SUMMARY
The variety and complexity of ocular infections have increased significantly in the last decade since the publication of Cumitech 13B, Laboratory Diagnosis of Ocular Infections (L. D. Gray, P. H. Gilligan, and W. C. Fowler, Cumitech 13B, Laboratory Diagnosis of Ocular Infections, 2010). The purpose of this practical guidance document is to review, for individuals working in clinical microbiology laboratories, current tools used in the laboratory diagnosis of ocular infections. This document begins by describing the complex, delicate anatomy of the eye, which often leads to limitations in specimen quantity, requiring a close working bond between laboratorians and ophthalmologists to ensure high-quality diagnostic care. Descriptions are provided of common ocular infections in developed nations and neglected ocular infections seen in developing nations. Subsequently, preanalytic, analytic, and postanalytic aspects of laboratory diagnosis and antimicrobial susceptibility testing are explored in depth.
KEYWORDS: endophthalmitis, eye infection, keratitis, ocular infection, retinitis, uveitis, Cumitech 13
INTRODUCTION
The diagnosis of many ocular infections is made clinically on the basis of ocular examination. Prior to topical and/or systemic treatment, small irreplaceable specimens are obtained and either inoculated at the bedside or transported to the microbiology laboratory. Because of the potentially major adverse effects of significant infections, including vision loss, blindness, eye evisceration, or enucleation, it is essential that the microbiology laboratory and treating clinicians work closely to ensure that optimal methodologies are used to establish the etiology of infection so that appropriate interventions are taken. In particular, the laboratory needs to clearly communicate the specimen requirements for the diagnosis of a wide array of pathogens that may be found in the eye and the clinical settings in which they will likely be found. In this document, we provide a brief primer on the anatomy of the eye so that the reader will understand the sites or regions from which different specimens are collected. Second, we review the different types of eye infections that are likely to be encountered and the organisms most likely to be associated with them. Third, we discuss specific testing approaches that have been successfully used to detect the wide array of infectious agents that cause ocular infections and the role of susceptibility testing in guiding topical/systemic treatment options.
ANATOMY OF THE EYE
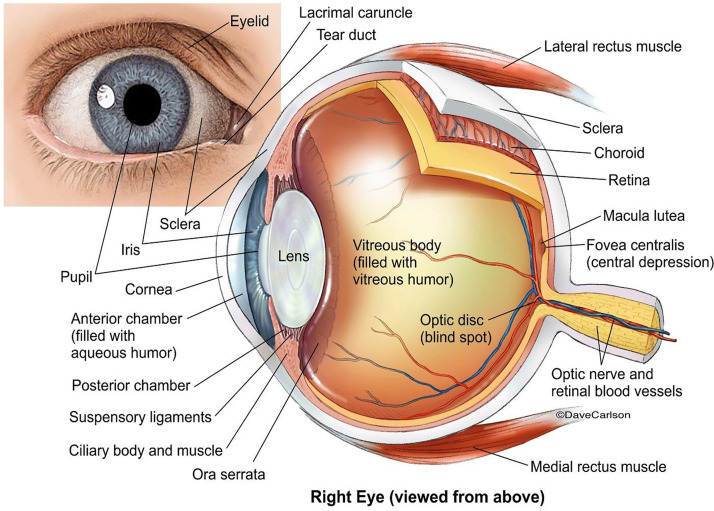
The complex anatomy of the eye presents important challenges to the diagnosis of ocular infections (Fig. 1). A brief overview of ocular anatomy is provided below. When thinking about laboratory diagnosis of ocular infections, it is helpful to distinguish the following groups of infections:
FIG 1.
Anatomy of the eye and surrounding tissues. (Licensed from https://www.carlsonstockart.com/photo/human-eye-anatomy-illustration-1/.)
-
1.
Group 1—outer eye: nonsterile outer eye structures, including conjunctivitis, dacryocystitis, blepharitis, canaliculitis, and preseptal and septal cellulitis
-
2.
Group 2—inner eye: sterile inner eye structures, including keratitis, endophthalmitis, uveitis, and retinitis
The main advantage of this dichotomous classification is to clearly identify ocular sites in which the isolation of commensal flora (Cutibacterium acnes and Staphylococcus epidermidis, etc.) may represent normal flora (group 1 [outer eye]) from tissues in which the isolation of these organisms is more likely to represent true infection (group 2 [inner eye]). Although the cornea is exposed to the environment and is anatomically considered part of the external eye, keratitis caused by various pathogens, including commensal flora, can lead to rapid loss of visual acuity and blindness. To simplify laboratory workflows and mitigate errors, cornea cultures are worked up as sterile sites and considered group 2 (inner eye) specimens.
Group 1—Outer Eye: the Eyelids, Conjunctiva, Lacrimal System, and Orbital and Periorbital Tissues
Eyelids.
The eyelids are delicate movable folds of skin overlying muscle (orbicularis), a firm plate (tarsus), and the membrane that limits the anterior extent of the orbit (orbital septum) and are lined with a mucous membrane (conjunctiva). Three main types of glands are present in the margins (edges) of the eyelids and empty on the border margins where the eyelashes emerge. Meibomian glands (which secrete lipids) are specialized sebaceous glands with ducts that empty at orifices, which can be seen as a line of ∼25 to 30 white dots along the inner margins of the eyelids. Lipids secreted by Meibomian and Zeis glands float on the surface of the cornea within the tear film and reduce its tendency to evaporate and avert “dry eye” surface breakdown.
Conjunctiva.
The conjunctiva is the thin, translucent, mucous membrane lining of the inner surfaces of the upper and lower eyelids (tarsal conjunctiva) and the surface of the anterior sclera (bulbar conjunctiva). The conjunctiva is continuous and essentially unchanging over the surfaces of the sclera and the inner surfaces of the upper and lower eyelids.
Lacrimal system.
Lacrimal glands are located within the bony orbit that surrounds the eyeball and produce tears, the aqueous-proteinaceous component of the tear film. Tears are secreted in the superior fornix onto the upper tarsal and bulbar conjunctiva, flow over and coat the cornea and sclera, and exit into the lacrimal puncta. The puncta (two per eye; one per eyelid) are two tiny openings close to the inner canthal regions on the eyelid margins nearest the nose. Tears flow into the puncta and drain through tiny ducts (canaliculi) and into the lacrimal sac, from which the tears eventually exit into the nasal cavity.
Orbital and periorbital soft tissue.
Fat, connective tissue, and muscle are contiguous with the eyeball both within (orbital) and outside (periorbital) the bony orbit that contains the eyeball.
Group 2—Inner Eye
Cornea.
The cornea is the transparent, central, anterior portion of the eyeball, with a shiny, convex surface that acts to refract (i.e., bend) light rays as they enter the eye. The regularly spaced and layered lamellar collagen fibers, which make up the bulk of the cornea, are responsible for the cornea’s transparency.
Iris.
The iris is the thin, colored, contractile diaphragm behind the cornea and is situated directly in front of the lens that dilates and constricts to appropriately regulate the amount of light entering the eye.
Anterior and posterior chambers.
The anterior chamber is the wider fluid-filled space between the cornea and the iris; the posterior chamber is the narrower fluid-filled space between the iris and the lens. Both chambers are filled with aqueous humor (or aqueous fluid), a colorless, watery, salty, low-protein-content, glucose-rich solution produced by the nonpigmented epithelium of the ciliary body. Its total volume is ∼250 μl, and it is replenished roughly every 2 h.
Lens.
The crystalline lens is a biconvex, avascular structure located directly behind the iris and pupil. It is held in place by ligamentous fibrils of the ciliary body, which contracts and relaxes to adjust the lens thickness to refract light rays precisely onto the retina.
Vitreous humor.
Most of the volume of the eyeball and the large space occupied between the lens and the retina is filled with approximately 4 ml of a transparent, viscous, jellylike structure called the vitreous humor.
Retina.
The retina is the neurosensory inner lining of the posterior of the eye and contains ∼120 million light-sensitive rods and cones (i.e., photoreceptors) along with neuroglial cells and neural tissues that function much like the film in a film camera or the charge-coupled-device (CCD) image sensor in a digital camera.
Macula.
The macula is a central 5- to 6-mm area of the posterior retina responsible for all of our central vision, most of our color vision, and visualization of fine details.
Fovea.
The fovea is a small central depression (∼1.5 mm) in the center of the macula. Its central foveola is ∼350 μm in diameter and has the highest concentration of cones corresponding to central fixation and our most acute vision.
Choroid.
The choroid is the highly vascular, pigmented inner layer of the eye located between the neurosensory retina and sclera.
Uvea.
The uveal tract refers to the combination of the choroid, iris, and ciliary body. All of these pigmented structures are continuous with one another.
Optic nerve.
The optic nerve is composed of ∼1.2 million nerve fibers, which transmit visual stimuli from the neurosensory retina to the brain.
CLINICAL DESCRIPTION OF OCULAR INFECTIONS
Ocular infections can be divided conveniently into two groups: those that occur in outer eye structures exposed to the environment (group 1) and those that occur in the inner eye structures (group 2) (1). An easy way to explain what ocular structures are associated with type 1 infections is that the structure can be touched with a finger or, for diagnostic purposes, with a swab. Structures associated with type 2 infections cannot be touched by a swab. Organisms causing group 1 outer eye infections may be introduced into the surface structure of the eye either from the environment or from the patient’s microbiome. Group 2 infection may also arise from the environment or the patient’s microbiome (exogenous infection) or as a result of systemic infection (endogenous infection). Clinical microbiology laboratory personnel should be familiar with the terminology that physicians use to describe ocular infections and inflammatory conditions. The following terms are often submitted as specimen descriptions. Knowledge of these terms could be helpful in determining the source of specimens, culture media to be used, usual organisms and potential pathogens, and the extent of identification (ID) and antimicrobial susceptibility testing (AST).
Group 1—Outer Eye
Blepharitis.
Blepharitis is characterized by erythema of the lid margin, fibrinous scale accumulation on eyelashes, and recurrent mild conjunctivitis leading to eye irritation and a foreign-body sensation (2). Staphylococcus aureus, C. acnes, and coagulase-negative Staphylococcus species (CNS) have been found in conjunction with blepharitis, with S. aureus being the most common and the target of antimicrobial therapy (3, 4).
Anterior blepharitis is usually seborrheic or staphylococcal in etiology (2). Posterior blepharitis (meibomitis) is characterized by thick, yellow or gray, turbid, cloudy secretory material that clogs the Meibomian gland orifices along the posterior lid margin, resulting in impaction, inflammation, and/or infection (2). A chalazion is a chronic lipogranulomatous inflammation of a Meibomian gland. A stye (hordeolum) is a painful nodule or pustule of the eyelid usually caused by staphylococcal infection of a sebaceous gland.
Anterior blepharitis requires lid scrubs and, if chronic, can require cultures and antimicrobial treatment. Meibomitis is treated by regular warm compresses to help liquefy viscous secretory material. When severe and/or chronic, oral antibiotics such as tetracyclines and azithromycin may be used for weeks, with treatment courses being repeated if patients do not respond adequately. In this setting, cycling of antimicrobials is recommended even though rigorous supporting clinical data are not available (2). When sizable chalazia result, steroid injections and/or surgical removal may be indicated.
The role of Demodex species (a type of mite) in blepharitis is uncertain since control populations often have a similar prevalence, although one recent study suggests that this mite is found more frequently and at higher numbers in older patients with blepharitis. These data contradict previous studies that did not see differences in Demodex species detection rates in individuals with and those without blepharitis (5). More data are needed to clarify whether Demodex spp. play a substantive role in blepharitis.
Lice (Phthirus pubis) have also been associated with blepharitis (2). Identification of nits and crusts on eyelashes is consistent with louse infection caused by either Pediculus humanus (body louse) or Phthirus pubis (pubic lice) (6, 7). Distinguishing the two is of great importance in children given that pubic lice are primarily sexually transmitted and may be representative of ongoing child abuse (6–8).
Myiasis.
The eye is involved in ∼5% of cases of human myiasis, which is caused by several fly genera, including Dermatobia species (human bot fly) (6). Larvae are deposited on the ocular surface, resulting in abrupt itching, pain, hyperemia, edema, soreness, lacrimation, and sensation of movement (6). Readers desiring more information on myiasis are referred to an excellent review by Francesconi and Lupi (9).
Canaliculitis.
Canaliculitis is low-level chronic inflammation of short channels (canaliculi) draining tears into the lacrimal sac. It is characterized by mucopurulent discharge and/or concretions from the punctum, tearing, eyelid erythema, and recurrent conjunctivitis. Primary infection is due to Gram-positive skin flora, including coagulase-negative staphylococci, streptococci, C. acnes, and Actinomyces species. Secondary infection of punctal plugs used to treat dry eye disease is additionally associated with environmental Gram-negative rods like Pseudomonas aeruginosa and rapidly growing mycobacteria (RGM) (10–12).
Dacryocystitis.
Dacryocystitis is inflammation of the lacrimal sac and is usually related to nasolacrimal duct obstruction (13, 14). Findings include tearing, redness, pain, tense and tender erythematous swelling over the lacrimal sac, expressible mucoid or purulent drainage from the punctum, and, in rare cases, periorbital cellulitis (14, 15). When expressible, the mucopurulent discharge can aid microbiological investigation, with S. aureus and CNS being the most common, followed by Streptococcus pneumoniae, C. acnes, P. aeruginosa, Haemophilus influenzae, and RGM (16–19).
Preseptal cellulitis.
Preseptal cellulitis is characterized by inflammation of the eyelids, conjunctiva, and surrounding skin without the involvement of deeper tissues and no pain with eye movements. Symptoms include eyelid erythema, warmth, tenderness, and fluctuant lymphedema or swelling that can extend over the nasal bridge to the opposite eyelids, usually accompanied by a low-grade fever and an elevated white blood cell (WBC) count. Often, there is a history of sinusitis, insect bite, dacryocystitis, local skin abrasion, laceration, dental abscess, or puncture wound (20). Culture of open wounds, weeping vesicles, purulent nasal drainage, and conjunctival discharge often yield S. aureus, beta-hemolytic streptococci, S. pneumoniae, H. influenzae, and/or P. aeruginosa, with rapid treatment often yielding a good outcome (21–23).
Orbital cellulitis.
All cases of orbital cellulitis should be considered potentially sight-threatening medical emergencies that require prompt diagnostic workup and treatment.
Orbital cellulitis is characterized by inflammation of deep periocular tissues resulting in severe clinical features like conjunctival edema and injection, restricted ocular motility, and pain on attempted eye movement that differentiate this condition from preseptal cellulitis. Additional symptoms include blurred vision, headache, double vision, eyelid edema, eyelid erythema, eyelid warmth, eyelid tenderness, and proptosis. Fever, purulent discharge, decreased periorbital sensation, decreased vision, retinal venous congestion, and optic neuropathy are worrisome signs that can also be present (21). Prevailing etiologies include direct extension from a paranasal sinus infection, focal periorbital infection, extension of a dental infection, sequelae of paranasal sinus surgery or orbital surgery, sequelae of trauma, seeding from systemic infection, facial cellulitis with vascular extension, or secondary inflammation and orbital venous stasis from a septic cavernous sinus thrombosis (15, 20, 22, 23).
Treatment of orbital cellulitis includes hospitalization, imaging studies, and broad-spectrum intravenous (i.v.) antibiotics. An immediate ophthalmology and/or otolaryngology, neurosurgery, or infectious disease consultation is indicated. If the orbit is taut and cramped, optic neuropathy is present, or the intraocular pressure is dangerously increased, then immediate surgical intervention may be needed. Bacterial causes include Staphylococcus species, Streptococcus species, H. influenzae, P. aeruginosa, Bacteroides species, and other environmental Gram-negative rods (14, 15, 24, 25). Failure to respond to i.v. antimicrobials and the formation of a subperiosteal abscess may necessitate surgical intervention to clear bacterial infection. Surgical intervention is also indicated for invasive mold infections such as sinonasal mucormycosis. This rapidly progressive and potentially fatal infection is a medical emergency and must be ruled out in patients with poorly controlled diabetes, high levels of comorbid conditions, and/or immunosuppression (23). All patients with orbital cellulitis must be monitored for complications, including cavernous sinus thrombosis, meningitis, and extension into the brain parenchyma (24).
Conjunctivitis.
Conjunctivitis can occur as an isolated condition or be a secondary finding in association with any type of ocular inflammation (26). A key clinical symptom and/or sign of infectious conjunctivitis is “red eye” (i.e., significant conjunctival hyperemia) over the bulbar conjunctiva and/or palpebral/tarsal conjunctiva often associated with irritation and discharge (26, 27). Diagnostic testing is rarely indicated since the usual clinical presentations are characteristic and fairly straightforward (26). Viral conjunctivitis is most commonly bilateral with serous, watery discharge and known sick contacts. Allergic conjunctivitis is uniformly bilateral with watery discharge and grayish, scant, stringy mucus with an associated situational exposure history. In contrast, bacterial conjunctivitis is typically unilateral with more purulent discharge, matting, and adherence of eyelids upon waking.
(i) Viral.
Adenovirus is the most common cause of viral conjunctivitis (34 to 80%) (27, 28). Epidemic keratoconjunctivitis outbreaks of adenovirus conjunctivitis due particularly to human adenovirus serotype 8 are well described in the literature and associated with ophthalmology clinics (29, 30). Risk factors include recent upper respiratory infection or direct contact with someone with respiratory illness or red eye in the previous 5 to 14 days. Both eyes can be affected simultaneously or in sequence up to 3 days apart, with infection progressing for the first 4 to 7 days and persisting for 2 to 3 weeks. Symptoms include eye irritation, watery discharge, conjunctival injection, preauricular adenopathy, and follicular inflammation. Pseudomembranes (inflammatory debris and fibrin) and subconjunctival hemorrhages indicate more severe disease. Inflammation can spread to the cornea, resulting in punctate keratitis leading to decreased vision, reduced contrast sensitivity, photosensitivity, and glare or haloes around bright lights (31, 32). Treatment of adenovirus conjunctivitis includes symptomatic relief and prevention of further transmission with avoidance of personal contacts, washing hands, and avoidance of sharing of personal items (31). Severe infections with membrane formation or cornea involvement may merit topical corticosteroids (31). Corticosteroids enhance viral replication, promote superinfection, delay viral clearance, and can facilitate higher numbers of community epidemics of viral conjunctivitis.
A less severe form of acute hemorrhagic viral conjunctivitis is due to two enterovirus serotypes, coxsackievirus type 24 and enterovirus 70 (33, 34). These viruses are highly contagious, and outbreaks in tropical countries in excess of 100,000 cases have been reported (33, 34). Patients present with red eyes and are treated symptomatically with eye drops, with spontaneous resolution (33, 34). Less commonly, herpes simplex virus (HSV) and varicella-zoster virus (VZV) reactivation can involve the conjunctiva, with severe infection meriting the use of systemic antiviral agents (32).
(ii) Bacterial.
Nongonococcal bacterial conjunctivitis is characterized by mild to moderate purulent discharge. Infections are often mild and self-limited. Only severe or recalcitrant infections are treated with topical antimicrobials (27). In adults, S. aureus, S. pneumoniae, and H. influenzae are the most frequent causes of bacterial conjunctivitis, while H. influenzae, S. pneumoniae, and Moraxella spp. are most common in children (27). Lack of vaccination for H. influenzae and S. pneumoniae in particular increases susceptibility to these infections. Chlamydia trachomatis is associated with neonatal and sexually transmitted conjunctivitis in developed nations and is the leading cause of infectious blindness (trachoma) in developing nations (35). Rare infections with Chlamydia pneumoniae and Chlamydia psittaci have also been reported (36, 37).
Neisseria gonorrhoeae conjunctivitis is a rapidly progressive medical emergency. High-risk individuals include neonates, infants, and sexually active adults with purulent conjunctivitis. Stat Gram stains accompanied by culture to determine if Gram-negative diplococci are present are essential to rapidly identify and treat this infection. Although in the United States, rates of neonatal N. gonorrhoeae ocular infections are low (estimated at 0.2/100,000), perinatal ocular antimicrobial prophylaxis has been abandoned in some industrialized countries, worldwide rates of N. gonorrhoeae infection are increasing, and strains have emerged that are resistant to all standard antimicrobial therapies (38–40).
Trachoma keratoconjunctivitis.
The leading infectious cause of blindness globally (∼2.2 million people) is keratoconjunctivitis due to C. trachomatis (35). Repeat infections lead to conjunctival scarring and abnormally positioned eyelids (trichiasis) that abrade the cornea, leading to scar formation and blindness. A global strategy to limit trachoma called SAFE focuses efforts on surgery to correct trichiasis, antibiotics to treat infection, facial cleanliness to decrease the risk of trachoma, and environmental improvements, including improved sanitation to reduce fly populations, which may act as a vector for trachoma (35). This meaningful approach has led to the elimination of trachoma in 4 countries: Laos, Cambodia, Morocco, and Mexico (41, 42). However, other populations have been more refractory to its elimination (43). In the industrialized world, vertical transmission of C. trachomatis from an infected mother to her infant may result in neonatal conjunctivitis (44). Infection may also occur through autoinoculation or sexual contact with individuals with genital tract infections (27).
Microsporidial keratoconjunctivitis.
Microsporidia are spore-like intracellular fungi that infect the conjunctiva and cornea in immunosuppressed individuals, causing a clinical presentation similar to that of viral keratoconjunctivitis. First reported in HIV-infected individuals, outbreaks in immunocompetent individuals have been associated with exposure to the pathogen in hot springs, swimming pools, soil, and contact sports (45–47).
Group 2—Inner Eye Infections
Keratitis.
All corneal inflammatory and infectious conditions should be considered potentially sight-threatening medical emergencies. All patients with suspected infectious keratitis should be referred to an ophthalmologist immediately for diagnosis and treatment.
Keratitis is characterized by inflammation of the cornea (48). It is the fourth leading cause of blindness globally and is associated with improper contact lens (CL) use, trauma, dry eye, chronic ocular surface disease, the use of topical corticosteroids, lid abnormalities, corneal hypesthesia, and iatrogenic postsurgical infection (49, 50). Symptoms of keratitis include redness with mild to severe pain, photophobia, decreased vision, and purulent discharge. If allowed to progress, severe scarring, thinning, perforation, or endophthalmitis may develop and progress to irreversible blindness and/or rupture of the globe resulting in evisceration or enucleation.
In the industrialized world, the most common predisposing factor for the development of infectious keratitis is the improper use or contamination of CL systems (50). Common risk factors include sleeping and swimming with CLs, poor hygiene, and using extended-wear lenses beyond the recommended time intervals (51, 52). Lens care solutions and cases exhibit transient colonization by environmental bacteria, commensal yeasts, molds, mycobacteria, and amoebae, but only a subset of these microbes cause keratitis.
(i) Posttrauma.
Trauma is a major route of cornea infection in agricultural settings within industrialized nations and a significant cause of blindness in developing nations. Disruption of the cornea epithelium enables access of commensal and environmental microbes to the cornea stroma and initiation of infection. Environmental bacteria and mold spores inoculated into the cornea stroma via trauma enable many different bacterial and fungal pathogens to cause significant destruction and vision loss in immunocompetent individuals (48).
(ii) Postsurgical.
Infectious keratitis is an infrequent complication of the most common corneal surgeries, keratoplasty (corneal transplants) and laser-assisted in situ keratomileusis (LASIK) surgery. Infection rates are relatively low following LASIK surgery (0.1%) and keratoplasty (1%), but the outcome can be devastating, with significant permanent vision loss (53, 54). Commonly encountered organisms in this setting include S. aureus, S. pneumoniae, C. acnes, coagulase-negative staphylococci, viridans group streptococci, beta-hemolytic streptococci, coryneform bacteria/diphtheroids, P. aeruginosa, Moraxella spp., Candida albicans, and Aspergillus spp. (49, 54–56). Two outbreaks of Mycobacterium chelonae have been described in the literature and are associated with contaminated water sources (57, 58).
(iii) Bacterial.
Bacterial keratitis is most frequently due to organisms that are part of the conjunctival microbiota, including CNS, S. aureus, S. pneumoniae, and C. acnes. Non-anthracis Bacillus spp. can be associated with the inoculation of organic matter into the eye (59). Moraxella catarrhalis infection can be severe, with reports of 5% of all M. catarrhalis-infected patients having evisceration or enucleation (60). Major bacterial pathogens in the setting of poor contact lens hygiene include P. aeruginosa and other enteric Gram-negative bacilli recoverable from water, such as Serratia marcescens (61–64).
(iv) Yeast.
Candida species, most commonly C. albicans, infect the corneas of individuals with preexisting ocular surface abnormalities or corticosteroid use (65). Cases of keratitis due to Cryptococcus neoformans (66), Cryptococcus laurentii (67), Cryptococcus albidus (68), and the dimorphic fungus Blastomyces dermatitidis (69) have been reported but are extremely rare.
Prior to transplantation, explanted donor corneas are stored in special media containing antibacterial agents but not antifungals. Bacterial growth in the storage solution is significantly mitigated such that when bacteria are identified as the cause of posttransplant infection, the same organism is isolated from solution in only 55% of cases, compared to >99% for Candida spp. (70). Despite infection occurring in up to 3 to 14% of patients receiving donor corneas with fungal contamination that had poor clinical outcomes, there are no consistent recommendations for routine fungal cultures on donor corneal rims (71, 72).
Although the addition of voriconazole to holding media reduces yeast growth and infection rates, high costs currently limit its widespread utilization (73). Amphotericin B is contraindicated given its significant endothelial cell toxicity (73, 74). Although research is ongoing, the utility of echinocandins may be limited by economic factors and poor activity against basidiomycetous yeasts, some Candida spp., and molds (75). As drug prices drop, economic analyses are needed to determine the cost/benefit ratio of supplementing media with antifungal agents.
(v) Mold.
Filamentous fungal keratitis is often associated with contact lenses in developed nations and trauma in developing nations (64). Although many molds are isolated from contact lens solutions, the most frequent cause of clinically significant infection are species in the Fusarium solani species complex (76–81). In the context of trauma, the most commonly isolated molds include Fusarium spp., Aspergillus spp., Scedosporium apiospermum, Paecilomyces spp., and Curvularia spp. (65). For a comprehensive list of other molds causing fungal keratitis, readers are referred to excellent reviews by Thomas and Kaliamurthy (65) and Kredics and colleagues (82).
(vi) Microsporidia.
Microsporidial (protozoon-like unicellular fungi) keratitis occurs worldwide in immunosuppressed individuals and immunocompetent individuals most often associated with trauma (45–47). Intractable Prototheca species keratitis (algae) has also been described in the literature, with variable treatment success with antifungal and antibacterial agents (83).
(vii) Acanthamoeba.
Acanthamoeba keratitis is associated with poor contact lens hygiene, soft contact lens use, and/or a history of trauma or exposure to water while wearing contact lenses. There are 20 different species of Acanthamoeba, with A. castellanii and A. polyphaga being the most frequently detected species in ocular infections (84). Notably, coinfections with bacteria have been reported. Treatment is difficult, and the long-term outcome is frequently severe, requiring transplantation. Fortunately, contact lens-associated Acanthamoeba keratitis is infrequent and is estimated to occur in approximately 1 to 2 contact lens wearers/100,000 in the United States, but infection rates are increasing and associated with deteriorating water pipe infrastructure in large cities (85, 86). Other free-living amoebae, including Hartmannella and vahlkamphid amoebae, are also associated with CL-associated keratitis (87).
(viii) Viral.
Viral infection of the cornea can be due to direct contact with virions or hematogenous dissemination (88). Due to the proximity of the cornea and conjunctiva, viral conjunctivitis caused by adenovirus serotypes 8 and 19 (89), coxsackievirus A24 (33), enterovirus 70 (90), and rubeola virus (91, 92) often leads to self-limited superficial keratitis (88). Mild keratitis in the setting of conjunctivitis has also been reported for Ebola virus (93) and arboviral infections, including Zika, dengue, and chikungunya viruses (94). In contrast, direct inoculation of the cornea with viral particles of HSV (95, 96), VZV (97), and vaccinia virus (cowpox) (98) results in more severe focal disease. Immunosuppression enhances susceptibility to certain viruses, including cytomegalovirus (CMV) (99), Epstein-Barr virus (EBV) (100), and human herpesvirus 6 (HHV6) (101, 102) and promotes the cyclic recurrence of latent viral infections involving HSV-1, HSV-2, and VZV resulting in progressive scarring and blindness (88).
