Introduction
Bruton’s tyrosine kinase (BTK) plays a pivotal role in B-cell proliferation and survival of leukemic cells. Ibrutinib, a molecule targeting BTK, was approved by the U.S. Food and Drug Administration in 2013 for treatment of mantle cell lymphoma and has since been approved for treatment of several other hematologic malignancies, including chronic lymphocytic leukemia (CLL), Waldenström macroglobulinemia, and marginal zone lymphoma (MZL) (1). Recently, Ibrutinib plus venetoclax have been found to be effective as first line treatment for high risk and older patients with CLL (2). Patients receiving ibrutinib are at increased risk for serious bacterial, fungal, and viral reactivation (3).
In this report, we describe the first case, to our knowledge, of HBV-associated liver failure after ibrutinib therapy in a patient with B-cell non-Hodgkin lymphoma. Additionally, we review the previously reported cases of HBV reactivation after ibrutinib therapy (3–7). We analyzed our patient reported below together with 5 of the 6 previously described patients for whom sufficient data about HBV reactivation were available.
HBV-related outcomes were defined according to the American Association for the Study of Liver Diseases (AASLD) 2018 hepatitis B guidance (8), and included past or chronic HBV infection, HBV reactivation, HBV-associated hepatitis, and HBV-associated liver failure
Case
A 68-year-old man who was diagnosed with MZL in March 2016. In 2014, he had been diagnosed with diffuse large B-cell lymphoma at another institution, had been treated with a rituximab-based regimen, and had achieved a complete remission. In 2015, he had been treated at another institution with sofosbuvir and ribavirin for chronic hepatitis C virus (HCV) infection and had achieved a sustained virologic response at week 24 without antivirals, regarded as virologic cure of HCV.
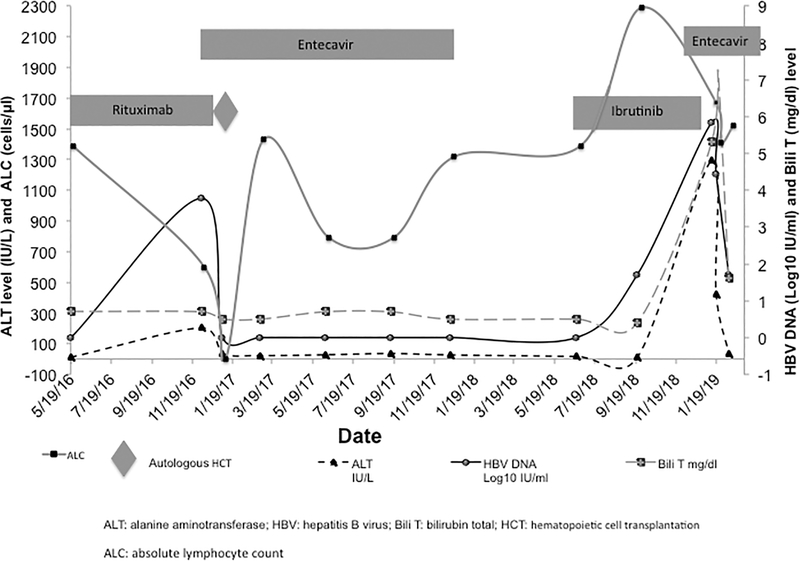
After the diagnosis of MZL at our center in 2016, HBV screening showed that the patient had past HBV infection: test results were positive for antibody to hepatitis B core antigen (anti-HBc), negative for antibody to hepatitis B surface antigen (anti-HBs), negative for hepatitis B surface antigen (HBsAg), and negative for hepatitis Be antigen, and HBV DNA was undetectable. Bendamustine and rituximab were initiated for treatment of MZL. The patient refused anti-HBV prophylaxis, and he was closely monitored for viral reactivation. In December 2016, while receiving chemotherapy, he developed HBV reactivation with reverse seroconversion without liver failure (Figure 1). Bendamustine and rituximab were stopped, and the patient was started on entecavir 0.5 mg orally daily. In early January 2017, HBV DNA became undetectable, and the patient underwent an autologous hematopoietic cell transplant (HCT) with conditioning chemotherapeutic regimen consisting of carmustine, etoposide, cytarabine, melphalan, and rituximab. HBV DNA remained undetectable during entecavir treatment. Entecavir was stopped in December 2017, 12 months after the last rituximab dose. Upon stopping entecavir, his serologic profile remained negative for HBsAg and HBsAb.
