Abstract
Microgravity and elevated CO2 levels are two important environmental spaceflight stressors that can adversely affect astronaut cognitive performance and jeopardize mission success. This study investigated the effects of 6° head-down tilt bed rest (HDBR) with (n = 11 participants, 30-day HDBR) and without (n = 8 participants, 60-day HDBR) elevated ambient (3.73 mmHg) CO2 concentrations on cognitive performance. Participants of both groups performed all 10 tests of NASA’s Cognition battery and a brief alertness and mood survey repeatedly before, during, and after the HDBR period. Test scores were adjusted for practice and stimulus set effects. Concentrating on the first 30 days of HDBR, a modest but statistically significant slowing across a range of cognitive domains was found in both groups (controls: −0.37 SD; 95% CI −0.48, −0.27; adjusted P < 0.0001; CO2: −0.25 SD; 95% CI −0.34, −0.16; adjusted P < 0.001), most prominently for sensorimotor speed. These changes were observed early during HDBR and did not further deteriorate or improve with increasing time in HDBR. The study found similar cognitive effects of HDBR irrespective of CO2 levels, suggesting that elevated CO2 neither ameliorated nor worsened the HDBR effects. In both groups, cognitive performance after 15 days of recovery was statistically indistinguishable from pre-HDBR performance. However, subjects undergoing 60 days of HDBR rated themselves as feeling more sleepy, tired, physically exhausted, stressed, and unhealthy during recovery compared to their 30-day counterparts.
NEW AND NOTEWORTHY This study investigated the effects of prolonged head-down tilt bed rest with and without elevated (3.73 mmHg) levels of ambient CO2 on cognitive performance across a range of cognitive domains and is one of the few studies investigating combined effects of environmental stressors prevalent in spaceflight. The study showed moderate declines in cognitive speed induced by head-down tilt bed rest and suggests that exposure to elevated levels of ambient CO2 did not modify this effect.
Keywords: cognition, CO2, microgravity, performance, spaceflight
INTRODUCTION
Successful human spaceflight requires sustained high levels of astronaut cognitive performance to increase the likelihood of mission success (1, 2). Several environmental, operational, physiological, and psychological stressors prevalent in spaceflight may adversely affect cognitive performance and thereby pose risks to astronaut safety and health. Space agencies use ground-based analogs to investigate the effects of common spaceflight stressors on human physiology and performance. Most of these studies focus on individual risk factors, thus neglecting the complex interactions between multiple stressors experienced simultaneously in spaceflight.
Two stressors that have been implicated in the development of ocular and vision changes in spaceflight [spaceflight-associated neuro-ocular syndrome (SANS)] are microgravity and elevated CO2 levels (3, 4). Microgravity induces a physical body unloading and a head-ward fluid shift (5). Structural brain changes observed in astronauts after return from International Space Station (ISS) missions have included an upward shift of the brain (6–8), changes in gray matter volume (9), and cerebrospinal fluid volume increases in the third and lateral ventricles (7, 10–12). However, the functional consequences of these physiologic and anatomical changes remain largely unknown (8).
Increased CO2 blood concentrations elicit a number of physiologic responses triggered by a pH-induced stimulation of central and peripheral chemoreceptors, including increases in heart rate and minute ventilation, cerebral arterial vasodilation, and central nervous system (CNS) arousal (13–16). When exposed to increasing CO2 concentrations, symptoms escalate from dizziness, headaches, and shortness of breath to chest pain, anxiety and sweating to convulsions, unconsciousness, and ultimately death, although this does not occur until inspired CO2 concentrations are more than ten times the levels used in the current study (17). Several studies have investigated the effects of high CO2 concentrations on cognitive performance (18), but the physiological and cognitive effects of CO2 concentrations operationally relevant for spaceflight (≤ 4 mmHg) are poorly understood and understudied (18, 19). Two exceptions to this gap are the studies by Allen et al. (20) and Satish et al. (21) that found exposure response-like effects of CO2 concentrations below 1.9 mmHg on higher-order decision making. However, these results could not be replicated in an astronaut surrogate population exposed to CO2 levels of up to 4 mmHg (19). NASA currently controls CO2 levels on the ISS to ∼2–4 mmHg, which is higher than CO2 levels typically observed outdoors (∼0.3 mmHg) or in well-ventilated rooms (∼0.5 mmHg) on Earth. CO2-related symptoms such as headaches have been reported at lower CO2 levels in spaceflight than would be expected terrestrially (17).
Head-down tilt bed rest (HDBR) has been used for at least 50 yr as a ground-based analog for microgravity-induced physiological changes (22). Findings of studies investigating the effects of HDBR on cognitive performance have been inconclusive so far, likely due to the diversity of cognitive test batteries used, protocol differences (e.g., exposure to stressors, degrees of head-down tilt, duration), practice effect confounds, circadian time of testing, low sample size, and inadequate control groups (23). Also, it is not known whether cognitive changes that occur in HDBR are similar to, or arise from the same mechanisms, as those observed during spaceflight. Several recent event-related potential (ERP) studies have, however, demonstrated that HDBR induced changes in the processing of emotional (24, 25) and pain (26) stimuli. Moreover, it is possible that in previous studies—when subjects were allowed to lift their head and upper torso using pillows during meals—these latter behaviors may have resulted in variability in the effects of the head-ward fluid shift. Therefore, subjects participating in the studies reported on here strictly maintained HDBR throughout bed rest to replicate the sustained head-ward fluid shift that occurs in weightlessness more accurately and to create a more consistent and uniform stimulus (27).
We are aware of only one study that investigated the effects of HDBR with and without elevated levels of CO2 on cognitive performance (28). This study found a CO2-induced change in response strategy (i.e., slower but more accurate performance). However, the small effect size (<0.2), the extreme (12°) and short (1.5 days) HDBR period, and the small sample size (n = 6 subjects) raise uncertainty relative to the interpretation of the findings. Here, we report findings from a study on the effects of a 30-day 6° HDBR period with (n = 11 subjects) and without (n = 8 subjects) elevated (3.73 mmHg) CO2 concentrations on cognitive performance. It is one of few studies that investigated combined effects of common spaceflight stressors on astronaut behavioral health.
METHODS
Study Design and Participants
This analysis includes data from two bed rest studies performed in the envihab at the German Aerospace Center (DLR) in Cologne, Germany, a research facility that allows for investigating up to 12 subjects concurrently under controlled environmental conditions. In the first study titled Visual impairment intracranial pressure and Psychological:envihab Research (VaPER), 12 participants spent 30 consecutive days in strict 6° HDBR with an elevated atmospheric CO2 concentration of 3.73 mmHg, levels similar to the levels experienced on the ISS (17, 19). CO2 concentration was measured in the research facility at 15-min intervals throughout the 30-day HDBR period and averaged 3.73 mmHg (standard deviation: 0.10 mmHg; the targeted CO2 concentration was just below 0.5% or 3.8 mmHg, equivalent to the occupational health limit in Germany). In the second study entitled Artificial Gravity Bed Rest study by the European Space Agency (AGBRESA), 24 subjects spent 60 consecutive days in strict 6° HDBR with standard atmospheric CO2 concentration (0.04%). Two groups of eight participants were subjected to a countermeasure consisting of intermittent or continuous centrifugation, respectively. This analysis uses data from only the remaining eight AGBRESA subjects that served as a control group and, except for the different CO2 concentrations and bed rest duration, underwent comparable procedures as VaPER participants.
