Abstract
Achilles tendinopathy is a debilitating condition affecting the entire spectrum of society and a condition that increases the risk of tendon rupture. Effective therapies remain elusive, as anti‐inflammatory drugs and surgical interventions show poor long-term outcomes. Eccentric loading of the Achilles muscle-tendon unit is an effective physical therapy for treatment of symptomatic human tendinopathy. Here, we introduce a novel mouse model of hindlimb muscle loading designed to achieve a tissue-targeted therapeutic exercise. This model includes the application of tissue (muscle and tendon)-loading “doses,” coupled with ankle dorsiflexion and plantarflexion, inspired by human clinical protocols. Under computer control, the foot was rotated through the entire ankle joint range of motion while the plantar flexors simultaneously contracted to simulate body mass loading, consistent with human therapeutic exercises. This approach achieved two key components of the heel drop and raise movement: ankle range of motion coupled with body mass loading. Model development entailed the tuning of parameters such as footplate speed, number of repetitions, number of sets of repetitions, treatment frequency, treatment duration, and treatment timing. Initial model development was carried out on uninjured mice to define a protocol that was well tolerated and nondeleterious to tendon biomechanical function. When applied to a murine Achilles tendinopathy model, muscle loading led to a significant improvement in biomechanical outcome measures, with a decrease in cross-sectional area and an increase in material properties, compared with untreated animals. Our model facilitates the future investigation of mechanisms whereby rehabilitative muscle loading promotes healing of Achilles tendon injuries.
NEW & NOTEWORTHY We introduce a novel mouse model of hindlimb muscle loading designed to achieve a tissue-targeted therapeutic exercise. This innovative model allows for application of muscle loading “doses,” coupled with ankle dorsiflexion and plantarflexion, inspired by human loading clinical treatment. Our model facilitates future investigation of mechanisms whereby rehabilitative muscle loading promotes healing of Achilles tendon injuries.
Keywords: Achilles, eccentric, muscle loading, preclinical, tendinopathy
INTRODUCTION
Achilles tendinopathy is a debilitating condition affecting people of all ages and lifestyles. Tendinopathy is widely believed to result from repetitive mechanical overload (1) and increases a patient’s risk of Achilles tendon rupture (1–3). Effective therapies to treat tendinopathies remain elusive, as anti‐inflammatory drugs show poor long‐term outcomes (4). Surgical options are limited and are predicated on patient compliance during recovery (5). Furthermore, inadequate healing or wound complications following surgery can lead to tissue morbidity in the form of infection, suture granulomas, or suture abscesses, leading to additional surgeries (6, 7).
Eccentric loading of the Achilles muscle-tendon unit (muscle lengthens while it contracts) is an effective physical therapy for treatment of symptomatic human tendinopathy (8–14). Typically, the patients support themselves on the toes of the affected limb, with the heel extended over a ledge (e.g., a stair tread) and then alternate heel drops (dorsiflexion) and heel raises (plantarflexion). Despite the clinical efficacy of this exercise, the biological and structural mechanisms by which the presumed healing occurs are poorly understood (10, 15). Eccentric exercise in clinical cases of Achilles tendinopathy reduces pathological tendon thickening and restores normal tendon architecture (16). However, the available evidence indicates that structural changes of the tendon are not the primary mechanism by which eccentric loading promotes Achilles tendon healing, as there are no short-term increases in organized tendon structure following exercise (17, 18). Furthermore, the selection of clinical treatment protocols (e.g., number of repetitions per session and number of weeks of therapy) required to achieve symptomatic relief and restoration of function are likely dependent on a number of factors, such as patient age, body mass index, and the severity of the tendinopathy (19, 20).
Several preclinical rat studies have demonstrated that mechanical loading can promote healing of surgically transected and repaired tendons (21) and improve biomechanical properties of uninjured tendons (22). For example, eccentric loading has been reported to increase the ultimate load capacity of partially transected rat patellar tendons (23). However, these models generally implement treadmill running rather than tissue-targeted muscle stimulation to emulate relevant rehabilitative loading treatments. Although treadmill exercise can be readily implemented in rodent models, it can be difficult to quantify the relative eccentric and concentric loading regimens that change throughout gait phases (24). Additionally, many studies that characterize the importance of muscle loading to tendon healing suppress muscle activity by paralysis or immobilization rather than increasing muscle activation (25–27). It is important to note that, whereas immobilization is effective in the healing process for injuries of the rotator cuff or severe ankle sprains (28), immobilization can be deleterious in various types of injuries of tendons, such as long-term immobilization of the knee (29–32).
Existing animal models of mechanical loading of injured tendons (33, 34) do not enable precise control of muscle loading “doses.” These methods target qualitative muscle activation patterns, which do not aim to replicate loading mechanisms associated with clinical rehabilitation protocols. To circumvent these limitations, we present a novel mouse model of hindlimb muscle loading that mimics human heel drop and raise exercises. We anticipate that in future studies this preclinical model will elucidate molecular mechanisms of heel drop and raise movements that promote healing of Achilles tendinopathy.
METHODS
Animals
Under IACUC approval, 12-wk-old C57BL/6 male mice (Jackson Laboratories, stock no. 000664) were used for all studies. Mice were placed on a 12-h:12-h light-dark cycle, were provided standard mouse chow and water ad libitum, and were active in the cage before and following loading treatments. After the in vivo protocols, mice were euthanized, with the use of carbon dioxide inhalation and cervical dislocation, for tissue biomechanical and histological assessments.
