Recurrent high-grade gliomas are aggressive brain tumors with a poor prognosis. They remain an unmet medical need, in part because of an incomplete understanding of the immunosuppressive tumor microenvironment. Although immune checkpoint inhibitors targeting programmed death 1 (PD-1) may have a therapeutic role, their efficacy may be limited by a paucity of tumor-infiltrating lymphocytes. Recently, the Controlled IL-12 gene therapy system has shown promise in increasing glioma immunogenicity. This system stimulates local IL-12 production through a locally administered adenoviral vector, which delivers genetic information for IL-12 and a transcription switch. An orally administered activator ligand, veledimex (VDX), controls transcription levels. IL-12 activity leads to downstream production of IFN-γ and increases tumor immunogenicity.1 A recent phase 1 clinical trial showed that Controlled IL-12 therapy was associated with increased expression of PD-1 and PD-L1 in tumor-associated T-cell infiltrates in glioma.1 This supports the concept of investigating Controlled IL-12 in combination with immune checkpoint inhibitor therapy. Here, we describe the case of a patient with a recurrent grade 4 astrocytoma in which post-temozolomide (TMZ) DNA mismatch repair (MMR)-deficient tumor subclones disappeared following administration of Controlled IL-12 with PD-1 blockade. These data demonstrate that the immune system can be engaged to target high-grade glioma.
Case Report
This male patient’s disease course began when, at 18 years old, he developed seizures. Imaging revealed a left-sided cerebral lesion (outside institution, no other details available). He underwent resection at an outside institution, with a pathologic diagnosis of anaplastic astrocytoma, WHO grade 3.
Twenty months later, the patient underwent re-resection of a recurrent tumor, followed by proton beam therapy, then another resection of recurrent tumor 5 years thereafter. By the third resection, the tumor had progressed to astrocytoma, isocitrate dehydrogenase (IDH)-mutant, WHO grade 4. He was then treated with TMZ and the poly(ADP-ribose) polymerase inhibitor, pamiparib.
Fifteen months later, he had a third recurrence diagnosed radiologically (Supplementary Figure 1A and B) and was seen in consultation at Northwestern Memorial Hospital, where he enrolled in phase 2 clinical trial ATI001-204 (NCT 04006119). Within the context of this trial, he received a dose of the PD-1 antibody cemiplimab, followed 4 days later by a fourth surgical resection. This type of neoadjuvant approach has been described with other PD-1 antibodies.2,3 After bulk resection, the remaining tumor was injected with an adenoviral vector (Ad-RTS-hIL-12) for gene therapy used for regulated local IL-12 production. Immediately prior to that surgery, the patient had also received 20 mg of oral VDX, followed by postoperative once-daily doses of VDX for 14 days, as described previously.1 At the time of this fourth resection, the tumor contained numerous mutations, including inactivating mutations in DNA MMR genes MSH2, MSH6, and PMS2, suggestive of a hypermutator phenotype. The patient continued with treatment on-study, resuming cemiplimab on day 14 postop and continuing this on an every 3-week cycle. He tolerated therapy well, with only mild fatigue.
Two months later, imaging demonstrated the development of left frontal enhancement adjacent to the resection cavity, as well as a diffuse leptomeningeal enhancement (Supplementary Figure 1C and D). Cerebrospinal fluid (CSF) analysis showed no evidence of tumor spread to the CSF (cell count and cytology, not shown). The patient then underwent a fifth (and final) resection. Remarkably, after local IL-12 and anti-PD-1 combination therapy (1 preoperative dose and 3 postoperative doses), the previously detected alterations in MSH2, MSH6, and PMS2 were no longer detectable.
After the fifth resection, the patient was treated with radiation therapy, lomustine (CCNU), and pembrolizumab. Unfortunately, subsequent imaging again suggested tumor progression with concomitant worsening of cognition. Thus, pembrolizumab was discontinued, and the patient was treated with bevacizumab and everolimus, followed by temporary cognitive improvement. However, the tumor eventually progressed even further, with the patient developing severe vomiting that was alleviated with stereotactic radiation. The tumor soon progressed again and was unresponsive to trials of TMZ, everolimus, and carboplatin. He developed progressive motor and speech deterioration and passed away 13 months after his final resection. The total disease course, from the original diagnosis to death, was 9 years.