Figure 1.
Changes in alanine aminotransferase, bilirubin, and hepatitis B viral load levels after cancer therapy in a patient with lymphoma and hepatitis B virus—associated liver failure. Labels on the x-axis indicate calendar dates in month/date/year format. ALT, alanine aminotransferase; Bili T, total bilirubin; HBV, hepatitis B virus; HCT, hematopoietic cell transplant.
In June 2018, 17 months after autologous HCT and the last dose of rituximab (used in the conditioning regimen), the patient had a relapse of MZL. Salvage therapy with ibrutinib (560 mg orally daily) was initiated in July 2018, and the patient was closely monitored every 3 months for HBV reactivation consisted of clinical evaluation, liver function test and HBV DNA (Figure 1).
In January 2019, 6 months after initiation of ibrutinib, 13 months after the last dose of entecavir, and 24 months after autologous HCT and the last dose of rituximab, the patient was hospitalized with fatigue, anorexia, pruritus, right upper quadrant pain, and jaundice. Laboratory values were as follows: alanine aminotransferase, 1,293 IU/L; aspartate aminotransferase, 872 IU/L; alkaline phosphatase, 237 IU/L; total bilirubin, 5.3 mg/dL; direct bilirubin, 4.4 mg/dL; absolute lymphocyte count, 1670 cells/μL; and absolute neutrophil count, 3,510/μL. Further testing revealed HBsAg positivity and HBV DNA level 674,000 IU/mL (5.83 log10 IU/mL). Other causes of liver failure were excluded, including infectious [herpes simplex virus, hepatitis A virus, HCV, hepatitis D virus, hepatitis E virus, cytomegalovirus, adenovirus, and bacterial and fungal infections] and noninfectious causes (drugs, biliary tract or hepatic veno-occlusive diseases). HBV reactivation (reverse seroconversion) and liver failure (total bilirubin level 5.3 mg/dl) were diagnosed, and entecavir 0.5 mg daily was initiated, resulting in normalization of liver function tests and reduction of HBV DNA viral load (Figure 1). Entecavir resistance mutations were not identified.
Discussion
To our knowledge, this is the first reported case of a patient with non-Hodgkin lymphoma who developed HBV-associated liver failure after anti-cancer therapy most recently with ibrutinib. In light of our case, life-threatening HBV reactivation could be considered to be one of the potential infectious complications of ibrutinib.
Six patients with HBV reactivation related to ibrutinib have been reported (Table 1). All 6 patients were men and 5 had CLL, and most (5, or 83%) had past HBV infection (Table 1). Only 1 patient had occult HBV infection (5). Three patients developed HBV-associated hepatitis, which in 2 of them progressed to liver failure (4,5). HBV reactivation was diagnosed at a median of 9.7 months (range, 1.5–42) after the initial dose of ibrutinib, and in 4 patients, reactivation was diagnosed at least 6 months after initiation of ibrutinib. Four patients with HBV reactivation had detectable anti-HBs at baseline (median, 51.7 IU/L; range, 10–85 IU/L). Four patients were pretreated with rituximab (anti-CD20). In all 4 of these patients, rituximab treatment was completed at least 24 months before HBV reactivation. Two of the 4 patients on rituximab received anti-HBV prophylaxis that was stopped 12 months after the completion of therapy; the other 2 patients were followed with HBV monitoring.
Table 1.
Cases of HBV reactivation in patients treated with ibrutinib
| Ref | Age, yr | Sex | Cancer | Chemoa | Anti-CD20 | Ibrutinib dose, mg/d | Months from initial ibrutinib dose to reactivation | Baseline | Post-ibrutinib | HBV-associated hepatitisc | HBV-associated liver failure | Rxd | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-HBs | Anti-HBc | HBs Ag | HBV DNA, log10 IU/mL | HBsAg | Anti-HBs | HBV DNA, log10 IU/mL | |||||||||||
| 3 | 79 | M | CLL | No | No | 420 | 13.5 | + | + | − | − | N/A | − | 6.27 | Yes | Yes | TDF |
| 4 | 80 | M | CLL | Yes | No | 280 | 5 | + | + | − | 2.62 | + | + | 7.36 | Yes | No | ETV |
| 5 | 57 | M | CLL | No | Yesb | N/A | 42 | + | + | − | − | − | − | 2.96 | No | No | No |
| 5 | 75 | M | CLL | No | Yesb | N/A | 31 | + | + | − | − | + | N/A | 8.23 | No | No | ETV |
| 6 | 59 | M | CLL | No | Yesb | N/A | 1.5 | − | + | − | − | N/A | − | 2.86 | No | No | ETV |
| Current case | 68 | M | MZL | No | Yesb | 560 | 6 | − | + | − | − | + | − | 5.83 | Yes | Yes | ETV |
Anti-HBc, hepatitis B core antibody; Anti-HBs, hepatitis B surface antibody; Chemo, chemotherapy; CLL, chronic lymphocytic leukemia; ETV, entecavir; F, female; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; M, male; MZL, marginal zone lymphoma; N/A, not available; Ref, reference; Rx, treatment; TDF, tenofovir disoproxil fumarate; +, positive; −, negative.