Experimental Design
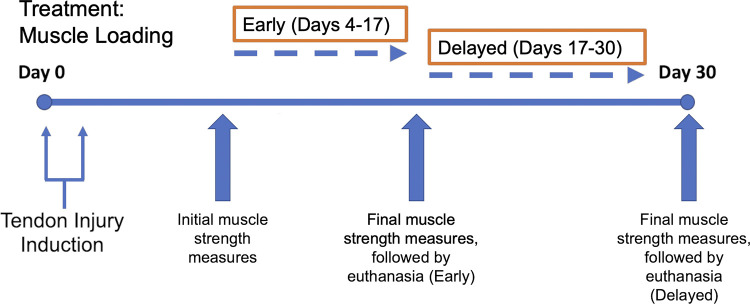
An initial set of loading parameters was identified for model development based on clinical consultation: dorsiflexion range of motion, footplate speed, number of repetitions, number of sets of repetitions, treatment frequency, treatment duration, and treatment timing, aiming to mimic the human treatment in a murine model (Fig. 1). Initial model development was carried out on uninjured mice to define a protocol that was well tolerated and nondeleterious to tendon biomechanical function. Subsequently, this protocol was applied to investigate the potential therapeutic efficacy of loading an injured tendon using an established murine model of Achilles tendinopathy (4, 6, 34, 35). All animals were randomized to treatment or naïve groups using MATLAB (Mathworks).
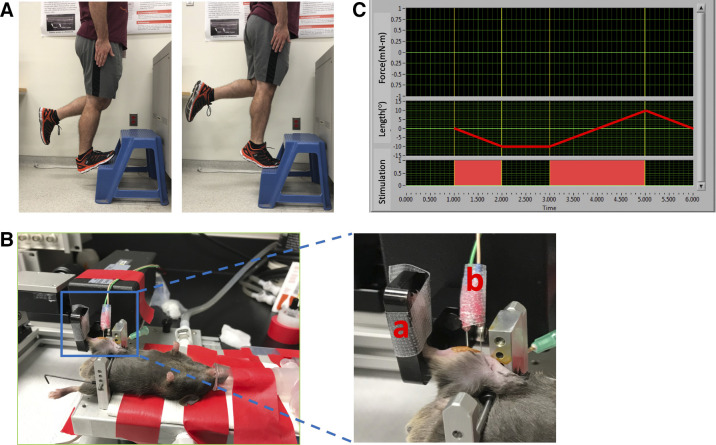
Figure 1.
A: image of the human exercise treatment modeled by the murine protocol showing dorsiflexion (“heel drop,” left) and plantarflexion (“heel raise,” right). B: murine heel drop/raise experimental setup: a: footplate to rotate ankle through range of motion, b: electrodes to control nerve stimulation. C: overview of the foot plate movement and electrode stimulation profiles. Electrodes were stimulated in conjunction with the foot plate movement to replicate body weight loading treatment. “Length” corresponds to the movement of the footplate, with a negative value denoting dorsiflexion and positive denoting plantarflexion. Profile shown illustrates one repetition of the exercise protocol.
Hindlimb Muscle Loading
Mice were deeply anesthetized with 3% isoflurane in oxygen in a sealed anesthesia box for 3 min before transfer to the temperature-controlled platform (37°C) of a 305 C-5N Dual-Mode Muscle Lever System (Aurora Scientific; Fig. 1A). Isoflurane was maintained at 1.5–3% via nose cone (36). For each mouse, the right hindlimb was clipped and hair remover applied. The foot was secured to the foot pedal so the femur was oriented 90° to the tibia and the tibia 90° to the foot. To prevent corneal drying, petrolatum ophthalmic lubricant was applied. Electrical stimulations were delivered (Aurora Scientific, 701 C stimulator) via subcutaneous F-E2 platinum-tipped needle electrodes (Natus Manufacturing Ltd.) stimulating the tibial nerve, inducing plantar flexion (36). Electrodes were inserted ∼0.5–1 mm deep with the proximal electrode inserted ∼1 mm distal and lateral to the knee while the distal electrode was placed in line with the proximal electrode ∼3–4 mm along the tibial nerve. Under computer control, the foot was rotated through the entire ankle joint range of motion while the plantar flexors simultaneously contracted to simulate body mass loading, consistent with human therapeutic exercises. This approach achieved two key components of the heel drop and raise movement: ankle range of motion with body mass loading.
Isometric twitch torque responses for each mouse were maximized by identifying the optimal stimulation current (typically 35–55 mA), which was then used throughout the experiment. Each individual repetition of the heel drop and raise exercise consisted of the foot pedal beginning at the neutral position of 90° (defined as the angle between the foot and tibial axes), rotation to 18° of dorsiflexion (as determined from pilot studies and in consideration of equipment capabilities), followed by a full plantarflexion (37), and return to the neutral position of 90°. Throughout the dorsiflexion (eccentric) and plantarflexion (concentric) movements, electrode stimulation was continuously delivered at the specified current and frequency (Fig. 1C). An additional group of naïve controls was included. These mice were treated as described above but without electrode insertion or muscle electrical stimulation. All naïve control animals were placed under anesthesia for equal duration and frequency (number of sessions) to the treatment groups. Following in vivo exercise, mice recovered in a sealed chamber with a flow of 1 L/min O2 and were returned to a clean cage once fully awake and mobile. Mice were monitored for 24 h following treatment to ensure there were no signs of distress, changes in appetite and activity level, or obvious impairments in gait, rearing, or ambulation.
Muscle Strength Measures (Torque-Frequency)
In vivo muscle strength was determined immediately before the initial, and 24 h following, the final loading session (Fig. 2), as described by Childers et al. (38), using the Aurora system described above. Identical measurements were performed for naïve, treated, and injured/untreated mice. Briefly, hindlimb torque values were obtained by electrically stimulating the plantarflexor muscle group over a range of stimulation frequencies (i.e., torque-frequency). Eleven frequencies were used, ranging from 1 to 200 Hz. Each stimulation frequency was delivered for 0.5 s at 30-s intervals. At each frequency, torque output measures were recorded to create a torque-frequency profile for each animal.
Figure 2.
Tendinopathy induction and treatment timeline. Each treatment session consisted of 3 sets of 10 repetitions (100 Hz stimulation) and was administered twice weekly.
