Abstract
Tuberculosis (TB), which is caused by Mycobacterium tuberculosis (MTB), is a serious infectious disease with high infection and mortality rates and is a public health problem around the world. According to the World Health Organization (WHO) report, one-third of the world's population is latently infected with MTB, and 5 to 10% of those with latent TB infection (LTBI) have the potential to develop active TB once in their lifetime. Therefore, TB management for promptly distinguishing LTBI from active TB and for proper treatment is important. LTBI is currently diagnosed using the tuberculin skin test (TST) and interferon gamma (IFN-γ) release assay (IGRA). However, this test is substantially limited by its inability to distinguish active TB from LTBI. It is necessary to discover indicators that can be used for effective TB management and to develop diagnostic methods. In the present study, we used IGRA and complete blood count (CBC) analysis for discrimination of active TB, LTBI, and healthy control groups. The results showed that the number of WBC was significantly increased in the group with active TB (p < 0.0100) and level of hemoglobin (Hb) was significantly decreased (p < 0.0010) in the CBC than those of the healthy control and LTBI groups. In the WBC differential count, the number of neutrophils and monocytes were increased (p < 0.0010) in active TB group, where as those of lymphocytes were significantly decreased (p < 0.0100) in active TB group compared healthy control group. Results verified that the levels of total WBC, Hb, neutrophils, lymphocytes and monocytes were statistically significant (p < 0.0500) and the AUC was approximately 0.8613. In addition, receiver operating characteristic (ROC) curve analysis was performed to confirm the clinical usefulness between active TB and healthy control groups. In conclusion, based on these data demonstrated that the usefulness of these potential indicators for differential diagnosis, according to the result can be provided for effective diagnosis and treatment by comparing the expression patterns of the markers in the whole blood of the active TB, LTBI, and healthy control groups. Furthermore, this study needs to investigate a larger number of clinical specimens later to develop biomarkers according to the state of infection with MTB such as LTBI and active TB, as well as after treatment.
Keywords: Active tuberculosis, Latent tuberculosis infection, Complete blood count, White blood cell differential count, Biomarker
1. Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (MTB) represents a major global public health problem [1], that cause high infection and mortality rates and constitute one of the most serious infectious disease [2]. Although TB in the Republic of Korea is on the decline, the country’s TB incidence and mortality rates in 2020 were 49.4 and 3.3 per 100,000 people, respectively, which was the highest among Organization for Economic Cooperation and Development (OECD) countries [3], [4]. To date, almost one third of the world’s population has LTBI, about 10% of whom progress to active TB during their lifetimes [5]. Co-infection of MTB and human immunodeficiency virus (HIV) promotes a serious condition, and people with HIV are known to be easily infected with mycobacteria [6].
Recent diagnostic methods for LTBI have used the tuberculin skin test (TST) or interferon gamma (IFN-γ) release assay (IGRA) with chest X-ray (CXR), physical examination, TB exposure, and medical history data [7]. TST is based on intradermal injection of whole MTB-antigens and subsequent confirmation of the delayed type hypersensitivity at the injection site [8]. However, TST is affected by bacillus Calmette-Guérin (BCG) vaccination, nontuberculous mycobacteria (NTM) infections, and immunosuppression-with low sensitivity, resulting in false-negative or false-positive results [9]. IGRA is a whole blood test of cell-mediated immune responses and is used for the measurement of IFN-γ released from CD4+ T-lymphocytes exposed to the MTB-antigens, a test faster and more sensitive than TST [10]. However, both TST and IGRA tests are incapable of distinguishing active TB from LTBI [11]. Therefore, it is necessary to distinguish these disease states and make a rapid examination. Recently, various studies have been conducted to distinguish between active TB, LTBI, and non-infected (healthy) populations using peripheral whole blood easy to collect, screening and identifying biomarkers in serum or plasma is an effective way to diagnose the disease [12].
Technological high-throughput screening has evolved rapidly and offers an extensive platform for TB research and biomarker discovery [12]. Recent studies have also reported a specific correlation between total white blood cell (WBC) count in diagnosing active TB [13]. In another study, when MTB is infected, circulating immune cells become active and granulocytes, lymphocytes, and macrophages interact to inhibit the growth of MTB in the host [14]. Routine testing with both TST and IGRA tests currently being conducted to diagnose active TB and LTBI are limited [15]. In order to differentiate active TB from LTBI, assistive biomarkers need to be developed and effective TB diagnosis, treatment and management are needed.
In the present study, combination of IGRA and complete blood count (CBC) analysis were investigated for discrimination of active TB, LTBI, and healthy control groups with a total of 126 whole blood and serum samples.
