Abstract
Context
Omega-3, a long-chain polyunsaturated fatty acid (LC-PUFA), may help promote healthy sleep outcomes. However, evidence from randomized controlled trials are inconclusive.
Objective
The objective of this systematic review and meta-analysis was to explore the impact of omega-3 LC-PUFA supplementation and related dietary intervention in clinical trials as well as omega-3 LC-PUFA exposure in longitudinal studies on human’s sleep-related outcome.
Data Sources
The PubMed, EMBASE, Cochrane Library, CINAHL, and AMED databases were searched from inception to November 2019. Randomized controlled trials, clinical trials that included a control group, and longitudinal studies that reported the intake of omega-3 LC-PUFA and sleep-related outcomes were included.
Study Selection
A total of 20 studies with 12 clinical trials and 8 longitudinal studies were identified for inclusion.
Data Extraction
Participant characteristics, study location, intervention information, and sleep-related outcome measurements were reported. Included studies were appraised with Cochrane risk-of-bias tools and the Newcastle-Ottawa Scale. Weighted mean differences (WMDs) and 95%CIs were pooled with fixed or random effect models.
Results
Omega-3 LC-PUFA may improve infants' sleep organization and maturity. It reduced the percentage of infants' active sleep (WMD = –8.40%; 95%CI, –14.50 to –2.29), sleep-wake transition (WMD = –1.15%; 95%CI, –2.09 to –0.20), and enhanced the percentage of wakefulness (WMD = 9.06%; 95%CI, 1.53–16.59) but had no effect on quiet sleep. Omega-3 reduced children’s total sleep disturbance score for those with clinical-level sleep problems (WMD = –1.81; 95%CI, –3.38 to –0.23) but had no effect on healthy children’s total sleep duration, sleep latency, or sleep efficiency. No effectiveness was found in adults’ total sleep duration, sleep latency, sleep efficiency, sleep quality, or insomnia severity.
Conclusion
Omega-3 LC-PUFA may improve certain aspects of sleep health throughout childhood. Additional robust studies are warranted to confirm the relationship between omega-3 LC-PUFA and sleep.
Keywords: meta-analysis, omega-3 LC-PUFA, sleep, systematic review
INTRODUCTION
Diet and nutrient implications on sleep have recently received much attention. Two systematic reviews demonstrated that macro- and micronutrients, energy intake, and dietary pattern affect healthy sleep.1,2 Observational studies have shown that higher proportions of carbohydrate and fat intake,3 lower consumption of foods in the Mediterranean diet,4 and deficiencies in micronutrients, including vitamin B1, folate, iron, zinc, and magnesium, are associated with shorter sleep duration and poorer sleep quality.5,6 Experimental studies have shown that carbohydrate-based, high–glycemic-index meals result in significant shortening of sleep latency in healthy populations,7 and an early introduction of solid foods into infants’ diet can facilitate longer sleep duration, fewer awakenings at night, and reductions in parent-reported sleep problems.8
Among various nutrients, the role of omega-3 long-chain polyunsaturated fatty acids (LC-PUFAs) on sleep has been increasingly studied, and different lines of evidence demonstrate the contribution of omega-3 LC-PUFA to sleep health. Animal studies have shown that omega-3 LC-PUFA may be involved in regulating the composition of melatonin and maintaining the structure of neuronal membrane, both of which are essential for sleep onset and sleep maintenance.9–12 These results are further reflected in human studies across various study designs. Observational studies show that consuming omega-3 LC-PUFA supplement and a diet rich in omega-3 LC-PUFAs (eg, fatty fish) is associated with earlier sleep onset, longer weekend sleep duration, and better sleep quality.13–15 Finally, intervention studies suggest omega-3 LC-PUFAs can improve sleep disturbances and overall sleep quality.16,17 The findings have been shown in children18 and adults.19
Sleep is essential for maintaining daily functioning and good health and well-being across the lifespan. Poor sleep is associated with a higher risk of incident cardiovascular disease,20 disrupted glucose metabolism,21 and even obesity in children22 and adults.23 It also has negative effects on cognition, including decreased alertness and attention24,25 and poor school performance in children.26,27 The prevalence of poor sleep is reported to be 10% in infants and toddlers,28 20% in preschool children,29 62% in school-aged children,30 26.0% to 28.3% in adolescents and young adults,31 and 13.1% among older adults.32 A person’s sleep health can be detected through several sleep outcomes, including total sleep duration (TSD), sleep latency (SL), sleep efficiency (SEff), and self-reported sleep quality (SQ),33 where TSD refers to the total sleep time between sleep onset and offset,34 SL refers to the time a person takes to fall asleep, and SEff is calculated as the total sleep duration divided by time spent in bed.18 Self-reported SQ is a person’s judgement of his or her sleep experience based on a series of parameters including TSD, feeling refreshed upon waking, and mood and daytime functioning, among others.35 Given the high prevalence of sleep problems across the lifespan and its detrimental effects on health, targeted interventions to improve sleep outcomes are warranted.
Although findings of the aforementioned studies suggest omega-3 LC-PUFA is a potentially promising nutrient supplement to improve adults’ and children’s sleep outcomes, inconsistent results have been found in other observational and experimental studies. Several clinical trials have shown that omega-3 LC-PUFA does not improve the sleep quality of adults with chronic insomnia or sleep disturbances in women with menopause.36,37 These mixed results may be due to methodological differences in study design, population, or measures. Thus, whether omega-3 LC-PUFA supplementation could improve sleep-related outcomes warrants additional investigation. Our aim for this systematic review and meta-analysis was to explore omega-3 LC-PUFA supplementation and dietary intervention in clinical trials and omega-3 LC-PUFA exposure in longitudinal studies on sleep-related outcome in humans.
METHODS
This review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (Appendix S1 in the Supporting Information online).38 The protocol of this study was registered with the International Prospective Register of Systematic Reviews (registration no. CRD42020156826). The research question was defined using the Population, Intervention, Comparator, Outcomes, and Study design criteria (Table 1).
Table 1.
PICOS criteria for inclusion of studies
| Parameter domain | Inclusion criteria |
|---|---|
| Population | The general population: neonates/infants, children, and adults, regardless of health status |
| Intervention/exposure | Omega-3 LC-PUFA supplements with or without other nutrients (regardless of formulation [ie, capsule, pills, or syrup]), or diet rich in omega-3 LC-PUFAs |
| Comparator | Placebo, standardized diet, or no intervention. |
| Outcome |
Objective sleep-related outcomes, including total sleep duration, sleep latency, sleep efficiency, infant active sleep, sleep-wake transition, wakefulness, and quiet sleep, among others Subjective sleep-outcome information, including sleep quality, insomnia severity, sleep disturbance, among others, reported via questionnaire |
| Study design | Randomized controlled trials, clinical trials that included a control group, or longitudinal studies that investigated the impact of omega-3 LC-PUFA or diet rich in omega-3 LC-PUFA on sleep-related outcomes in humans |
Abbreviation: LC-PUFA, long-chain polyunsaturated fatty acid.
Data sources and search strategy
After consulting with a librarian, the PubMed, EMBASE, Cochrane Library, CINAHL, and AMED databases were searched from inception to November 2019. The search strategy of PubMed was as follows: ((((“fish”[All Fields] OR “fish oils”[MeSH Terms] OR (“fish”[All Fields] AND “oils”[All Fields]) OR “fish oils”[All Fields] OR (“fish”[All Fields] AND “oil”[All Fields]) OR “fish oil”[All Fields]) OR “Fish Oils”[Mesh]) OR (“fatty acids, omega-3”[MeSH Terms] OR (“fatty”[All Fields] AND “acids”[All Fields] AND “omega-3”[All Fields]) OR “omega-3 fatty acids”[All Fields] OR “omega 3 fatty acids”[All Fields])) OR “Fatty Acids, Omega-3”[Mesh]) OR “Fatty Acids, Unsaturated”[Mesh] AND ((((“sleep”[MeSH Terms] OR “sleep”[All Fields]) OR “Sleep”[Mesh] OR “circadian rhythm”[All Fields]) OR (“circadian rhythm”[MeSH Terms] OR (“circadian”[All Fields] AND “rhythm”[All Fields]) OR “circadian rhythm”[All Fields])) OR “Circadian rhythm”[Mesh]). The search strategy was adapted according to the indexing systems of other databases. No language or study design filters were used in the initial search to enhance the comprehensibility of the literature search. Reference lists of included studies and existing systematic reviews were screened for additional relevant studies. One key author was contacted for sleep outcome data that were not reported in their published paper but was relevant to this review.
Two rounds of screening were conducted. First, 2 reviewers independently screened the titles and abstracts of acquired articles for eligibility. Relevant articles that were in line with the inclusion criteria were included for full-text screening. Articles that were evaluated as relevant after 2 rounds of screening were included for data extraction and quality appraisal. Disagreements between the 2 authors were resolved by discussion.
Inclusion criteria
Randomized controlled trials (RCTs), or clinical trials that included a control group, and longitudinal studies that investigated the impact of omega-3 LC-PUFA or diet rich in omega-3 LC-PUFA on sleep-related outcomes in humans were included. The main components of omega-3 LC-PUFA—eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—either singly or mixed, were included. Multiple papers generated from the same data source were reviewed and only relevant data were included.
Exclusion criteria
Reviews, conference proceedings, and studies with outcome measurements focusing on sleep apnea were excluded. Non–English-language papers were also excluded because of lack of time and funding to use professional translations.
Data extraction
A standardized data extraction form was developed that included the following information: year of publication, country, study design, age and sex of participants, number of participants included for analysis, content of intervention and control, duration of intervention, time of follow-up, and sleep outcome measurements. The primary outcomes were TSD, SL, SEff, and SQ; other sleep-related outcomes were considered secondary outcomes.
The statistical data on primary and secondary outcomes in each study were extracted and transcribed into an Excel spreadsheet (Microsoft, Redmond, WA) by 1 author (Y.D.) and double checked by another author (J.L.). Two outcome measurements (ie, TSD and SL) in the included studies were presented in hours or minutes, and those presented in hours were converted into minutes. The following data were also extracted to the spreadsheet when reported: the number of participants at baseline and included for analysis in each group, baseline and end point outcome measures and their variability (ie, reported as standard deviation [SD], standard error of the mean, or 95% CI), and both within and between groups.