Male and female participants were recruited by the DLR. Study eligibility criteria included age between 24 and 55 yr, nonsmokers, body mass index between 19 and 30 kg/m2, no elevated risk of thrombosis, no recent history of bone fractures, and no history of chronic pain, hypertension, hyperlipidemia, arthritis, diabetes, obesity, hepatic disease, eye conditions, or a calcium/bone metabolism disorder. Subjects were screened to ensure they were psychologically healthy before participation. They were empaneled 14 days before the start and discharged 14 days after the end of the HDBR period. In contrast to most previous studies where participants were allowed to use a pillow during bed rest to elevate slightly the head relative to the rest of the body, both VaPER and AGBRESA strictly enforced the head-town tilt (HDT) position during the course of the experiment. A specially designed neck support was allowed when subjects were on their sides during sleep, although many chose not to use the special neck support (see Ref. 29 for images). Subjects participated in several scientific investigations that were scheduled throughout the day, interrupted by meals that reflected a controlled diet. Participants were provided a daily 8-h sleep opportunity between 2230 and 0630. They were compensated for participating in the study, which was approved by the local ethics committee (Ärztekammer Nordrhein) and by the Institutional Review Board of NASA Johnson Space Center. Subjects provided written informed consent before participation and were allowed to discontinue the study at any time. The study was registered at the German Clinical Trials Register (DRKS) under 00015677 (AGBRESA) and 00023491 (VaPER).
One VaPER subject dropped out on the first HDBR day and was excluded from data analysis. The remaining 11 subjects (55% male; mean ± SD, 33.6 ± 7.9 yr; range, 25–50 yr) were comparable in age but had a more balanced sex distribution relative to the eight AGBRESA control subjects (75% male; mean ± SD, 34.9 ± 7.9 yr; range, 24–46 yr).
Cognition Test Battery
The following description of the Cognition battery was modified from Basner et al. (1, 28). It contains a subset of tests from a widely used and validated neurocognitive battery, the Penn Computerized Neurocognitive Battery (PennCNB) (30–32), as well as a number of additional tests with an emphasis on those that either have been used extensively in spaceflight or that assess cognitive domains of particular interest in spaceflight (such as spatial orientation, emotion recognition, and risk decision making) (33, 34). The 10 Cognition tests were modified to reflect the high aptitude and motivation of astronauts. They assess a range of cognitive domains, and the brain regions primarily recruited by each test have been previously established (35). A detailed overview of Cognition can be found in Ref. 1. Here, we provide a brief description of each test.
The motor praxis task (MP) was administered at the start of testing to ensure that participants have sufficient command of the computer interface and immediately thereafter as a measure of sensorimotor speed (36). Participants were instructed to click on squares that appear randomly on the screen, with each successive square smaller and thus more difficult to track. Performance was assessed by the speed with which participants click each square.
The visual object learning test (VOLT) assessed participant memory for complex figures (37). Participants were asked to memorize 10 sequentially displayed 3-D figures. Later, they were instructed to select those objects they memorized from a set of 20 such objects also sequentially presented, half of them from the learning set and half new.
The fractal 2-back (F2B) is a nonverbal variant of the Letter 2-Back. N-back tasks have become standard probes of the working memory system and activate canonical working memory brain areas (38). The F2B consists of the sequential presentation of a set of figures (fractals), each potentially repeated multiple times. Participants are instructed to respond when the current stimulus matches the stimulus displayed two figures ago. The current implementation used 62 consecutive stimuli.
The abstract matching (AM) test is a validated measure of the abstraction and flexibility components of executive function, including an ability to discern general rules from specific instances (39). The test paradigm presents subjects with two pairs of objects at the bottom left and right of the screen, varied on perceptual dimensions (e.g., color and shape). Subjects are presented with a target object in the upper middle of the screen that they have to classify as belonging more with one of the two pairs, based on a set of implicit, abstract rules. The current implementation used 30 consecutive stimuli.
The line orientation test (LOT) is a measure of spatial orientation and derived from the well-validated judgment of line orientation test (40). The LOT format consists of presenting two lines at a time, one stationary, whereas the other could be rotated by clicking an arrow. Participants can rotate the movable line until they perceive it to be parallel to the stationary line. The implementation used in this study had 12 consecutive line pairs that varied in length and orientation.
The emotion recognition task (ERT) is a measure of facial emotion recognition (31). It presents subjects with photographs of professional actors (adults of varying age and ethnicity) portraying emotional facial expressions of varying types and intensities (biased toward lower intensities, and with the prevalence of emotion categories balanced within each version of the test). Subjects are given a set of emotion labels (happy; sad; angry; fearful; and no emotion) and have to select the label that correctly describes the expressed emotion. The implementation used in the study had 40 consecutive stimuli, with eight stimuli each representing one of the five emotion categories.
The matrix reasoning test (MRT) is a measure of abstract reasoning and consists of increasingly difficult pattern matching tasks (36). It is analogous to Raven’s progressive matrices; recruits prefrontal, parietal, and temporal cortices (41); and is based on a well-known measure of general intelligence. The test consists of a series of patterns, overlaid on a grid. One element from the grid is missing, and the participant has to select the element that fits the pattern from a set of alternative options. The implementation used in the study applied 12 consecutive stimuli.
The digit symbol substitution task (DSST) is a computerized adaptation of a paradigm used in the Wechsler adult intelligence scale (WAIS-III) (34). The DSST required the participant to refer to a displayed legend relating each of the digits one through nine to specific symbols. One of the nine symbols appears on the screen, and the participant has to select the corresponding number as quickly as possible. The test duration is fixed at 90 s, and the legend key is randomly re-assigned with each administration.
The balloon analog risk test (BART) is a validated assessment of risk-taking behavior (42). The BART requires participants to either inflate an animated balloon or stop inflating and collect a reward. Participants are rewarded in proportion to the final size of each balloon, but a balloon pops after a hidden number of pumps that changed across stimuli, in which case the reward is voided. The implementation used in the study had 30 consecutive stimuli. The average tendency of balloons to pop is varied systematically between test administrations. This required subjects to adjust the level of risk based on the behavior of the balloons. It prevented subjects from identifying a strategy during the first administrations of the battery and carrying it through to later administrations.
The psychomotor vigilance test (PVT) records reaction times (RT) to visual stimuli that occur at random interstimulus intervals (43). The PVT is a sensitive measure of vigilant attention and sensitive to acute and chronic sleep deprivation as well as circadian misalignment (44). Subjects are instructed to monitor a box on the screen and press the space bar once a millisecond counter appears in the box and starts incrementing. The reaction time is displayed for 1 s. Subjects were instructed to be as fast as possible without hitting the spacebar in the absence of a stimulus (i.e., false starts or errors of commission). In the current study, Cognition contained a validated 3-min brief PVT-B with 2- to 5-s interstimulus intervals and a 355-ms lapse threshold (45).
The Cognition software implemented a brief survey before each administration of the test battery. Participants entered the time they went to bed and got up, which was used as an estimate of their sleep duration. They then indicated their current status on the following thirteen 11-point Likert scales (anchors are provided in parenthesis after each question; the middle point was labeled “neutral”): (20) What was the quality of your sleep? (good-poor); (10) What was today’s workload? (very high-very low); How are you feeling right now? (44) (not sleepy at all-very sleepy); (43) (happy-unhappy); (46) (sick-healthy); (45) (energetic-physically exhausted); (28) (mentally sharp-mentally fatigued); (1) (not stressed at all-very stressed); (47) (tired-fresh, ready to go); (40) (very depressed-not depressed at all); (48) (very bored-not bored at all); (24) (not lonely at all-very lonely); and (13) What is your current everyday life like? (very monotonous-not monotonous at all).
