Abstract
Olfactory receptor (Olfr) 78 is expressed in the carotid bodies (CB) and participates in CB responses to acute hypoxia. Olfr78 is also expressed in the kidney, which is a major site of erythropoietin (Epo) production by hypoxia. The present study examined the role of Olfr78 in cardiorespiratory and renal Epo gene responses to hypobaric hypoxia (HH), simulating low O2 condition experienced at high altitude. Studies were performed on adult, male wild-type (WT) and Olfr78 null mice treated with 18 h of HH (0.4 atmospheres). HH-treated WT mice exhibited increased baseline breathing, augmented hypoxic ventilatory response, elevated blood pressure, and plasma norepinephrine (NE) levels. These effects were associated with increased baseline CB sensory nerve activity and augmented CB sensory nerve response to subsequent acute hypoxia. In contrast, HH-treated Olfr78 null mice showed an absence of cardiorespiratory and CB sensory nerve responses, suggesting impaired CB-dependent cardiorespiratory adaptations. WT mice responded to HH with activation of the renal Epo gene expression and elevated plasma Epo levels, and these effects were attenuated or absent in Olfr78 null mice. The attenuated Epo activation by HH was accompanied with markedly reduced hypoxia-inducible factor (HIF)-2α protein and reduced activation of HIF-2 target gene Sod-1 in Olfr78 null mice, suggesting impaired transcriptional activation of HIF-2 contributes to attenuated Epo responses to HH. These results demonstrate a hitherto uncharacterized role for Olfr78 in cardiorespiratory adaptations and renal Epo gene activation by HH such as that experienced at high altitude.
NEW & NOTEWORTHY In this study, we delineated a previously uncharacterized role for olfactory receptor 78 (Olfr78), a G-protein-coupled receptor in regulation of erythropoietin and cardiorespiratory responses to hypobaric hypoxia. Our results demonstrate a striking loss of cardiorespiratory adaptations accompanied by an equally striking absence of carotid body sensory nerve responses to hypobaric hypoxia in Olfr78 null mice. We further demonstrate a hitherto uncharacterized role for Olfr78 in erythropoietin activation by hypobaric hypoxia.
Keywords: carotid body, erythropoietin, high-altitude adaptation, hypertension, sympathetic nervous system
INTRODUCTION
Humans and mammals experience hypobaric hypoxia (HH) at high altitude. HH initiates a series of physiological adaptations to maintain homeostasis. One such adaptation is ventilatory acclimatization to hypoxia (VAH), manifested as increased resting ventilation (1) and enhanced hypoxic ventilatory response (HVR) (2, 3). HH also increases blood pressure (BP) due to vasoconstriction mediated by the sympathetic nervous system (4). While VAH facilitates O2 intake to compensate for hypoxia, elevated BP ensures an adequate supply of oxygenated blood to vital organs by increasing perfusion pressure. Thus, cardiorespiratory responses represent important physiological adaptations to HH. Carotid body (CB)-ablated animals (5–7) and rodents with selective loss of CB activation by hypoxia (2, 8) exhibit remarkable loss of cardiorespiratory responses to HH. Moreover, prolonged hypoxia increases CB sensory activity (9) and augments CB sensory nerve response to subsequent acute hypoxia (10). These studies suggest that activation of the CB chemo reflex is essential for eliciting cardiorespiratory adaptations to HH.
Olfactory receptors (ORs) are G protein-coupled receptors expressed in the olfactory epithelium and were originally thought to be involved in sensing odorants (11). Emerging evidence suggests some ORs are expressed in tissues other than the olfactory epithelium. For instance, olfactory receptor (Olfr)-78 is expressed in glomus cells, the primary O2-sensing cells of the CB (12, 13). Recent studies showed that Olfr78 is an integral component of the CB sensory nerve response to a wide range of low-oxygen levels (12, 14) but not to severe hypoxia (14, 15). These studies implicate Olfr78 in sensing stimuli other than odorants. The role of Olfr78 in CB-dependent cardiorespiratory adaptations to HH is not known.
In addition to cardiorespiratory adaptations, HH also activates the Epo gene, which encodes the glycoprotein hormone erythropoietin (Epo). Epo facilitates O2 transport in blood through stimulation of red blood cell production and thereby provides a defense against hypoxia (16). In adult mammals, the kidney is a major source of Epo production (16). Hypoxia-induced Epo gene activation requires hypoxia-inducible factor (HIF)-2, a member of the HIF family of transcriptional activators (17–23). Given that Olfr78 is also expressed in the kidney (24) and hypoxia stimulates Epo gene expression, whether Olfr78 participates in the renal Epo gene activation by HH remains an intriguing possibility.
The objectives of the present study are to test the hypotheses that Olfr78 participates in 1) CB-mediated cardiorespiratory adaptations to HH and 2) Epo gene activation by HH involving HIF-2. These possibilities were tested in age- and gender-matched wild-type (WT) and Olfr78 null mice exposed to 18 h of 0.4 atmospheres simulating an altitude of ∼6,400 m, which is equivalent to 9.4% inspired O2. Our results showed that Olfr78 null mice exhibit striking impairment of CB sensory nerve responses and attenuated or absence of cardiorespiratory adaptations to HH. We further found remarkable attenuation of renal Epo gene activation and absence of elevated plasma Epo protein levels in Olfr78 null mice treated with HH.
METHODS
Preparation of Animals
Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago (Protocol No. ACUP 71811, approved on February 27, 2019). Experiments were performed on age-matched adult, male, wild-type (WT, n = 33) and Olfr78 null (n = 34) mice (weights 20–27 g) on a C57BL/6 background, a gift from Dr. J. Pluznick, Johns Hopkins University.
Exposure to Hypobaric Hypoxia
Mice along with their standard housing cages were placed in a hypobaric hypoxia chamber for 18 h (between 17:00 PM and 11:00 AM next day). The hypobaric chamber contained an inlet port for entry of room air and an outlet port for administration of vacuum for induction of 0.4 ATM environments. The mice were unrestrained and fed ad libitum. Blood pressure and breathing were measured 1 h after terminating HH treatment. BP and breathing were monitored by tail-cuff method and whole body plethysmography, respectively. Measurements were made in the same mouse before and after HH, such that each mouse served as its own control. Mice were acclimatized to the BP measuring instrument or plethysmograph chamber for at least 1 h. All measurements were made between 12 AM and 1 PM to exclude potential confounding influence from circadian variations. CB sensory nerve activity was measured 2–3 h following termination of HH.
