admission and medical management as well as indirect cost of loss of wages and productivity loss. Unfortunately, there is limitation in available long-term treatment options. Thus, there is a large unmet need in the UF space for noninvasive therapeutics. Elagolix is an oral GnRH antagonist that suppresses steroidal hormones production in a dose-dependent manner. Elagolix with add-back therapy is the first oral FDA approved drug for management of UF associated HMB up to 24 months due to the risk of continued bone loss.
4. Expert opinion
Alternative therapies have developed proposing women other options for symptomatic control (pharmacological treatment) rather than a definite cure with a side damage (surgical intervention). Many of these innovative therapies exploit UF dependence on ovarian hormones. Medical therapy aims either as a short term goal to temporarily relieve women for symptoms until their surgical intervention is sought as or as long term one until there is a natural regression of UF after menopause [81]. Unfortunately, traditional hormonal medical therapies carry intrinsic risks of their own and often fail in long-term control symptoms [82].
Classically, combined oral contraceptive pills (COCs) was an attracting option for gynecologists to control UF related bleeding, considering their low cost and relatively good safety profile [83]. However, COCs showed limited efficacy especially in tumor shrinkage outcome[83]. Also, FDA approved the use of levonorgestrel-releasing intrauterine system in 2009 to treat women with HMB. However, studies have shown conflicting results on its efficacy in controlling UF related bleeding [84–87].
Similarly, GnRH agonist LA was one of the first used medical anti-UF treatment. In 1999, FDA approved its utility as a preoperative short-term additive in women with symptomatic UF for anemia improvement due to HMB [4, 83, 88, 89]. However, acting as agonist is accompanied with delayed onset of action as well as worsening the UF related symptoms due to the initial estrogen flare effect. Additionally, its prolonged hypoestrogenism effect resulted in higher risk of BMD loss. Fortunately, this BMD changes are reversible only if treatment course is limited to 6 months. Nonetheless, continued therapy resulted in progressive and irreversible BMD loss [90]. In addition, classic menopausal side-effects such as hot flashes, vaginal dryness, and headaches limit the treatment to 6 months as well without add-back therapy [91].
Selective progesterone receptor modulators (SPRMs) have been studied extensively in the last few years with initial promising effects in induction of amenorrhea and shrinking tumor size [16]. SPRMs are synthetic ligands targeting the progesterone receptor (PR) with mixed agonist and antagonist activities [92]. Their indication as emergency contraception, termination of pregnancy, premenstrual syndrome and assisted reproduction are due their tissue-selective effects [93]. Moreover, expression of PR in endometrial and fibroid cells encouraged SPRMs investigations against UF, AUB, dysmenorrhea and endometriosis [82]. SPRMs family includes Mifepristone, Asoprisnil, Onapristone, ulipristal acetate (UPA), Lonaprisan, Vilaprisan and Telapristone. Notably, enthusiasm about SPRMs was attributed to their minimal effect on serum estrogen levels therefore they are not expected to induce menopausal-like symptoms or subsequent bone loss [92–95]. Vilaprisan dosed in 1–5 mg daily for 12 week induced profound bleeding cessation in women with UFs which supported further advancement of clinical trials to evaluate vilaprisan in women with symptomatic UFs [96]. However, long-term toxicological animal studies using high doses revealed safety concerns, therefore all current trials were paused [97]. A main known risk with the use of SPRMs is their association with a reversible morphological change in the endometrial lining known as progesterone-receptor modulator-associated endometrial changes (PAECs) after prolonged exposure [82, 98]. UPA was approved and prescribed in Europe, Canada and Australia for few years but recent studies found that UPA could cause liver failure that may require liver transplantation [17, 18] and EMA called for temporary suspension of UPA (Esmya™) and generic medicines sales. Off note, The EMA’s review is restricted only to UPA 5mg for the treatment of UFs and does not affect its use in 30mg as a single-dose medicine (ellaOne) for EC, as there is currently no concern about liver injury with the latter [99].
