Abstract
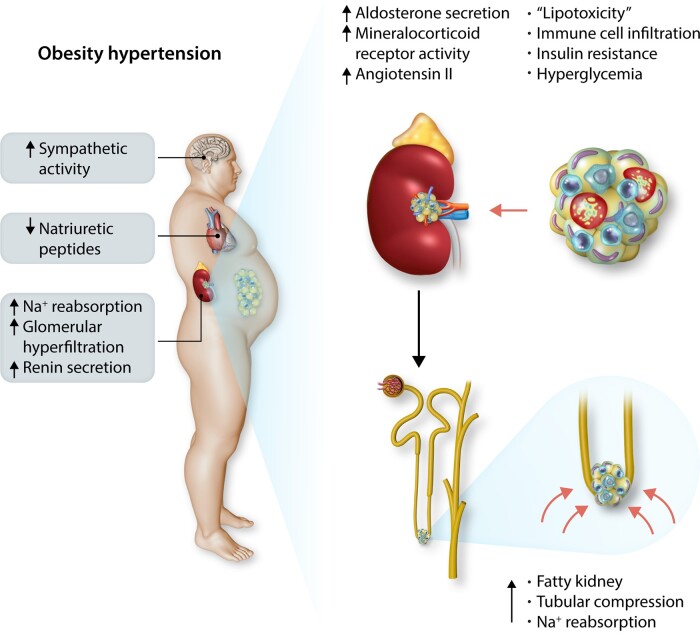
Obesity contributes 65–75% of the risk for human primary (essential) hypertension (HT) which is a major driver of cardiovascular and kidney diseases. Kidney dysfunction, associated with increased renal sodium reabsorption and compensatory glomerular hyperfiltration, plays a key role in initiating obesity-HT and target organ injury. Mediators of kidney dysfunction and increased blood pressure include (i) elevated renal sympathetic nerve activity (RSNA); (ii) increased antinatriuretic hormones such as angiotensin II and aldosterone; (iii) relative deficiency of natriuretic hormones; (iv) renal compression by fat in and around the kidneys; and (v) activation of innate and adaptive immune cells that invade tissues throughout the body, producing inflammatory cytokines/chemokines that contribute to vascular and target organ injury, and exacerbate HT. These neurohormonal, renal, and inflammatory mechanisms of obesity-HT are interdependent. For example, excess adiposity increases the adipocyte-derived cytokine leptin which increases RSNA by stimulating the central nervous system proopiomelanocortin-melanocortin 4 receptor pathway. Excess visceral, perirenal and renal sinus fat compress the kidneys which, along with increased RSNA, contribute to renin–angiotensin–aldosterone system activation, although obesity may also activate mineralocorticoid receptors independent of aldosterone. Prolonged obesity, HT, metabolic abnormalities, and inflammation cause progressive renal injury, making HT more resistant to therapy and often requiring multiple antihypertensive drugs and concurrent treatment of dyslipidaemia, insulin resistance, diabetes, and inflammation. More effective anti-obesity drugs are needed to prevent the cascade of cardiorenal, metabolic, and immune disorders that threaten to overwhelm health care systems as obesity prevalence continues to increase.
Keywords: Adipose, Blood pressure, Sympathetic activity, Renin–angiotensin–aldosterone system, Immune cells, Leptin, Melanocortins, Chronic kidney disease
Graphical Abstract
1. Introduction
The worldwide prevalence of obesity has more than tripled since 1975, with a parallel trend in type 2 diabetes (T2D).1,2 Over 1.9 billion adults were overweight or obese in 2016 and >60% of people with obesity live in developing countries where obesity-associated cardiometabolic disorders, including hypertension (HT), are rapidly increasing.3 These high rates of obesity will continue growing since >340 million children and adolescents aged 5–19 and >38 million children under the age of 5 are overweight/obese, and obese children usually suffer from obesity as adults. Reasonable projections suggest that nearly 50% of adults in the USA will be obese by 20304 and similar trends are forecast for global obesity.
One of the most significant consequences of obesity is HT, a major driver of cardiovascular disease (CVD), stroke and kidney disease. Epidemiology studies indicate that 65–75% of the risk for primary (essential) HT is due to overweight/obesity.5 At least 72% of patients with end-stage renal disease (ESRD) have HT and/or T2D, both driven largely by obesity. Obesity is also a risk factor for ESRD independent of HT and T2D.6
Although obesity is an established cause of HT, the mechanisms involved are multifactorial and not fully understood. However, progress is being made towards elucidating interactions of renal, neurohormonal, and inflammatory factors that link obesity with elevated blood pressure (BP) and end-organ injury. In this review, we highlight key mechanisms that initiate obesity-HT and the potential role of immunity/inflammation and obesity-associated metabolic disorders in exacerbating HT by causing kidney injury.
1.1 Cardiorenal and metabolic risk associated with obesity
Although body mass index (BMI, weight in kg/height in meters2) is often used to gauge obesity in clinical studies, BMI does not differentiate muscle from adipose tissue, or visceral adipose tissue (VAT) from subcutaneous adipose tissue (SAT). Considerable evidence indicates that excess VAT conveys greater risk for insulin resistance, T2D, dyslipidaemia, HT, and cardiorenal disorders than SAT.7 Thus, individuals with the highest VAT and ectopic fat in organs have higher cardiometabolic risk compared to equally obese subjects with less VAT.7 Excess fat accumulation in and around the kidneys (renal sinus and perirenal fat) is also associated with HT even after adjusting for overall adiposity, BMI, and other traditional risk factors.8 Although VAT and BMI are correlated in populations, variations in body fat distribution explain some of the sex, ethnic, and age-related differences reported for associations of HT with BMI.
1.2 Excess adiposity and blood pressure
There is a linear relationship between BP and obesity indices in diverse populations. Approximately 60–76% of overweight/obese subjects have HT, the most common comorbid condition associated with obesity.9 These estimates are based on HT defined as BP ≥ 140/90 mmHg whereas recent guidelines define stage 1 HT as systolic/diastolic BP of 130–139/80–89 mmHg.10 These new criteria substantially increase the percentage of people with obesity who are considered for HT treatment.
Some people with obesity have BPs not considered as ‘hypertensive’. This observation has often been interpreted as evidence that genetic/epigenetic predisposition or other factors are required for obesity to increase BP in some people. However, most studies have not measured VAT, renal sinus fat, and perirenal fat which may be increased in people with HT who have a BMI <30 and are sedentary with sarcopenia. Visceral obesity displaces the BP frequency distribution towards higher pressures, regardless of whether a person’s BP resides in the hypertensive range. Thus, weight loss and decreased adiposity in obese people generally reduces BP even when they are ‘normotensive’.8
Duration of obesity also influences BP. Nyamdorj et al.11 found in persons 25–74 years old that a 1-SD increase in BMI over a 5-year period led to 30% greater risk of HT compared with people whose weight did not change. In the Nurses' Health Study and the Health Professionals Follow-up Study, weight gain during 10 years of follow-up was associated with higher risk of HT, even for BMIs considered normal.12 In the Johns Hopkins Precursors Study, obesity in young adults conferred a three-fold greater risk of HT at 46 years of follow-up, after accounting for lifestyle changes over the life course.13 Thus, HT risk increases with increasing obesity duration.
1.2.1 Ethnic and sex differences in hypertension associated with obesity
Although obesity is a major risk factor for HT in all populations that have been studied, the quantitative impact of obesity on BP may depend, at least in part, on age, sex, and ethnicity. In most countries, including the USA, more women than men are obese but a greater percentage of men have HT than women.14 After menopause, however, HT prevalence in women rises to the same level or higher, compared with men, and comparable increases in BMI cause greater increases in systolic BP in women than in men.15,16
Ethnicity also influences the association of obesity and HT. In the USA where the overall prevalence of obesity in adults was 42.4% in 2018, non-Hispanic blacks had the highest age-adjusted prevalence of obesity (49.6%), followed by Hispanics (44.8%), non-Hispanic Whites (42.2%) and non-Hispanic Asians (17.4%).17 Obesity prevalence was lowest in non-Hispanic Asian women (17.2%) and highest in non-Hispanic black women (56.9%) compared with all other groups.17 High BP is also more common in non-Hispanic black adults (54%) than in non-Hispanic White adults (46%), non-Hispanic Asian adults (39%), or Hispanic adults (36%).
Differences in body fat distribution among women and men likely explain some of the variations in BMI and BP associations.18,19 Women generally have greater body fat percentage than men with preferential accumulation of adipose tissue in the gluteofemoral region, whereas men usually have more VAT.18 Higher levels of oestrogens and lower testosterone in women than men may account for sex differences in VAT before menopause. After menopause, however, increased VAT may be a major driver of increased BP and cardiovascular risk in women as well as in men.
Differences in VAT also have important clinical implications for ethnic/race variations in cardiometabolic risk. Compared with non-Hispanic Whites, Asian Americans have greater VAT and risk for HT and T2D mellitus at lower BMIs.20–22 These findings have led to recommendations for race/ethnicity-specific cut-offs for waist circumference in the diagnosis of metabolic syndrome.23 Thus, ethnicity, sex, age, duration of obesity, and location of adipose tissue are all important factors to consider in associating obesity with increased BP and cardiometabolic risk.
1.2.2 Are some people with obesity ‘metabolically healthy’?
Several organizations, including the World Obesity Federation, the Obesity Society, and the American Medical Association, have declared that obesity is a chronic, relapsing progressive disease.24 Others have proposed the concept of metabolically healthy obesity (MHO) and metabolically unhealthy obesity (MUO) based on clinical observations that a subgroup of people with BMI ≥ 30 do not exhibit obvious cardiometabolic abnormalities.25,26
Fat location is important in determining cardiometabolic health and accumulation of VAT and ectopic organ fat carries greater risk than SAT.7,18 Also, whether MHO represents a distinct or stable phenotype is debatable. The Atherosclerosis Risk in Communities study showed that people with MHO had greater increases in BP over a 3-year period compared to normal weight participants.27 A 30-year follow-up of >90000 women revealed that most obese women who were considered metabolically healthy eventually developed metabolically unhealthy phenotypes associated with increased CVD risk.28 These and other observations suggest that ‘metabolically healthy’ obese subjects often convert to unhealthy phenotypes over time.28
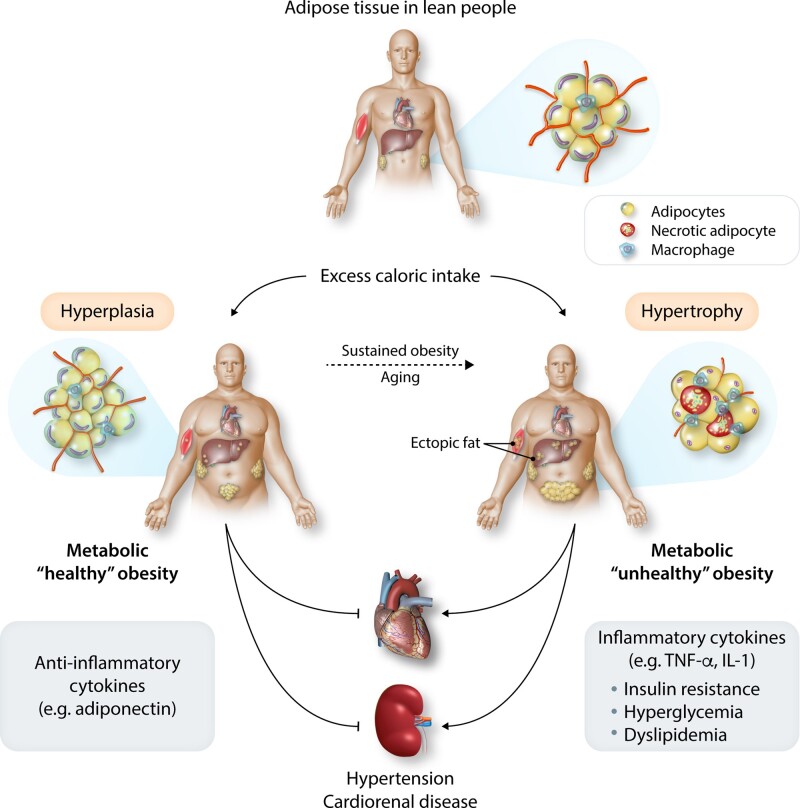
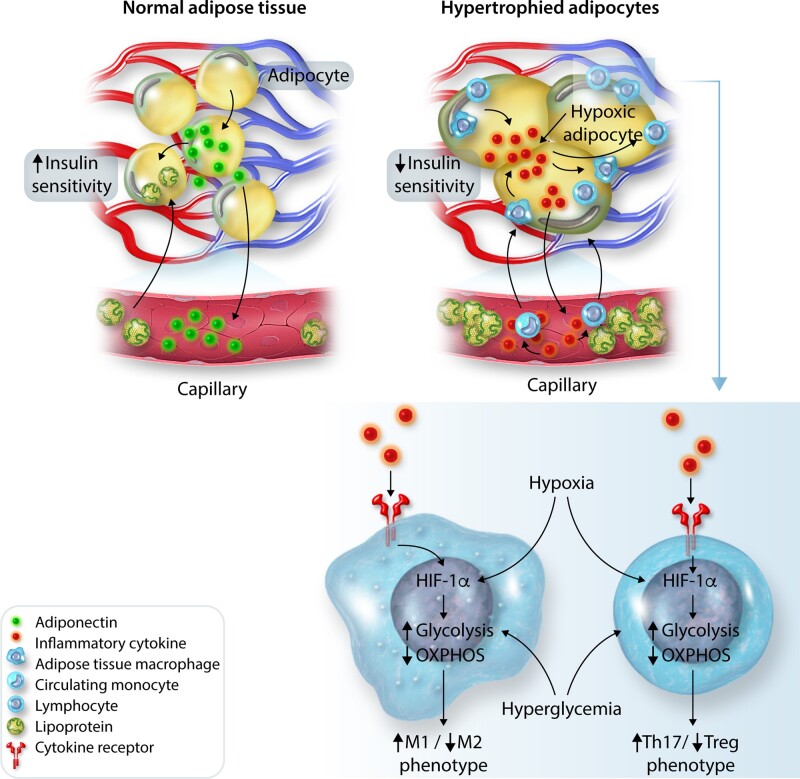
What causes conversion from MHO to MUO? Increasing the number of small adipocytes (hyperplasia) is considered as a healthy way of storing fat since small adipocytes have adequate supplies of blood and oxygen, normal secretion of the anti-inflammatory hormone adiponectin, and normal insulin sensitivity. In contrast, excessively large, hypertrophied adipocytes have inadequate vascularization, hypoxia, and decreased adiponectin secretion (Figure 1).29 Some large hypoxic adipocytes may undergo necrosis, causing immune cell infiltration of adipose tissue, inflammation, and insulin resistance. This cascade may ultimately lead to MUO associated with elevated circulating lipids and glucose and ectopic deposition of lipids (‘lipotoxicity’), and inflammation in the liver, muscle, heart, kidneys, pancreas, and other tissues.29,30
Figure 1.