Measurements of Breathing
Ventilation was monitored by whole body plethysmograph (Buxco, DSI, St. Paul, MN) as described (2, 14). Oxygen consumption () and CO2 production () were determined by the open-circuit method as described (25). The following equations were used to calculate and : and wherein “i” denotes ingoing gas and “e” denotes outgoing gas, the flow, and F fractional concentration (25). Breathing was recorded while the mice breathed room air or 12% O2-balanced with N2. O2 consumption and CO2 production were measured at the end of 5 min of breathing either room air or hypoxic gas. Sighs, sniffs, and movement-induced changes in breathing were excluded in the analysis. All recordings were made at an ambient temperature of 25 ± 1°C. Minute ventilation (V˙e = Vt × RR, where Vt is tidal volume and RR is respiratory rate) was calculated and normalized to body weight and expressed as a ratio to O2 consumption (V˙e/V˙o2).
Measurements of Blood Pressure
Blood pressure was monitored by the tail-cuff method in unanesthetized mice using a noninvasive BP system (IITC Life Science Inc., Woodland Hills, CA) as described previously (26). Mice were placed in the restrainer provided by the manufacturer and allowed to acclimate at least 1 h before the measurements of BP.
Measurements of Plasma Norepinephrine
Plasma norepinephrine levels were analyzed by high-performance liquid chromatography (HPLC) coupled with electrochemical detection (ECD) as described previously (26). Briefly, blood samples (∼300 μL) were collected from anaesthetized (urethane, 1.2 g/kg, i.p.) mice by cardiac puncture and placed in heparinized (30 U/mL of blood) ice-cold microcentrifuge tubes. Plasma was separated by centrifugation and stored at −80°C until further analysis. Plasma norepinephrine (NE) was determined by HPLC-ECD using dihydroxybenzylamine as an internal standard (27). The NE levels were corrected for recovery loss and expressed as nanograms of NE per 100 mL of plasma.
Measurements of Carotid Body Sensory Nerve Activity
The sensory nerve activity was recorded from ex vivo CBs as described previously (14, 28). Briefly, CBs along with the sinus nerves were harvested from anesthetized (urethane, 1.2 g/kg, i.p.) mice, placed in a recording chamber (volume, 250 μL) and superfused with warm physiological saline (35°C) at a rate of 3 mL/min. The composition of the medium was (mM): NaCl, 125; KCl, 5; CaCl2, 1.8; MgSO4, 2; NaH2PO4, 1.2; NaHCO3, 25; d-Glucose, 10; Sucrose, 5. The solution was bubbled with 21% O2/5% CO2. Hypoxic challenges were achieved by switching the perfusate to physiological saline equilibrated with desired levels of O2. Oxygen levels in the medium were continuously monitored by a platinum electrode placed next to the CB using a polarographic amplifier (Model 1900, A-M Systems, Sequim, WA). To facilitate recording of clearly identifiable action potentials, the sinus nerve was treated with 0.1% collagenase for 5 min. Action potentials (1–3 active units) were recorded from one of the nerve bundles with a suction electrode (band pass 30 Hz–10 kHz) and stored in a computer via a data acquisition system (PowerLab/8P). “Single” units were sorted based on the shape, height, and duration of the individual action potentials using the spike discrimination module.
Measurements of mRNA in the Kidney by Quantitative Real-Time PCR
Real-time polymerase chain reaction (RT-PCR) assay was performed using a MiniOpticon system (Bio-Rad Laboratories) with SYBR Green ER two-step qRT-PCR kit (No. 11764-100, Invitrogen) as described (29). Briefly, kidneys were harvested from anesthetized mice, and RNA was extracted using TRIZOL reagent and reverse transcribed using iScript reverse transcriptase super mix (Bio-Rad). The mRNA abundance was calculated with the comparative threshold (CT) method using the formula “2−ΔCT” where ΔCT is the difference between the threshold cycle of the given target cDNA expressed in normoxia and HH kidneys. The CT value was taken as a fractional cycle number at which the emitted fluorescence of the sample passes a fixed threshold above the baseline. Values were compared with the internal standard 18S gene. Purity and specificity of all products were confirmed by omitting the template and performing a standard melting curve analysis. Primers used in the experiments are listed in Table 1.
Table 1.
Primer sequences for real time RT-PCR amplification
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Epo | 5′- CACCCTGCTGCTTTTACTCT-3′ | 5′- AACCCATCGTGACATTTTCT-3′ |
| Sod1 | 5′- AGAAGGCAAGCGGTGAACCAGT-3′ | 5′- CACATTGCCCAGGTCTCCAACATG-3′ |
| VEGF | 5′- GGAAGAAGAGGCCTGGTAATGGC-3′ | 5′- TGTGTGAGTGGCTTACCCTTCC-3′ |
| 18s | 5′- GTAACCCGTTGAACCCCATT-3′ | 5′- CCATCCAATCGGTAGTAGCG |
Epo, erythropoietin; Sod1, superoxide dismutase-1; VEGF, vascular endothelial growth factor; 18S, 18S rRNA.
Measurements of Plasma Epo Protein
Plasma erythropoietin (EPO) levels were measured using the Quantikine ELISA mouse erythropoietin immunoassay kit (MEP00B, R&D Systems, Inc. Minneapolis, MN). The ELISA assay was based on the double-antibody sandwich method. Briefly, blood was collected from anesthetized mice via cardiac puncture in heparinized tubes and plasma was separated and stored at −80°C until further analysis. A total of 70 µL of each sample was diluted (twofold) with Calibrator Diluent (MEP00B, R&D Systems, Inc. Minneapolis, MN). A total of 50 µL diluted sample was mixed with 50 µL Assay Diluent (MEP00B, R&D Systems, Inc. Minneapolis, MN) and loaded into a microplate well precoated with mouse EPO-specific monoclonal antibody. After washing unbound proteins, 100 µL enzyme-linked EPO-specific monoclonal antibody was introduced to each well (Mouse EPO conjugate, MEP00B, R&D Systems, Inc. Minneapolis, MN) and allowed to incubate for 2 h at room temperature on a shaker bath. Following removal of unbound reagent, 100 µL substrate solution (MEP00B, R&D Systems, Inc. Minneapolis, MN) was added to each well and the plate was incubated for 30 min at room temperature while being protected from light. The enzymatic reaction between enzyme and substrate induced a blue-colored product that changed to yellow color after addition of 100 µL stop solution (MEP00B, R&D Systems, Inc. Minneapolis, MN). The intensity of the yellow color was measured by a microplate reader at 450 nm and compared with a mouse EPO Standard (MEP00B, R&D Systems, Inc. Minneapolis, MN). The intensity of the yellow color was proportional to the amount of EPO within each well. The detection limit of the ELISA EPO assay was 18 pg/mL of plasma.