The utility of new-generation oral GnRH-antagonists, such as elagolix, relugolix and linzagolix is offering a new potential opportunity for the future therapy of UFs: elagolix has been the most studied drug of this class for treating benign gynecological diseases including endometriosis and UFs, for which it has been approved in 2018 and 2020 respectively [100, 101]. Notably, elagolix is the only and first FDA approved oral treatment for UF associated bleeding with advantage over the injectable antagonists that it offers less frequent regimen and better safety profile. Studies explored different dosage regimens of elagolix to balance the efficacy and safety and concluded 300 mg twice daily in combination with add back therapy to fit the best based on Phase III ELARIS studies as well as population-based PK studies. Further studies will identify best population to benefit from the therapy in addition to long term safety profile. Interestingly, upon drug discontinuation, hormonal level was found to return rapidly to normal values along with restoration of menstruation which represent an advantage for women pursuing future fertility [102]. Recently, a case report was published showing a debulking effect of elagolix on a uterine adenomyoma [103].
Other GnRH-antagonist, relugolix is recently licensed in Japan for UF related symptoms management following several Phase III clinical trials showing it efficacy in UF associated pain [102, 104]. In United States, extensive phase III studies are currently exploring its efficacy in UF and prostate cancer. For prostate cancer, relugolix, as oral androgen deprivation therapy, achieved rapid, sustained suppression of testosterone levels that was superior to that with leuprolide, with a 54% lower risk of major adverse cardiovascular events [105]. For UF, ongoing phase III 24 week trials (Liberty 1 and 2) are conducted in women with UF associated HMB exploring combination therapy of relugolix 40 mg, E2 1 mg, NETA 0.5 mg, or delayed relugolix combination therapy (relugolix 40 mg monotherapy followed by relugolix combination therapy, each for 12 weeks) compared to placebo control (LIBERTY 1, NCT03049735; LIBERTY 2, NCT03103087). Of note, relugolix has longer t1/2 that allows for once daily administration and may improve patients’ compliance in long-term therapy [106].
The recent explored GnRH antagonist is linzagolix (OBE2109), alone and in combination with add-back therapy in 2 ongoing prospective, randomized, parallel-group, double-blind, placebo-controlled phase III studies for treatment of UF associated HMB in premenopausal women(NCT03070951 and NCT03070899).
Article highlights:
Uterine Fibroid has a profound impact on patients’ quality of life and health care costs worldwide.
The mechanism underlying uterine fibroids pathogenesis remains unclear, which in turn limits its effective treatment. Current investigated treatment options aim to halt their hormonal dependent growth including gonadotropin releasing hormone (GnRH) analogs (agonists and antagonists).
If possible, many women with UFs would choose pharmacological treatment over a surgical treatment to secure their future family plans and avoid major surgeries.
Elagolix is an orally bioavailable, second-generation, non-peptide GnRH antagonist recently approved by the US Food and Drug Administration (FDA) in combination with estradiol/norethindrone acetate for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women up to 24 months.
Following elagolix discontinuation, hormonal level was found to return rapidly to normal values along with restoration of menstruation which represent an advantage for women pursuing future fertility.
Funding
This study was supported in part by the National Institutes of Health grants: R01 HD094378-04, R01 ES 028615-02, R01 HD100367-01, U54 MD007602 and R01 HD094380-02
Footnotes
Declaration of interest
A. Al-Hendy has declared consultancy for Allergan, Bayer, Repros and Myovant Sciences, as well as AbbVie. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed having served on advisory boards for Bayer AG and Gedeon Richter. AbbVie provided a scientific accuracy review at the request of the journal editor. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest * or of considerable interest ** to readers.