Comparative Muscle Loading Protocols
After consulting clinical protocols shown to relieve symptomatic tendinopathy in human patients (13, 39), we implemented three sets of 20 repetitions of treatment (with 2 min of recovery between sets of treatment), three times per week for 2 wk. The stimulation frequency was set at 50 Hz, corresponding to 50% body mass loading. Body mass loading magnitude was determined before any loading bouts (day 0) by stimulating the muscle and measuring the torque output on the foot pedal. A full murine dorsiflexion range of motion (18°) and a footplate speed of 5 mm/s were used. Treated mice exhibited pronounced lameness when ambulating in the cage that did not resolve within 4 days. All animals were therefore quickly transitioned to a treatment plan of three sets of 10 repetitions with all additional parameters unchanged (Protocol 1). Subsequently, model parameters were reduced to three sets of 10 repetitions of treatment, twice weekly for 3 wk, with the remaining parameters identical to Protocol 1 (Protocol 2). These protocols did not result in lameness. Further model development tuned the stimulation frequency to ensure that the applied frequency elicited an appropriate hindlimb force output equivalent to the body mass loading of the animal. Therefore, stimulation frequencies of 50 Hz and 100 Hz (corresponding to half and full body mass loading, respectively) were used with three sets of 10 repetitions twice weekly for 2 wk (Protocol 3). Details of all treatment protocols are provided in Table 1.
Table 1.
Overview of parameters utilized in each treatment protocol
| Sets | Repetitions | Frequency | Duration | Stimulation Frequency | Footplate Speed | |
|---|---|---|---|---|---|---|
| Protocol 1 | 3 | 10 | 3×/wk | 2 wk | 50 Hz | 5 mm/s |
| Protocol 2 | 3 | 10 | 2×/wk | 3 wk | 50 Hz | 5 mm/s |
| Protocol 3 | 3 | 10 | 2×/wk | 2 wk | 100 Hz | 5 mm/s |
Muscle Loading Treatment of Tendon Injury
To assess efficacy of the developed model for treatment of tendon injury, we induced unilateral Achilles tendinopathy using our established mouse model (4, 34, 35). Briefly, two injections of 100 ng of active recombinat human transforming growth factor-β1 (rhTGFβ1 (Peprotech, Inc.) in 6 µL of PBS were delivered, 48 h apart, into the midportion of the Achilles tendon of 12-wk-old C57BL/6 male mice. The 28-G needle, secured to a 0.5-mL syringe, was inserted through the gastrocnemius complex and directed distally into the Achilles tendon midbody, where the rhTGFβ1 bolus injection was delivered. To investigate whether the functional quality of the healing response was dependent on the timing of the loading treatment, treatment was initiated either 2 days (“early”) or 2 wk (“delayed”) following the second TGFβ1 injection. The loading treatment was implemented twice weekly for 2 wk (Protocol 3), after which mice were euthanized by a CO2 overdose, followed by cervical dislocation, and assessed for biomechanical outcome measures (n = 8 mice/group; Fig. 2). Separate age-matched injured groups (“Injury early no treatment” and “Injury delayed no treatment”) were similarly anesthetized and secured to the pedal but received no loading treatment. An uninjured naïve group of age-matched mice was also included. Protocol 3 (3 sets of 10 repetitions, 2×/wk, 100 Hz frequency) was chosen as the final treatment protocol (Fig. 2).
Biomechanical Testing
Tendon tensile properties were the primary outcome measurements to assess treatment effects. For those mice receiving loading treatment, only the limb receiving the treatment was assessed for biomechanical outcomes. Biomechanical testing and quantification of mechanical properties were performed as previously described (40, 41). Briefly, the Achilles tendon/calcaneus complex was dissected from the surrounding tissues. Measurements of tendon thickness and width were performed with a laser displacement sensor (model no. LK-G82, Keyence Corp.) and precision caliper, respectively. Cross-sectional area (CSA) was computed assuming an elliptical geometry. The dissected foot was potted in dental cement while the proximal end of the Achilles tendon was secured between sandpaper-lined grips and mounted onto a material testing system (MTS Insight 10). Tensile testing (in a saline-filled chamber) consisted of preconditioning followed by a load-to-failure test at 0.05 mm/s.
Histology
Following completion of Protocol 3 (loading of uninjured tendons), hindlimbs from three mice per experimental group were dissected immediately after euthanasia and prepared for histological assessment (4, 6, 40). Briefly, tissues were fixed in 10% formaldehyde for 1 wk, decalcified using ethylenediaminetetraacetic acid (5% wt/vol in distilled H2O) for 4 wk, processed and embedded in paraffin, and sectioned at 5 µm thickness. Tissues were stained with hematoxylin and eosin (H&E) to evaluate qualitative changes in cellularity and overall structure of the muscle-tendon-bone unit.
Statistical Analysis
Multivariate analysis was carried out to determine correlations between muscle outcomes (maximum muscle strength) and tendon geometric and biomechanical outcomes (CSA, maximum load, stiffness, maximum stress, and elastic modulus). For this analysis, pooled data from injured and uninjured tendons were analyzed separately for treated (muscle-loaded) and untreated animals. A correlation coefficient >0.7 was considered a strong correlation, between 0.5 and 0.7 considered a moderate correlation, and <0.5 considered a weak or no correlation. All statistical analyses were completed using JMP Pro 15 (SAS). For all biomechanical outcome measures, n = 6–8 animals (i.e., one limb each from 6–8 mice) were assessed for each treatment group within each experiment. Due to the destructive nature of the biomechanical assay, a separate group of n = 3 mice was allocated to histological outcome measures. All animals were randomized to either a treatment or naïve group. For biomechanical outcomes, any individual samples deemed to be statistical outliers (as identified from Grubbs’ test, α level 0.05) were removed from the analysis. Biomechanical outcome measures were analyzed using a one-way ANOVA with Tukey’s post hoc tests. Statistical significance was set at P < 0.05.
RESULTS
Animal Tolerance to Procedures
As noted above, mice subjected to the initial protocol (3 sets of 20 repetitions, 3×/wk, 50 Hz) exhibited pronounced lameness that did not resolve within 4 days. These animals were transitioned to a milder protocol (Protocol 1) only to confirm that lameness did not continue and was resolved. However, these data were not considered further. Subsequent protocols were well tolerated by the mice and no visible gait issues (impairment, lameness) were observed for 24 h and beyond following any treatment session.