Possible mechanisms for metabolically healthy and unhealthy obesity. Storage of excess calories by adipocyte hyperplasia is considered to be healthy because the tissue maintains adequate vascularization and secretion of anti-inflammatory adipokines. Excessive hypertrophy of existing adipocytes is considered unhealthy since cells become inadequately vascularized, hypoxic, and dysfunctional, leading to macrophage invasion and secretion of inflammatory cytokines that may contribute to insulin resistance and other metabolic disorders, exacerbating hypertension, and cardiorenal disease.
With sustained obesity and ageing, conversion from MHO to MUO may also be related to a gradual decline in adipogenic potential associated with senescent preadipocytes and decreased capability of SAT to undergo hyperplasia and effectively store lipids.29 In women, menopause is associated with redistribution of adipose tissue from SAT to VAT and increased ectopic fat accumulation, partly due to loss of oestrogens which promote adipocyte hyperplasia and help prevent excessive enlargement of adipocytes. Excessively hypertrophied adipocytes lead to inflammation, insulin resistance, glucose intolerance, dyslipidaemia, accumulation of ectopic fat, and increased cardiometabolic risk.31
2. General mechanisms of obesity-hypertension
The mechanisms that initiate and maintain obesity-HT are complex and time-dependent. Overfeeding rapidly activates the sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS), even before large increases in body weight or VAT occur.30 Conversely, reductions in caloric intake following bariatric surgery cause rapid reductions in BP, SNS activity and correction of some metabolic disorders before significant weight loss.32–34
Activation of the SNS and RAAS in obesity is modest but sufficient to cause increased renal sodium reabsorption, impaired pressure natriuresis, and expansion of extracellular fluid volume. Renal blood flow and glomerular filtration rate are increased (hyperfiltration) in obesity prior to substantial glomerular injury. Blood flows in most other tissues and organs are also elevated in obesity, thereby increasing venous return and cardiac output.30
As visceral, perirenal and renal sinus fat accumulate and intra-abdominal pressure increases, the kidneys become compressed, further increasing sodium reabsorption and BP.8,30 Excessive adipocyte hypertrophy leads to activation of resident macrophages, infiltration of macrophages, and secretion of proinflammatory cytokines that adversely influence kidney function and vascular function throughout the body.29 When obesity-induced increases in BP, metabolic disorders, and inflammatory mediators coexist and are sustained, gradual injury to end-organs occurs, initiating a cascade that exacerbates HT, making it more resistant to treatment.
3. Sympathetic nervous system activation in obesity
Studies in humans and experimental models indicate that SNS overactivation contributes to initiation and maintenance of obesity-HT.35,36 Increased sympathetic nerve activity (SNA) in obesity is often mild and non-uniform in various tissues. Muscle SNA (MSNA) and renal SNA (RSNA), assessed by kidney norepinephrine spillover, are higher in obese compared with non-obese normotensive humans.37 In rabbits RSNA increases within a few days after starting a high-fat diet.38 In contrast, cardiac norepinephrine spillover may be normal or reduced in obesity-HT, with increased heart rate (HR) due mainly to decreased parasympathetic activity.37,39
Age, sex, ethnicity, and differences in fat distribution contribute to variability of SNA and BP among obese subjects. Increased muscle SNA is more closely associated with VAT than with SAT or overall adiposity.40 Unfortunately, accurate assessments of SNA in various organs in younger and older men and women of different ethnicities are limited and essentially all measurements have been made under quiet, resting conditions which may not reflect SNA during normal daily activities.41
Despite mild increases in SNA, sympathetic blockade in obese humans or experimental animals with dietary-induced obesity consistently reduces BP.8,30 These findings indicate that increased SNA contributes importantly to obesity-HT in experimental animals and humans.
3.1 Renal denervation attenuates obesity-hypertension
Renal nerves mediate much of the BP effects of sympathetic activation in obese subjects. In obese dogs, renal denervation (RDN) greatly attenuated sodium retention and increased BP.42 RDN also nearly normalized BP in established obesity-HT.43,44
In obese humans with resistant HT, catheter-based radiofrequency RDN significantly lowered BP for up to 3 years.45 However, most clinical trials were confounded by failure to determine the extent of RDN and because patients were already on ≥3 BP medications, including antagonists of the RAAS which contributes to BP effects of RSNA. In hypertensive patients who were not taking antihypertensive medications, RDN significantly reduced 24-h ambulatory systolic BP at 2–3 months compared to the sham procedure, although RDN adequacy was not verified.46 These results underestimate BP effects of RDN since catheter-based RDN typically causes <40–50% renal nerve ablation unless nerves in renal segmental arteries are ablated.44,47
3.2 Potential mediators of SNS activation in obesity
Several mediators of increased SNS activity in obesity have been proposed.37,40,48 In this review, we focus on (i) impaired baroreceptor reflexes, (ii) chemoreceptor activation, (iii) activation of the central nervous system (CNS) leptin-proopiomelanocortin (POMC) pathway, (iv) hyperinsulinaemia, and (v) inflammation of hypothalamic and/or brainstem neurons.
3.3 Impaired baroreflexes in obesity-hypertension
Cardiac and RSNA baroreflex sensitivities are reduced in obesity-HT.38,39,49 Iliescu et al.49 found decreases in 24-h cardiac baroreflex sensitivity within a few days of starting a high-fat diet in dogs, even before substantial increases in adiposity or BP, although these responses escalated with progressive weight gain and HT. Similar results were found in rabbits fed a high-fat diet.38
Obesity also blunts cardiopulmonary reflexes activated by volume expansion. Administration of tacrolimus, an immunosuppressive drug that inhibits calcineurin, production of interleukin-2, and proliferation of T cells, restored renal sympatho-inhibition during acute volume expansion.50 Whether inflammation-induced attenuation of cardiopulmonary-renal reflexes contributes to obesity-HT is uncertain.
Although the importance of diminished baroreflexes in initiating obesity-HT is uncertain, chronic baroreflex activation, by electrically stimulating the carotid sinuses, restored cardiac baroreflex sensitivity and BP to control levels in obese dogs.44 Sustained BP reductions during chronic baroreceptor stimulation occurred concurrently with decreases in plasma renin activity and renal sodium reabsorption, highlighting the importance of the renal nerves and renin secretion in obesity-HT. Although RDN also decreased BP to normal levels in obese hypertensive dogs, RDN did not improve baroreflex sensitivity.44 These results also indicate that BP reductions can be achieved by RDN independently of changes in baroreflex sensitivity in obesity-HT.
Clinical studies also indicate that chronic baroreflex stimulation, electrically or mechanically with an endovascular device, diminishes central sympathetic outflow and reduces BP in patients with treatment-resistant HT.44 However, randomized, controlled trials have not been completed to demonstrate long-term safety and efficacy of baroreflex activation for treating HT.44
3.4 Role of hypoxaemia and chemoreflexes in obesity-hypertension
Obesity-induced hypoxaemia may activate peripheral chemoreceptors, causing increases in SNA that raise BP. Obese dogs were hypoxaemic but eucapnic and appeared to have tonic activation of carotid chemoreceptors associated with increases in BP and respiratory rate.51 Chronic electrical stimulation of the carotid baroreflex or carotid body denervation attenuated increases in BP and respiratory rate despite worsening hypoxaemia in obese dogs. Since obesity increases metabolic rate and impairs respiratory mechanics, hypoxaemia may be more common than previously appreciated, even in the absence of obstructive sleep apnoea (OSA).
Obesity-HT is frequently associated with OSA, intermittent nocturnal hypoxia, and elevated SNA.48,52 Suppression of chemoreflex activity by breathing 100% oxygen reduces SNA, HR, and BP.53 Chemoreflex-induced sympathetic responses to hypoxia and enhanced central chemoreflex responses to hypercapnia are exaggerated in patients with obesity and OSA.52,53 The mechanisms underlying central chemoreflex potentiation in obesity are unclear, but may be partly related to increased leptin produced by adipocytes.54
The observation that obesity causes chemoreflex activation, even without OSA, has stimulated interest in carotid body resection as a potential treatment for HT although this procedure may worsen hypoxaemia.55 Currently, studies on the role of peripheral or central chemoreceptors in humans with obesity-HT are scarce and usually related to respiratory rather than BP responses.
3.5 Role of leptin in SNS activation and obesity-hypertension
Leptin, a cytokine secreted by adipocytes, may contribute to obesity-HT.30 Chronic leptin infusion causes modest, slowly-developing increases in BP in rodents,56,57 consistent with mild SNS activation that increases renal sodium reabsorption but is insufficient to directly cause vasoconstriction. Leptin’s chronic effect on BP in male rats was completely abolished by α/β-adrenergic receptor blockade, suggesting mediation by the SNS.57 However, in female rodents leptin may also raise BP by stimulating aldosterone secretion.58
Inhibition of nitric oxide (NO) synthesis potentiates leptin’s BP effects despite decreased food intake and weight loss.59 Because obesity causes endothelial injury, decreased NO bioavailability, and resistance to leptin’s anorexic effects, obesity may enhance leptin’s BP effects if SNS activation is preserved, as previously reported.60
Leptin receptor (LepR) blockade reduces SNA and BP in obese rabbits, further supporting a potential role of leptin in obesity-HT.61 Also, ob/ob mice with leptin deficiency have morbid obesity, insulin resistance, hyperinsulinaemia, and dyslipidaemia but are not hypertensive, compared to lean control mice.62 Moreover, a 3-day leptin infusion increased systolic BP in ob/ob mice notwithstanding substantial weight loss.63 Thus, leptin contributes to elevated BP in obese rodents. However, leptin is not the only factor driving increased SNA in obesity since obese Zucker rats with defective leptin signalling may have increased sympathetic activity and mild increases in BP, possibly related to impaired baroreflexes as well as activation of the CNS melanocortin pathway.64,65
Leptin’s role in human obesity-HT is less clear. Intravenous leptin infusion stimulates SNA in humans,66 similar to findings in rodents. However, subcutaneous injections of recombinant leptin for 12 weeks in overweight/obese humans did not raise BP, although BP was not a primary outcome and leptin had no significant effects on body weight.67 In a study of four severely obese patients with leptin gene mutations, only one had high BP, possibly due to underlying adrenocorticotrophic hormone excess.68 Humans with leptin-deficiency also exhibited impaired autonomic function, reduced BP responses to hypertensive stimuli, and decreases in BP and RAAS activation during upright posture. Thus, available data in humans, although limited, suggest that leptin increases SNA and in the absence of leptin, even severe obesity, insulin resistance, hyperinsulinaemia, and dyslipidaemia may not increase BP.