HSV-1 infection is more common in adults due to proximity to the oral mucosa; however, neonatal infection is most often due to HSV-2 inoculated at the time of birth (103). After initial infection of the highly innervated cornea, HSV and VZV undergo retrograde axonal transport to neuronal cell bodies in the trigeminal nerve ganglion and lie dormant until reactivation (103). Triggers for reactivation include immunosuppression (iatrogenic, stress, HIV, or diabetes), altered homeostasis (fever, advancing age, or menses), radiation exposure (sunlight), and ocular irritants (contact lens wear or foreign body) (103, 104). Anterograde transport of HSV or VZV along arborizing nerves in the ophthalmic branch of the trigeminal nerve results in a characteristic neurotrophic ulcer, which can spread to involve neighboring epithelial cells forming a larger geographic ulcer with irregular angulated borders (95, 96). Severe cases can result in corneal perforation or spread to posterior segments of the eye (95, 96). Recurrent ulcerations lead to corneal scarring and vision loss requiring transplantation, and graft failure is common due to immunological rejection of the inflamed donor cornea and/or herpetic disease recurrence (95, 96, 105).
Recurrent VZV infection exhibits a dermatomal distribution affecting multiple tissues innervated by the ophthalmic branch of the trigeminal nerve (97, 106). This syndrome, known as herpes zoster ophthalmicus, often begins with a prodrome of unilateral pain or hypesthesia followed by vesicular eruptions of the skin, corneal ulceration, uveitis, and/or acute retinal necrosis (97, 107). Cornea involvement is seen in two-thirds of affected patients, yielding a punctate or dendritic appearance resembling that with HSV infection (97, 108).
Individuals receiving smallpox vaccinations (live nonattenuated vaccinia virus [cowpox]) develop an infectious blister at the inoculation site with viable virions that can spread by contact to other body sites, including the cornea (98). Corneal pathology is dependent on the dose and ranges from mild superficial keratitis to perforation (98). Although uncommon, molluscum contagiosum infection of the palpebral conjunctiva results in nodular lesions that may rarely abrade the corneal epithelium, similar to trachoma, resulting in keratitis, progressive scarring, and vision loss (109, 110). Finally, there is growing evidence that human papillomavirus infection of corneal epithelial cells can cause ocular surface papillomas or squamous cell carcinoma, particularly in immunosuppressed individuals (111, 112).
Endophthalmitis.
All cases of endophthalmitis should be considered potentially sight-threatening medical emergencies and require prompt diagnostic workup and treatment.
Endophthalmitis is characterized by inflammation within the vitreous (113) and is remarkable for its severity. The etiology of endophthalmitis can be infectious or noninfectious, but most cases are due to infection (113, 114). Symptoms include pain, decreased vision, eyelid and/or corneal edema, conjunctival chemosis, severe anterior chamber reaction, intense conjunctival injection, vitritis, and/or hypopyon (a microscopic, but sometimes grossly visible, inferiorly layered pool of inflammatory white blood cells settled in the inferior aspect of the anterior chamber behind the cornea). Endophthalmitis can be caused by a direct extension of a local infection (exogenous) or seeding from the bloodstream (endogenous).
(i) Exogenous.
Exogenous endophthalmitis is due to penetrating trauma to the eye either due to injury caused by foreign objects or secondary to surgical procedures or intravitreal injection (113, 115). Most exogenous cases (70%) are associated with recent eye surgery, including cataract surgery, LASIK, keratoplasty, trabeculectomy, and glaucoma drainage implant surgery (115). Fortunately, these infections are uncommon but, because of the high rates of blindness associated with them, are particularly devastating (113).
Endophthalmitis caused by foreign objects is typically due to endogenous microflora of the skin such as CNS, Streptococcus spp., S. aureus, and Bacillus spp. and environmental organisms such as P. aeruginosa and Nocardia spp. (113, 116, 117). Trauma with plant material increases the likelihood of inoculating fungal spores with frequent progression to endophthalmitis (65, 113, 118, 119).
Cataract surgery is the most widely performed ocular surgery globally. In the United States, approximately 1.5 million surgeries are performed annually, with an ∼0.04% infection rate (120). The most common causes of infection are indigenous Gram-positive cocci: coagulase-negative staphylococci, streptococci, enterococci, and S. aureus. P. aeruginosa, Stenotrophomonas maltophilia, and the Burkholderia cepacia complex are less common (120–122). Candida species, Nocardia species, RGM, and mold infections have also been reported but are fortunately rare (117, 122–124).
Intravitreal drug injections, such as humanized monoclonal antibodies to treat macular degeneration and diabetic retinopathy, are increasingly more common. They are associated with a low infection rate of 0.013%, with staphylococci and streptococci accounting for over 90% of infections (124, 125). However, Pseudomonas, Acinetobacter, Stenotrophomonas, Burkholderia, Enterobacterales, Fusarium spp., and Bipolaris spp. have all been associated with outbreaks caused by contaminated drug solutions (126–132).
Approximately 20% of corneal transplants (keratoplasty) are performed in patients with corneal damage due to infectious keratitis (133). In North America, donor corneas are typically stored in a preservative medium solution such as Optisol-GS, which contains both gentamicin and streptomycin but no antifungals, and are typically held in storage at 2°C to 8°C for up to 1 week prior to transplant. Although posttransplant infection rates are low, with endophthalmitis occurring in 0.028% and keratitis occurring in 0.019%, infection progression and/or organ rejection can be severe, often requiring a second transplant (115). In endophthalmitis, Candida species was recovered in 65% of cases, Enterococcus was recovered in 13%, and Streptococcus spp. were recovered in 11% (115). In keratitis, Candida spp. are recovered in 81% of cases (115).
Surgical procedures to drain aqueous humor and alleviate intraocular pressure in patients with glaucoma are associated with a 0.4% to 1.2% prevalence of endophthalmitis. Trabeculectomy procedures involve the creation of a small outflow chamber space or “bleb” between the anterior chamber and the subconjunctiva to alleviate pressure, increasing the risk of infection with skin flora, particularly CNS and streptococci (134, 135). Placement of an intraocular tube or shunt is associated with biofilm formation with C. acnes and RGM (123, 134).
(ii) Endogenous.
Endogenous endophthalmitis is a rare condition and is the result of hematogenous spread to the eye during disseminated infection (136). Risk factors include indwelling venous catheters, immunosuppression, intravenous drug use, diabetes, and liver abscesses (136, 137). Candida species, particularly C. albicans, are the most common cause of endogenous endophthalmitis in intravenous drug users (137). Infections with echinocandin-resistant Rhodotorula spp. (138); Cryptococcus spp. (139, 140) and other yeasts, including Geotrichum spp. (141), Malassezia spp. (142), Sporobolomyces spp. (143), and Saccharomyces spp. (144); Trichosporon spp. (145–147); and algae like Prototheca spp. have been implicated (83, 148, 149).
Endogenous endophthalmitis due to molds is rare, occurring in immunosuppressed individuals in the setting of disseminated infections due to Aspergillus spp., Fusarium spp., Scedosporium spp. (rarely other molds), and dimorphic fungi including Histoplasma capsulatum (150–152), Coccidioides spp. (153–155), B. dermatitidis (156), Paracoccidioides spp. (157), and the Sporothrix schenckii complex (158, 159). Diagnosis is based on the detection of fluffy white chorioretinal lesions with or without vitritis in the setting of systemic infection with a known pathogen and does not require ocular sampling prior to treatment initiation (113).
Bacterial causes of endogenous endophthalmitis include oral cavity and skin commensals such as staphylococci, particularly methicillin-resistant S. aureus (MRSA) and oral streptococci such as Streptococcus mitis. Less frequent bacterial causes include Bacillus cereus, S. pneumoniae, Neisseria meningitidis, P. aeruginosa, and Escherichia coli (137, 160). Hyperviscous Klebsiella pneumoniae, known for its rich capsule production and liver abscess formation, is also a frequent cause of endogenous endophthalmitis in Southeast Asia (137, 161, 162).
Uveitis.
Uveitis is inflammation of any portion of the uveal tract composed of the choroid, iris, and ciliary body and is categorized anatomically as follows: anterior uveitis, where the anterior chamber is the primary site of inflammation; intermediate uveitis, involving primarily the peripheral retina with overlying vitreous inflammation; and posterior uveitis, which principally involves the posterior retina, vitreous body, and/or choroid. In panuveitis, all uveal structures are involved. Symptoms include eye redness, pain, photophobia, blurred vision, and floaters (163).
Uveitis can be caused by trauma, autoimmune disorders, neoplasia, idiopathic inflammation, and infection (163). Infectious uveitis due to microbial seeding from the bloodstream is most commonly caused by Toxoplasma gondii, CMV, HSV, or VZV (163). Other less commonly encountered viruses include West Nile, dengue, chikungunya, Rift Valley fever, Zika, and Ebola viruses (164–166). Tick-borne pathogens, including Borrelia burgdorferi and Rickettsia rickettsii, are unusual causes of uveitis (167). Ocular syphilis rates are increasing in men who have sex with men (168–171), and although infrequent, Mycobacterium tuberculosis complex (TB) uveitis remains a global health issue (172, 173).
Retinitis.
Retinitis is inflammation of the neurosensory retina. Symptoms include photophobia, blurred vision, ocular pain, and floaters. T. gondii and CMV are the most common etiological agents of infectious retinitis (174–176). Toxoplasmosis accounts for roughly 90% of all cases of focal necrotizing retinitis. Fundoscopic identification of focal retinal necrosis surrounded by choroidal edema and vitritis yielding a characteristic “headlight in the fog” appearance with a favorable therapeutic response is diagnostic for ocular toxoplasmosis without the need for additional tests. T. gondii infection occurs via ingestion of oocysts in contaminated products, tissue cysts in undercooked meats, blood products, and in utero vertical transmission (176).
Panophthalmitis.
Panophthalmitis is a medical emergency and can involve N. meningitidis, S. pneumoniae, Streptococcus species, B. cereus, and Clostridium species (59, 177–179).
Panophthalmitis is inflammation of the entire eye, including the sclera and the adjacent extraocular tissues (177). Infection is due to direct extension from a periocular focus or seeding from the bloodstream. Beta-hemolytic streptococci, S. aureus, P. aeruginosa, and K. pneumoniae, particularly hyperviscous strains, are the most common causes (59, 177–179). Symptoms include severe eyelid edema, conjunctival chemosis, proptosis, fixed pupil, and limited ocular movement. If scleral involvement is substantial, thinning and perforation may occur, resulting in loss of the eye. Although enucleation or evisceration may be prevented with prompt initiation of antimicrobials and steroids, the prognosis for recovery of sight is dismal (177).
Ocular parasitic infections.
Many nematodes can reside intraocularly or subconjunctivally, including Acanthocheilonema spp., Angiostrongylus spp., Baylisascaris spp., Dirofilaria spp., Gnathostoma spp., Loaina spp., and Trichinella spp., but only adult worms of Ancylostoma spp. Toxocara spp., and zoonotic Onchocerca spp. (O. cervicalis and O. gutturosa) have been reported within the cornea stroma (6, 180–182). In contrast to adult worms, the microfilariae from Onchocerca volvulus (6) and Mansonella ozzardi (183) migrate from the skin throughout the body and can lodge within the cornea. Interestingly, O. volvulus keratitis develops when the helminths die and release endosymbiotic Wolbachia species bacteria, leading to corneal inflammation and scar formation (184, 185). Some nematodes like Thelazia spp. (the eye worm) reside on the surface of the eye and physically abrade the corneal epithelium, resulting in secondary infection and scarring (186). Likewise, although Loa loa can reside in the subconjunctiva or within the eye, it does not penetrate the cornea to cause keratitis (6, 182).
The cornea stroma is a dense collagenous matrix not readily invaded by large helminths. However, intraocular cysticerci and hydatid cysts from Taenia solium and echinococci, respectively, cause proptosis, exposure keratitis, and secondary infection (6, 182, 187, 188). Although flukes, including Fasciola hepatica and Alaria spp., have been identified in ocular compartments, their large size, soft bodies, and inability to penetrate collagenous tissues limit their ability to access the cornea stroma (6). Likewise, although adult schistosomes may reside in limbal venules, the avascular cornea limits access for the deposition of eggs (187). Schistosome cercariae have been experimentally shown to penetrate the cornea stroma, with self-limited resolution and subclinical illness (189).
LABORATORY DIAGNOSIS
Specimen Collection and Transport
Challenges associated with the diagnosis of ocular infections include the following:
-
•
Limited sample volume
-
•
Irreplaceable specimens
-
•
Bedside specimen inoculation
-
•
Frequent antimicrobial exposure prior to sample collection
-
•
Diverse pathogens requiring specific collection and transport conditions
-
•
Logistics solutions required for prompt sample transport from remote sites
-
•
Limited ocular anatomy/pathophysiology training in clinical microbiology laboratories
Frequent interaction of the microbiology laboratory with ophthalmology clinics is essential to address these challenges. Implementation of the following recommendations may enable streamlined communication and optimize ocular diagnostic approaches:
-
•
Annual ophthalmology resident education focused on explaining the test menu, proper specimen collection, detailed labeling, medium inoculation, and specimen transport
-
•
Establishment of an ophthalmology clinic contact and system to restock media
-
•
Implementation of a courier service to ensure prompt delivery of specimens
-
•
Development of an ophthalmology-specific order requisition to capture unique data associated with ocular specimens and reduce the need to contact providers after-hours
-
•
Availability of consultative services to advise physicians on special diagnostic testing
Specimen collection.
Methods for collecting ocular specimens are straightforward and have not changed appreciably in the last 50 years. Table 1 lists the recommended collection method and transport device based on ocular anatomy. Clinicians should be aware of the potential for ocular dyes and anesthetics to inhibit viral culture (190–192). Prior to sample collection, the eye should be thoroughly rinsed with sterile, nonbacteriostatic saline or water when dyes and anesthetics are used to ensure the optimal detection of ocular pathogens. Physicians should also be encouraged to collect comparable lid and conjunctival specimens from both eyes, even if only one eye is affected. Comparison of microbial growth from the nonaffected eye with that from the affected eye may allow the determination of the etiology of infection.
TABLE 1.
Ocular sample collection
| Group | Sample site(s) | Disease(s) | Specimen collection method | Specimen transport device(s)a |
|---|---|---|---|---|
| 1—outer eye | Eyelid, lid margin, conjunctiva, lacrimal system | Blepharitis, canaliculitis, conjunctivitis, dacryoadenitis | Collect swab of discharge/purulent materialb | Culturette, manufacturer-provided collection devicec |
| Periorbital tissue | Dacryocystitis, preseptal cellulitis | Collect tissue and/or discharge/purulent materialb | Sterile container, syringe with needle removed | |
| 2—inner eye | Orbital cavity | Orbital cellulitis | Collect tissue and/or discharge/purulent materialb | Sterile container, syringe with needle removed |
| Cornea | Keratitis | Collect scrapings with a blade or spatulab,d | Sterile container | |
| Anterior chamber, vitreous humor, iris, uvea, sclera, lens | Endophthalmitis, uveitis, retinitis, panophthalmitis | Collect ocular fluide | Original collection device (needle removed), sterile container | |
| Eye | Foreign object, helminth infection, arthropod | Collect foreign object, helminth, arthropod | Sterile container | |
Swabs are acceptable only for the eyelids, lid margin, conjunctiva, and samples from the superficial lacrimal system. Specimens should be transported to the laboratory immediately at room temperature, <2 h after collection. If this cannot be achieved, specimens may need to be placed into transport media or refrigerated, dependent upon the desired testing.
If sufficient material is available, providers should consider bedside inoculation; stain upon request.
Contact the testing laboratory for the most appropriate collection and transport media.
Blades or spatulas are preferred for the collection of tissue to isolate viruses, fungi, Chlamydia, and free-living amoebae.
Ocular fluids from large-volume washes require cytocentrifugation in the laboratory; stain upon request.
(i) Group 1—outer eye.
The use of a Kimura/platinum spatula with a gentle scraping motion is most helpful for trying to detect intracellular organisms, fungi, and amoebae. Platinum spatulas are preferable because of their rapid heating and cooling capabilities when flame sterilized. However, swabs are much gentler on the patient, yield higher rates of bacterial recovery, and are typically preferred by ophthalmologists (193). Sterile swabs moistened with saline or laboratory-supplied broth enable optimal adsorption. Limited data exist on the use of flocked swabs for ocular cultures (194). The use of cotton and calcium alginate swabs is discouraged because the fatty acids in the cotton fibers and the glue in calcium alginate swabs may inhibit bacterial growth.
If a direct examination is indicated, physicians should place some of each specimen onto a 1-cm2 outlined area on a glass slide. Such marked indications help the clinical microbiologist focus the microscopic examination, mitigating confusion with contaminating surface debris introduced during processing. One slide should be submitted for each requested stain.
All specimens should be inoculated immediately onto fresh solid medium and/or broth using a method that is mutually acceptable to both the physician and the testing laboratory. Table 2 lists the media commonly used for ocular cultures, which include blood, chocolate, fungal, and Centers for Disease Control and Prevention (CDC) anaerobic agars or thioglycolate broth (175, 195, 196). Traditionally, ophthalmologists have inoculated group 1 outer eye specimens onto single solid plate media in distinct patterns to indicate the source of the specimen: for right and left conjunctiva, horizontal and vertical streaks, respectively; for right and left lid margins, “R” and “L” patterns, respectively; and for cornea, “C” pattern. Alternatively, separate plates are inoculated for each tissue site. Clinical microbiologists should encourage the eye care specialist to be more concerned about accurate recovery than artistic inoculation.
TABLE 2.
Primary culture media for ocular specimenso
| Organism or type of infection | Primary culture medium or suggested combination of primary media |
|---|---|
| Organism(s) | |
| Bacteria | 5% chocolate agara |
| 5% sheep blood agara | |
| Brain heart infusion agar with 5% BA | |
| MacConkey agarb | |
| Thioglycolate brotha,c | |
| Anaerobes | CDC anaerobic blood agar |
| Thioglycolate brotha | |
| Fungi | Brain heart infusion agar ± BA |
| Sabouraud dextrose agar | |
| Inhibitory mold agar | |
| Potato dextrose agar | |
| Potato flake agar | |
| Mycobacteria | Lowenstein-Jensen medium |
| Middlebrook 7H10 | |
| Viruses | Universal transport media/cell culture |
| NAATd | |
| Acanthamoebae | Nonnutritive medium with bacterial overlay |
| NAATd | |
| Toxoplasma gondii | NAATd |
| Chlamydia trachomatis | NAATd,f |
| Neisseria gonorrhoeae | NAATd,f |
| 5% chocolate agar and/or N. gonorrhoeae media (e.g., Thayer-Martin agar, Martin-Lewis agar)g | |
| Type(s) of infection | |
| Group 1h | |
| Stye (hordeolum) | Not routinely cultured |
| Conjunctivitis, blepharitis | BA, CHOC, (MAC),b (TMA)g |
| Canaliculitis, dacryocystitis, dacryoadenitis | BA, CHOC, (MAC),m (CDC-ANA)n |
| Preseptal cellulitis | BA, CHOC, (FUNG),i (CDC-ANA)j |
| Group 2h | |
| Orbital cellulitis | BA, CHOC, FUNG,i MAC, CDC-ANA |
| Keratitis | BA, CHOC, MAC, FUNG,i (MYCO),l (CDC-ANA)j |
| Contact lens-associated keratitis | BA, CHOC, FUNG,i (CDC-ANA)b |
| LASIK-associated keratitis | BA, CHOC, FUNG,i MYCO,l CDC-ANAb |
| Foreign objectk | BA, CHOC, FUNG,i (CDC-ANA)j |
| Endophthalmitis | BA, CHOC, MAC, FUNG,i MYCO,l CDC-ANAb |
Medium is commonly supplied to ophthalmologists.
Optional; should be considered for hospitalized patients.
Thioglycolate broth or CDC anaerobic blood agar.
Contact the testing laboratory for the most appropriate transport medium.
Culture of nonclinical samples should be discouraged. However, if indicated, contact the reference laboratory for details regarding specimen type, transport, and testing method.
Manufacturer collection device may be needed. Contact the testing laboratory for the necessary collection kit.
Thayer-Martin medium inoculated if N. gonorrhoeae is suspected. It must be at room temperature at the time of inoculation and placed immediately into a CO2-generating system or bag or a CO2 incubator with moisture after inoculation.
Gram staining should be performed if requested and if the specimen quantity is sufficient. Positive results from group 2 samples and conjunctival samples showing Gram-negative diplococci (suggestive of N. gonorrhoeae) should be handled as a critical value.
Direct fungal staining or examination should be performed if the quantity of the specimen is sufficient. Positive results should be handled as a critical value.
The anaerobic medium of choice, e.g., thioglycolate broth or CDC anaerobic medium, is not usually supplied to physicians. Perform anaerobic cultures only if the physician suspects an anaerobic infection, if the quantity of the specimen is sufficient, and if the specimen has been submitted in the original syringe, in an anaerobic transport device, or in another acceptable anaerobic manner.
Culture only foreign objects if indicated. Most can be cultured in the original thioglycolate or an equivalent broth.
Add media if mycobacteria are suspected, and stain (either auramine-rhodamine or Kinyoun stain) if the quantity of the specimen is sufficient. Positive results should be handled as a critical value.
Add a MacConkey agar plate if infection is secondary to trauma.
Add thioglycolate broth or CDC anaerobic medium if the infection is chronic.
Abbreviations: BA, 5% sheep blood agar or brain heart infusion agar with 5% sheep blood; CHOC, 5% chocolate agar; FUNG, preferred fungal culture medium from the list above; THIO, thioglycolate broth; TMA, Thayer-Martin agar; CDC-ANA, CDC anaerobic blood agar; MYCO, preferred mycobacterial culture medium from the list above; NAAT, nucleic acid amplification test. Parentheses indicate optional media to include.
Swabs for nucleic acid amplification tests (NAATs) can be submitted to the laboratory at room temperature if delivered within 2 h of collection. Dacron, rayon, and flocculated (but not cotton) swabs are acceptable for NAATs. If delays are anticipated, it is advisable to place dry swabs in universal transport media under refrigerated conditions for transport (175). If viral culture is indicated, samples should be submitted in universal transport media and transported at room temperature (175, 196). However, flocculated swabs are associated with increased cell cytotoxicity and should not be used for viral culture (197, 198).
(ii) Group 2—inner eye.
Group 2 specimens are obtained via invasive methods and include tissues, biopsy specimens, scrapings, aspirates, ocular fluids, and surgical irrigation fluid. Scalpel blades, Kimura platinum spatulas, and 25-, 27-, or 30-gauge needles (sometimes bent at the tip) are often utilized and can be used to inoculate liquid or solid media directly. Although a recent study comparing cellulose impression disk culture with corneal scraping showed a high bacterial recovery rate of 40% versus 27%, it is not widely used (196). Depending on the patient presentation and relevant history, the potential etiology may include more diverse pathogens than group 1 outer eye specimens, including fungi, mycobacteria, and Acanthamoeba. The clinical microbiology laboratory and the ophthalmology practice should establish an institution-specific process for providing specialized media for near-patient plating within the expiration date. Factors to consider include the following. Should specialized media be provided routinely or only via special request? Can the quality of a broadened medium supply be maintained, and is there a system to prevent incorrect usage? Are there cultures or tests for which media will not be supplied and submission of the specimen to the laboratory will not be required?
Given limited ocular fluid volumes and an increasingly complex and expanding test menu, submitting the sample to the laboratory and triaging within the lab is the preferred option. In this approach, the clinical microbiology laboratory can triage the specimen to provide maximum diagnostic coverage. It can be helpful to prioritize testing in terms of drops, with ∼50 μl constituting one free-falling drop. Optimized utility can be achieved by limiting the number of solid-medium plates constituting a culture, discussing the need for smears (e.g., Gram staining may be of low priority given the limited expected utility and high likelihood of empirical antibiotic administration), and reducing the volume needed for molecular testing.
Specimen transport.
Almost all ocular specimens submitted for culture are extremely small and can be hidden and easily lost in containers or moist gauze. Therefore, physicians should inform the testing laboratory exactly what is being submitted and the initial test prioritization. This can be accomplished by calling the laboratory or indicating on the requisition accompanying the specimen, but as electronic health records become more user-friendly, a standardized electronic solution is preferred. Given the irreplaceable nature of many ocular specimens, the laboratory should contact the ordering provider if the specimen does not appear as described, prior to canceling the test.
Table 1 includes details on the transport of ocular specimens. All specimens must be transported to the testing laboratory immediately (196). If a significant delay is anticipated, the laboratory should be contacted to determine the appropriate storage conditions. Swab specimens should be transported at room temperature in laboratory-supplied broth or in the original swab transport device. Fluids should be transported in their original containers (syringe with needles removed) or expressed into a sterile specimen container. Tissues should be transported intact and moistened with sterile saline. Foreign objects, artificial lenses, and specimens collected on filter paper should also be transported as if they were tissues. If anaerobic culture is desired, the tissue should be placed in an anaerobic transport device if transit will take >2 h. If viral culture is warranted, direct specimens, such as tissues and aspirates, should be transported at room temperature (1, 175).
Organism Identification and Reporting
The source of specimen, type of specimen, and suspected infection are essential to triage small-volume specimens to achieve clinically actionable results. If such detailed information is not received, the ordering physician must be contacted immediately for additional clarification and relevant detailed information to allow optimal microbiological processing, preparation, examination, and culture of these often-irreplaceable specimens.
Approaches to detect ocular pathogens in the microbiology laboratory include the following:
-
•
Direct examination
-
•
Antigen tests
-
•
Culture
-
•
NAATs
-
•
Serology
Direct examination.
Glass slides prepared at the bedside or in the laboratory can be stained in the microbiology laboratory, enabling rapid microscopic identification of infectious agents to direct appropriate therapy. Bedside inoculation of glass slides is most commonly performed for cases of suspected conjunctivitis, keratitis, or cellulitis. Ocular fluids require cytocentrifugation in the laboratory and are not amenable to bedside inoculation. To optimize rapid diagnostic opportunities, communication between the clinical microbiology laboratory and the ophthalmologist is essential.
(i) Gram stain.
Gram stain results from ocular specimens generally have a high positive predictive value but low sensitivity due to limited sample volume and prior antimicrobial treatment (199, 200). All conjunctival and lacrimal duct exudate specimens should be Gram stained to identify Gram-negative diplococci characteristic of Neisseria species as this medical emergency necessitates the addition of parenteral therapy (Fig. 2A) (201). In non-N. gonorrhoeae cases, the positive predictive value of conjunctival Gram stains is less certain (202). The detection of large, boxy Gram-positive rods (Fig. 2B) consistent with Bacillus or true hyphae consistent with molds (Fig. 2C) should raise concern for rapidly progressive infections. Corneal scrapings and vitreous biopsies/aspirates are done at some risk to the patient with low specimen mass; therefore, Gram stains should be performed on these specimens only at a physician’s request. Gram stain of corneal scraping and vitreous fluid is typically less sensitive (30 to 55%) than culture (203–205).
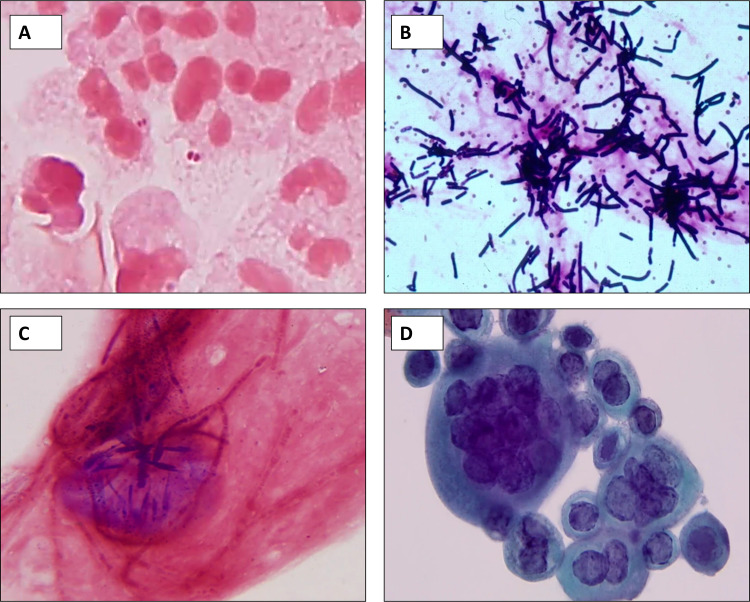
FIG 2.
(A to C) Gram staining of cornea tissue infected with pathogens requiring rapid medical intervention. (A) Neisseria gonorrhoeae; (B) Bacillus cereus (note the iris melanin granules [reprinted from reference 394]); (C) hyaline septate mold (scopulariopsis). (D) Papanicolaou stain of HSV infection in the cornea.