Muscle Loading Effects on Uninjured Tendons
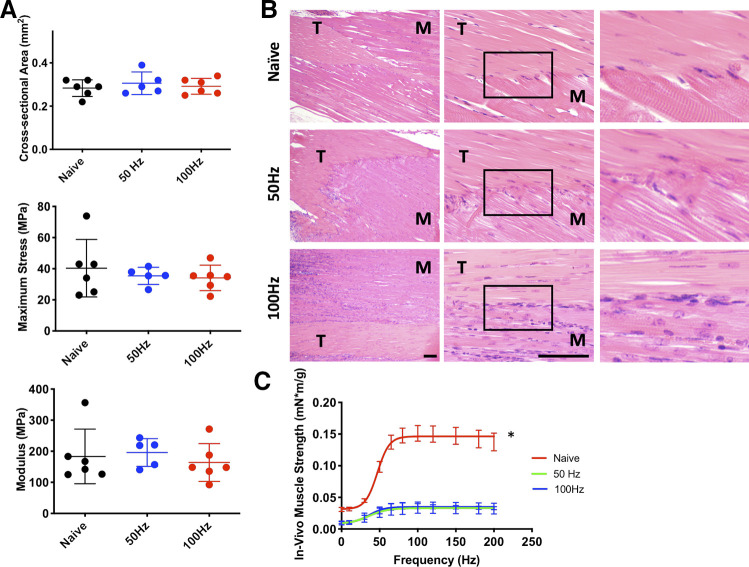
Following 3 wk of muscle loading treatment of uninjured tendons (Protocol 2: 3 sets of 10 repetitions, 2×/wk, 50 Hz frequency), no significant changes were observed in structural, geometric, or material properties relative to naïve animals (Fig. 3). No differences were noted for gastrocnemius CSA (calculated from width and thickness) and mass (data not shown). Model development investigating the effect of stimulation frequencies at either 50 or 100 Hz (Protocol 3: 3 sets of 10 repetitions, 2×/wk, 100 Hz) showed similar tendon geometric and mechanical properties. Neither treatment group exhibited significant differences compared with naïve untreated animals (Fig. 4). Histologically, there were no overt changes to the structure or cellularity of the Achilles tendon itself. However, compared with the naïve group, increased cellularity was observed at the myotendinous junction in the 50-Hz treatment group with a similar, more pronounced, increased cellularity in the 100-Hz group (Fig. 4B). The increased cellularity was primarily localized along the demarcation between muscle and tendon in the 50-Hz group but extended over a broader area into both the muscle and tendon tissue adjacent to the myotendinous junction in the 100-Hz group (Fig. 4B). The predominant cell morphology was large, oval cells with abundant cytoplasm and small, indistinct nuclei organized along lines of tension parallel to tendon fibroblasts and in many cases organized end to end in small groups with tendon fibroblasts. These areas of increased cellularity were not associated with the locations in the muscle where the electrodes were inserted. No evidence of muscle fiber or tendon degeneration was noted in either group. Muscle strength was significantly decreased in normalized (to animal body mass) force output (P = 0.001) following loading treatment in both 50-Hz and 100-Hz groups (Fig. 4C).
Figure 3.
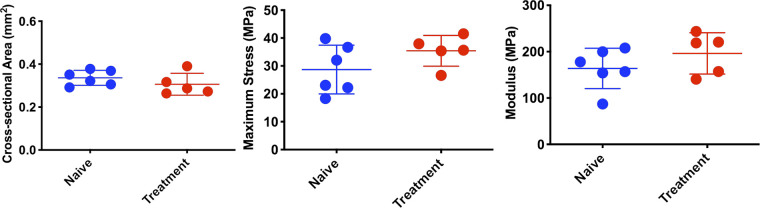
Biomechanical outcome measures following 3 wk of muscle loading treatment (twice weekly). Treatments showed no statistically significant changes in structural, geometric, or material properties compared with naïve animals. Error bars denote means ± SD. Each scatterplot symbol denotes data from an individual mouse Achilles tendon. P < 0.05 was deemed to be statistically significant.
Figure 4.
A: biomechanical outcome measures for 2 stimulation frequencies (50 vs. 100 Hz, applied to uninjured tendon). No significant differences between either treatment group compared with naïve untreated animals. B: hematoxylin-eosin (H&E) images of the myotendinous region. Top: sham control. Middle: 50-Hz stimulation. Bottom: 100-Hz stimulation. Left panel: 10x; middle panel: 40x; right panel: higher magnification insets of area in box in middle panel. M, muscle; T, tendon. Scale bars, 100 µm. C: muscle strength outcomes following 2 wk of treatment. *Significant differences from other groups. Errors bars denote means ± SD. Each scatterplot symbol denotes data from an individual mouse Achilles tendon. P < 0.05 was deemed to be statistically significant.
Muscle Loading Effects on Injured Tendons
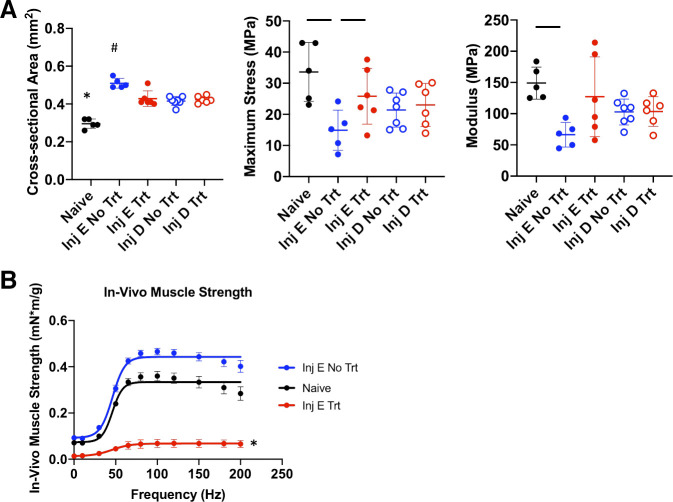
Early loading treatment of Achilles tendinopathy significantly decreased CSA (P = 0.004) and increased material properties (P = 0.050) relative to untreated tendinopathy 2 wk postinjury (Fig. 5A). In contrast, no significant changes in CSA or biomechanical properties were observed following delayed loading treatment. A significant decrease in normalized force output was observed following 2 wk of treatment compared with the naïve group (P = 0.001; Fig. 5B).