Cognition Procedures
Subjects first watched a standardized familiarization video. They then performed the full Cognition battery twice for task familiarization 13 and 11 days before the start for the HDBR period. They were required to perform a brief practice version of 8 of the 10 tests during the first familiarization bout. Cognition was performed three more times on days 9, 7, and 6 before bed rest. These administrations served as baseline. Cognition then was performed on days 1, 3, 5, 14, and 28 after the initiation of the bed rest period. In AGBRESA, two more Cognition batteries were administered on days 42 and 57, but these administrations were not used for the analyses presented here as they had no equivalent in the VaPER study. Finally, Cognition was administered on days 1, 5, and 12 during the recovery period following bed rest.
Cognition (version 2.6.0.4 in VaPER and version 3.0.9 in AGBRESA, both using the version 2 ERT with 40 stimuli) was administered on Dell laptop computers (12.5 in. screen diagonal, 1,366 × 768 resolution) calibrated for timing accuracy in the afternoon (means ± SD of administration time across all study periods: 17:24 ± 0:28). It was performed in the seated upright position before and after the bed rest period. For testing in the HDT position, laptops were mounted vertically on an adjustable swivel arm and positioned in chest height in front of the participants (see Supplemental Fig. S1, all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13075958). Participants used the laptop’s track pad and integrated mouse button to operate the arrow on the screen.
Cognition Outcome Variables
Analyses concentrated on one main accuracy and one main speed outcome for each Cognition test. Congruent with descriptions in (46), all accuracy outcomes ranged from 0% to 100% with 100% representing best possible performance. For all speed outcomes, lower values reflect shorter response times and thus higher speed. Average response time (ms) was the speed outcome for all tests except the PVT. In the latter, 10 minus reciprocal response time (1/RT) was used as the speed outcome, as this metric was shown to be a superior outcome for the PVT relative to average RT (43). Percentage correct was the accuracy outcome for five Cognition tests. For the MP, the distance from the center of each square (in pixels) was averaged across all responses. The center of the square translates to 100% accuracy, 50 pixels, or more away from the center translate to an accuracy score of 0%, with linear scaling between 0 and 50 pixels. For the F2B, the percentage correct for matches and nonmatches was averaged to avoid subjects achieving good accuracy scores even if they never hit the spacebar. For the LOT, the accuracy measure was calculated as 3 minus the average number of clicks off, which was then divided by three (subjects are on average ∼0.8 clicks off). For tests with more than three clicks off on average, the accuracy score was set to 0%. For the ERT and MRT, stimuli that loaded negatively in an item response theory (IRT) analysis were excluded for the calculation of both speed and accuracy (see Ref. 46 for a list of excluded stimuli). For each pump on the BART, a value of 1 divided by the number of possible pumps across all 30 balloons was added to the risk score. This risk score therefore takes into account that different sets of balloons popped at different inflation rates. Here, we list BART risk taking as an accuracy outcome despite the fact that it inherently measures risk taking. For the PVT, the accuracy score was calculated as 1 – [(Number of Lapses + Number of False Starts)/(Number of Stimuli + Number of False Starts)]. Any response time not falling in between the false start threshold (100 ms) and the lapse threshold (355 ms) thus decreased accuracy on the PVT. All outcomes were corrected for practice and stimulus set difficulty affects according to (46) before statistical analysis based on an administration interval of 5 days or less.
All Cognition outcomes were also z-transformed based on the average and standard deviation of baseline performance scores (9, 7, and 6 days before bed rest) across study subjects and conditions. Summary scores for accuracy and speed were calculated by averaging across z-transformed scores within the accuracy and speed domain, respectively. The BART reflects risk taking rather than accuracy and was thus not included in the accuracy summary score. Speed summary scores were multiplied by −1 so that higher scores reflected higher speed. An efficiency score was calculated by averaging the accuracy and speed summary (z) scores.
Statistical Analyses
Data were analyzed with SAS (SAS Institute, Cary, NC; version 9.4). Linear mixed effect models with random subject effect were used to account for the clustered nature of the data. All models were adjusted for sex and age (continuous variable). Furthermore, all models were adjusted for baseline performance, with the exception of models for subjective outcomes and sleep duration (unless otherwise noted). P values were adjusted for multiple testing according to the false discovery rate method (47) for the 23 Cognition outcomes (one standard speed and accuracy outcome for each test plus accuracy, speed and efficiency across tests; i.e., n = 23 comparisons) and for the 13 subjective outcomes and sleep duration (i.e., n = 13 comparisons). We provide both unadjusted P values and confidence intervals as well as the alpha level that survived after adjustment (i.e., P < 0.05, P < 0.01, P < 0.001, P < 0.0001).
The following analyses were performed on both cognitive and subjective outcomes: 1) the difference in baseline assessments between the two groups was assessed: control (6° HDBR only; AGBRESA) and CO2 (6° HDBR plus 3.73 mmHg CO2 concentration; VaPER); 2) marginal means were estimated for the control and CO2 group during the HDBR phase and the recovery phase using observed marginal means for sex, age, and baseline performance. As z-transformation was performed using baseline data only, estimated marginal means reflect the difference of cognitive test scores during HDBR and recovery to baseline cognitive test scores, adjusted for potential differences in baseline performance between the two groups; 3) the control and CO2 groups were contrasted separately for the bed rest and recovery phase; 4) it was investigated whether assessments changed linearly with time in HDBR, and whether the slope differed significantly between groups (i.e., group × time interaction). Model 4 was the only model that allowed for random intercepts and random slopes (unstructured covariance).
RESULTS
Data were extracted and visualized for each subject. A few individual test bouts were excluded from data analysis. In the CO2 group, three tests were excluded due to technical difficulties and two tests due to participant nonadherence. The data were 99.7% complete (1,425 out of 1,430 expected tests). In the control group, one test was excluded due to technical difficulties and another test due to nonadherence. The data were 99.8% complete (1,198 out of 1,200 expected tests).
The CO2 group and the control group did not differ significantly at baseline in terms of cognitive performance, sleep duration, or survey responses (Supplemental Table S1). Self-reported sleep duration averaged 7.54 h (95% CI 7.31–7.76 h) in the control and 7.51 h (95% CI 7.32–7.70 h) in the CO2 group, respectively. Subjects in both groups reported average levels of tiredness, sleepiness, sleep quality, and workload; low levels of monotony, boredom, loneliness, depression, stress, mental fatigue, and physical exhaustion; and high levels of health and happiness at baseline.