When a Gram stain is indicated, both the organism and the surrounding host inflammatory response should be reported. Morphological identification of large, boxy Gram-positive rods consistent with Bacillus species, ghost cells consistent with mycobacteria, Gram-negative diplococci consistent with Neisseria spp., filamentous bacteria, yeast/pseudohyphae consistent with Candida spp., and true hyphae consistent with mold infection may necessitate a prompt change in medical therapy. Semiquantitation of white blood cells helps inform decisions on true infection versus contamination. Further differentiation into granulocytes and lymphocytes may inform bacterial versus viral infection but is difficult to perform on a Gram stain. Upon request, Giemsa-stained slides may enable leukocyte differentiation, but the tissue quantity is often limited, and this staining is not routinely performed.
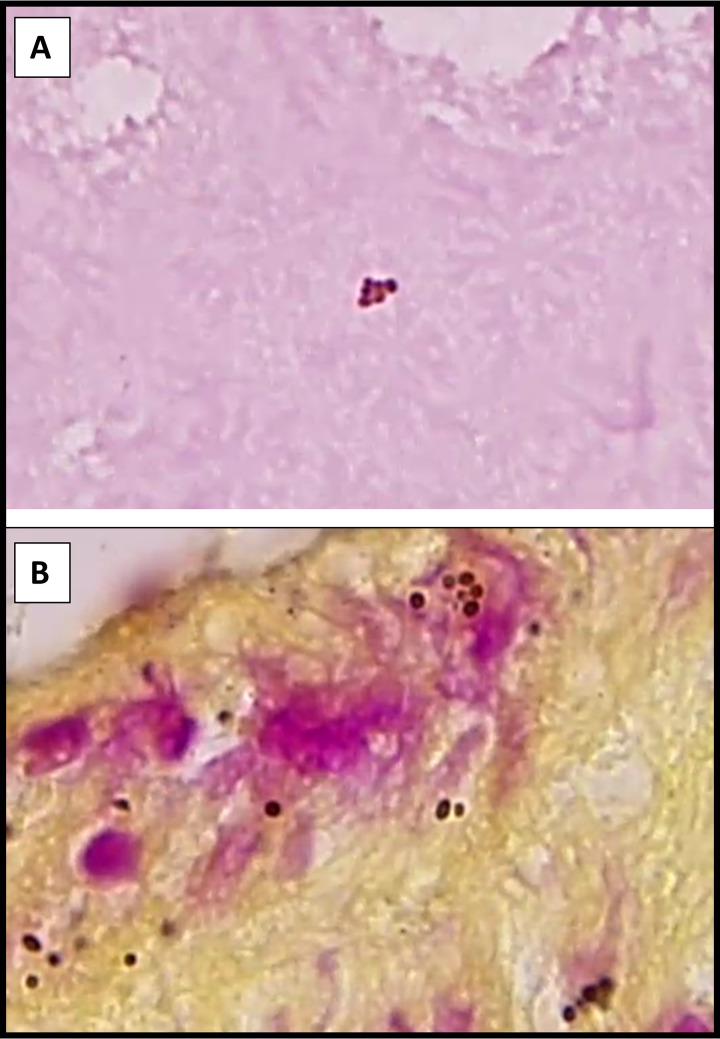
Small round pigmented melanin granules derived from the iris are frequently mistaken for bacterial cocci in ocular specimens (Fig. 3). Technologists should be aware of this entity and note the brownish tinge, irregular edges, lack of cluster/chain formation, and lack of an appropriate host response to identify this mimic.
FIG 3.
Gram (A)- and hematoxylin and eosin (H&E) (B)-stained sections of cornea tissue with melanized granules from the iris mimicking bacterial cocci. Note the brownish pigmentation, irregular edges to some of the granules, and the lack of an appropriate host response.
(ii) Acid-fast stains.
Acid-fast stains should be performed on ocular specimens only at a physician’s request. Ziehl-Neelsen and Kinyoun stains yield equivalent detection of mycobacteria, but modified acid-fast stains are required for organisms like Nocardia with smaller amounts of mycolic acid in their cell wall. Situations in which this stain should be strongly considered include the presence of ghost cells upon Gram staining, samples from patients with active TB, and samples from patients with possible RGM infection such as post-LASIK surgery or traumatic keratitis unresponsive to standard antimicrobials (58, 172, 173, 206).
(iii) Calcofluor white.
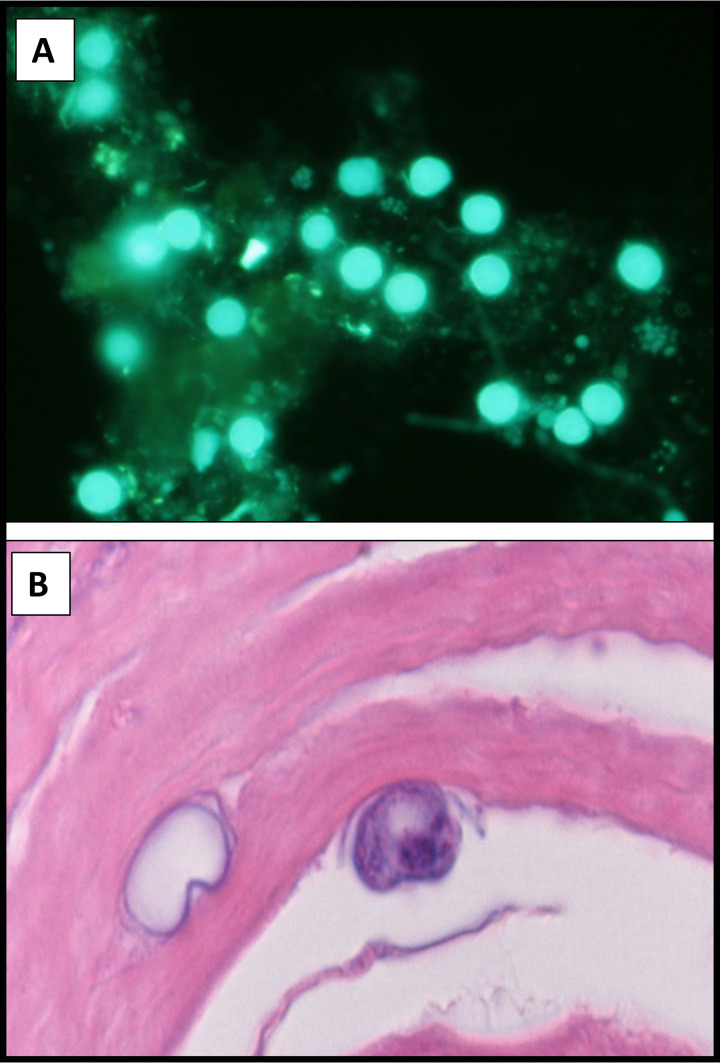
A Gram stain will reveal the presence of yeasts, hyphae, and amoebae. However, if there is high clinical suspicion, the high fluorescent signal-to-noise ratio of the calcofluor white stain significantly improves sensitivity for fungi, including microsporidia (207–212) and Acanthamoeba (Fig. 4A) (213, 214). Technologists should be aware that cotton fibers and any cellulose- or chitin-containing substance will also fluoresce with calcofluor white and may be confused with fungal elements. Additionally, the quality, performance, and specificity of commercially prepared formulations of calcofluor white can vary widely. Readers are advised to evaluate the product from more than a single supplier.
FIG 4.
Calcofluor white-stained cornea scraping (A) and H&E-stained section (B) of cornea tissue showing Acanthamoeba cysts in a patient with contact lens-associated keratitis.
(iv) Giemsa and Papanicolaou stains.
Viral cytopathic effect and intracellular inclusion bodies associated with Chlamydia infection are difficult to assess with stains commonly used in the microbiology laboratory. However, Giemsa and Papanicolaou stains enable the detection of multinucleation, molding, and margination consistent with HSV and VZV infection (Fig. 2D); smudgy nuclei associated with adenovirus; and intracellular inclusion bodies (215, 216). Given small tissue volumes, the routine use of Giemsa stains to evaluate host cells in ocular specimens is most often of limited clinical utility.
Antigen tests.
Clinical Laboratory Improvement Amendments (CLIA)-waived enzyme immunoassays (EIAs) are available to detect adenovirus from ocular fluid (89, 217, 218). Although not often performed because of the self-limited nature of the disease, a rapid and accurate positive result restricts inappropriate antibiotic use and enables confident utilization of topical steroids (89, 219). Additionally, a negative result prompts clinicians to seek alternative diagnoses and can clear patients to safely return to work (220).
The CLIA-waived AdenoPlus point-of-care (POC) test (Quidel) (formerly the RPS Adeno detector) is a single-use, handheld, membrane-based EIA that captures and detects adenovirus antigens within 10 min. This assay has undergone several upgrades, and two published studies have evaluated its test performance characteristics. A multicenter blind prospective trial (n = 128) detected a sensitivity of 85% and a specificity of 98% compared to PCR (217), and a subsequent smaller prospective study (n = 46) showed a sensitivity of 50% and a specificity of 92% (218). The results of both studies indicate that a positive result with the AdenoPlus POC assay should be acted upon and that a negative result should be reflexed to PCR, should clinical concern persist. The majority of adenovirus-negative keratoconjunctivitis cases caused by certain coxsackievirus and enterovirus serotypes are mild and self-limited and do not merit additional testing (220). However, in the setting of more severe disease, especially in the context of an ongoing epidemic of acute hemorrhagic conjunctivitis, testing for infectious agents like enterovirus 70 and coxsackievirus A24 may be indicated and available at regional public health laboratories and/or the CDC (221, 222).
Culture.
Open communication with ocular health providers and flexibility in the microbiology laboratory are essential to obtain clinically relevant data from ocular cultures. Table 3 lists pathogens that infect ocular tissues. The laboratory should inform providers that it is their responsibility to alert the laboratory of any concern for organisms that might require extended incubation or unique culture conditions. Additionally, a detailed clinical history of underlying clinical diseases, risk of sexually transmitted infection and TB, travel and immigration history, and vaccination status are essential for the laboratory to identify rare infectious etiologies. Preinoculated media should be incubated as received and should not be streaked. The remainder of the specimens should be processed as described below.
TABLE 3.
Examples of more common and less common ocular pathogensa
| Disease(s) | More common pathogens | Less common pathogens |
|---|---|---|
| Group 1—outer eye | ||
| Canaliculitis | S. pneumoniae, H. influenzae, Actinomyces spp. | RGM, P. aeruginosa |
| Dacryocystitis | S. aureus, S. pyogenes, C. albicans, S. pneumoniae, Actinomyces spp., P. aeruginosa, H. influenzae | RGM |
| Blepharitis | S. aureus, coagulase-negative staphylococci, C. acnes, HSV | Demodex folliculorum, Pthirus pubis |
| Conjunctivitis | S. pneumoniae, H. influenzae, S. aureus, S. pyogenes, Moraxella spp., C. trachomatis, adenovirus, coxsackievirus, enterovirus 70 | Neisseria gonorrhoeae, Toxocara canis, microfilariae, microsporidia, Ebola virus, Zika virus, dengue virus, chikungunya virus |
| Preseptal cellulitis | S. aureus, beta-hemolytic streptococci, H. influenzae, S. pneumoniae | |
| Group 2—inner eye | ||
| Keratitis | P. aeruginosa, S. aureus, coagulase-negative staphylococci, S. pneumoniae, viridans group streptococci, beta-hemolytic streptococci, Moraxella spp., S. marcescens, C. acnes, B. cereus, B. subtilis, Enterobacterales, Fusarium spp., Aspergillus spp., C. albicans, adenovirus, coxsackie virus, enterovirus, HSV-1 and -2, VZV, Acanthamoeba | N. gonorrhoeae, N. meningitidis, Nocardia spp., RGM, Exophiala spp., Scedosporium spp., Paecilomyces spp., Curvularia spp., Bipolaris spp., Acremonium spp., microsporidia, microfilaria, Hartmannella spp., vahlkamphid amoebae |
| Endophthalmitis, panophthalmitis | S. aureus, coagulase-negative staphylococci, S. pneumoniae, alpha-hemolytic streptococci, beta-hemolytic streptococci, Enterococcus spp., C. acnes, E. coli, Klebsiella pneumoniae, Enterobacterales, B. cereus, B. subtilis, P. aeruginosa, Acinetobacter, Bacillus spp., C. albicans, Aspergillus spp. | H. influenzae, Burkholderia cepacia complex, N. meningitidis, Nocardia spp., RGM, Exserohilum spp., Fusarium spp., Aspergillus spp., Acremonium spp., Paecilomyces spp., Scedosporium spp., HSV, VZV, CMV, T. gondii, Taenia solium, Echinococcus spp. |
| Infectious uveitis/retinitis | C. albicans, T. gondii, HSV, VZV, CMV | T. pallidum, M. tuberculosis, B. burgdorferi, West Nile virus, dengue virus, Rift Valley fever virus, Ebola virus, Zika virus, chikungunya virus |
| Orbital cellulitis | S. pyogenes, alpha-hemolytic streptococci, P. aeruginosa, Enterobacterales, H. influenzae, Bacteroides spp., Mucorales spp. | Aspergillus spp., Scedosporium spp. |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; VZV, varicella-zoster virus; RGM, rapidly growing mycobacteria.
(i) Processing tissues.
Corneal tissue and biopsy specimens should be minced using sterile scissors or a scalpel and inoculated on media appropriate for the organisms suspected. If unsure of how to proceed, clarification should be obtained from the ordering physician.
(ii) Processing fluid specimens.
Most fluid specimens will be very small in quantity (usually 0.1 to 0.2 ml of fluid in a 1-ml syringe). Distribute the fluid evenly onto solid medium, and streak for isolation. If enough fluid is received (e.g., wash or irrigation fluid), centrifuge the fluid, and use the sediment to inoculate the medium.
Alternatively, some laboratories concentrate vitreous wash specimens by passing the specimen through a 0.45- or 0.22-μm sterile membrane filter and collecting nonfilterable materials, including bacteria and fungi, on the surface of the membrane filter. The membrane filter is then aseptically divided using a sterile scalpel, placed on the agar surface, and incubated. Filtered vitreous wash fluids are more sensitive for the diagnosis of bacterial and fungal endophthalmitis than vitreous biopsy specimens, but the combination of both is the most sensitive diagnostic option (223).
Culture of vitreous fluid (0.1 to 3 ml) in blood culture bottles has an increased sensitivity compared to traditional culture (219, 224–228). Blood culture bottles were more likely to detect Gram-positive organisms, especially C. acnes (228). Although these data are promising, clinical validation of this off-label use is difficult given the limited access to appropriate clinical specimens.
Intraocular fluids destined for molecular or serological studies do not require additional processing and should be analyzed directly for the presence of infectious agents (1). Testing for emerging pathogens with serious public health implications, such as Ebola virus, requires special precautions (229, 230). Clinicians with suspected patients must communicate with the laboratory as early as possible, and notifications should be sent to regional public health centers within the laboratory response network (229). Samples should be handled by as few individuals as possible, labeled and triple packaged at the site of collection, transported with cold packs at 2°C to 8°C, and sent to the regional public health laboratory for analysis (230).
(iii) Inoculation and incubation.
To simplify culture approaches, group 1 and 2 specimens should be inoculated onto blood agar, chocolate agar, fungal agar, and thioglycolate broth and incubated for the same length of time. Suitable options for fungal media are provided in Table 2. Most bacteria and yeasts associated with ocular infections (Table 3) will grow within 48 to 72 h. A 4-day incubation of bacterial cultures at 35°C to 37°C in 5 to 7% CO2, a 7-day incubation in thioglycolate broth in ambient air, and a 3- to 4-week incubation of fungal cultures of group 1 and 2 specimens at 30°C meet the community standard of care (231). However, suspicion for slower-growing organisms, including C. acnes (10 to 14 days) and mycobacteria (7 to 14 days), and invasive surgically collected specimens may merit longer incubation of bacterial cultures (231). Likewise, if informed of a suspected iatrogenic infection from surgery or intravitreal injection, bacterial cultures should be extended for up to 14 days to isolate RGM and C. acnes (123, 134, 232).
(iv) Bacterial and yeast culture workup.
Bacterial cultures should be examined daily, and technologists should be prepared to encounter the rare ocular pathogens shown in Table 3. The probable identity and relative quantity (rare, few, moderate, or many) of each morphotype should be determined. The isolation of the following ocular pathogens in any concentration should be reported: N. gonorrhoeae, P. aeruginosa, B. cereus, S. aureus, beta-hemolytic streptococci, RGM, and molds.
Group 1 outer eye specimens should be worked up similarly to specimens from other nonsterile tissues. It can be difficult to determine if isolations of commensal flora such as coagulase-negative staphylococci, viridans group streptococci, Corynebacterium species, Actinomyces spp., and C. acnes are contaminants or the true cause of infection. The presence of organisms with the expected morphology and white cells on a direct Gram stain may be helpful in determining clinical significance (233). Pure culture of a single organism merits identification (ID) and antimicrobial susceptibility testing (AST), with the exception of coagulase-negative staphylococci (not Staphylococcus lugdunensis) and diphtheroids, which do not require AST unless requested. Full ID and AST are indicated for up to 2 predominant pathogens with minimal morphological ID for nonpredominant pathogens and flora. Importantly, S. aureus and P. aeruginosa merit ID and AST at any quantity. Likewise, B. cereus, beta-hemolytic streptococci, RGM, and molds merit ID at any quantity. If 3 or more potential pathogens are present with none predominating, ID and AST should be performed for up to 2 pathogens with minimal morphological ID (no AST) of other pathogens and flora.
In contrast, group 2 specimens should be treated as a sterile site with performance of ID and AST on any potential pathogen. The term “flora” should not be used for these specimen types. At a minimum, all growth should be quantitated, and minimal morphological ID should be reported.
The most commonly encountered bacterial and yeast pathogens in ocular infections, including RGM, are reliably identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (234). In contrast, the identification of Bacillus species by MALDI-TOF MS can be challenging. This has potential implications for the optimal treatment of endophthalmitis given the increased resistance in B. cereus compared to Bacillus subtilis (59, 235, 236). Other conventional methods, including biochemical kits, are also reliable for most ocular bacterial and yeast pathogens (237).
(v) Mold culture workup.
Most molds that cause ocular infections, including Fusarium spp. and Aspergillus spp., will grow on bacterial cultures. Fungal cultures should be incubated for 4 weeks and evaluated with a standard mycology plate-reading schedule to identify slow-growing molds, dimorphic pathogens, filamentous bacteria, and rare algal and oomycete infections. For more information on mold ID by morphology, readers are referred to the excellent text Medically Important Fungi: a Guide to Identification, 6th ed., written by Davise Larone (238). Given the wide variety of molds that cause ocular infection, the isolation of any mold from an ocular culture should be reported unless clearly deemed a contaminant.
The Vitek MS and Bruker MALDI-TOF MS platforms exhibit similar accuracies (77% versus 79%) in identifying molds to the species level (239, 240) and readily detect the most common molds and rare pathogens present in the database. This technology is particularly powerful in enabling the early detection of molds lacking fruiting bodies (mycelia sterilia) and distinguishing between species with overlapping morphologies. However, rapid user-friendly extraction protocols and expansion of curated databases will likely be required prior to widespread implementation (241). The potential role for this technology in identifying ocular isolates is not clear given that a plethora of environmental molds cause infection (82); species-level identification is often not required to guide therapy (65); the identification of the most important pathogen group, Fusarium spp., can be problematic (240); and the majority of infections occur in settings without access to MALDI-TOF MS (82).
(vi) Corneal rim.
There are no consistent recommendations for routine fungal cultures on donor corneal rims. The decision to culture corneal rims should be made in consultation with the ophthalmology groups serviced by the laboratory. The rationales for not performing them are the very low rate of postsurgical corneal transplant infections and the expense of culturing all donor rims estimated in the late 1990s as being between $2 million and $6 million (70). Updated economic analyses are needed to determine the current cost/benefit ratio of culturing transplanted corneal rims. Since endothelial keratoplasty is more closely associated with posttransplant fungal infections, some groups may wish to limit culture to this specific type of explanted tissue (71). If explanted tissue culture is indicated, the holding medium should be inoculated onto chocolate agar (4 days) and Sabouraud dextrose agar (4 weeks) and worked up as described above for group 2 specimens. Positive cultures should be reported immediately, enabling the judicious use of prophylactic antimicrobials in transplant recipients.
(vii) Contact lenses and foreign bodies.
The accurate diagnosis of contact lens-related infectious keratitis requires organism isolation from infected tissue. Culturing of accessory items should be discouraged given the high rates of transient colonization of lens care products (77, 242) and the potential to erroneously ascribe infection to an organism requiring a different therapy regimen. Although a discussion with the treating clinician is warranted, microbiologists must state that the standard of care is to culture clinical material and that culture of nonclinical material is not a surrogate for a clinical specimen. The same rationale should also be considered when requests are made for culture of foreign bodies associated with traumatic eye injuries such as pieces of wood or metal (243).
(viii) Acanthamoeba.
Culturing specimens for Acanthamoeba species is technically complex and not commonly performed in clinical laboratories. Culture is available in select reference and public health laboratories, including the CDC. Corneal scrapings, corneal biopsy specimens, and vitreous fluid from individuals with protracted keratitis and documented vision loss can be cultured on nonnutritive solid media covered with a confluent lawn of dead Gram-negative rods such as E. coli or Enterobacter aerogenes (188, 244). Culture of nonclinical specimens such as contact lens solutions, lenses, and cases is controversial. Cultures should be incubated in a moist environment at 37°C for at least 10 days and examined daily under 10× and 40×objective light, phase, or interference microscopes. Acanthamoeba will feed on the bacteria, move over the surface of the medium, and produce irregular tracks that are visible under a dissecting microscope in only a day or so; after 2 or 3 days, the amoebae usually form visible cysts (87). Active trophozoites measuring 15 to 40 μm with a large contractile vacuole, a single nucleus, and fine tapering pseudopodia and double-walled cysts measuring 10 to 20 μm with a characteristic wrinkled outer wall are diagnostic (Fig. 4). Acanthamoeba amoebae and cysts are infectious, and cysts have been documented to remain viable for over 20 years (245, 246). Always wear gloves when performing Acanthamoeba cultures and wash hands thoroughly after such work.
(ix) Viral culture.
Viral culture requires significant laboratory resources and specialized expertise and is not routinely performed on ocular specimens unless antiviral resistance is suspected in the setting of recurrent HSV infection (95). Most laboratories send this testing out to specialized referral centers. For detailed information on viral culture, consult the Clinical Microbiology Procedures Handbook (247).
Nucleic acid amplification tests.
There are no FDA-approved NAATs for ocular specimens, and most clinical laboratories lack access to sufficient quantities of positive clinical samples to validate in-house or commercial assays. However, commercial reference laboratories and a subset of hospital-based laboratories servicing large ophthalmology practices offer molecular testing on ocular specimens utilizing validated laboratory-developed tests (LDTs) or modified commercial assays, some of which are FDA approved for other specimen types. Laboratories interested in this approach can find continuously updated information on FDA-approved diagnostic tests for infectious diseases on the FDA website. The modification of any FDA-approved commercial assay renders it an LDT, requiring thorough validation prior to implementation for patient care.
NAAT identification of ocular pathogens from conjunctival swabs, cornea scrapings, aqueous humor, and vitreous humor exhibits increased sensitivity compared to traditional culture (248–250). NAAT specificity for pathogens that are not a part of the normal ocular surface flora (for example, C. trachomatis) exceeds 99%. However, the molecular detection of a commensal organism from an inner or outer eye specimen (for example, C. acnes) may represent true infection or contamination during specimen collection (251). NAATs are particularly helpful in deciphering the etiology of culture-negative intraocular infections with fastidious organisms, and non-FDA-approved singleplex assays for the following pathogens have been reported primarily in research settings: bacteria (C. acnes [251], C. trachomatis [252–258], M. tuberculosis complex [206, 259, 260], and N. gonorrhoeae [252]), fungi (Candida spp. [261], Aspergillus spp. [261], Fusarium spp. [261], and Microsporidia spp. [262]), parasites (Acanthamoeba spp. [263, 264] and T. gondii [265–267]), and viruses (adenovirus [222, 268], arboviruses [94], CMV [269], coxsackievirus [270], Ebola virus [229], EBV [269], enterovirus 70 [222], HHV6 [227], HSV-1 [269], HSV-2 [269], rubeola virus [271], vaccinia virus [272], and VZV [273]).
(i) Tuberculosis.
In most clinical settings, the diagnosis of ocular TB, typically either uveitis or retinitis, is based on ophthalmological examination and evidence of pulmonary TB by standard methods, including a positive chest radiograph coupled with a positive skin tuberculin test or interferon gamma release assay (IGRA) or a positive acid-fast bacillus smear, culture, or NAAT on a nonocular specimen (172).
However, a significant number of patients with ocular TB do not have evidence of pulmonary disease (172). Current approaches to diagnose ocular TB rely on culture or NAATs on ocular fluid (172). Laboratory-developed NAATs for TB use different genes and amplification conditions, contributing to the high variability in test performance (274). In the largest study to date, NAATs were positive in only 56% (33/59) of specimens from individuals who met the study criteria for ocular TB. Particularly concerning is that NAATs were negative in 8 patients with disseminated TB (274). It is not clear if this insensitivity is due to the paucibacillary nature of ocular TB or the variability in NAAT performance.
A commercial PCR assay, Cepheid Xpert MTB/RIF, has been widely adopted globally for the diagnosis of TB from lung specimens but is not FDA approved for ocular specimens (275). For now, NAAT performance on ocular specimens cannot be recommended. Large centers are urged to freeze any excess ocular fluid specimens that could subsequently be used for NAAT validation studies not only for M. tuberculosis but also for other difficult-to-detect microbes.
(ii) Chlamydia trachomatis.
Commercially available NAATs targeting C. trachomatis are highly sensitive and specific but are FDA approved only for screening genital tract specimens (35). In the United States, off-label use of commercial NAATs on ocular specimens requires access to appropriate samples in sufficient volumes for clinical validation. Combination tests that include gonococcus may enable cost-effective implementation at some large academic medical centers, but most testing is limited to reference laboratories.
Although potentially useful for global trachoma eradication efforts, most diagnoses are based on clinical findings, and macrolide treatment is initiated without laboratory diagnostics. NAATs requiring cold reagent storage and/or expensive and complex equipment are cold chain and cost prohibitive in geographic regions with a high trachoma burden. In contrast, the field-tested Cepheid Xpert C. trachomatis assay with >99% sensitivity bypasses cold chain issues and may prove more cost-effective, particularly in local laboratories already using Cepheid Xpert MTB/RIF PCR (253).
(iii) Treponema pallidum.
A small series (n = 40) of a nested Treponema pallidum PCR assay performed on cerebrospinal fluid (CSF) from patients diagnosed with neurosyphilis showed a modest sensitivity of only 43%. Over half of the patients had physical findings on ocular examination consistent with ocular syphilis (276). Based on these very limited data, PCR assays currently do not have a role in the diagnosis of ocular syphilis.
(iv) Acanthamoeba.
There are no FDA-approved commercial molecular diagnostic tests for Acanthamoeba. Laboratory-developed PCR assays targeting Acanthamoeba are significantly easier to perform than Acanthamoeba culture and are at a minimum as sensitive as culture (244, 277). Quantitative PCR may have prognostic significance (278). These assays are available at the CDC and select reference laboratories.
(v) Toxoplasma gondii.
Serology coupled with clinical findings is the most widely used approach to diagnose T. gondii uveitis and retinitis (279). However, during early acute infection or in immunocompromised hosts when antibody levels are low and the organism burden is high, NAATs exhibit higher sensitivity than serology (280–284). There are no currently available FDA-approved NAATs targeting T. gondii; however, commercial assays and LDTs are available. To optimize analytical sensitivity, these assays target repetitive gene sequences in the T. gondii genome, including the B1 gene (repeated ∼30-fold) (284) and the 529-bp repeat element (∼300-fold) (266, 284). Both assays are >99% specific for T. gondii; however, increased analytical sensitivity is observed for assays targeting the 529-bp repeat element (266, 284). Reported PCR sensitivities in the literature range from 28 to 57% in immunocompetent individuals (266, 282, 285, 286) to 72% in immunosuppressed hosts (281, 282). Multiplex PCR panels targeting multiple ocular pathogens, including T. gondii, have been developed, but comparative studies with intraocular serology and singleplex PCR assays are not currently available (287, 288).
(vi) Viruses.