2. Materials and methods
2.1. Clinical samples
A total of 126 human whole blood samples were obtained from April 2018 to March 2019 at the Department of Laboratory Medicine, Good Samsun Hospital, Busan, the Republic of Korea. The present study was approved by the Institutional Review Board (IRB) of the Catholic University of Pusan (IRB Approval No.: CUP IRB-2019-01-010) (Table 1).
Table 1.
Demographic and clinical characteristics of study subjects.
| Demographic and clinical characteristics | Active TB | LTBI | Healthy control |
|---|---|---|---|
| Total number | 22 | 29 | 58 |
| Median age (range), years | 55.2 (23–89) | 44.6 (21–70) | 33.2 (22–61) |
| Gender, male/female | 15/7 | 6/23 | 12/46 |
| AFB stain results | |||
| + positive, n (%) | 2 (9.1) | NA | NA |
| ++ positive, n (%) | 4 (18.2) | NA | NA |
| +++ positive, n (%) | 4 (18.2) | NA | NA |
| ++++ positive, n (%) | 4 (18.2) | NA | NA |
| Negative | 8 (36.4) | NA | NA |
| AFB culture results | |||
| Positive, n (%) | 19 (86.4) | NA | NA |
| Negative, n (%) | 3 (13.6) | NA | NA |
| MTB-PCR results | |||
| Positive, n (%) | 21 (95.5) | NA | NA |
| Negative, n (%) | 1 (4.5) | NA | NA |
| CXR | |||
| Positive, n (%) | 22 (100.0) | 4 (13.8) | 0 (0) |
| Negative, n (%) | 0 (0) | 25 (86.2) | 58 (100.0) |
| IGRA test results | |||
| Positive, n (%) | NA | 29 (100.0) | 0 (0) |
| Negative, n (%) | NA | 0 (0) | 58 (100.0) |
| General characteristics of the groups involved in the study showing the number of subjects per group (n), the mean age, gender, AFB stain results, AFB culture results, MTB-PCR results, chest X-ray (CXR) results; n: number. Negative: Non-infected healthy group; LTBI: Latent tuberculosis infection group; Active TB: Active pulmonary tuberculosis group, NA: not applied. | |||
2.2. Whole blood collection
Whole blood samples were collected using a VACUETTE® EDTA (Greiner Bio-One, Frickenhausen, Austria) blood collection tube containing EDTA anticoagulant used for the CBC and WBC differential count test. Whole blood was tested within 4 hr of collection.
2.3. Complete blood count and WBC differential count analysis
The automated hematology analyzer XN-1000™ (Sysmex Corp., Kobe, Japan) was used for the CBC count containing total WBC count, red blood cell (RBC) count, hemoglobin (Hb) concentration, and platelet (PLT) count and the WBC differential count containing neutrophils, lymphocytes, monocytes, eosinophils, and basophils. White cell nucleated channel (WNR) was used for the CBC and white cell differential channel (WDF) was used for the WBC differential count. The analyzer and sampler checked the ready status. The rack was then placed in the sampler pool and the test proceeded. For the data interpretation, XN_Software v. UR (Sysmex) was used.
2.4. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Differences in CBC and WBC differential counts and APP markers among the active TB, LTBI, and the healthy control groups were statistically analyzed, and 95% confidential intervals were also calculated. An unpaired t-test was used to compare these three groups. Additionally, a receiver operator characteristic (ROC) curve analysis was conducted to confirm the clinical usefulness and to determine the cut-off value, specificity, and sensitivity of the assays. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Comparison of complete blood count analysis results among the active TB, LTBI, and healthy control groups
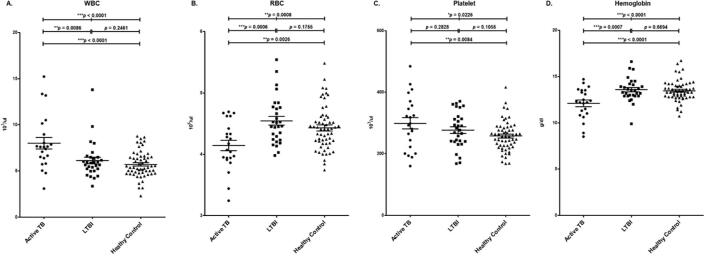
Based on the CBC analysis, the mean total WBC counts were 8.01 ± 2.94 (1 × 103/μL) for TB group, 6.13 ± 1.96 (1 × 103/μL) for LTBI group, and 5.70 ± 1.41 (1 × 103/μL) for healthy control group, respectively. The mean RBC counts were 4.14 ± 0.40 (1 × 106/μL) for the active TB group, 4.54 ± 0.38 (1 × 106/μL) for LTBI group, and 4.40 ± 0.36 (1 × 106/μL) for healthy control group, respectively. The mean PLT counts were 298.80 ± 86.65 (1 × 103/μL) for TB group, 276.72 ± 58.56 (1 × 103/μL) for LTBI group, and 257.30 ± 48.57 (1 × 103/μL) for healthy control group, respectively. The mean Hb values were 12.10 ± 1.66 g/dL for TB group, 13.60 ± 1.27 g/dL for LTBI group, and 13.50 ± 1.14 g/dL for healthy control group, respectively. Compared to the LTBI and healthy control groups, the mean WBC and PLT values of the active TB were significantly higher, and its RBC and Hb mean values were significantly lower (Table 2, Fig. 1).