Received within 12 months of HBV reactivation.
Anti-CD20 (rituximab) therapy was completed at least 24 months HBV reactivation in all cases.
ALT with median of 103 and range of 70–987 lU/l. We reported the peak values of ALT and HBV DNA.
All patients recovered after treatment.
None of the 6 patients treated with ibrutinib were receiving anti-HBV prophylaxis. However, 5 (83%) were started on anti-HBV treatment at the first sign of viral reactivation. Four patients were treated with entecavir and 1, with tenofovir disoproxil fumarate. The patient not started on anti-HBV treatment was monitored closely as he had a normal alanine aminotransferase level and a low viral load (HBV DNA, 920 IU/mL, log10 2.96 IU/mL) (6). All 5 patients treated with antivirals recovered from HBV reactivation and achieved undetectable viral load. Ibrutinib was successfully resumed in 3 of the 6 patients, including 2 with HBV-associated hepatitis. Ibrutinib was never discontinued in the patient who was only monitored. Ibrutinib was stopped 6 weeks before the HBV reactivation occurs in one patient and 22 months in the other patient (4,6). No patient died of HBV reactivation or associated outcomes after ibrutinib therapy.
Our patient had other potential risk factors besides ibrutinib that could contribute to HBV reactivation, including the use direct-acting antivirals for HCV, autologous HCT, and previous rituximab. HBV reactivation in HBV/HCV co-infected patients has been reported during direct-acting antiviral therapy, but not 36 months after such therapy, as occurred in our case (9). As for chemotherapy and HCT, the period of 24 months between chemotherapy with autologous HCT and onset of HBV reactivation makes these therapeutic interventions a less likely sole contributor to reactivation. Most cases of rituximab-associated HBV reactivation occur within 12 months after such treatment. Rituximab-induced profound lymphopenia can lead to HBV reactivation; however, our patient was not lymphopenic at the time of reactivation. All predictors considered, the temporal association between ibrutinib therapy and HBV reactivation supports that ibrutinib is a likely contributing cause of the HBV reactivation in our case; however, the long-term immunosuppressive effects of chemotherapy and HCT would also have contributed to the HBV-related outcome observed.
The exact mechanism by which ibrutinib leads to HBV reactivation is likely more complex than simple B-cell depletion, especially in the absence of lymphopenia. Probably the mechanism is related to the gradual decline in protective immunity (anti-HBs) and/or modulation of B cell—T cell interactions similar to the one induced by rituximab (10,11). In addition to secreting antibodies, B cells play a crucial role in activating T cells. Hence, inhibition of B cells affects both cell-mediated and humoral immunity (12). Ibrutinib also reduces the secretion of immunoglobulin G in CLL patients (13).
In HBsAg-negative and anti-HBc—positive patients, anti-HBV prophylaxis is recommended for at least 12 months after completion of anti-CD20 therapies (8) because such therapies increase the risk of reactivation in patients with past or chronic HBV infection (14,15). In our analysis of ibrutinib-related HBV reactivation, 2 of 4 patients receiving rituximab received also anti-HBV prophylaxis, and in both, anti-HBV prophylaxis was completed 12 months after rituximab therapy. It remains unknown if anti-HBV prophylaxis should be extended beyond 12 months after rituximab when the use of ibrutinib is considered, as a more prolonged or severe B-cell depletion cannot be completely excluded and remote rituximab therapy may act synergistically to increase the risk of HBV reactivation in patients treated with ibrutinib. Further research is needed to determine the risk of HBV reactivation in patients receiving second-generation BTK inhibitors such as acalabrutinib. However, from a practical standpoint, and as is the case with rituximab, ibrutinib therapy can be safely continued if HBV is appropriately monitored.