- 1.Ulin M, et al. , Uterine fibroids in menopause and perimenopause. Menopause, 2020. 27(2): p. 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulun SE, Uterine fibroids. N Engl J Med, 2013. 369(14): p. 1344–55. [DOI] [PubMed] [Google Scholar]
- 3.Stewart EA, Clinical practice. Uterine fibroids. N Engl J Med, 2015. 372(17): p. 1646–55. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo ER, et al. , The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol, 2012. 206(3): p. 211 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Q, Diamond MP, and Al-Hendy A, Converting of Myometrial Stem Cells to Tumor-Initiating Cells: Mechanism of Uterine Fibroid Development. Cell Stem Cells Regen Med, 2016. 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke-Pearson DL and Geller EJ, Complications of hysterectomy. Obstet Gynecol, 2013. 121(3): p. 654–73. [DOI] [PubMed] [Google Scholar]
- 7.Lonnee-Hoffmann R. and Pinas I, Effects of Hysterectomy on Sexual Function. Curr Sex Health Rep, 2014. 6(4): p. 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook H, et al. , The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol, 2010. 62(3): p. 225–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Owen C. and Armstrong AY, Clinical management of leiomyoma. Obstet Gynecol Clin North Am, 2015. 42(1): p. 67–85. [DOI] [PubMed] [Google Scholar]
- 10.Ali M, Chaudhry ZT, and Al-Hendy A, Successes and failures of uterine leiomyoma drug discovery. Expert Opin Drug Discov, 2018. 13(2): p. 169–177. [DOI] [PubMed] [Google Scholar]
- 11.Lethaby A, Vollenhoven B, and Sowter M, Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev, 2001(2): p. CD000547. [DOI] [PubMed] [Google Scholar]
- 12.Lethaby A, Vollenhoven B, and Sowter M, Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG, 2002. 109(10): p. 1097–108. [DOI] [PubMed] [Google Scholar]
- 13.Sabry M. and Al-Hendy A, Medical treatment of uterine leiomyoma. Reprod Sci, 2012. 19(4): p. 339–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P. and Sharma A, Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J Hum Reprod Sci, 2014. 7(3): p. 170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conn PM and Ulloa-Aguirre A, Pharmacological chaperones for misfolded gonadotropin-releasing hormone receptors. Adv Pharmacol, 2011. 62: p. 109–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali M. and Al-Hendy A, Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol Reprod, 2017. 97(3): p. 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnez J, et al. , Liver safety parameters of ulipristal acetate for the treatment of uterine fibroids: a comprehensive review of the clinical development program. Expert Opin Drug Saf, 2018. 17(12): p. 1225–1232. [DOI] [PubMed] [Google Scholar]
- 18.Meunier L, et al. , Acute liver failure requiring transplantation caused by ulipristal acetate. Clin Res Hepatol Gastroenterol, 2020. 44(3): p. e45–e49. [DOI] [PubMed] [Google Scholar]
- 19.EMA, Ulipristal acetate for uterine fibroids: EMA recommends restricting use. https://www.ema.europa.eu/en/medicines/human/referrals/ulipristal-acetate-5mg-medicinal-products, 2020: p. Accessed February 2021. [Google Scholar]
- 20.Lamb YN, Elagolix: First Global Approval. Drugs, 2018. 78(14): p. 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz K, et al. , The role of pharmacotherapy in the treatment of endometriosis across the lifespan. Expert Opin Pharmacother, 2020. 21(8): p. 893–903. [DOI] [PubMed] [Google Scholar]
- 22.Chen C, et al. , Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4 -methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J Med Chem, 2008. 51(23): p. 7478–85. [DOI] [PubMed] [Google Scholar]
- 23.Ng J, et al. , Dose-Dependent Suppression of Gonadotropins and Ovarian Hormones by Elagolix in Healthy Premenopausal Women. J Clin Endocrinol Metab, 2017. 102(5): p. 1683–1691. [DOI] [PubMed] [Google Scholar]
- 24.Shebley M, et al. , Clinical Pharmacology of Elagolix: An Oral Gonadotropin-Releasing Hormone Receptor Antagonist for Endometriosis. Clin Pharmacokinet, 2020. 59(3): p. 297–309.* Article with lots of information about elagolix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winzenborg I, et al. , Population Pharmacokinetics of Elagolix in Healthy Women and Women with Endometriosis. Clin Pharmacokinet, 2018. 57(10): p. 1295–1306. [DOI] [PubMed] [Google Scholar]