Table 2.
Complete blood cell count analysis results between the active TB, LTBI, and healthy control groups.
| Cell Type | Acitve TB, mean count ± SD (range) | LTBI, mean count ± SD (range) | Healthy control, mean count ± SD (range) |
|---|---|---|---|
| White blood cell (1 × 103/μL) | 8.01 ± 2.94 (3.09–15.22) | 6.13 ± 1.96 (3.34–13.81) | 5.70 ± 1.41 (2.29–8.75) |
| Red blood cell (1 × 106/μL) | 4.14 ± 0.40 (3.24–4.69) | 4.54 ± 0.38 (3.98–5.54) | 4.40 ± 0.36 (3.74–5.48) |
| Platelet (1 × 103/μL) | 298.80 ± 86.65 86.65(160.00–484.00) | 276.72 ± 58.56 (168.00–371.00) | 257.30 ± 48.57 (168.00–416.00) |
| Hemoglobin (g/dL) | 12.10 ± 1.66 (8.50–14.70) | 13.58 ± 1.27 (9.90–16.60) | 13.50 ± 1.14 (10.70–16.70) |
SD: Standard Deviation.
Fig. 1.
Comparison of complete blood cell count analysis results between the active TB, LTBI, and healthy control groups A. White blood cell (WBC), B. Red blood cell (RBC), C. Hemoglobin (Hb), D. Platelet (PLT).
The active TB and LTBI groups were statistically significant with a p values of 0.0086 in WBC, 0.0006 in RBC, and 0.0007 in Hb. There were no statistically significant differences in WBC, RBC, Hb, or PLT between the LTBI and healthy control groups. The active TB and healthy control groups were statistically significant with p values under 0.0001 in WBC, 0.0026 in RBC, under 0.0001 in Hb, and 0.0084 in PLT. All three groups had p values under 0.0001 in WBC, 0.0008 in RBC, under 0.0001 in Hb and 0.0226 in PLT, all of which were statistically significant (Table 3).
Table 3.
Statistical data of complete blood cell count analysis between the active TB, LTBI, and healthy control groups.
| Cell type | Active TB vs. LTBI | LTBI vs. healthy control | Active TB vs. healthy control | Active TB vs. LTBI vs. healthy control |
|---|---|---|---|---|
| White blood cell | 0.0086** | 0.2461 | < 0.0001*** | < 0.0001*** |
| Red blood cell | 0.0006*** | 0.1755 | 0.0026** | 0.0008*** |
| Platelet | 0.2828 | 0.1055 | 0.0084** | 0.0226* |
| Hemoglobin | 0.0007*** | 0.6694 | < 0.0001*** | < 0.0001*** |
p < 0.0500; **p < 0.0100; ***p < 0.0010.