Figure 5.
A: comparison of tendon biomechanical properties following early and delayed treatment of tendinopathy. Symbols above groups denote statistically significant differences (P < 0.05) compared with all other groups. Horizontal lines denote significant differences between 2 individual groups. Inj, injury; E, early; D, delayed; Trt, treatment. Early muscle-loading treatment significantly decreased cross-sectional area (CSA) and increased material properties relative to untreated injury. No significant differences were observed for structural properties, stiffness, and maximum load (data not shown). B: muscle strength outcomes following 2 wk of early treatment. Errors bars denote means ± SD. Each scatterplot symbol denotes data from an individual mouse Achilles tendon. P < 0.05 was deemed to be statistically significant. *,#Significant differences from all other groups.
Correlation Analysis between Muscle and Tendon Outcomes
For untreated mice, multivariate analysis revealed a strong correlation between maximum muscle strength and tendon CSA (correlation coefficient: 0.91, P < 0.001), maximum stress (−0.85, P < 0.01), and elastic modulus (−0.93, P < 0.001). For treated mice, muscle strength was moderately correlated (P > 0.05) with maximum load (0.53), stiffness (0.64), and tendon modulus (0.57).
DISCUSSION
We successfully developed and applied an innovative, tissue-targeted muscle-tendon loading method to treat Achilles tendinopathy in mice. This loading paradigm, bioinspired by human clinical protocols, constitutes the first preclinical, in vivo investigation of a common physical therapy exercise (heel drop and raise) to treat Achilles tendinopathy. Using electrical stimulation of the plantarflexor complex while simultaneously moving the ankle joint through a physiological range of motion, we identified a combination of loading variables that were nondeleterious to healthy tendon biomechanical function, were well tolerated by the animals, and promoted restoration of tensile properties of tendinopathic tissue, consistent with clinical findings (42). Specifically, we have shown that early implementation of the loading protocol decreased tendon CSA while improving material properties relative to the untreated injury.
Although our described methodology was designed to simulate a specific rehabilitation exercise, the experimental system allows for the development of a variety of treatment protocols combining both ankle movement and muscle electrical stimulation. The system can be adapted to study any desired combination of eccentric and concentric loading regimens. To our knowledge, the Aurora in vivo system has primarily been utilized in a “diagnostic” capacity, quantifying muscle functional measures in states of health and disease (36, 38, 43, 44) along with recent work that mimicked human gait with murine muscles (45). Therefore, the current study presents the first application of the system for muscle loading-based therapeutic treatment of musculoskeletal injuries and allows for the investigation of myriad loading regimens. While it has been widely accepted that the eccentric component of these muscle loading exercises drive improvements in symptom relief (46–48), other loading regimens, such as isolated concentric training (49), heavy-slow resistance (50, 51), and concentric/eccentric progressing to eccentric training (52, 53), have also been shown to improve outcomes. Adaptation of this system for treatment of hindlimb injuries is also not limited to the stimulation of the plantar flexor muscle groups through the tibial nerve. Targeting the peroneal nerve, for example, to contract the dorsiflexors of the hindlimb can be readily implemented (54).
No significant differences were apparent following delayed treatment (relative to untreated tendons), as both groups showed improved biomechanical outcomes at day 30 postinjury. These results suggest that tendon injury without treatment may have resolved within the examined time course (i.e., 4 wk following injury), consistent with our previous observations (4, 40). Muscle strength was correlated strongly with tendon CSA (positive correlation), maximum stress (negative correlation), and elastic modulus (negative correlation) in those mice that received the tendon injury but no muscle loading treatment. The positive correlation with CSA in the untreated animals could potentially be attributed to gastrocnemius hypertrophy following tendon injury, a characteristic likely not seen in the treated mice, possibly due to the apparent muscle damage caused by the treatment.
Whereas healthy tendon functional properties were not compromised by the loading treatments, the adjoining muscle complex exhibited functional and cellular changes. Histological examination revealed increased cellularity along the demarcation of the myotendinous junction in treated animals, most marked in the 100-Hz group. Immunohistochemistry would be required to definitively identify these additional cells not present in the untreated animals. The large size, indistinct morphology, and lack of clear nuclear staining, along with their proximity to and alignment with mature tendon fibroblasts is of note. One possible cell type is mesenchymal progenitor cells (55), which may have originated from proliferation of tendon fibroblasts, tendon progenitor cells, migration from the vasculature, and/or satellite cells from the muscle. Their appearance is not consistent with immune cells, such as tissue macrophages, and there is no evidence of tissue degeneration as described in a murine model of electroporation (56). The infiltrate was not neutrophilic in nature. The increased cellularity may represent an early adaptive response to the increased loads. There is a possibility that the increased cellularity follows a pattern similar to that seen in healing tendon, where cellularity increases in response to injury and gradually decreases back to baseline cellularity with elongated tendon fibroblasts predominating (55).
Consistent with these histological findings, muscle strength measures were significantly decreased in torque output following loading treatment. Of note, the decrease in muscle strength was not evident when comparing naïve animals with the TGFβ1-injured but untreated animals, suggesting that the growth factor injection was not deleterious to muscle function. The impaired muscle force output suggests the likelihood of tissue structural damage. However, these alterations did not appear to induce pain or compromise ambulation or cage activities of mice following the treatments. Furthermore, the decrease in muscle strength may be indicative of an early adaptive response to the loading protocol, consistent with the loss of strength human patients often experience when beginning a new rehabilitative exercise or treatment regimen (57). Whereas the emphasis of the current study quantified tendon biomechanical properties as the functional phenotype of tendon adaptation to muscle loading, our future studies will examine whether the decrease in muscle strength is a transient or more persistent outcome. Although there is the potential for the reduction in muscle strength to result in tendon unloading, all visual assessments indicate the animals exhibited no signs of distress, changes to appetite and activity level, or obvious impairments in gait, rearing, or ambulation, indicating that dramatic unloading of the muscle-tendon unit does not occur. Further studies will incorporate more frequent assessments to temporally examine muscle strength recovery following muscle loading treatments. Decreased activation level of the muscle can also be investigated to identify a loading threshold that promotes Achilles tendon healing with minimal compromise of muscle strength.