Measurements of HIF-2α Protein by Western Blot Assay
Kidneys were harvested from anesthetized mice. For each assay, kidney tissue was homogenized in 800 µL of RIPA buffer, supplemented with 1× protease inhibitor cocktail (Sigma, P3840), by homogenizer (Wheaton No. 903475). Cell debris was pelleted by centrifugation at 4°C, centrifuged at 12,000 × g for 10 min, and the supernatant was transferred to a test tube on ice. Protein quantification was performed by BCA assay (Thermo, No. 23227). Samples were prepared for loading after heating at 99°C for 5 min with 4× Laemmli sample buffer (Bio-Rad, No. 1610747) supplemented with 10% β-mercaptoethanol. SDS-PAGE analysis was performed with 50 µg protein equivalent of each sample loaded onto 8% polyacrylamide gels and was transferred to a PVDF membrane using tank transfer for overnight at 30 V. Membranes were blocked for one hour at room temperature in tris-buffered saline-Tween 20 (TBS-T) + 4% nonfat dry milk. We screened several anti HIF1α and HIF2α antibodies in various cells and mouse tissues from a sample kit obtained from Novus Biologicals. Based on this analysis, we used the following antibodies: HIF-2α (Novus, NB100-132; dilution 1:1,000) and HIF-1α (Novus, NB100-123, dilution 1:1,000). Under identical experimental conditions, HIF-1α could not be detected in kidney samples (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.13489659.v1), whereas HIF-2α protein was detected (Fig. 5). In the initial experiments, we first incubated the kidney samples overnight with anti-HIF-2α (Novus, NB100-132) antibody diluted (1:1,000) in blocking solution at 4°C with gentle shaking, followed by five washes in TBS-T. Membranes were then incubated for 2 h at room temperature with HRP-conjugated secondary antibody (HRP-linked goat anti-mouse; Cell Signaling Technology, No. 7076) followed by five TBS-T washes. Blots were incubated with Clarity (Bio-Rad, No. 1705061) and imaged by Odyssey Imagers from Li-Cor. The blots were stripped of HIF-2α anti body and then reprobed with anti-HIF-1α anti body (Novus, NB100-123, dilution 1:1,000). However, as shown in Supplemental Fig. S1 (see https://doi.org/10.6084/m9.figshare.13489659.v1), this procedure yielded false-positive HIF-1α signal, perhaps due to incomplete stripping of HIF-2α antibody. Therefore, in all further experiments, HIF-2α and HIF-1α proteins were analyzed separately using tubulin as a house keeping protein (Sigma-Aldrich, T6199, 1:1,000). Densitometry analysis was performed with LI-COR software (image studio v. 2.1).
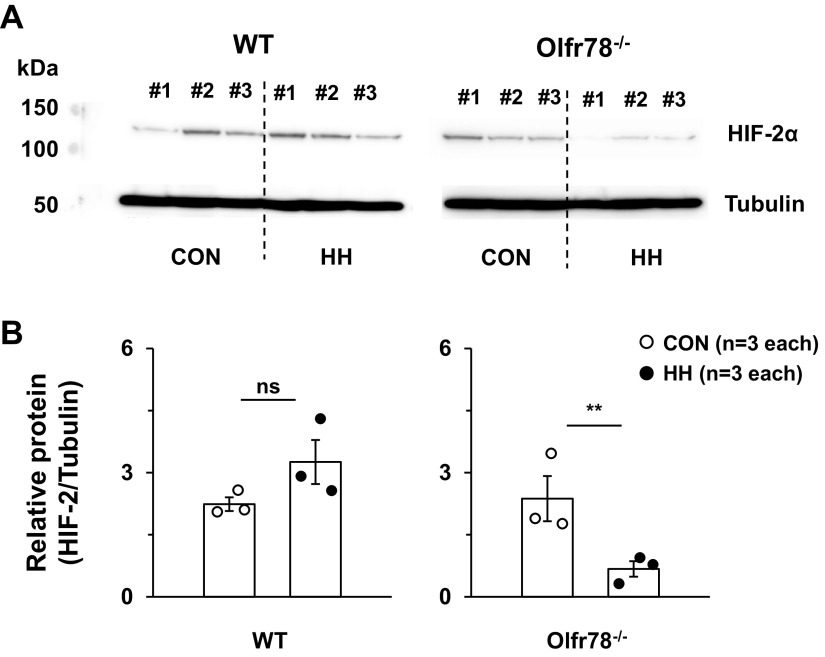
Figure 5.
HIF-2α protein abundance in kidneys of wild-type (WT) and Olfr78 null (Olfr78−/−) mice reared in room air (control; CONT) or exposed to hypobaric hypoxia (HH) for 18 h. A: kidneys were harvested from anesthetized mice of both genotypes reared in either room air (control, CON) or exposed to HH for 18 h. HIF2α protein was analyzed by western blot assay using HIF-2a antibody (Novus, NB100-132; 1:1,000 dilution) as described in methods. Tubulin protein was determined simultaneously, and the HIF-2a protein was normalized to tubulin. B: corresponding densitometric analysis presented as individual data points along with mean ± SE. Same blot was used for determining HIF-2α and tubulin proteins. **P < 0.01; ns, not significant, P > 0.05 compared with CON analyzed by “t” test. Data presented in A, the blot was cut and probed with HIF-2α above 75 kd and probed with tubulin antibody below the below 75 kd. For the sake of presentation, both blots were aligned in A and original blots were presented in Supplemental Fig. S2. Under identical experimental conditions, HIF-1α protein could not be detected in the same kidney samples run separately (Supplemental Fig. S3), indicating specificity of HIF-2α antibody. HIF-2α, hypoxia-inducible factor-2α.
Data Analysis
The following respiratory variables were analyzed in unanesthetized mice: tidal volume (VT; μL/g body weight); respiratory rate (RR/min); minute ventilation (VE; ml/min·g body weight); O2 consumption (VO2; mL/min·g body weight); and CO2 production (VCO2; mL/min · g body weight). VT and VE were normalized to the body weight of the animals. Each data point represents the average of two trials in each animal for a given gas challenge. BP measurements represent average of five measurements in each mouse. CB sensory nerve activity (impulse frequency from “single” units) was averaged during 3 min of baseline and during the entire 3 min hypoxic challenge and expressed as impulses per second unless otherwise stated. Changes in CB sensory nerve activity were expressed as differences from baseline values. Average data are presented as mean ± SEM. Statistical significance was assessed by one-way analysis of variance (ANOVA) on Ranks followed by Dunn’s or Student-Newman-Keuls’s test, or t-test, or two-way ANOVA with repeated measures followed by Tukey's test using Sigma Plot (v. 11). P values <0.05 were considered significant.
RESULTS
Olfr78 Null Mice Exhibit Impaired Cardiorespiratory Responses to HH
Effect of HH on breathing.
Baseline breathing before HH was comparable between WT and Olfr78 null mice (Fig. 1, A and B). HH increased breathing in WT mice due to increased respiratory rate and tidal volume (Fig. 1, A, C, and D; Table 2) and, as a consequence, minute ventilation (VE) was elevated (Fig. 1E). The magnitude of breathing stimulation by HH was less pronounced in Olfr78 null mice compared WT controls (Fig. 1, B, C, E, and F; Table 2).
Figure 1.