3.5.1 Brain sites of leptin action
Leptin increases SNA and BP by activation of neurons in the hypothalamus and brainstem. After deletion of LepR in the hypothalamic arcuate nucleus (ARC) leptin failed to increase RSNA.69 Also, deletion of LepR in POMC neurons of the ARC and brainstem completely abolished the chronic effects of leptin to increase BP.70 Leptin injections into the brainstem nucleus tractus solitarii (NTS) increased RSNA and BP.60 Thus, leptin’s effects on SNA involve hypothalamic and brainstem neuronal populations.30,71
3.5.2 Possible ‘selective’ leptin resistance in obesity
Although obesity attenuates leptin’s anorexic effects, stimulation of RSNA by leptin is maintained.60 The chronic effects of leptin on BP or HR were also preserved in obese rodents fed a high-fat diet whereas leptin-induced anorexia was attenuated.72 Similar studies in humans, however, have not been reported and the mechanisms that mediate this ‘selective’ resistance to leptin in obesity are still uncertain.60
3.5.3 Molecular pathways for differential effects of leptin on BP and metabolic functions
In the CNS, leptin stimulates janus tyrosine kinase 2 (JAK2) activity and three intracellular signalling pathways.73 Signal transducers and activators of transcription 3 (STAT3) plays a major role in mediating the effects of leptin on food intake and overall energy balance.30,35 Insulin receptor substrate 2 (IRS2)-phosphatidylinositol 3-kinase (PI3K) signalling mediates leptin’s effects on SNA and BP but is much less important in regulating energy balance.74 Src homology-2 tyrosine phosphatase (SHP2) signalling in the forebrain appears to be important in controlling food intake, energy balance, blood glucose and BP.75 Moreover, the effects of leptin on BP and glucose due to forebrain SHP2 signalling are mediated mainly by POMC neurons, although effects on food intake are mediated mainly by other forebrain neurons.75,76
It is possible that obesity differentially affects these three pathways, although this hypothesis has not been adequately tested. Also, different neuronal populations may contribute to differential control of cardiovascular and metabolic functions in obesity. For example, POMC neuron activation is essential for leptin’s effects on glucose regulation, RSNA and BP, whereas other neurons appear to be more critical for leptin’s actions on energy balance. However, interactions of hypothalamic and brainstem neurons, regulation of their signalling pathways, and how selective leptin resistance occurs in obesity have not been fully elucidated.
3.5.4 Can partial leptin reduction be beneficial in obesity-HT?
Leptin antagonism has not been seriously considered for treating HT since this could exacerbate obesity. However, some studies suggest that hyperleptinaemia per se causes leptin resistance and that partial leptin reduction may restore leptin sensitivity and, counterintuitively, reduce obesity. Zhao et al.77 showed in obese mice that a leptin neutralizing antibody, restored hypothalamic leptin sensitivity, reduced food intake, increased energy expenditure, and improved insulin sensitivity. This finding, if confirmed in humans, implies that there is an inflection point for leptin concentration, perhaps influenced by obesity-associated metabolic factors, where further increases cause desensitization of hypothalamic neurons, additional weight gain, and worsening metabolic disorders. In this circumstance, partial leptin reduction may restore leptin sensitivity, reduce adiposity, and improve the metabolic profile.
Because chronic hyperleptinaemia also promotes activation of innate and adaptive immunity as well as inflammation,78 partial leptin reduction may also reduce inflammation which could have additional beneficial effects, as discussed later. This provocative prediction that partial leptin reduction therapies would be beneficial for control of adiposity, glucose, inflammation, and BP will require testing in humans.
3.6 Brain melanocortins contribute to SNS activation in obesity-hypertension
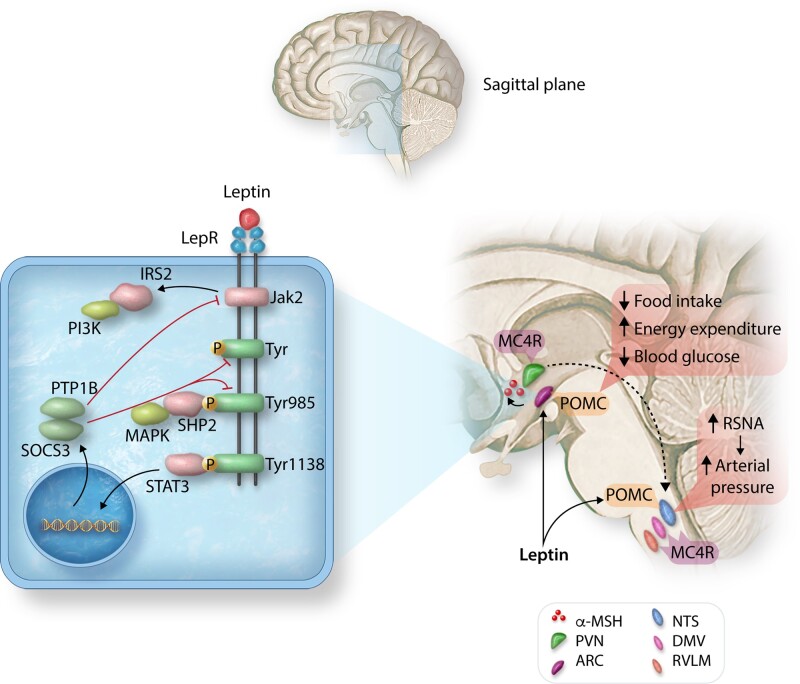
The CNS POMC-melanocortin 4 receptor (MC4R) system regulates energy balance, glucose metabolism, SNS activity and BP among other actions.8,79,80 Neurons that express POMC are located in the brainstem and ARC and project to second order neurons involved in BP regulation, such as the paraventricular nucleus (PVN), where their axons release α-melanocyte stimulating hormone (α-MSH), the endogenous ligand for MC4R (Figure 2).79,80
Figure 2.
Activation of the CNS leptin-melanocortin system differentially controls food intake, energy expenditure, blood glucose, and arterial pressure by activating distinct signalling pathways in the hypothalamic arcuate nucleus (ARC) and brainstem. Stimulation of leptin receptors (LepR) activates JAK2 tyrosine (Tyr) kinase, causing autophosphorylation of tyrosine residues on JAK2 and phosphorylation of Tyr985 and Tyr1138. Phosphorylation of Tyr985 activates SHP2/MAPK and phosphorylation of Tyr1183 activates STAT3 which mediates several effects of leptin while increasing transcription of SOCS3, which attenuates LepR-mediated signalling. PTP1B is increased in obesity and reduces leptin signalling by dephosphorylation of JAK2. LepR activation of POMC neurons stimulates release of α-MSH, which activates MC4R in neurons in the paraventricular nucleus (PVN), and nucleus tractus solitaries (NTS) and dorsal motor nucleus of the vagus (DMV). ARC, arcuate nucleus; IRS2, insulin receptor substrate 2; RSNA, renal sympathetic nerve activity; and RVLM, rostral ventrolateral medulla.
Hyperphagia and increased adiposity stimulate secretion of leptin, insulin and other factors which, in turn, activate POMC neurons and MC4R signalling while energy deficits and weight loss reduce MC4R signalling. Pharmacological activation of MC4R increases BP, an effect abolished by α/β-adrenergic blockade, despite reducing food intake and body weight.81,82 Conversely, MC4R antagonism or genetic disruption of MC4R causes hyperphagia, increased adiposity, insulin resistance and dyslipidaemia, but normal or reduced BP.83,84 Thus, in rodents MC4R signalling may be essential for obesity to increase SNA and BP.
Humans with MC4R deficiency are also extremely obese, insulin resistant and dyslipidaemic but have lower prevalence of HT and reduced norepinephrine excretion, compared to control subjects.85 Similar to results in rodents, treatment of obese people with synthetic MC4R agonists raises BP and HR, effects that have limited use of these drugs for treatment of common obesity.85 However, MC4R agonists cause reductions in hunger and substantial weight loss in patients with deficiency of POMC or LepR.86,87 Overall, current evidence suggests that the brain POMC-MC4R system plays a key role not only in body weight regulation but also in stimulating SNA and raising BP in obesity.
3.6.1 Other factors besides leptin activate the POMC-MC4R pathway in obesity
Although leptin is a key link between increased adiposity and POMC-MC4R activation, other factors also activate this pathway in obesity. For example, MC4R blockade reduced BP in obese Zucker rats with defective LepR signalling.64 MC4R antagonism also markedly decreased BP in spontaneously hypertensive rats (SHR) which are not obese or hyperleptinaemic but have increased SNA.83 HT associated with inhibition of NO synthesis was also diminished by MC4R blockade88 and the rise in MSNA during hypoxic stress was reduced in obese humans with MC4R mutations compared to control subjects.89 These observations indicate that multiple factors can activate CNS POMC-MC4R signalling to mediate SNS activation and increased BP in obesity, and that this system may also be important in regulation of SNA and BP in non-obese subjects.
4. Hormonal mechanisms of obesity-hypertension
Other hormone systems besides leptin also contribute to altered kidney function and increased BP in obesity.
4.1 RAAS activation in obesity-hypertension
The importance of the RAAS in obesity-HT has been previously reviewed90,91 and is briefly considered in this review. Experimental animals and people with obesity generally have mild increases in most components of the RAAS, including angiotensin II (AngII), and aldosterone.8,91 RAAS activation occurs despite high BP and sodium retention which normally suppress renin secretion, AngII formation, and aldosterone secretion.
4.1.1 Role of AngII
Although adipocytes produce angiotensinogen, whether they make enough AngII to increase BP in obesity is uncertain. Current evidence suggests that elevated AngII in obesity is driven largely by increased renal renin secretion due to SNS activation, kidney compression, and perhaps other factors.
Despite mild increases in renin and AngII formation, RAAS blockade attenuates sodium retention and obesity-HT.8 Obesity enhances BP sensitivity to AngII8,30 and even low levels of AngII stimulate renal sodium reabsorption via direct effects on multiple NaCl transporters and by efferent arteriolar constriction which increases peritubular capillary reabsorption.91 Constriction of efferent arterioles also raises glomerular capillary pressure, aggravating the adverse effects of HT on glomerular stress.91
RAAS blockade with angiotensin-converting enzyme (ACE) inhibitors or AngII receptor blockers (ARBs) lowers BP effectively in most obese patients with HT.8,30 While major HT clinical trials have generally included RAAS blockers, randomized trials comparing the effectiveness of RAAS blockers with other antihypertensive agents in lean and obese patients have not been conducted. However, clinical trials have demonstrated that RAAS antagonism attenuates progression of kidney injury in obese patients with HT and T2D.6,8,30
4.1.2 Role of aldosterone and mineralocorticoid receptor activation
Although AngII stimulates aldosterone secretion in obesity, leptin may contribute to sex-specific increases in aldosterone secretion. LepR antagonism reduced plasma aldosterone and mineralocorticoid receptor (MR) blockade improved endothelial function in obese female, but not male, mice.92 Yet, the role of leptin in controlling aldosterone secretion in obese humans, male or female, is still unclear.