As with any murine model of human musculoskeletal disease, an inherent limitation arises due to fundamental differences in anatomy and limb function between the human and the mouse. Since developing a voluntary, single-limb exercise regimen inspired by the human clinical heel drop and raise is not feasible in mice, we implemented a concurrent muscle stimulation and ankle range of motion methodology. Although published human protocols often consist of several hundred (or more) repetitions of the heel drop and raise motion (3) per week, our initial experiments revealed that mice could not well tolerate 180 weekly repetitions. Moreover, the relatively brief timeline of the tendon injury models also does not allow for a training program to be implemented in the mice before the therapeutic treatment implementation. Although an exact replication of human exercise protocols is therefore not possible in our model, our approach can be readily modified to mimic a variety of muscle-loading patterns. In its current form, the plantarflexors are externally activated during the entirety of the heel drop and raise portions of the exercise. Additionally, future work will implement the strategy of Bukovec et al. (45) to develop a more accurate muscle stimulation pattern. Briefly, through the use of OpenSim or custom-written software, the human exercise can be modeled and scaled to the murine system described herein to more accurately match the muscle stimulation patterns. Concentric exercise shows greater electromyography activation than eccentric exercises in normal muscles, whereas eccentric exercises induce a higher maximum voluntary contraction in the gastrocnemius muscle in human subjects (58). These components can therefore be incorporated into the electrode stimulation profile in future work.
Our new experimental methodology will facilitate further studies aimed at understanding the biological and molecular basis for functional improvement and understanding neuromuscular mechanisms underlying tendon healing. While we have thus far focused our analyses on tendon biomechanical properties, we will further utilize this model to include characterization of structural (e.g., matrix organization), functional (muscle contractile properties), and cellular (gene expression) behavior of the entire musculotendinous unit. The Aurora system can be adapted to study numerous combinations of eccentric and concentric loading regimens with precise parameter control. Therefore, this model offers a unique opportunity to contribute novel insights into cross-talk among muscle, tendon, and bone in the context of rehabilitative loading and to further study mechanisms of musculotendinous adaptation under different muscle loading protocols.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR63144 (V.M.W.), The Edward Via College of Osteopathic Medicine/Virginia-Maryland College of Veterinary Medicine Center for One Health Research Seed Grant (L.A.D. and P.G.B.), and the Interdisciplinary Graduate Education Program in Regenerative Medicine at Virginia Tech (S.N.R.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N.R., A.E.N., P.G.B., and V.M.W. conceived and designed research; S.N.R. performed experiments; S.N.R., A.E.N., R.W.G., and L.A.D. analyzed data; S.N.R., A.E.N., R.W.G., L.A.D., P.G.B., and V.M.W. interpreted results of experiments; S.N.R. prepared figures; S.N.R. drafted manuscript; S.N.R., A.E.N., R.W.G., L.A.D., P.G.B., and V.M.W. edited and revised manuscript; S.N.R., A.E.N., R.W.G., L.A.D., P.G.B., and V.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Shelby Hamm and Kate Bukovec from the Grange lab for their assistance with the Aurora system, Colleen Valentine for assistance with histological analyses, and Morgan Herrera for assistance with the in vivo experiments.
REFERENCES
- 1.de Jonge S, van den Berg C, de Vos RJ, van der Heide HJ, Weir A, Verhaar JA, Bierma-Zeinstra SM, Tol JL. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med 45: 1026–1028, 2011. doi: 10.1136/bjsports-2011-090342. [DOI] [PubMed] [Google Scholar]
- 2.Yasui Y, Tonogai I, Rosenbaum AJ, Shimozono Y, Kawano H, Kennedy, JG. The risk of Achilles tendon rupture in the patients with Achilles tendinopathy: healthcare database analysis in the United States. Biomed Res Int 2017: 7021862, 2017. doi: 10.1155/2017/7021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maffulli N. Rupture of the Achilles tendon. J Bone Joint Surg Am 81: 1019–1036, 1999. doi: 10.2106/00004623-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Bittermann A, Gao S, Rezvani S, Li J, Sikes KJ, Sandy J, Wang V, Lee S, Holmes G, Lin J, Plaas A. Oral ibuprofen interferes with cellular healing responses in a murine model of Achilles tendinopathy. J Musculoskelet Disord Treat 4: 049, 2018. doi: 10.23937/2572-3243.1510049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paavola M, Orava S, Leppilahti J, Kannus P, Järvinen M. Chronic Achilles tendon overuse injury: complications after surgical treatment. An analysis of 432 consecutive patients. Am J Sports Med 28: 77–82, 2000. doi: 10.1177/03635465000280012501. [DOI] [PubMed] [Google Scholar]
- 6.Sikes KJ, Li J, Gao SG, Shen Q, Sandy JD, Plaas A, Wang VM. TGF-b1 or hypoxia enhance glucose metabolism and lactate production via HIF1A signaling in tendon cells. Connect Tissue Res 59: 458–471, 2018. doi: 10.1080/03008207.2018.1439483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena A, Maffulli N, Nguyen A, Li A. Wound complications from surgeries pertaining to the Achilles tendon: an analysis of 219 surgeries. J Am Podiatr Med Assoc 98: 95–101, 2008. doi: 10.1016/j.ptsp.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Frizziero A, Vittadini F, Fusco A, Giombini A, Masiero S. Efficacy of eccentric exercise in lower limb tendinopathies in athletes. J Sports Med Phys Fitness 56: 1352–1358, 2016. [PubMed] [Google Scholar]
