Abstract
Congenital complete heart block is a potentially fatal complication that can occur in neonates whose mothers have autoimmune disorders; it has rarely been reported in the presence of Sjögren syndrome. Pacemaker implantation is recommended to treat rhythm abnormalities in these neonates. We report the case of a late-preterm infant with Sjögren-syndrome-antibody–induced complete heart block who underwent temporary bipolar epicardial pacing as a bridge to permanent pacemaker implantation. Soon after the pacemaker was implanted, takotsubo cardiomyopathy developed. To our knowledge, this is the first report of reversible cardiomyopathy after pacemaker implantation in an infant.
Keywords: Auto-antibodies/analysis; cardiac pacing, artificial; heart block/congenital/therapy; infant, newborn; maternal-fetal exchange; postoperative complications/therapy; Sjögren's syndrome; treatment outcome; takotsubo cardiomyopathy/etiology; ventricular dysfunction, left/etiology/therapy
Congenital complete heart block (CHB), a rare and potentially severe fetal complication of maternal autoimmune conditions such as Sjögren syndrome, is suspected to result from maternal antibody transmission.1 Permanent pacemaker (PPM) placement is definitive treatment for CHB in the presence of symptomatic bradycardia and a resting heart rate <55 beats/min without a structural cardiac defect, or <70 beats/min with a defect.2 Whereas pacemaker-induced cardiomyopathy has been reported in adults after chronic right ventricular (RV) pacing or rhythm changes,3 it occurs only rarely in pediatric patients, especially in the immediate postoperative period.4,5 We report the case of a preterm infant with antibody-induced congenital CHB who underwent PPM treatment, after which takotsubo cardiomyopathy developed.
Case Report
A 32-year-old woman, pregnant for the first time, underwent evaluation of a low fetal heart rate detected on a routine sonogram. An echocardiogram confirmed fetal bradycardia with no structural cardiac abnormality. After testing, the patient was found to have anti-Ro/SSA antibodies and Sjögren syndrome. As a result of placental abruption, she underwent a cesarean section at 34 weeks + 1 day of gestation. The female infant weighed 4 lb, and her Apgar scores were 2 at one min, 6 at 5 min, and 6 at 10 min. The infant was hemodynamically unstable because of symptomatic bradycardia, and she underwent brief intubation for stabilization. An electrocardiogram (ECG) confirmed CHB (atrial rate, 160 beats/min; ventricular rate, 40–50 beats/min) (Fig. 1). Laboratory tests revealed that the infant had anti-Ro/SSA antibodies. An echocardiogram showed mildly depressed RV function and a small patent ductus arteriosus with left-to-right shunt flow, so she was placed on isoproterenol to maintain cardiac output.
Fig. 1.
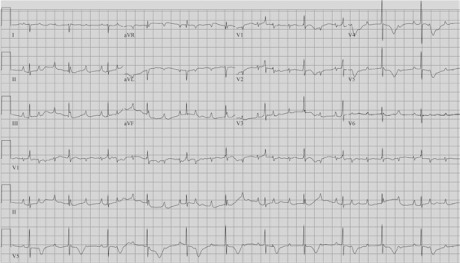
Electrocardiogram on patient's first day of life shows complete heart block.
On day of life (DOL) 7, the patient was to undergo PPM implantation, but her physical limitations and friable tissue prompted us to initiate temporary epicardial pacemaker support as a bridge to PPM. We placed 2 wires each in the right atrium and RV (Fig. 2). The pacing setting was in DDD mode at a heart rate of 120 to 188 beats/min. The pacemaker was checked daily, and the pacing and sensing thresholds were monitored daily. The sensitivity threshold of one atrial wire worsened until atrial capture ceased on DOL 37; unipolar atrial pacing was used thereafter. The bipolar ventricular pacing was reliable.
Fig. 2.