Study quality
Randomized controlled studies were assessed by the Cochrane risk-of-bias tool, which appraises the quality of RCTs from 6 domains of potential biases.39 The quality of cohort studies was evaluated by the Newcastle-Ottawa Scale, which evaluates the quality of cohort studies from 3 dimensions: selection, comparability, and exposure.40
Data analysis
Because of the heterogenous nature of sleep-health outcomes among different age groups, only the results of participants in the same age group were combined. The weighted mean difference (WMD) was pooled as the effect size of omega-3 LC-PUFAs on each continuous sleep outcome. When the included studies did not directly report the means and their corresponding standard deviations but reported medians and quantiles or means with confidence intervals, the means and standard deviations were calculated following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.39 Heterogeneity and variation in the pooled estimations were computed by Cochrane’s Q test and I2, respectively, with P < 0.1 considered statistically significant. Following the recommendations of the Cochrane handbook, when considerable heterogeneity was found (ie, I2 > 75% and P < 0.1), the pooled estimate is not presented.39
The statistical synthesis was conducted with Stata, version 14 (Stata Corp, College Station, TX). The “metan” syntax was used to pool the effect sizes. Two studies were conducted by the same research team in the same geographic area reporting the same outcome measurements.41,42 It was assumed the samples in the 2 studies were from the same population, and thus the effect sizes were pulled by the fixed-effect model.43 The effect sizes of other studies were pooled by the random-effects model. Originally, subgroup analysis was planned on the basis of participants’ age range and type of disease when considerable heterogeneity was present (P < 0.1). However, because of limited included studies (n < 2) in each subgroup, it was not appropriate to conduct subgroup analysis. Similarly, because there were few included studies (n < 3) in each sleep outcome, sensitivity analysis and publication bias analysis were not suitable. Narrative synthesis was conducted when statistical synthesis was not appropriate.
RESULTS
Study characteristics
A total of 869 papers were retrieved from the literature search. After removing duplicates and initial screening of titles and abstracts, 89 papers were included for full-text screening. From this group, 69 papers were excluded because of their irrelevance to the topic of this study, lack of control group, or lack of sleep-related outcomes. Thus, 20 studies (n = 12 RCTs; n = 8 cohort studies) were included for quality appraisal and data synthesis (Figure 1). Two articles44,45 reported the results of 1 study; only results relevant to the current systematic review were included. The included studies were conducted in the United States41,42,44–49 (n = 10), Europe18,50–54 (n = 6), and Asia19,55–57 (n = 4). Five studies focused on infants and toddlers (0–3 years old), among which 2 studies41,42 focused on the impact of maternal omega-3 LC-PUFA intake on neonates’ sleep outcome. Five studies focused on children (3–18 years old),18,50,54,56,57 and the other 10 studies focused on adults (> 18 years old).19,44,47–49,51,55,58–60 The primary research aims of only 4 RCTs18,46,50,57 and 5 cohort studies42,52,53,56,59 were to investigate the relationship between omega-3 LC-PUFA and sleep outcomes. The characteristics of included RCTs and cohort studies are listed in Table 218,41,44,46–49and Table 3, 42,52–54,56,58–60respectively.
Figure 1.
Flow diagram of the literature search process.
Table 2.
Characteristics of included controlled intervention studies
| No. | Reference (year) | Country | Study design | Population | Intervention | Control | Duration | Sleep outcome measurement | Main results |
|---|---|---|---|---|---|---|---|---|---|
| Infants and toddlers (aged 0–3 y) | |||||||||
| 1 | Judge et al (2012)41 | United States | RCT | Healthy pregnant women and their newborn babies (n = 48) | Cereal bars contained fish oil (300 mg DHA/d) | Corn oil | Started from 24 wk gestation and continued until delivery (38–40 wk): 98–112 d | Neonates’ sleep outcome including arousals, QS, AS, active-quiet sleep transition, and sleep-wake transition measured by actigraph | On postnatal day 1, infants of mothers in the DHA intervention group had significantly fewer arousals in QS (t = 2.17, P < 0.05) and arousals in AS (t = 2.21, P < 0.05) compared with infants born to mothers in the placebo group. On postnatal day 2, QS: I: 12.70 ± 5.85, C: 13.70 ± 4.76; active-quiet sleep transition: I: 0.47 ± 0.30, C: 0.41 ± 0.27; sleep-wake transition: I: 51.57 ± 14.54, C: 51.70 ± 11.13. Infants of mothers in the treatment group had significantly fewer arousals in QS (F = 5.72, P < 0.05) than the placebo group when controlling for maternal total weight gain during pregnancy, because maternal weight was significantly correlated with the infant sleep measure. |
| 2 | Boone et al (2019)46 | United States | RCT | Children (n = 377) aged 10–16 mo born at < 35 wk gestation | DHA 200 mg+AA 200 mg/d supplementation | Corn oil (400 mg/d) | 180 d | Nocturnal and daytime sleep duration, and sleep-onset time measured by caregiver-reported BISQ | Nocturnal sleep duration (h) I: 10.0 ± 1.6, C: 9.9 ± 1.5, difference in change (95%CI): 0.24 (−0.05 to 0.53), effect size = 0.16, P = 0.11; TSD (h) I: 12.1 ± 1.8, C: 12.3 ± 1.8, difference in change (95%CI): 0.14 (−0.23 to 0.51), effect size = 0.07, P = 0.32; sleep onset time (min) I: 34.4 ± 42.8, C: 32.1 ± 35.5, difference in change (95%CI): 3.50 (−4.80 to 11.79), effect size = 0.09, P = 0.35; night wakefulness (min): I: 28.6 ± 72.3, C: 26.8 ± 61.1, difference in change −0.95 (95%CI, −15.80 to 13.90), effect size = −0.01, P = 0.82. Although there is no evidence of an overall effect of DHA+AA supplementation on child sleep, exploratory post hoc analyses identified that boys and children whose caregivers had depressive symptomatology may benefit more from the supplementation. |
| Children (aged 4–18 y) | |||||||||
| 3 | Hysing et al (2018)50 | Finland | RCT | Preschoolers between ages 4 and 6 y (n = 232) | Three warm lunch meals per week containing fatty fish. Each meal contained 50–80 g of fatty fish. | Three warm lunch meals per week containing meat. Each meal contained 50–80 g of meat. | 112 d | Parent-reported bedtime and rise time, time in bed, sleep latency, wake after sleep onset, sleep efficiency (ratio of duration of sleep to time in bed). | Time in beda (min) I: 678 ± 42, C: 672 ± 34, change of mean score: I: −5.3 (95%CI, −11.8 to 0.3); C: −5.3 (95%CI, −11.2 to 0.6), P = 0.905. TSD (min) I: 653 ± 45, C: 644 ± 36; change of mean score, I: −1.2 (95%CI, −8.3 to 5.9), C: −1.8 (95%CI, −8.8 to 5.1), P = 0.893. Sleep latency (min): I: 23.0 ± 16.0, C: 26.3 ± 16.8; change of mean score: I: −1.9 (95%CI, −4.7 to 0.9). There were no statistically significant differences between the fish and the meat groups on any of the included sleep measures. |
| 4 | Montgomery et al (2014)18 | United Kingdom | RCT | Healthy children aged 7–9 y (n = 362) who were under performing in reading from mainstream UK schools | Three capsules containing a total of 600 mg of algal DHA/d | Three capsules /d containing corn or soybean oil, matched with the active treatment for taste and color | 112 d |
Behavioral and medical sleep problems were measured by CSHQ. TSD, SL, SEff, frequency and length of wakefulness during night were measured by sleep diary and actigraphy. |
Slight but nonsignificant improvements were seen in both groups for all but 1 CSHQ subscale (sleep duration). Actigraphy results showed TSD increased by 58 min more in the active group than in the control group. TSD (h): I: 3.94 ± 1.214, C: 3.88 ± 1.202, z = −0.724, P = 0.469. Sleep onset delay: I : 1.66 ± 0.676, C: 1.62 ± 0.677, z = 0.71, P = 0.478. Bedtime resistance: I : 6.99 ± 1.575, C: 7.33 ± 2.02, z = 0.998, P = 0.318. Daytime sleepiness: I: 9.56 ± 2.555, C: 9.69 ± 2.766, z = −0.128, P = 0.898. TSD: I : 40.48 ± 6.166, C: 40.87 ± 6.084, z = −0.682, P = 0.495. Actigraphy score: TSD (min): I: 639 ± 52, C: 611 ± 66, t = 0.6, P = 0.551. SEff (ratio): I: 0.8 ± 0.098, C: 0.09 ± 0.117, t = 2.000, P = 0.052. Wake episodes: I: 12.86 ± 3.93, C: 15.78 ± 6.521, t = −2.59, P = 0.013. SL (min): I: 14 ± 22, C : 25 ± 33, z = 0379, P = 0.704. |
| 5 | Yehuda et al, (2011)57 | Israel | CT | Children aged 9–12 y (n = 78) and diagnosed with ADHD with onset of sleep deprivation | Capsules containing 720 g/d linoleic acid and 180 g/d ALA. | Placebo composed of mineral oil in identical capsule. Two capsules/d | 70 d | Self-reported SQ measured on a 5-point Likert-scale question | SQ: I: 3.8 ± 0.7, C: 1.4 ± 0.8. Polyunsaturated acid administration was associated with significant improvement in quality of life, ability to concentrate, SQ, and hemoglobin levels. |
| Adults (aged > 18 y) | |||||||||
| 6 | Yehuda et al (2005)19 | Israel | RCT | Undergraduate male students (n = 126) with test anxiety | Two capsules/d containing 450 mg of ALA and linoleic acid in a 1:4 ratio | Mineral oil | 21 d | Self-reported SQ on a 5-point Likert-scale question | Participants in the intervention group reported better sleep than those who received placebo. SQ: I: 3.6 ± 1.0, C: 1.8 ± 1.1. |
| 7 | Dretsch et al (2014)47 | United States | RCT | US deployed soldiers aged 18–55 y (n = 106) | One capsule/d containing 2500 mg EPA+DHA | Identical corn oil capsules containing 12% palmitic acid, 28% oleic acid, and 56% linoleic acid | 60 d | Self-reported SQ was measured by PSQI. Daytime sleepiness was measured by ESS. | PSQI score: I: 7.1 ± 3.4, C: 7.1 ± 3.7, effect sizes (Cohen d = 0.10), P = 0.663; ESS score I: 10.7 ± 4.5, C: 10.0 ± 4.3, Cohen d = 0.26, P = 0.247. A change in the HS-Omega-3 Index was a significant predictor of the change in ESS scores, F(1, 77) = 7.25, P = 0.009, suggesting that as omega-3 levels increased, daytime sleepiness decreased. |