Head-down Tilt Bed Rest Effects
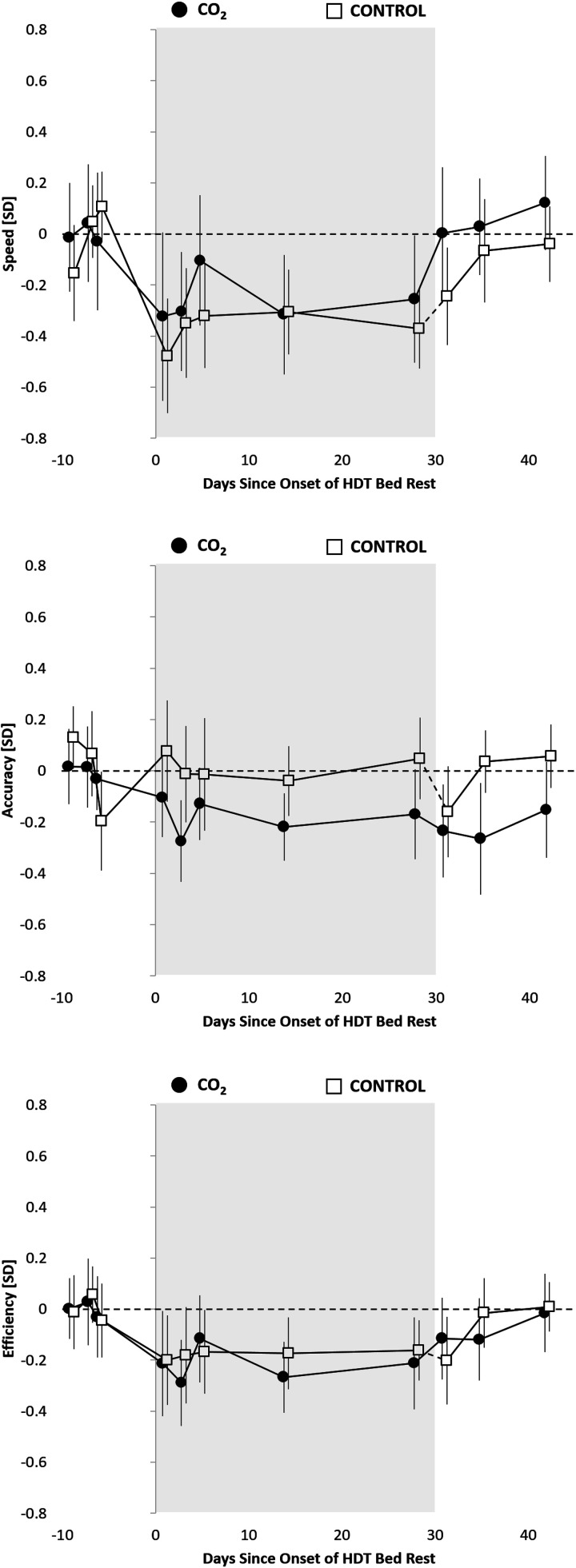
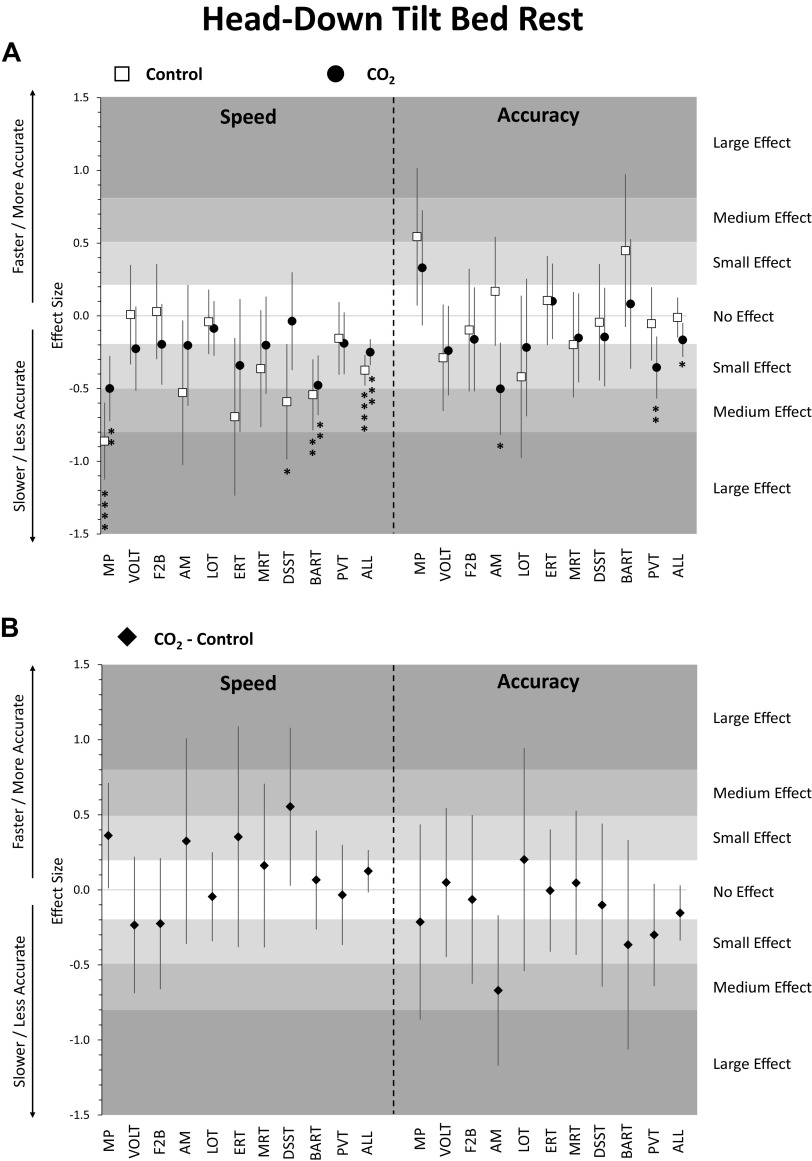
Compared to baseline performance, there was a moderate but significant decrease in speed across cognitive domains in both the control group (−0.37 SD; adjusted P < 0.0001) and the CO2 group (−0.25 SD; adjusted P < 0.0001) during the bed rest period (see Figs. 1 and 2A and Supplemental Table S2; trajectories for individual subjects are shown in Supplemental Fig. S2). Accuracy across cognitive domains was only significantly lower in the CO2 group (−0.17 SD; adjusted P < 0.05; trajectories for individual subjects are shown in Supplemental Fig. S3) and did not differ from baseline in the control group (−0.01 SD, adjusted P > 0.05). Cognitive efficiency was significantly lower relative to baseline and comparable in the control (−0.19 SD; adjusted P < 0.01) and the CO2 group (−0.21; adjusted P < 0.001). Neither cognitive speed, accuracy, nor efficiency differed significantly between control and CO2 groups across cognitive domains (Supplemental Table S2).
Figure 1.
Cognitive speed, accuracy, and efficiency across cognitive domains relative to the 30-day head-down tilt bed rest (HDBR) period (gray background) for the CO2 group (3.73 mmHg CO2, n = 11, 6 men) and the control group (ambient CO2, n = 8, 6 men). Estimates reflect unadjusted means ± standard error z-transformed based on baseline (pre-HDBR) performance within each of the 10 Cognition tests and then averaged across tests. To reflect the analytical approach (adjusting for baseline performance), means were shifted within groups to reflect a pre-HDBR baseline performance of 0. The dashed line between the last HDBR administration and the first recovery phase administration in the control group indicates that this group spent 30 additional days in HDBR relative to the CO2 group. HDBR, head-down tilt bed rest.
Figure 2.