Pathology
Histologic examination of the original tumor showed a moderately cellular, infiltrating astrocytic tumor with increased mitotic activity, but no necrosis or microvascular proliferation. Data on IDH status were not available from that outside institution. The second resected tumor (first recurrence) was also not available for examination.
The third resected tumor (second recurrence) resembled the first tumor but had increased cellularity, as well as necrosis and microvascular proliferation. By immunohistochemistry (IHC), the third tumor was reportedly positive for IDH1-R132H and had a loss of ATRX expression.
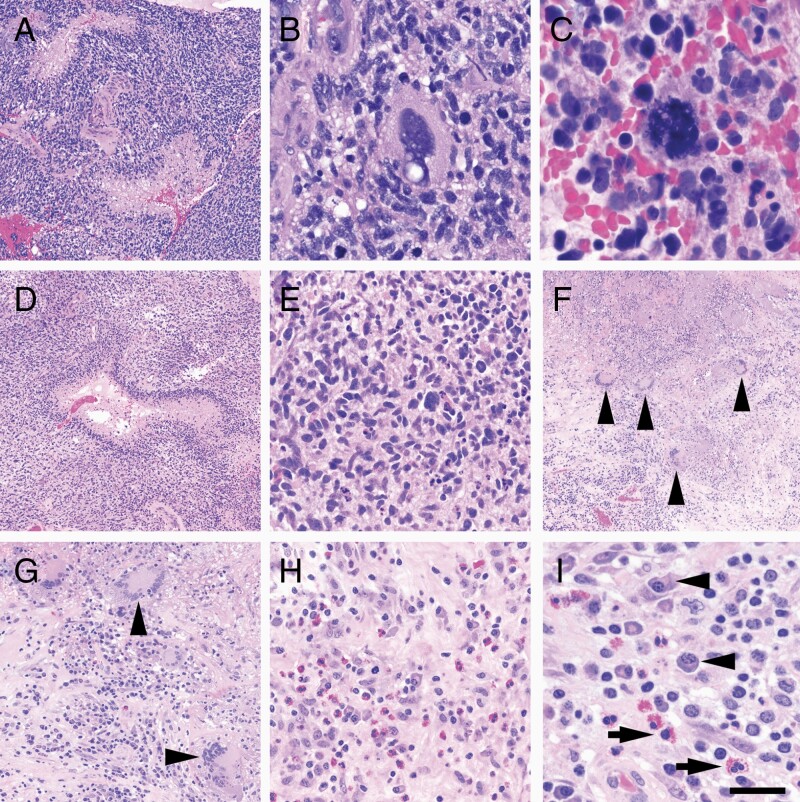
The fourth resected tumor (third recurrence, following a neoadjuvant dose of cemiplimab and prior to IL-12 adenoviral vector injection) still showed grade 4 features, including palisading necrosis (Figure 1A). Extreme nuclear atypia was present (Figure 1B), along with numerous atypical mitotic figures (Figure 1C). Intratumoral CD3+ T cells were relatively sparse, scored at 1.2 cells per mm2 (Supplementary Figure 2A). PD-L1 expression was 0.46 cells per mm2 (not shown).
Figure 1.
Histologic features of glioma, pre- and post-combination immunotherapy. Hypermutated glioma prior to immunotherapy: (A) hypercellular neoplasm with palisading necrosis. (B) Many cells showed extreme nuclear atypia. (C) Markedly atypical mitoses were also present. Recurrent glioma post-immunotherapy: (D) The majority of resected tissue showed features consistent with a recurrent tumor, including palisading necrosis. (E) Nuclear atypia was present but was not as severe as in the pre-immunotherapy sample, and atypical mitoses were not identified. (F) Approximately 20–30% of the resected tumor showed therapy-related necrosis and granulomas (arrowheads). (G) Granulomas were non-caseating, with numerous multinucleated giant cells (arrowheads), positive for CD68 and CD163 (not shown). (H and I) Accompanying the granulomas was a brisk mixed inflammatory infiltrate containing numerous plasma cells (I, arrowheads) and eosinophils (I, arrows). Length of scale bar in (I) is equivalent to 100 μm in (A), (D), and (F), 40 μm in (G), 20 μm in (B), (E), and (H), and 10 μm in (C).