| 8 | Hansen et al (2014)48 | United States | RCT | Male forensic patients aged 21–60 years (n = 95) from a secure forensic inpatient facility in the USA | 300 gram of Atlantic salmon that contain 4.8 g of EPA+DHA was served three times a week; however during the final 4 wk of the study, only 150 g of salmon were served each time. | Meat (eg, chicken, pork, beef) meals three times a week | 180 d | SL, SE, TSD, and actual wake time were measured by actigraph. Self-reported SQ was measured by sleep diary. | Actigraph: SL (min): I: 23.30 ± 20.38, C: 30.89 ± 18.93, main effects between two groups: F = 0.198, P = 0.66; effects between pre- and post-test conditions: F = 4.14, P = 0.05; interaction between groups and conditions: F = 4.11, P = 0.05. SEff (min): I: 70.37 ± 10.33, C: 69.64 ± 7.1, effect between groups: not significant; main effects of pre- and post-conditions: F = 32.84, P < 0.001; interaction between groups and conditions: F = 1.63, P = 0.21. Actual wake time (min): I: 110.04 ± 59.09, C: 107.49 ± 31.1, with a significant effect of pre- and post-test conditions (F = 19.83, P < 0.001), and a significant increase in actual wake time from pre- to post-test (P < 0.001, d = 0.43). TSD (min): I: 328.78 ± 52.84, C: 325.53 ± 67.09; main effect of pre- and post-test conditions: F = 7.44, P = 0.008. Self-reported SQ: 3.52 ± 0.6, C: 3.41 ± 0.8, with no effect of groups (F = 0.20, P = 0.66); no effect of pre- and post-test conditions (F = 0.26, P = 0.61). Daily functioning score: I: 3.35 ± 0.86, C: 2.85 ± 0.62, with significant main effect of groups, F = 54.63, P = 0.03, no effects of the pre- and post-test conditions (F = 0.49, P = 0.49). |
| 9 | Watanabe et al (2018)55 | Japan | RCT | Female nurses aged 20–59 y (n = 80) and worked in inpatient wards at hospitals | Omega-3 PUFA capsules containing 1200 mg EPA and 60 mg DHA per day | Identical capsules contained rapeseed oil (47%), soybean oil (25%), olive oil (25%), and fish oil (3%) | 91 d of capsule intake and 52 wk of follow-up | Insomnia severity was measured by ISI | ISI score at the week 52: I: 6.07 (95%CI, 4.96–7.18), C: 5.34 (95%CI, 4.16–6.51), group by time interaction, −0.24 (95%CI, −1.96 to 1.49), P = 0.786. Statistically significant superiority was observed in favor of the omega-3 PUFA group in terms of the ISI at 13 wk (95%CI, −6.29 to −12.56, and 0.02; P = 0.049), but no significant differences were found in other time periods. |
| 10 | Doornbos et al (2009)51 | Netherlands | RCT | Healthy pregnant women (n = 119) | DHA 220 mg or DHA+AA 220 mg/d | Soybean oil | From second trimester to 3 wk after delivery | Quantity and quality of sleep were assessed using sleep diaries. | No between-group effects were noted. The indices of SQ did not change significantly over time in any group. In a significant regression model, F = 15.240; P < 0.001. Efficient sleep at week 4 postpartum (min) median (25th;75th percentile): I: 487 ( 420–540), C = 450 ( 370, 490), SEff (%) median (25th;75th percentile): I: 88.03 ( 81.28–90.39), C: 84.62 ( 78.22–87.50), P > 0.05 for all. |
| 11 | Judge et al (2014)49 | United States | RCT (pilot trial) | Pregnant women aged 18–35 years (n = 42) without self-reported significant medical history | One fish oil capsule that contains 300 mg DHA, 5 d weekly | Corn oil | From 24 wk gestation to delivery | Sleep disturbance was measured with a 5-point Likert item contained in the postpartum depressive symptomatology. | Sleep disturbance at 6 mo (mean ± SD): I: 6.80 ± 3.44, C: 7.00 ± 2.67, > 0.05. |
| 12 | Cohen et al (2014)44 and Reed et al (2014)45 were the same study | United States | RCT | Women aged 40–62 y (n = 355) experiencing the menopausal or postmenopausal transition | Fish oil capsule containing a total omega-3 dose of 615 mg, including EPA 425 mg and DHA 100 mg, along with other assorted omega-3 PUFA (90 mg) | Matching placebo capsule containing olive oil | 84 d | Self-reported SQ was measured by PSQI. Insomnia severity was measured by ISI. | The mean PSQI score reduction was 2.1 for the omega-3 group and 1.7 for the placebo group (P = 0.09). The mean ISI reduction was 3.8 for the omega-3 group and 3.7 for the placebo group (P = 0.73). |
Time in bed was calculated by subtracting bedtime from rise time.
Abbreviations: AA, arachidonic acid; ADHD, attention deficit hyperactivity disorder; ALA, α-linolenic acid; AS, active sleep; BISQ, Brief Infant Sleep Questionnaire; C, control group; CSHQ, Children’s Sleep Habits Questionnaire; CT, controlled trial; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ESS, Epworth Sleepiness Scale; I, intervention group; ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; PUFA, polyunsaturated fatty acid; QS, quiet sleep; RCT, randomized controlled trial; SEff, sleep efficiency; SL, sleep latency; SQ, sleep quality; TSD, total sleep duration.
Table 3.
Characteristics of included cohort studies
| No. | Study (year) | Country | Population | Exposure | Sleep outcome | Adjusted variables | NOS scorea | Main result |
|---|---|---|---|---|---|---|---|---|
| Infants and toddlers (aged 0–3 y) | ||||||||
| 1 | Cheruku et al (2002)42 | United States | Healthy pregnant women (n = 17) and their new-born children | High DHA level: Maternal plasma phospholipid fatty acid concentration > 3.0% by weight of total fatty acids | Neonates’ AS, QS, sleep-wake transition, and wakefulness measured with actigraph | Maternal age, race, parity, length of gestation, maternal education, infant birth weight, infant birth length, infant head circumference, 1-min Apgar score, 5-min Apgar score |
Total: 5 S: 4, C: 1, O: 0 |
On postpartum day 1, the ratio of maternal n-6 to n-3 fatty acids in maternal plasma was negatively associated with QS and positively associated with arousals in QS. On postpartum day 2, maternal n-6: n-3 was positively associated with AS (r = 0.53, P < 0.05), sleep-wake transition (r = 0.52, P < 0.05), and AS: QS (r = 0.52, P < 0.05). On postpartum day 2, maternal DHA concentration was negatively associated with AS (r = 0.49, P < 0.05), AS: QS (r = 0.55, P < 0.05), and sleep-wake transition (r = 0.49, P < 0.05) and positively associated with wakefulness (r = 0.51, < 0.05). |
| 2 | Zornoza-Moreno et al (2014)52 | Spain | Pregnant women (n = 63) both healthy and with GDM, and their infants | Venous cord plasma DHA percentages in women with GDM treated with diet and insulin | Infants’ sleep rhythm maturation (ie, IS, and CFI) estimated from body temperature and physical activity | Exposure duration: the whole pregnancy period. Follow-up time points: at birth; 15 d; 1, 3, and 6 mo after birth; but only data at 3 and 6 mo were reported |
Total: 7 S: 4, C: 1, O: 2 |
Maternal DHA level at recruitment correlated with children’s better sleep rhythm maturation at 6 mo of age, as indicated by higher IS (r = 0.383, P = 0.007) and CFI (r = 0.340, P = 0.018). |
| 3 | Kocevska et al (2016)53 | Netherlands | Children (n = 3465) aged 1–3 y | Daily nutrient intake | Parent-reported questionnaires regarding total sleep duration, number of night awakenings, usual bedtime, wake-up time, and daytime napping | Maternal parity, age, marital status, education, household income, maternal smoking during pregnancy, children’s sex and birth weight, ethnic group, breastfeeding history, child behavior problems, family regularity, time spent watching television at age 2 y |
Total: 6 S: 3, C: 1, O: 2 |
Substituting unsaturated fat intake with saturated fat was associated with 7 min (95%CI, −13 to −1 min) shorter TSD at age 3 y for each 5% of energy from saturated fat. Vice versa, substituting saturated with unsaturated fat was associated with 5 min (95%CI, 2–8 min) longer nighttime sleep duration at age 3 y |
| Children (aged 4–18 y) | ||||||||
| 4 | Huss et al (2010)54 | Germany | Children aged between 5 and 12 y (n = 810) and referred to primary care settings for attentional and behavioral problems | Food supplement containing a combination of omega-3 (EPA 400 mg +DHA 40 mg) and omega-6 fatty acids (60 mg) as well as magnesium (80 mg) and zinc (5 mg) | Pediatricians’ documentation of sleep-related problems (ie, problems falling asleep, sleeping through the night, and impaired sleep quality) | Child sex and age groups |
Total: 6 S: 3, C: 1, O: 2 |
The number of children evaluated as having problems falling asleep, having problems to sleep through the night, and having impaired sleep quality were 305 (38.3%) and 182 (22.9%), 148 (18.6%) and 87 (10.9%), and 186 (23.7%) and 107 (13.6%), before and after intervention, respectively (P < 0.001 for all). Improvement of sleep-related symptoms was similar in all age groups but more statistically significant for girls regarding to difficulty falling asleep. |
| 5 | Liu et al (2017)56 | China | Chinese children aged 9–11 y (n = 541) | Fish consumption data at age 9–11 y were obtained from a self-administrated food frequency questionnaire | Sleep quality was measured by the total sleep disturbance score derived from parental report of sleep patterns in the CSHQ | Parental education, occupation, marital status, maternal age at childbirth, home location, breastfeeding history, child sex and siblings, and breakfast consumption |
Total: 6 S: 3, C: 1, O: 2 |
Sleep disturbance score in children who frequently ate fish (≥ 1/wk) was 4.49 (P = 0.001, Cohen d = 0.221) and in children who sometimes ate fish (2–3 times per mo) was 3.01 (P = 0.019, Cohen d = 0.132), lower than those who never or seldom ate fish. |
| Adults (aged > 18 y) | ||||||||
| 6 | Christian et al (2016)59 | United States | Pregnant women (n = 135) | RBC PUFA status | Pregnant women’s self-reported sleep quality measured by PSQI | Age, race/ethnicity, education, annual household income, gravidity, and parity, Pre-pregnancy BMI, |
Total: 6 S: 4, C: 1, O: 1 |
Higher RBC DHA levels were associated with significantly better overall sleep quality, as indicated by lower total scores on the PSQI (b = −1.00, P = 0.012), longer sleep duration (P = 0.019), and better sleep efficiency (P = 0.047). Neither EPA nor AA was associated with overall sleep quality (P ≥ 0.34). After adjusting for covariates, higher DHA:AA ratios were associated with better overall sleep quality (b = −15.4, P = 0.005), shorter sleep latency (P = 0.033), longer sleep duration (P = 0.019), and better habitual sleep efficiency (P = 0.026). |
| 7 | Lotrich et al (2016)58 | United States | Nondepressed adult patients aged between 18 and 80 y with HCV (n = 104) who received IFN-α therapy | RBC PUFA status | Patients’ self-reported sleep quality measured by PSQI | Sex, race, age, weight, baseline Beck Depression Inventory score |
Total: 6 S: 4, C: 1, O: 1 |
The PSQI score was 6.6 ± 4.1; the correlation with AA/(EPA+DHA) was 0.31 (P < 0.05). |
| 8 | Ford et al (2016)60 | United States | Participants (n = 8771) older than 30 y who completed the AHS-2 and the PsyMRS cohort studies | Daily dietary intake | Participants reported on questionnaires the duration of sleep at night categorized as < 6, 7–8, and ≥ 9 h. | Age, sex, ethnicity, BMI, education level, frequency of vigorous exercise, alcohol intake, Mediterranean diet pattern, total energy intake |
Total: 7 S: 4, C: 1, O: 2 |
The correlation coefficient between hours of sleep and omega-3 PUFA exposure was β = 0.24, B = 0.15 (95%CI, 0.08–0.22), P < 0.001. |
The NOS checklist was used to evaluate the quality of included cohort studies.
