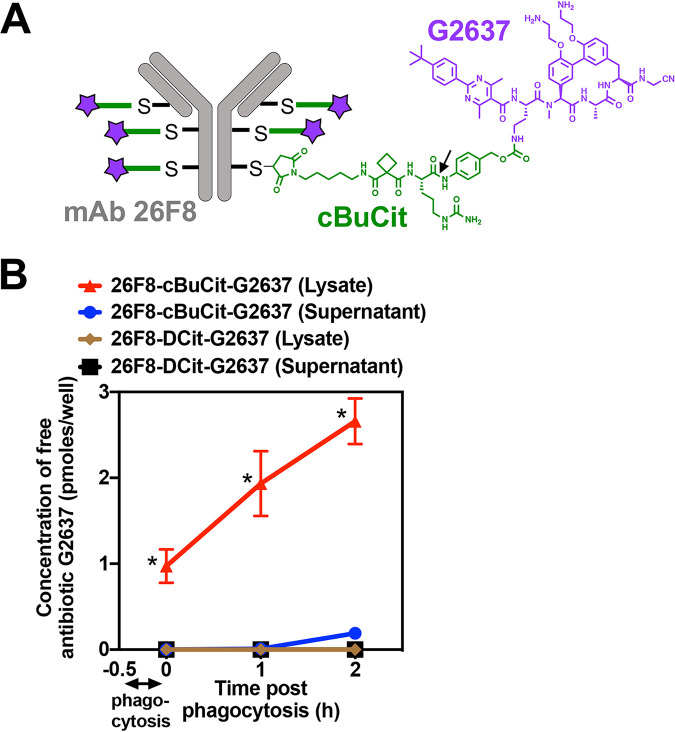
FIG 2.
Composition of the AAC molecule and delivery of high AAC-released intracellular concentrations of free G2637 antibiotic into macrophages. (A) Schematic of the 26F8-cBuCit-G2637 AAC molecule, composed of MAb 26F8 (gray), which recognizes the LPS O antigen of P. aeruginosa PA14, cathepsin-cleavable cBuCit linker (green), and the arylomycin analog antibiotic G2637 (purple), with a drug-to-antibody ratio of 6. The arrow indicates the cathepsin cleavage site. (B) Determination of the amount of free AAC-released antibiotic G2637 in cell lysates or supernatants after phagocytosis of AAC-preincubated P. aeruginosa PA14 WT by LC-MS/MS analysis. When PA14 WT bacteria were incubated with 26F8-cBuCit-G2637, free G2637 was detected in lysates immediately after 30 min of phagocytosis by macrophages and removal of extracellular bacteria (0 h); this value increased during 2 h of subsequent incubation following phagocytosis, suggesting continued intracellular cleavage of the linker. Free G2637 was hardly detectable in the extracellular cell supernatant, indicating prolonged intracellular retention of the free AAC-released antibiotic. The AAC 26F8-DCit-G2637, which contains the noncleavable DCit linker, did not release detectable intracellular G2637. Value are averages ± SD for technical triplicates; asterisks indicate significant differences (P < 0.01) from values for 26F8-DCit-G2637.