Change in cognitive performance in the head-down tilt bed rest (HDBR) period relative to pre-HDBR baseline. Estimates reflect z-scores based on the mean and standard deviation of pre-HDBR baseline performance. Error bars reflect unadjusted 95% confidence intervals. A: estimates for the control group (ambient CO2, n = 8, 6 men) and the CO2 group (3.73 mmHg CO2, n = 11, 6 men); B: estimates for the difference between the CO2 group and the control group; *adjusted P < 0.05; **adjusted P < 0.01; ***adjusted P < 0.001; ****adjusted P < 0.0001. ALL, scores averaged across cognitive domains; AM, abstract matching; BART, balloon analog risk test; DSST, digit symbol substitution test; ERT, emotion recognition test; F2B, fractal 2-back; HDBR, head-down tilt bed rest; LOT, line orientation test; MP, motor praxis; MRT, matrix reasoning test; PVT, psychomotor vigilance test; VOLT, visual object learning test.
Focusing on individual tests, speed was significantly lower on the MP and BART for both the control and CO2 groups compared to baseline (Fig. 2A and Supplemental Table S2). Speed on the DSST was significantly lower in the control group only. Accuracy was significantly lower for AM and PVT in the CO2 group only. However, none of the speed or accuracy outcomes differed in a direct comparison between control and CO2 groups for any of the 10 Cognition tests after adjusting for multiple testing (Fig. 2B).
Sleep duration did not differ significantly between bed rest and baseline periods for either the control or CO2 group (Supplemental Table S2). Analyses of the survey responses suggest that both control and CO2 groups experienced significantly lower levels of workload during the bed rest period compared to baseline (Fig. 3A, Supplemental Table S2). Additionally, the control group rated itself significantly less healthy and reported experiencing significantly higher levels of monotony, whereas the CO2 group reported lower levels of happiness. However, none of the survey responses differed in a direct comparison between control and CO2 groups during the bed rest period after adjusting for multiple testing.
Figure 3.
Change in survey responses during head-down tilt bed rest (HDBR) (A) and post-HDBR recovery (B) relative to pre-HDBR baseline for the CO2 group (3.73 mmHg CO2, n = 11, 6 men) and the control group (ambient CO2, n = 8, 6 men). Estimates reflect points on an 11-point scale (variables are listed by anchors for high values). Error bars reflect unadjusted 95% confidence intervals. *adjusted P < 0.05; **adjusted P < 0.01; ***adjusted P < 0.001; ****adjusted P < 0.0001. HDBR, head-down tilt bed rest.
With the exception of self-reported monotony, which significantly increased with days in HDBR in the CO2 group only (adjusted P < 0.05), none of the other survey responses or cognitive test outcomes changed significantly with days in HDBR (all adjusted P > 0.05; Supplemental Table S4). Also, time in HDBR slopes did not differ significantly between groups (all adjusted P > 0.05 for time × group interaction).
Recovery Effects
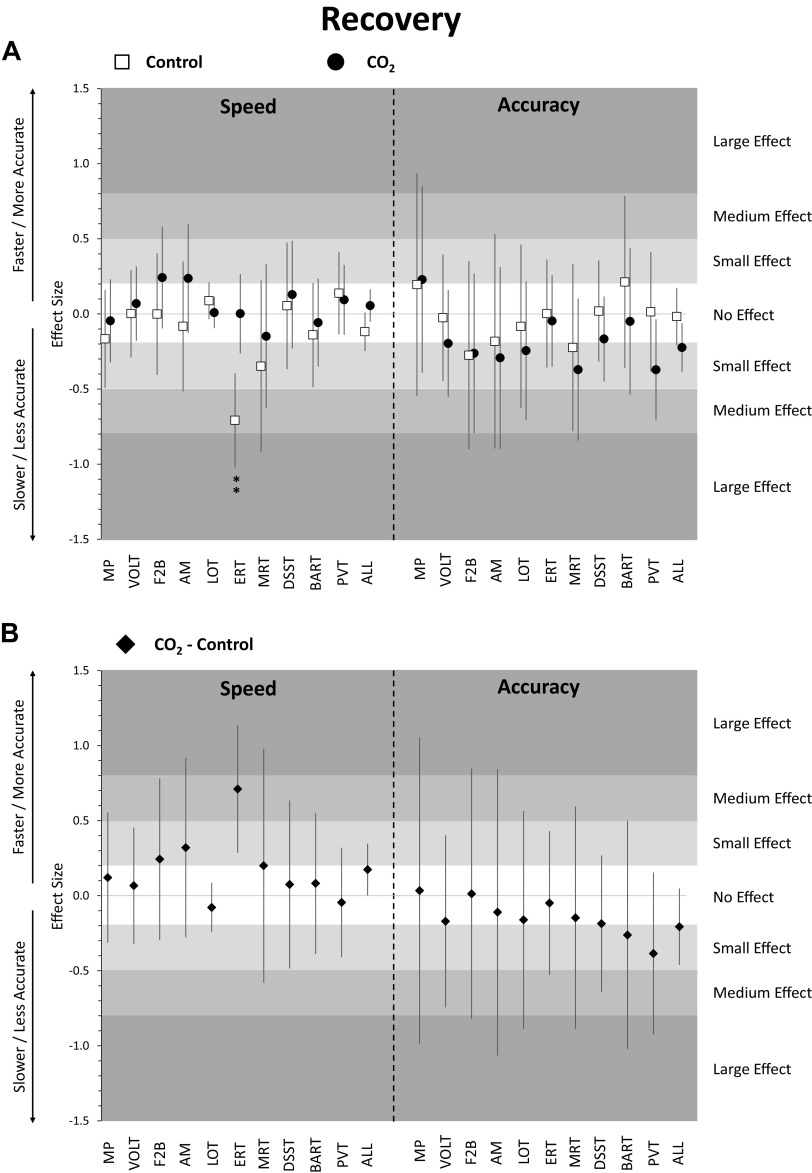
Cognitive slowing across cognitive domains observed during the bed rest period recovered immediately to pre-bed rest levels in the CO2 group, whereas it was comparable to baseline only starting with the second administration in the recovery period in the control group (Fig. 1). The subtle decline in accuracy across cognitive domains observed in the CO2 group during bed rest persisted during the recovery phase, but was no longer statistically significant. Neither cognitive speed, accuracy, nor efficiency differed significantly between control and CO2 groups across cognitive domains during the recovery phase (Supplemental Table S3).
Focusing on individual tests, only ERT speed in the control group was significantly lower than baseline performance (Fig. 4A and Supplemental Table S3). None of the other tests differed from baseline in either of the two groups for both speed and accuracy. In addition, none of the speed and accuracy outcomes differed in a direct comparison between control and CO2 groups for any of the 10 Cognition tests in the recovery phase after adjustment for multiple testing (Fig. 4B).
Figure 4.
Change in cognitive performance in post-head-down tilt bed rest (HDBR) recovery period relative to pre-HDBR baseline. Estimates reflect z-scores based on the mean and standard deviation of pre-HDBR baseline performance. Error bars reflect unadjusted 95% confidence intervals. A: estimates for the control group (ambient CO2, n = 8, 6 men) and the CO2 group (3.73 mmHg CO2, n = 11, 6 men); B: estimates for the difference between the CO2 group and the control group; **adjusted P < 0.01. ALL, scores averaged across cognitive domains; AM, abstract matching; BART, balloon analog risk test; DSST, digit symbol substitution test; ERT, emotion recognition test; F2B, fractal 2-back; HDBR, head-down tilt bed rest; LOT, line orientation test; MP, motor praxis; MRT, matrix reasoning test; PVT, psychomotor vigilance test; VOLT, visual object learning test.