Abbreviations: AHS-2, Adventist Health Study-2; AS, active sleep; BMI, body mass index; C, comparability; CFI, Circadian Function Index; CSHQ, Children’s Sleep Habits Questionnaire; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GDM, gestational diabetes mellitus; HCV, hepatitis C virus; IFN-α, interferon-α; IS, inter-daily stability of the rhythm; O, outcome; NOS, Newcastle-Ottawa Scale; PSQI, Pittsburgh Sleep Quality Index; PsyMRS, Psychosocial Manifestations of Religion Sub-Study; PUFA, polyunsaturated fatty acid; QS, quiet sleep; RBC, red blood cell; S, selection; TSD, total sleep duration.
Intervention or exposure
The intervention or exposure for infants and toddlers involved omega-3 LC-PUFA–intake by pregnant women)41,42,52 and interventions directly targeting infants themselves (ie, daily intake of DHA and arachidonic acid [AA] supplementation).46 Children and adults received capsules that contained various compositions of LC-PUFA supplementation (DHA,49 or DHA plus AA,51 or DHA plus EPA55) or meals rich in omega-3 LC-PUFA.48,56,60
The interventions or exposures varied in terms of dose and duration. The daily intake dose of omega-3 LC-PUFA for infants and children in the included RCTs ranged from 300 mg49 to 1800 mg,52 and the duration lasted from 70 days57 to 180 days.46 Adults’ daily intake dose of omega-3 LC-PUFA supplementation ranged from 220 mg51 to 2500 mg,47 and the duration ranged from 21 days50 to 180 days.48 The proportion of omega-3 LC-PUFA and other nutrients contained in daily diet was measured by a self-reported food frequency questionnaire.53,56,60
Sleep outcome measurement
Objective measurements (via actigraph and wearable devices) and subjective measurements, including self-reported questionnaires and sleep diaries, were used to evaluate participants’ sleep outcomes. Three studies used actigraphs18,41,42 and 1 study used a wearable device48 to detect participants’ physical activities and body temperature, respectively, and transferred these data into sleep outcomes with a specific algorithm. Only 1 study used both actigraphy and parent-report questionnaires to measure children’s sleep outcomes.18 The Brief Infant Sleep Questionnaire46 and the Child Sleep Habits Questionnaire (CSHQ)18,56 were used to measure infants’ and children’s sleep disturbance, SL, sleep onset time, among other parameters. The Pittsburgh Sleep Quality Index (PSQI),47,58,59 the Epworth Sleepiness Scale,47 and the Insomnia Severity Index (ISI)44,55 were used to measure adults’ SQ, daily sleepiness, and severity of insomnia, respectively. Two studies used a 5-point Likert scale to rate participant SQ.19,57
Quality of included studies
The included RCTs were generally of high quality, with most having low risk of selection bias, performance bias, and detection bias (Table 4). Two cohort studies were evaluated as high quality,52,60 and the other 6 studies were evaluated as moderate quality (Table 3).
Table 4.
Risk of bias of included randomized controlled trials
| No. | Reference (year) | Risk level |
|||||
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Performance bias | Attrition bias | Detection bias | Reporting bias | ||
| 1 | Judge et al (2012)41 | Low | Low | Low | Low | Low | Low |
| 2 | Boone et al (2019)46 | Low | Low | Low | Low | Low | Low |
| 3 | Hysing et al (2018)50 | Unclear | Unclear | Unclear | Low | Unclear | Low |
| 4 | Montgomery et al (2014)18 | Low | Low | Low | Low | Low | Low |
| 5 | Yehuda et al (2011)57 | High | High | Low | Low | Unclear | Low |
| 6 | Yehuda et al (2005)19 | High | High | Low | Low | Unclear | Low |
| 7 | Dretsch et al (2014)47 | Low | Low | Low | Low | Low | Low |
| 8 | Hansen et al (2014)48 | Low | Unclear | Unclear | Low | Low | Low |
| 9 | Watanabe et al (2018)55 | Low | Low | Low | Low | Low | Low |
| 10 | Doornbos et al (2009)51 | Low | Low | Unclear | Low | Low | Low |
| 11 | Judge et al (2014)49 | Low | Low | Low | Low | Low | Low |
| 12 | Cohen et al (2014)44 and Reed et al (2014)45 | Low | Low | Low | Low | Low | Low |
Neonate sleep indicators measured with the motility monitoring system
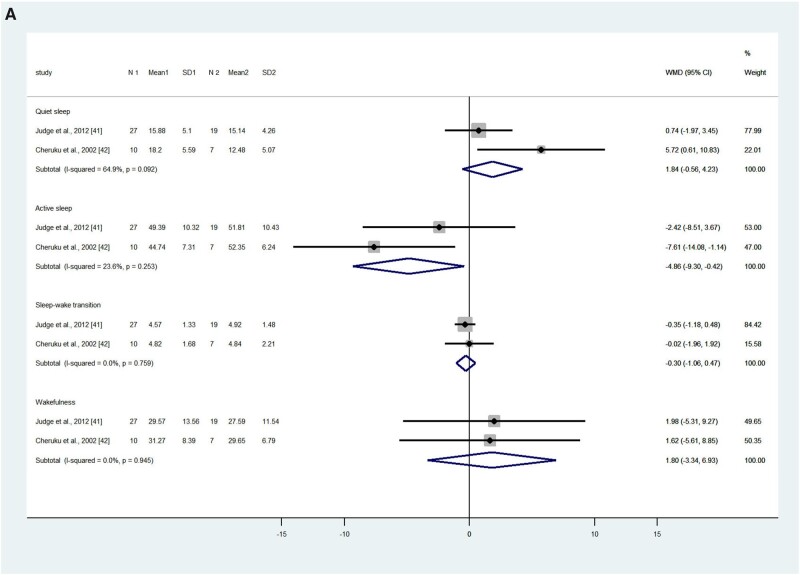
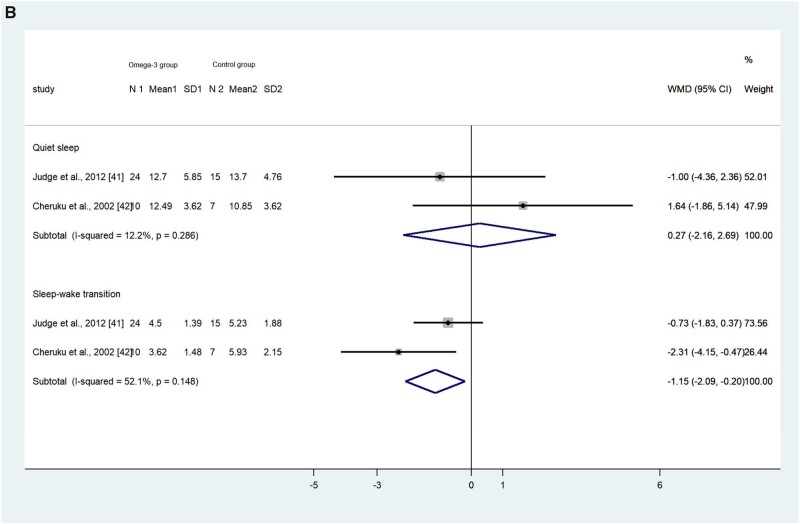
Judge et al41 and Cheruku et al42 used the same nonintrusive motility monitoring system to obtain neonates’ sleep data during the first and second day after birth. The pooled results showed that on the first day after birth, neonates in the intervention or higher exposure group (hereafter, the intervention group) had less active sleep (measured as a percentage) compared with the control or low-exposure group (hereafter, the control group; WMD, –4.86%; 95%CI, –9.30 to –0.42; P = 0.032; I2 = 23.6%), but no significant differences were found in terms of the percentages of quiet sleep (WMD, 2.74%; 95%CI, –2.04 to 7.53; P = 0.133; I2 = 64.9%), sleep-wake transition (WMD, –0.30%; 95%CI, –1.06 to 0.47; P = 0.445; I2 = 0), and wakefulness (WMD, 1.80; 95%CI, –3.34 to 6.93; P = 0.492; I2 = 0; Figure 2A).41,42
Figure 2.