3.2. ROC curve analysis based on results of complete blood count analysis
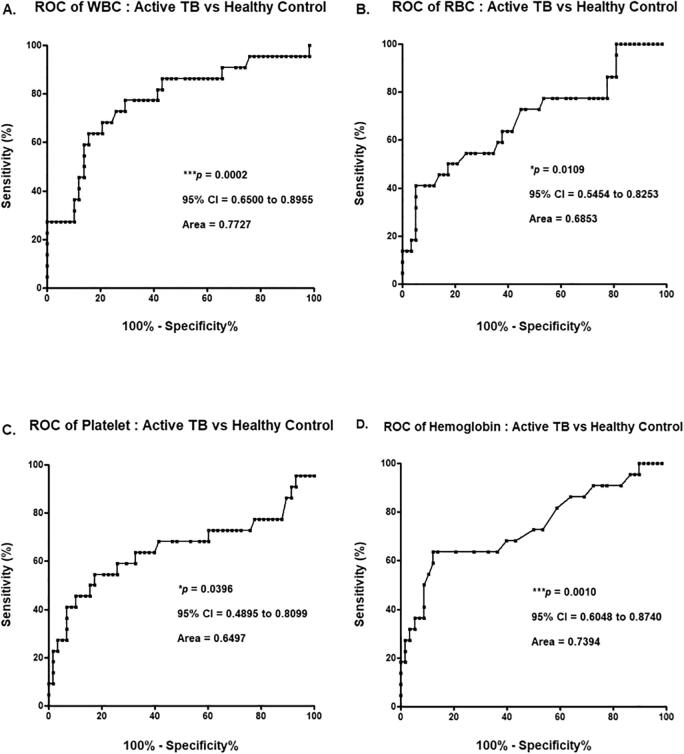
ROC curve analysis was performed to ensure that results from the CBC analysis were clinically applicable. The p value of the ROC curve for the total WBC count was 0.0002, and the area under the curve (AUC) was 0.7727. The p value of the ROC curve for the RBC count was 0.0109, and the AUC was 0.6853. The p value of the ROC curve for the PLT count was 0.0396, and the AUC was 0.6497. The p value of the ROC curve for the Hb concentration was 0.0010, and the AUC was 0.7394. Of the CBC analysis results, the p values of WBC, RBC, PLT counts, and Hb concentration were statistically significant (p < 0.0500), and the AUC was approximately 0.7117 (Fig. 2).
Fig. 2.
ROC curve analysis based on results of complete blood cell count analysis between the acitve TB and healthy control groups A. White blood cell (WBC), B. Red blood cell (RBC), C. Platelet (PLT), D. Hemoglobin (Hb).
3.3. Comparison of WBC differential count analysis results among the active TB, LTBI, and healthy control groups
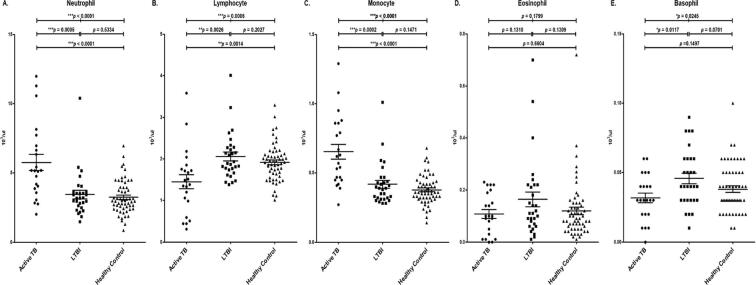
Based on the WBC differential count analysis, the mean of neutrophil counts were 5.77 ± 2.76 × 103/μL (70.00%) for the active TB group,3.44 ± 1.62 × 103/μL (54.80%) for the LTBI group, and 3.25 ± 1.21 × 103/μL (55.61%), for the healthy group respectively. The mean of lymphocyte counts were 1.45 ± 0.79 × 103/μL (19.31%) for the active TB group, 2.06 ± 0.57 × 103/μL (34.75%) for the LTBI group, and 1.91 ± 0.44 × 103/μL (34.73%) for the healthy control group, respectively. The mean of monocyte counts were 0.65 ± 0.26 × 103/μL (8.70%) for the active TB group, 0.42 ± 0.15 × 103/μL (6.94%) for the LTBI group, and 0.38 ± 0.10 × 103/μL (6.80%) for the healthy control group, respectively. The mean of eosinophil counts were 0.11 ± 0.08 × 103/μL (1.53%) for the active TB group, 0.16 ± 0.16 × 103/μL (2.74%) for the LTBI group, and 0.12 ± 0.11 × 103/μL (2.17%) for the healthy control group, respectively. The mean of basophil counts were 0.03 ± 0.02 × 103/μL (0.43%) for the active TB group, 0.05 ± 0.02 × 103/μL (0.78%) for the LTBI group, and 0.04 ± 0.02 × 103/μL (0.69%) for the healthy control group, respectively. Compared to the LTBI and healthy control groups, the mean values of neutrophils and monocytes in the active TB were significantly higher, and the mean values of lymphocytes and basophils were significantly lower (Fig. 3, Table 4).
Fig. 3.
Comparison of WBC differential count analysis results between the active TB, LTBI, and healthy control groups A. Neutrophil, B. Lymphocyte, C. Monocyte, D. Eosinophil, E. Basophil.
Table 4.