Even though there are usually mild increases in plasma aldosterone concentration in obesity-HT, MR blockade reduces BP and end-organ injury. In obese dogs, MR antagonism blunted renal sodium reabsorption, glomerular hyperfiltration, and HT by >50%.93 MR antagonists also decreased BP in obese patients with treatment-resistant HT although BP reductions did not correlate with plasma aldosterone concentration.94 Also, spironolactone reduced BP and urinary albumin excretion in obese hypertensive subjects even after blockade of AngII formation.95
Compared to other antihypertensive drugs, the MR antagonist spironolactone was superior for treating obese patients with resistant HT who were already on ≥3 antihypertensive medications, usually including an ARB or ACE inhibitor.96 The antihypertensive benefit of MR blockade appears to be mainly due to reduced renal sodium reabsorption and the resulting natriuresis and diuresis96,97
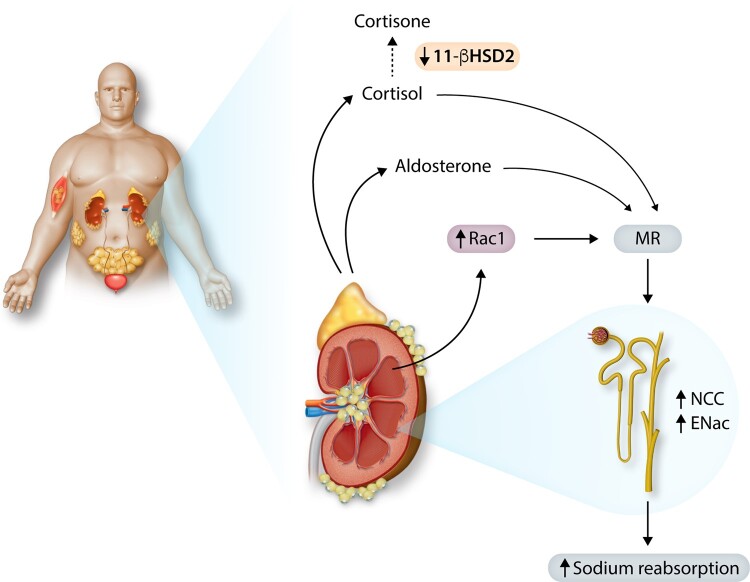
The observation that MR antagonism is effective in reducing BP and protecting against end-organ injury in obesity-HT, despite normal or even reduced plasma aldosterone, suggests that other factors besides aldosterone may activate MR. Several potential mechanisms have been suggested for aldosterone-independent MR activation (Figure 3). The GTP-binding protein Rac1, a member of the Rho family of GTPases, stimulates MR signalling and is increased in renal epithelial cells in obesity.98 Adipocyte-derived cytokines, hyperglycaemia and oxidative stress have been proposed to induce Rac1 expression in obesity.98 Fujita et al.98 also reported that Rac1 inhibitors ameliorate proteinuria and kidney injury in obesity and in salt-sensitive HT.
Figure 3.
Possible mechanisms of renal tubular mineralocorticoid receptor (MR) activation, increased renal sodium reabsorption, and hypertension in obesity. Obesity increases angiotensin II which stimulates secretion of aldosterone, normally the primary agonist of renal tubular MR. Adipokines, such as leptin, may also stimulate aldosterone secretion and obesity may cause MR activation via aldosterone-independent mechanisms such as increased renal tubular expression of Rac 1, a small GTP-binding protein member of the Rho family of GTPases. Cortisol may also activate MR in obesity via down-regulation of 11β-HSD2, which normally converts cortisol to cortisone, a glucocorticoid that does not avidly bind MR.
Glucocorticoids may also activate MR in obesity if 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) is reduced (Figure 3). Although cortisol normally has a high affinity for MR, 11β-HSD2 is co-localized with the MR in renal collecting tubules and converts cortisol to cortisone which is relatively inactive at the MR. Supporting the possibility that 11β-HSD2 deficiency may permit glucocorticoids to activate MR in obesity is the finding that renal 11β-HSD2 expression is reduced by 60-70% in obese dogs.8 Thus, increased oxidative stress, changes in the renal epithelial intracellular redox state, increased Rac1, and decreased 11β-HSD2 could all contribute to MR activation in obesity.8,99 However, the importance of these factors, in addition to aldosterone, in MR activation in obesity-HT is still unclear.
4.2 Are natriuretic peptides deficient in obesity-hypertension?
Blood volume expansion and cardiomyocyte stretch normally stimulate secretion of cardiac natriuretic peptides (NP) which circulate to the kidneys, reducing sodium reabsorption and attenuating volume expansion and increased BP. Obese hypertensive men, however, have lower plasma pro-atrial NP levels even though they have higher sodium intake and larger left atria than normotensive lean men.100 Also, NP responses to volume loading are impaired in obese subjects.101 Participants in the Framingham Heart Study who were obese had reduced circulating N-terminal pro-atrial NP concentration compared with those with normal BMI,102 although no significant correlations between BMI and plasma NP concentrations were reported in some other studies .103
Since obesity usually causes increased cardiac filling pressures, higher NP secretion is expected. However, a relative NP deficiency in obesity may be due to increased adipocyte-derived neprilysin, an endopeptidase that degrades NP. Obesity may also increase NP receptor-C (NPR-C) a scavenger receptor that facilitates cellular NP uptake and degradation without contributing to increased guanylyl cyclase activity.104 Thus, higher expression of neprilysin and NPR-C may cause a relative natriuretic handicap in obesity despite increased cardiac filling pressures which would normally stimulate NP secretion. However, the overall significance of abnormal secretion and clearance of NP in obesity-HT is still uncertain.104,105
4.3 Role of gut hormones and microbiota in obesity-hypertension
People with obesity often have decreased gut microbiota (GM) diversity along with metabolic disorders such as T2D.106 Although changes in diet and subsequent weight gain can alter GM, a causal role for GM dysbiosis in obesity has also been suggested by GM transplantation experiments.107
Complex interactions among GM, the immune system, inflammation, and vascular dysfunction in the kidneys and brain have been hypothesized for HT.108 For example, high-salt intake alters GM in mice and Lactobacillus murinus administration attenuates BP responses to high salt intake.109 The BP effects of GM dysbiosis were proposed to be mediated by induction of T helper 17 (TH17) cells and an autoimmune response.109 In healthy humans, high-salt intake for 2 weeks decreased intestinal survival of Lactobacillus and increased BP, although a cause and effect relationship was not established.109 However, BP reduction in SHR after ACE inhibition reduced mucosal permeability, inflammation, and dysbiosis110 suggesting that GM changes may be secondary to HT or RAAS activation which contributes to HT in SHR.
GM are the body’s only source of short chain fatty acids (SCFA), including acetate, proprionate and butyrate, produced by GM digestion of dietary fibre. The SCFA products of fibre digestion may influence the immune and nervous systems, epithelial cells, and blood vessels and mediate decreased HT risk with consumption of diets rich in fibre.108 Although SCFA production offers a reasonable connection between GM and BP regulation, evidence for a causal relationship in obesity-HT is still limited.
Another potential connection between BP regulation and GM is the enteric nervous system (ENS) which influences autonomic nervous system activity in coordination with gut motility.108 The ENS communicates with the CNS via sympathetic and parasympathetic input and gut hormones. The vagus nerve sends sensory signals to the NTS, a major CNS centre for cardiometabolic regulation. However, the importance of vagal afferent signals, induced by changes in GM, for BP control is uncertain.
Several gut hormones are influenced by GM, or their metabolites, and have various actions that could alter BP.111 For example, glucagon-like peptide-1 (GLP-1) increases renal sodium excretion while stimulating insulin secretion, increasing insulin sensitivity, and reducing food.112 Dipeptidyl peptidase-4 inhibitors, which attenuate degradation of GLP-1, as well as GLP-1 agonists slightly reduce BP in obese animals and hypertensive humans with T2D.111 Gastrin, an enterokine secreted by gastric G-cells, may also promote natriuresis which could reduce BP.111
While the possible role of GM metabolites and gut hormones in cardiometabolic regulation has stimulated substantial interest, there is still limited evidence for a major role in obesity-HT or that GM manipulation (e.g. through fecal transplants, antibiotics, or probiotics) has major beneficial effects for HT therapy. However, this research field is rapidly advancing and the multiple effects of GM and their metabolites on nervous, hormonal, and immune systems of the body are consistent with a potential role for cardiometabolic regulation.
5. Obesity, inflammation, and insulin resistance
Immune/inflammatory cells and their interactions with other cells serve as defenses against pathogens and injury, and are important for tissue homeostasis. Immune cells also respond to changes in their nutrient environment and energy supply in various ways, including release of inflammatory cytokines. Some of these cytokines circulate in the blood and influence metabolism in other tissues and in tissues that release them. Infection-related changes in metabolism as well as metabolic/energetic programming of immune responses are recognized as critical components of immunometabolism in adipose tissue and the liver.113,114 Immunometabolic pathways, including glycolysis and oxidative phosphorylation, are critical for phenotypic switching of various immune cells, and are heavily influenced by obesity-induced metabolic changes such as hyperglycaemia and hyperlipidaemia.115
This crosstalk between immune and metabolic systems permits tissues to eliminate pathogens, adapt to changing energy needs, and is essential for tissue repair and remodelling after injury.114 However, immune-inflammatory responses must be spatially and temporally regulated to maintain homeostasis. If inflammatory responses are excessive or sustained too long, they may damage tissues and cause metabolic derangements.
As discussed previously, excess energy is stored mainly as fat in adipocytes which undergo hypertrophy and hyperplasia. Visceral adipocytes have limited hyperplastic potential and undergo hypertrophy when storing additional lipids. As these cells enlarge, they become inadequately vascularized and hypoxic, leading to cell stress, apoptosis, immune cell infiltration, and increased secretion of inflammatory cytokines (Figure 4). As adipocytes become inflamed and dysfunctional, lipids are stored in other tissues such as the liver, skeletal muscle, pancreas, kidneys, and heart leading to ‘lipotoxicity’ and a cascade of cardiometabolic disorders.
Figure 4.
Possible mechanisms of inflammation and insulin resistance associated with excessive adipocyte hypertrophy. Small adipocytes secrete adiponectin locally and into the circulation, promoting insulin sensitivity and lipid storage. Hypertrophied adipocytes become hypoxic, leading to secretion of inflammatory cytokines and recruitment of circulating monocytes/lymphocytes into the tissue, which also secrete inflammatory cytokines. Within the microenvironment of hypertrophied adipocytes, immune cells are also hypoxic, leading to metabolic reprogramming that supports pro-inflammatory phenotypic switching and inflammation. This inflammation decreases insulin sensitivity, leading to hyperglycaemia and hyperlipidaemia. HIF-1α, hypoxia-inducible factor 1-alpha; OXPHOS, oxidative phosphorylation; Th17, T helper subset 17 lymphocyte; Treg, T regulatory lymphocyte.
The molecular pathways leading to inflammation and insulin resistance in obesity have been the subject of considerable research and previously reviewed.29,114 The innate immune system, which is highly regulated by infiltrating and tissue resident macrophages, plays a critical role in adipose tissue inflammation. Obesity is associated with activation and infiltration of macrophages into adipose tissue, especially VAT. Hypertrophied VAT secretes pro-inflammatory cytokines such as IL-1β and ΤΝF-α as well as chemokines that attract circulating monocytes into adipose tissue where they become activated macrophages. The hypoxic environment and exposure to other factors, such hyperglycaemia, impair macrophage and T lymphocyte oxidative metabolism and promote glycolytic metabolism via hypoxia-inducible factor 1α activation, thus polarizing macrophages to a pro-inflammatory M1-like phenotype and T lymphocytes to a pro-inflammatory Th17 phenotype, and causing systemic low grade inflammation.115 Activation of the inflammasome complex enables maturation of pro-inflammatory cytokines IL-1β and IL-18 and is also an integral part of innate immunity that may contribute to metabolic disorders in obesity.114
The adaptive immune system also plays a role in adipose tissue dysfunction. There are increases in CD4+ and CD8+ T cells in VAT of obese humans and mice.116 Conversely, regulatory T (Treg) cells are reduced in VAT of insulin resistant subjects and adoptive transfer of Treg cells improves glucose homeostasis in obese mice.117 The beneficial effect of Treg cells apparently requires expression of peroxisome proliferator-activated receptor gamma (PPARγ). B lymphocytes also accumulate in VAT of obese mice and produce inflammatory cytokines.118
Immune cell-mediated inflammation in VAT, liver, and skeletal muscle has been proposed to play a major role in obesity-associated metabolic disorders, including insulin resistance and T2D.29,114 Several agents have been used to block specific components of the immune-inflammatory systems, including tumour necrosis factor (TNF)-α, interleukins, Janus kinase enzyme, and T cells. Some studies suggest that these agents reduce risk of insulin resistance and diabetes in patients with chronic inflammatory conditions such as rheumatoid arthritis and systemic lupus erythematosus (SLE).119 However, there has been limited success in using anti-inflammatory drugs to improve insulin sensitivity and overall metabolic function in obese humans. Challenges in interpreting these results have been previously reviewed.114,119 In patients with obesity and T2D, anti-inflammatory therapies may not improve insulin sensitivity, except for high doses of salicylate. In clinical trials, anti-inflammatory interventions such as anti-TNFα antibodies, statins, glucocorticoids, and IL1β receptor antagonists, either failed to improve or worsened insulin sensitivity.119,120
A moderate level of inflammation in adipocytes appears to be important for maintaining normal adipose tissue function and insulin sensitivity.121 These observations highlight a beneficial role of inflammation in adipocytes and illustrate the challenge of modulating, temporally and spatially, immune-inflammatory responses as a treatment for metabolic disorders.