Effects of omega-3 long-chain polyunsaturated fatty acid exposure on neonates’ quiet sleep, active sleep, sleep-wake transition, and wakefulness on (A) first day after birth and (B) second day after birth, measured by motility monitoring systems. Fixed-effect models were used to calculate the pooled estimate of the differences in means and 95%CI. Mean, mean sleep duration; WMD, weighted mean difference. N 1 and N 2 refer to the number of participants in the intervention/exposure group and control group respectively. Mean 1 and Mean 2 refer to the mean sleep duration of the intervention/exposure group and control group respectively. SD1 and SD2 refer to the standard deviation of sleep duration of the intervention/exposure group and control group respectively.
On the second day after birth, neonates in the intervention group had less active sleep (WMD, –8.40%; 95%CI, –14.50 to –2.29; P = 0.007; I2 = 89.2%), less sleep-wake transition (WMD, –1.15%; 95%CI, –2.09 to –0.20; P = 0.017; I2 = 52.1%), and more wakefulness (WMD, 9.06%; 95%CI, 1.53–16.59; P = 0.018; I2 = 81.3%) compared with the control group. There were no statistical differences between the groups in terms of the percentage of neonates’ quiet sleep (WMD, 0.27%; 95%CI, –2.16 to 2.69; P = 0.286; I2 = 12.2%; Figure 2B).41,42
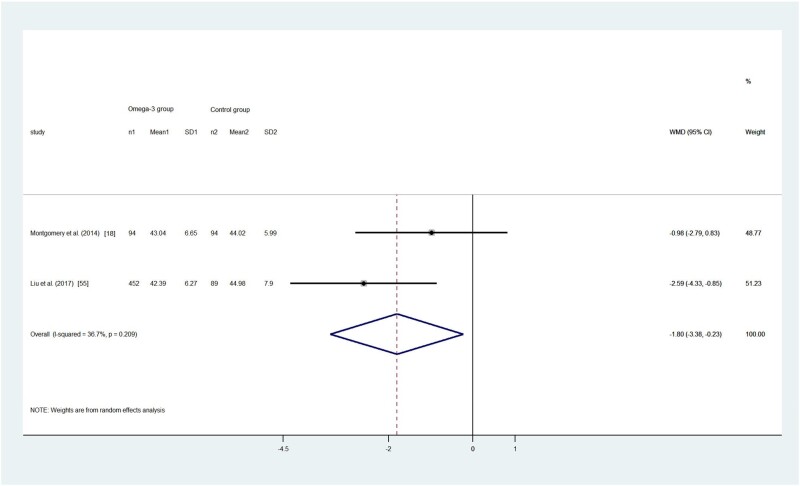
Children’s total sleep disturbance score
One RCT18 and 1 cohort study56 used the CSHQ to measure children’s sleep disturbances and found an inconsistent relationship between omega-3 LC-PUFA intake and children’s total sleep disturbance score. Montgomery et al18 found no statistically significant difference in the change of total sleep disturbance scores between the omega-3 LC-PUFA group and control groups in the school-aged children in general (P = 0.495), whereas omega-3 LC-PUFA supplementation was effective in a subgroup of children with clinical-level sleep problems (total CSHQ scores > 41; P = 0.049).18 The children recruited in the cohort study reported total CSHQ scores > 41 in all groups, and the researchers found that a greater amount of fish consumption (≥ 2–3 times/mo) was associated with fewer sleep disturbances.56 Only children with CSHQ scores > 41 were included to pool the effect size. The result showed that children with clinical-level sleep problems who received omega-3 LC-PUFA intervention or exposure had a lower sleep disturbance score (WMD, –1.81; 95%CI, –3.38 to –0.23; P = 0.025; I2 = 36.7%; Figure 3).18,56
Figure 3.
Effects of omega-3 long-chain polyunsaturated fatty acid on children’s sleep disturbance evaluated with the Children’s Sleep Habits Questionnaire (CSHQ). A random-effects model was used to calculate the pooled estimate of the differences in means and 95%CI. Weights are from random-effects analysis. In the Liu et al study,56 3 groups were reported (ie, children who never or seldom ate fish, children who sometimes ate fish, and children who often ate fish). The latter 2 groups were combined as 1 group of children with higher levels of omega-3 exposure. Mean, mean CSHQ score; WMD, weighted mean difference.
Total sleep duration
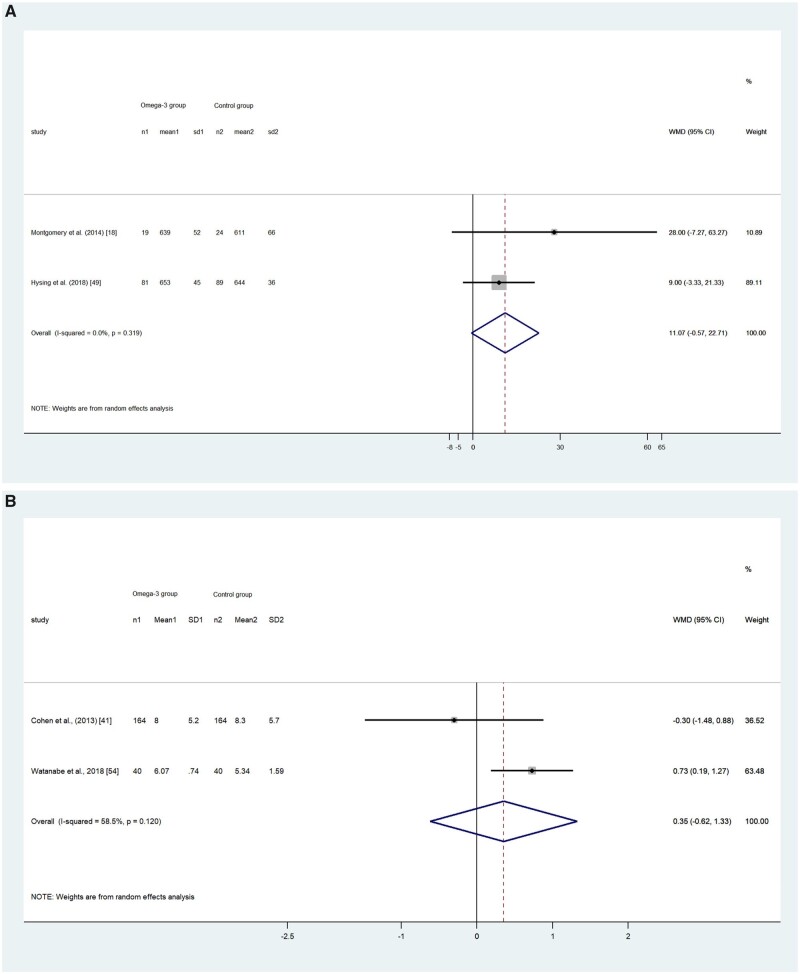
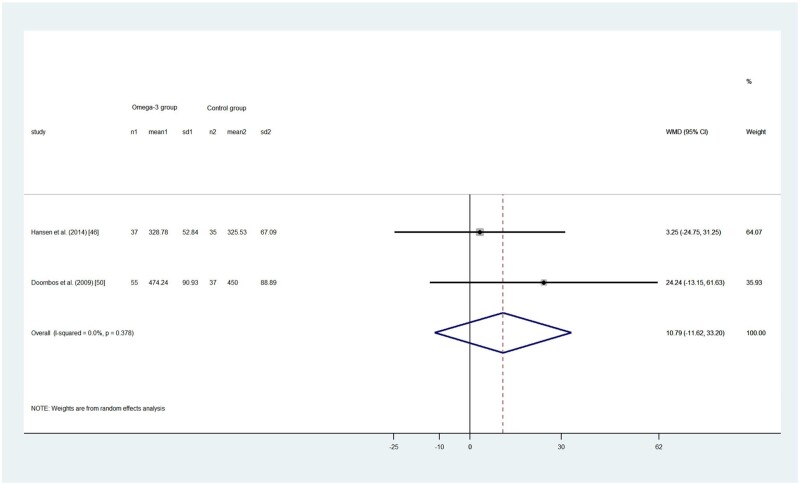
Five RCTs18,46,48,50,51 reported a total of 679 participants’ TSD, among which 1 study46 found that infants (aged 10–16 mo) in the control group had longer TSD than those in the intervention group (738 ± 108 and 726 ± 108 min, respectively; P = 0.32). The other 4 studies generally found the intervention group had longer TSD than the control group, although the differences were not statistically significant. The pooled effect size by the random-effects model showed no statistical difference between the omega-3 LC-PUFA intervention group and control group in children (WMD, 11.07; 95%CI, –0.57 to 22.71; P = 0.062; I2 = 0; Figure 4A) and18,50 adults (WMD, 10.79; 95%CI, –11.62 to 33.20; P = 0.345; I2 = 0; Figure 4B).48,51
Figure 4.
(A) Effect of omega-3 long-chain polyunsaturated fatty acid exposure on (A) children’s and (B) adults’ total sleep duration (minutes). A random-effects model was used to calculate the pooled estimate of the differences in means and 95%CI. Weights are from random-effects analysis. Mean, mean of sleep duration; WMD, weighted mean difference.
Two longitudinal studies reported the relationship between omega-3 LC-PUFA intake and sleep duration.53,59 Kocevska et al53 (2016) analyzed the subtypes of macronutrients eaten by toddlers and found that replacing saturated fats with unsaturated fats was associated with 5 minutes (95%CI, 2–8) longer nighttime sleep duration in toddlerhood. Christian et al59 collected sleep duration data via a self-reported questionnaire and found that higher DHA levels were associated with longer sleep duration in pregnant women (P = 0.019).