HSV and VZV are often not readily distinguished by direct examination; however, NAATs can differentiate them and exhibit higher sensitivity and specificity than EIAs (289, 290). There are many commercial HSV NAATs available (291), and most clinical laboratories using HSV PCR on tissue and cerebrospinal fluid may choose to additionally validate ocular specimens on these platforms. Both the Lyra direct PCR assay (Quidel) (292) and the Solana isothermal helicase-based amplification assay (Quidel) (293) target HSV-1, HSV-2, and VZV. The AmpliVue 1+2 assay (Quidel) is a disposable point-of-care lateral flow isothermal amplification assay targeting HSV-1 and HSV-2 with a reported sensitivity of 84% and a specificity of 100% on ocular specimens (294, 295). Recently, the CLIA moderate-complexity DiaSorin Simplexa HSV1 and -2 assay performed on the Liaison MDX platform gained FDA approval for the detection of HSV-1 and HSV-2 within 1 h from ocular swabs. To date, no studies evaluating its test performance characteristics have been published, but FDA submission data showed accurate detection of HSV-1 in two positive samples and no detection in nine negative ocular specimens.
The diagnosis of ocular infection caused by ubiquitous commensal viruses such as CMV, EBV, and HHV6 requires careful clinical correlation as their detection is expected in both active infection and latency (290, 296, 297). Acute visual changes and retinal lesions in the setting of documented viremia are often sufficient to diagnose ocular infection (296, 298–301). Should additional evidence be required, a quantitative NAAT on ocular fluids (aqueous or vitreous humor) is indicated and should be reported out with a quantitative value and/or statement to assist clinicians in determining whether findings are representative of active infection or commensal latency (296, 300). LDT cutoffs vary depending on the specific assay and lack interlaboratory standardization.
Ocular infection with measles (91, 92), Zika (302), dengue (94), chikungunya (94), and Ebola (93) viruses will most often present in the context of a characteristic clinical syndrome. In this setting, viral detection and/or serological confirmation in other specimen types (usually blood) is sufficient evidence for ocular involvement. However, if there is suspicion for persistent ocular involvement in the absence of systemic symptoms, such as with survivors of Ebola virus infection, serology should be performed to confirm exposure (if not already done), and positive results should be followed up with organism identification by NAATs on an ocular specimen (229, 230). Regional public health laboratories and/or the CDC offers PCR for all of these infectious agents. Any clinical suspicion for Ebola virus, measles virus, and other highly communicable pathogens should trigger rapid and open communication and appropriate infection prevention measures (230).
Multiplex assays that simultaneously target several ocular pathogens with overlapping clinical syndromes enable the optimized utilization of limited sample volumes with minimal loss in sensitivity (287, 303). Laboratory-developed (289, 304) and commercial (292) multiplex assays targeting recurrent ocular infections caused by HSV and VZV have been described. Multiplex PCR assays targeting coxsackievirus and enterovirus 70 have been used to identify the cause of acute hemorrhagic conjunctivitis (222). Multiplex assays additionally targeting adenovirus and C. trachomatis aim to cover the most common causes of ocular surface pathology (304). Nakano and colleagues recently developed a novel multiplex strip PCR assay targeting 24 common ocular pathogens with the incorporation of the 16S rRNA and 28S rRNA for panbacterial and panfungal detection (288). Other groups have utilized PCR amplification of 16S, 18S, or 28S rRNA genes to screen for organisms with subsequent identification by sequencing (305–307). Although complex and time-consuming, this approach works well on most ocular specimens given the high signal-to-noise ratio in sterile sites lacking commensal organisms. Comprehensive algorithmic approaches utilizing an initial multiplex PCR targeting HHV1 to -8 and T. gondii followed by reflex 16S rRNA and 28S rRNA gene PCR and Sanger sequencing exhibit high sensitivity (>90%) for all clinically relevant bacteria and fungi and the most common viral and parasitic pathogens (307).
Advances in precision medicine and mutation-specific drug therapy have ushered in the era of next-generation sequencing (NGS) in the molecular pathology laboratory (308–310). High costs and low test volumes limit implementation in most clinical microbiology laboratories; however, specialized laboratories associated with academic institutions and commercial reference laboratories have validated NGS for clinical use (310–313). To grasp the potential utility of NGS for infectious diseases, it is important to understand the two most common general approaches (314). Targeted amplicon sequencing requires preexisting knowledge of the target of interest (for example, the 16S rRNA gene) enabling primer-mediated PCR amplification or probe-specific selection prior to sequencing and the identification of suspected pathogens with a high signal-to-noise ratio (314). In contrast, shotgun metagenomic approaches sequence all nucleic acids in a sample without a selective amplification step, and bioinformatic analyses are used to match nonhuman sequences to curated databases for organism identification (314). Metagenomic data can be difficult to interpret in nonsterile sites (cornea and conjunctiva) due to background genomic material from commensal organisms; however, the relative abundance of reads can be used to ascertain the predominating species, and a minimal commensal burden would be expected for the majority of intraocular specimens (313, 314). Both approaches are therefore well suited for the detection of ocular pathogens given the wide array of infectious agents and the small sample size available to render a diagnosis (303). The literature exhibits several examples of the target-agnostic potential of NGS to identify unsuspected pathogens, including intraocular rubella virus infection in an individual with a >20-year history of chronic bilateral idiopathic uveitis (315).
Serological tests.
Serological diagnosis of ocular infection is done primarily in patients who have retinitis or uveitis secondary to systemic infection. The main ocular pathogens diagnosed in this manner are T. pallidum, T. gondii, and B. burgdorferi.
(i) Treponema pallidum.
The early diagnosis/prevention of ocular syphilis is dependent on screening patients at risk for syphilis infection, including men who have sex with men, commercial sex workers, and HIV-positive individuals, with testing every 3 months being optimal (316).
CDC diagnostic guidelines for neurosyphilis apply to ocular syphilis. Visual dysfunction in the context of a positive Venereal Disease Research Laboratory (VDRL) test from a nonbloody CSF specimen is 60% sensitive but highly specific and should be considered diagnostic of ocular syphilis (317, 318). When a CSF VDRL test is negative, reactive serological test results, abnormal CSF cell counts (>5 WBCs/μl [>20 more specific]), and/or elevated CSF protein levels should prompt a CSF fluorescent treponemal antibody absorption (FTA-ABS) test. If the test is negative, ocular syphilis can be ruled out. However, a positive CSF FTA-ABS test result may represent nothing more than a past infection and should be interpreted with caution (317, 318).
Unfortunately, a recent ocular syphilis series reported that only half of the patients had a lumbar puncture performed (318). In patients who do not have lumbar punctures, eye examinations and serum positive for T. pallidum antibodies are used to establish the diagnosis of ocular syphilis.
(ii) Toxoplasma gondii.
In immunocompetent individuals, serology is the most widely used approach to diagnose T. gondii uveitis and retinitis (279). FDA-approved assays are available on automated platforms and as manual enzyme-linked immunosorbent assays (ELISAs) (319–322). The use of FDA-approved kits is recommended given their high accuracy (323). Negative IgG serology rules out ocular infection in an immunocompetent host (324–327). However, serology in immunocompromised individuals is susceptible to false-negative results and should not be used to rule out infection (281, 327).
Clinicians seeking additional evidence to confidently render a diagnosis of ocular toxoplasmosis should obtain anterior chamber or vitreous fluid for additional testing (291). Unlike sera, diagnostic testing is limited to specialized reference laboratories that have validated the analysis of ocular specimens (280–282, 328). ELISAs are utilized to quantify T. gondii-specific antibody concentrations in ocular and serum specimens, with a ratio of intraocular/serum antibodies (Goldmann-Witmer coefficient [GWC]) of >3 considered diagnostic of ocular involvement (281). Alternatively, qualitative immunoblot (IB) assays are performed (329, 330). Rothova and colleagues identified variability in the sensitivity of the GWC for immunocompetent patients (93%) compared to immunosuppressed individuals (57%) (282). Fekkar and colleagues showed a sensitivity of 81% for both assays compared to a 38% sensitivity with PCR (329). The sensitivity increased to 92% if both GWC and IB were performed and 97% if PCR testing was added (329). Multiple studies confirm a specificity of >99% for the GWC, IB, and PCR (330, 331).
(iii) Borrelia burgdorferi.
Confirmation of retinitis or uveitis due to B. burgdorferi is best accomplished by performing a two-tier antibody test similar to the approach used for HIV (332). The patient should have had prior tick exposure and an appropriate clinical course since B. burgdorferi antibodies may be seen in 10% of individuals (333).
Diagnosis of ocular helminth infections.
(i) Nematodes.
O. volvulus (cause of river blindness) is spread by the bite of the tsetse fly and resides subcutaneously in the skin, with the adult worm periodically releasing microfilariae that migrate through tissues (not seen in blood), including the cornea stroma, intraocular fluid, and retina (6, 334). Diagnosis is based on exposure history, characteristic ocular lesions, and microscopic identification of the adult worm with microfilaria upon skin histopathology (335, 336). Like O. volvulus, there are reports primarily in South America of Mansonella ozzardi (spread by biting midges) microfilariae inducing keratitis and conjunctivitis, with diagnosis requiring the identification of unsheathed microfilariae on a nocturnal blood smear (183, 335). Loa loa (African eye worm) is transmitted through the bite of the Chrysops deer fly, with adult worms migrating through the skin at a rate of 1 cm/min and occasionally traversing the conjunctiva or being suspended in intraocular fluid (6). Adult worms release sheathed microfilariae (characteristic nuclei extending to the end of the tail [“lower lower”]) into systemic circulation in the daytime, enabling optimal diagnosis on diurnal blood smears (334). Gross identification of any adult worm in the eye merits an attempt at in toto extraction to minimize subsequent inflammatory damage to delicate intraocular structures. Similarly, Thelazia spp. (oriental eye worms) (secretophagous flies) are commonly located on the conjunctival surface but do not penetrate tissue or produce microfilariae, are smaller than Loa loa, are confined to the ocular surface, and lack accompanying diagnostic tests (6, 182). Microfilariae of Dirofilaria spp. (Dirofilaria immitis, dog heartworm) are ingested by mosquitoes during a blood meal, which subsequently inject larvae into human skin (334). Few sterile larvae survive and migrate through various tissues (predominantly lung, heart, and subcutaneous tissue), including the conjunctiva and intraocular fluid (6, 334). Diagnosis entails gross identification of characteristic long (<30-cm) threadlike adult worms in the eye or histopathological identification of intact/degenerated parasitic tissue within excised nodules (334, 335). Although rare, adult worms of Wuchereria bancrofti and Brugia malayi have been isolated from conjunctival and intraocular fluids (6). In this setting, diagnosis requires the examination of nocturnal blood smears for sheathed microfilariae (W. bancrofti, nuclei do not extend into the tail; B. malayi, significant gap between the distal 2 nuclei [334]).
Toxocariasis (Toxocara canis [dogs] and T. cati [cats]) is acquired by the ingestion of embryonated eggs in the environment or encysted larvae in undercooked meat (336). Larvae penetrate the small intestine and wander, depositing most commonly in the liver, lung, brain, and eye (6, 336). Unilateral chorioretinitis, granuloma formation, and vitritis in a patient with an exposure history and positive serology are diagnostic (6). High concentrations of infectious Baylisascaris (raccoon roundworm) eggs are located in communal raccoon defecation sites, resulting in the ingestion of a high inoculum and larval spread to the lungs, liver, central nervous system, and eye (337, 338). Visualization of migrating larvae (∼2 mm; 5-fold larger than Toxocara spp.) in the eye and serology (available at the CDC) may be useful for diagnosis (6). Angiostrongylus (rat lungworm) is acquired by ingesting vegetables contaminated with mollusk secretions or infected crustaceans (336). The infective larvae are neurotropic, residing within the subarachnoid space or brain parenchyma, and occasionally traverse the optic nerve to enter the posterior eye (6, 336, 339). Ocular involvement in the absence of central nervous system infection is extremely rare; therefore, PCR on cerebrospinal fluid (offered by the CDC) may be helpful in rendering a diagnosis (6, 336). Encysted Trichinella larvae are ingested in undercooked pork or game meat and excyst in the intestines, maturing into adults that release larvae for ∼4 weeks before death (336). The larvae (approximately the size of hookworms) enter the systemic circulation and penetrate tissue (skeletal/cardiac muscle and central nervous system), including the posterior eye and conjunctiva, rendering conjunctivitis the presenting illness in a large percentage of patients (6, 336). Diagnosis of trichinellosis is aided by exposure history (hunters eating wild game), eosinophilia, elevated creatine kinase levels, and positive serology (6). Ocular gnathostomiasis (Gnathostoma spp.) occurs via the ingestion of infected copepods or undercooked meats with subsequent spread to the eye (182, 336). Gross and microscopic observation of a short, thick nematode with a characteristic hooked-radial head is diagnostic for this entity (182). Serology testing is not currently available in the United States, but specimens can be sent via the CDC to laboratories in countries where the disease is endemic (Thailand and Japan).
(ii) Cestodes.
Ingestion of Taenia solium (pork tapeworm) eggs enables larvae to penetrate the gut wall and deposit in tissues (brain, muscles, eye, and subcutaneous tissue) as translucent masses with a single dense white spot (scolex) known as cysticerci (335, 340). Fundoscopic identification and extraction of cysticerci followed by histopathological examination of the characteristic 3-layered bladder wall, single scolex, and calcareous corpuscles (pathognomonic for cestodes) are diagnostic of ocular cysticercosis (6, 335). Additional diagnostic features include exposure history, positive serology, and characteristic ova in stool (if the adult worm is present in the gastrointestinal [GI] tract [taeniasis]) (7, 340). Early extraction of an intact organism (true for all ocular cestode infections) and imaging studies to rule out brain involvement (which necessitates hospitalization) prior to anthelminthic treatment are crucial to mitigate inflammatory damage to vital intraocular structures (6). Similarly, fundoscopic identification, extraction, and histopathological identification of a multiloculated cyst with multiple scoleces are diagnostic for coenurosis caused by Taenia multiceps (canine tapeworm) (335). Identification of single (Echinococcus granulosus) or multiloculated (Echinococcus multilocularis) cysts with debris (hydatid sand) upon imaging, positive serology, and multiple brood capsules and scoleces upon histopathological examination are diagnostic for ocular echinococcosis caused by the ingestion of eggs from infected canines (6, 335, 340). Snails release Spirometra species cercariae into water, enabling them to attach to aquatic vegetation or encyst within frogs and snakes, resulting in human infection (sparganosis) upon consumption (340). The identification of an intact flatworm (not cyst; measuring ∼50 mm) in the conjunctiva or periorbital tissue (rarely intraocular) with an exposure history is diagnostic for this entity (335).
(iii) Trematodes.
Ingestion of aquatic plants contaminated with snail-derived ciliated metacercariae from Fasciola hepatica and Fasciola gigantica results in fluke infection of the extrahepatic and intrahepatic bile ducts, chronic cholestasis, and hepatic injury (335). Occasionally, flukes disseminate via the systemic circulation into other tissues, including intraocular/periorbital structures (6, 341). Identification, extraction, and gross examination of organisms with a characteristic broadly flattened disk-shaped morphology (<30 mm long); histopathological examination of internal structures; positive serology; and large operculated eggs in stool or tissue are diagnostic for ocular fascioliasis (7, 336, 342). Snail-derived Schistosoma species cercariae in contaminated water penetrate human epithelia, including periocular and conjunctival tissues (6, 182, 342). Schistosomula migrate through the skin to venules, with species-specific preferences for unique anatomic sites (Schistosoma haematobium, bladder; S. mansoni, distal mesentery; S. japonicum, proximal mesentery) (343, 344); however, localization is not absolute, resulting in the rare deposition of proinflammatory eggs in tissues including the conjunctiva, eyelids, posterior eye, and subretinal space (6, 182). Accessible granulomas can be surgically excised, and ocular schistosomiasis can be diagnosed by the identification of eggs with characteristic morphology in tissue or stool (6, 335, 340). Likewise, the identification of trematode tegument upon histopathology and sequencing can identify pathogens like Procerovum varium in conjunctival granulomas acquired by swimming in snail-infested waters (333, 343, 345–347).
(iv) Arthropods.
The diagnosis of human myiasis caused by Dermatobia species (human bot fly) is rendered by direct observation of tiny translucent worms with dark heads crawling on the ocular surface or residing within chalazion-like lesions (ophthalmomyiasis externa) (6). Prompt removal is indicated to mitigate larval penetration into the eye (ophthalmomyiasis interna), orbital contents (orbital myiasis), or brain (6). Laboratory identification of larvae is based on characteristic morphological features and enables providers to choose optimal treatment approaches and understand how the infestation occurred to prevent reoccurrence (9).
Identification of nits and crusts on eyelashes followed by direct microscopic observation of a 2-mm-long broad crablike louse, P. pubis (pubic lice), is diagnostic for ocular phthiriasis (6, 7). Distinguishing this entity from eyebrow infestation by the more elongated body louse P. humanus is of great importance in children given that pubic lice are primarily sexually transmitted and may be representative of ongoing child abuse (6–8).
Antimicrobials and Susceptibility Testing
Ocular drug delivery.
Antimicrobials used to treat ocular infections can be administered via many routes, including topical, oral, intracameral, intrastromal/intracorneal, intravenous, subconjunctival, subretinal, periocular, and intraocular routes (among others). Topical agents are instilled directly onto the conjunctiva and corneal surface and are immediately available; however, they are rapidly diluted by tears and quickly drained away by the lacrimal system. Similarly, the anterior chamber’s entire aqueous humor volume of ∼250 μl is replaced roughly every 2 h. Hence, the retention time of topical agents on or within the anterior eye is relatively short, and frequent dosing is required for maximizing therapeutic management. Similarly, intravenous agents or oral therapeutics (which are less commonly used) must diffuse from scleral capillaries into the avascular cornea or from inner ocular capillaries into inner eye tissues. Alternatively, intraocular agents are injected intracamerally or surgically placed into the anterior or posterior chambers and are typically immediately available.
The most common ocular infections, bacterial keratitis and conjunctivitis, are typically treated with topical antimicrobial agents (348); important exceptions are N. gonorrhoeae and C. trachomatis ocular infections. Because the pharmacokinetic and pharmacodynamic factors of the topical delivery mode for antimicrobials are quite different than those of systematically delivered antimicrobials, defining antimicrobial interpretive breakpoints as susceptible, intermediate, or resistant needs to be done cautiously since susceptibility breakpoints are based on achievable serum concentrations (349–351). Fortunately, most topical agents for keratitis and conjunctivitis can be applied in concentrations considerably higher than achievable blood levels; however, such high concentrations are possible only after repeated and frequent administrations of the agents. As a result, the most likely susceptibility error in this setting is reporting an isolate to be resistant when it is actually susceptible rather than reporting an isolate as being susceptible when it is resistant. The result of these systematic errors in interpretation is that antimicrobials that might have activity will be precluded from clinical use because of medicolegal concerns despite the fact that antimicrobials applied topically can be active in vivo at ocular concentrations that are often some multiple higher than achievable serum concentrations (344, 348, 351).
For patients with bacterial endophthalmitis who are treated with systemic antimicrobials, achievable ocular drug concentrations will be only a fraction of those achieved in serum, thus making the use of intravitreal antimicrobial agents mandatory (352). The value of systemic antimicrobials in endophthalmitis is controversial because the very limited pharmacokinetic data that are available indicate that vitreal penetration mimics penetration across the blood-brain barrier, meaning that antimicrobials such as tobramycin and vancomycin penetrate poorly, while ceftriaxone, cefazolin, and carbapenems show better penetration and may be useful (352).
Antimicrobial formulations.
Several classes of antibacterial agents are commercially available to treat patients with ocular infections: cephalosporins, sulfonamides, macrolides, aminoglycosides, carbapenems, linezolid, daptomycin, rifampicin, and fluoroquinolones (352). Many of these agents are specially formulated for ophthalmic use and have the appropriate pH, buffers, and preservatives required by the Food and Drug Administration. Because a consequence of ocular infection can be blindness, ophthalmologists typically choose the most potent antibiotic with the broadest spectrum of activity. At present, the fluoroquinolones, especially moxifloxacin, gatifloxacin, and besifloxacin, are among some of the more widely used ocular agents (344, 352–354). Ophthalmic surgeons rely on these agents for both prophylaxis and treatment of ocular infections.
Compounding pharmacists are invaluable in the preparation of many special and exotic drugs to treat serious ocular diseases. In the case of severe suppurative keratitis, specialist pharmacists will often compound “fortified” agents that are prepared from i.v. or intramuscular (i.m.) solutions and diluted with artificial tears. For example, gentamicin is used at 15 mg/ml instead of 3 mg/ml. The cephalosporins do not possess long-term stability in solution and are not commercially available; however, they are valuable in the treatment of Gram-positive ocular infections, and ophthalmologists will typically use compounded ceftazidime or cefazolin at 50 mg/ml as eye drops.
Compounding pharmacists often make preparations of special and exotic agents. There are several examples of such preparations. Baquacil, a swimming pool sanitizer, contains polyhexamethylene biguanide. It can be compounded with chlorhexidine for ocular use; together, the combination is a useful option for treating Acanthamoeba keratitis (355). Natamycin is the only FDA-approved antifungal agent for ocular use; however, many other antifungal agents can be compounded and formulated (for example, amphotericin B and voriconazole) (356, 357). Similarly, vancomycin can be specifically compounded for topical, subconjunctival, or intraocular use to treat MRSA (358); clarithromycin can be compounded for use to treat mycobacterial infections (359); and linezolid (0.2%) or fortified bacitracin (10,000 U/ml) can be specially formulated as alternative topical antimicrobial agents in challenging infections.
In a situation where drug choices can be limited, the treating clinician, supervisory clinical microbiologist(s), and participating pharmacy personnel can develop an “inhibitory quotient” for determining which agent(s) might potentially be most active against the infecting pathogen(s) (348, 351). The inhibitory quotient is the ratio of the achievable antimicrobial tissue concentration divided by the MIC90 for the organism in question or, alternatively, the actual MIC of the infecting organism; the higher this quotient, the greater the potential potency of the particular antimicrobial against the infecting pathogen.
Important factors affecting the choice of antimicrobial agents include variability in ocular tissue penetration, the availability and stability of an ocular antimicrobial formulation, toxicity, and the likelihood of compliance with the treatment regimen (349–352). Emerging data support the observations that treatment of keratitis caused by S. aureus, Pseudomonas, and members of the Enterobacterales with low fluoroquinolone MIC values is associated with good clinical outcomes. No such correlation was seen with similar infections caused by Streptococcus spp. and coagulase-negative staphylococci (350, 351).
Bacterial susceptibility testing.
In nonacademic settings and unless a patient has a sight-threatening infection, cultures are not routinely performed; patients are empirically given broad-spectrum agents either topically, subconjunctivally, or by injection directly into the anterior chamber, posterior chamber, or vitreous. However, when a definitive diagnosis is required, culturing the affected tissue or fluid is mandatory, and treatment is started immediately after appropriate specimens have been collected.
As ocular and nonocular isolates exhibit the same levels of antimicrobial resistance, local hospital antibiogram data can provide useful information on expected susceptibility patterns. However, it is essential to keep in mind that the breakpoints used to render interpretations of the antibiogram are exclusively based on the outcome of patients with nonocular infections and systemic drug administration. As topical and intraocular drug concentrations exceed achievable blood concentrations, many of the isolates reported as resistant on the antibiogram may be susceptible to higher local drug concentrations used to treat ocular infections.
Until guidelines for AST and ocular-specific interpretative breakpoints are developed for ocular isolates (not at all likely in the near future), clinical microbiology laboratories should consider testing ocular isolates and reporting MIC values and their interpretations as is done for nonocular isolates. A clinical comment should be included informing physicians that the reported interpretations are based on achievable blood levels and not on achievable ocular levels. Many drugs used to treat ocular infection, particularly topical agents, are not included on commercial susceptibility testing platforms, are not routinely tested in clinical laboratories, and lack interpretive breakpoints (360, 361).
Two recent multicenter national surveys of bacterial antimicrobial resistance, one of isolates from any ocular infection (362) and one of patients with conjunctivitis (363), indicate the following:
-
1.
Oxacillin resistance was found in 34% and 43% of S. aureus isolates. Oxacillin resistance was higher in coagulase-negative staphylococci (50% and 49%), and there was significant azithromycin and fluoroquinolone resistance among those isolates; no vancomycin-resistant organisms were detected.
-
2.
There was significant azithromycin resistance among S. pneumoniae isolates (36% and 34%) but essentially no resistance to the fluoroquinolones (0 and 1%).
-
3.
H. influenzae isolates were highly susceptible to azithromycin and fluoroquinolones.
-
4.
Fewer than 10% of isolates were resistant to the tested fluoroquinolones (ciprofloxacin and levofloxacin), tobramycin, and carbapenems.
Antibiotic-resistant N. gonorrhoeae is also emerging as a potentially problematic organism in ocular infections, with recent studies showing 50% of isolates from a European survey being fluoroquinolone resistant and 7% being azithromycin resistant (38, 364).
Antifungal agents.
The recommended empirical treatment for filamentous fungal keratitis is 5% natamycin topical eye drops (356, 365). Natamycin is the only FDA-approved drug to treat fungal keratitis with in vitro activity against the majority of frequently encountered molds (365). Mycotic Ulcer Treatment Trial 1 (MUTT-1) was a National Eye Institute-supported randomized, double-blind, multicenter clinical trial (n = 326) performed at the Aravind Eye Hospital in Madurai, Tamil Nadu, India, which showed the superiority (improved visual acuity and decreased perforation) of 5% natamycin compared to 1% voriconazole, particularly for infections with Fusarium spp. (356). Subsequent studies and meta-analyses confirmed these findings (357, 363). The MUTT-2 trial (n = 240) showed no additional advantage of systemic voriconazole compared to placebo as an adjunct to topical natamycin for most fungi (366), except for a possible benefit in the treatment of Fusarium species infections (367). Similarly, adjunctive treatment strategies targeting fungal iron acquisition (368), antioxidant defenses (369), and zinc homeostasis (370) have shown efficacy in experimental models but are currently limited to the research setting.
Fungal susceptibility testing.
CLSI documents M27 and M38 provide standardized broth microdilution and disk diffusion testing methodologies for yeasts and molds, respectively (371, 372). Likewise, CLSI document M60 contains several breakpoints for yeasts, whereas document M61 contains a single breakpoint for Aspergillus fumigatus: voriconazole. Epidemiological cutoff values (ECVs) for yeasts and molds can be found in CLSI document M59 (373).
Many laboratories perform yeast antimicrobial susceptibility testing. Similar to bacteria, yeast MIC values should be reported with a comment indicating that interpretations are based on achievable blood, not ocular, levels. In contrast to yeasts, mold susceptibility testing is limited to a few major academic medical centers and reference laboratories. Given the severe consequences (loss of visual acuity or enucleation) of failed therapy (65) and the relatively few cases at most institutions, laboratory directors should develop protocols for susceptibility testing on ocular isolates in conjunction with the ophthalmologist groups that they serve. At a minimum, susceptibility testing for natamycin, voriconazole, and relevant formulary antifungals should be made accessible via in-house or send-out testing at the request of the treating clinician with MIC values other than for A. fumigatus: voriconazole reported out with “no interpretation.”
Utilizing CLSI methodologies, many investigators have reported MIC data on mold keratitis isolates (82, 332, 374, 395–398), which, as expected, show no difference in susceptibility patterns from organisms isolated from other tissue sites with no prior exposure to antifungals. However, as most infections worldwide are due to trauma, with vegetative material increasing, azole resistance associated with agricultural fungicides in environmental molds, including Aspergillus spp., is alarming (375). If available, mold isolates from individuals with a history of trauma in an agricultural setting merit an evaluation of azole resistance testing.
Although infection with species in the F. solani complex is associated with worse clinical outcomes and increased voriconazole resistance than infection with other Fusarium spp., identification to the species level is not reliable by morphology alone, requiring genetic analysis (238, 376). Compared to sequencing, it is often less expensive and of increased clinical relevance to report the organism to the genus level and perform susceptibility testing on that particular isolate.