WBC differential counts analysis results between the active TB, LTBI, and healthy control groups.
| Cell type | Acitve TB, mean count (range) | LTBI, mean count (range) | Healthy control, mean count (range) | |
|---|---|---|---|---|
| Neutrophil | (1 × 103/μL) | 5.77 ± 2.76 (2.01–11.99) | 3.44 ± 1.62 (1.48–10.40) | 3.25 ± 1.21 (0.85–6.97) |
| % | 70.00 (44.50–90.70) | 54.80 (41.50–75.30) | 55.61 (37.10–79.60) | |
| Lymphocyte | (1 × 103/μL) | 1.45 ± 0.79 (0.31–3.58) | 2.06 ± 0.57 (1.38–4.01) | 1.91 ± 0.44 (1.01–3.29) |
| % | 19.31 (4.90–44.60) | 34.75 (16.10–50.00) | 34.73 (12.70–50.10) | |
| Monocyte | (1 × 103/μL) | 0.65 ± 0.26 (0.27–1.29) | 0.42 ± 0.15 (0.28–1.01) | 0.38 ± 0.10 (0.14–0.68) |
| % | 8.70 (4.30–15.30) | 6.94 (4.60–12.80) | 6.80 (3.80–14.00) | |
| Eosinophil | (1 × 103/μL) | 0.11 ± 0.08 (0.00–0.23) | 0.16 ± 0.16 (0.01–0.70) | 0.12 ± 0.11 (0.01–0.72) |
| % | 1.53 (0.00–3.50) | 2.74 (0.10–12.40) | 2.17 (0.20–9.40) | |
| Basophil | (1 × 103/μL) | 0.03 ± 0.02 (0.00–0.06) | 0.05 ± 0.02 (0.01–0.09) | 0.04 ± 0.02 (0.01–0.10) |
| % | 0.43 (0.00–1.20) | 0.78 (0.20–1.50) | 0.69 (0.20–1.60) | |
Active TB and LTBI groups were statistically significant with p values of 0.0005 in neutrophils, 0.0026 in lymphocytes, 0.002 in monocytes, and 0.0117 in basophils. There were no statistically significant differences in neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts between the LTBI and healthy control. The active TB and healthy control groups were statistically significant with p values under 0.0001 in neutrophils, 0.0014 in lymphocytes, and under 0.0001 in monocyte. The active TB, LTBI, and healthy control groups all had p values under 0.0001 in neutrophils, 0.0006 in lymphocyte, under 0.0001 in monocytes, 0.0245 in basophils. There was no statistically significant difference in eosinophil counts among the active TB, LTBI, and healthy control groups (Table 5).
Table 5.
Statistical data of WBC differential count analysis between the active TB, LTBI, and healthy control groups.
| Cell type | Active TB vs. LTBI | LTBI vs. healthy control | Active TB vs. healthy control | Active TB vs. LTBI vs. healthy control |
|---|---|---|---|---|
| Neutrophil | 0.0005*** | 0.5334 | < 0.0001*** | < 0.0001*** |
| Lymphocyte | 0.0026** | 0.2027 | 0.0014** | 0.0006*** |
| Monocyte | 0.0002*** | 0.1471 | < 0.0001*** | < 0.0001*** |
| Eosinophil | 0.1310 | 0.1309 | 0.6604 | 0.1799 |
| Basophil | 0.0117* | 0.0701 | 0.1497 | 0.0245* |
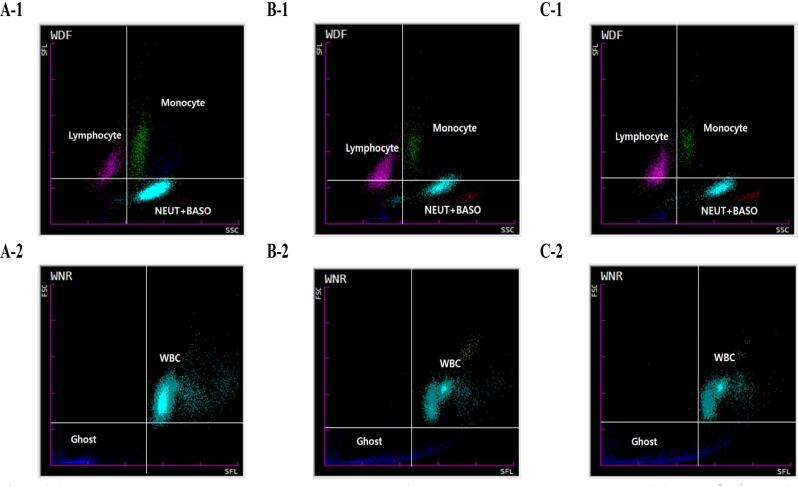
Automatic hematology analyzer scatter gram of active TB, LTBI and healthy control (Fig. 4). Scatter size of sky blue NEUT + BASO and green monocytes was increased in the WDR scatter gram of the active TB group (Fig. 4A-1). Scatter size of light blue WBC was also increased in the WNR scatter gram of the active TB group (Fig. 4A-2). WNR scatter grams of the LTBI and healthy control groups showed smaller scatter plots of light blue NEUT + BASO and green monocytes, and increased pink Lymphocyte scatter plots compared to the WNR scatter gram of the active TB group (Fig. 4B-1, Fig. 4C-1). The WDF scatter grams of LTBI and healthy control groups have smaller scatter sizes of light blue WBCs than the WDF scatter grams of active TB groups (Fig. 4B-2 and Fig. 4C-2).