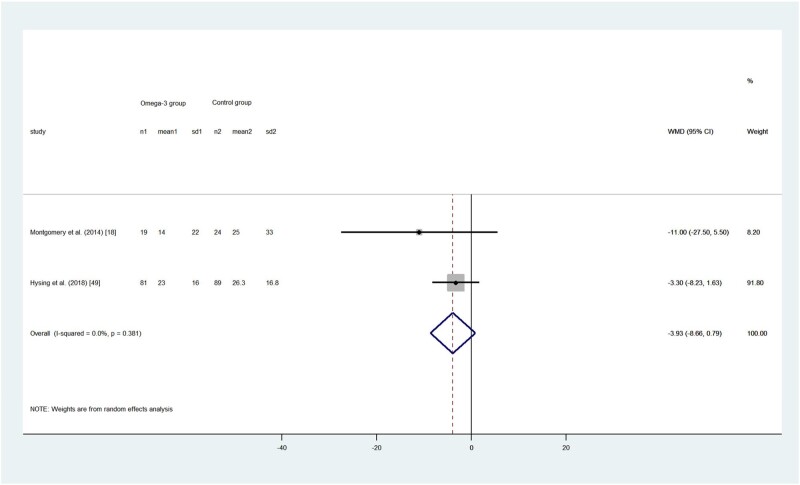
Sleep latency
Two RCTs reported the SL (in minutes) in children.18,50 The pooled effect size showed no difference between omega-3 LC-PUFA effect in the intervention group and control group (WMD, –3.93; 95%CI, –8.66 to 0.79; P = 0.103; I2 = 0; Figure 5). Only18,50 1 RCT reported the SL in adults and found no main effect between the intervention and control groups (F = 0.1980; P = 0.66).48 However, a post hoc test showed that there was a significant SL increase between pre- and postintervention tests in the control group but not in omega-3 LC-PUFA group (P = 0.04; Cohen d = 0.60).48 Only 1 included cohort study reported SL; the researchers found that a higher DHA-to-AA ratio was associated with shorter SL in pregnant women (P = 0.033) when potential covariates were adjusted, including maternal age, race, education level, household income, and body mass index before pregnancy.59
Figure 5.
Effects of omega-3 long-chain polyunsaturated fatty acid intervention on children’s sleep latency (minutes). A random-effects model was used to calculate the pooled estimate of the differences in means and 95%CI. Weights are from random-effects analysis. Mean, mean of sleep latency; WMD, weighted mean difference.
Sleep efficiency
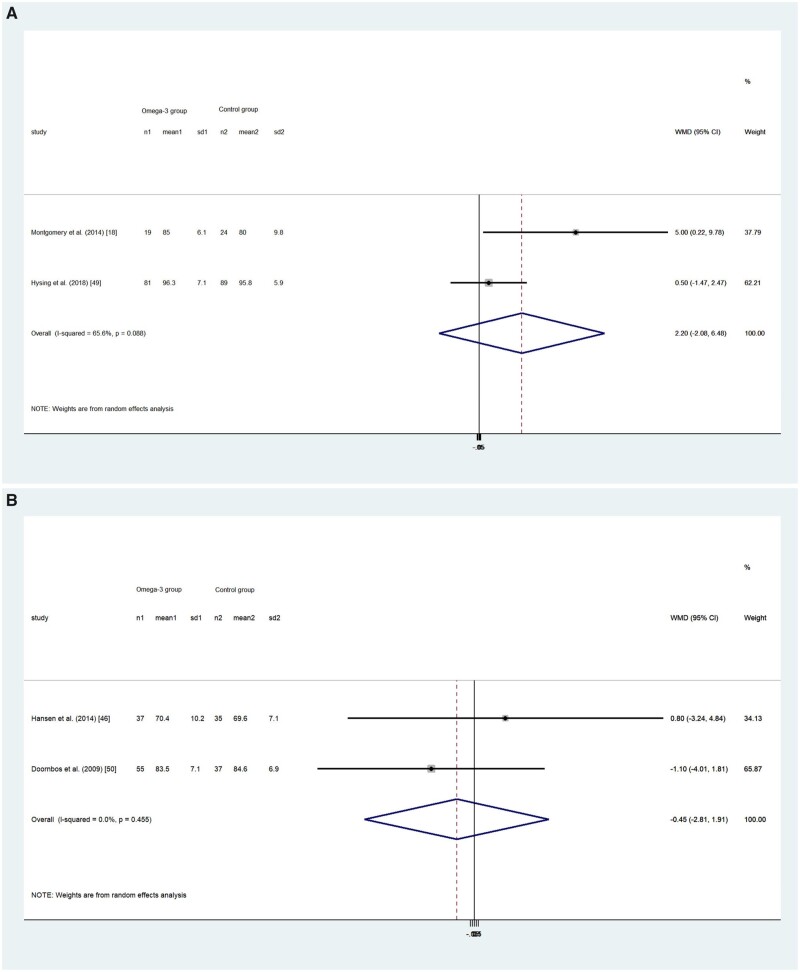
Four RCTs including a total of 377 participants reported SEff as an outcome.18,48,50,51 Only 1 study18 found slightly higher SEff in the omega-3 LC-PUFA and control groups after intervention (increase of 9% and 1%, respectively; t = 2.000; P = 0.052), whereas the 3 other studies all reported negative change in SEff in both groups after intervention.48,50,51 The pooled results showed no difference in SEff improvement between children (WMD, 0.022; 95%CI, –0.021 to 0.065; P = 0.313; I2 = 65.6%; Figure 6A) and18,50 the adult population (WMD, –0.45; 95%CI, –2.81 to 1.91; P = 0.708; I2 = 0; Figure 6B).48,51
Figure 6.
(A) Effects of omega-3 long-chain polyunsaturated fatty acid exposure on (A) children’s and (B) adults’ sleep efficiency (%). A random-effects model was used to calculate the pooled estimate of the differences in means and 95%CI. Weights are from random-effects analysis. Mean, mean sleep efficiency; WMD, weighted mean difference.
Meanwhile, only 1 cohort study explored the relationship between DHA exposure and pregnant women’s SEff.59 The researchers found that after adjusting covariates including age, race/ethnicity, education, annual household income, gravidity, parity, and prepregnancy body mass index, a higher DHA-to-AA ratio was associated with better SEff (P = 0.026).59
Sleep quality
Only 1 RCT explored the SQ of children with attention deficit hyperactivity disorder, measured with a 5-point Likert scale.57 The researchers found significant improvement in the omega-3 LC-PUFA group (mean ± SD: omega-3 LC-PUFA group [n = 40 participants], 3.8 ± 0.7; vs control group [n = 38 participants], 1.4 ± 0.8; no P value was reported in the article).57
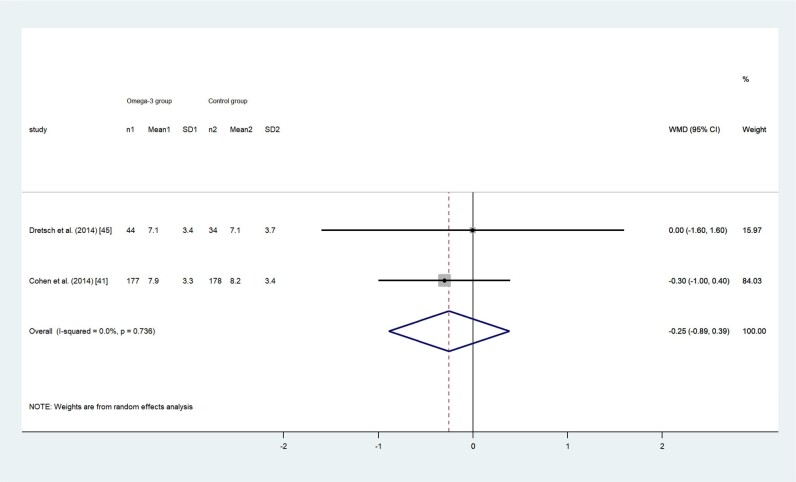
Adult participants’ SQ was measured with either the PSQI or on a 5-point Likert scale. Two RCTs used the PSQI to evaluate adults’ SQ and both reported a decrease in the PSQI total score after intervention, indicating participants in both the intervention and control groups had improved SQ, although not statistically significantly (P = 0.663 and 0.093, respectively).44,47 The pooled PSQI total score using the random-effects model showed no difference between the 2 groups (WMD, –0.25; 95%CI, –0.89 to 0.39; P = 0.439; I2 = 0; Figure 7). In44,47 2 other RCTs, researchers measured adults’ SQ on a 5-point Likert scale.19,48 Hansen et al48 found no difference in SQ between the omega-3 LC-PUFA group (mean ± SD: 3.52 ± 0.6) and control group (mean ± SD: 3.41 ± 0.8) after intervention (P = 0.50).48 Yehuda et al19 reported improved SQ in children with attention deficit hyperactivity disorder who received omega-3 supplementation (mean ± SD: 3.6 ± 1.0) compared with children received placebo (mean ± SD: 1.8 ± 1.1). Because of the high heterogeneity (I2 > 75%) in the 2 studies, the effect size is not presented.
Figure 7.
Effects of omega-3 long-chain polyunsaturated fatty acid exposure on adults’ sleep quality, measured with the Pittsburgh Sleep Quality Index. A random-effects model was used to calculate the pooled estimate of the differences in means and 95%CI. Weights are from random-effects analysis. Mean, mean Pittsburgh Sleep Quality Index score; WMD, weighted mean difference.
Insomnia severity index
Two RCTs assessed adult participants’ insomnia severity with the ISI.44,55 Watanabe et al55 collected participants’ outcome at multiple times, whereas Cohen et al44 only collected outcomes at week 12 after the intervention. To make the outcomes in these 2 studies comparable, only the follow-up data at week 13 in the Watanabe et al study55 were included for comparison. The pooled result showed that the change of the insomnia severity had no significant difference between the intervention and control groups (WMD, 0.35; 95%CI, –0.62 to 1.33; P = 0.475; I2 = 58.5%; Figure 8). No44,55 included longitudinal studies measured participants’ ISI.
Figure 8.
Effects of omega-3 long-chain polyunsaturated fatty acid exposure on adults’ Insomnia Severity Index score. A random-effects model was used to calculate the pooled estimate of the differences in means and 95%CI. Weights are from random-effects analysis. Mean, mean Insomnia Severity Index score; WMD, weighted mean difference.
DISCUSSION
In this meta-analysis, we investigated the role of omega-3 LC-PUFA in human sleep outcomes. Overall, omega-3 LC-PUFA may benefit certain aspects of sleep health throughout childhood. Maternal intake or exposure to omega-3 LC-PUFA during pregnancy improve infants' sleep maturity and organization. Specifically, it reduced infants’ active sleep and sleep-wake transition and enhanced wakefulness on their second day of age but had no effect on quiet sleep. Furthermore, omega-3 reduced total sleep disturbance score for children with clinical-levels of sleep problems but had no effect on healthy children’s TSD, SL, or SEff. For the adult population, no effectiveness was found in TSD, SL, SEff, SQ, or insomnia severity.