Olfr78 null mice exhibit impaired breathing responses to hypobaric hypoxia (HH). A and B: representative tracings of breathing in unanesthetized wild type (WT) (A) and Olfr78 null (Olfr78−/−) mice (B) while breathing room air (21% O2) or hypoxia (12% O2) before and after HH (0.4 atmospheres) for 18 h. Tracings represent breathing in same mice before and after HH. C–F: individual data points of respiratory rate (RR; breaths/min; C), tidal volume (VT, µL/g of body weight; D), and minute ventilation (VE, mL/g · min; E) normalized to body weight and oxygen consumption (VE/VO2; F). G–J: individual data points show hypoxic ventilatory responses (HVR) to 12% O2 breathing in WT and Olfr78−/− mice before and after HH. HVR was determined in the same mice before and after HH. Respiratory rate (breaths/min; G); tidal volume (VT, µL/g of body weight; H), and minute ventilation (VE, mL/g · min; I) VE normalized to body weight and oxygen consumption (VE/VO2; J). Numbers in parenthesis represent the number of mice. **P < 0.01; n.s., not significant, P > 0.05; compared to WT, two-way ANOVA with repeated measures followed by Tukey’s test.
Table 2.
Respiratory and metabolic responses before and after HH in WT and Olfr78−/− mice
| Wild-Type Mice (n = 7) |
Olfactory Receptor 78-Null Mice (n = 7) |
|||
|---|---|---|---|---|
| 21% O2 | 12% O2 | 21% O2 | 12% O2 | |
| Pre-HH | ||||
| RR, breaths/min | 176 ± 4 | 221 ± 3a | 171 ± 3 | 204 ± 3a,c |
| V̇t, µL/g body wt | 2.13 ± 0.09 | 2.60 ± 0.08a | 2.15 ± 0.07 | 2.49 ± 0.07a |
| V̇e, mL/g body wt/min | 0.38 ± 0.02 | 0.57 ± 0.02a | 0.37 ± 0.01 | 0.51 ± 0.02a,b |
| V̇o2, mL/g body wt/min | 0.053 ± 0.001 | 0.029 ± 0.001a | 0.051 ± 0.002 | 0.029 ± 0.001a |
| V̇co2, mL/g body wt/min | 0.038 ± 0.001 | 0.027 ± 0.002a | 0.037 ± 0.001 | 0.026 ± 0.002a |
| V̇e/V̇o2 | 7.1 ± 0.4 | 20.7 ± 0.8a | 7.3 ± 0.4 | 16.9 ± 0.7a,b |
| Post-HH | ||||
| RR, breaths/min | 213 ± 4e | 260 ± 5a,e | 184 ± 2c,d | 225 ± 4a,c,e |
| V̇t, µL/g body wt | 2.59 ± 0.08e | 3.24 ± 0.09a,e | 2.48 ± 0.07d | 2.90 ± 0.09a,b,e |
| V̇e, mL/g body wt/min | 0.55 ± 0.03e | 0.84 ± 0.03a,e | 0.46 ± 0.01c,e | 0.65 ± 0.02a,c,e |
| V̇o2, mL/g body wt/min | 0.057 ± 0.001d | 0.031 ± 0.001a | 0.058 ± 0.002d | 0.031 ± 0.002a |
| V̇co2, mL/g body wt/min | 0.039 ± 0.001 | 0.027 ± 0.002a | 0.037 ± 0.002 | 0.027 ± 0.001a |
| V̇e/V̇o2 | 9.8 ± 0.3 | 27.5 ± 1.5a,e | 8.0 ± 0.4 | 21.5 ± 1.2a,c,e |
Values are means ± SE; n, number of mice of each genotype. HH, hypobaric hypoxia; Pre-HH, prehypobaric hypoxia; post-HH, posthypobaric hypoxia; Olfr78−/− mice, olfactory receptor 78 null mice; RR, respiratory rate; V̇t, tidal volume; V̇e, minute ventilation; V̇o2, oxygen consumption; V̇co2, CO2 production; V̇e/V̇o2, ratio of minute ventilation/oxygen consumption; WT, wild-type. Statistical analysis was analyzed by two-way ANOVA with repeated measures followed by a Tukey’s test.
P < 0.01 compared with values with breathing at 21% O2; bP < 0.05 and cP < 0.01 compared with wild-type mice; dP < 0.05 and eP < 0.01 compared with pre-HH.
The effect of HH on hypoxic ventilatory response (HVR) to 12% O2 was determined. WT mice treated with HH showed augmented HVR, and this effect was less pronounced in HH treated Olfr78 null mice (P < 0.01; Fig. 1, A, B, and G–J, Table 2).
Effect of HH on blood pressure.
Baseline systolic BP tended to be lower in Olfr78 null mice compared with WT controls (wild type = 109 ± 1 mmHg vs. Olfr78 null mice = 105 ± 1 mmHg), but the difference was not significant (P > 0.05; n = 6). Both genotypes showed comparable diastolic and mean BP. HH-treated WT mice showed elevated systolic and mean BP, whereas diastolic BP was unaltered (Fig. 2, A–C). In striking contrast, BP was unaltered in Olfr78 null mice treated with HH (Fig. 2, A–C). Although diastolic and mean BPs tended to be reduced in HH treated Olfr78 null mice, the difference did not reach statistical significance compared with pre-HH controls (P > 0.05; n = 6).
Figure 2.

Olfr78 null mice exhibit absence of blood pressure (BP) (A–C) and plasma norepinephrine (NE, D) responses to hypobaric hypoxia (HH). BP was determined by tail-cuff method in the same unsedated wild-type WT and Olfr78 null (Olfr78−/−) mice before and after HH. A–C: individual data points of systolic (SBP; A), diastolic (DBP; B) and mean BP (MBP) in mmHg (C). Results represent individual data points along with mean ± SE. Numbers in parenthesis represent the number of mice. D: plasma norepinephrine (NE) responses to HH in wild-type (WT) and Olfr78−/− mice. Arterial blood was collected from anesthetized mice, and plasma NE was measured by high-performance liquid chromatography coupled with electrochemical detector (HPLC-ECD). Data represent individual data points along with mean ± SE. Numbers in parenthesis represent the number of mice. **P < 0.01; *P < 0.05; n.s., not significant; two-way ANOVA with repeated measures followed by Tukey’s test in A–C, and one-way ANOVA on Ranks followed by Dunn’s test in D. CON, control.
Plasma Norepinephrine Measurement in WT and Olfr78 Null Mice
HH increases sympathetic nerve activity (SNA) in humans (30). To assess whether HH increases SNA, plasma norepinephrine (NE) levels were determined as an index of SNA. Baseline plasma NE levels were comparable between WT and Olfr78 null mice (Fig. 2D). Plasma NE levels were elevated in HH-treated WT but not in Olfr78 null mice (Fig. 2D).