The fifth resected tumor (fourth recurrence, post-cemiplimab, and Controlled IL-12 combination) continued to show widespread palisading necrosis (Figure 1D). However, unlike the fourth tumor, neither extreme nuclear atypia nor atypical mitotic figures were observed (Figure 1E). Unlike the previous specimens, therapy-related geographic necrosis was present, along with non-caseating granulomas, plasma cells, and eosinophils (Figure 1F–I). Intratumoral CD3+ T-cell infiltration was markedly increased relative to the fourth resection, at 13.3 cells per mm2 (Supplementary Figure 2B). PD-L1 expression was 0.31 cells per mm2 (not shown).
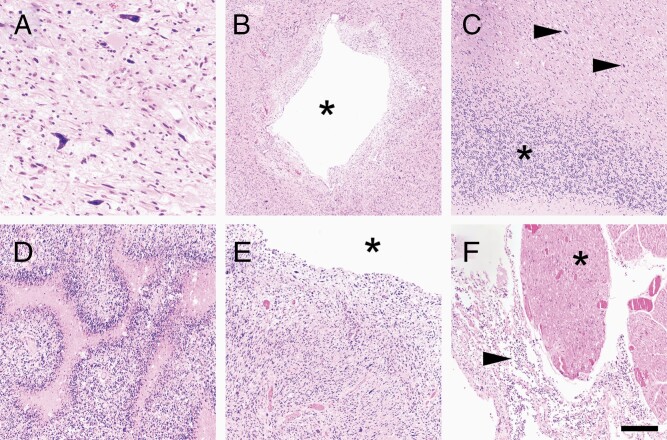
Postmortem analysis, performed 13 months after the fifth and final surgery, showed tumor grossly spreading throughout the brain, including the corpus callosum (Supplementary Figure 1E) and extending downward into the pons (Supplementary Figure 1F). On histologic examination, tumor cells were abundant near the original site, including highly atypical tumor cells (Figure 2A). Tumor was present in all sections examined, including down into the midbrain (Figure 2B), cerebellum (Figure 2C), pons (Figure 2D), and medulla (Figure 2E). Leptomeningeal dissemination was present all the way down into the spinal cord (Figure 2F). Intratumoral CD3+ T-cell infiltration was only 0.81 cells per mm2 (Supplementary Figure 2C).
Figure 2.
Postmortem findings. (A) Postmortem examination of the original tumor resection cavity showed abundant viable tumor cells, including markedly atypical cells, as was seen prior to immunotherapy. Granulomas, eosinophils, and plasma cells were no longer present anywhere in the brain. Tumor spread diffusely throughout all brain regions sampled, including the midbrain (B, asterisk = central aqueduct), cerebellum (C, asterisk = granular neurons, arrows = tumor cells), pons (D), and medulla (E, asterisk = fourth ventricle). Leptomeningeal dissemination was present, all the way down into the spinal cord, around the nerve rootlets in the thoracic region (F, arrow = tumor, asterisk = nerve rootlet). Scale bar in (F) = 80 μm in (A), 400 μm in (B), 200 μm in (C–F).
Molecular Diagnostics
In-house next-generation sequencing (NGS) of the fourth and fifth tumors showed the same mutations in IDH1, ATRX, and TP53, as well as a homozygous loss of CDKN2A/B and MGMT promoter methylation (Table 1). (Because the NGS panel covered only a very small portion of the total genome, tumor mutation burden data were not available.) However, while the fourth tumor sample (post-pamiparib, following a neoadjuvant dose of cemiplimab and prior to the IL-12 adenoviral vector injection) had inactivating alterations in the DNA MMR genes MSH2, MSH6, and PMS2 (hemizygous deletion), those alterations were not detectable in the fifth tumor sample (post-cemiplimab and Controlled IL-12 combination). Likewise, whereas the fourth tumor had many areas in which glioma cells lost expression of those 3 MMR proteins (Supplementary Figure 3A–C), the fifth tumor had far fewer malignant cells in which Msh2 and Msh6 proteins were still lost (Supplementary Figure 3D and E). Only Pms2 remained heterogeneously absent in both tumors (Supplementary Figure 3F).