Isolation of pathogens intrinsically resistant to voriconazole, such as all Mucorales, should trigger a report alerting ophthalmologists to this resistance pattern (377, 378). However, identifications of fungi known to harbor intrinsic resistance to the polyene amphotericin B are more likely to exhibit elevated MICs to natamycin due to decreased expression of ergosterol in the cell membrane (379). Therefore, the isolation of Acremonium strictum, Aspergillus terreus, Lomentospora prolificans, Purpureocillium lilacinus, S. apiospermum, and other fungi with intrinsic amphotericin B resistance merits susceptibility testing for natamycin, and clinicians should be alerted to the possibility of polyene resistance, enabling treatment modification (377–379). For example, a recent case series of P. lilacinus (a nematophagous fungus used as a “green” pesticide in the plant industry) keratitis identified in vitro natamycin resistance in all tested strains, with worse visual outcomes in patients treated with natamycin, prompting the authors to recommend topical voriconazole upon the isolation of this pathogen (378).
The increased incidence of infection with the fungus-like oomycete Pythium species (pythiosis [swamp cancer]) is of particular concern as this organism morphologically mimics filamentous fungi but exhibits diminished expression of ergosterol in the cell membrane, resulting in resistance to most antifungal agents, including natamycin and voriconazole (380, 381).
Antiviral agents.
Cases of HSV and VZV keratitis are treated with either a 3% topical acyclovir ointment, oral acyclovir, or oral valacyclovir until 1 week after symptom resolution. Intraocular HSV/VZV infections require hospitalization and i.v. dosing given the high risk of acute retinal necrosis. Sight-threatening intraocular CMV infections necessitate intraocular ganciclovir or foscarnet and either oral valganciclovir, i.v. ganciclovir, or i.v. foscarnet. Non-sight-threatening infections do not require intravitreal injection (296, 382).
Viral susceptibility testing.
Antiviral susceptibility testing is limited to large academic medical centers and reference laboratories. Molecular detection of resistance mutations via viral genome sequencing is becoming increasingly more common. Identification of antiviral resistance can optimize therapeutic regimens in patients with recurrent HSV, VZV, and CMV ocular infections not responding to first-line therapy (97, 296, 383). Prior to testing, exclusion of clinical resistance due to other factors such as pharmacokinetics and missed doses is essential (382). If indicated, concurrent evaluation of second-line agents (foscarnet/cidofovir) is recommended given the long turnaround time and high complexity of test performance (382).
During HSV infection, acyclovir and ganciclovir are phosphorylated by HSV thymidine kinase (TK), forming guanosine analogues that inhibit nucleic acid synthesis, whereas foscarnet and cidofovir act directly on viral DNA polymerase (vDP) (103, 384). Mutations in the HSV TK gene (UL23) and vDP gene (UL30) are responsible for ∼95% and ∼5% of acyclovir resistance, respectively (103, 382). Mutations in these enzymes confer antiviral resistance and are detected either phenotypically via drug susceptibility testing or genotypically via sequencing (103, 382).
HSV strains with both TK and vDP mutations have been reported but have fitness costs and are extremely rare (385, 386). Sanger sequencing can identify these mutations from virus-infected tissue or culture isolates (382). NGS assays are available in the research setting (387).
HSV infection of a new host is most commonly but not always due to a strain lacking acyclovir resistance mutations (388–390). The acyclovir resistance rate of 0.11% for nonneonatal ocular infections is similar to rates for orofacial HSV (0.11%). In contrast, neonatal ocular HSV acyclovir resistance rates range from 3.5 to 8.6% (103). This difference is likely due to acyclovir exposure and selection for the development of resistance mutations in mothers previously treated with acyclovir. Likewise, individuals previously treated with acyclovir are more likely to develop infection with acyclovir-resistant HSV. Therefore, HSV susceptibility testing should be limited to neonates and individuals with refractory disease while on treatment (103, 382).
VZV acyclovir resistance is less common than HSV resistance and is associated with repeat drug exposure in immunosuppressed patients (107, 391). Although VZV is not easily cultured, specialized reference laboratories can perform susceptibility testing (97, 382), and sequencing of VZV TK and vDP is available in the research setting (392).
CMV molecular testing is indicated in an immunosuppressed patient with rising viremia on ganciclovir therapy (296, 393). Susceptibility testing via sequencing has a shorter turnaround time than AST, can be performed on blood, is the test of choice for systemic disease, and should be interpreted as representative of the ocular pathology (296, 382). Focal ocular involvement in the absence of systemic disease is an indication for invasive sampling (296). Detection of well-characterized mutations in the CMV phosphotransferase gene (UL97) and vDP gene (UL54) merits an empirical trial with second-line agents such as foscarnet or cidofovir and adjustment of the immunosuppressive regimen (296, 382).
CONCLUSION
The variety and complexity of ocular infections will no doubt continue to increase with the advancement of ophthalmological interventions, emerging ocular pathogens, increasing antimicrobial resistance, and novel antimicrobial agents. This document provides a foundation to understand the essential contributions of the modern clinical microbiology laboratory to the diagnosis and treatment of ocular infections. Despite all of the advancements, there remains significant work to be done, including the establishment of bacterial breakpoints relevant to ocular infection, standardization of molecular tests, economic impact studies on cornea rim cultures, expansion of mold susceptibility testing, investigation of neglected ocular infections such as fungal keratitis, and development and implementation of rapid diagnostics to aid in the elimination of trachoma. Of the utmost importance for the continued provision of high-quality ocular diagnostic care is communication, adaptation, development, and implementation of novel diagnostics to meet the growing demand and needs of our patients and ocular health colleagues.
ACKNOWLEDGMENT
We have no conflicts of interest to report.
Biographies

Sixto M. Leal, Jr., is the Director of Clinical Microbiology and the Fungal Reference Lab at the University of Alabama at Birmingham. He has a broad background in microbiology, immunology, molecular biology, and medicine with residency training in pathology and subspecialty fellowship training in medical microbiology. He received his M.D./Ph.D. training in the Medical Scientist Training Program (MSTP) at Case Western Reserve University, where he studied host-pathogen interactions mediating fungal keratitis. He then pursued pathology residency training at the University of North Carolina at Chapel Hill and fellowship training at the Cleveland Clinic. He is certified by the American Board of Pathology in Clinical Pathology and Medical Microbiology and is a Diplomate of the American Board of Medical Microbiology. He serves on the CLSI antifungal subcommittee and CAP Microbiology Resource Committee and has special clinical and research interests in mycology, parasitology, molecular diagnostics, ID histopathology, host-pathogen interactions, and ocular infections.

Kyle G. Rodino, Ph.D., D.(A.B.M.M.), is an Assistant Professor at the Perelman School of Medicine and the Assistant Director of Clinical Microbiology at the Hospital of the University of Pennsylvania. He started in the clinical microbiology laboratory as an undergraduate researcher, before obtaining his bachelor’s degree in Clinical Laboratory Science and working as a medical technologist, all at the University of North Carolina at Chapel Hill. He received his Ph.D. in Microbiology and Immunology from the Virginia Commonwealth University School of Medicine. He completed an ASM CPEP fellowship in Clinical Microbiology at Mayo Clinic and is a Diplomate of the American Board of Medical Microbiology. His interests include the development, implementation, and clinical utility of advanced molecular diagnostics as well as diagnostic stewardship, vector-borne diseases, and ocular infections.

W. Craig Fowler, M.D., is a Professor and Chair of Surgery at the Campbell University School of Osteopathic Medicine. He is an Associate Professor at the University of North Carolina (UNC) School of Medicine and Medical Director Emeritus and Medical Advisory Board Chair for Miracles in Sight. He completed medical school at the Medical College of Virginia and was the chief Ophthalmology resident at George Washington University prior to completing a cornea and external eye disease fellowship at the Dean McGee Eye Institute at the University of Oklahoma with special emphasis on refractive surgery, ocular microbiology, and electron microscopy. Additional training in ocular pathology was obtained at the Armed Forces Institute of Pathology. He began the refractive surgery program at Duke University and obtained tenure at UNC prior to transitioning to Chair the Department of Surgery at Campbell University. He has significant experience diagnosing and treating eye infections and coauthored Cumitech 13B, Laboratory Diagnosis of Ocular Infections.

Peter H. Gilligan is Director emeritus of the Clinical Microbiology-Immunology Laboratories at the University of North Carolina Hospitals and Professor emeritus of Pathology and Laboratory Medicine at the University of North Carolina. A Diplomate emeritus of the American Board of Medical Microbiology and Fellow of the American Academy of Microbiology, he currently is a member of the Board of Editors of mBio and the Editorial Board of Clinical Infectious Diseases and is a former editor of the Journal of Clinical Microbiology and Clinical Microbiology Reviews. He is a coauthor of Cumitech 13B, Laboratory Diagnosis of Ocular Infections.
REFERENCES
- 1.Gray LD, Gilligan PH, Fowler WC. 2010. Cumitech 13B, Laboratory diagnosis of ocular infections. Coordinating ed, Snyder J. ASM Press, Washington, DC. [Google Scholar]
- 2.Amescua G, Akpek EK, Farid M, Garcia-Ferrer FJ, Lin A, Rhee MK, Varu DM, Musch DC, Dunn SP, Mah FS, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel . 2019. Blepharitis preferred practice patterns. Ophthalmology 126:P56–P93. 10.1016/j.ophtha.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 3.McCulley JP, Shine WE. 2000. Changing concepts in the diagnosis and management of blepharitis. Cornea 19:650–658. 10.1097/00003226-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Viswalingam M, Rauz S, Morlet N, Dart JK. 2005. Blepharokeratoconjunctivitis in children: diagnosis and treatment. Br J Ophthalmol 89:400–403. 10.1136/bjo.2004.052134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biernat MM, Rusiecka-Ziółkowska J, Piątkowska E, Helemejko I, Biernat P, Gościniak G. 2018. Occurrence of Demodex species in patients with blepharitis and in healthy individuals: a 10-year observational study. Jpn J Ophthalmol 62:628–633. 10.1007/s10384-018-0624-3. [DOI] [PubMed] [Google Scholar]
- 6.Padhi TR, Das S, Sharma S, Rath S, Rath S, Tripathy D, Panda KG, Basu S, Besirli CG. 2017. Ocular parasitoses: a comprehensive review. Surv Ophthalmol 62:161–189. 10.1016/j.survophthal.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Luo B, Xiang N, Liu R, Wang W, Li Y, Qi X. 2020. Phthiriasis palpebrarum, thelaziasis, and ophthalmomyiasis. Int J Infect Dis 96:511–516. 10.1016/j.ijid.2020.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Ryan MF. 2014. Phthiriasis palpebrarum infection: a concern for child abuse. J Emerg Med 46:e159–e162. 10.1016/j.jemermed.2013.11.090. [DOI] [PubMed] [Google Scholar]
- 9.Francesconi F, Lupi O. 2012. Myiasis. Clin Microbiol Rev 25:79–105. 10.1128/CMR.00010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y-Y, Yu W-K, Tsai C-C, Kao S-C, Kau H-C, Liu CJ-L. 2016. Clinical features, microbiological profiles and treatment outcome of lacrimal plug-related canaliculitis compared with those of primary canaliculitis. Br J Ophthalmol 100:1285–1289. 10.1136/bjophthalmol-2015-307500. [DOI] [PubMed] [Google Scholar]
- 11.Kaliki S, Ali MJ, Honavar SG, Chandrasekhar G, Naik MN. 2012. Primary canaliculitis: clinical features, microbiological profile and management outcomes. Ophthalmic Plast Reconstr Surg 28:355–360. 10.1097/IOP.0b013e31825fb0cd. [DOI] [PubMed] [Google Scholar]
- 12.Fowler AM, Dutton JJ, Fowler WC, Gilligan P. 2008. Mycobacterium chelonae canaliculitis associated with Smart Plug use. Ophthalmic Plast Reconstr Surg 24:241–243. 10.1097/IOP.0b013e3181724302. [DOI] [PubMed] [Google Scholar]
- 13.O’Callaghan RJ. 2018. The pathogenesis of Staphylococcus aureus eye infections. Pathogens 7:9. 10.3390/pathogens7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wladis EJ, Shinder R, LeFebvre DR, Sokol JA, Boyce M. 2016. Clinical and microbiologic features of dacryocystitis-related orbital cellulitis. Orbit 35:258–261. 10.1080/01676830.2016.1176214. [DOI] [PubMed] [Google Scholar]
- 15.Branson SV, McClintic E, Yeatts RP. 2019. Septic cavernous sinus thrombosis associated with orbital cellulitis: a report of 6 cases and a review of the literature. Ophthalmic Plast Reconstr Surg 35:272–280. 10.1097/IOP.0000000000001231. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Fu T, Gu H, Jie Y, Sun Z, Jiang D, Yu J, Zhu X, Xu J, Hong J. 2018. Trends in dacryocystitis in China. Medicine (Baltimore) 97:e11318. 10.1097/MD.0000000000011318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung SY, Rafailov L, Turbin RE, Langer PD. 2019. The microbiologic profile of dacryocystitis. Orbit 38:72–78. 10.1080/01676830.2018.1466901. [DOI] [PubMed] [Google Scholar]
- 18.Pornpanich K, Luemsamran P, Leelaporn A, Santisuk J, Tesavibul N, Lertsuwanroj B, Vangveeravong S. 2016. Microbiology of primary acquired nasolacrimal duct obstruction: simple epiphora, acute dacryocystitis, and chronic dacryocystitis. Clin Ophthalmol 10:337–342. 10.2147/OPTH.S100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills DM, Bodman MG, Meyer DR, Morton AD, III.. 2007. The microbiologic spectrum of dacryocystitis: a national study of acute versus chronic infection. Ophthalmic Plast Reconstr Surg 23:302–306. 10.1097/IOP.0b013e318070d237. [DOI] [PubMed] [Google Scholar]
- 20.Santos JC, Pinto S, Ferreira S, Maia C, Alves S, da Silva V. 2019. Pediatric preseptal and orbital cellulitis: a 10-year experience. Int J Pediatr Otorhinolaryngol 120:82–88. 10.1016/j.ijporl.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Pepose J, Holland G, Wilhelmus K. 1996. Ocular infection and immunity, p 1081–1087, 1287–1297. Mosby, St Louis, MO. [Google Scholar]
- 22.Ekhlassi T, Becker N. 2017. Preseptal and orbital cellulitis. Dis Mon 63:30–32. 10.1016/j.disamonth.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Sagiv O, Thakar SD, Kandl TJ, Kontoyiannis DP, Debnam JM, Esmaeli B. 2018. Clinical curse of preseptal and orbital cellulitis in 50 immunocompromised patients with cancer. Ophthalmology 125:318–320. 10.1016/j.ophtha.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Bilyk JR. 2007. Periocular infection. Curr Opin Ophthalmol 18:414–423. 10.1097/ICU.0b013e3282dd979f. [DOI] [PubMed] [Google Scholar]
- 25.McKinley SH, Yen MT, Miller AM, Yen KG. 2007. Microbiology of pediatric orbital cellulitis. Am J Ophthalmol 144:497–501. 10.1016/j.ajo.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 26.Tarff A, Behrens A. 2017. Ocular emergencies: red eye. Med Clin North Am 101:615–639. 10.1016/j.mcna.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Azari AA, Barney NP. 2013. Conjunctivitis: a systemic review of diagnosis and treatment. JAMA 310:1721–1729. 10.1001/jama.2013.280318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalski RP, Nayyar SV, Romanowski EG, Shanks RMQ, Mammen A, Dhaliwal DK, Jhanji V. 2020. The prevalence of bacteria, fungi, viruses, and Acanthamoeba from 3,004 cases of keratitis, endophthalmitis, and conjunctivitis. Eye Contact Lens 46:265–268. 10.1097/ICL.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 29.OYong K, Killerby M, Pan C-Y, Huynh T, Green NM, Wadford DA, Terashita D. 2018. Outbreak of epidemic keratoconjunctivitis caused by human adenovirus type D53 in an eye clinic—Los Angeles County, 2017. MMWR Morb Mortal Wkly Rep 67:1347–1349. 10.15585/mmwr.mm6748a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Zhao N, Sha J, Wang C, Jin X, Amer S, Liu S. 2016. Virology and epidemiology analyses of global adenovirus-associated conjunctivitis outbreaks, 1953-2013. Epidemiol Infect 144:1661–1672. 10.1017/S0950268815003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowler WC, Fowler AM. 2009. Common anterior segment and red eye problems for the primary care specialist: diagnosis and management, p 1112–1125. In Runge MS, Greganti MA (ed), Netter’s internal medicine, 2nd ed. Saunders, Elsevier, Philadelphia, PA. [Google Scholar]
- 32.Varu DM, Rhee MK, Akpek EK, Amescua G, Farid M, Garcia-Ferrer FJ, Lin A, Musch DC, Mah FS, Dunn SP, American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel . 2019. Conjunctivitis preferred practice pattern. Ophthalmology 126:P94–P169. 10.1016/j.ophtha.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Chansaenroj J, Vongpunsawad S, Puenpa J, Theamboonlers A, Vuthitanachot V, Chattakul P, Areechokchai D, Poovorawan Y. 2015. Epidemic outbreak of acute haemorrhagic conjunctivitis caused by coxsackievirus A24 in Thailand 2014. Epidemiol Infect 143:3087–3093. 10.1017/S0950268815000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margeritie N, Brottet E, Pages F, Jaffar-Bandjee MC, Schuffenecker I, Jossett L, Vilain P, Filleul L. 2016. A major outbreak of conjunctivitis caused by coxsackievirus A24, Réunion, January to April 2015. Euro Surveill 21:30271. 10.2807/1560-7917.ES.2016.21.26.30271. [DOI] [PubMed] [Google Scholar]
- 35.Taylor HR, Burton MJ, Haddad D, West S, Wright H. 2014. Trachoma. Lancet 384:2142–2152. 10.1016/S0140-6736(13)62182-0. [DOI] [PubMed] [Google Scholar]
- 36.Vandendriessche S, Rybarczyk J, Schauwvlieghe PP, Accou G, Van den Abeele AM, Vanrompay D. 2019. A bird’s-eye view of chronic unilateral conjunctivitis: remember about Chlamydia psittaci. Microorganisms 7:118. 10.3390/microorganisms7050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krasny J, Tomasova-Borovanska J, Hruba D. 2005. The relationship between Chlamydia trachomatis and Chlamydia pneumoniae as the cause of neonatal conjunctivitis (ophthalmia neonatorum). Ophthalmologica 219:232–236. 10.1159/000085733. [DOI] [PubMed] [Google Scholar]
- 38.Blank S, Daskalakis DC. 2018. Neisseria gonorrhoeae—rising infection rates, dwindling treatment options. N Engl J Med 379:1795–1797. 10.1056/NEJMp1812269. [DOI] [PubMed] [Google Scholar]
- 39.Kreisel K, Weston E, Braxton J, Llata E, Torrone E. 2017. Keeping an eye on chlamydia and gonorrhoeae conjunctivitis in infants in the United States, 2010-2015. Sex Transm Dis 44:356–358. 10.1097/OLQ.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAnena L, Knowles SJ, Curry A, Cassidy L. 2015. Prevalence of gonococcal conjunctivitis in adults and neonates, 2015. Eye (Lond) 29:875–880. 10.1038/eye.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien KS, Emerson P, Hooper PJ, Reingold AL, Dennis EG, Keenan JD, Lietman TM, Oldenburg CE. 2019. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis 19:e14–e25. 10.1016/S1473-3099(18)30444-4. [DOI] [PubMed] [Google Scholar]
- 42.Lietman TM, Pinsent A, Liu F, Deiner M, Hollingsworth TD, Porco TC. 2018. Models of trachoma transmission and their policy implications: from control to elimination. Clin Infect Dis 66(Suppl 4):S275–S280. 10.1093/cid/ciy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keenan JD, Tadesse Z, Gebresillasie S, Shiferaw A, Zerihun M, Emerson PM, Callahan K, Cotter SY, Stoller NE, Porco TC, Oldenburg CE, Lietman TM. 2018. Mass azithromycin distribution for hyperendemic trachoma following a cluster-randomized trial: a continuation study of randomly reassigned subclusters (TANA II). PLoS Med 15:e1002633. 10.1371/journal.pmed.1002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honkila M, Renko M, Pokka T, Wikstrom E, Uhari M, Tapiainen T. 2018. Symptoms, signs and long-term prognosis of vertically transmitted Chlamydia trachomatis infections. Pediatr Infect Dis J 37:930–933. 10.1097/INF.0000000000001925. [DOI] [PubMed] [Google Scholar]
- 45.Chen JS, Hsu TK, Hsu BM, Chao SC, Huang TY, Ji DD, Yang PY, Huang IH. 2019. Swimming pool-associated Vittaforma-like microsporidia linked to microsporidial keratoconjunctivitis outbreak, Taiwan. Emerg Infect Dis 25:2100–2103. 10.3201/eid2511.181483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalitha P, Seitzman GD, Kotecha R, Hinterwirth A, Chen C, Zhong L, Cummings S, Lebas E, Sahoo MK, Pinsky BA, Lietman TM, Doan T. 2019. Unbiased pathogen detection and host gene profiling for conjunctivitis. Ophthalmology 126:1090–1094. 10.1016/j.ophtha.2019.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan J, Lee P, Lai Y, Hishamuddin P, Tay J, Tan AL, Chan KS, Lin R, Tan D, Cutter J, Goh KT. 2013. Microsporidial keratoconjunctivitis after rugby tournament, Singapore. Emerg Infect Dis 19:1484–1486. 10.3201/eid1909.121464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas PA, Geraldine P. 2007. Infectious keratitis. Curr Opin Infect Dis 20:129–141. 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 49.Khor WB, Prajna VN, Garg P, Mehta JS, Xie L, Liu Z, Padilla MDB, Joo CK, Inoue Y, Goseyarakwong P, Hu FR, Nishida K, Kinoshita S, Puangsricharern V, Tan AL, Beuerman R, Young A, Sharma N, Haaland B, Mah FS, Tu EY, Stapleton FJ, Abbott RL, Tan DT, ACSIKS Group . 2018. The Asia Cornea Infectious Keratitis Study: a prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol 195:161–170. 10.1016/j.ajo.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 50.Cope JR, Konne NM, Jacobs DS, Dhaliwal DK, Rhee MK, Yin J, Steinemann TL. 2018. Corneal infections associated with sleeping in contact lenses—six cases, United States, 2016-2018. MMWR Morb Mortal Wkly Rep 67:877–881. 10.15585/mmwr.mm6732a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cope JR, Collier SA, Nethercut H, Jones JM, Yates K, Yoder JS. 2017. Risk behaviors for contact lens-related eye infections among adults and adolescents—United States, 2016. MMWR Morb Mortal Wkly Rep 66:841–845. 10.15585/mmwr.mm6632a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masters J, Kocak M, Waite A. 2017. Risk of microbial keratitis: comparative metaanalysis of contact lens wearer and post-laser in situ keratomileusis patients. J Cataract Refract Surg 43:67–73. 10.1016/j.jcrs.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 53.Mozayan A, Madu A, Channa P. 2011. Laser in situ keratomileusis infection: review and update of current practices. Curr Opin Ophthalmol 22:233–237. 10.1097/ICU.0b013e3283479ed4. [DOI] [PubMed] [Google Scholar]
- 54.Okonkwo ACO, Siah WF, Hogg HDJ, Anwar H, Figueiredo FC. 2018. Microbial keratitis in corneal grafts: predisposing factors and outcomes. Eye (Lond) 32:775–781. 10.1038/eye.2017.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB, Colby K, Tuli SS, Patel SR, Lee SM, Irvine J, Stulting RD, Mauger TF, Schein OD. 2010. Trends in fungal keratitis in the United States, 2001-7. Ophthalmology 117:2263–2267. 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 56.Durrani AF, Faith SC, Kowalski RP, Yu M, Romanowski E, Shanks R, Dhaliwal D, Jhanji V. 2019. Moraxella keratitis: analysis of risk factors, clinical characteristics, management, and treatment outcomes. Am J Ophthalmol 197:17–22. 10.1016/j.ajo.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 57.Nascimento H, Viana-Niero C, Nogueira CL, Martins Bispo PJ, Pinto F, de Paula Pereira Uzam C, Matsumoto CK, Oliveira Machado AM, Leão SC, Höfling-Lima AL, de Freitas D. 2018. Identification of the infection source of an outbreak of Mycobacterium chelonae after laser in situ keratomileusis. Cornea 37:116–122. 10.1097/ICO.0000000000001423. [DOI] [PubMed] [Google Scholar]
- 58.Edens C, Liebich L, Halpin AL, Moulton-Meissner H, Eitniear S, Zgodzinski E, Vasko L, Grossman D, Perz JF, Mohr MC. 2015. Mycobacterium chelonae eye infections associated with humidifier use in outpatient LASIK clinic—Ohio, 2015. MMWR Morb Mortal Wkly Rep 64:1177. 10.15585/mmwr.mm6441a4. [DOI] [PubMed] [Google Scholar]
- 59.Drobniewski FA. 1993. Bacillus cereus and related species. Clin Microbiol Rev 6:324–338. 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zafar H, Tan SZ, Walkden A, Fullwood C, Au L, Brahma A, Carley F. 2018. Clinical characteristic and outcomes of Moraxella keratitis. Cornea 37:1551–1554. 10.1097/ICO.0000000000001749. [DOI] [PubMed] [Google Scholar]
- 61.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. 2003. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol 87:834–838. 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green M, Apel A, Stapleton F. 2008. Risk factors and causative organisms in microbial keratitis. Cornea 27:22–27. 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 63.Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. 2001. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol 85:842–847. 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ni N, Nam EM, Hammersmith KM, Nagra PK, Azari AA, Leiby BE, Dai Y, Cabrera FA, Ma JF, Lambert CE, Jr, Honig SE, Rapuano CJ. 2015. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea 34:296–302. 10.1097/ICO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 65.Thomas PA, Kaliamurthy J. 2013. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 19:210–220. 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 66.Perry HD, Donnenfeld ED. 1990. Cryptococcal keratitis after keratoplasty. Am J Ophthalmol 110:320–321. 10.1016/s0002-9394(14)76362-7. [DOI] [PubMed] [Google Scholar]
- 67.Ritterband DC, Seedor JA, Shah MK, Waheed S, Schorr I. 1998. A unique case of Cryptococcus laurentii keratitis spread by a rigid gas permeable contact lens in a patient with onychomycosis. Cornea 17:115–118. 10.1097/00003226-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 68.de Castro LE, Sarraf OA, Lally JM, Sandoval HP, Solomon KD, Vroman DT. 2005. Cryptococcus albidus keratitis after corneal transplantation. Cornea 24:882–883. 10.1097/01.ico.0000157404.34774.1a. [DOI] [PubMed] [Google Scholar]
- 69.Rodrigues M, Laibson P. 1973. Exogenous mycotic keratitis caused by Blastomyces dermatitidis. Am J Ophthalmol 75:782–789. 10.1016/0002-9394(73)90882-9. [DOI] [PubMed] [Google Scholar]