- 26.Orilissa™, [United States package insert]. North Chicago, IL. AbbVie Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210450s000lbl.pdf, 2018: p. Accessed February 2021. [Google Scholar]
- 27.Ng J, et al. , Elagolix Pharmacokinetic Profiles in Women With Renal or Hepatic Impairment. Clin Pharmacol Drug Dev, 2019. 8(8): p. 1053–1061. [DOI] [PubMed] [Google Scholar]
- 28.Archer DF, et al. , Elagolix Suppresses Ovulation in a Dose-Dependent Manner: Results From a 3-Month, Randomized Study in Ovulatory Women. J Clin Endocrinol Metab, 2020. 105(3). [DOI] [PubMed] [Google Scholar]
- 29.Struthers RS, et al. , Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab, 2009. 94(2): p. 545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archer DF, et al. , Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertil Steril, 2017. 108(1): p. 152–160 e4. [DOI] [PubMed] [Google Scholar]
- 31.Carr BR, et al. , Elagolix Alone or With Add-Back Therapy in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas: A Randomized Controlled Trial. Obstet Gynecol, 2018. 132(5): p. 1252–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winzenborg I, et al. , Effect of Elagolix Exposure on Clinical Efficacy End Points in Phase III Trials in Women With Endometriosis-Associated Pain: An Application of Markov Model. CPT Pharmacometrics Syst Pharmacol, 2020. 9(8): p. 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Review EM, US FDA Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210450Orig1s000MultiD.pdf, 2018. Accessed January 2021. [Google Scholar]
- 34.Chiney MS, et al. , Quantitative Assessment of Elagolix Enzyme-Transporter Interplay and Drug-Drug Interactions Using Physiologically Based Pharmacokinetic Modeling. Clin Pharmacokinet, 2020. 59(5): p. 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polepally AR, et al. , Assessment of Clinical Drug-Drug Interactions of Elagolix, a Gonadotropin-Releasing Hormone Receptor Antagonist. J Clin Pharmacol, 2020. 60(12): p. 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nader A, et al. , Drug-Drug Interaction Studies of Elagolix with Oral and Transdermal Low-Dose Hormonal Add-Back Therapy. Clin Pharmacokinet, 2020. [DOI] [PubMed] [Google Scholar]
- 37.Taylor HS, Dun EC, and Chwalisz K, Clinical evaluation of the oral gonadotropin-releasing hormone-antagonist elagolix for the management of endometriosis-associated pain. Pain Manag, 2019. 9(5): p. 497–515. [DOI] [PubMed] [Google Scholar]
- 38.Diamond MP, et al. , Elagolix treatment for endometriosis-associated pain: results from a phase 2, randomized, double-blind, placebo-controlled study. Reprod Sci, 2014. 21(3): p. 363–71. [DOI] [PubMed] [Google Scholar]
- 39.Carr B, et al. , Elagolix, an Oral GnRH Antagonist for Endometriosis-Associated Pain: A Randomized Controlled Study. J Endometr Pelvic Pain Disord, 2013. 5(3): p. 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor HS, et al. , Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N Engl J Med, 2017. 377(1): p. 28–40.** Phase III trial of elagolix in endometriosis associated pain management [DOI] [PubMed] [Google Scholar]
- 41.Surrey ES, et al. , Impact of elagolix treatment on fatigue experienced by women with moderate to severe pain associated with endometriosis. Fertil Steril, 2019. 112(2): p. 298–304 e3. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal SK, et al. , Clinically Meaningful Reduction in Dyspareunia Is Associated With Significant Improvements in Health-Related Quality of Life Among Women With Moderate to Severe Pain Associated With Endometriosis: A Pooled Analysis of Two Phase III Trials of Elagolix. J Sex Med, 2020. 17(12): p. 2427–2433. [DOI] [PubMed] [Google Scholar]
- 43.Vercellini P, et al. , Elagolix for endometriosis: all that glitters is not gold. Hum Reprod, 2019. 34(2): p. 193–199. [DOI] [PubMed] [Google Scholar]
- 44.Archer DF, et al. , Elagolix in the treatment of endometriosis: impact beyond pain symptoms. Ther Adv Reprod Health, 2020. 14: p. 2633494120964517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor HS, Use of Elagolix in Gynaecology. J Obstet Gynaecol Can, 2018. 40(7): p. 931–934. [DOI] [PubMed] [Google Scholar]