5.1 Does inflammation promote hypertension by causing insulin resistance and hyperinsulinaemia?
Evidence that insulin resistance and hyperinsulinaemia, initiated by inflammation or other mechanisms, can cause HT has been controversial.122,123 Acute studies demonstrated that hyperinsulinaemia increases SNS activity and renal sodium retention, effects that could cause HT, if sustained.122,124 However, chronic studies in dogs and humans indicate that hyperinsulinaemia, caused by insulin infusion or insulinoma, does not elevate BP.122,124 In fact, insulin infusion in humans and in dogs caused peripheral vasodilation due to its metabolic effects. In rodents, however, hyperinsulinaemia may increase BP modestly,122,124 raising the possibility that there may be pathophysiologic conditions that could unmask a hypertensive action of insulin. Yet, multiple studies in other species have failed to find increased BP during chronic hyperinsulinaemia even under pathophysiological conditions of elevated Ang II, increased adrenergic activity, high-salt intake and reduced kidney function.122,123
Although insulin resistance attenuates insulin’s vasodilator actions, chronic insulin infusion does not raise BP in obese insulin resistance dogs.122,123 Also, obese humans and rodents with genetic leptin deficiency or mutations of the POMC-MC4R pathway have severe insulin resistance and hyperinsulinaemia but do not have increases in SNS activity or BP, compared to control subjects.8,30
Taken together, these observations do not support the concept that insulin resistance and hyperinsulinaemia, caused by activation the immune-inflammatory system or other mechanisms, initiate obesity-induced HT. However, these findings do not rule out the possibility that that hyperglycaemia, dyslipidaemia, and other metabolic disorders associated with obesity and insulin resistance, may interact with increased BP to mediate cardiorenal injury, exacerbating tissue inflammation and HT initiated by other factors.
5.2 Obesity and hypothalamic inflammation
In addition to causing metabolic disorders via adipose, muscle and liver inflammation, obesity may also cause inflammation of the hypothalamus which plays a key role in regulating energy balance, glucose metabolism, BP, and many other physiological functions. Thaler et al.125 reported that reactive gliosis and markers of neuron injury occurred in ARC of rats and mice after one week of a high-fat diet and in the mediobasal hypothalamus of obese humans, as assessed by magnetic resonance imaging.
Factors that link obesity with hypothalamic inflammation and obesity-induced HT has been largely unexplored. Mechanisms proposed to explain obesity-induced hypothalamic inflammation include cytokines released from adipose or other peripheral tissues, activation of toll-like receptor 4 (TLR4), induction of endoplasmic reticulum (ER) stress, and activation of the inhibitor of nuclear factor-kβ kinase subunit β (IKKβ)/nuclear factor-κβ (NF-κβ) pathway.126
Purkayastha et al.127 showed that constitutive activation of IKK-β (IKK-βCA) and NF-κB in the mediobasal hypothalamus of mice increased BP without altering body weight. They also reported that NF-κB inhibition in the mediobasal hypothalamus attenuated obesity-HT independent of changes in body weight and that POMC neurons appeared to be crucial for the hypertensive effects of hypothalamic IKK-β and NF-κB activation. A potential link between obesity and activation of the NF-κB pathway in POMC neurons is increased TNF-α secreted by activated macrophages in VAT.
Despite evidence that obesity causes inflammation and injury of hypothalamic neurons that regulate BP, there is limited evidence that hypothalamic inflammation drives obesity-HT. If inflammation causes injury to hypothalamic neurons and impairs leptin signalling, how would this cause increased BP if activation of these excitatory neurons is critical for obesity-HT? One possibility is that inflammation may exert time-dependent effects on neuronal activity, initially activating hypothalamic POMC neurons and contributing to increased BP, but eventually causing neuronal injury, reduced sensitivity to leptin, and exacerbation of obesity.
Another possibility is that hypothalamic inflammation may cause ‘selective’ leptin resistance, whereby leptin’s effects to increase SNA and BP are preserved while appetite suppression is attenuated.60 Two negative regulators of leptin signalling that attenuate leptin’s anorexic effect and are influenced by inflammation are suppressor of cytokine signalling 3 (SOCS3) and protein tyrosine phosphatase 1B (PTP1B).8,30 Activation of NF-κB and hypothalamic inflammation have been suggested to up-regulate SOCS3 and PTP1B, providing a potential mechanism by which hypothalamic inflammation could contribute to feeding dysregulation. Although increased expression of SOCS3 and PTP1B may contribute to the blunted anorexic effects of leptin in obese mice, deletion of SOCS3 or PTP1B signalling in POMC neurons did not significantly alter BP or leptin’s chronic effects on BP.128,129
In some forms of severe experimental HT, hypothalamic neuronal inflammation may increase BP. For example, Inhibition of NF-κβ in the PVN attenuates AngII-HT by reducing pro-inflammatory cytokines and reactive oxygen species (ROS).130 Other studies also demonstrated that AngII-HT is associated with increased pro-inflammatory cytokines, ER stress, increased ROS in the PVN, and that blockade of these changes can attenuate increased BP. Yet, it is difficult to extrapolate results from studies in which high doses of Ang II have been infused in rodents to obesity-induced HT or to primary HT in humans. Currently, the role of hypothalamic inflammation in initiating or maintaining obesity-HT is still uncertain.
5.3 Inflammation and obesity-hypertension—which comes first?
Excessive, prolonged activation of immune-inflammatory mechanisms in conditions such as in SLE clearly causes injury to the kidneys, which ultimately leads to HT. Thus, in patients and experimental models of SLE, rheumatoid arthritis, and psoriasis, immunosuppressive drugs attenuated HT.131,132
There is also little doubt that many forms of experimental and human HT, including obesity-HT, are associated with increased immune cells and inflammatory mediators in blood vessels, kidneys, and other tissues. Chronic HT due to administration of AngII or deoxycorticosterone acetate plus high-salt intake (DOCA-salt) induces T cell and macrophage accumulation, as well as injury to blood vessels and kidneys.133,134 Seminal studies by Guzik, Harrison et al.134,135 reported that mice lacking recombination activating gene 1 (Rag1−/− mice), required for maturation and development of T and B cells, are protected from oxidative stress, vascular dysfunction and HT during infusion of AngII, norepinephrine, or DOCA-salt. Adoptive transfer of T cells, but not B cells, restored BP responses in Rag1−/− mice. Recent studies by Seniuk et al.,136 however, reported that AngII-induced HT and end-organ injury were not attenuated in Rag1−/− mice,136 challenging the concept that T and B lymphocytes are critically involved in AngII-mediated HT. These conflicting observations are especially perplexing since the same Rag1−/− mouse strain and control littermates were used as in the original experiments which demonstrated protection from AngII-HT.
Despite inconsistencies in studies using Rag1−/− mice, there is compelling evidence that immune-inflammatory mechanisms contribute to, or exacerbate, increased BP and cardiorenal injury in several forms of experimental HT.135,137,138 For example, deletion of Rag 1 attenuated HT and renal injury in Dahl salt-sensitive rats fed a high-salt diet.139 Genetic deletion or pharmacological inhibition of interleukin-17A also decreases BP as well as renal and vascular damage in mice infused with AngII. Genetic deletion or pharmacological inhibition of IL-21, produced by Th17 cells, also attenuated HT and end-organ damage in Ang II-HT.138 However, most of these studies used lean animals, and whether inflammation contributes to obesity-HT in the same manner is intriguing but uncertain.
5.3.1 Role of increased blood pressure in causing immune cell activation and inflammation
Activation of immune/inflammatory pathways may occur secondary to HT and pressure-induced tissue damage. For example, in Dahl salt sensitive rats, kidney infiltration of ED1+ macrophages and monocytes and tissue damage were greatly attenuated when renal perfusion pressure was servo-controlled and prevented from increasing during development of HT induced by feeding a high-salt diet.140 After 7 days of high-salt intake, the numbers of total CD3+ T cells, CD4+ TH cells and CD8+ cytotoxic T cells were all lower in servo-controlled kidneys than in kidneys in which renal perfusion pressure was permitted to increase as HT developed. Also, CD45R+ B cells, CD11b/c+ monocytes, macrophages, and histological injury were increased in kidneys with elevated BP, compared to servo-controlled kidneys which were protected from injury. Other studies indicate that mechanical stretch of endothelial cells promotes monocyte differentiation associated with reduced NO and increased release of IL-6 and hydrogen peroxide.141
Thus, increased BP and excessive mechanical stretch promote vascular and renal injury, cytokine release, and infiltration of immune cells which can exacerbate tissue damage. Tissue injury may stimulate formation or presentation of antigens/neoantigens that subsequently trigger an immune response, infiltration of macrophages and T lymphocytes that stimulate release of inflammatory mediators.
The mechanisms by which infiltrating immune cells in the kidneys exacerbate HT are uncertain. Multiple effects of cytokines and ROS released by immune cells have been suggested, including increased tubular sodium reabsorption, increased vascular resistance, and reduced glomerular filtration rate, and ultimately loss of nephrons due tissue injury.135,137,142
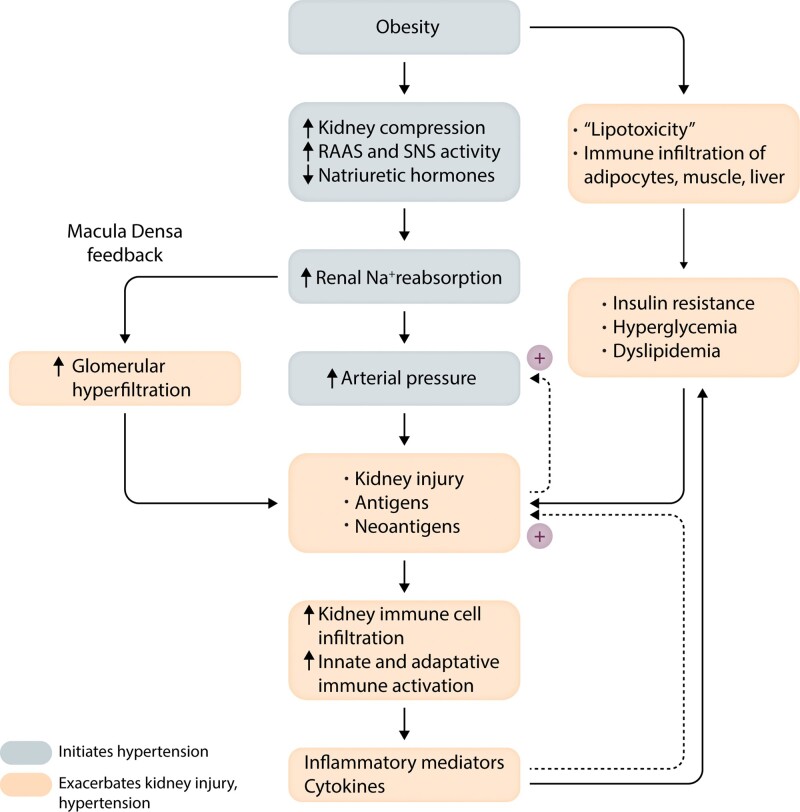
HT and metabolic disorders associated with obesity clearly contribute to cardiorenal injury which, in turn, can initiate immune responses and inflammation. These responses are critical for repair and remodelling of damaged tissues but sustained, excessive immune activation and inflammation may exacerbate kidney injury and amplify HT severity (Figure 5).