- 9.Habets B, van Cingel RE. Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols. Scand J Med Sci Sports 25: 3–15, 2015. doi: 10.1111/sms.12208. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill S, Barry S, Watson P. Plantarflexor strength and endurance deficits associated with mid-portion Achilles tendinopathy: the role of soleus. Phys Ther Sport 37: 69–76, 2019. doi: 10.1016/j.ptsp.2019.03.002. ]. [DOI] [PubMed] [Google Scholar]
- 11.McAuliffe S, Tabuena A, McCreesh K, O'Keeffe M, Hurley J, Comyns T, Purtill H, O'Neill S, O'Sullivan K. Altered strength profile in Achilles tendinopathy: a systematic review and meta-analysis. J Athl Train 54: 889–900, 2019. doi: 10.4085/1062-6050-43-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfredson H, Lorentzon R. Chronic Achilles tendinosis: recommendations for treatment and prevention. Sports Med 29: 135–146, 2000. doi: 10.2165/00007256-200029020-00005. [DOI] [PubMed] [Google Scholar]
- 13.Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med 26: 360–366, 1998. doi: 10.1177/03635465980260030301. [DOI] [PubMed] [Google Scholar]
- 14.Fahlström M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc 11: 327–333, 2003. doi: 10.1007/s00167-003-0418-z. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill S, Watson PJ, Barry S. Why are eccentric exercises effective for Achilles tendinopathy? Int J Sports Phys Ther 10: 552–562, 2015. . [PMC free article] [PubMed] [Google Scholar]
- 16.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med 38: 8–11, 2004. doi: 10.1136/bjsm.2001.000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vos RJ, Heijboer MP, Weinans H, Verhaar JA, van Schie JT. Tendon structure's lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J Sport Rehabil 21: 34–43, 2012. doi: 10.1123/jsr.21.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Scott A, Docking S, Vicenzino B, Alfredson H, Murphy RJ, Carr AJ, Zwerver J, Lundgreen K, Finlay O, Pollock N, Cook JL, Fearon A, Purdam CR, Hoens A, Rees JD, Goetz TJ, Danielson, P. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med 47: 536–544, 2013. [Erratum in Br J Sports Med 47: 774, 2013]. doi: 10.1136/bjsports-2013-092329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Plas A, de Jonge S, de Vos RJ, van der Heide HJ, Verhaar JA, Weir A, Tol JL. A 5-year follow-up study of Alfredson's heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med 46: 214–218, 2012. doi: 10.1136/bjsports-2011-090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silbernagel KG. Does one size fit all when it comes to exercise treatment for Achilles tendinopathy? J Orthop Sports Phys Ther 44: 42–44, 2014. doi: 10.2519/jospt.2014.0103. [DOI] [PubMed] [Google Scholar]
- 21.Galloway MT, Lalley AL, Shearn JT. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am 95: 1620–1628, 2013. doi: 10.2106/JBJS.L.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaux JF, Drion P, Libertiaux V, Colige A, Hoffmann A, Nusgens B, Besancon B, Forthomme B, Le GC, Franzen R, Defraigne JO, Cescotto S, Rickert M, Crielaard JM, Croisier JL. Eccentric training improves tendon biomechanical properties: a rat model. J Orthop Res 31: 119–124, 2013. doi: 10.1002/jor.22202. [DOI] [PubMed] [Google Scholar]
- 23.Kaux JF, Libertiaux V, Leprince P, Fillet M, Denoel V, Wyss C, Lecut C, Gothot A, Le Goff C, Croisier JL, Crielaard JM, Drion P. Eccentric training for tendon healing after acute lesion: a rat model. Am J Sports Med 45: 1440–1446, 2017. doi: 10.1177/0363546517689872. [DOI] [PubMed] [Google Scholar]
- 24.Allen KD, Mata BA, Gabr MA, Huebner JL, Adams SB Jr, Kraus VB, Schmitt DO, Setton LA. Kinematic and dynamic gait compensations resulting from knee instability in a rat model of osteoarthritis. Arthritis Res Ther 14: R78, 2012. doi: 10.1186/ar3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res 26: 1611–1617, 2008. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J, Shen J, Smith BP, Ritting A, Smith TL, Koman LA. Bioprotection of tendon repair: adjunctive use of botulinum toxin A in Achilles tendon repair in the rat. J Bone Joint Surg Am 89: 2241–2249, 2007. doi: 10.2106/JBJS.D.03054. [DOI] [PubMed] [Google Scholar]
- 27.Hettrich CM, Rodeo SA, Hannafin JA, Ehteshami J, Shubin Stein BE. The effect of muscle paralysis using Botox on the healing of tendon to bone in a rat model. J Shoulder Elbow Surg 20: 688–697, 2011. doi: 10.1016/j.jse.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Popp D, Weber J, Kerschbaum M, Schicho A, Baumann F, Hilber F, Krutsch W, Alt V, Pfeifer C. Early functional treatment or trivialization? Current treatment strategies in lateral ligament injuries of the ankle. Eur J Sport Sci Jan 1: 1–8, 2021. doi: 10.1080/17461391.2020.1845813. [DOI] [PubMed] [Google Scholar]
- 29.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg 18: 669–675, 2009. doi: 10.1016/j.jse.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther 18: 80–85, 2005. doi: 10.1197/j.jht.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am 7: 170–175, 1982. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 32.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 21: 228–237, 2012. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammerman M, Blomgran P, Dansac A, Eliasson P, Aspenberg P. Different gene response to mechanical loading during early and late phases of rat Achilles tendon healing. J Appl Physiol (1985) 123: 800–815, 2017. doi: 10.1152/japplphysiol.00323.2017. [DOI] [PubMed] [Google Scholar]