- 70.Wilhelmus KR, Hassan SS. 2007. The prognostic role of donor corneoscleral rim cultures in corneal transplantation. Ophthalmology 114:440–445. 10.1016/j.ophtha.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 71.Rauen MP, Goins KM, Sutphin JE, Kitzmann AS, Schmidt GA, Wagoner MD. 2012. Impact of eye bank lamellar tissue cutting for endothelial keratoplasty on bacterial and fungal corneoscleral donor rim cultures after corneal transplant. Cornea 31:376–379. 10.1097/ICO.0b013e31823cbee3. [DOI] [PubMed] [Google Scholar]
- 72.Mian SI, Aldave AJ, Tu EY, Ayres BD, Jeng BH, Macsai MS, Nordlund ML, Penta JG, Pramanik S, Szczotka-Flynn LB, Ayala AR, Liang W, Maguire MG, Lass JH. 2018. Incidence and outcomes of positive donor rim cultures and infections in the cornea preservation time study. Cornea 37:1102–1109. 10.1097/ICO.0000000000001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritterband DC, Shah MK, Meskin SW, Seedor JA, Koplin RS, Perez W, Yang R, Hu DN, Dahl P. 2007. Efficacy and safety of voriconazole as an additive on Optisol GS. Cornea 26:343–347. 10.1097/ICO.0b013e31802d82e8. [DOI] [PubMed] [Google Scholar]
- 74.Layer N, Cevallos V, Maxwell AJ, Hoover C, Keenan JD, Jeng BH. 2014. Efficacy and safety of antifungal additives in Optisol-GS corneal storage medium. JAMA Ophthalmol 132:832–837. 10.1001/jamaophthalmol.2014.397. [DOI] [PubMed] [Google Scholar]
- 75.Brothers KM, Shanks RMQ, Hurlbert S, Kowalski RP, Tu EY. 2017. Association between fungal contamination and eye bank-prepared endothelial keratoplasty material. JAMA Ophthalmol 135:1184–1190. 10.1001/jamaophthalmol.2017.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imamura Y, Chandra J, Mukherjee PK, Lattif AA, Szczotka-Flynn LB, Pearlman E, Lass JH, O’Donnell K, Ghannoum MA. 2008. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother 52:171–182. 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gray TB, Cursons RT, Sherwan JF, Rose PR. 1995. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol 79:601–605. 10.1136/bjo.79.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Centers for Disease Control and Prevention. 2006. Update: Fusarium keratitis—United States, 2005-2006. MMWR Morb Mortal Wkly Rep 55:563–564. [PubMed] [Google Scholar]
- 79.Cheung N, Nagra P, Hammersmith K. 2016. Emerging trends in contact lens-related infections. Curr Opin Ophthalmol 27:327–332. 10.1097/ICU.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 80.Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder JS, Beach MJ, Centers for Disease Control and Prevention . 2014. Estimated burden of keratitis—United States, 2010. MMWR Morb Mortal Wkly Rep 63:1027–1030. [PMC free article] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. 2006. Fusarium keratitis—multiple states, 2006. MMWR Morb Mortal Wkly Rep 55:400–401. [PubMed] [Google Scholar]
- 82.Kredics L, Narendran V, Shobana CS, Vagvolgyi C, Manikandan P, Indo-Hungarian Fungal Keratitis Working Group . 2015. Filamentous fungal infections of the cornea: a global overview of epidemiology and drug sensitivity. Mycoses 58:243–260. 10.1111/myc.12306. [DOI] [PubMed] [Google Scholar]
- 83.Ng J, Minckler D, Walsh TJ, Farid M. 2016. An intractable case of Prototheca keratitis and chronic endophthalmitis in Stevens-Johnson syndrome with Boston type 1 keratoprosthesis. Cornea 35:1257–1260. 10.1097/ICO.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 84.Maycock NJ, Jayaswal R. 2016. Update on Acanthamoeba keratitis: diagnosis, treatment, and outcomes. Cornea 35:713–720. 10.1097/ICO.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 85.Stehr-Green JK, Bailey TM, Visvesvara GS. 1989. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol 107:331–336. 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 86.Ross J, Roy SL, Mathers WD, Ritterband DC, Yoder JS, Ayers T, Shah RD, Samper ME, Shih CY, Schmitz A, Brown AC. 2014. Clinical characteristics of Acanthamoeba keratitis infections in 28 states, 2008 to 2001. Cornea 33:161–168. 10.1097/ICO.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 87.Pinna A, Porcu T, Boscia F, Cano A, Erre G, Mattana A. 2017. Free-living amoeba keratitis. Cornea 36:785–790. 10.1097/ICO.0000000000001226. [DOI] [PubMed] [Google Scholar]
- 88.Barnes SD, Pavan-Langston D, Azar DT. 2015. Microbial keratitis, p 1402–1414. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 8th ed. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 89.Kaufman HE. 2011. Adenovirus advances: new diagnostic and therapeutic options. Curr Opin Ophthalmol 22:290–293. 10.1097/ICU.0b013e3283477cb5. [DOI] [PubMed] [Google Scholar]
- 90.Palacios G, Oberste MS. 2005. Enteroviruses as agents of emerging infectious diseases. J Neurovirol 11:424–433. 10.1080/13550280591002531. [DOI] [PubMed] [Google Scholar]
- 91.Rota PA, Moss WJ, Takeda M, de Swart RL, Thompson KM, Goodson JL. 2016. Measles. Nat Rev Dis Primers 2:16049. 10.1038/nrdp.2016.49. [DOI] [PubMed] [Google Scholar]
- 92.Moss WJ. 2017. Measles. Lancet 390:2490–2502. 10.1016/S0140-6736(17)31463-0. [DOI] [PubMed] [Google Scholar]
- 93.Shantha JG, Crozier I, Yeh S. 2017. An update on ocular complications of Ebola virus disease. Curr Opin Ophthalmol 28:600–606. 10.1097/ICU.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Andrade GC, Ventura CV, Mello Filho PA, Maia M, Vianello S, Rodrigues EB. 2017. Arboviruses and the eye. Int J Retina Vitreous 3:4. 10.1186/s40942-016-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Azher TN, Yin XT, Tajfirouz D, Huang AJ, Stuart PM. 2017. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol 11:185–191. 10.2147/OPTH.S80475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaye S, Choudhary A. 2006. Herpes simplex keratitis. Prog Retin Eye Res 25:355–380. 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Vrcek I, Choudhury E, Durairaj V. 2017. Herpes zoster ophthalmicus: a review for the internist. Am J Med 130:21–26. 10.1016/j.amjmed.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 98.Pepose JS, Margolis TP, LaRussa P, Pavan-Langston D. 2003. Ocular complications of smallpox vaccination. Am J Ophthalmol 136:343–352. 10.1016/s0002-9394(03)00293-9. [DOI] [PubMed] [Google Scholar]
- 99.Faith SC, Durrani AF, Jhanji V. 2018. Cytomegalovirus keratitis. Curr Opin Ophthalmol 29:373–377. 10.1097/ICU.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 100.Matoba AY, Wilhelmus KR, Jones DB. 1986. Epstein-Barr viral stromal keratitis. Ophthalmology 93:746–751. 10.1016/s0161-6420(86)33668-6. [DOI] [PubMed] [Google Scholar]
- 101.Boto-de-los-Bueis A, Romero Gomez MP, del Hierro Zarzuelo A, Sanchez EG, Mediero S, Noval S. 2015. Recurrent ocular surface inflammation associated with human herpesvirus 6 infection. Eye Contact Lens 41:e11–e13. 10.1097/ICL.0b013e3182a70a1b. [DOI] [PubMed] [Google Scholar]
- 102.Okuno T, Hooper LC, Ursea R, Smith J, Nussenblatt R, Hooks JJ, Hayashi K. 2011. Role of human herpes virus 6 in corneal inflammation alone or with human herpesviruses. Cornea 30:204–207. 10.1097/ICO.0b013e3181e2e9be. [DOI] [PubMed] [Google Scholar]
- 103.Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Jameel S, Hossain P, Anderson D. 2017. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents—comment. Clin Exp Ophthalmol 45:932. 10.1111/ceo.12991. [DOI] [PubMed] [Google Scholar]
- 104.Butler TKH, Spencer NA, Chan CCK, Singh Gilhotra J, McClellan K. 2005. Infective keratitis in older patients: a 4 year review, 1998-2002. Br J Ophthalmol 89:591–596. 10.1136/bjo.2004.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serna-Ojeda JC, Loya-Garcia D, Navas A, Lichtinger A, Ramirez-Miranda A, Graue-Hernandez EO. 2017. Long-term outcomes of pediatric penetrating keratoplasty for herpes simplex virus keratitis. Am J Ophthalmol 173:139–144. 10.1016/j.ajo.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 106.Taylor MP, Enquist LW. 2015. Axonal spread of neuroinvasive viral infections. Trends Microbiol 23:283–288. 10.1016/j.tim.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schoenberger SD, Kim SJ, Thorne JE, Mruthyunjaya P, Yeh S, Bakri SJ, Ehlers JP. 2017. Diagnosis and treatment of acute retinal necrosis: a report by the American Academy of Ophthalmology. Ophthalmology 124:382–392. 10.1016/j.ophtha.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 108.Shaikh S, Ta CN. 2002. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician 66:1723–1730. [PubMed] [Google Scholar]
- 109.Schornack MM, Siemsen DW, Bradley EA, Salomao DR, Lee HB. 2006. Ocular manifestations of molluscum contagiosum. Clin Exp Optom 89:390–393. 10.1111/j.1444-0938.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- 110.Butler NJ, Thorne JE. 2012. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol 23:517–522. 10.1097/ICU.0b013e328358ba85. [DOI] [PubMed] [Google Scholar]
- 111.Hanbazazh M, Gyure KA. 2018. Ocular human papillomavirus infections. Arch Pathol Lab Med 142:706–710. 10.5858/arpa.2017-0571-RA. [DOI] [PubMed] [Google Scholar]
- 112.Leal SM, Jr, Gulley ML. 2017. Current and emerging molecular tests for human papillomavirus-related neoplasia in the genomic era. J Mol Diagn 19:366–377. 10.1016/j.jmoldx.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Durand ML. 2017. Bacterial and fungal endophthalmitis. Clin Microbiol Rev 30:597–613. 10.1128/CMR.00113-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. 2003. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine (Baltimore) 82:97–105. 10.1097/00005792-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 115.Edelstein SL, DeMatteo J, Stoeger CG, Macsai MS, Wang CH. 2016. Report of the Eye Bank Association of America review subcommittee on adverse reactions reported from 2007-2014. Cornea 35:917–926. 10.1097/ICO.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 116.Cornut P-L, Youssef EB, Bron A, Thuret G, Gain P, Burillon C, Romanet J-P, Vandenesch F, Maurin M, Creuzot-Garcher C, Chiquet C, French Institutional Endophthalmitis Study (FRIENDS) Group . 2013. A multicentre prospective study of post-traumatic endophthalmitis. Acta Ophthalmol 91:475–482. 10.1111/j.1755-3768.2011.02349.x. [DOI] [PubMed] [Google Scholar]
- 117.Dave VP, Pathengay A, Sharma S, Naveen N, Basu S, Pappuru RR, Das T. 2019. Diagnosis, clinical presentations, and outcomes of Nocardia endophthalmitis. Am J Ophthalmol 197:53–58. 10.1016/j.ajo.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 118.Fox AR, Houser KH, Morris WR, Walton RC. 2016. Dematiaceous fungal endophthalmitis: report of a case and review of the literature. J Ophthalmic Inflamm Infect 6:43. 10.1186/s12348-016-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Homa M, Manikandan P, Saravanan V, Revathi R, Anita R, Narendran V, Panneerselvam K, Shobana CS, Aidarous MA, Galgoczy L, Vagvolgyi C, Papp T, Kredics L. 2018. Exophiala dermatitidis endophthalmitis: case report and literature review. Mycopathologia 183:603–609. 10.1007/s11046-017-0235-4. [DOI] [PubMed] [Google Scholar]
- 120.Pershing S, Lum F, Hsu S, Kelly S, Chiang MF, Rich WL, III, Parke DW, II.. 2020. Endophthalmitis after cataract surgery in the United States. Ophthalmology 127:151–158. 10.1016/j.ophtha.2019.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yannuzzi NA, Si N, Relhan N, Kuriyan AE, Albini TA, Berrocal AM, Davis JL, Smiddy WE, Townsend J, Miller D, Flynn HW, Jr.. 2017. Endophthalmitis after clear corneal cataract surgery: outcomes over two decades. Am J Ophthalmol 174:155–159. 10.1016/j.ajo.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 122.Jeong SH, Cho HJ, Kim HS, Han JI, Lee DW, Kim CG, Kim JW. 2017. Acute endophthalmitis after cataract surgery: 164 consecutive cases treated at a referral center in South Korea. Eye (Lond) 31:1456–1462. 10.1038/eye.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shah M, Relhan N, Kuriyan AE, Davis JL, Albini TA, Pathengay A, Miller D, Flynn HW, Jr.. 2016. Endophthalmitis caused by non-tuberculous mycobacterium: clinical features, antimicrobial susceptibilities, and treatment outcomes. Am J Ophthalmol 168:150–156. 10.1016/j.ajo.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 124.Jindal A, Pathengay A, Jalali S, Mathai A, Pappuru RR, Narayanan R, Chhablani J, Sharma S, Das T, Flynn HW, Jr.. 2015. Microbiologic spectrum and susceptibility of isolates in delayed post-cataract surgery endophthalmitis. Clin Ophthalmol 9:1077–1079. 10.2147/OPTH.S82852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yannuzzi NA, Gregori NZ, Rosenfeld PJ, Relhan N, Patel NA, Si N, Miller D, Dubovy SR, Smiddy WE, Schwartz SG, Flynn HW, Jr.. 2018. Endophthalmitis associated with intravitreal injections of anti-VEGF agents at a tertiary referral center: in-house and referral cases. Ophthalmic Surg Lasers Imaging Retina 49:313–319. 10.3928/23258160-20180501-04. [DOI] [PubMed] [Google Scholar]
- 126.Mishra C, Lalitha P, Rameshkumar G, Agrawal R, Balne PK, Iswarya M, Kannan NB, Ramasamy K. 2018. Incidence of endophthalmitis after intravitreal injections: risk factors, microbiology profiles, and clinical outcomes. Ocul Immunol Inflamm 26:559–568. 10.1080/09273948.2018.1430238. [DOI] [PubMed] [Google Scholar]
- 127.Brooks RB, Mitchell PK, Miller JR, Vasquez AM, Havlicek J, Lee H, Quinn M, Adams E, Baker D, Greeley R, Ross K, Daskalaki I, Walrath J, Moulton-Meissner H, Crist MB, Burkholderia cepacia Workgroup . 2019. Multistate outbreak of Burkholderia cepacia complex bloodstream infections after exposure to contaminated saline flush syringes—United States, 2016-2017. Clin Infect Dis 69:445–449. 10.1093/cid/ciy910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cilli F, Nazli-Zeka A, Arda B, Sipahi OR, Aksit-Barik S, Kepeli N, Ozinel MA, Gulay Z, Ulusoy S. 2019. Serratia marcescens sepsis outbreak caused by contaminated propofol. Am J Infect Control 47:582–584. 10.1016/j.ajic.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 129.Beri S, Shandil A, Garg R. 2017. Stenotrophomonas maltophilia: an emerging entity for cluster endophthalmitis. Indian J Ophthalmol 65:1166–1171. 10.4103/ijo.IJO_314_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Centers for Disease Control and Prevention. 2012. Notes from the field: multistate outbreak of postprocedural fungal endophthalmitis associated with a single compounding pharmacy—United States, March-April 2012. MMWR Morb Mortal Wkly Rep 61:310–311. [PubMed] [Google Scholar]
- 131.Small KW, Chan CK, Silva-Garcia R, Walsh TJ. 2014. Onset of an outbreak of Bipolaris hawaiiensis fungal endophthalmitis after intravitreal injections of triamcinolone. Ophthalmology 121:952–958. 10.1016/j.ophtha.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 132.Sheyman AT, Cohen BZ, Friedman AH, Ackert JM. 2013. An outbreak of fungal endophthalmitis after intravitreal injection of compounded combined bevacizumab and triamcinolone. JAMA Ophthalmol 131:864–869. 10.1001/jamaophthalmol.2013.88. [DOI] [PubMed] [Google Scholar]
- 133.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G. 2016. Global survey of corneal transplants and eye banking. JAMA Ophthalmol 134:167–173. 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 134.Medina CA, Butler MR, Deobhakta AA, Banitt MR, Albini TA, Smiddy WE, Berrocal AM, Gedde SJ, Flynn H, Jr.. 2016. Endophthalmitis associates with glaucoma drainage implants. Ophthalmic Surg Lasers Imaging Retina 47:563–569. 10.3928/23258160-20160601-08. [DOI] [PubMed] [Google Scholar]
- 135.Yassin SA. 2016. Bleb-related infections revisited: a literature review. Acta Ophthalmol 94:122–134. 10.1111/aos.12805. [DOI] [PubMed] [Google Scholar]
- 136.Jackson TL, Paraskevopoulos T, Georgalas I. 2014. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv Ophthalmol 59:627–635. 10.1016/j.survophthal.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 137.Luong PM, Tsui E, Batra NN, Zegans ME. 2019. Endogenous endophthalmitis and other ocular manifestations of injection drug use. Curr Opin Ophthalmol 30:506–512. 10.1097/ICU.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 138.Pinna A, Carta F, Zanetti S, Sanna S, Sechi LA. 2001. Endogenous Rhodotorula minuta and Candida albicans endophthalmitis in an injecting drug user. Br J Ophthalmol 85:754. 10.1136/bjo.85.6.754-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin CJ, Chen WL, Lin JM, Tien PT, Tsai YY. 2017. Endogenous endophthalmitis caused by Cryptococcus neoformans var. gattii mimicking choroidal tumor: from positron-emission tomography/computed tomography to histopathology. Indian J Ophthalmol 65:526–528. 10.4103/ijo.IJO_543_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vela JI, Diaz-Cascajosa J, Sanchez F, Rosello N, Buil JA. 2009. Management of endogenous cryptococcal endophthalmitis with voriconazole. Can J Ophthalmol 44:e61–e62. 10.3129/i09-153. [DOI] [PubMed] [Google Scholar]
- 141.Myint T, Dykhuizen MJ, McDonald CH, Ribes JA. 2015. Post operative fungal endopthalmitis [sic] due to Geotrichum candidum. Med Mycol Case Rep 10:4–6. 10.1016/j.mmcr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Augsten R, Pfister W, Wildner K, Voigt U, Oelzner P, Konigsdorffer E. 2013. Endogenous malassezia endophthalmitis. Klin Monbl Augenheilkd 230:536–537. 10.1055/s-0032-1328420. [DOI] [PubMed] [Google Scholar]
- 143.Sharma V, Shankar J, Kotamarthi V. 2006. Endogeneous endophthalmitis caused by Sporobolomyces salmonicolor. Eye (Lond) 20:945–946. 10.1038/sj.eye.6702051. [DOI] [PubMed] [Google Scholar]
- 144.Kirsch LS, Brownstein S, Deschenes J, Sorgini C, Jackson WB. 1999. Saccharomyces keratitis and endophthalmitis. Can J Ophthalmol 34:229–232. [PubMed] [Google Scholar]
- 145.Walia H, Tucci VT, Greene JN, Tordilla-Wadia J, Kelty P, Walia S. 2009. A case of endogenous Trichosporon endophthalmitis treated with micafungin and voriconazole. J Glob Infect Dis 1:71–74. 10.4103/0974-777X.52987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Slocumb RW, Elner SG, Hall EF. 2010. Chronic postoperative fungal endophthalmitis caused by Trichosporon asahii. Retin Cases Brief Rep 4:366–367. 10.1097/ICB.0b013e3181b5ef61. [DOI] [PubMed] [Google Scholar]
- 147.Gonul S, Gedik S, Ozturk BT, Bakbak B, Koktekir BE, Okudan S, Dagi HT. 2015. Postoperative fungal endophthalmitis caused by Trichosporon asahii treated with voriconazole. Arq Bras Oftalmol 78:252–254. 10.5935/0004-2749.20150065. [DOI] [PubMed] [Google Scholar]
- 148.Relhan N, Schwartz SG, Flynn HW, Jr.. 2017. Endogenous fungal endophthalmitis: an increasing problem among intravenous drug users. JAMA 318:741–742. 10.1001/jama.2017.10585. [DOI] [PubMed] [Google Scholar]
- 149.Tirpack AR, Duker JS, Baumal CR. 2017. An outbreak of endogenous fungal endophthalmitis among intravenous drug abusers in New England. JAMA Ophthalmol 135:534–540. 10.1001/jamaophthalmol.2017.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gonzales CA, Scott IU, Chaudhry NA, Luu KM, Miller D, Murray TG, Davis JL. 2000. Endogenous endophthalmitis caused by Histoplasma capsulatum var. capsulatum: a case report and literature review. Ophthalmology 107:725–729. 10.1016/s0161-6420(99)00179-7. [DOI] [PubMed] [Google Scholar]
- 151.Schlaen A, Ingolotti M, Couto C, Jacob N, Pineda G, Saravia M. 2015. Endogenous Histoplasma capsulatum endophthalmitis in an immunocompetent patient. Eur J Ophthalmol 25:e53–e55. 10.5301/ejo.5000550. [DOI] [PubMed] [Google Scholar]
- 152.Leung C, Farmer JP, Zoutman DE, Urton TE, Gale JG. 2010. Histoplasma capsulatum endophthalmitis in southeastern Ontario. Can J Ophthalmol 45:90–91. 10.3129/i09-196. [DOI] [PubMed] [Google Scholar]
- 153.Cheng ML, Leibowitz M, Ha E. 2012. Coccidioidal endophthalmitis in immunocompetent person, California, USA. Emerg Infect Dis 18:1015–1016. 10.3201/eid1806.111765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ling JJ, Bays DJ, Thompson GR, III, Moshiri A, Mannis MJ. 2017. Favorable outcome in Coccidioides endophthalmitis—a combined medical and surgical treatment approach. Cornea 36:1423–1425. 10.1097/ICO.0000000000001353. [DOI] [PubMed] [Google Scholar]
- 155.Reed DC, Shah KH, Hubschman JP. 2013. Resolution of Coccidioides immitis endophthalmitis with an aggressive surgical and medical therapeutic approach. Semin Ophthalmol 28:251–252. 10.3109/08820538.2013.788677. [DOI] [PubMed] [Google Scholar]
- 156.Li S, Perlman JI, Edward DP, Weiss R. 1998. Unilateral Blastomyces dermatitidis endophthalmitis and orbital cellulitis. A case report and literature review. Ophthalmology 105:1466–1470. 10.1016/S0161-6420(98)98030-7. [DOI] [PubMed] [Google Scholar]
- 157.Silva MR, Mendes RP, Lastoria JC, Barraviera B, Marques SA, Kamegasawa A. 1988. Paracoccidioidomycosis: study of six cases with ocular involvement. Mycopathologia 102:87–96. 10.1007/BF00437445. [DOI] [PubMed] [Google Scholar]
- 158.Kurosawa A, Pollock SC, Collins MP, Kraff CR, Tso MO. 1988. Sporothrix schenckii endophthalmitis in a patient with human immunodeficiency virus infection. Arch Ophthalmol 106:376–380. 10.1001/archopht.1988.01060130402030. [DOI] [PubMed] [Google Scholar]
- 159.Cartwright MJ, Promersberger M, Stevens GA. 1993. Sporothrix schenckii endophthalmitis presenting as granulomatous uveitis. Br J Ophthalmol 77:61–62. 10.1136/bjo.77.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Balaskas K, Potamitou D. 2009. Endogenous endophthalmitis secondary to bacterial meningitis from Neisseria meningitidis: a case report and review of the literature. Cases J 2:149. 10.1186/1757-1626-2-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Russo TA, Olson R, Fang C-T, Stoesser N, Miller M, MacDonald U, Hutson A, Barker JH, La Hoz RM, Johnson JR, Backer M, Bajwa R, Catanzaro AT, Crook D, de Almeda K, Fierer J, Greenberg DE, Klevay M, Patel P, Ratner A, Wang J-T, Zola J, for the Hypervirulent Klebsiella pneumoniae Investigator Group . 2018. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol 56:e00776-18. 10.1128/JCM.00776-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fang C-T, Lai S-Y, Yi W-C, Hsueh P-R, Liu K-L, Chang S-C. 2007. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 45:284–293. 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 163.Lin P. 2015. Infectious uveitis. Curr Ophthalmol Rep 3:170–183. 10.1007/s40135-015-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.PREVAIL III Study Group, Sneller MC, Reilly C, Badio M, Bishop RJ, Eghrari AO, Moses SJ, Johnson KL, Gayedyu-Dennis D, Hensley LE, Higgs ES, Nath A, Tuznik K, Varughese J, Jensen KS, Dighero-Kemp B, Neaton JD, Lane HC, Fallah MP. 2019. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med 380:924–934. 10.1056/NEJMoa1805435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Furtado JM, Espósito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. 2016. Uveitis associated with Zika virus infection. N Engl J Med 375:394–396. 10.1056/NEJMc1603618. [DOI] [PubMed] [Google Scholar]
- 166.Khairallah M, Kahloun R, Ben Yahia S, Jelliti B, Messaoud R. 2013. New infectious etiologies for posterior uveitis. Ophthalmic Res 49:66–72. 10.1159/000344009. [DOI] [PubMed] [Google Scholar]
- 167.Sathiamoorthi S, Smith WM. 2016. The eye and tick-borne disease in the United States. Curr Opin Ophthalmol 27:530–537. 10.1097/ICU.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 168.Mathew RG, Goh BT, Westcott MC. 2014. British ocular syphilis study (BOSS): 2-year national surveillance study for intraocular inflammation secondary to ocular syphilis. Invest Ophthalmol Vis Sci 55:5394–5400. 10.1167/iovs.14-14559. [DOI] [PubMed] [Google Scholar]
- 169.Oliver SE, Cope AB, Rinsky JL, Williams C, Liu G, Hawks S, Peterman TA, Markowitz L, Fleischauer AT, Samoff E, Ocular Syphilis Disease Investigation Specialists Workgroup . 2017. Increases in ocular syphilis—North Carolina, 2014-5. Clin Infect Dis 65:1676–1682. 10.1093/cid/cix604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pratas AC, Goldschmidt P, Lebeaux D, Aguilar C, Ermak N, Benesty J, Charlier C, Benveniste E, Merabet L, Sedira N, Hope-Rapp E, Chaumeil C, Bodaghi B, Héron E, Sahel JA, Lortholary O, Errera MH. 2018. Increase in ocular syphilis cases at ophthalmologic reference center, France, 2012-5. Emerg Infect Dis 24:193–200. 10.3201/eid2402.171167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tuddenham S, Ghanem KG. 2016. Ocular syphilis: opportunities to address important unanswered questions. Sex Transm Infect 92:563–565. 10.1136/sextrans-2016-052570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ang M, Vasconcelos-Santos DV, Sharma K, Accorinti M, Sharma A, Gupta A, Rao NA, Chee SP. 2018. Diagnosis of ocular tuberculosis. Ocul Immunol Inflamm 26:208–216. 10.1080/09273948.2016.1178304. [DOI] [PubMed] [Google Scholar]
- 173.Basu S, Monira S, Modi RR, Choudhury N, Mohan N, Padhi TR, Balne PK, Sharma S, Panigrahi SR. 2014. Degree, duration, and causes of visual impairment in eyes affected with ocular tuberculosis. J Ophthalmic Inflamm Infect 4:3. 10.1186/1869-5760-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Joye A, Gonzales JA. 2018. Ocular manifestations of cytomegalovirus in immunocompetent hosts. Curr Opin Ophthalmol 29:535–542. 10.1097/ICU.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 175.Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S, III, Theel ES, Thomson RB, Jr, Weinstein MP, Yao JD. 2018. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67:e1–e94. 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Montoya JC, Boothroyd JC, Kovacs JA. 2015. Toxoplasma gondii, p 3122–3153. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 8th ed. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 177.Chen KJ, Chen YP, Chao AN, Wang NK, Wu WC, Lai CC, Chen TL. 2017. Prevention of evisceration or enucleation in endogenous bacterial panophthalmitis with no light perception and scleral abscess. PLoS One 12:e0169603. 10.1371/journal.pone.0169603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hagiya H, Semba T, Morimoto T, Yamamoto N, Yoshida H, Tomono K. 2018. Panophthalmitis caused by Streptococcus dysgalactiae subsp. equisimilis: a case report and review of the literature. J Infect Chemother 24:936–940. 10.1016/j.jiac.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 179.Guedira G, Taright N, Blin H, Fattoum T, Leroy J, El Samad Y, Milazzo S, Hamdad F. 2018. Clostridium perfringens panophthalmitis and orbital cellulitis: a case report. BMC Ophthalmol 18:88. 10.1186/s12886-018-0751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Burr WE, Jr, Brown MF, Eberhard ML. 1998. Zoonotic Onchocerca (Nematoda: Filarioidea) in the cornea of a Colorado resident. Ophthalmology 105:1494–1497. 10.1016/S0161-6420(98)98035-6. [DOI] [PubMed] [Google Scholar]
- 181.Ament CS, Young LH. 2006. Ocular manifestations of helminthic infections: onchocersiasis [sic], cysticercosis, toxocariasis, and diffuse unilateral subacute neuroretinitis. Int Ophthalmol Clin 46:1–10. 10.1097/00004397-200604620-00003. [DOI] [PubMed] [Google Scholar]