Neonate sleep indicators measured with the motility monitoring system
Neonates’ sleep architecture has its unique characteristics in that it includes a total of 10 behavioral states and acts in complex and dynamic ways to influence the neonates’ response to external stimuli.61 In infancy, active sleep and quiet sleep can be observed as rapid eye movement sleep and nonrapid eye movement sleep, respectively,62 and sleep-wake transition indicates the duration a neonate needs to shift from the sleep state to the awake state.61 The pooled results based on 2 studies showed that on the second day of age, neonates in the omega-3 LC-PUFA intervention group had significantly less active sleep, less sleep-wake transition, and more wakefulness than control groups.
It is noteworthy that the intervention or exposure to omega-3 in these 2 studies targeted mothers.41,42 Fetuses and neonates rely on maternal transfer of fatty acids through the placenta and human milk. However, studies have shown that genetic control, namely the fatty acid desaturase genotype, contributes to maternal transfer of DHA regardless of dietary intake.63,64 A recent systematic review found that the fatty acid desaturase genotype can affect infants’ PUFA status and their neurodevelopmental outcome.65 Given the short follow-up duration (ie, only after the immediate birth of the neonates) and the uncertain interacting direction between epigenetic programming and diet, the pooled results of omega-3 LC-PUFA effect on neonatal sleep outcomes should be considered with caution.
Children’s sleep disturbances
Omega-3 LC-PUFA intervention or exposure can reduce the sleep disturbance score in children with clinical-level sleep problems. This is in line with the findings of another observational study in which children’s erythrocyte omega-3 PUFA was inversely associated with Chinese children’s and adolescents’ sleep disturbance prevalence.66 However, the inconsistent effectiveness of omega-3 LC-PUFA intervention on children with or without clinical-level sleep problems found in the included RCT18 suggests omega-3 LC-PUFAs may be effective mainly for reducing sleep disturbance in children with more severe sleep problems. The moderating effect of the fatty acid desaturase genotype on children’s neurocognitive outcomes65 may be another possible reason that omega-3 LC-PUFA only benefited certain groups of children.
TSD and SL
The impact omega-3 LC-PUFA has on humans’ TSD and SL is inconsistent between observational studies and RCTs. The longitudinal studies included in this meta-analysis suggest both a positive association between omega-3 LC-PUFA or fish consumption and sleep duration and SL, which is in line with findings from other cross-sectional studies.14,67,68 However, the pooled results of the included RCTs showed no causal relationship between omega-3 LC-PUFA and TSD or SL in both child and adult populations. One potential reason is that other factors, such as the genetic polymorphisms within the fatty acid gene cluster and elongation of very-long-chain fatty acids family, can influence LC-PUFA accumulation in human body.69 Another possible reason is that the included studies did not take in account other nutrient statuses that could influence sleep outcomes. Studies have shown that different micronutrient statuses and the proportion of macronutrient intake can influence sleep duration.70,71 Thus, additional investigation with more consideration of wider potential confounding factors is needed.
Sleep efficiency
The effect of omega-3 LC-PUFA intake on SEff is inconsistent within the included RCTs in this study. One possible reason is that most included studies only used self- or parent-reported questionnaires to collect data on participants’ sleep duration and total time spent in bed, which can yield inaccurate information due to recall bias regarding time to fall asleep, because one cannot know the exact time one loses consciousness and falls asleep. The only RCT that used both the actigraph and parent-reported questionnaire to collect children’s sleep data found inconsistencies between subjective and objective data, which proved somewhat the inaccuracy of self-reported data in sleep.18 Another possible reason is the relatively short duration of the intervention in healthy participants with normal omega-3 LC-PUFA blood levels at baseline, which could limit the effect of the intervention.50
Sleep quality
Adults’ SQ reveals controversial results, as well. Although several cross-sectional studies reported that improved SQ (assessed by PSQI) is associated with omega-3 LC-PUFA consumption,15,72 the pooled results in the current study revealed no effectiveness of omega-3 LC-PUFA on adults’ SQ. Meanwhile, the high heterogeneity in the 2 studies19,48 using a single 5-point Likert scale to assess adult SQ prevented us from pooling the estimated effect size. The conclusion between these 2 studies remained inconsistent. One study48 reported no difference between the omega-3 LC-PUFA intervention and control groups, whereas another study reported higher SQ in the omega-3 LC-PUFA group.19 The inconsistency may result from different SQ evaluation tools, limited intervention duration, and sample characteristics and size.47
Insomnia severity
One RCT that reported adults insomnia severity recruited female nurses44 and another recruited women in peri- or postmenopause55; the pooled results showed no effects of omega-3 LC-PUFA intake on improving the participants’ insomnia severity. It is noteworthy that the included nurses had a low rate of subthreshold and clinical insomnia at baseline (ISI score ≥ 8 or 15, respectively) and the results showed omega-3 LC-PUFA intake only was effective in improving the included nurses’ ISI at 13 weeks postintervention but not at other times.55 The participants in another study, namely women in peri- or postmenopause, had more severe ISI baseline scores (all > 8) and the results showed no beneficial effect of omega-3 LC-PUFA intake on these women.44 Night-shift work can alter human circadian rhythms73 and different psychological distress during the menopausal period can mediate insomnia severity.45 However, the small number of included studies prevented us from adjusting for these potential confounders in the analysis.
Strengths and limitations
This study has several limitations. First, because of the limited number of included studies, subgroup analysis based on participant sex and health status was not feasible to further explore whether omega-3 LC-PUFA intake has influences different health statuses. Similarly, sensitivity analysis was not appropriate to explore heterogeneity and publication bias of all outcomes. Another limitation is that only studies published in English were included, so there may be language bias. Despite these limitations, a strengths of this study included incorporation of both longitudinal studies and RCTs, comprehensive literature searches were conducted, and studies of moderate to high quality were included.
CONCLUSION
In this systematic and meta-analytic review of clinical trials and longitudinal observational studies in participants across various age groups, we conclude that omega-3 LC-PUFA exposure may improve several aspects of infants’ sleep architecture and reduce the total sleep disturbance score for children with clinical levels of sleep problems. However, this review did not find that omega-3 LC PUFA has effect on sleep outcome for healthy children and adult population. Due to small number of available studies, the impact of omega-3 LC-PUFA on humans’ sleep architecture and sleep quality warrants additional investigation. Future studies should take into consideration participant health status, nutrient intake, genetic polymorphisms, and psychological factors, among other potential confounders.
Supplementary Material
Acknowledgments
The authors thank student RA Meg Gladieux for her help in proofreading the manuscript.
Author contributions. J.L. conceptualized the work and contributed to the development of the systematic review protocol. Y.D. designed and conducted the literature search. Both authors screened and evaluated studies for eligibility. Y.D. extracted the data, conducted the statistical analysis, and drafted the manuscript. J.L. double-checked the extracted data and critically reviewed the draft. Both authors approved the final version of the manuscript.
Funding. This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant no.: NIH/NICHD 5R01HD087485), and National Institutes of Health National Institute of Environmental Health Sciences (grant no.: P30-ES013508).The funders had no role in the undertaking, data analyses, or reporting of this work.
Declaration of interest. The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA 2009 checklist
References
- 1. Frank S, Gonzalez K, Lee-Ang L, et al. Diet and sleep physiology: public health and clinical implications. Front Neurol. 2017;8: 393. doi:10.3389/fneur.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peuhkuri K, Sihvola N, Korpela R.. Diet promotes sleep duration and quality. Nutr Res. 2012;32:309–319. [DOI] [PubMed] [Google Scholar]
- 3. He F, Bixler EO, Liao J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med. 2015;16:1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castro-Diehl C, Wood AC, Redline S, et al. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41(11):zsy158. doi:10.1093/sleep/zsy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ji X, Liu J.. Associations between blood zinc concentrations and sleep quality in childhood: a cohort study. Nutrients. 2015;7:5684–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grandner MA, Jackson N, Gerstner JR, et al. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afaghi A, O’Connor H, Chow CM.. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007;85:426–430. [DOI] [PubMed] [Google Scholar]
- 8. Perkin MR, Bahnson HT, Logan K, et al. Association of early introduction of solids with infant sleep a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2018;172:e180739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catalá A. The function of very long chain polyunsaturated fatty acids in the pineal gland. Biochim Biophys Acta. 2010;1801:95–99. [DOI] [PubMed] [Google Scholar]
- 10. Zhang H, Hamilton JH, Salem JN, et al. N-3 fatty acid deficiency in the rat pineal gland: effects on phospholipid molecular species composition and endogenous levels of melatonin and lipoxygenase products. J Lipid Res. 1998;39:1397–1403. [PubMed] [Google Scholar]
- 11. Kim HY, Edsall L, Garcia M, et al. The release of polyunsaturated fatty acids and their lipoxygenation in the brain. Adv Exp Med Biol. 1999;447:75–85. [DOI] [PubMed] [Google Scholar]
- 12. Yehuda S, Rabinovitz S, Mostofsk DI.. Essential fatty acids and sleep: mini-review and hypothesis. Med Hypotheses. 1998;50:139–145. [DOI] [PubMed] [Google Scholar]
- 13. Dashti HS, Follis JL, Smith CE, et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jansen EC, Conroy DA, Burgess HJ, et al. Consumption of the long-chain fatty acid docosahexaenoic acid in relation to sleep timing and duration in adolescents: an actigraphy-based study in Mexico City. Sleep. 2019;42:A99–A100. [Google Scholar]
- 15. Del Brutto OH, Mera RM, Ha JE, et al. Dietary fish intake and sleep quality: a population-based study. Sleep Med. 2016;17:126–128. [DOI] [PubMed] [Google Scholar]