Figure 5.
Summary of mechanisms by which obesity initiates development of hypertension and renal injury. Metabolic abnormalities interact synergistically with hypertension to cause renal injury which, in turn, may expose antigens/neoantigens and activate immune/inflammatory mechanisms that exacerbate kidney injury and hypertension. SNS, Sympathetic nervous system; RAAS, renin–angiotensin–aldosterone system.
5.4 Targeting immune/inflammatory mechanisms as a strategy for treating obesity-hypertension?
Although low-grade inflammation occurs in many forms of HT, anti-inflammatory therapies have not yet proved to be efficacious for reducing BP in obesity-HT or primary human HT.143 In fact, some anti-inflammatory therapies (non-steroidal anti-inflammatory drugs), and some immunosuppressive therapies may raise BP and exacerbate cardiorenal injury. The impact of immune modulators and anti-inflammatory drugs on BP and target organ injury may depend on when the drugs are administered during the evolution of HT and tissue injury.143,144
Although considerable evidence indicates that immune cells and inflammation are involved in HT-associated vascular injury and target organ damage, their role in human primary HT remains unclear. The discrepancies found in various experimental models and the paucity of clinical evidence linking altered inflammation and immune function to primary HT emphasize the importance of further research before firm conclusions can be made regarding the question of whether inflammation precedes or follows HT, and if targeting the immune-inflammatory systems might be beneficial in obesity-HT.
6. Obesity and salt-sensitivity of blood pressure
Obesity is often associated with increased salt intake145 which could contribute to HT in salt-sensitive subjects.146 Although many consider obesity a salt-sensitive form of HT, BP may not be highly sensitive to short-term changes in sodium intake in some people with obesity. For example, increasing sodium intake 2.5-fold for 5 days was associated with unchanged BP in a small number of patients with severe obesity and average ages of 39–43 years.147 Egan et al.148,149 also reported that BP was not salt-sensitive in obese patients <45 years old when sodium intake was increased from 20 to 200 mmol/day for 1 week. In contrast, Rocchini et al.150 found that obese adolescents were salt-sensitive compared with non-obese adolescents during successive 2-week periods of high (>250 mmol/day) and low sodium (<30mmol/day) diets; after a 20-week weight loss program, 70% of the subjects who lost more than 1 kg of weight had reduced salt-sensitivity while in the remaining subjects BP was still salt-sensitive. In obese postmenopausal women, moderate sodium restriction caused dramatic reduction in BP (∼16 mmHg in systolic BP).151 In larger clinical trials, sodium restriction reduced BP, albeit modestly and with significant variability, in obese individuals.152,153
These differences in salt-sensitivity are perhaps predictable, considering the multiple factors that determine BP response to changes in sodium intake and the different protocols used to assess this phenotype. Salt-sensitivity of BP is a continuous, rather than bimodal, phenotype and varies with aging, loss of functional nephrons, inability to appropriately modulate the RAAS, inflammation, duration of high salt intake, and other factors.146 With progressive nephron loss, especially in older patients with long-term obesity and onset of T2D, BP becomes more salt-sensitive and the BP effects of weight loss and decreased sodium intake are partly additive. For example, in obese men and women aged 60–80 years, combining weight loss with sodium restriction decreased the hazard ratio for combined outcomes (HT, use of antihypertensive medications, or adverse cardiovascular events) significantly more than did weight loss or decreased sodium intake alone.154 Moderating salt intake also increases the antihypertensive efficacy of RAAS blockers, similar to the effect of adding a thiazide diuretic to an ACE inhibitor or ARB.155 Since many obese patients with HT are treated with RAAS blockers which also increase salt-sensitivity of BP, moderating salt intake often facilitates effective control of BP.
7. Progression of chronic kidney injury and resistant hypertension in obesity
Although excessive weight gain and increased adiposity initially cause modest increases in BP, obesity-induced metabolic and inflammatory disorders interact with HT to promote gradual injury to blood vessels and various organs, including the heart and kidneys.6,120,156,157 The impact of obesity on chronic kidney disease (CKD) is especially apparent when considering that T2D and HT, which are driven largely by obesity, account for at least 70–75% of ESRD. Although rarely considered a distinct cause of CKD, obesity is also an independent risk factor for CKD beyond its effects to cause HT and T2D.6 In 320 252 adults followed for 15–35 years, ESRD increased progressively as BMI rose even after adjustment for BP, diabetes, smoking, age, and several other variables.158 Similar results were obtained in a study of more than 100 000 individuals in Japan where the risk for incident ESRD rose for those with a BMI >25 kg/m2 even after adjusting for confounders such as age, sex, HT, and proteinuria. Reductions in estimated glomerular filtration rate and CKD were associated with higher BMI and waist circumference in 6500 non-diabetic participants159 and higher risk of renal insufficiency was associated with abdominal obesity in patients with essential HT after adjustment for dyslipidaemia, and hyperglycaemia. Thus, studies in different populations indicate that kidney disease risk is closely associated with increased BMI and abdominal obesity even after adjustment for other known risk factors.
Early in obesity development, there is microalbuminuria, interstitial fibrosis, increased mesangial matrix, glomerulomegaly, focal glomerulosclerosis, and podocyte abnormalities associated with glomerular hyperfiltration.160–162 When obesity-HT and metabolic abnormalities are maintained over several years, gradual nephron loss eventually reduces overall glomerular filtration rate despite continuing hyperfiltration and increased glomerular pressure in surviving nephrons.6 Nephron loss also increases salt-sensitivity of BP.146,162 In patients with kidney injury caused by other insults (e.g. unilateral nephrectomy, unilateral renal agenesis, and immunoglobulin A nephropathy), obesity accelerates CKD.161,162
Although the multiple factors that mediate obesity-induced kidney damage, in addition to HT and T2D, are still uncertain, several mechanisms have been proposed including mitochondrial dysfunction, oxidative stress, inflammation, dyslipidaemia and ‘lipotoxicity’ caused by fat infiltration into and around the kidneys.162 As discussed previously, these mechanisms are closely interrelated. Hypertrophied adipose tissue is a source of adipokines and inflammatory mediators that may have important effects on kidney function and BP. Elevated BP, interacting with metabolic disorders, contributes to kidney injury which activates immune/inflammatory mechanisms that exacerbate HT and kidney injury, leading to gradual declines in kidney function and treatment resistant HT (Figure 5).163
Weight loss, whether achieved by lifestyle modification or bariatric surgery, causes rapid remission of major risk factors for CKD, including HT, T2D, dyslipidaemia, inflammation, glomerular hyperfiltration, and proteinuria.6,33,164 The Action for Health in Diabetes (Look AHEAD) trial, which randomized 5145 obese patients with T2D and mainly normal baseline kidney function to intensive lifestyle intervention or usual care,165 found that an average weight loss of only 4 kg was associated with a 31% reduction of developing high-risk CKD over an 8-year follow-up period. Multiple studies have shown that Roux-en-Y gastric bypass or sleeve gastrectomy produces more robust and sustainable reductions in adiposity, compared to lifestyle interventions, and rapidly reduces BP, albumin excretion, and other risk factors for CKD.33 Although these surgical procedures slow CKD progression in diabetic or non-diabetic patients with severe obesity, there are few randomized controlled trials that have included sufficient numbers of patients with pre-existing CKD and statistical power to detect differences in hard outcomes (e.g. ESRD, hospitalization, death). Also, whether weight loss with bariatric surgery can reverse CKD or delay ESRD progression when kidney injury is severe is uncertain. Large, randomized prospective studies with longer follow-up are needed to address these questions.
8. Summary and perspectives
Excess adiposity, especially when associated with increased VAT and ectopic fat, are major causes of HT and target organ injury, contributing 65–75% of the risk for primary HT and >75% of ESRD. Obesity-HT is initiated by increased renal sodium reabsorption and attenuated renal-pressure natriuresis due to moderate increases in RAAS and SNS activity, as well as kidney compression by perirenal and renal sinus fat (Figure 5). Obesity-associated inflammation and metabolic disorders interact with elevated BP to exacerbate kidney damage, making HT more severe and resistance to therapy.
For many obese patients, therapy is directed primarily at treating HT, dyslipidaemia, insulin resistance and diabetes, although evidence-based guidelines for effective management of obesity-HT have not been developed. This treatment approach is costly, with many patients on three or more drugs to control BP as well as multiple drugs to manage their metabolic disorders.
The most important therapeutic goal for patients with obesity-HT should be to improve their physical fitness and more effectively manage their underlying causes of obesity. Unfortunately, most obese patients are unable to adequately reduce body weight and adiposity through lifestyle modifications. Also, there are currently few drugs available that can be used to safely and cost-effectively produce sufficient long-term weight loss. A major challenge for developing effective treatment is understanding the complex interplay of behavioural, genetic and physiological factors that influence energy balance and lead to obesity.
Despite rapid advances in obesity-related research in recent years and discovery of many adipokines, hormones, and nervous system pathways that regulate food intake and energy expenditure, development of safe and effective anti-obesity drugs has been challenging. Several relatively new anti-obesity drugs are available for clinical use, but they are often expensive, only moderately effective, and some have challenging safety profiles. The most powerful potential targets for reducing adiposity, such as leptin and the CNS melanocortin system, tend to raise HR and BP when activated. Bariatric surgery, sometimes called ‘cardiometabolic surgery’ because of its effectiveness in rapidly reversing cardiovascular and metabolic disorders, is an option but is currently recommended for only a small percentage of obese patients. Additional research will be required to better understand the complex physiology that links metabolic and cardiovascular regulation, but the rapid progress occurring in this field is promising and suggests that more efficacious therapies for obesity and the accompanying cascade of inflammatory, metabolic and cardiorenal disorders will be forthcoming.
Acknowledgements
We greatly appreciate the excellent assistance of Stephanie Lucas in preparing this manuscript.
Conflict of interest: none declared.
Funding
The authors’ research was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) [P01HL051971]; the National Institute of General Medical Sciences [P20GM104357, U54GM115428]; the National Institute of Diabetes and Digestive and Kidney Diseases [R01 DK121411 and R00 DK113280].
References
- 1. Afshin A, Reitsma MB, Murray CJL.. Health effects of overweight and obesity in 195 countries. N Engl J Med 2017;377:1496–1497. [DOI] [PubMed] [Google Scholar]
- 2. Gregg EW, Shaw JE.. Global health effects of overweight and obesity. N Engl J Med 2017;377:80–81. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization—Obesity and Overweight—Key Facts. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (30 September 2020, date last accessed).
- 4. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL.. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440–2450. [DOI] [PubMed] [Google Scholar]
- 5. Garrison RJ, Kannel WB, Stokes J III, Castelli WP.. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 1987;16:235–251. [DOI] [PubMed] [Google Scholar]
- 6. Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE.. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014;7:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piche ME, Tchernof A, Despres JP.. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res 2020;126:1477–1500. [DOI] [PubMed] [Google Scholar]
- 8. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME.. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, Hoefler M, Unger T, Sharma AM.. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens 2004;17:904–910. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 11. Nyamdorj R, Qiao Q, Soderberg S, Pitkaniemi J, Zimmet P, Shaw J, Alberti G, Nan H, Uusitalo U, Pauvaday V, Chitson P, Tuomilehto J.. Comparison of body mass index with waist circumference, waist-to-hip ratio, and waist-to-stature ratio as a predictor of hypertension incidence in Mauritius. J Hypertens 2008;26:866–870. [DOI] [PubMed] [Google Scholar]
- 12. Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, Colditz GA.. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581–1586. [DOI] [PubMed] [Google Scholar]
- 13. Shihab HM, Meoni LA, Chu AY, Wang NY, Ford DE, Liang KY, Gallo JJ, Klag MJ.. Body mass index and risk of incident hypertension over the life course: the Johns Hopkins Precursors Study. Circulation 2012;126:2983–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention—Facts About Hypertension. https://www.cdc.gov/bloodpressure/facts.htm (30 September 2020, date last accessed).
- 15. Wilsgaard T, Schirmer H, Arnesen E.. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986. Arch Intern Med 2000;160:2847–2853. [DOI] [PubMed] [Google Scholar]
- 16. Colafella KMM, Denton KM.. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018;14:185–201. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention—Adult Obesity Prevalence Maps. https://www.cdc.gov/obesity/data/adult.html (30 September 2020, date last accessed).