Fig. 4.
Automated hematology analyzer scattergram between the active TB, LTBI, and healthy control groups A-1. WDF of active TB, A-2. WNR of active TB, B-1. WDF of LTBI, B-2. WNR of LTBI, C-1. WDF of healthy control, C-2. WNR of healthy control.
3.4. ROC curve analysis based on results of WBC differential count analysis
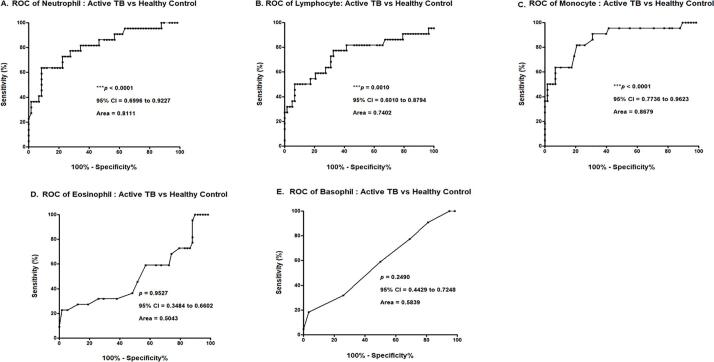
The analysis of ROC curve was performed to ensure that the results were clinically applicable. The p value of the ROC curve for neutrophils was under 0.0001 and the AUC was 0.8111. The p value of the ROC curve for lymphocytes was 0.0010, and the AUC was 0.7402. The p value of the ROC curve for monocytes was under 0.0001, and the AUC was 0.8679. There were no statistically significance differences in the ROC curves of the eosinophils, or basophil (Fig. 5). Of the WBC differential count results, the p values of neutrophils, lymphocytes, and monocytes were all statistically significant (p < 0.0500), and the AUC was approximately 0.8064 (Fig. 5).
Fig. 5.
ROC curve analysis of WBC differential counts between the active TB and healthy control groups A. Neutrophil, B. Lymphocyte, C. Monocyte, D. Eosinophil, E. Basophil.
4. Discussion
TB is a serious infectious disease with high infection and mortality rates [2]. According to the 2019 WHO report, approximately half a million (range, 417,000–556,000) new cases of rifampicin-resistant TB (of which 78% are multi-drug-resistant TB) are diagnosed, and this increasing resistance rates is a major concern in TB treatment and management (WHO, 2020). Among the OECD, the Republic of Korea has a high prevalence and mortality rate of TB [16]. TB management is important for the rapid differentiation of LTBI from active TB and appropriate anti-TB treatment.
The most commonly used diagnostic tool for tuberculosis is a simple skin test, though blood tests are becoming more commonplace. Currently, TST or IGRA tests are used alone to diagnose LTBI (Korean Guidelines for tuberculosis, 2017). However, it is difficult to distinguish between LTBI and active TB with a single test among the two tests mentioned, and it may result in false negative results for reasons such as immunosuppression. However, additional screening tests, such as the CBC and WBC differential counts and additional inflammatory mediator test are expected to improve the quality of TB diagnosis. This study aims to compare the CBC and WBC differential counts of whole blood samples to differentiate active TB and LTBI.
According to one study, TB is associated with the activation of the immune system and with the number and form of WBCs that release various cytokines [17]. In the early stages of MTB infection, MTB moves and accumulates in lung lesions, there by increasing the number of WBCs associated with the host's innate immune mechanism [18]. In another study, a group exposed to MTB and developing TB (home contact) showed statistically significantly higher levels (p < 0.0500) of leukocytes than did a community control group [14]. In the present study, the CBC of active TB group had a statistically significant increase in WBC (p < 0.0100, AUC = 0.7727) than did the LTBI and healthy control groups. Chronic infection caused by MTB reduces chlorine and factors that stimulate erythrocytes, such as hepcidin; consequently, iron imbalance occurs, and the regulation of sTfR mechanisms is inhibited, thus resulting in anemia [19]. In another study, 31.9% of patients diagnosed with TB had anemia, and the Hb concentrations in 45 patients were <10 g/dL [20]. In another study, TB in the elderly continues to be a problem worldwide, and a retrospective evaluation of data from patients diagnosed with pulmonary TB over 65 years of age reported that there were many cases of treatment failure such as weight loss and dyspnea [21]. Therefore, in order to diagnose patients who are vulnerable to TB management, there is a need for a new diagnostic marker that can be commonly diagnosed in clinical practice.