- 16. Zick SM, Colacino J, Cornellier M, et al. Fatigue reduction diet in breast cancer survivors: a pilot randomized clinical trial. Breast Cancer Res Treat. 2017;161:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jahangard L, Sadeghi A, Ahmadpanah M, et al. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders—results from a double-blind, randomized and placebo-controlled clinical trial. J Psychiatr Res. 2018;107:48–56. [DOI] [PubMed] [Google Scholar]
- 18. Montgomery P, Burton JR, Sewell RP, et al. Fatty acids and sleep in UK children: subjective and pilot objective sleep results from the DOLAB study—a randomized controlled trial. J Sleep Res. 2014;23:364–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yehuda S, Rabinovitz S, Mostofsky DI.. Mixture of essential fatty acids lowers test anxiety. Nutr Neurosci. 2005;8:265–267. [DOI] [PubMed] [Google Scholar]
- 20. Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41:zsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kay DB, Karim HT, Soehner AM, et al. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep. 2016;39:1779–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang F, Liu H, Wan Y, et al. Sleep duration and overweight/obesity in preschool-aged children: a prospective study of up to 48,922 children of the Jiaxing Birth Cohort. Sleep. 2016;39:2013–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jean-Louis G, Williams NJ, Sarpong D, et al. Associations between inadequate sleep and obesity in the US adult population: analysis of the national health interview survey (1977-2009). BMC Public Health. 2014;14:290–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J, Manousakis J, Fielding J, et al. Alcohol and sleep restriction combined reduces vigilant attention, whereas sleep restriction alone enhances distractibility. Sleep. 2015;38:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louca M, Short MA.. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep. 2014;37:1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Liu X, Ji X, et al. Sleep disordered breathing symptoms and daytime sleepiness are associated with emotional problems and poor school performance in children. Psychiatry Res. 2016;242:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Zhou G, Wang Y, et al. Sleep problems, fatigue, and cognitive performance in Chinese kindergarten children. J Pediatr. 2012;161:520–525.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Byars KC, Yolton K, Rausch J, et al. Prevalence, patterns, and persistence of sleep problems in the first 3 years of life. Pediatrics. 2012;129:e276–e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi M, Adachi M, Yasuda S, et al. Prevalence of sleep problems in Japanese preschoolers in a medium-sized city: community-based survey using the Children’s Sleep Habits Questionnaire. Pediatr Int. 2017;59:747–750. [DOI] [PubMed] [Google Scholar]
- 30. Spruyt K, O’Brien LM, Cluydts R, et al. Odds, prevalence and predictors of sleep problems in school‐age normal children. J Sleep Res. 2005;14:163–176. [DOI] [PubMed] [Google Scholar]
- 31. Fatima Y, Doi SAR, Najman JM, et al. Continuity of sleep problems from adolescence to young adulthood: results from a longitudinal study. Sleep Health. 2017;3:290–295. [DOI] [PubMed] [Google Scholar]
- 32. Sagayadevan V, Subramaniam M, Abdin E, et al. Prevalence and correlates of sleep problems among older Singaporeans. Sleep Med. 2015;16:S182–S182. [DOI] [PubMed] [Google Scholar]
- 33. Shrivastava D, Jung S, Saadat M, et al. How to interpret the results of a sleep study. J Commun Hosp Int Med Perspect. 2014;4:24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colten HR, Altevogt BM.. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 35. Smith DJ, Sarris J, Dowling N, et al. Adjunctive low-dose docosahexaenoic acid (DHA) for major depression: an open-label pilot trial. Nutr Neurosci. 2018;21:224–228. [DOI] [PubMed] [Google Scholar]
- 36. Guthrie KA, Larson JC, Ensrud KE, et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and self-reported sleep quality in women with hot flashes: a pooled analysis of individual participant data from four MsFLASH trials. Sleep. 2018;41:zsx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cornu C, Remontet L, Noel-Baron F, et al. A dietary supplement to improve the quality of sleep: a randomized placebo controlled trial. BMC Complement Altern Med. 2010;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati AF, Tetzlaff J,, Altman DG; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:1756–1833.doi:10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Oxford: Ottawa Hospital Research Institute; 2014.
- 41. Judge MP, Cong X, Harel O, et al. Maternal consumption of a DHA-containing functional food benefits infant sleep patterning: an early neurodevelopmental measure. Early Hum Dev. 2012;88:531–537. [DOI] [PubMed] [Google Scholar]
- 42. Cheruku SR, Montgomery-Downs HE, Farkas SL, et al. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–613. [DOI] [PubMed] [Google Scholar]
- 43. Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Method. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 44. Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 for vasomotor symptoms treatment: a randomized controlled trial. Menopause .2014;21:1–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reed SD, Guthrie KA, Newton KM, et al. Menopausal quality of life: RCT of yoga, exercise, and omega-3 supplements. Am J Obstet Gynecol. 2014;210:244.e241–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boone KM, Rausch J, Pelak G, et al. Docosahexaenoic acid and arachidonic acid supplementation and sleep in toddlers born preterm: secondary analysis of a randomized clinical trial. J Clin Sleep Med. 2019;15:1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dretsch MN, Johnston D, Bradley RS, et al. Effects of omega-3 fatty acid supplementation on neurocognitive functioning and mood in deployed U.S. soldiers: a pilot study. Military Med. 2014;179:396–403. [DOI] [PubMed] [Google Scholar]
- 48. Hansen AL, Dahl L, Olson G, et al. Fish consumption, sleep, daily functioning, and heart rate variability. J Clin Sleep Med. 2014;10:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Judge MP, Beck CT, Durham H, et al. Pilot trial evaluating maternal docosahexaenoic acid consumption during pregnancy: decreased postpartum depressive symptomatology. Int J Nurs Sci. 2014;1:339–345. [Google Scholar]
- 50. Hysing MKvestad IKjellevold M, . et al. Fatty fish intake and the effect on mental health and sleep in preschool children in FINS-KIDS, a randomized controlled trial. Nutrients 2018;10:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Doornbos B, van Goor SA, Dijck-Brouwer DA, et al. Supplementation of a low dose of DHA or DHA+AA does not prevent peripartum depressive symptoms in a small population based sample. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:49–52. [DOI] [PubMed] [Google Scholar]
- 52. Zornoza-Moreno M, Fuentes-Hernández S, Carrión V, et al. Is low docosahexaenoic acid associated with disturbed rhythms and neurodevelopment in offsprings of diabetic mothers? Eur J Clin Nutr. 2014;68:931–937. [DOI] [PubMed] [Google Scholar]
- 53. Kocevska D, Voortman T, Dashti HS, et al. Macronutrient intakes in infancy are associated with sleep duration in toddlerhood. J Nutr. 2016;146:1250–1256. [DOI] [PubMed] [Google Scholar]
- 54. Huss M, Völp A, Stauss-Grabo M.. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems—an observational cohort study. Lipids Health Dis. 2010;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Watanabe N, Matsuoka Y, Kumachi M, et al. Omega-3 fatty acids for a better mental state in working populations—Happy Nurse Project: a 52-week randomized controlled trial. J Psychiatr Res. 2018;102:72–80. [DOI] [PubMed] [Google Scholar]
- 56. Liu J, Cui Y, Li L, et al. The mediating role of sleep in the fish consumption—cognitive functioning relationship: a cohort study. Sci Rep. 2017;7:17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yehuda S, Rabinovitz-Shenkar S, Carasso RL.. Effects of essential fatty acids in iron deficient and sleep-disturbed attention deficit hyperactivity disorder (ADHD) children. Eur J Clin Nutr. 2011;65:1167–1169. [DOI] [PubMed] [Google Scholar]
- 58. Lotrich FE, Sears B, McNamara RK.. Polyunsaturated fatty acids moderate the effect of poor sleep on depression risk. Prostaglandins Leukot Essent Fatty Acids. 2016;106:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Christian LM, Blair LM, Porter K, et al. Polyunsaturated fatty acid (PUFA) status in pregnant women: associations with sleep quality, inflammation, and length of gestation. PLoS One. 2016;11:e0148752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ford PA, Jaceldo-Siegl K, Lee JW, et al. Trans fatty acid intake is related to emotional affect in the Adventist Health Study-2. Nutr Res. 2016;36:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thoman EB. Sleeping and waking states in infants: a functional perspective. Neurosci Biobehav Rev. 1990;14:93–107. [DOI] [PubMed] [Google Scholar]
- 62. Bat-Pitault F, Sesso G, Deruelle C, et al. Altered sleep architecture during the first months of life in infants born to depressed mothers. Sleep Med. 2017;30:195–203. [DOI] [PubMed] [Google Scholar]
- 63. Cheatham CL, Lupu DS, Niculescu MD.. Genetic and epigenetic transgenerational implications related to omega-3 fatty acids. Part II: maternal FADS2 rs174575 genotype and DNA methylation predict toddler cognitive performance. Nutr Res. 2015;35:948–955. [DOI] [PubMed] [Google Scholar]
- 64. Niculescu MD, Lupu DS, Craciunescu CN.. Maternal α-linolenic acid availability during gestation and lactation alters the postnatal hippocampal development in the mouse offspring. Int J Dev Neurosci. 2011;29:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Conway MC, McSorley EM, Mulhern MS, et al. Influence of fatty acid desaturase (FADS) genotype on maternal and child polyunsaturated fatty acids (PUFA) status and child health outcomes: a systematic review. Nutr Rev. 2020;78:627–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tang J, Yan Y, Zheng JS, et al. Association between erythrocyte membrane phospholipid fatty acids and sleep disturbance in Chinese children and adolescents. Nutrients. 2018;10:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Komada YNarisawa HUeda F, . et al. Relationship between self-reported dietary nutrient intake and self-reported sleep duration among Japanese adults. Nutrients 2017;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bennett CJ, Truby H, Zia Z, et al. Investigating the relationship between sleep and macronutrient intake in women of childbearing age. Eur J Clin Nutr. 2017;71:712–717. [DOI] [PubMed] [Google Scholar]
- 69. Zhang JY, Kothapalli KSD, Brenna JT.. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr Opin Clin Nutr Metab Care. 2016;19:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ji X, Grandner MA, Liu J.. The relationship between micronutrient status and sleep patterns: a systematic review. Public Health Nutr. Mar 2017;20:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Norouzi M, Hosseini B, Yaseri M, et al. The association between sleep pattern and nutrients intake pattern in healthy overweight and obese adults. Sleep Biol Rhythms. 2018;16:55–61. [Google Scholar]
- 72. Javadi M, Alimoradi F, Avani A, et al. Association between sleep quality and intake of macronutrients and micronutrients in adolescents. J Mazandaran Univ Med Sci. 2018;27:205–210. [Google Scholar]
- 73. Weatherly DG. Lighting and the circadian rhythm of night shift workers. ASME J Risk Uncertainty Part B. 2020;6(1):011007. doi:10.1115/1.4044788. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.