- 18. Neeland IJ, Poirier P, Despres JP.. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 2018;137:1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerdts E, Regitz-Zagrosek V.. Sex differences in cardiometabolic disorders. Nat Med 2019;25:1657–1666. [DOI] [PubMed] [Google Scholar]
- 20. Deurenberg-Yap M, Deurenberg P.. Is a re-evaluation of WHO body mass index cut-off values needed? The case of Asians in Singapore. Nutr Rev 2003;61:S80–S87. [DOI] [PubMed] [Google Scholar]
- 21. Colin Bell A, Adair LS, Popkin BM.. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol 2002;155:346–353. [DOI] [PubMed] [Google Scholar]
- 22. Nazare JA, Smith JD, Borel AL, Haffner SM, Balkau B, Ross R, Massien C, Almeras N, Despres JP.. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am J Clin Nutr 2012;96:714–726. [DOI] [PubMed] [Google Scholar]
- 23. Alberti KG, Zimmet P, Shaw J, Group I.. The metabolic syndrome–a new worldwide definition. Lancet 2005;366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 24. Jastreboff AM, Kotz CM, Kahan S, Kelly AS, Heymsfield SB.. Obesity as a disease: the Obesity Society 2018 position statement. Obesity (Silver Spring) 2019;27:7–9. [DOI] [PubMed] [Google Scholar]
- 25. Bluher M. Metabolically healthy obesity. Endocr Rev 2020;41: 405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith GI, Mittendorfer B, Klein S.. Metabolically healthy obesity: facts and fantasies. J Clin Invest 2019;129:3978–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cui Z, Truesdale KP, Bradshaw PT, Cai J, Stevens J.. Three-year weight change and cardiometabolic risk factors in obese and normal weight adults who are metabolically healthy: the atherosclerosis risk in communities study. Int J Obes 2015;39:1203–1208. [DOI] [PubMed] [Google Scholar]
- 28. Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB.. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses' Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol 2018;6:714–724. [DOI] [PubMed] [Google Scholar]
- 29. Ghaben AL, Scherer PE.. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol 2019;20:242–258. [DOI] [PubMed] [Google Scholar]
- 30. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME.. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol 2019;15:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol 2020;8:616–627. [DOI] [PubMed] [Google Scholar]
- 32. Samson R, Ayinapudi K, Le Jemtel TH, Oparil S.. Obesity, hypertension, and bariatric surgery. Curr Hypertens Rep 2020;22:46. [DOI] [PubMed] [Google Scholar]
- 33. Hall JE, Hall ME.. Cardiometabolic surgery for treatment of hypertension? Hypertension 2019;73:543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL.. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol 2018;71:670–687. [DOI] [PubMed] [Google Scholar]
- 35. Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE.. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010;285:17271–17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grassi G, Biffi A, Seravalle G, Trevano FQ, Dell’Oro R, Corrao G, Mancia G.. Sympathetic neural overdrive in the obese and overweight state. Hypertension 2019;74:349–358. [DOI] [PubMed] [Google Scholar]
- 37. Grassi G, Mark A, Esler M.. The sympathetic nervous system alterations in human hypertension. Circ Res 2015;116:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA.. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 2012;60:163–171. [DOI] [PubMed] [Google Scholar]
- 39. Van Vliet BN, Hall JE, Mizelle HL, Montani JP, Smith MJ Jr.. Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol 1995;269:H629–H637. [DOI] [PubMed] [Google Scholar]
- 40. Davy KP, Hall JE.. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol 2004;286:R803–R813. [DOI] [PubMed] [Google Scholar]
- 41. Hart EC, Head GA, Carter JR, Wallin BG, May CN, Hamza SM, Hall JE, Charkoudian N, Osborn JW.. Recording sympathetic nerve activity in conscious humans and other mammals: guidelines and the road to standardization. Am J Physiol Heart Circ Physiol 2017;312:H1031–H1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP.. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995;25:893–897. [DOI] [PubMed] [Google Scholar]
- 43. Henegar JR, Zhang Y, Rama RD, Hata C, Hall ME, Hall JE.. Catheter-based radiorefrequency renal denervation lowers blood pressure in obese hypertensive dogs. Am J Hypertens 2014;27:1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lohmeier TE, Hall JE.. Device-based neuromodulation for resistant hypertension therapy. Circ Res 2019;124:1071–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mahfoud F, Bohm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, Schlaich M, Williams B, Fahy M, Mancia G.. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J 2019;40:3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sardar P, Bhatt DL, Kirtane AJ, Kennedy KF, Chatterjee S, Giri J, Soukas PA, White WB, Parikh SA, Aronow HD.. Sham-Controlled randomized trials of catheter-based renal denervation in patients with hypertension. J Am Coll Cardiol 2019;73:1633–1642. [DOI] [PubMed] [Google Scholar]
- 47. Henegar JR, Zhang Y, Hata C, Narciso I, Hall ME, Hall JE.. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens 2015;28:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dewan NA, Nieto FJ, Somers VK.. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iliescu R, Tudorancea I, Irwin ED, Lohmeier TE.. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol 2013;305:H1080–H1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khan SA, Sattar MZA, Abdullah NA, Rathore HA, Ahmad A, Abdulla MH, Johns EJ.. Improvement in baroreflex control of renal sympathetic nerve activity in obese Sprague Dawley rats following immunosuppression. Acta Physiol 2017;221:250–265. [DOI] [PubMed] [Google Scholar]
- 51. Lohmeier TE, Iliescu R, Tudorancea I, Cazan R, Cates AW, Georgakopoulos D, Irwin ED.. Chronic interactions between carotid baroreceptors and chemoreceptors in obesity hypertension. Hypertension 2016;68:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mansukhani MP, Kara T, Caples SM, Somers VK.. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep 2014;16:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK.. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017;69:841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ciriello J, Moreau JM.. Leptin signaling in the nucleus of the solitary tract alters the cardiovascular responses to activation of the chemoreceptor reflex. Am J Physiol Regul Integr Comp Physiol 2012;303:R727–736. [DOI] [PubMed] [Google Scholar]
- 55. Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JF, Ponikowski P.. Carotid body resection for sympathetic modulation in systolic heart failure: results from first-in-man study. Eur J Heart Fail 2017;19:391–400. [DOI] [PubMed] [Google Scholar]
- 56. Shek EW, Brands MW, Hall JE.. Chronic leptin infusion increases arterial pressure. Hypertension 1998;31:409–414. [DOI] [PubMed] [Google Scholar]
- 57. Carlyle M, Jones OB, Kuo JJ, Hall JE.. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension 2002;39:496–501. [DOI] [PubMed] [Google Scholar]
- 58. Faulkner JL, Bruder-Nascimento T, Belin de Chantemèle EJ.. The regulation of aldosterone secretion by leptin: implications in obesity-related cardiovascular disease. Curr Opin Nephrol Hypertens 2018;27:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kuo JJ, Jones OB, Hall JE.. Inhibition of NO synthesis enhances chronic cardiovascular and renal actions of leptin. Hypertension 2001;37:670–676. [DOI] [PubMed] [Google Scholar]
- 60. Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol 2013;305:R566–R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lim K, Burke SL, Head GA.. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension 2013;61:628–634. [DOI] [PubMed] [Google Scholar]
- 62. do Carmo JM, da Silva AA, Gava FN, Moak SP, Dai X, Hall JE.. Impact of leptin deficiency compared with neuronal-specific leptin receptor deletion on cardiometabolic regulation. Am J Physiol Regul Integr Comp Physiol 2019;317:R552–R562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K.. Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 2000;105:1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. do Carmo JM, da Silva AA, Rushing JS, Hall JE.. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 2012;302:R561–R567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chaudhary P, Schreihofer AM.. Improved glucose homeostasis in male obese Zucker rats coincides with enhanced baroreflexes and activation of the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 2018;315:R1195–R1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Machleidt F, Simon P, Krapalis AF, Hallschmid M, Lehnert H, Sayk F.. Experimental hyperleptinemia acutely increases vasoconstrictory sympathetic nerve activity in healthy humans. J Clin Endocrinol Metab 2013;98:E491–E496. [DOI] [PubMed] [Google Scholar]
- 67. Zelissen PM, Stenlof K, Lean ME, Fogteloo J, Keulen ET, Wilding J, Finer N, Rossner S, Lawrence E, Fletcher C, McCamish M, Author G; on behalf of the author group. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes Metab 2005;7:755–761. [DOI] [PubMed] [Google Scholar]
- 68. Ozata M, Ozdemir IC, Licinio J.. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 1999;84:3686–3695. [DOI] [PubMed] [Google Scholar]
- 69. Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K.. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 2011;108:808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE.. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 2011;57:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K.. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension 2009;53:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dubinion JH, da Silva AA, Hall JE.. Chronic blood pressure and appetite responses to central leptin infusion in rats fed a high fat diet. J Hypertens 2011;29:758–762. [DOI] [PubMed] [Google Scholar]
- 73. Pan WW, Myers MG Jr.. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 2018;19:95–105. [DOI] [PubMed] [Google Scholar]
- 74. do Carmo JM, da Silva AA, Wang Z, Freeman NJ, Alsheik AJ, Adi A, Hall JE.. Regulation of blood pressure, appetite, and glucose by leptin after inactivation of insulin receptor substrate 2 signaling in the entire brain or in proopiomelanocortin neurons. Hypertension 2016;67:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. do Carmo JM, da Silva AA, Sessums PO, Ebaady SH, Pace BR, Rushing JS, Davis MT, Hall JE.. Role of Shp2 in forebrain neurons in regulating metabolic and cardiovascular functions and responses to leptin. Int J Obes (Lond )2014;38:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. do Carmo JM, da Silva AA, Ebaady SE, Sessums PO, Abraham RS, Elmquist JK, Lowell BB, Hall JE.. Shp2 signaling in POMC neurons is important for leptin's actions on blood pressure, energy balance, and glucose regulation. Am J Physiol Regul Integr Comp Physiol 2014;307:R1438–R1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhao S, Li N, Zhu Y, Straub L, Zhang Z, Wang MY, Zhu Q, Kusminski CM, Elmquist JK, Scherer PE.. Partial leptin deficiency confers resistance to diet-induced obesity in mice. Mol Metab 2020;37:100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, Mera A, Lago F, Gómez R, Gualillo O.. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol 2017;13:100–109. [DOI] [PubMed] [Google Scholar]
- 79. do Carmo JM, da Silva AA, Wang Z, Fang T, Aberdein N, Perez de Lara CE, Hall JE.. Role of the brain melanocortins in blood pressure regulation. Biochim Biophys Acta 2017;1863:2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. da Silva AA, do Carmo JM, Wang Z, Hall JE.. The brain melanocortin system, sympathetic control, and obesity hypertension. Physiology (Bethesda) 2014;29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kuo JJ, Silva AA, Hall JE.. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 2003;41:768–774. [DOI] [PubMed] [Google Scholar]
- 82. Kuo JJ, da Silva AA, Tallam LS, Hall JE.. Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation. Hypertension 2004;43:370–375. [DOI] [PubMed] [Google Scholar]
- 83. da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE.. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 2008;51:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tallam LS, da Silva AA, Hall JE.. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 2006;48:58–64. [DOI] [PubMed] [Google Scholar]
- 85. Greenfield JR. Melanocortin signalling and the regulation of blood pressure in human obesity. J Neuroendocrinol 2011;23:186–193. [DOI] [PubMed] [Google Scholar]
- 86. Clément K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C, Puder L, Fiedorek F, Gottesdiener K, Kleinau G, Heyder N, Scheerer P, Blume-Peytavi U, Jahnke I, Sharma S, Mokrosinski J, Wiegand S, Müller A, Weiß K, Mai K, Spranger J, Grüters A, Blankenstein O, Krude H, Kühnen P.. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med 2018;24:551–555. [DOI] [PubMed] [Google Scholar]
- 87. Kühnen P, Clément K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, Mai K, Blume-Peytavi U, Grüters A, Krude H.. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med 2016;375:240–246. [DOI] [PubMed] [Google Scholar]
- 88. da Silva AA, do Carmo JM, Dubinion JH, Bassi M, Mokhtarpouriani K, Hamza SM, Hall JE.. Chronic central nervous system MC3/4R blockade attenuates hypertension induced by nitric oxide synthase inhibition but not by angiotensin II infusion. Hypertension 2015;65:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H.. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J Clin Endocrinol Metab 2010;95:1998–2002. [DOI] [PubMed] [Google Scholar]