In the present study, a result of CBC revealed that the Hb concentration (p < 0.0010, AUC = 0.7394) and WBC differential count of lymphocytes (p < 0.0100, AUC = 0.7402) in the active TB group were significantly decreased than those in the LTBI and healthy control groups. Besides, WBC differential count of neutrophils (p < 0.0010, AUC = 0.8111) and monocytes (p < 0.0010, AUC = 0.8679) in active TB had a statistically significant increase than those in the LTBI and healthy control groups.
In conclusion, total WBCs, neutrophils, lymphocytes and monocytes were statistically significant biomarkers for the differentiation of active TB, LTBI and healthy control groups. By comparing the expression levels of these markers in whole blood of active TB, LTBI, and healthy control groups, it will be useful as an indicator for differential diagnosis and provide basic data for effective diagnosis and treatment. Further studies with larger number of clinical samples and populations than those in the current work are needed to confirm the importance of this study and to improve the accuracy of discrimination between the active TB and LTBI.
CRediT authorship contribution statement
Yun-Jeong Kang: Conceptualization, Formal analysis, Writing - original draft, Investigation. Heechul Park: Conceptualization, Formal analysis, Writing - original draft, Investigation. Sung-Bae Park: Data curation, Visualization, Validation. Junseong Kim: Data curation, Visualization, Validation. Jiyoung Lee: Data curation, Visualization, Validation. Jungho Kim: Data curation, Visualization, Validation. Sunyoung Park: Data curation, Visualization, Validation. Yong Sung Lee: Supervision. Sunghyun Kim: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1C1B108888 and -2020R1C1007169) and the Brain Busan 21 Plus project and the Center for Women In Science, Engineering and Technology (WISET) and WISET Regional Agency of PKNU Grant funded by the Ministry of Science and ICT (MSIT) under the Program for Returners into R&D.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Availability of data and materials
The data analyzed during this study are included in this paper. Some of the datasets are available from the corresponding author upon reasonable request.
Author’s contributions
SHK and YSL conceptualized and designed the paper. YJK and HCP analyzed the data, wrote and drafted the paper. YJK, HCP, SBP, JSK, JYL, JHK and SYP collected the data and reviewed the paper critically. All authors read and approved the final paper.
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the institutional ethics committee of Catholic University of Pusan (approval numbers CUPIRB-2019-01-010).
Consent for publication
Not applicable.
Contributor Information
Yong Sung Lee, Email: ichlys@naver.com.
Sunghyun Kim, Email: shkim0423@cup.ac.kr.
References
- 1.Zambuzi F.A., Cardoso-Silva P.M., Espindola M.S., Soares L.S., Galvão-Lima L.J., Brauer V.S. Identification of promising plasma immune biomarkers to differentiate active pulmonary tuberculosis. Cytokine. 2016;88:99–107. doi: 10.1016/j.cyto.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Ren N, JinLi J, Chen Y, Zhou X, Wang J, Ge P, et al. Identification of new diagnostic biomarkers for Mycobacterium tuberculosis and the potential application in the serodiagnosis of human tuberculosis, Microb Biotechnol 2018;11(5):893–904. [DOI] [PMC free article] [PubMed]
- 3.World Health Organization. Global tuberculosis Report; 2020. httls://www.who.int./tb/publications/global_report/en/.