Absence of Carotid Body Response to Hypobaric Hypoxia in Olfr78 Null Mice
We next determined the mechanism(s) underlying cardiorespiratory responses to HH. Earlier studies suggest that CB activation mediates cardiorespiratory responses to HH (1, 31, 32). Given that Olfr78 null mice exhibit impaired CB sensory nerve response to acute hypoxia (12, 14), we hypothesized that attenuated or absence of CB sensory responses to HH in Olfr78 null mice contributes to impaired cardiorespiratory responses. To begin to assess the effects of HH on the CB, we first confirmed the deletion of the Olfr78 gene in CBs of Olfr78 null mice. Quantitative RT-PCR assay showed the presence of Olfr78 mRNA in CBs of room air-treated WT but not in Olfr78 null mice (Fig. 3A). HH had no effect on Olfr78 mRNA abundance either in CBs of WT or Olfr78 null mice (Fig. 3A).
Figure 3.

Olfr78 null (Olfr78−/−) mice show absence of carotid body (CB) sensory nerve response to hypobaric hypoxia (HH). Sensory nerve activity was recorded from carotid body ex vivo in wild-type (WT) and Olfr78 null mice reared in room air (21% O2) or treated with 18 h of HH (0.4 atmosphere). A: Olfr78 mRNA was determined in CBs of wild-type (WT) and Olfr78 null mice by quantitative RT-PCR assay. Shown are the individual data points of Olfr78 mRNA abundance in CBs of WT and Olfr78 null mice reared in room air or treated with HH for 18 h. Olfr78 mRNA was normalized to 18S mRNA. N represents the number of CBs; two CBs/mouse. B and C: examples of baseline CB sensory nerve activity and response to acute hypoxia (Hx) before (CON) and after HH in wild-type (WT; B), and Olfr78 null (Olfr78−/−) mice (C). The pO2 represents partial pressure of O2 (mmHg) in the medium. Action potentials (A.P. top) and integrated action potential frequency of the CB sensory nerve activity presented as impulses per second (CB sensory nerve activity; imp/s; bottom). D: individual data points along with mean ± SE of baseline CB sensory nerve activity before and after HH. E and F: average data (mean ± SE) of CB sensory nerve response to graded hypoxia in WT and Ofr78−/− mice before (CON) and after HH exposure. In E and F, numbers represent the number of fibers (f) and number of carotid bodies (CB). **P < 0.01; n.s. not significant, compared with CON, one-way ANOVA on Ranks followed by Student–Newman–Keuls’s test in A, one-way ANOVA on Ranks followed by Dunn’s test in D, two-way ANOVA with repeated measures followed by Tukey’s test in E and F.
CB sensory nerve activity was recorded ex vivo. We chose the ex vivo CB preparation to exclude the confounding influence of BP in intact mice. Prior to HH treatment, baseline CB sensory nerve activity was comparable between WT and Olfr78 null mice (Fig. 3, B–D). HH increased baseline CB sensory nerve activity in WT but not in Olfr78 null mice (Fig. 3, B and D).
We next determined CB sensory nerve responses to graded hypoxia. Following HH, the magnitude of CB sensory nerve excitation for a given Po2 was greater in WT CB compared with room air-treated controls (Fig. 3, B and E). Room air-treated Olfr78 null mice showed blunted CB sensory nerve response to graded hypoxia compared with WT controls (Fig. 3, B, C, E, and F). HH was ineffective in augmenting CB sensory nerve response to hypoxia in Olfr78 null mice (Fig. 3, C and F).
Olfr78 Null Mice Exhibit Impaired Epo Activation by HH
Activation of the Epo gene and the resulting increase in circulating Epo levels defend against HH by facilitating blood O2 transport through increasing red blood cell production (16). Olfr78 is expressed in the kidney (24), which is a major site of Epo production in adult rodents. To begin to assess the potential role of Olfr78 in HH-evoked Epo gene activation, we first confirmed the deletion of the Olfr78 gene in kidneys of Olfr78 null mice. WT but not Olfr78 null kidneys expressed Olfr78 mRNA, and HH had no effect on Olfr78 mRNA either in WT or Olfr78 null mice (Fig. 4A).
Figure 4.

Absence of Olfr78 mRNA and impaired erythropoietin (Epo) responses to hypobaric hypoxia (HH) in Olfr78 null (Olfr78−/−) mice. A and B: abundance of Olfr78 (A) and Epo (B) mRNAs were measured in the kidneys of wild type (WT) and Olfr78−/− mice by quantitative real time reverse transcriptase polymerase chain reaction (qRT-PCR) in the kidneys of wild type (WT) and Olfr78−/− mice exposed to room air (Control; CON) or hypobaric hypoxia (HH; 0.4 atmospheres) for 18 h. mRNA abundances were normalized to 18S mRNA. Individual data points along with mean ± SE are presented. Near absence of renal Olfr78 mRNA confirms Olfr78 gene knockdown in the kidneys of mutant mice and lack of Olfr78 gene responses to HH. C: plasma Epo protein abundance was measured by ELISA assay in WT and Olfr78−/− mice reared in room air (control = CON) or treated with 18 h of HH. Number in parenthesis represents number of mice in each group. **P < 0.01 compared with WT, one-way ANOVA on Ranks followed by Student–Newman–Keuls’s test.
Basal renal Epo mRNA abundance was low in both WT and Olfr78 null mice (Fig. 4B). HH increased Epo mRNA abundance in WT mice, and this effect was significantly attenuated in Olfr78 null mice (P < 0.01; Fig. 4B). Consistent with mRNA expression, basal plasma EPO levels were low in both groups of mice (Fig. 4C). HH increased plasma Epo protein levels in WT mice, but this effect was absent in Olfr78 null mice (P < 0.01; Fig. 4C).
Effect of HH on Renal HIF-2α Protein Expression
We then explored mechanism(s) underlying impaired Epo response to HH in Olfr78 null mice. Hypoxia-evoked Epo gene activation requires hypoxia-inducible factor (HIF)-2, also known as endothelial PAS (EPAS)-1, a member of the HIF family of transcriptional activators (17, 18, 20–23). HIFs are heterodimeric proteins comprising α and β subunits. Accumulation of the α subunit is generally necessary for HIF activation (33). We asked whether altered HIF-2α accumulation accounted for the impaired Epo gene activation by HH in Olfr78 null mice. To this end, HIF-2α protein abundance was determined by Western blot assay in kidney homogenates of WT and Olfr78 null mice. HIF-2α abundance was markedly reduced in kidneys of HH treated Olfr78 null mice compared with room air controls in all three mice tested (Fig. 5, A and B; original blot presented in Supplemental Fig. S3; see https://doi.org/10.6084/m9.figshare.13489659.v1). Under identical experimental conditions, HIF-1α protein could not be detected in the kidneys of either WT or Olfr78 null mice treated with either room air or HH (Supplemental Fig. S1B; https://doi.org/10.6084/m9.figshare.13489659.v1).