- 90. Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM.. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 2002;324:127–137. [DOI] [PubMed] [Google Scholar]
- 91. Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME.. Hypertension: physiology and pathophysiology. Compr Physiol 2012;2:2393–2442. [DOI] [PubMed] [Google Scholar]
- 92. Davel AP, Jaffe IZ, Tostes RC, Jaisser F, Belin de Chantemèle EJ.. New roles of aldosterone and mineralocorticoid receptors in cardiovascular disease: translational and sex-specific effects. Am J Physiol Heart Circ Physiol 2018;315:H989–H999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. de Paula RB, da Silva AA, Hall JE.. Aldosterone antagonism attenuates obesity-induced hypertension and glomerular hyperfiltration. Hypertension 2004;43:41–47. [DOI] [PubMed] [Google Scholar]
- 94. Dudenbostel T, Calhoun DA.. Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. Am J Hypertens 2017;30:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bomback AS, Muskala P, Bald E, Chwatko G, Nowicki M.. Low-dose spironolactone, added to long-term ACE inhibitor therapy, reduces blood pressure and urinary albumin excretion in obese patients with hypertensive target organ damage. Clin Nephrol 2009;72:449–456. [DOI] [PubMed] [Google Scholar]
- 96. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society's PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Calhoun DA. Fluid retention, aldosterone excess, and treatment of resistant hypertension. Lancet Diabetes Endocrinol 2018;6:431–433. [DOI] [PubMed] [Google Scholar]
- 98. Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 2014;25:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Funder JW. Apparent mineralocorticoid excess. J Steroid Biochem Mol Biol 2017;165:151–153. [DOI] [PubMed] [Google Scholar]
- 100. Asferg CL, Andersen UB, Linneberg A, Goetze JP, Jeppesen JL.. Obese hypertensive men have lower circulating proatrial natriuretic peptide concentrations despite greater left atrial size. Am J Hypertens 2018;31:645–650. [DOI] [PubMed] [Google Scholar]
- 101. Savoia C, Volpe M, Alonzo A, Rossi C, Rubattu S.. Natriuretic peptides and cardiovascular damage in the metabolic syndrome: molecular mechanisms and clinical implications. Clin Sci (Lond) 2010;118:231–240. [DOI] [PubMed] [Google Scholar]
- 102. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS.. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 103. Abdulle AM, Nagelkerke NJ, Adem A, Abouchacra S, Pathan JY, Al-Rukhaimi M, Suleiman MN, Mathew MC, Nicholls MG, Obineche EN.. Plasma N terminal pro-brain natriuretic peptide levels and its determinants in a multi-ethnic population. J Hum Hypertens 2007;21:647–653. [DOI] [PubMed] [Google Scholar]
- 104. Jordan J, Birkenfeld AL, Melander O, Moro C.. Natriuretic peptides in cardiovascular and metabolic crosstalk: implications for hypertension management. Hypertension 2018;72:270–276. [DOI] [PubMed] [Google Scholar]
- 105. Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP.. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol 2014;11:403–412. [DOI] [PubMed] [Google Scholar]
- 106. Aydin O, Nieuwdorp M, Gerdes V.. The gut microbiome as a target for the treatment of type 2 diabetes. Curr Diab Rep 2018;18:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI.. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013;341:1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yang T, Richards EM, Pepine CJ, Raizada MK.. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 2018;14:442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN.. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017;551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ, Raizada MK.. Hypertension-linked pathophysiological alterations in the gut. Circ Res 2017;120:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Afsar B, Vaziri ND, Aslan G, Tarim K, Kanbay M.. Gut hormones and gut microbiota: implications for kidney function and hypertension. J Am Soc Hypertens 2016;10:954–961. [DOI] [PubMed] [Google Scholar]
- 112. Muskiet MHA, Tonneijck L, Smits MM, van Baar MJB, Kramer MHH, Hoorn EJ, Joles JA, van Raalte DH.. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017;13:605–628. [DOI] [PubMed] [Google Scholar]
- 113. Lee YS, Wollam J, Olefsky JM.. An integrated view of immunometabolism. Cell 2018;172:22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–185. [DOI] [PubMed] [Google Scholar]
- 115. Mouton AJ, Li X, Hall ME, Hall JE.. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res 2020;126:789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD.. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 2010;185:1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ilan Y, Maron R, Tukpah AM, Maioli TU, Murugaiyan G, Yang K, Wu HY, Weiner HL.. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci USA 2010;107:9765–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, Allen J, Bouchard J, Toraldo G, Jasuja R, Obin MS, McDonnell ME, Apovian C, Denis GV, Nikolajczyk BS.. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci USA 2013;110:5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014;13:465–476. [DOI] [PubMed] [Google Scholar]
- 120. Zhu Q, Scherer PE.. Immunologic and endocrine functions of adipose tissue: implications for kidney disease. Nat Rev Nephrol 2018;14:105–120. [DOI] [PubMed] [Google Scholar]
- 121. Zhu Q, An YA, Kim M, Zhang Z, Zhao S, Zhu Y, Asterholm IW, Kusminski CM, Scherer PE.. Suppressing adipocyte inflammation promotes insulin resistance in mice. Mol Metab 2020;39:101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hall JE. Hyperinsulinemia: a link between obesity and hypertension? Kidney Int 1993;43:1402–1417. [DOI] [PubMed] [Google Scholar]
- 123. da Silva AA, do Carmo JM, Li X, Wang Z, Mouton AJ, Hall JE.. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol 2020;36:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hall JE, Brands MW, Hildebrandt DA, Mizelle HL.. Obesity-associated hypertension. Hyperinsulinemia and renal mechanisms. Hypertension 1992;19:I45–I55. [DOI] [PubMed] [Google Scholar]
- 125. Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW.. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Khor S, Cai D.. Hypothalamic and inflammatory basis of hypertension. Clin Sci (Lond) 2017;131:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Purkayastha S, Zhang G, Cai D.. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med 2011;17:883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wang Z, do Carmo JM, da Silva AA, Bailey KC, Aberdein N, Moak SP, Hall JE.. Role of SOCS3 in pomc neurons in metabolic and cardiovascular regulation. Am J Physiol Regul Integr Comp Physiol 2019;316:R338–R351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Aberdein N, Dambrino RJ, do Carmo JM, Wang Z, Mitchell LE, Drummond HA, Hall JE.. Role of PTP1B in POMC neurons during chronic high-fat diet: sex differences in regulation of liver lipids and glucose tolerance. Am J Physiol Regul Integr Comp Physiol 2018;314:R478–R488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J.. Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension 2012;59:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B.. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. JASN 2006;17:S218–S225. [DOI] [PubMed] [Google Scholar]
- 132. Taylor EB, Ryan MJ.. Immunosuppression with mycophenolate mofetil attenuates hypertension in an experimental model of autoimmune disease. J Am Heart Assoc 2017;6:e005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Muller DN, Mervaala EMA, Schmidt F, Park J-K, Dechend R, Genersch E, Breu V, Löffler B-M, Ganten D, Schneider W, Haller H, Luft FC.. Effect of bosentan on NF-kappaB, inflammation, and tissue factor in angiotensin II-induced end-organ damage. Hypertension 2000;36:282–290. [DOI] [PubMed] [Google Scholar]
- 134. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG.. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Norlander AE, Madhur MS, Harrison DG.. The immunology of hypertension. J Exp Med 2018;215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Seniuk A, Thiele JL, Stubbe A, Oser P, Rosendahl A, Bode M, Meyer-Schwesinger C, Wenzel UO, Ehmke H.. B6.Rag1 knockout mice generated at the Jackson Laboratory in 2009 show a Rrbust wild-type hypertensive phenotype in response to ANG II (angiotensin II). Hypertension 2020;75:1110–1116. [DOI] [PubMed] [Google Scholar]
- 137. Drummond GR, Vinh A, Guzik TJ, Sobey CG.. Immune mechanisms of hypertension. Nat Rev Immunol 2019;19:517–532. [DOI] [PubMed] [Google Scholar]
- 138. Madhur MS, Kirabo A, Guzik TJ, Harrison DG.. From rags to riches: moving beyond RAG1 in studies of hypertension. Hypertension 2020;75:930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H.. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 2013;304:R407–R414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr.. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. JASN 2008;19:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, Elijovich F, Laffer CL, Gnecco JS, Noonan J, Maffia P, Jasiewicz-Honkisz B, Czesnikiewicz-Guzik M, Mikolajczyk T, Sliwa T, Dikalov S, Weyand CM, Guzik TJ, Harrison DG.. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res 2018;114:1547–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 2019;15:290–300. [DOI] [PubMed] [Google Scholar]
- 143. Donath MY, Dinarello CA, Mandrup-Poulsen T.. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol 2019;19:734–746. [DOI] [PubMed] [Google Scholar]
- 144. Wu H, Ballantyne CM.. Metabolic inflammation and insulin resistance in obesity. Circ Res 2020;126:1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ma Y, He FJ, MacGregor GA.. High salt intake: independent risk factor for obesity? Hypertension 2015;66:843–849. [DOI] [PubMed] [Google Scholar]
- 146. Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation 2016;133:894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bonfils PK, Taskiran M, Damgaard M, Goetze JP, Floyd AK, Funch-Jensen P, Kristiansen VB, Gadsbøll N.. The influence of high versus low sodium intake on blood pressure and haemodynamics in patients with morbid obesity. J Hypertens 2013;31:2220–2229; discussion 2229. [DOI] [PubMed] [Google Scholar]
- 148. Egan BM, Stepniakowski KT.. Adverse effects of short-term, very-low-salt diets in subjects with risk-factor clustering. Am J Clin Nutr 1997;65:671S–677S. [DOI] [PubMed] [Google Scholar]
- 149. Egan BM, Stepniakowski K, Goodfriend TL.. Renin and aldosterone are higher and the hyperinsulinemic effect of salt restriction greater in subjects with risk factors clustering. Am J Hypertens 1994;7:886–893. [DOI] [PubMed] [Google Scholar]
- 150. Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M.. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med 1989;321:580–585. [DOI] [PubMed] [Google Scholar]
- 151. Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA.. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol 2001;38:506–513. [DOI] [PubMed] [Google Scholar]
- 152. Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith WD, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J.. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001;134:1–11. [DOI] [PubMed] [Google Scholar]
- 153. Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J; GenSalt Collaborative Research Group. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet 2009;373:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA.. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998;279:839–846. [DOI] [PubMed] [Google Scholar]
- 155. Singer DR, Markandu ND, Cappuccio FP, Miller MA, Sagnella GA, MacGregor GA.. Reduction of salt intake during converting enzyme inhibitor treatment compared with addition of a thiazide. Hypertension 1995;25:1042–1044. [DOI] [PubMed] [Google Scholar]
- 156. Jia G, Hill MA, Sowers JR.. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 2018;122:624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Alpert MA, Karthikeyan K, Abdullah O, Ghadban R.. Obesity and cardiac remodeling in adults: mechanisms and clinical implications. Prog Cardiovasc Dis 2018;61:114–123. [DOI] [PubMed] [Google Scholar]
- 158. Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS.. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006;144:21–28. [DOI] [PubMed] [Google Scholar]
- 159. Burton JO, Gray LJ, Webb DR, Davies MJ, Khunti K, Crasto W, Carr SJ, Brunskill NJ.. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol Dial Transplant 2012;27:1860–1866. [DOI] [PubMed] [Google Scholar]
- 160. Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE.. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 2001;12:1211–1217. [DOI] [PubMed] [Google Scholar]
- 161. Amann K, Benz K.. Structural renal changes in obesity and diabetes. Semin Nephrol 2013;33:23–33. [DOI] [PubMed] [Google Scholar]
- 162. Hall JE, Henegar JR, Dwyer TM, Liu J, da Silva AA, Kuo JJ, Tallam L.. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther 2004;11:41–54. [DOI] [PubMed] [Google Scholar]
- 163. Egan BM, Li J.. Role of aldosterone blockade in resistant hypertension. Semin Nephrol 2014;34:273–284. [DOI] [PubMed] [Google Scholar]
- 164. Heffron SP, Parham JS, Pendse J, Aleman JO.. Treatment of obesity in mitigating metabolic risk. Circ Res 2020;126:1646–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Look ARG. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2014;2:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]