- 182.Otranto D, Eberhard ML. 2011. Zoonotic helminths affecting the human eye. Parasit Vectors 4:41. 10.1186/1756-3305-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lima NF, Veggiani Aybar CA, Dantur Juri MJ, Ferreira MU. 2016. Mansonella ozzardi: a neglected New World filarial nematode. Pathog Glob Health 110:97–107. 10.1080/20477724.2016.1190544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tamarozzi F, Halliday A, Gentil K, Hoerauf A, Pearlman E, Taylor MJ. 2011. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev 24:459–468. 10.1128/CMR.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gillette-Ferguson I, Daehnel K, Hise AG, Sun Y, Carlson E, Diaconu E, McGarry HF, Taylor MJ, Pearlman E. 2007. Toll-like receptor 2 regulates CXC chemokine production and neutrophil recruitment to the cornea in Onchocerca volvulus/Wolbachia-induced keratitis. Infect Immun 75:5908–5915. 10.1128/IAI.00991-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huh K, Choi JH. 2016. Worms in the eye. N Engl J Med 375:e30. 10.1056/NEJMicm1515440. [DOI] [PubMed] [Google Scholar]
- 187.Mills SE (ed). 2012. Histology for pathologists, 4th ed, vol 1. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 188.Klotz SA, Penn CC, Negvesky GJ, Butrus SI. 2000. Fungal and parasitic infections of the eye. Clin Microbiol Rev 13:662–685. 10.1128/cmr.13.4.662-685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lester RJ, Freeman RS. 1975. Eye penetration by cercariae of Schistosoma mansoni. J Parasitol 61:970–972. 10.2307/3279255. [DOI] [PubMed] [Google Scholar]
- 190.Badenoch PR, Coster DJ. 1982. Antimicrobial activity of topical anaesthetic preparations. Br J Ophthalmol 66:364–367. 10.1136/bjo.66.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chodosh J, Dix RD, Howell RC, Stroop WG, Tseng SC. 1994. Staining characteristics and antiviral activity of sulforhodamine B and lissamine green B. Invest Ophthalmol Vis Sci 35:1046–1058. [PubMed] [Google Scholar]
- 192.Roat MI, Romanowski E, Araullo-Cruz T, Gordon YJ. 1987. The antiviral effects of rose Bengal and fluorescein. Arch Ophthalmol 105:1415–1417. 10.1001/archopht.1987.01060100117039. [DOI] [PubMed] [Google Scholar]
- 193.Benson WH, Lanier JD. 1992. Comparison of techniques for collecting corneal ulcers. Ophthalmology 99:800–804. 10.1016/S0161-6420(92)31897-4. [DOI] [PubMed] [Google Scholar]
- 194.Pakzad-Vaezi K, Levasseur SD, Schendel S, Mark S, Mathias R, Roscoe D, Holland SP. 2015. The corneal ulcer one-touch study: a simplified microbiological specimen collection method. Am J Ophthalmol 159:37–43.e1. 10.1016/j.ajo.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 195.Church DL. 2016. Ocular cultures, p 3.10.1–3.10.10. In Leber AL (ed), Clinical microbiology procedures handbook, 4th ed. ASM Press, Washington, DC. [Google Scholar]
- 196.Thomson RB. 2007. Specimen collection, transport, and processing: bacteriology, p 291–333. In Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA (ed), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC. [Google Scholar]
- 197.Trebbien R, Andersen B, Ronn J, McCauley J, Fischer TK. 2014. ESwab challenges virus propagation in cell cultures. Euro Surveill 19:20995. 10.2807/1560-7917.es2014.19.50.20995. [DOI] [PubMed] [Google Scholar]
- 198.Indevuyst C, Beuselinck K, Lagrou K. 2012. eSwab flocked swabs unfit for viral culture. J Clin Virol 55:282–283. 10.1016/j.jcv.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 199.Bharathi MJ, Ramakrishnan R, Meenakshi R, Mittal S, Shivakumar C, Srinivasan M. 2006. Microbiological diagnosis of infective keratitis: comparative evaluation of direct microscopy and culture results. Br J Ophthalmol 90:1271–1276. 10.1136/bjo.2006.096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yeh DL, Stinnett SS, Afshari NA. 2006. Analysis of bacterial cultures in infectious keratitis, 1997 to 2004. Am J Ophthalmol 142:1066–1068. 10.1016/j.ajo.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 201.Day AC, Ramkissoon YD, George S, Corbett MC. 2006. Don’t forget gonococcus! Eye (Lond) 20:1400–1402. 10.1038/sj.eye.6702249. [DOI] [PubMed] [Google Scholar]
- 202.Gigliotti F, Williams WT, Hayden FG, Hendley JO, Benjamin J, Dickens M, Gleason C, Perriello VA, Wood J. 1981. Etiology of acute conjunctivitis in children. J Pediatr 98:531–536. 10.1016/s0022-3476(81)80754-8. [DOI] [PubMed] [Google Scholar]
- 203.Kehrmann J, Chapot V, Buer J, Rating P, Bornfeld N, Steinmann J. 2018. Diagnostic performance of blood culture bottles for vitreous culture compared to conventional microbiological cultures in patients with suspected endophthalmitis. Eur J Clin Microbiol Infect Dis 37:889–895. 10.1007/s10096-017-3182-6. [DOI] [PubMed] [Google Scholar]
- 204.Hsu HY, Ernst B, Schmidt EJ, Parihar R, Horwood C, Edelstein SL. 2019. Laboratory results, epidemiological features and outcomes analyses of microbial keratitis: a 15-year review from Saint Louis. Am J Ophthalmol 198:54–62. 10.1016/j.ajo.2018.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bhende M, Raman R, Jain M, Shah PK, Sharma T, Gopal L, Bhende PS, Srinivasan S, Jambulingam M, Sankara Nethralaya Vitreoretinal Study Group (SNVR-Study Group) . 2018. Incidence, microbiology, and outcomes of endophthalmitis after 111,876 pars plana vitrectomies at a single, tertiary eye care hospital. PLoS One 13:e0191173. 10.1371/journal.pone.0191173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Agarwal A, Agrawal R, Gunasekaran DV, Raje D, Gupta B, Aggarwal K, Murthy SL, Westcott M, Chee SP, McCluskey P, Ling HS, Teoh S, Cimino L, Biswas J, Narain S, Agarwal M, Mahendradas P, Khairallah M, Jones N, Tugal-Tutkun I, Babu K, Basu S, Carreno E, Lee R, Al-Dhibi H, Bodaghi B, Invernizzi A, Goldstein DA, Herbort CP, Barisani-Asenbauer T, Gonzalez-Lopez JJ, Androudi S, Bansal R, Moharana B, Mahajan S, Esposti S, Tasiopoulou A, Nadarajah S, Agarwal M, Abraham S, Vala R, Singh R, Sharma A, Sharma K, Zierhut M, Kon OM, Cunningham E, Nguyen QD, Pavesio C, Gupta V. 2019. The Collaborative Ocular Tuberculosis Study (COTS)-1 report 3. Polymerase chain reaction in the diagnosis and management of tubercular uveitis: global trends. Ocul Immunol Inflamm 27:465–473. 10.1080/09273948.2017.1406529. [DOI] [PubMed] [Google Scholar]
- 207.Harrington BJ, Hageage GJ. 2003. Calcofluor white: a review of its uses and applications in clinical mycology and parasitology. Lab Med 34:361–366. 10.1309/EPH2TDT8335GH0R3. [DOI] [Google Scholar]
- 208.Wilhelmus KR, Osato MS, Font RL, Robinson NM, Jones DB. 1986. Rapid diagnosis of Acanthamoeba keratitis using calcofluor white. Arch Ophthalmol 104:1309–1312. 10.1001/archopht.1986.01050210063026. [DOI] [PubMed] [Google Scholar]
- 209.McDonnell PJ. 1996. Empirical or culture guided therapy for microbial keratitis? A plea for data. Arch Ophthalmol 114:84–87. 10.1001/archopht.1996.01100130080013. [DOI] [PubMed] [Google Scholar]
- 210.CLSI. 2012. CLSI standard M54: principles and procedures for detection of fungi in clinical specimens—direct examination and culture; approved guidelines, vol 32. CLSI, Wayne, PA. [Google Scholar]
- 211.Chander J, Chakrabarti A, Sharma A, Saini JS, Panigarhi D. 1993. Evaluation of calcofluor staining in the diagnosis of fungal corneal ulcer. Mycoses 36:243–245. 10.1111/j.1439-0507.1993.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 212.Gupta MK, Chandra A, Prakash P, Banerjee T, Maurya OP, Tilak R. 2015. Fungal keratitis in north India; spectrum and diagnosis by calcofluor white stain. Indian J Med Microbiol 33:462–463. 10.4103/0255-0857.158609. [DOI] [PubMed] [Google Scholar]
- 213.Gatti S, Rama P, Matuska S, Berrilli F, Cavallero A, Carletti S, Bruno A, Maserati R, Di Cave D. 2010. Isolation and genotyping of Acanthamoeba strains from corneal infections in Italy. J Med Microbiol 59:1324–1330. 10.1099/jmm.0.019786-0. [DOI] [PubMed] [Google Scholar]
- 214.Peretz A, Geffen Y, Socea SD, Pastukh N, Graffi S. 2015. Comparison of fluorescence microscopy and different growth media culture methods for Acanthamoeba keratitis diagnosis. Am J Trop Med Hyg 93:316–318. 10.4269/ajtmh.15-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Milner D. 2015. Diagnostic pathology: infectious diseases, 1st ed. Elsevier, Philadelphia, PA. [Google Scholar]
- 216.Procop GW, Pritt BS. 2014. Pathology of infectious diseases, 1st ed. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 217.Sambursky R, Trattler W, Tauber S, Starr C, Friedberg M, Boland T, McDonald M, DellaVecchia M, Luchs J. 2013. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol 131:17–22. 10.1001/2013.jamaophthalmol.513. [DOI] [PubMed] [Google Scholar]
- 218.Kam KY, Ong HS, Bunce C, Ogunbowale L, Verma S. 2015. Sensitivity and specificity of the AdenoPlus point-of-care system in detecting adenovirus in conjunctivitis patients at an ophthalmic emergency department: a diagnostic accuracy study. Br J Ophthalmol 99:1186–1189. 10.1136/bjophthalmol-2014-306508. [DOI] [PubMed] [Google Scholar]
- 219.Tan HS, Ghyczy-Carlborg EA, Spanjaard L, de Smet MD. 2011. The additional value of blood cultures in the diagnosis of endophthalmitis. Eye (Lond) 25:1069–1073. 10.1038/eye.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Everitt HA, Little PS, Smith PWF. 2006. A randomised controlled trial of management strategies for acute infective conjunctivitis in general practice. BMJ 333:321. 10.1136/bmj.38891.551088.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu D, Ke CW, Mo YL, Sun LM, Li H, Chen QX, Zou LR, Fang L, Huang P, Zhen HY. 2008. Multiple outbreaks of acute hemorrhagic conjunctivitis due to a variant of coxsackievirus A24: Guangdong, China, 2007. J Med Virol 80:1762–1768. 10.1002/jmv.21288. [DOI] [PubMed] [Google Scholar]
- 222.Xiao XL, Wu H, Li YJ, Li HF, He YQ, Chen G, Zhang JW, Yang H, Li XF, Yang XQ, Yu YG. 2009. Simultaneous detection of enterovirus 70 and coxsackievirus A24 variant by multiplex real-time RT-PCR using an internal control. J Virol Methods 159:23–28. 10.1016/j.jviromet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 223.Sharma S, Jalali S, Adiraju MV, Gopinathan U, Das T. 1996. Sensitivity and predictability of vitreous cytology, biopsy, and membrane filter culture in endophthalmitis. Retina 16:525–529. 10.1097/00006982-199616060-00010. [DOI] [PubMed] [Google Scholar]
- 224.Eser I, Kapran Z, Altan T, Eren H, Yilmaz OF. 2007. The use of blood culture bottles in endophthalmitis. Retina 27:971–973. 10.1097/IAE.0b013e31802bfe04. [DOI] [PubMed] [Google Scholar]
- 225.Pongsachareonnont P, Honglertnapakul W, Chatsuwan T. 2017. Comparison of methods for identifying causative bacterial microorganisms in presumed acute endophthalmitis: conventional culture, blood culture, and PCR. BMC Infect Dis 17:165. 10.1186/s12879-017-2264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tanaka T, Oliveira LMDF, Ferreira BFDA, Kato JM, Rossi F, Correa KDLG, Pimentel SLG, Yamamoto JH, Almeida JN, Jr.. 2017. Bactec blood culture bottles allied to MALDI-TOF mass spectroscopy: rapid etiologic diagnosis of bacterial endophthalmitis. Diagn Microbiol Infect Dis 88:222–224. 10.1016/j.diagmicrobio.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 227.Thariya P, Yospaiboon Y, Sinawat S, Sanguansak T, Bhoomibunchoo C, Laovirojjanakul W. 2016. Blood culture bottles are superior to conventional media for vitreous culture. Clin Exp Ophthalmol 44:488–491. 10.1111/ceo.12707. [DOI] [PubMed] [Google Scholar]
- 228.Rachitskaya AV, Flynn HW, Jr, Wong J, Kuriyan AE, Miller D. 2013. A 10-year study of membrane filter system versus blood culture bottles in culturing vitrectomy cassette vitreous in infectious endophthalmitis. Am J Ophthalmol 156:349–354.e2. 10.1016/j.ajo.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 229.Broadhurst MJ, Brooks TJ, Pollock NR. 2016. Diagnosis of Ebola virus disease: past, present, and future. Clin Microbiol Rev 29:773–793. 10.1128/CMR.00003-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.CDC. 2015. Infection prevention and control recommendations for hospitalized patients under investigation (PUIs) for Ebola virus disease (EVD) in U.S. hospitals. CDC, Atlanta, GA. https://www.cdc.gov/vhf/ebola/clinicians/evd/infection-control.html. Accessed 16 April 2020. [Google Scholar]
- 231.Carson JA. 2016. Wound cultures, p 3.13.1.1–3.13.2.4. In Leber AL (ed), Clinical microbiology procedures handbook, 4th ed. ASM Press, Washington, DC. [Google Scholar]
- 232.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol 47:1878–1884. 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Leal SM, Jr, Jones M, Gilligan PH. 2016. Clinical significance of commensal Gram-positive rods routinely isolated from patient samples. J Clin Microbiol 54:2928–2936. 10.1128/JCM.01393-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Panagea T, Pincus DH, Grogono D, Jones M, Bryant J, Parkhill J, Floto RA, Gilligan P. 2015. Mycobacterium abscessus complex identification with matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 53:2355–2358. 10.1128/JCM.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Celandroni F, Salvetti S, Gueye SA, Mazzantini D, Lupetti A, Senesi S, Ghelardi E. 2016. Identification and pathogenic potential of clinical Bacillus and Paenibacillus isolates. PLoS One 11:e0152831. 10.1371/journal.pone.0152831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ikeda M, Yagihara Y, Tatsuno K, Okazaki M, Okugawa S, Moriya K. 2015. Clinical characteristics and antimicrobial susceptibility of Bacillus cereus blood stream infections. Ann Clin Microbiol Antimicrob 14:43. 10.1186/s12941-015-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Alby K, Gilligan PH. 2013. Identification of pathogens by classical clinical tests, p 1–45. In Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (ed), The prokaryotes, 4th ed, vol 5. Human microbiology. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 238.Larone DH. 2018. Medically important fungi: a guide to identification, 6th ed. ASM Press, Washington, DC. [Google Scholar]
- 239.Schulthess B, Ledermann R, Mouttet F, Zbinden A, Bloemberg GV, Bottger EC, Hombach M. 2014. Use of the Bruker MALDI Biotyper for identification of molds in the clinical mycology laboratory. J Clin Microbiol 52:2797–2803. 10.1128/JCM.00049-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McMullen AR, Wallace MA, Pincus DH, Wilkey K, Burnham CA. 2016. Evaluation of the Vitek MS matrix-assisted laser desorption ionization–time of flight mass spectrometry system for identification of clinically relevant filamentous fungi. J Clin Microbiol 54:2068–2073. 10.1128/JCM.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Luethy PM, Zelazny AM. 2018. Rapid one-step extraction method for the identification of molds using MALDI-TOF MS. Diagn Microbiol Infect Dis 91:130–135. 10.1016/j.diagmicrobio.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Szczotka-Flynn LB, Pearlman E, Ghannoum M. 2010. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens 36:116–129. 10.1097/ICL.0b013e3181d20cae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li X, Zarbin MA, Langer PD, Bhagat N. 2018. Postraumatic endophthalmitis—an 18-year case series. Retina 38:60–71. 10.1097/IAE.0000000000001511. [DOI] [PubMed] [Google Scholar]
- 244.Kowalski RP, Melan MA, Karenchak LM, Mammen A. 2015. Comparison of validated polymerase chain reaction and culture isolation for routine detection of Acanthamoeba from ocular samples. Eye Contact Lens 41:341–343. 10.1097/ICL.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 245.Sriram R, Shoff M, Booton G, Fuerst P, Visvesvara GS. 2008. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J Clin Microbiol 46:4045–4048. 10.1128/JCM.01903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Visvesvara GS, Schuster FL. 2008. Opportunistic free-living amoebae, part I. Clin Microbiol Newsl 30:151–158. 10.1016/j.clinmicnews.2008.09.004. [DOI] [Google Scholar]
- 247.St George K. 2016. Chapter 10: Viruses and chlamydia. In Leber AL (ed), Clinical microbiology procedures handbook, 4th ed. ASM Press, Washington, DC. [Google Scholar]
- 248.Kim E, Chidambaram JD, Srinivasan M, Lalitha P, Wee D, Lietman TM, Whitcher JP, Van Gelder RN. 2008. Prospective comparison of microbial culture and polymerase chain reaction in the diagnosis of corneal ulcer. Am J Ophthalmol 146:714–723.e1. 10.1016/j.ajo.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 249.Eleinen KG, Mohalhal AA, Elmekawy HE, Abdulbaki AM, Sherif AM, El-Sherif RH, Abdul Rahman EM. 2012. Polymerase chain reaction-guided diagnosis of infective keratitis—a hospital-based study. Curr Eye Res 37:1005–1011. 10.3109/02713683.2012.698357. [DOI] [PubMed] [Google Scholar]
- 250.Taravati P, Lam D, Van Gelder RN. 2013. Role of molecular diagnostics in ocular microbiology. Curr Ophthalmol Rep 1:181–189. 10.1007/s40135-013-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu DTL, Li C-L, Lee VYW. 2006. The presence of Propionibacterium spp. in the vitreous fluid of uveitis patients with sarcoidosis. Acta Ophthalmol Scand 84:152–153. 10.1111/j.1600-0420.2005.00638.x. (Reply, 84:153.) [DOI] [PubMed] [Google Scholar]
- 252.Fontana C, Favaro M, Cicchetti O, Minelli S, Pistoia ES, Favalli C. 2005. Performance of strand displacement amplification assay in the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Jpn J Infect Dis 58:283–288. [PubMed] [Google Scholar]
- 253.Jenson A, Dize L, Mkocha H, Munoz B, Lee J, Gaydos C, Quinn T, West SK. 2013. Field evaluation of the Cepheid GeneXpert Chlamydia trachomatis assay for detection of infection in a trachoma endemic community in Tanzania. PLoS Negl Trop Dis 7:e2265. 10.1371/journal.pntd.0002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kowalski RP, Thompson PP, Kinchington PR, Gordon YJ. 2006. Evaluation of the SmartCycler II system for real-time detection of viruses and Chlamydia from ocular specimens. Arch Ophthalmol 124:1135–1139. 10.1001/archopht.124.8.1135. [DOI] [PubMed] [Google Scholar]
- 255.Yang JL, Hong KC, Schachter J, Moncada J, Lekew T, House JI, Zhou Z, Neuwelt MD, Rutar T, Halfpenny C, Shah N, Whitcher JP, Lietman TM. 2009. Detection of Chlamydia trachomatis ocular infection in trachoma-endemic communities by rRNA amplification. Invest Ophthalmol Vis Sci 50:90–94. 10.1167/iovs.08-2247. [DOI] [PubMed] [Google Scholar]
- 256.Dize L, West S, Williams JA, Van Der Pol B, Quinn TC, Gaydos CA. 2013. Comparison of the Abbott m2000 RealTime CT assay and the Cepheid GeneXpert CT/NG assay to the Roche Amplicor CT assay for detection of Chlamydia trachomatis in ocular samples from Tanzania. J Clin Microbiol 51:1611–1613. 10.1128/JCM.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yang JL, Schachter J, Moncada J, Habte D, Zerihun M, House JI, Zhou Z, Hong KC, Maxey K, Gaynor BD, Lietman TM. 2007. Comparison of an rRNA-based and DNA-based nucleic acid amplification test for the detection of Chlamydia trachomatis in trachoma. Br J Ophthalmol 91:293–295. 10.1136/bjo.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kowalski RP, Karenchak LM, Raju LV, Ismail N. 2015. The verification of nucleic acid amplification testing (Gen-Probe Aptima assay) for Chlamydia trachomatis from ocular samples. Ophthalmology 122:244–247. 10.1016/j.ophtha.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 259.Biswas J, Kazi MS, Agarwal VA, Alam MS, Therese KL. 2016. Polymerase chain reaction for Mycobacterium tuberculosis DNA detection from ocular fluids in patients with various types of choroiditis in a referral eye center in India. Indian J Ophthalmol 64:904–907. 10.4103/0301-4738.198857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gupta V, Gupta A, Rao NA. 2007. Intraocular tuberculosis—an update. Surv Ophthalmol 52:561–587. 10.1016/j.survophthal.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 261.Jaeger EE, Carroll NM, Choudhury S, Dunlop AA, Towler HM, Matheson MM, Adamson P, Okhravi N, Lightman S. 2000. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. J Clin Microbiol 38:2902–2908. 10.1128/JCM.38.8.2902-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Reddy AK, Balne PK, Gaje K, Garg P. 2011. PCR for the diagnosis and species identification of microsporidia in patients with keratitis. Clin Microbiol Infect 17:476–478. 10.1111/j.1469-0691.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- 263.Pasricha G, Sharma S, Garg P, Aggarwal RK. 2003. Use of 18S rRNA gene-based PCR assay for diagnosis of Acanthamoeba keratitis in non-contact lens wearers in India. J Clin Microbiol 41:3206–3211. 10.1128/jcm.41.7.3206-3211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Maubon D, Dubosson M, Chiquet C, Yera H, Brenier-Pinchart MP, Cornet M, Savy O, Renard E, Pelloux H. 2012. A one-step multiplex PCR for Acanthamoeba keratitis diagnosis and quality samples control. Invest Ophthalmol Vis Sci 53:2866–2872. 10.1167/iovs.11-8587. [DOI] [PubMed] [Google Scholar]
- 265.Montoya JG, Parmley S, Liesenfeld O, Jaffe GJ, Remington JS. 1999. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology 106:1554–1563. 10.1016/S0161-6420(99)90453-0. [DOI] [PubMed] [Google Scholar]
- 266.Steeples LR, Guiver M, Jones NP. 2016. Real-time PCR using the 529 bp repeat element for the diagnosis of atypical ocular toxoplasmosis. Br J Ophthalmol 100:200–203. 10.1136/bjophthalmol-2015-306801. [DOI] [PubMed] [Google Scholar]
- 267.Bourdin C, Busse A, Kouamou E, Touafek F, Bodaghi B, Le Hoang P, Mazier D, Paris L, Fekkar A. 2014. PCR-based detection of Toxoplasma gondii DNA in blood and ocular samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol 52:3987–3991. 10.1128/JCM.01793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kinchington PR, Turse SE, Kowalski RP, Gordon YJ. 1994. Use of polymerase chain amplification reaction for the detection of adenoviruses in ocular swab specimens. Invest Ophthalmol Vis Sci 35:4126–4134. [PubMed] [Google Scholar]
- 269.Fox GM, Crouse CA, Chuang EL, Pflugfelder SC, Cleary TJ, Nelson SJ, Atherton SS. 1991. Detection of herpesvirus DNA in vitreous and aqueous specimens by the polymerase chain reaction. Arch Ophthalmol 109:266–271. 10.1001/archopht.1991.01080020112054. [DOI] [PubMed] [Google Scholar]
- 270.Mitchell SM, Fox JD, Tedder RS, Gazzard BG, Lightman S. 1994. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol 43:336–340. 10.1002/jmv.1890430404. [DOI] [PubMed] [Google Scholar]
- 271.Shinoda K, Kobayashi A, Higashide T, Shirao Y, Sakurai M, Shirota Y, Kagaya M. 2002. Detection of measles virus genomic RNA in tear samples from a patient with measles keratitis. Cornea 21:610–612. 10.1097/00003226-200208000-00017. [DOI] [PubMed] [Google Scholar]
- 272.Lewis FM, Chernak E, Goldman E, Li Y, Karem K, Damon IK, Henkel R, Newbern EC, Ross P, Johnson CC. 2006. Ocular vaccinia infection in laboratory worker, Philadelphia, 2004. Emerg Infect Dis 12:134–137. 10.3201/eid1201.051126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yamamoto S, Pavan-Langston D, Kinoshita S, Nishida K, Shimomura Y, Tano Y. 1996. Detecting herpesvirus DNA in uveitis using the polymerase chain reaction. Br J Ophthalmol 80:465–468. 10.1136/bjo.80.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Agrawal R, Gunasekeran DV, Raje D, Agarwal A, Nguyen QD, Kon OM, Pavesio C, Gupta V, Collaborative Ocular Tuberculosis Study Group . 2018. Global variation and challenges with tubercular uveitis in the Collaborative Ocular Tuberculosis Study. Invest Ophthalmol Vis Sci 59:4162–4171. 10.1167/iovs.18-24102. [DOI] [PubMed] [Google Scholar]
- 275.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Vanhaecke C, Grange P, Benhaddou N, Blanche P, Salmon D, Parize P, Lortholary O, Caumes E, Pelloux I, Epaulard O, Guinard J, Dupin N, on behalf of the Neurosyphilis Network . 2016. Clinical and biological characteristics of 40 patients with neurosyphilis and evaluation of Treponema pallidum nested polymerase chain reaction in cerebrospinal fluid samples. Clin Infect Dis 63:1180–1186. 10.1093/cid/ciw499. [DOI] [PubMed] [Google Scholar]
- 277.Lau R, Cunanan M, Jackson J, Ali IK, Chong-Kit A, Gasgas J, Tian J, Ralevski F, Boggild AK. 2015. Reevaluation of an Acanthamoeba molecular diagnostic algorithm following an atypical case of amoebic keratitis. J Clin Microbiol 53:3213–3218. 10.1128/JCM.01607-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ikeda Y, Miyazaki D, Yakura K, Kawaguchi A, Ishikura R, Inoue Y, Mito T, Shiraishi A, Ohashi Y, Higaki S, Itahashi M, Fukuda M, Shimomura Y, Yagita K. 2012. Assessment of real-time polymerase chain reaction detection of Acanthamoeba and prognosis determinants of Acanthamoeba keratitis. Ophthalmology 119:1111–1119. 10.1016/j.ophtha.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 279.Greigert V, Di Foggia E, Filisetti D, Villard O, Pfaff AW, Sauer A, Candolfi E. 2019. When biology supports clinical diagnosis: review of techniques to diagnose ocular toxoplasmosis. Br J Ophthalmol 103:1008–1012. 10.1136/bjophthalmol-2019-313884. [DOI] [PubMed] [Google Scholar]
- 280.Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. 2007. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am J Ophthalmol 144:781–785. 10.1016/j.ajo.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 281.Ozgonul C, Besirli CG. 2017. Recent developments in the diagnosis and treatment of ocular toxoplasmosis. Ophthalmic Res 57:1–12. 10.1159/000449169. [DOI] [PubMed] [Google Scholar]
- 282.Rothova A, de Boer JH, Ten Dam-van Loon NH, Postma G, de Visser L, Zuurveen SJ, Schuller M, Weersink AJL, van Loon AM, de Groot-Mijnes JDF. 2008. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology 115:306–311. 10.1016/j.ophtha.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 283.Talabani H, Asseraf M, Yera H, Delair E, Ancelle T, Thulliez P, Brezin AP, Dupouy-Camet J. 2009. Contributions of immunoblotting, real-time PCR, and the Goldmann-Witmer coefficient to diagnosis of atypical toxoplasmic retinochoroiditis. J Clin Microbiol 47:2131–2135. 10.1128/JCM.00128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cassaing S, Bessieres MH, Berry A, Berrebi A, Fabre R, Magnaval JF. 2006. Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR. J Clin Microbiol 44:720–724. 10.1128/JCM.44.3.720-724.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.De Groot-Mijnes JD, Rothova A, Van Loon AM, Schuller M, Ten Dam-Van Loon NH, De Boer JH, Schuurman R, Weersink AJ. 2006. Polymerase chain reaction and Goldmann-Witmer coefficient analysis are complimentary for the diagnosis of infectious uveitis. Am J Ophthalmol 141:313–318. 10.1016/j.ajo.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 286.Villard O, Filisetti D, Roch-Deries F, Garweg J, Flament J, Candolfi E. 2003. Comparison of enzyme-linked immunosorbent assay, immunoblotting, and PCR for diagnosis of toxoplasmic chorioretinitis. J Clin Microbiol 41:3537–3541. 10.1128/jcm.41.8.3537-3541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Doan T, Pinsky BA. 2016. Current and future molecular diagnostics for ocular infectious diseases. Curr Opin Ophthalmol 27:561–567. 10.1097/ICU.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 288.Nakano S, Sugita S, Tomaru Y, Hono A, Nakamuro T, Kubota T, Takase H, Mochizuki M, Takahashi M, Shimizu N. 2017. Establishment of multiplex solid-phase strip PCR test for detection of 24 ocular infectious disease pathogens. Invest Ophthalmol Vis Sci 58:1553–1559. 10.1167/iovs.16-20556. [DOI] [PubMed] [Google Scholar]