Attenuated HIF-2-Dependent Gene Expression by HH in Olfr78 Null Mice
The above-described studies indicated insufficient HIF-2-mediated transcription resulting from reduced HIF-2α accumulation might account for impaired Epo response to HH in Olfr78 null mice. To further assess this possibility, the effect of HH on Sod-1, a HIF-2 regulated gene (34), was determined. Basal expression of renal Sod1 mRNA expression was indistinguishable between both genotypes. Sod1 mRNA abundance increased in HH-treated WT but not in Olfr78 null mice (Fig. 6). VEGF gene abundance, which is a target gene of HIF-1, was unaffected by HH in both WT and in Olfr78 null mice (Supplemental Fig. S2; https://doi.org/10.6084/m9.figshare.13489659.v1).
Figure 6.
Impaired Sod1 gene activation by hypobaric hypoxia (HH), a HIF-2 target gene, in Olfr78 null mice kidneys. Data represent individual data points along with mean ± SE. **P < 0.01; ns, not significant, P > 0.05; compared with CON, one-way ANOVA on Ranks followed by Student–Newman–Keuls’s test. HIF-2α, hypoxia-inducible factor-2α.
DISCUSSION
The present results demonstrate striking absence of CB sensory nerve responses to HH with equally striking loss of cardiorespiratory responses in Olfr78 null mice. Our results further revealed a hitherto uncharacterized loss of renal Epo gene activation by HH in Olfr78 null mice.
Consistent with earlier reports (2, 3, 35), HH-treated WT mice exhibited ventilatory adaptations manifesting as increased baseline breathing and augmented HVR. In contrast, Olfr78 null mice showed markedly reduced ventilatory adaptations primarily due to their inability to increase respiratory rate compared to WT mice. Consistent with early reports (12, 14), room air-treated Olfr78 null mice exhibited blunted HVR, and HH had no effect on HVR. In contrast, HVR was augmented in HH-treated WT mice. Hypoxia reduces body metabolism (VO2) and, as a consequence, HVR remains either reduced or unaltered (25). It is conceivable that markedly reduced metabolism by HH might account for the absence of augmented HVR in Olfr78 null mice. However, changes in O2 consumption (VO2), an index of metabolism, were indistinguishable between WT and Ofr78 null mice treated with HH (Table 2). It follows that the absence of enhanced HVR by HH is not secondary changes in metabolism rather due to selective loss of breathing response to hypoxia in Olfr78 null mice. Ventilatory adaptations to HH have important physiological consequences. For example, Brown-Norway rats, which exhibit absence of ventilatory adaptations, develop pulmonary edema in response to HH, which is a manifestation of acute mountain sickness (8). Whether Olfr78 null mice also exhibit pulmonary edema in response to HH remains to be investigated. Nonetheless, these results demonstrate that Olfr78 contributes to ventilatory adaptations to HH.
In addition to stimulation of breathing, HH increases BP. For instance, elevated systolic and diastolic BP was reported in human subjects experiencing HH as a consequence of ascent to high altitude (36). However, HH-treated WT mice showed only elevated systolic but not diastolic BP. It may be that BP responses to HH differ between mice and humans. Alternatively, mice may require more than 18 h of HH treatment to increase both systolic and diastolic BP. BP responses were strikingly absent in HH treated Olfr78 null mice. In fact, BP tends to be reduced, albeit not significantly, in HH-treated Olfr78 null mice compared with room air controls. Studies in human subjects suggest that increased sympathetic nerve activity (SNA) mediates HH-induced elevated BP (30). Consistent with this possibility, elevated BP in WT mice is associated with increased plasma norepinephrine (NE) levels, which is an index of increased SNA, and this response was remarkably absent in Olfr78 mice. The absence of HH-evoked BP response is likely due to lack of increased SNA in Olfr78 null mice. BP elevation by HH is physiologically important because by increasing perfusion pressure it ensures adequate flow of oxygenated blood to vital organs. Together, these findings suggest a role for Olfr78 in cardiovascular adaptations to HH.
Earlier studies reported cardiorespiratory responses to HH were absent in CB-ablated animals (5–7) and in mice with impaired CB sensitivity to hypoxia (2, 8). Moreover, prolonged hypoxia such as HH increases baseline CB sensory nerve activity (9) and augments CB sensory nerve response to subsequent acute hypoxia (10). These studies suggest that activation of the CB chemo reflex is essential for evoking cardiorespiratory adaptations to HH. Consistent with earlier studies (9, 10), we also found increased baseline CB sensory nerve activity and augmented CB sensory nerve response to subsequent acute hypoxia in HH-treated WT mice. The role of Olfr78 in CB response to acute hypoxia has been controversial. On one hand, Chang et al. reported impaired CB sensory nerve response to modest hypoxia (pO2 ∼60 mmHg) in Olfr78 null mice (12). On the other, glomus cell [Ca2+]i and neurotransmitter secretory responses to severe hypoxia (pO2 ∼10–15 mmHg) were unaltered in Olfr78 null mice (15). Re-evaluation of the role of Olfr78 showed that wide range of hypoxia levels (up to pO2 ∼40 mmHg) markedly stimulate CB sensory nerve activity, whereas severe hypoxia (pO2 ≤15 mmHg) produces modest CB sensory nerve excitation, and Olfr78 null mice exhibit attenuated CB sensory nerve activity in response to modest (pO2 ∼40 mmHg) but not that elicited by severe hypoxia (14). These findings suggested that Olfr78 participates in the CB sensory nerve response to modest but not to severe hypoxia. Remarkably, CB sensory nerve responses to HH were absent in Olfr78 null mice, suggesting that impaired cardiorespiratory adaptations to HH are due to loss of CB sensory nerve excitation by HH. It follows that Olfr78 participates not only CB sensory nerve excitation by acute hypoxia as reported previously (12, 14) but also by prolonged hypoxia such as that seen with HH as shown in the present study.
An intriguing finding of the present study is the attenuated renal Epo gene activation by HH in Olfr78 null mice. Does the impaired CB activation account for the impaired Epo gene response to HH in Olfr78 null mice? Earlier studies reported that CB ablation has either no effect (37, 38) or augments hypoxia-induced erythropoiesis (39). Therefore, it is unlikely impaired hypoxic sensing by the CB accounts for the reduced Epo gene activation. Hypoxia is a major determinant of Epo synthesis in the kidney (40). Current evidence suggests that hypoxia, by activating HIF-2-dependent transcription, mediates the Epo gene activation (17, 18, 20–23). Accumulation of the O2-sensitive α subunit by hypoxia is generally considered an essential prerequisite for transcriptional activation of HIFs by low O2 (19, 41). However, much of the evidence supporting this view comes from studies on cell cultures. In this study, HIF-2α protein levels were unaltered in HH-treated WT null mice. Similar lack of HIF-2α protein accumulation by hypoxia was also reported in kidneys of WT mice, although these mice exhibited elevated Epo protein (42). In contrast, HH-treated Olfr78 null mice exhibited markedly reduced HIF-2α abundance compared with pre-HH controls, and this effect was associated with attenuated Epo as well as Sod1, a HIF-2 target gene (34). These results suggest that impaired transcriptional activation of HIF-2 accounts for attenuated Epo gene activation by HH in Olfr78 null mice. Emerging evidence suggests that in addition to prolyl hydroxylase-dependent accumulation of HIF-2α subunit, posttranslational modifications of HIF-α subunit by either acetylation (42), sumolylation (43), or protein phosphorylation (44) can also activate HIFs. Whether Olfr78 contributes to HIF activation through posttranslational mechanisms induced under the setting of HH remains to be investigated.