Conclusion
Life-threatening HBV reactivation can occur following ibrutinib therapy in patients with past or chronic HBV infection. As the clinical indications for ibrutinib therapy may evolve, the number of patients at risk for HBV-associated liver failure after ibrutinib may increase. Patients to be started on ibrutinib could be considered for HBV screening. At this time, data are insufficient to support a recommendation of universal anti-HBV prophylaxis for patients with past or chronic HBV infection treated with ibrutinib, but physicians should be aware of the potential risk of HBV reactivation in these patients. Large studies are needed to define the risk of reactivation in patients receiving BTK inhibitors and optimal management strategies to prevent HBV reactivation.
Clinical Practice Points:
Hepatitis B virus (HBV) reactivation is seen after hematopoietic cell transplantation or following therapy with monoclonal anti-CD20 antibody (rituximab) in patients with past or chronic HBV infection. HBV reactivation can be associated with hepatitis, liver failure, and death.
The current case describes a patient with B-cell non-Hodgkin lymphoma who received anti-cancer treatment with ibrutinib as the most recent agent and subsequently developed HBV—associated liver failure.
As the clinical indications for ibrutinib therapy are evolving, the number of patients at risk for HBV-associated liver failure may increase. Clinicians are encouraged to monitor for reactivation in patients receiving multiple anticancer therapies, including ibrutinib.
Acknowledgments
The authors thank Stephanie Deming of the Department of Scientific Publications at MD Anderson for editorial assistance.
Conflict of Interest
Dr Torres is or has been the principal investigator for research grants from Gilead Sciences and Merck & Co., Inc., with all funds paid to MD Anderson. He also is or has been a paid scientific advisor for AbbVie, Inc., Gilead Sciences, Merck & Co., Inc., and Dynavax Technologies. The terms of these arrangements are managed by MD Anderson in accordance with its conflicts of interest policies. Dr Hwang has received research funding from Merck & Co., Inc., and Gilead Sciences. All other authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain N, Keating M, Thompson P, et al. Ibrutinib and Venetoclax for First-Line Treatment of CLL. N Engl J Med. 2019;380(22):2095–2103. [DOI] [PubMed] [Google Scholar]
- 3.Varughese T, Taur Y, Cohen N, et al. Serious Infections in Patients Receiving Ibrutinib for Treatment of Lymphoid Cancer. Clin Infect Dis. 2018;67(5):687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herishanu Y, Katchman H, Polliack A. Severe hepatitis B virus reactivation related to ibrutinib monotherapy. Ann Hematol. 2017;96(4):689–690. [DOI] [PubMed] [Google Scholar]
- 5.de Jésus Ngoma P, Kabamba B, Dahlqvist G, et al. Occult HBV reactivation induced by ibrutinib treatment: a case report. Acta Gastroenterol Belg. 2015;78(4):424–426. [PubMed] [Google Scholar]
- 6.Hammond SP, Chen K, Pandit A, Davids MS, Issa NC, Marty FM. Risk of hepatitis B virus reactivation in patients treated with ibrutinib. Blood. 2018;131(17):1987–1989. [DOI] [PubMed] [Google Scholar]
- 7.Innocenti I, Morelli F, Autore F, et al. HBV reactivation in CLL patients with occult HBV infection treated with ibrutinib without viral prophylaxis. Leuk Lymphoma. February 2019:1–3. [DOI] [PubMed] [Google Scholar]
- 8.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mücke MM, Mücke VT, Peiffer K-H, et al. Absence of HBV Reactivation in Patients With Resolved HBV Infection Following DAA Therapy for Hepatitis C: A 1-Year Follow-up Study. Open forum Infect Dis. 2019;6(1):ofy340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouaziz J-D, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104(52):20878–20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110(8):2924–2930. [DOI] [PubMed] [Google Scholar]
- 12.Mikulska M, Lanini S, Gudiol C, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin Microbiol Infect. 2018;24:S71–S82. [DOI] [PubMed] [Google Scholar]
- 13.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126(19):2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozessohn L, Chan KKW, Feld JJ, Hicks LK. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. 2015. [DOI] [PubMed]
- 15.Tang Z, Li X, Wu S, et al. Risk of hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA after treatment with rituximab for lymphoma: a meta-analysis. Hepatol Int. 2017;11(5):429–433. [DOI] [PubMed] [Google Scholar]