Sleep duration did not differ significantly between recovery and baseline periods for either the control or the CO2 group (Supplemental Table S3). CO2 group participants rated themselves significantly less happy in the recovery phase relative to baseline (Fig. 3B and Supplemental Table S3). In contrast, the control group, which spent 30 days more in HDBR compared to the CO2 group, felt significantly sleepier, unhealthy, physically exhausted, mentally fatigued, stressed, and tired during recovery relative to baseline. Survey responses were significantly worse in the control group relative to the CO2 group for sleepiness, health, physical exhaustion, stress, and tiredness during recovery.
DISCUSSION
This study investigated the effects of a 30-day 6° HDBR period with and without elevated CO2 concentrations on cognitive performance across a range of cognitive performance domains. We found a small but reliable slowing of cognitive speed across a range of cognitive domains in both the control and CO2 groups. Eighteen out of 20 speed point estimates across the two groups were negative indicating slower performance relative to baseline. The slowing was most prominent for MP, a measure of sensorimotor speed that probes the sensorimotor cortex. Both spaceflight (6–8) and bed rest studies (49) have demonstrated an upward shift of the brain with increased brain tissue density at the vertex, which includes the somatosensory cortex and could be the cause for the observed slowing. Some of the reductions seen in the other nine tests also may be explained by reduced sensorimotor speed, because all Cognition tests naturally have a sensorimotor component. Notably, accuracy on the MP increased during HDBR compared with baseline. This is in contrast to a strategy adopted by subjects who repeatedly perform the task, where they increasingly click in the square periphery (i.e., decrease accuracy) to increase response speed (46). The data of this study therefore suggest that the decreased sensorimotor speed may in part be due to a decreased willingness or ability to adopt faster response strategies.
Speed on the DSST was 0.55 SD faster in the CO2 than in the control group during HDBR. This difference was statistically significant (P = 0.0405) but did not survive adjustment for multiple testing. It is consistent with findings by Lee at al. (50) who contrasted data from the VaPER study with another HDBR study without elevated levels of CO2 and found a significantly higher cognitive throughput on the DSST in the elevated CO2 group. Cerebrovascular reactivity to hypercapnia differs regionally (51), which could explain the differential vulnerability of the 10 Cognition tests in the speed and accuracy domain. Bhogal et al. (48) found the highest peak cerebrovascular reactivity to hypercapnia in the frontal lobe, which is recruited by the DSST. However, the participants of the CO2 group showed no change in arterialized and end-tidal PCO2 levels, cerebrovascular response to CO2, and the hypercapnic ventilatory response, suggesting there was no direct link between the increased ambient CO2 levels and a physiologic response that could explain the cognitive function findings reported here (27, 29).
Other HDBR studies also found a response slowing for selected cognitive domains (23, 52). One short-term HDBR study that investigated both EEG power spectral density and response speed on a simple reaction time task found an increase in delta and theta EEG frequencies coupled with slower reaction times induced by HDBR (53). The slower reaction times were thus interpreted as signs of cortical inhibition induced by central hypervolemia. That response slowing is not a more consistent finding across HDBR studies can potentially be attributed to practice effect confounds in studies that did not have the ability to adjust for practice effects or did not employ proper ambulatory controls. In addition, to our knowledge, VaPER and AGBRESA were the first two long-term HDBR studies with a strict HDT position of the head, which could have played a role.
Accuracy was significantly decreased in the CO2 group only, but the size of this decline is considered “no effect” according to generally accepted standards (i.e., effect size < 0.2) (54). Significant declines in accuracy for individual tests were observed for abstraction and mental flexibility (AM) and psychomotor vigilance (PVT) in the CO2 group only, with small and medium effect sizes, respectively. Importantly, in a direct comparison between the CO2 and the control groups, there was no evidence for a difference in speed or accuracy on the 10 Cognition tests after adjustment for multiple testing. This is in line with findings of a ground-based study where a stepwise increase of CO2 up to 3.80 mmHg was not associated with relevant performance deficits on the Cognition test battery (19). Our results suggest slightly faster speed and lower accuracy in the CO2 compared to the control group during HDBR, which contradicts the findings of a study in six subjects performing Cognition on an iPad in a short-term 12° HDBR study (28). That study found slightly slower speed and higher accuracy across cognitive domains when subjects were additionally exposed to 3.73 mmHg CO2 during bed rest. However, in both that study (28) and the current study, effects were negligible.
Surprisingly, in both the control and the CO2 groups, cognitive speed and accuracy did not change systematically with time in HDBR on any of the 10 Cognition tests—thus, any change observed on bed rest day 1 remained stable until bed rest day 28. This not only suggests that the changes induced by HDBR were instantaneous, but also that they neither ameliorated nor further deteriorated over a period of 30 days.
The cognitive slowing observed in the bed rest period returned to baseline levels on day 1 of the recovery period for the CO2 group only, whereas it was comparable to baseline only starting with the second administration in the recovery period in the control group. This difference can likely be explained by a bed rest period twice as long for the control group. The effect was driven by a significant decline in emotion recognition speed relative to baseline. In contrast, accuracy did not recover post-bed rest in the CO2 group. It remained 0.22 SD below baseline performance, whereas a stable baseline accuracy level remained throughout the bed rest and recovery period for the control group. However, in a direct comparison between the CO2 and control groups, none of the observed differences were statistically significant, suggesting similar performance during the recovery phase between the two groups.
Study participants of both groups showed healthy survey responses before the bed rest period. During the bed rest period, subjective assessments of workload, happiness, and health decreased, whereas perceived monotony increased. It is unclear to what degree these changes contributed to changes in cognitive performance (a motivated behavior). These changes were comparable between the CO2 and control groups. During the recovery phase, a >2-point deterioration relative to baseline was observed for health, physical exhaustion, and stress in the control group only. The CO2 group felt significantly less sleepy, less physically exhausted, less stressed, less tired, and healthier than the control group. These differences are likely explained by the longer bed rest period of the control group, and they suggest that participants exposed to 60 days of HDBR had more negative survey responses and recovered more slowly compared to participants exposed to 30 days of HDBR.
Strength and Limitations
To our knowledge, this is the first study investigating the effects of combined exposure to 6° HDBR with elevated (3.73 mmHg) CO2 concentration over a longer period of time (i.e., 30 days). We were able to use an HDBR only control group from another study performed in the same location by the same study team. The breadth of the Cognition test battery, the near completeness of the data, and the ability to adjust for practice and stimulus set effects are strengths of this study. Practice effects in the absence of proper ambulatory controls may have restricted the interpretability of cognitive test data obtained in most bed rest studies performed to date (23).
However, there are also several limitations. First and foremost, HDBR is a spaceflight analog, and as such, an imperfect replication of the conditions caused by microgravity and other stressors in spaceflight. It is therefore unclear how well the findings of this study translate to the spaceflight environment. Although the change in gravity vector is the most plausible explanation for the observed effects, we cannot rule out other contributing factors. For example, performing the cognitive tests in the unusual HDT position could have accounted for some of the observed effects, especially in the sensorimotor speed domain. However, it was not possible to quantify the contribution of individual factors, as they were perfectly confounded with the HDBR intervention. Similar limitations apply to cognitive testing in spaceflight. Second, differences in number, type, and schedule of scientific experiments between the VaPER and AGBRESA study may explain some of the observed differences between groups. However, both studies were performed in the same research facility, by the same study team and with identical methods and schedules for cognitive testing, which increases comparability relative to previously published research (50). Also, it is unknown if subjects volunteering for a 60-day bed rest study may differ systematically from those volunteering for a 30-day study, and their mindset may be different with the expectation of spending 60 days in HDBR. Finally, as evidenced by the large 95% confidence intervals in Fig. 2B, we were likely underpowered to find small effect sizes statistically significant in this between-subject design with group sizes of n = 11 and n = 8. Larger studies are needed to more conclusively test CO2 as a mediator of cognitive performance deficits induced by HDBR.