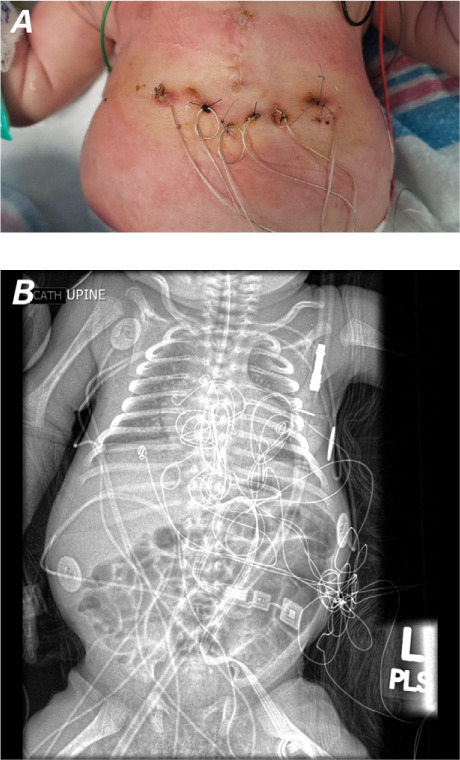
A) Photograph shows positions of the temporary epicardial pacing wires 2 weeks after placement. B) Radiograph shows internal view of the pacing wires.
On DOL 84, when the patient weighed 7.5 lb, an ADDRS1 Adapta S DR pacemaker (Medtronic) was implanted, and bipolar leads were placed in the RV and right atrium. The patient was monitored closely for weight gain during transition to an oral diet.
On postoperative day (POD) 20, a routine echocardiogram revealed focal hypokinesia with left ventricular (LV) apical ballooning and a systolic LV ejection fraction (LVEF) of 39% (Fig. 3); of note, the patient had a stable ECG, normal cardiac troponin and brain natriuretic peptide levels, and anti-Ro/SSA levels that had become nearly undetectable over time (Table I). To help reduce LV afterload, she was prescribed captopril, titrated to 0.3 mg/kg/dose 3×/d. On POD 23, an echocardiogram revealed mild LV depression (LVEF, 53%). No mitral regurgitation was ever apparent.
Fig. 3.
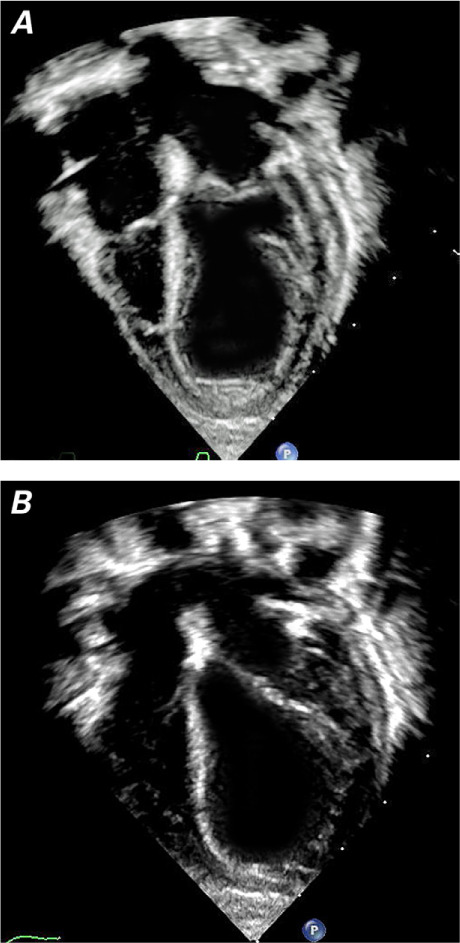
Transthoracic echocardiograms (apical 4-chamber views) show decreased left ventricular function and apical ballooning at A) end-systole and B) end-diastole.
Supplemental motion image is available for Figure 3.
TABLE I.
Serial Anti-Ro/SSA Titers
* Reference range, 0–0.9 U.
** Value on postoperative day 20.
On POD 26, the patient had gained weight on a fully oral diet. She had stable vital signs, and her oxygen saturation level on room air was ≥98%. She was discharged from the hospital with her PPM in DDD mode at a rate of 80 to 120 beats/min and prescribed captopril. At periodic follow-up examinations, she was well, with improved LV systolic function and apical septal motion apparent on an echocardiogram (Fig. 4). At 10 months of age, her LV systolic function had continued to improve (LVEF, 60%).
Fig. 4.