- 289.Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, Mochizuki M. 2008. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol 92:928–932. 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Harper TW, Miller D, Schiffman JC, Davis JL. 2009. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol 147:140–147.e2. 10.1016/j.ajo.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Johnston C, Corey L. 2016. Current concepts for genital herpes simplex virus infection: diagnostics and pathogenesis of genital tract shedding. Clin Microbiol Rev 29:149–161. 10.1128/CMR.00043-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Heaton PR, Espy MJ, Binnicker MJ. 2015. Evaluation of 2 multiplex real-time PCR assays for the detection of HSV-1/2 and varicella zoster virus directly from clinical samples. Diagn Microbiol Infect Dis 81:169–170. 10.1016/j.diagmicrobio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 293.Nikolic D, Kohn D, Yen-Lieberman B, Procop GW. 2019. Detection of herpes simplex virus and varicella-zoster virus by traditional and multiplex molecular methods. Am J Clin Pathol 151:122–126. 10.1093/ajcp/aqy111. [DOI] [PubMed] [Google Scholar]
- 294.Granato PA, Alkins BR, Yen-Lieberman B, Greene WH, Connolly J, Buchan BW, Ledeboer NA. 2015. Comparative evaluation of AmpliVue HSV 1+2 assay with ELVIS culture for detecting herpes simplex virus 1 (HSV-1) and HSV-2 in clinical specimens. J Clin Microbiol 53:3922–3925. 10.1128/JCM.01905-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Kowalski RP, Karenchak LM, Dhaliwal DK, Mammen A. 2018. AmpliVue is a practical and timely test for the detection of HSV from keratitis specimens. Eye Contact Lens 44(Suppl 1):S244–S248. 10.1097/ICL.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 296.Port AD, Orlin A, Kiss S, Patel S, D’Amico DJ, Gupta MP. 2017. Cytomegalovirus retinitis: a review. J Ocul Pharmacol Ther 33:224–234. 10.1089/jop.2016.0140. [DOI] [PubMed] [Google Scholar]
- 297.Papageorgiou E, Ch’ng S, Kulkarni A, Anwar S, Empeslidis T. 2014. Fourth cranial nerve palsy and bilateral acute retinal necrosis following human herpesvirus 6 infection of the central nervous system. Ocul Immunol Inflamm 22:228–232. 10.3109/09273948.2013.856533. [DOI] [PubMed] [Google Scholar]
- 298.Holland GN, Vaudaux JD, Shiramizu KM, Yu F, Goldenberg DT, Gupta A, Carlson M, Read RW, Novack RD, Kuppermann BD, Southern California HIV/Eye Consortium . 2008. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in the era of highly active antiretroviral therapy (1997 to 2000). Am J Ophthalmol 145:12–22. 10.1016/j.ajo.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 299.Holland GN, Vaudaux JD, Jeng SM, Yu F, Goldenberg DT, Folz IC, Cumberland WG, McCannel CA, Helm CJ, Hardy WD, UCLA CMV Retinitis Study Group . 2008. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994). Am J Ophthalmol 145:5–11. 10.1016/j.ajo.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 300.Zhang Y, Ruan X, Yang W, Li L, Xian Z, Feng Q, Mo W. 2017. High ocular CMV copies and mismatched receipts may predict poor visual prognosis in CMV retinitis patients following allogeneic haematopoietic stem cell transplantation. BMC Ophthalmol 17:224. 10.1186/s12886-017-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Durand ML. 2015. Infectious causes of uveitis, p 1423–1431. In Bennett JE, Dolin R, Blaser MJ (ed), Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 8th ed. Elsevier/Saunders, Philadelphia, PA. [Google Scholar]
- 302.de Paula Freitas B, Ventura CV, Maia M, Belfort R, Jr.. 2017. Zika virus and the eye. Curr Opin Ophthalmol 28:595–599. 10.1097/ICU.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 303.Hong BK, Lee CS, Van Gelder RN, Garg SJ. 2015. Emerging techniques for pathogen discovery in endophthalmitis. Curr Opin Ophthalmol 26:221–225. 10.1097/ICU.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bennett S, Carman WF, Gunson RN. 2013. The development of a multiplex real-time PCR for the detection of herpes simplex virus 1 and 2, varicella zoster virus, adenovirus and Chlamydia trachomatis from eye swabs. J Virol Methods 189:143–147. 10.1016/j.jviromet.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Embong Z, Wan Hitam WH, Yean CY, Rashid NHA, Kamarudin B, Abidin SKZ, Osman S, Zainuddin ZF, Ravichandran M. 2008. Specific detection of fungal pathogens by 18S rRNA gene PCR in microbial keratitis. BMC Ophthalmol 8:7. 10.1186/1471-2415-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mazoteras P, Bispo PJ, Hofling-Lima AL, Casaroli-Marano RP. 2015. DNA extraction methods for panbacterial and panfungal PCR detection in intraocular fluids. Curr Eye Res 40:697–706. 10.3109/02713683.2014.957327. [DOI] [PubMed] [Google Scholar]
- 307.Sugita S, Ogawa M, Shimizu N, Morio T, Ohguro N, Nakai K, Maruyama K, Nagata K, Takeda A, Usui Y, Sonoda KH, Takeuchi M, Mochizuki M. 2013. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology 120:1761–1768. 10.1016/j.ophtha.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 308.Weck KE. 2017. The next generation of molecular pathology is here: validation of next-generation sequencing technology for clinical molecular testing across multiple different disciplines. Arch Pathol Lab Med 141:749–750. 10.5858/arpa.2017-0042-ED. [DOI] [PubMed] [Google Scholar]
- 309.Weinstock GM, Goldberg B, Ledeboer N, Rubin E, Sichtig H, Geyer C. 2016. Applications of clinical microbial next-generation sequencing. American Society for Microbiology, Washington, DC. [PubMed] [Google Scholar]
- 310.Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. 2015. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio 6:e01888-15. 10.1128/mBio.01888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Schrijver I, Aziz N, Jennings LJ, Richards CS, Voelkerding KV, Weck KE. 2014. Methods-based proficiency testing in molecular genetic pathology. J Mol Diagn 16:283–287. 10.1016/j.jmoldx.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 312.Duncavage EJ, Abel HJ, Merker JD, Bodner JB, Zhao Q, Voelkerding KV, Pfeifer JD. 2016. A model study of in silico proficiency testing for clinical next-generation sequencing. Arch Pathol Lab Med 140:1085–1091. 10.5858/arpa.2016-0194-CP. [DOI] [PubMed] [Google Scholar]
- 313.Schlaberg R, Chiu CY, Miller S, Procop GW, Weinstock G, Professional Practice Committee and Committee on Laboratory Practices of the American Society for Microbiology, Microbiology Resource Committee of the College of American Pathologists . 2017. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch Pathol Lab Med 141:776–786. 10.5858/arpa.2016-0539-RA. [DOI] [PubMed] [Google Scholar]
- 314.Lefterova MI, Suarez CJ, Banaei N, Pinsky BA. 2015. Next-generation sequencing for infectious disease diagnosis and management: a report of the Association for Molecular Pathology. J Mol Diagn 17:623–634. 10.1016/j.jmoldx.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 315.Doan T, Wilson MR, Crawford ED, Chow ED, Khan LM, Knopp KA, O’Donovan BD, Xia D, Hacker JK, Stewart JM, Gonzales JA, Acharya NR, DeRisi JL. 2016. Illuminating uveitis: metagenomic deep sequencing identifies common and rare pathogens. Genome Med 8:90. 10.1186/s13073-016-0344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cantor AG, Pappas M, Daeges M, Nelson HD. 2016. Screening for syphilis: updated evidence report and systematic review for the US Preventive Services task force. JAMA 315:2328–2337. 10.1001/jama.2016.4114. [DOI] [PubMed] [Google Scholar]
- 317.Workowski KA, Bolan GA, Centers for Disease Control and Prevention . 2015. Sexually transmitted disease treatment guidelines 2015. MMWR Recommend Rep 64(RR-3):1–179. [Google Scholar]
- 318.Reekie I, Reddy Y. 2018. Use of lumbar punctures in the management of ocular syphilis. Semin Ophthalmol 33:271–274. 10.1080/08820538.2016.1228986. [DOI] [PubMed] [Google Scholar]
- 319.US Food and Drug Administration. 20 April 2020, accession date. FDA approved devices—toxoplasma. US Food and Drug Administration, Silver Spring, MD. https://www.accessdata.fda.gov/cdrh_docs/reviews/K052499.pdf. [Google Scholar]
- 320.Villard O, Cimon B, L’Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, Paris L, Pelloux H, Villena I, Candolfi E. 2016. Help in the choice of automated or semiautomated immunoassays for serological diagnosis of toxoplasmosis: evaluation of nine immunoassays by the French National Reference Center for Toxoplasmosis. J Clin Microbiol 54:3034–3042. 10.1128/JCM.01193-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Owen WE, Martins TB, Litwin CM, Roberts WL. 2006. Performance characteristics of six IMMULITE 2000 TORCH assays. Am J Clin Pathol 126:900–905. 10.1309/KUA926D3YAPFYQG8. [DOI] [PubMed] [Google Scholar]
- 322.Vlaspolder F, Singer P, Smit A, Diepersloot RJ. 2001. Comparison of Immulite with Vidas for detection of infection in a low-prevalence population of pregnant women in The Netherlands. Clin Diagn Lab Immunol 8:552–555. 10.1128/CDLI.8.3.552-555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Lopez A, Dietz VJ, Wilson M, Navin TR, Jones JL. 2000. Preventing congenital toxoplasmosis. MMWR Recommend Rep 49:59–68. [PubMed] [Google Scholar]
- 324.Holliman RE, Stevens PJ, Duffy KT, Johnson JD. 1991. Serological investigation of ocular toxoplasmosis. Br J Ophthalmol 75:353–355. 10.1136/bjo.75.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Rothova A, van Knapen F, Baarsma GS, Kruit PJ, Loewer-Sieger DH, Kijlstra A. 1986. Serology in ocular toxoplasmosis. Br J Ophthalmol 70:615–622. 10.1136/bjo.70.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Roh M, Yasa C, Cho H, Nicholson L, Uchiyama E, Young LH, Lobo AM, Papaliodis GN, Durand ML, Sobrin L. 2016. The role of serological titres in the diagnosis of ocular toxoplasmosis. Acta Ophthalmol 94:521–522. 10.1111/aos.12851. [DOI] [PubMed] [Google Scholar]
- 327.Rajput R, Denniston AK, Murray PI. 2018. False negative Toxoplasma serology in an immunocompromised patient with PCR positive ocular toxoplasmosis. Ocul Immunol Inflamm 26:1200–1202. 10.1080/09273948.2017.1332769. [DOI] [PubMed] [Google Scholar]
- 328.Chronopoulos A, Roquelaure D, Souteyrand G, Seebach JD, Schutz JS, Thumann G. 2016. Aqueous humor polymerase chain reaction in uveitis—utility and safety. BMC Ophthalmol 16:189. 10.1186/s12886-016-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Fekkar A, Bodaghi B, Touafek F, Le Hoang P, Mazier D, Paris L. 2008. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol 46:1965–1967. 10.1128/JCM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mathis T, Beccat S, Seve P, Peyron F, Wallon M, Kodjikian L. 2018. Comparison of immunoblotting (IgA and IgG) and the Goldmann-Witmer coefficient for diagnosis of ocular toxoplasmosis in immunocompetent patients. Br J Ophthalmol 102:1454–1458. 10.1136/bjophthalmol-2017-311528. [DOI] [PubMed] [Google Scholar]
- 331.Streilein JW. 2003. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 3:879–889. 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 332.Theel ES, Aguero-Rosenfeld ME, Pritt B, Adem PV, Wormser GP. 2019. Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the United States. J Clin Microbiol 57:e01406-18. 10.1128/JCM.01406-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Jones MK, Keiser J, McManus DP. 2019. Trematodes, p 2590–2605. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 334.Nutman TB, Norice-Tra CT. 2019. Filarial nematodes, p 2570–2581. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 335.Orihel TC, Ash LR. 1995. Parasites in human tissues, p 149–209. ASCP Press, Chicago, IL. [Google Scholar]
- 336.Sheorey H, Biggs B-A, Ryan N. 2019. Nematodes, p 2551–2569. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 337.Sircar AD, Abanyie F, Blumberg D, Chin-Hong P, Coulter KS, Cunningham D, Huskins WC, Langelier C, Reid M, Scott BJ, Shirley DA, Babik JM, Belova A, Sapp SG, McAuliffe I, Rivera HN, Yabsley MJ, Montgomery SP. 2016. Raccoon roundworm infection associated with central nervous system disease and ocular disease—six states, 2013-2015. MMWR Morb Mortal Wkly Rep 65:930–933. 10.15585/mmwr.mm6535a2. [DOI] [PubMed] [Google Scholar]
- 338.Graeff-Teixeira C, Morassutti AL, Kazacos KR. 2016. Update on baylisascariasis, a highly pathogenic zoonotic infection. Clin Microbiol Rev 29:375–399. 10.1128/CMR.00044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Feng Y, Nawa Y, Sawanyavisuth K, Lv Z, Wu ZD. 2013. Comprehensive review of ocular angiostrongyliasis with special reference to optic neuritis. Korean J Parasitol 51:613–619. 10.3347/kjp.2013.51.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Garcia HH, Jiminez JA, Escalante H. 2019. Cestodes, p 2582–2589. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 341.Ash LR, Orihel TC. 2007. Atlas of human parasitology , 5th ed. ASCP Press, Chicago, IL. [Google Scholar]
- 342.Dalimi A, Jabarvand M. 2005. Fasciola hepatica in the human eye. Trans R Soc Trop Med Hyg 99:798–800. 10.1016/j.trstmh.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 343.Rathinam S, Fritsche TR, Srinivasan M, Vijayalakshmi P, Read RW, Gautom R, Namperumalsamy P, Rao NA. 2001. An outbreak of trematode-induced granulomas of the conjunctiva. Ophthalmology 108:1223–1229. 10.1016/s0161-6420(01)00604-2. [DOI] [PubMed] [Google Scholar]
- 344.Mather R, Karenchak LM, Romanowski EG, Kowalski RP. 2002. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol 133:463–466. 10.1016/S0002-9394(02)01334-X. [DOI] [PubMed] [Google Scholar]
- 345.Arya LK, Rathinam SR, Lalitha P, Kim UR, Ghatani S, Tandon V. 2016. Trematode fluke Procerovum varium as cause of ocular inflammation in children, South India. Emerg Infect Dis 22:192–200. 10.3201/eid2202.150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Rathinam SR, Usha KR, Rao NA. 2002. Presumed trematode-induced granulomatous anterior uveitis: a newly recognized cause of intraocular inflammation in children from south India. Am J Ophthalmol 133:773–779. 10.1016/s0002-9394(02)01435-6. [DOI] [PubMed] [Google Scholar]
- 347.Rathinam SR, Arya LK, Usha KR, Prajna L, Tandon V. 2012. Novel etiological agent: molecular evidence for trematode-induced anterior uveitis in children. Arch Ophthalmol 130:1481–1484. 10.1001/archophthalmol.2012.729. [DOI] [PubMed] [Google Scholar]
- 348.Brockhaus L, Goldblum D, Eggenschwiler L, Zimmerli S, Marzolini C. 2019. Revisiting systemic treatment of bacterial endophthalmitis: a review of intravitreal penetration of systemic antibiotics. Clin Microbiol Infect 25:1364–1369. 10.1016/j.cmi.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 349.Chen A, Prajna L, Srinivasan M, Mahalakshmi R, Whitcher JP, McLeod S, Lietman TM, Acharya NR. 2008. Does in vitro susceptibility predict clinical outcome in bacterial keratitis? Am J Ophthalmol 145:409–412. 10.1016/j.ajo.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kowalski RP, Yates KA, Romanowski EG, Karenchak LM, Mah FS, Gordon YJ. 2005. An ophthalmologist’s guide to understanding antibiotic susceptibility testing and minimum inhibitory concentration data. Ophthalmology 112:1987. 10.1016/j.ophtha.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 351.Asbell PA, Colby KA, Deng S, McDonnell P, Meisler DM, Raizman MB, Sheppard JD, Jr, Sahm DF. 2008. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol 145:951–958. 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 352.Scoper SV. 2008. Review of third- and fourth-generation fluoroquinolones in ophthalmology: in-vitro and in-vivo efficacy. Adv Ther 25:979–994. 10.1007/s12325-008-0107-x. [DOI] [PubMed] [Google Scholar]
- 353.Smith A, Pennefather PM, Kaye SB, Hart CA. 2001. Fluoroquinolones: place in ocular therapy. Drugs 61:747–761. 10.2165/00003495-200161060-00004. [DOI] [PubMed] [Google Scholar]
- 354.Sanfilippo CM, Allaire CM, DeCory HH. 2017. Besifloxacin ophthalmic suspension 0.6% compared with gatifloxacin ophthalmic solution 0.3% for the treatment of bacterial conjunctivitis in neonates. Drugs R D 17:167–175. 10.1007/s40268-016-0164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Siddiqui R, Aqeel Y, Khan NA. 2016. The development of drugs against Acanthamoeba infections. Antimicrob Agents Chemother 60:6441–6450. 10.1128/AAC.00686-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, Srinivasan M, Raghavan A, Oldenburg CE, Ray KJ, Zegans ME, McLeod SD, Porco TC, Acharya NR, Lietman TM, Mycotic Ulcer Treatment Trial Group . 2013. The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol 131:422–429. 10.1001/jamaophthalmol.2013.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sharma S, Das S, Virdi A, Fernandes M, Sahu SK, Kumar Koday N, Ali MH, Garg P, Motukupally SR. 2015. Re-appraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol 99:1190–1195. 10.1136/bjophthalmol-2014-306485. [DOI] [PubMed] [Google Scholar]
- 358.Chang VS, Dhaliwal DK, Raju L, Kowalski RP. 2015. Antibiotic resistance in the treatment of Staphylococcus aureus keratitis: a 20-year review. Cornea 34:698–703. 10.1097/ICO.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hyon JY, Joo MJ, Hose S, Sinha D, Dick JD, O’Brien TP. 2004. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarithromycin in treatment of experimental Mycobacterium chelonae keratitis. Arch Ophthalmol 122:1166–1169. 10.1001/archopht.122.8.1166. [DOI] [PubMed] [Google Scholar]
- 360.CLSI. 2020. CLSI standard M100—performance standards for antimicrobial susceptibility testing, 30th ed. CLSI, Wayne, PA. [Google Scholar]
- 361.CLSI. 2016. CLSI standard M45—methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd ed. CLSI, Wayne, PA. [Google Scholar]
- 362.Asbell PA, Sanfilippo CM, Sahm DF, DeCory HH. 2020. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol 138:439–450. 10.1001/jamaophthalmol.2020.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Asbell PA, DeCory HH. 2018. Antibiotic resistance among bacterial conjunctival pathogens collected in the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. PLoS One 13:e0205814. 10.1371/journal.pone.0205814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Harris SR, Cole MJ, Spiteri G, Sánchez-Busó L, Golparian D, Jacobsson S, Goater R, Abudahab K, Yeats CA, Bercot B, Borrego MJ, Crowley B, Stefanelli P, Tripodo F, Abad R, Aanensen DM, Unemo M, Euro-GASP Study Group . 2018. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis 18:758–768. 10.1016/S1473-3099(18)30225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.FlorCruz NV, Evans JR. 2015. Medical interventions for fungal keratitis. Cochrane Database Syst Rev 2015:CD004241. 10.1002/14651858.CD004241.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Prajna NV, Krishnan T, Rajaraman R, Patel S, Srinivasan M, Das M, Ray KJ, O’Brien KS, Oldenburg CE, McLeod SD, Zegans ME, Porco TC, Acharya NR, Lietman TM, Rose-Nussbaumer J, for the Mycotic Ulcer Treatment Trial II Group . 2016. Effect of oral voriconazole on fungal keratitis in the Mycotic Ulcer Treatment Trial II (MUTT II): a randomized clinical trial. JAMA Ophthalmol 134:1365–1372. 10.1001/jamaophthalmol.2016.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Prajna NV, Krishnan T, Rajaraman R, Patel S, Shah R, Srinivasan M, Devi L, Das M, Ray KJ, O’Brien KS, Oldenburg CE, McLeod SD, Zegans ME, Acharya NR, Lietman TM, Rose-Nussbaumer J, Mycotic Ulcer Treatment Trial Group . 2017. Adjunctive oral voriconazole treatment of Fusarium keratitis: a secondary analysis from the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol 135:520–525. 10.1001/jamaophthalmol.2017.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Leal SM, Jr, Roy S, Vareechon C, Carrion SD, Clark H, Lopez-Berges MS, diPietro A, Schrettl M, Beckmann N, Redl B, Haas H, Pearlman E. 2013. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog 9:e1003436. 10.1371/journal.ppat.1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Leal SM, Jr, Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, Pearlman E. 2012. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest 122:2482–2498. 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Clark HL, Minns MS, Sun Y, de Jesus T, Ghannoum MG, Pearlman E. 2018. Atovaquone impairs growth of Aspergillus and Fusarium keratitis isolates by modulating mitochondrial function and zinc homeostasis. Invest Ophthalmol Vis Sci 59:1589–1598. 10.1167/iovs.17-22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.CLSI. 2017. CLSI standard M38—reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed. CLSI, Wayne, PA. [Google Scholar]
- 372.CLSI. 2017. CLSI standard M27—reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed. CLSI, Wayne, PA. [Google Scholar]
- 373.CLSI. 2018. CLSI standard M59—epidemiologic cutoff values for antifungal susceptibility testing, 2nd ed. CLSI, Wayne, PA. [Google Scholar]
- 374.Lalitha P, Vijaykumar R, Prajna NV, Fothergill AW. 2008. In vitro natamycin susceptibility of ocular isolates of Fusarium and Aspergillus species: comparison of commercially formulated natamycin eye drops to pharmaceutical-grade powder. J Clin Microbiol 46:3477–3478. 10.1128/JCM.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Lockhart SR, Beer K, Toda M. 2020. Azole-resistant Aspergillus fumigatus: what you need to know. Clin Microbiol Newsl 42:1–6. 10.1016/j.clinmicnews.2019.12.003. [DOI] [Google Scholar]
- 376.Oechsler RA, Feilmeier MR, Miller D, Shi W, Hofling-Lima AL, Alfonso EC. 2013. Fusarium keratitis: genotyping, in vitro susceptibility and clinical outcomes. Cornea 32:667–673. 10.1097/ICO.0b013e318277ac74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Lockhart SR, Berkow E. 2019. Antifungal agents, p 2319–2333. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 378.Ali TK, Amescua G, Miller D, Suh LH, Delmonte DW, Gibbons A, Alfonso EC, Forster RK. 2017. Contact-lens-associated Purpureocillium keratitis: risk factors, microbiologic characteristics, clinical course, and outcomes. Semin Ophthalmol 32:157–162. 10.3109/08820538.2015.1011342. [DOI] [PubMed] [Google Scholar]
- 379.Perlin DS. 2019. Mechanisms of resistance to antifungal agents, p 2334–2350. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 380.Bagga B, Sharma S, Madhuri Guda SJ, Nagpal R, Joseph J, Manjulatha K, Mohamed A, Garg P. 2018. Leap forward in the treatment of Pythium insidiosum keratitis. Br J Ophthalmol 102:1629–1633. 10.1136/bjophthalmol-2017-311360. [DOI] [PubMed] [Google Scholar]
- 381.Mittal R, Jena SK, Desai A, Agarwal S. 2017. Pythium insidiosum keratitis: histopathology and rapid novel diagnostic staining technique. Cornea 36:1124–1132. 10.1097/ICO.0000000000001244. [DOI] [PubMed] [Google Scholar]
- 382.Huang DD, Pinsky BA, Bankowski MJ. 2015. Susceptibility test methods: viruses, p 1985–2006. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 383.Roozbahani M, Hammersmith KM. 2018. Management of herpes simplex virus epithelial keratitis. Curr Opin Ophthalmol 29:360–364. 10.1097/ICU.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 384.Santos CAQ, Lurain N. 2019. Antiviral agents, p 1937–1961. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 385.Snoeck R, Andrei G, Gerard M, Silverman A, Hedderman A, Balzarini J, Sadzot-Delvaux C, Tricot G, Clumeck N, De Clercq E. 1994. Successful treatment of progressive mucocutaneous infection due to acyclovir- and foscarnet-resistant herpes simplex virus with (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC). Clin Infect Dis 18:570–578. 10.1093/clinids/18.4.570. [DOI] [PubMed] [Google Scholar]
- 386.Blot N, Schneider P, Young P, Janvresse C, Dehesdin D, Tron P, Vannier JP. 2000. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant 26:903–905. 10.1038/sj.bmt.1702591. [DOI] [PubMed] [Google Scholar]
- 387.Fujii H, Kakiuchi S, Tsuji M, Nishimura H, Yoshikawa T, Yamada S, Omura N, Inagaki T, Shibamura M, Harada S, Taniguchi S, Saijo M. 2018. Application of next-generation sequencing to detect acyclovir-resistant herpes simplex virus type 1 variants at low frequency in thymidine kinase gene of the isolates recovered from patients with hematopoietic stem cell transplantation. J Virol Methods 251:123–128. 10.1016/j.jviromet.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 388.van Velzen M, van de Vijver DA, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. 2013. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis 208:1359–1365. 10.1093/infdis/jit350. [DOI] [PubMed] [Google Scholar]
- 389.Duan R, de Vries RD, van Dun JM, van Loenen FB, Osterhaus AD, Remeijer L, Verjans GM. 2009. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis 200:1402–1414. 10.1086/606028. [DOI] [PubMed] [Google Scholar]
- 390.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. 2008. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis 198:659–663. 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 391.Schafer R, Boivin G, Chou S. 2019. Mechanisms of resistance to antiviral agents, p 1962–1984. In Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 12th ed. ASM Press, Washington, DC. [Google Scholar]
- 392.Andrei G, Topalis D, Fiten P, McGuigan C, Balzarini J, Opdenakker G, Snoeck R. 2012. In vitro-selected drug-resistant varicella-zoster virus mutants in the thymidine kinase and DNA polymerase genes yield novel phenotype-genotype associations and highlight differences between antiherpesvirus drugs. J Virol 86:2641–2652. 10.1128/JVI.06620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Jabs DA, Van Natta ML, Holland GN, Danis R, Studies of the Ocular Complications of AIDS Research Group . 2017. Cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome after initiating antiretroviral therapy. Am J Ophthalmol 174:23–32. 10.1016/j.ajo.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Bottone EJ. 2010. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23:382–398. 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Shobana CS, Mythili A, Homa M, Galgoczy L, Priya R, Babu Singh YR, Panneerselvam K, Vagvolgyi C, Kredics L, Narendran V, Manikandan P. 2015. In vitro susceptibility of filamentous fungi from mycotic keratitis to azole drugs. J Mycol Med 25:44–49. 10.1016/j.mycmed.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 396.Krizsan K, Toth E, Nagy LG, Galgoczy L, Manikandan P, Chandrasekaran M, Kadaikunnan S, Alharbi NS, Vagvolgyi C, Papp T. 2015. Molecular identification and antifungal susceptibility of Curvularia australiensis, C. hawaiiensis and C. spicifera isolated from human eye infections. Mycoses 58:603–609. 10.1111/myc.12367. [DOI] [PubMed] [Google Scholar]
- 397.Xie L, Zhai H, Zhao J, Sun S, Shi W, Dong X. 2008. Antifungal susceptibility for common pathogens of fungal keratitis in Shandong Province, China. Am J Ophthalmol 146:260–265. 10.1016/j.ajo.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 398.Lalitha P, Shapiro BL, Srinivasan M, Prajna NV, Acharya NR, Fothergill AW, Ruiz J, Chidambaram JD, Maxey KJ, Hong KC, McLeod SD, Lietman TM. 2007. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol 125:789–793. 10.1001/archopht.125.6.789. [DOI] [PubMed] [Google Scholar]