How might hypoxia activate Olfr78? Lactate was proposed as an activator of Olfr78 in the CB (12). Although HH might increase lactate production (12, 45–47), CB sensory nerve excitation and glomus cell response by exogenous lactate (as high as 30 mM) was unaltered in Olfr78 null mice compared with WT mice (14), negating a role for lactate as a mediator of Olfr78 activation at least in the CB. Short chain fatty acids (SCFA) such as acetate were also proposed as endogenous ligands for Olfr78 (48). Although acetate-induced activation of the CB was unaltered in Olfr78 null mice (14), cells and tissues subjected to a hypoxic challenge produce SCFA, particularly acetate. Acetylation of HIF-2 by acetate is required for maximal HIF-2 signaling in cells and mice (42, 49). Exogenous acetate augments Epo production in mice with acute anemia in an Acyl-coenzyme A synthetase, a short-chain family member 2 (Acss2), -dependent manner, while also augmenting Epo production in mice with chronic anemia associated with renal failure (42, 49). Acss2 null mice exhibit impaired HIF-2 associated molecular processes including HIF-2 acetylation, HIF-2/Cbp coactivator formation, HIF-2/Cbp-dependent chromatin recruitment and modification, and induction of Epo as well as other HIF-2 regulated target genes (42, 49). Olfr78 responds to acetate and propionate. Olfr78 null mice exhibit altered renin release and BP regulation in response to depletion of the gut microbiome, a major source of SCFA and particularly acetate in mammals (48). Further studies are necessary to determine if alterations in SCFA-mediated activation of HIF-2 signaling account for impaired renal Epo activation in Olfr78 null mice.
Limitations
The following are the limitations of the current study. First, our studies were performed on adult male mice only. Additional studies in female mice are necessary to further establish a role for Olfr78 in HH-evoked physiological adaptations. Second, BP was monitored by tail-cuff method. To minimize variability in BP measurements by tail-cuff method, mice were acclimatized to the BP measuring equipment for 1 h and at least five BP measurements were averaged in each mouse. Although it is desirable to measure BP by telemetry through placing a probe in the abdominal aorta, currently available telemetry probes are designed for the carotid artery. These probes are not suitable for our study because blood flow to the CB will be compromised by placing a probe in the carotid artery. Furthermore, our initial studies showed poor survival rates of mice by placing the probe in the abdominal aorta. These technical issues prompted us to utilize the tail-cuff method for measuring BP. Another limitation is that mice were exposed to only 18 h of HH. Consequently, the consequences of longer durations of HH greater than 18 h cannot be ascertained from the present results. High altitude in addition to HH also decreases environmental temperature, which may contribute to the physiological effects associated with high altitude in addition to hypoxia. Further studies are needed to elucidate potential posttranslational mechanism(s) associated with activation of renal HIF-2 by HH. Epo production is confined to a subset of specialized interstitial cells near the proximal convoluted tubule in the kidney (50, 51). Although kidneys express Olfr78 (48), whether this expression is localized to Epo-producing cells remains to be investigated. Moreover, we have not attempted to correlate the Epo gene activation to changes in hematocrit because an earlier study reported that several days of HH is required for increased erythropoiesis in mice (52).
Notwithstanding the above limitations, current results provide reasonable evidence for participation of Olfr78 in physiological adaptations to HH as evidenced by impaired CB-mediated cardiorespiratory adaptations as well as hitherto uncharacterized impairment of renal Epo gene activation. These findings taken together with earlier studies (12, 14) suggest a broad role for Olfr78 not only in CB sensory nerve responses to acute hypoxia but, more importantly, for maintaining O2 homeostasis under HH such as that experienced at high altitude.
GRANTS
This work was supported by the National Institutes of Health Grants P01-HL-44454.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R.P. conceived and designed research; B.W., Y-J.P., X.S., C.Z., J.S.N., and J.A.G. performed experiments; B.W., Y-J.P., X.S., C.Z., J.S.N., and J.A.G. analyzed data; B.W., Y-J.P., J.A.G., and N.R.P. interpreted results of experiments; B.W. and Y-J.P. prepared figures; N.R.P. drafted manuscript; B.W., Y-J.P., J.A.G., and N.R.P. edited and revised manuscript; B.W., Y-J.P., X.S., C.Z., J.A.G., and N.R.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Prof. J. Pluznick for providing the Olfr78/Bl6 mice and to Prof. Ganesh K. Kumar for constructive comments.