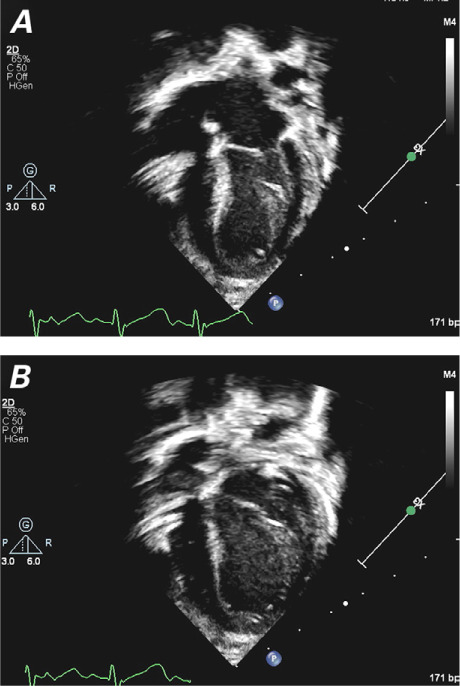
Transthoracic echocardiograms (apical 4-chamber views) at 2 months of age show improved left ventricular function and wall motion at A) end-systole and B) end-diastole.
Discussion
To our knowledge, this is the first report of reversible cardiomyopathy developing within a week of PPM implantation in a premature infant. The patient had CHB at birth and had been bridged to PPM with temporary bipolar epicardial pacing.
Takotsubo cardiomyopathy is characterized by transient LV dysfunction that is usually preceded by stress.5 Prolonged in utero exposure to maternally transmitted autoimmune antibodies is thought to cause calcium channel internalization, disrupt cytoplasmic calcium metabolism, and induce apoptosis. If this inflammatory process continues, calcification and fibrosis of the cardiac conduction system can cause atrioventricular block.6 The prevalence of anti-Ro/SSA antibodies during pregnancy is less than 1 in 100, and CHB occurs in only 2% of those cases.3 The risk of CHB in subsequent pregnancies increases 9-fold, leading to as many as 700 CHB cases per year in the United States.7,8 The overall mortality rate of CHB is 19% before 3 months of age, and fetal hydrops is the major cause of morbidity.9 More than 90% of surviving infants need pacemakers.
Our case illustrates the importance of using a bipolar pacing system as a bridge to PPM placement in selected patients. Temporary epicardial pacing can increase cardiac output and promote atrioventricular synchrony lost during complete CHB. Inflammation around epicardial pacing wires can be exacerbated by high energy from pacing, resulting in lead capture failure.10 Bipolar systems need lower energy than do unipolar systems and thus may last longer.
Using unipolar leads is technically advantageous in smaller patients and cost-effective when resources are limited. Unipolar systems have been used in cases of postoperative heart block to see whether the arrhythmia persists or reverts to sinus rhythm; if the patient is too small, the system can serve as a bridge to enable placement of a PPM.11,12 In addition, unipolar systems have often been used in infants who have CHB10; however, we found only 2 reports of bipolar pacing before PPM.13,14 The second lead attached to subcutaneous tissue enables bipolar systems to be converted to unipolar if one lead fails. Accordingly, we recommend bipolar epicardial pacing in patients like ours who may need longer temporary pacing before PPM implantation.
Differential Diagnosis. Shortly after PPM implantation, our seemingly well patient had apical hypokinesia, LV apical ballooning, and depressed LV function, all without cardiomegaly, ECG changes, or laboratory abnormalities. For that reason, dilated cardiomyopathy secondary to viral myocarditis was a less likely differential diagnosis. The prevalence of pacemaker-induced cardiomyopathy is 10% in patients who have congenital CHB; however, this condition is usually associated with chronic RV pacing or rhythm changes that were not present in our patient.3,15 Antibody-mediated cardiomyopathy was unlikely because our patient's antibody levels were undetectable at the time of cardiomyopathy. Takotsubo cardiomyopathy after PPM implantation is a diagnosis of exclusion and most often reported in adults.4 We found one case each in an infant16 and a neonate. 17 Left ventricular apical ballooning is seen on echocardiograms and typically resolves in days or weeks.15 Because our patient had been on temporary pacing support for 10 weeks, it is unclear why cardiomyopathy developed so soon after PPM placement. Although surgical stress is a reasonable pathophysiologic mechanism given our patient's clinical course, other causes cannot be ruled out, such as prematurity, infection, ventricular pacing, and complications of the underlying congenital CHB. The patient responded well to angiotensin-converting enzyme inhibition with substantial reversal of changes within days.