- 34.Bell R, Li J, Gorski DJ, Bartels AK, Shewman EF, Wysocki RW, Cole BJ, Bach BR Jr, Mikecz K, Sandy JD, Plaas AH, Wang VM. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J Biomech 46: 498–505, 2013. doi: 10.1016/j.jbiomech.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Bell R, Li J, Shewman EF, Galante JO, Cole BJ, Bach BR Jr, Troy KL, Mikecz K, Sandy JD, Plaas AH, Wang VM. ADAMTS5 is required for biomechanically-stimulated healing of murine tendinopathy. J Orthop Res 31: 1540–1548, 2013. doi: 10.1002/jor.22398. [DOI] [PubMed] [Google Scholar]
- 36.Capogrosso RF, Mantuano P, Cozzoli A, Sanarica F, Massari AM, Conte E, Fonzino A, Giustino A, Rolland JF, Quaranta A, De Bellis M, Camerino GM, Grange RW, De Luca A. Contractile efficiency of dystrophic mdx mouse muscle: in vivo and ex vivo assessment of adaptation to exercise of functional end points. J Appl Physiol (1985) 122: 828–843, 2017. doi: 10.1152/japplphysiol.00776.2015. [DOI] [PubMed] [Google Scholar]
- 37.Ashton-Miller JA, He Y, Kadhiresan VA, McCubbrey DA, Faulkner JA. An apparatus to measure in vivo biomechanical behavior of dorsi- and plantarflexors of mouse ankle. J Appl Physiol (1985) 72: 1205–1211, 1992. doi: 10.1152/jappl.1992.72.3.1205. [DOI] [PubMed] [Google Scholar]
- 38.Childers MK, Grange RW, Kornegay JN. In vivo canine muscle function assay. J Vis Exp 50: 2623, 2011. doi: 10.3791/2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med 39: 607–613, 2011. doi: 10.1177/0363546510384789. [DOI] [PubMed] [Google Scholar]
- 40.Rezvani SN, Chen J, Li J, Midura R, Cali V, Sandy JD, Plaas A, Wang VM. In-vivo efficacy of recombinant human hyaluronidase (rHuPH20) injection for accelerated healing of murine retrocalcaneal bursitis and tendinopathy. J Orthop Res 38: 59–69, 2020. doi: 10.1002/jor.24459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang VM, Bell RM, Thakore R, Eyre DR, Galante JO, Li J, Sandy JD, Plaas A. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J Orthop Res 30: 620–626, 2012. doi: 10.1002/jor.21558. [DOI] [PubMed] [Google Scholar]
- 42.Zellers JA, Christensen M, Kjær IL, Rathleff MS, Silbernagel KG. Defining components of early functional rehabilitation for acute Achilles tendon rupture: a systematic review. Orthop J Sports Med 7: 2325967119884071, 2019. doi: 10.1177/2325967119884071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tegeler CJ, Grange RW, Bogan DJ, Markert CD, Case D, Kornegay JN, Childers MK. Eccentric contractions induce rapid isometric torque drop in dystrophin-deficient dogs. Muscle Nerve 42: 130–132, 2010. doi: 10.1002/mus.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerlinger-Romero F, Addinsall AB, Lovering RM, Foletta VC, van der Poel C, Della-Gatta PA, Russell AP. Non-invasive assessment of dorsiflexor muscle function in mice. J Vis Exp 17: 143, 2019. doi: 10.3791/58696. [DOI] [PubMed] [Google Scholar]
- 45.Bukovec KE, Hu X, Borkowski M, Jeffery D, Blemker SS, Grange RW. A novel ex vivo protocol to mimic human walking gait: implications for Duchenne muscular dystrophy. J Appl Physiol (1985) 129: 779–791, 2020. doi: 10.1152/japplphysiol.00002.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfredson H, Lorentzon R. Intratendinous glutamate levels and eccentric training in chronic Achilles tendinosis: a prospective study using microdialysis technique. Knee Surg Sports Traumatol Arthrosc 11: 196–199, 2003. doi: 10.1007/s00167-003-0360-0. [DOI] [PubMed] [Google Scholar]
- 47.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med 15: 133–135, 2005. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 48.Croisier J-L, Forthomme B, Foidart-Dessalle M, Godon B, Crielaard J-M. Treatment of recurrent tendinitis by isokinetic eccentric exercises. IES 9: 133–141, 2001. doi: 10.3233/IES-2001-0077. [DOI] [Google Scholar]
- 49.Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surg Sports Traumatol Art 9: 42–47, 2001. doi: 10.1007/s001670000148. [DOI] [PubMed] [Google Scholar]
- 50.Beyer R, Kongsgaard M, Hougs Kjær B, Øhlenschlæger T, Kjær M, Magnusson SP. Heavy slow resistance versus eccentric training as treatment for chilles tendinopathy: a randomized controlled trial. Am J Sports Med 43: 1704–1711, 2015. doi: 10.1177/0363546515584760. [DOI] [PubMed] [Google Scholar]
- 51.Kongsgaard M, Kovanen V, Aagaard P, Doessing S, Hansen P, Laursen AH, Kaldau NC, Kjaer M, Magnusson SP. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports 19: 790–802, 2009. doi: 10.1111/j.1600-0838.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 52.Silbernagel KG, Thomeé R, Thomeé P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain–a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports 11: 197–206, 2001. doi: 10.1034/j.1600-0838.2001.110402.x. [DOI] [PubMed] [Google Scholar]
- 53.Silbernagel KG, Thomeé R, Eriksson BI, Karlsson J. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy: a randomized controlled study. Am J Sports Med 35: 897–906, 2007. doi: 10.1177/0363546506298279. [DOI] [PubMed] [Google Scholar]
- 54.Baima J, Krivickas L. Evaluation and treatment of peroneal neuropathy. Curr Rev Musculoskelet Med 1: 147–153, 2008. doi: 10.1007/s12178-008-9023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res 23: 84–92, 2005. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Roche JA, Ford-Speelman DL, Ru LW, Densmore AL, Roche R, Reed PW, Bloch RJ. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am J Physiol Cell Physiol 301: C1239–C1250, 2011. doi: 10.1152/ajpcell.00431.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cleak MJ, Eston RG. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br J Sports Med 26: 267–272, 1992. doi: 10.1136/bjsm.26.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu J. Comparison of lower limb muscle activity during eccentric and concentric exercises in runners with Achilles tendinopathy. J Phys Ther Sci 26: 1351–1353, 2014. doi: 10.1589/jpts.26.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]