Conclusion
This study found a modest but statistically significant slowing across a range of cognitive domains induced by 6° HDBR, most prominently for sensorimotor speed. These changes were observed early during HDBR and did not deteriorate further or improve with increasing time in HDBR. The study found similar cognitive effects of HDBR in the CO2 group and the control group. However, we do acknowledge the low power for finding a statistically significant difference between the CO2 and control groups. Subjects undergoing 60 days of HDBR showed significantly more negative survey responses on a range of outcomes during the recovery period compared to subjects undergoing 30 days of bed rest.
DATA AVAILABILITY
The datasets generated for this study can be found by request at the NASA Life Science Data Archive at https://lsda.jsc.nasa.gov/.
GRANTS
This study was supported by NASA through Grants 80NSSC18K0765 and NNJ14ZSA001N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.B., A.C.S., B.R.M., and S.S.L. conceived and designed research; M.B., A.C.S., J.N., and C.M. performed experiments; M.B. and J.N. analyzed data; M.B., A.C.S., D.F.D., T.M.M., R.C.G., C.M., B.R.M., and S.S.L. interpreted results of experiments; M.B. prepared figures; M.B. drafted manuscript; M.B., A.C.S., J.N., D.F.D., T.M.M., R.C.G., C.M., B.R.M., and S.S.L. edited and revised manuscript; M.B., A.C.S., J.N., D.F.D., T.M.M., R.C.G., C.M., B.R.M., and S.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the German Aerospace Center (DLR), Institute of Aerospace Medicine, and the European Space Agency for their support.
REFERENCES
- 1.Basner M, Savitt A, Moore TM, Port AM, McGuire S, Ecker AJ, Nasrini J, Mollicone DJ, Mott CG, McCann T, Dinges DF, Gur RC. Development and validation of the cognition test battery for spaceflight. Aerosp Med Hum Perform 86: 942–952, 2015. doi: 10.3357/AMHP.4343.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strangman GE, Sipes W, Beven G. Human cognitive performance in spaceflight and analogue environments. Aviat Space Environ Med 85: 1033–1048, 2014. doi: 10.3357/ASEM.3961.2014. [DOI] [PubMed] [Google Scholar]
- 3.Laurie SS, Lee SMC, Macias BR, Patel N, Stern C, Young M, Stenger MB. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol 138: 165–172, 2020. doi: 10.1001/jamaophthalmol.2019.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall-Goebel K, Damani R, Bershad EM. Brain physiological response and adaptation during spaceflight. Neurosurgery 85: E1138–E1139, 2019. doi: 10.1093/neuros/nyz374. [DOI] [PubMed] [Google Scholar]
- 5.Marshall-Goebel K, Laurie SS, Alferova IV, Arbeille P, Aunon-Chancellor SM, Ebert DJ, Lee SMC, Macias BR, Martin DS, Pattarini JM, Ploutz-Snyder R, Ribeiro LC, Tarver WJ, Dulchavsky SA, Hargens AR, Stenger MB. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw Open 2: e1915011, 2019. doi: 10.1001/jamanetworkopen.2019.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JK, Koppelmans V, Riascos RF, Hasan KM, Pasternak O, Mulavara AP, Bloomberg JJ, Seidler RD. Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol 76: 412–419, 2019. doi: 10.1001/jamaneurol.2018.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts DR, Albrecht MH, Collins HR, Asemani D, Chatterjee AR, Spampinato MV, Zhu X, Chimowitz MI, Antonucci MU. Effects of spaceflight on astronaut brain structure as indicated on MRI. N Engl J Med 377: 1746–1753, 2017. doi: 10.1056/NEJMoa1705129. [DOI] [PubMed] [Google Scholar]
- 8.Roberts DR, Brown TR, Nietert PJ, Eckert MA, Inglesby DC, Bloomberg JJ, George MS, Asemani D. Prolonged microgravity affects human brain structure and function. AJNR Am J Neuroradiol 40: 1878–1885, 2019. doi: 10.3174/ajnr.a6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD. Brain structural plasticity with spaceflight. NPJ Microgravity 2: 2, 2016. doi: 10.1038/s41526-016-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alperin N, Bagci AM, Lee SH. Spaceflight-induced changes in white matter hyperintensity burden in astronauts. Neurology 89: 2187–2191, 2017. doi: 10.1212/WNL.0000000000004475. [DOI] [PubMed] [Google Scholar]
- 11.Kramer LA, Hasan KM, Stenger MB, Sargsyan A, Laurie SS, Otto C, Ploutz-Snyder RJ, Marshall-Goebel K, Riascos RF, Macias BR. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology 295: 640–648, 2020. doi: 10.1148/radiol.2020191413 [DOI] [PubMed] [Google Scholar]
- 12.Van Ombergen A, Jillings S, Jeurissen B, Tomilovskaya E, Rumshiskaya A, Litvinova L, Nosikova I, Pechenkova E, Rukavishnikov I, Manko O, Danylichev S, Rühl RM, Kozlovskaya IB, Sunaert S, Parizel PM, Sinitsyn V, Laureys S, Sijbers J, zu Eulenburg P, Wuyts FL. Brain ventricular volume changes induced by long-duration spaceflight. Proc Natl Acad Sci USA 116: 10531–10536, 2019. doi: 10.1073/pnas.1820354116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brian JE Jr. Carbon dioxide and the cerebral circulation. Anesthesiology 88: 1365–1386, 1998. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol 518: 3883–3906, 2010. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langhorst P, Schulz B, Schulz G, Lambertz M. Reticular formation of the lower brainstem. A common system for cardiorespiratory and somatomotor functions: discharge patterns of neighboring neurons influenced by cardiovascular and respiratory afferents. J Auton Nerv Syst 9: 411–432, 1983. doi: 10.1016/0165-1838(83)90005-x. [DOI] [PubMed] [Google Scholar]
- 17.Law J, Van Baalen M, Foy M, Mason SS, Mendez C, Wear ML, Meyers VE, Alexander D. Relationship between carbon dioxide levels and reported headaches on the international space station. J Occup Environ Med 56: 477–483, 2014. doi: 10.1097/JOM.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic A, Alexander D, Oman CM, Schneiderman J. A review of cognitive and behavioral effects of increased carbon dioxide exposure in humans. Johnson Space Center, Houston, TX, 2016. [Google Scholar]
- 19.Scully RR, Basner M, Nasrini J, Lam C-W, Hermosillo E, Gur RC, Moore T, Alexander DJ, Satish U, Ryder VE. Effects of acute exposures to carbon dioxide on decision making and cognition in astronaut-like subjects. NPJ Microgravity 5: 17, 2019. doi: 10.1038/s41526-019-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen JG, MacNaughton P, Satish U, Santanam S, Vallarino J, Spengler JD. Associations of cognitive function scores with carbon dioxide, ventilation, and volatile organic compound exposures in office workers: a controlled exposure study of green and conventional office environments. Environ Health Perspect 124: 805–812, 2016. doi: 10.1289/ehp.1510037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satish U, Mendell MJ, Shekhar K, Hotchi T, Sullivan D, Streufert S, Fisk WJ. Is CO2 an indoor pollutant? Direct effects of low-to-moderate CO2 concentrations on human decision-making performance. Environ Health Perspect 120: 1671–1677, 2012. doi: 10.1289/ehp.1104789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986-2006). Eur J Appl Physiol 101: 143–194, 2007. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 23.Lipnicki DM, Gunga HC. Physical inactivity and cognitive functioning: results from bed rest studies. Eur J Appl Physiol 105: 27–35, 2009. doi: 10.1007/s00421-008-0869-5. [DOI] [PubMed] [Google Scholar]