REFERENCES
- 1.Dempsey JA, Forster HV. Mediation of ventilatory adaptations. Physiol Rev 62: 262–346, 1982. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- 2.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 99: 821–826, 2002. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik M, Peng Y, Kline D, Adhikary G, Prabhakar N. Impaired ventilatory acclimatization to hypoxia in mice lacking the immediate early gene fos B. Respir Physiol Neurobiol 145: 23–31, 2005. doi: 10.1016/j.resp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Parati G, Ochoa JE, Torlasco C, Salvi P, Lombardi C, Bilo G. Aging, high altitude, and blood pressure: a complex relationship. High Alt Med Biol 16: 97–109, 2015. doi: 10.1089/ham.2015.0010. [DOI] [PubMed] [Google Scholar]
- 5.Bouverot P, Bureau M. Ventilatory acclimatization and csf acid-base balance in carotid chemodenervated dogs at 3550 m. Pflugers Arch 361: 17–23, 1975. doi: 10.1007/BF00587335. [DOI] [PubMed] [Google Scholar]
- 6.Forster HV, Bisgard GE, Klein JP. Effect of peripheral chemoreceptor denervation on acclimatization of goats during hypoxia. J Appl Physiol Respir Environ Exerc Physiol 50: 392–398, 1981. doi: 10.1152/jappl.1981.50.2.392. [DOI] [PubMed] [Google Scholar]
- 7.Smith CA, Bisgard GE, Nielsen AM, Daristotle L, Kressin NA, Forster HV, Dempsey JA. Carotid bodies are required for ventilatory acclimatization to chronic hypoxia. J Appl Physiol (1985) 60: 1003–1010, 1986. doi: 10.1152/jappl.1986.60.3.1003. [DOI] [PubMed] [Google Scholar]
- 8.Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci USA 111: 1174–1179, 2014. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen AM, Bisgard GE, Vidruk EH. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. J Appl Physiol (1985) 65: 1796–1802, 1988. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 11.Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev 78: 429–466, 1998. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- 12.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 527: 240–244, 2015. doi: 10.1038/nature15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou T, Chien MS, Kaleem S, Matsunami H. Single cell transcriptome analysis of mouse carotid body glomus cells. J Physiol 594: 4225–4251, 2016. doi: 10.1113/JP271936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng YJ, Gridina A, Wang B, Nanduri J, Fox AP, Prabhakar NR. Olfactory receptor 78 participates in carotid body response to a wide range of low O2 levels but not severe hypoxia. J Neurophysiol 123: 1886–1895, 2020. doi: 10.1152/jn.00075.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Torrelo H, Ortega-Sáenz P, Macías D, Omura M, Zhou T, Matsunami H, Johnson RS, Mombaerts P, López-Barneo J. The role of Olfr78 in the breathing circuit of mice. Nature 561: E33–E40, 2018. doi: 10.1038/s41586-018-0545-9. [DOI] [PubMed] [Google Scholar]
- 16.Jelkmann W. Regulation of erythropoietin production. J Physiol 589: 1251–1258, 2011. doi: 10.1113/jphysiol.2010.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324: 1289–1293, 2009. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 18.Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116: 3039–3048, 2010. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92: 967–1003, 2012. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117: 1068–1077, 2007. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105: 3133–3140, 2005. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 22.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood 102: 1634–1640, 2003. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 23.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464, 2004. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- 24.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992. doi: 10.1152/ajpregu.1992.262.6.R1040. [DOI] [PubMed] [Google Scholar]
- 26.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan G, Peng YJ, Khan SA, Nanduri J, Singh A, Vasavda C, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR. H2S production by reactive oxygen species in the carotid body triggers hypertension in a rodent model of sleep apnea. Sci Signal 9: ra80, 2016. doi: 10.1126/scisignal.aaf3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol 546: 921–929, 2003. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisgard GE. The role of arterial chemoreceptors in ventilatory acclimatization to hypoxia. Adv Exp Med Biol 360: 109–122, 1994. doi: 10.1007/978-1-4615-2572-1_10. [DOI] [PubMed] [Google Scholar]
- 32.Weil JV. Ventilatory control at high altitude. In: Handbook of Physiology, edited by Cherniack NS, Widdicombe JG.. Bethesda: American Physiological Society, 1986, p. 703–728. [Google Scholar]
- 33.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90: 4304–4308, 1993. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet 35: 331–340, 2003. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 35.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- 36.Parati G, Bilo G, Faini A, Bilo B, Revera M, Giuliano A, Lombardi C, Caldara G, Gregorini F, Styczkiewicz K, Zambon A, Piperno A, Modesti PA, Agostoni P, Mancia G. Changes in 24 h ambulatory blood pressure and effects of angiotensin II receptor blockade during acute and prolonged high-altitude exposure: a randomized clinical trial. Eur Heart J 35: 3113–3122, 2014. doi: 10.1093/eurheartj/ehu275. [DOI] [PubMed] [Google Scholar]
- 37.Beynon G. The influence of the autonomic nervous system in the control of erythropoietin secretion in the hypoxic rat. J Physiol 266: 347–360, 1977. doi: 10.1113/jphysiol.1977.sp011771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillis DB, Mitchell RA. Erythropoiesis in carotid body resected cats. Blood 42: 907–912, 1973. doi: 10.1182/blood.V42.6.907.907. [DOI] [PubMed] [Google Scholar]
- 39.Paulo LG, Fink GD, Roh BL, Fisher JW. Influence of carotid body ablation on erythropoietin production in rabbits. Am J Physiol 224: 442–444, 1973. doi: 10.1152/ajplegacy.1973.224.2.442. [DOI] [PubMed] [Google Scholar]
- 40.Dunn A, Lo V, Donnelly S. The role of the kidney in blood volume regulation: the kidney as a regulator of the hematocrit. Am J Med Sci 334: 65–71, 2007. [Erratum in Am J Med Sci 335: 79, 2008]. doi: 10.1097/MAJ.0b013e318095a4ae. [DOI] [PubMed] [Google Scholar]
- 41.Semenza GL. The genomics and genetics of oxygen homeostasis. Annu Rev Genomics Hum Genet 21: 183–204, 2020. doi: 10.1146/annurev-genom-111119-073356. [DOI] [PubMed] [Google Scholar]
- 42.Xu M, Nagati JS, Xie J, Li J, Walters H, Moon YA, Gerard RD, Huang CL, Comerford SA, Hammer RE, Horton JD, Chen R, Garcia JA. An acetate switch regulates stress erythropoiesis. Nat Med 20: 1018–1026, 2014. doi: 10.1038/nm.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Y, Zuo Y, Zhang H, Kang X, Yue F, Yi Z, Liu M, Yeh ET, Chen G, Cheng J. Induction of SENP1 in endothelial cells contributes to hypoxia-driven VEGF expression and angiogenesis. J Biol Chem 285: 36682–36688, 2010. doi: 10.1074/jbc.M110.164236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bullen JW, Tchernyshyov I, Holewinski RJ, DeVine L, Wu F, Venkatraman V, Kass DL, Cole RN, Van Eyk J, Semenza GL. Protein kinase A-dependent phosphorylation stimulates the transcriptional activity of hypoxia-inducible factor 1. Sci Signal 9: ra56, 2016. doi: 10.1126/scisignal.aaf0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards HT. Lactic acid in rest and work at high altitude. Am J Physiol 116: 367–375, 1936. doi: 10.1152/ajplegacy.1936.116.2.367. [DOI] [Google Scholar]
- 46.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol 118: 691–728, 2018. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 47.Severinghaus JW, Mitchell RA, Richardson BW, Singer MM. Respiratory control at high altitude suggesting active transport regulation of CSF pH. J Appl Physiol 18: 1155–1166, 1963. doi: 10.1152/jappl.1963.18.6.1155. [DOI] [PubMed] [Google Scholar]
- 48.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen R, Xu M, Nagati JS, Hogg RT, Das A, Gerard RD, Garcia JA. The acetate/ACSS2 switch regulates HIF-2 stress signaling in the tumor cell microenvironment. PLoS One 10: e0116515, 2015. [Erratum in PLoS One 10: e0123612, 2015]. doi: 10.1371/journal.pone.0116515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koury ST, Bondurant MC, Koury MJ, Semenza GL. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood 77: 2497–2503, 1991. doi: 10.1182/blood.V77.11.2497.2497. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberger C, Mandriota S, Jürgensen JS, Wiesener MS, Hörstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002. doi: 10.1097/01.ASN.0000017223.49823.2A. [DOI] [PubMed] [Google Scholar]
- 52.Abbrecht PH, Littell JK. Plasma erythropoietin in men and mice during acclimatization to different altitudes. J Appl Physiol 32: 54–58, 1972. doi: 10.1152/jappl.1972.32.1.54. [DOI] [PubMed] [Google Scholar]