This case highlights the challenges in managing congenital CHB in a preterm infant with maternal Sjögren syndrome. We found that temporary bipolar epicardial pacing can safely serve as a bridge to PPM placement, and that rare complications such as takotsubo cardiomyopathy can occur.
Supplementary Material
Footnotes
Author contributions: Drs. Iqbal and Umapathi contributed equally to this report and should be considered joint first authors.
References
- 1.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6–75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Balaji S, Sreeram N. The development of pacing induced ventricular dysfunction is influenced by the underlying structural heart defect in children with congenital heart disease. Indian Heart J. 2017;69(2):240–3. doi: 10.1016/j.ihj.2016.11.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Hata T et al. Persistent left ventricular dysfunction in takotsubo cardiomyopathy after pacemaker implantation. Circ J. 2006;70(5):641–4. doi: 10.1253/circj.70.641. [DOI] [PubMed] [Google Scholar]
- 5.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929–38. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 6.Taylor PV, Scott JS, Gerlis LM, Esscher E, Scott O. Maternal antibodies against fetal cardiac antigens in congenital complete heart block. N Engl J Med. 1986;315(11):667–72. doi: 10.1056/NEJM198609113151103. [DOI] [PubMed] [Google Scholar]
- 7.Brucato A, Frassi M, Franceschini F, Cimaz R, Faden D, Pisoni MP et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44(8):1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Llanos C, Izmirly PM, Katholi M, Clancy RM, Friedman DM, Kim MY, Buyon JP. Recurrence rates of cardiac manifestations associated with neonatal lupus and maternal/fetal risk factors. Arthritis Rheum. 2009;60(10):3091–7. doi: 10.1002/art.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeggi ET, Hamilton RM, Silverman ED, Zamora SA, Hornberger LK. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block: a single institution's experience of 30 years. J Am Coll Cardiol. 2002;39(1):130–7. doi: 10.1016/s0735-1097(01)01697-7. [DOI] [PubMed] [Google Scholar]
- 10.Reade MC. Temporary epicardial pacing after cardiac surgery: a practical review: part 1: general considerations in the management of epicardial pacing [published erratum appears in Anaesthesia 2007;62(6):644] Anaesthesia. 2007;62(3):264–71. doi: 10.1111/j.1365-2044.2007.04950.x. [DOI] [PubMed] [Google Scholar]
- 11.Batra AS, Balaji S. Post operative temporary epicardial pacing: when, how and why? Ann Pediatr Cardiol. 2008;1(2):120–5. doi: 10.4103/0974-2069.43877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippi L, Vangi V, Murzi B, Moschetti R, Colella A. Temporary epicardial pacing in an extremely low-birth-weight infant with congenital atrioventricular block. Congenit Heart Dis. 2007;2(3):199–202. doi: 10.1111/j.1747-0803.2007.00098.x. [DOI] [PubMed] [Google Scholar]
- 13.Deloof E, Devlieger H, Van Hoestenberghe R, Van den Berghe K, Daenen W, Gewillig M. Management with a staged approach of the premature hydropic fetus due to complete congenital heart block. Eur J Pediatr. 1997;156(7):521–3. doi: 10.1007/s004310050652. [DOI] [PubMed] [Google Scholar]
- 14.Di Mauro A, Caroli Casavola V, Favia Guarnieri G, Calderoni G, Cicinelli E, Laforgia N. Antenatal and postnatal combined therapy for autoantibody-related congenital atrioventricular block. BMC Pregnancy Childbirth. 2013;13:220. doi: 10.1186/1471-2393-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelliccia F, Parodi G, Greco C, Antoniucci D, Brenner R, Bossone E et al. Comorbidities frequency in takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med. 2015;128(6):654.e11–9. doi: 10.1016/j.amjmed.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Okada S, Azuma Y, Kaneyasu H, Mizutani M, Korenaga Y, Kittaka S et al. Takotsubo (stress) cardiomyopathy and obstructive renal stones in an infant with norovirus gastroenteritis. Int J Cardiol. 2015;186:233–5. doi: 10.1016/j.ijcard.2015.03.199. [DOI] [PubMed] [Google Scholar]
- 17.Greco CA, De Rito V, Petracca M, Garzya M, Donateo M, Magliari F. Takotsubo syndrome in a newborn. J Am Soc Echocardiogr. 2011;24(4):471.e5–7. doi: 10.1016/j.echo.2010.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