Table 1.
Comparison of Key Mutations in Tumor Pre- and Post-Cemiplimab and Controlled IL-12 Therapies
| Fourth Resection (Neoadjuvant Cemiplimab and Pre-Controlled IL-12), % Allelic Fraction | Fifth Resection (Post-cemiplimab and Post-Controlled IL-12), % Allelic Fraction | Postmortem Tissue, % Allelic Fraction |
|---|---|---|
| IDH1 mutation, 49.75% | IDH1 mutation, 52.83% | IDH1 mutation, 23.00% |
| ATRX mutation, 92.31% | ATRX mutation, 94.9% | ATRX mutation, 54.00% |
| TP53 mutation, 92.45% | TP53 mutation, 91.08% | TP53 mutation, 48.00% |
| MSH2 mutation, 3.00% | No MSH2 mutation identified | No MSH2 mutation identified |
| MSH6 mutation, 7.19% | No MSH6 mutation identified | No MSH6 mutation identified |
| PMS2 mutation, 79.49% | No PMS2 mutation identified | No PMS2 mutation identified |
| PMS2 deletion | No PMS2 deletion identified | No PMS2 deletion identified |
| CDKN2A/B deletion | CDKN2A/B deletion | CDKN2A/B deletion |
NGS on postmortem tissue revealed the same mutations in IDH1, ATRX, and TP53, as well as deletion of CDKN2A/B. Again, no alterations in MSH2, MSH6, or PMS2 were identified (Table 1). However, those MMR proteins were only variably immunopositive in tumor cells (Supplementary Figure 3G–I).
Discussion
Immune checkpoint inhibitor therapy has improved survival in select groups of patients with hypermutated malignancies.4,5 Defects in DNA MMR genes, and loss of immunostaining of DNA MMR repair enzymes by immunohistochemistry, correspond well to hypermutation.6 In the current case, the MMR defects and hypermutation arose during the course of the disease, after exposure to TMZ. This glioma had 2 key features that have been associated with an increased likelihood of hypermutation after TMZ, IDH mutation and MGMT promoter methylation.6–8 Since MMR defects seem to confer TMZ resistance,8 the obvious inference is that TMZ favors the deleterious selection of MMR-deficient, hypermutated subclones, particularly in gliomas that are especially sensitive to TMZ because of IDH mutation and MGMT promoter methylation. However, our previous study showed that such “hypermutated” gliomas still retain subsets of tumor cells with intact MMR expression.6 This was confirmed by another group, in a different cohort of patients, using single-cell RNA sequencing.8 Together, these data suggest that most “hypermutated” gliomas are in fact heterogeneous (even after selective pressure by TMZ) and contain a mixture of MMR-deficient and MMR-intact subclones. In the current case, low-population subclones with MSH2 and MSH6 mutations were present in the fourth resection sample, following neoadjuvant (brief exposure) cemiplimab, but pre-Controlled IL-12 tumor specimen, but not in the fifth resection, post-cemiplimab, and Controlled IL-12 (Table 1). This suggests that combining Controlled IL-12 with systemic cemiplimab may have stimulated the immune system to preferentially target the MMR-deficient subclones. For glioma patients with germline DNA repair defects, where 100% of tumor cells are MMR-deficient, this type of combinatorial immunotherapeutic approach may be of even greater benefit.9
Of additional interest are the unusual histologic features of the fifth, post-cemiplimab, and IL-12 tumor sample, including numerous granulomas, plasma cells, and eosinophils (Figure 1). None of those features are common in gliomas, either before or after standard therapy. However, there have been many reports of systemic granulomatous disease associated with PD-1 blockade therapy in patients with advanced cancer.10 One patient on ipilimumab for metastatic melanoma even developed granulomas in the sella turcica.11 While the significance of plasma cells in the context of checkpoint inhibitors and Controlled IL-12 is unclear, eosinophilic inflammation has repeatedly been described in response to checkpoint inhibitors,12,13 and eosinophilia may correlate with treatment response.14
Postmortem examination and testing revealed 3 key findings. First, DNA MMR gene alterations were still undetectable on NGS (Table 1), even though MMR IHC was only positive in some cells (Supplementary Figure 3). Second, all the pro-inflammatory effects of the immunotherapy that had been observed in the fifth resection, including increased CD3+ T cells, granulomas, plasma cells, and eosinophils, had completely disappeared over the ensuing year (Figure 2 and Supplementary Figure 2). Third, the extensive infiltration of tumors into the brainstem is consistent with our previous study of end-stage gliomas, particularly those with a protracted disease course.15
In summary, this case demonstrates the potential therapeutic effect of immune checkpoint inhibitors in combination with Controlled IL-12 for recurrent high-grade glioma. An antitumor effect was noted, including immune-mediated selection against MMR-deficient subclones, and induction of inflammation rich in granulomas, eosinophils, and plasma cells.