- 24.Brauns K, Werner A, Gunga HC, Maggioni MA, Dinges DF, Stahn A. Electrocortical evidence for impaired affective picture processing after long-term immobilization. Sci Rep 9: 16610, 2019. doi: 10.1038/s41598-019-52555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benvenuti SM, Bianchin M, Angrilli A. Posture affects emotional responses: a head down bed rest and ERP study. Brain Cogn 82: 313–318, 2013. doi: 10.1016/j.bandc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Spironelli C, Angrilli A. Influence of body position on cortical pain-related somatosensory processing: an ERP study. PLoS One 6: e24932, 2011. doi: 10.1371/journal.pone.0024932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurie SS, Macias BR, Dunn JT, Young M, Stern C, Lee SMC, Stenger MB. Optic disc edema after 30 days of strict head-down tilt bed rest. Ophthalmology 126: 467–468, 2019. doi: 10.1016/j.ophtha.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 28.Basner M, Nasrini J, Hermosillo E, McGuire S, Dinges DF, Moore TM, Gur RC, Rittweger J, Mulder E, Wittkowski M, Donoviel D, Stevens B, Bershad EM. Effects of −12° head-down tilt with and without elevated levels of CO2 on cognitive performance: the SPACECOT study. J Appl Physiol 124: 750–760, 2017. doi: 10.1152/japplphysiol.00855.2017. [DOI] [PubMed] [Google Scholar]
- 29.Laurie SS, Christian K, Kysar J, Lee SMC, Lovering AT, Macias BR, Moestl S, Sies W, Mulder E, Young M, Stenger MB. Unchanged cerebrovascular CO2 reactivity and hypercapnic ventilatory response during strict head-down tilt bed rest in a mild hypercapnic environment. J Physiol 598: 2491–2505, 2020. doi: 10.1113/JP279383. [DOI] [PubMed] [Google Scholar]
- 30.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology 26: 251–265, 2012. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods 187: 254–262, 2010. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology 29: 235–246, 2015. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann NY Acad Sci 1129: 305–322, 2008. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 34.Usui N, Haji T, Maruyama M, Katsuyama N, Uchida S, Hozawa A, Omori K, Tsuji I, Kawashima R, Taira M. Cortical areas related to performance of WAIS Digit Symbol Test: a functional imaging study. Neurosci Lett 463: 1–5, 2009. doi: 10.1016/j.neulet.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 35.Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliott MA, Gallagher RS, Almasy L, Pogue-Geile MF, Prasad K, Wood J, Nimgaonkar VL, Gur RC. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology 28: 161–176, 2014. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology 25: 766–776, 2001. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 37.Glahn DC, Gur RC, Ragland JD, Censits DM, Gur RE. Reliability, performance characteristics, construct validity, and an initial clinical application of a visual object learning test (VOLT). Neuropsychology 11: 602–612, 1997. doi: 10.1037//0894-4105.11.4.602. [DOI] [PubMed] [Google Scholar]
- 38.Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology 16: 370–379, 2002. [PMC free article] [PubMed] [Google Scholar]
- 39.Glahn DC, Cannon TD, Gur RE, Ragland JD, Gur RC. Working memory constrains abstraction in schizophrenia. Biol Psychiatry 47: 34–42, 2000. doi: 10.1016/s0006-3223(99)00187-0. [DOI] [PubMed] [Google Scholar]
- 40.Benton AL, Varney NR, Hamsher KD. Visuospatial judgment. A clinical test. Arch Neurol 35: 364–367, 1978. doi: 10.1001/archneur.1978.00500300038006. [DOI] [PubMed] [Google Scholar]
- 41.Perfetti B, Saggino A, Ferretti A, Caulo M, Romani GL, Onofrj M. Differential patterns of cortical activation as a function of fluid reasoning complexity. Hum Brain Mapp 30: 497–510, 2009. doi: 10.1002/hbm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). J Exp Psychol Appl 8: 75–84, 2002. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 43.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep 34: 581–591, 2011. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barger LK, Flynn-Evans EE, Kubey A, Walsh L, Ronda JM, Wang W, Wright KP, Czeisler CA. Prevalence of sleep deficiency and hypnotic use among astronauts before, during and after spaceflight: an observational study. Lancet Neurol 13: 904–912, 2014. doi: 10.1016/s1474-4422(14)70122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut 69: 949–959, 2011. doi: 10.1016/j.actaastro.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basner M, Hermosillo E, Nasrini J, Saxena S, Dinges DF, Moore TM, Gur RC. Cognition test battery: adjusting for practice and stimulus set effects for varying administration intervals in high performing individuals. J Clin Exp Neuropsychol 42: 516–529, 2020. doi: 10.1080/13803395.2020.1773765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Ser B (Methodological) 57: 289–300, 1995. doi: 10.2307/2346101. [DOI] [Google Scholar]
- 48.Bhogal AA, Philippens ME, Siero JC, Fisher JA, Petersen ET, Luijten PR, Hoogduin H. Examining the regional and cerebral depth-dependent BOLD cerebrovascular reactivity response at 7T. Neuroimage 114: 239–248, 2015. doi: 10.1016/j.neuroimage.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Roberts DR, Zhu X, Tabesh A, Duffy EW, Ramsey DA, Brown TR. Structural brain changes following long-term 6° head-down tilt bed rest as an analog for spaceflight. AJNR Am J Neuroradiol 36: 2048–2054, 2015. doi: 10.3174/ajnr.A4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JK, De Dios Y, Kofman I, Mulavara AP, Bloomberg JJ, Seidler RD. Head down tilt bed rest plus elevated CO2 as a spaceflight analog: effects on cognitive and sensorimotor performance. Front Hum Neurosci 13: 355, 2019. doi: 10.3389/fnhum.2019.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan AP, Evans KC, Stout JN, Rosen BR, Adalsteinsson E. Regional quantification of cerebral venous oxygenation from MRI susceptibility during hypercapnia. Neuroimage 104: 146–155, 2015. doi: 10.1016/j.neuroimage.2014.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Q, Zhou R, Chen S, Tan C. Effects of head-down bed rest on the executive functions and emotional response. PLoS One 7: e52160, 2012. doi: 10.1371/journal.pone.0052160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaitl D, Gruppe H, Stark R, Possel P. Simulated micro-gravity and cortical inhibition: a study of the hemodynamic-brain interaction. Biol Psychol 42: 87–103, 1996. doi: 10.1016/0301-0511(95)05148-1. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum, 1988. [Google Scholar]
- 55.Raven J. The Raven’s progressive matrices: change and stability over culture and time. Cogn Psychol 41: 1–48, 2000. doi: 10.1006/cogp.1999.0735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study can be found by request at the NASA Life Science Data Archive at https://lsda.jsc.nasa.gov/.