- 46.Barra F, Scala C, and Ferrero S, Elagolix sodium for the treatment of women with moderate to severe endometriosis-associated pain. Drugs Today (Barc), 2019. 55(4): p. 237–246. [DOI] [PubMed] [Google Scholar]
- 47.Ford B, Elagolix (Orilissa) for Endometriosis Pain. Am Fam Physician, 2019. 100(8): p. 502–504. [PubMed] [Google Scholar]
- 48.Melis GB, et al. , Overview of elagolix for the treatment of endometriosis. Expert Opin Drug Metab Toxicol, 2016. 12(5): p. 581–8. [DOI] [PubMed] [Google Scholar]
- 49.Leyland N, et al. , A Clinician’s Guide to the Treatment of Endometriosis with Elagolix. J Womens Health (Larchmt), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ezzati M. and Carr BR, Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain. Womens Health (Lond), 2015. 11(1): p. 19–28. [DOI] [PubMed] [Google Scholar]
- 51.Bradley CA, Reproductive endocrinology: Elagolix in endometriosis. Nat Rev Endocrinol, 2017. 13(8): p. 439. [DOI] [PubMed] [Google Scholar]
- 52.Poulos C, et al. , Patient preferences for elagolix and leuprolide for treating endometriosis-related pain in the United States. Expert Rev Pharmacoecon Outcomes Res, 2020: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 53.Dun EC and Taylor HS, Elagolix: a promising oral GnRH antagonist for endometriosis-associated pain. Oncotarget, 2017. 8(59): p. 99219–99220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor HS, et al. , Health-Related Quality of Life Improvements in Patients With Endometriosis Treated With Elagolix. Obstet Gynecol, 2020. 136(3): p. 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alessandro P, et al. , Research development of a new GnRH antagonist (Elagolix) for the treatment of endometriosis: a review of the literature. Arch Gynecol Obstet, 2017. 295(4): p. 827–832. [DOI] [PubMed] [Google Scholar]
- 56.Surrey ES, et al. , Real-World Characterization of Women with Diagnosed Endometriosis Initiating Therapy with Elagolix Using a US Claims Database. Clinicoecon Outcomes Res, 2020. 12: p. 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barra F, et al. , A comprehensive review of hormonal and biological therapies for endometriosis: latest developments. Expert Opin Biol Ther, 2019. 19(4): p. 343–360. [DOI] [PubMed] [Google Scholar]
- 58.Pokrzywinski R, et al. , Psychometric assessment of the PROMIS Fatigue Short Form 6a in women with moderate-to-severe endometriosis-associated pain. J Patient Rep Outcomes, 2020. 4(1): p. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pokrzywinski RM, et al. , Responsiveness and thresholds for clinically meaningful changes in worst pain numerical rating scale for dysmenorrhea and nonmenstrual pelvic pain in women with moderate to severe endometriosis. Fertil Steril, 2021. 115(2): p. 423–430. [DOI] [PubMed] [Google Scholar]
- 60.Barra F, et al. , Investigational drugs for the treatment of endometriosis, an update on recent developments. Expert Opin Investig Drugs, 2018. 27(5): p. 445–458. [DOI] [PubMed] [Google Scholar]
- 61.Winzenborg I, Soliman AM, and Shebley M, A Personalized Medicine Approach Using Clinical Utility Index and Exposure-Response Modeling Informed by Patient Preferences Data. CPT Pharmacometrics Syst Pharmacol, 2021. 10(1): p. 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pokrzywinski R, et al. , Responsiveness Evaluation and Recommendation for Responder Thresholds for Endometriosis Health Profile-30: Analysis of Two Phase III Clinical Trials. J Womens Health (Larchmt), 2020. 29(2): p. 253–261. [DOI] [PubMed] [Google Scholar]
- 63.Pokrzywinski RM, et al. , Achieving clinically meaningful response in endometriosis pain symptoms is associated with improvements in health-related quality of life and work productivity: analysis of 2 phase III clinical trials. Am J Obstet Gynecol, 2020. 222(6): p. 592 e1–592 e10. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal SK, et al. , Endometriosis-Related Pain Reduction During Bleeding and Nonbleeding Days in Women Treated with Elagolix. J Pain Res, 2021. 14: p. 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pokrzywinski RM, et al. , Impact of elagolix on work loss due to endometriosis-associated pain: estimates based on the results of two phase III clinical trials. Fertil Steril, 2019. 112(3): p. 545–551. [DOI] [PubMed] [Google Scholar]