Supplementary Material
Acknowledgments
Not applicable.
Funding
This work was supported in part by the National Institutes of Health grant R01NS102669, R01NS117104, and R01NS118039 (to C.H.), as well as by the Northwestern SPORE in Brain Cancer (P50CA221747). The clinical trial is funded by Ziopharm Oncology, Inc. Investigational Ad-RTS-hIL-12 + veledimex was supplied by Ziopharm, and cemiplimab was provided by Regeneron Pharmaceuticals, Inc.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Competing interests: N.D. and J.B. are employees of, and have equity ownership in, Ziopharm Oncology, Inc. A.G. is an employee of, and have equity ownership in, Bristol-Myers Squibb and former employee of, and has equity ownership in, Ziopharm Oncology, Inc. The other authors declare that they have no competing interests.
Authorship Statement. M.M., R.V.L., C.H., and A.G. prepared the text and figures.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
References
- 1. Chiocca EA, Yu JS, Lukas RV, et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci Transl Med. 2019;11(505). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019;25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 4. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lukas RV, Rodon J, Becker K, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol. 2018;140(2):317–328. [DOI] [PubMed] [Google Scholar]
- 6. McCord M, Steffens A, Javier R, Kam KL, McCortney K, Horbinski C. The efficacy of DNA mismatch repair enzyme immunohistochemistry as a screening test for hypermutated gliomas. Acta Neuropathol Commun. 2020;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang J, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouffet E, Larouche V, Campbell BB, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol. 2016;34(19):2206–2211. [DOI] [PubMed] [Google Scholar]
- 10. Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy KP, Kennedy MP, Barry JE, O’Regan KN, Power DG. New-onset mediastinal and central nervous system sarcoidosis in a patient with metastatic melanoma undergoing CTLA4 monoclonal antibody treatment. Oncol Res Treat. 2014;37(6):351–353. [DOI] [PubMed] [Google Scholar]
- 12. Bui AN, Nelson CA, Lian CG, Canales AL, LeBoeuf NR. Eosinophilic fasciitis induced by nivolumab therapy managed without treatment interruption or systemic immunosuppression. JAAD Case Rep. 2020;6(8):693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watanabe H, Asada K, Shirai T, Torii H, Yoshimura K, Kusafuka K. Eosinophilic airway inflammation and eosinophilic chronic rhinosinusitis during nivolumab and ipilimumab. Respirol Case Rep. 2020;8(7):e00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohashi H, Takeuchi S, Miyagaki T, Kadono T. Increase of lymphocytes and eosinophils, and decrease of neutrophils at an early stage of anti-PD-1 antibody treatment is a favorable sign for advanced malignant melanoma. Drug Discov Ther. 2020;14(3):117–121. [DOI] [PubMed] [Google Scholar]
- 15. Drumm MR, Dixit KS, Grimm S, et al. Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas. Neuro Oncol. 2020;22(4):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.