- 4.Won E.-J., Choi J.-H., Cho Y.-N., Jin H.-M., Kee H.J., Park Y.-W. Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. J Infect. 2017;74(3):281–293. doi: 10.1016/j.jinf.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Cao S., Chen Y., Yong S., Yang L., Zheng S., Zhang Z. Screening of serum biomarkers for distinguishing between latent and active tuberculosis using proteome microarray. Biomed Environ Sci. 2018;31(7):515–526. doi: 10.3967/bes2018.069. [DOI] [PubMed] [Google Scholar]
- 6.Chen C., Yan T., Liu L., Wang J., Jin Q. Identification of a novel serum biomarker for tuberculosis infection in Chinese HIV patients by iTRAQ-based quantitative proteomics. Front Microbiol. 2018;9:330–342. doi: 10.3389/fmicb.2018.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell J.R., Krot J., Elwood K., Cook V., Marra F. A systematic review on TST and IGRA tests used for diagnosis of LTBI in immigrants. Mol Diagn Ther. 2015;19(1):9–24. doi: 10.1007/s40291-014-0125-0. [DOI] [PubMed] [Google Scholar]
- 8.James P.M., Ganaie F.A., Kadahalli R.L. The performance of quantiferon-TB gold in-tube (QFT-IT) test compared to tuberculin skin test (TST) in detecting latent tuberculosis infection (LTBI) in the presence of HIV coinfection in a high TB-burden area with BCG-vaccinated population. J Int Assoc Providers AIDS Care (JIAPAC) 2014;13(1):47–55. doi: 10.1177/2325957412469687. [DOI] [PubMed] [Google Scholar]
- 9.Bozkanat E., Kaya H., Sezer O., Caliskan T., Kilic E., Ciftci F. Comparison of tuberculin skin test and quantiferon-TB gold in tube test for diagnosis of latent tuberculosis infection in health care workers: a cross sectional study. JPMA. 2016;66:270–274. [PubMed] [Google Scholar]
- 10.Pai M., Denkinger C.M., Kik S.V., Rangaka M.X., Zwerling A., Oxlade O. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamishi S., Mahmoudi S., Banar M., Hosseinpour Sadeghi R., Marjani M., Pourakbari B. Diagnostic accuracy of interferon (IFN)-γ inducible protein 10 (IP-10) as a biomarker for the discrimination of active and latent tuberculosis. Mol Biol Rep. 2019;46(6):6263–6269. doi: 10.1007/s11033-019-05067-0. [DOI] [PubMed] [Google Scholar]
- 12.Sun H., Pan L., Jia H., Zhang Z., Gao M., Huang M. Label-free quantitative proteomics identifies novel plasma biomarkers for distinguishing pulmonary tuberculosis and latent infection. Front Microbiol. 2018;9 doi: 10.3389/fmicb.2018.0126710.3389/fmicb.2018.01267.s00110.3389/fmicb.2018.01267.s00210.3389/fmicb.2018.01267.s00310.3389/fmicb.2018.01267.s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding R., Zhang H. Effect of linezolid on serum PCT, ESR, and CRP in patients with pulmonary tuberculosis and pneumonia. Medicine. 2018;97:37–40. doi: 10.1097/MD.0000000000012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakotosamimanana N., Richard V., Raharimanga V., Gicquel B., Doherty T.M., Zumla A. Biomarkers for risk of developing active tuberculosis in contacts of TB patients: a prospective cohort study. Eur Respir J. 2015;46(4):1095–1103. doi: 10.1183/13993003.00263-2015. [DOI] [PubMed] [Google Scholar]
- 15.Shim T. Special review: diagnosis and treatment of latent tuberculosis infection. Korean J Med. 2012;82(3):284–290. [Google Scholar]
- 16.Cho K.S. Tuberculosis control in the Republic of Korea. Epidemiol Health. 2018;40:e2018036. doi: 10.4178/epih.e2018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seebach J.D., Morant R., Rüegg R., Seifert B., Fehr J. The diagnostic value of the neutrophil left shift in predicting inflammatory and infectious disease. Am J Clin Pathol. 1997;107(5):582–591. doi: 10.1093/ajcp/107.5.582. [DOI] [PubMed] [Google Scholar]
- 18.Park J., Lee H., Kim Y.-K., Kim K.H., Lee W., Lee K.-Y. Automated screening for tuberculosis by multiparametric analysis of data obtained during routine complete blood count. Int J Lab Hematol. 2014;36(2):156–164. doi: 10.1111/ijlh.12148. [DOI] [PubMed] [Google Scholar]
- 19.Hella J., Cercamondi C.I., Mhimbira F., Sasamalo M., Stoffel N., Zwahlen M. Anemia in tuberculosis cases and household controls from Tanzania: contribution of disease, coinfections, and the role of hepcidin. PLoS ONE. 2018;13(4):e0195985. doi: 10.1371/journal.pone.0195985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.W., Kang Y.A., Yoon Y.S., Um S.-W., Lee S.M., Yoo C.-G. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci. 2006;21(6):1028. doi: 10.3346/jkms.2006.21.6.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Gennaro F, Vittozzi P, Gualano G, Musso M, Mosti S, et al. Active Pulmonary Tuberculosis in Elderly Patients: a 2016-2019 Retrospective Analysis from an Italian Referral Hospital. Antibiotics (Basel, Switzerland) 2020;9(8):489. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during this study are included in this paper. Some of the datasets are available from the corresponding author upon reasonable request.