- 66.Surrey ES, et al. , Impact of Elagolix on Workplace and Household Productivity Among Women with Moderate to Severe Pain Associated with Endometriosis: A Pooled Analysis of Two Phase III Trials. Patient, 2019. 12(6): p. 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlaff WD, et al. , Elagolix for Heavy Menstrual Bleeding in Women with Uterine Fibroids. N Engl J Med, 2020. 382(4): p. 328–340.** Phase III trial of elagolix in endometriosis associated pain management [DOI] [PubMed] [Google Scholar]
- 68.Simon JA, et al. , Elagolix Treatment for Up to 12 Months in Women With Heavy Menstrual Bleeding and Uterine Leiomyomas. Obstet Gynecol, 2020. 135(6): p. 1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slomski A, Elagolix Reduces Fibroid-Related Heavy Menstrual Bleeding Long-term. JAMA, 2020. 324(8): p. 733. [DOI] [PubMed] [Google Scholar]
- 70.Neri M, et al. , Clinical Utility Of Elagolix As An Oral Treatment For Women With Uterine Fibroids: A Short Report On The Emerging Efficacy Data. Int J Womens Health, 2019. 11: p. 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angioni S, D’Alterio MN, and Daniilidis A, Highlights on Medical Treatment of Uterine Fibroids. Curr Pharm Des, 2021.* Comprehensive article of medical treatment of uterine fibroids [DOI] [PubMed] [Google Scholar]
- 72.Farris M, et al. , Uterine fibroids: an update on current and emerging medical treatment options. Ther Clin Risk Manag, 2019. 15: p. 157–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perricos A. and Wenzl R, Efficacy of elagolix in the treatment of endometriosis. Expert Opin Pharmacother, 2017. 18(13): p. 1391–1397. [DOI] [PubMed] [Google Scholar]
- 74.Review EM, US FDA Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210450Orig1s000MultiD.pdf., 2018. Accessed February 2021. [Google Scholar]
- 75.ORIAHNN™, ORIAHNN™ (elagolix) [United States package insert]. North Chicago, IL. AbbVie Inc. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213388s000lbl.pdf, 2020. Last accessed January 2021. [Google Scholar]
- 76.Abbas Suleiman A, et al. , Exposure-Safety Analyses Identify Predictors of Change in Bone Mineral Density and Support Elagolix Labeling for Endometriosis-Associated Pain. CPT Pharmacometrics Syst Pharmacol, 2020. 9(11): p. 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surrey E, et al. , Long-Term Outcomes of Elagolix in Women With Endometriosis: Results From Two Extension Studies. Obstet Gynecol, 2018. 132(1): p. 147–160. [DOI] [PubMed] [Google Scholar]
- 78.Kilpatrick RD, et al. , Estimating the Effect of Elagolix Treatment for Endometriosis on Postmenopausal Bone Outcomes: A Model Bridging Phase III Trials to an Older Real-World Population. JBMR Plus, 2020. 4(12): p. e10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Hendy A, et al. , Predictors of response for elagolix with add-back therapy in women with heavy menstrual bleeding associated with uterine fibroids. Am J Obstet Gynecol, 2021. 224(1): p. 72 e1–72 e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang ST, et al. , Cost-effectiveness of elagolix versus leuprolide acetate for treating moderate-to-severe endometriosis pain in the USA. J Comp Eff Res, 2019. 8(5): p. 337–355. [DOI] [PubMed] [Google Scholar]
- 81.Kashani BN, et al. , Role of Medical Management for Uterine Leiomyomas. Best Pract Res Clin Obstet Gynaecol, 2016. 34: p. 85–103. [DOI] [PubMed] [Google Scholar]
- 82.Wagenfeld A, et al. , Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets, 2016. 20(9): p. 1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh SS and Belland L, Contemporary management of uterine fibroids: focus on emerging medical treatments. Curr Med Res Opin, 2015. 31(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- 84.Mercorio F, et al. , The effect of a levonorgestrel-releasing intrauterine device in the treatment of myoma-related menorrhagia. Contraception, 2003. 67(4): p. 277–80. [DOI] [PubMed] [Google Scholar]
- 85.Machado RB, et al. , The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecol Endocrinol, 2013. 29(5): p. 492–5. [DOI] [PubMed] [Google Scholar]
- 86.Maruo T, et al. , Effects of levonorgestrel-releasing IUS and progesterone receptor modulator PRM CDB-2914 on uterine leiomyomas. Contraception, 2007. 75(6 Suppl): p. S99–103. [DOI] [PubMed] [Google Scholar]
- 87.Song H, et al. , Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev, 2013(10): p. CD009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doherty L, et al. , Uterine fibroids: clinical manifestations and contemporary management. Reprod Sci, 2014. 21(9): p. 1067–92. [DOI] [PubMed] [Google Scholar]
- 89.Segars JH, et al. , Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update, 2014. 20(3): p. 309–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Surrey ES and Hornstein MD, Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstet Gynecol, 2002. 99(5 Pt 1): p. 709–19. [DOI] [PubMed] [Google Scholar]
- 91.Moroni RM, et al. , Add-back therapy with GnRH analogues for uterine fibroids. Cochrane Database Syst Rev, 2015(3): p. CD010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lusher SJ, et al. , Structural basis for agonism and antagonism for a set of chemically related progesterone receptor modulators. J Biol Chem, 2011. 286(40): p. 35079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bouchard P, Selective progesterone receptor modulators: a class with multiple actions and applications in reproductive endocrinology, and gynecology. Gynecol Endocrinol, 2014. 30(10): p. 683–4. [DOI] [PubMed] [Google Scholar]
- 94.Bouchard P, Chabbert-Buffet N, and Fauser BC, Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril, 2011. 96(5): p. 1175–89. [DOI] [PubMed] [Google Scholar]
- 95.Donnez J, et al. , Current management of myomas: the place of medical therapy with the advent of selective progesterone receptor modulators. Curr Opin Obstet Gynecol, 2015. 27(6): p. 422–31. [DOI] [PubMed] [Google Scholar]
- 96.Schutt B, et al. , Pharmacodynamics and safety of the novel selective progesterone receptor modulator vilaprisan: a double-blind, randomized, placebo-controlled phase 1 trial in healthy women. Hum Reprod, 2016. 31(8): p. 1703–12. [DOI] [PubMed] [Google Scholar]
- 97.Ciebiera M, et al. , Vilaprisan, a New Selective Progesterone Receptor Modulator in Uterine Fibroid Pharmacotherapy-Will it Really be a Breakthrough? Curr Pharm Des, 2020. 26(3): p. 300–309. [DOI] [PubMed] [Google Scholar]
- 98.Murji A, et al. , Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev, 2017. 4: p. CD010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.(EMA), E.M.A., Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk, 2020. Accessed January 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fantasia HC, Elagolix as a Novel Treatment for Endometriosis-Related Pain. Nurs Womens Health, 2019. 23(4): p. 366–369. [DOI] [PubMed] [Google Scholar]
- 101.Barra F, et al. , The potential role of elagolix for treating uterine bleeding associated to uterine myomas. Expert Opin Pharmacother, 2020. 21(12): p. 1419–1430. [DOI] [PubMed] [Google Scholar]
- 102.Osuga Y, et al. , Oral Gonadotropin-Releasing Hormone Antagonist Relugolix Compared With Leuprorelin Injections for Uterine Leiomyomas: A Randomized Controlled Trial. Obstet Gynecol, 2019. 133(3): p. 423–433. [DOI] [PubMed] [Google Scholar]
- 103.Kavoussi SK, Esqueda AS, and Jukes LM, Elagolix to medically treat a uterine adenomyoma: A case report. Eur J Obstet Gynecol Reprod Biol, 2020. 247: p. 266–267. [DOI] [PubMed] [Google Scholar]
- 104.Osuga Y, et al. , Relugolix, a novel oral gonadotropin-releasing hormone antagonist, in the treatment of pain symptoms associated with uterine fibroids: a randomized, placebo-controlled, phase 3 study in Japanese women. Fertil Steril, 2019. 112(5): p. 922–929 e2. [DOI] [PubMed] [Google Scholar]
- 105.Shore ND, et al. , Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med, 2020. 382(23): p. 2187–2196. [DOI] [PubMed] [Google Scholar]
- 106.Barra F, et al. , Relugolix for the treatment of uterine fibroids. Drugs Today (Barc), 2019. 55(8): p. 503–512. [DOI] [PubMed] [Google Scholar]