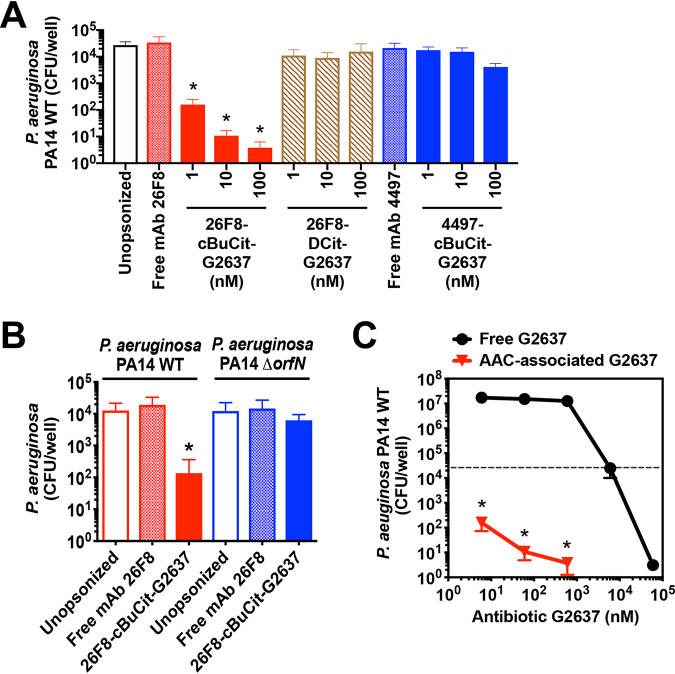
FIG 3.
26F8-cBuCit-G2637 AAC induces potent intracellular killing of P. aeruginosa in macrophages. To determine anti-P. aeruginosa potency of AAC, P. aeruginosa bacteria were preincubated with AAC (1, 10, or 100 nM) or with free MAb (100 nM) and added to macrophages to induce phagocytosis. After addition of gentamicin to remove extracellular bacteria, macrophages were subsequently incubated for an additional 6 h at 37°C to enable bacterial killing, followed by macrophage lysis and CFU enumeration. (A) The 26F8-cBuCit-G2637 AAC (solid red bars) induced potent and dose-dependent intracellular killing of P. aeruginosa PA14 WT bacteria. In contrast, the viability of intracellular P. aeruginosa was hardly affected when bacteria were left unopsonized (empty bar) or preincubated with noncleavable 26F8-DCit-G2637 DAR6 AAC (hatched bars) or 4497-cBuCit-G2637 DAR6, which contains anti-S. aureus MAb 4497 (solid blue bars), or with free anti-P. aeruginosa MAb 26F8 (cross-hatched red bar) or free MAb 4497 (cross-hatched blue bar). (B) The 26F8-cBuCit-G2637 AAC promoted intracellular killing of P. aeruginosa PA14 WT (solid red bar) but not of the P. aeruginosa PA14 ΔorfN mutant (solid blue bar), which lacks the LPS O-antigen required for binding of MAb 26F8 (Fig. 1D and E). (A and B) Data are averages and SD for biological triplicates; asterisks indicate statistical significance (P < 0.05) compared to the CFU value of each condition immediately after phagocytosis (values are plotted in Fig. S3). (C) PA14 WT bacteria were preincubated with 26F8-cBuCit-G2637 AAC, followed by determination of killing 6 h postphagocytosis as for panel A (red triangles), or were incubated with free G2637 antibiotic for 6 h in the same medium without macrophages (black circles); viability of bacteria is expressed as CFU per well. 26F8-cBuCit-G2637-associated G2637 antibiotic required a molar concentration to kill P. aeruginosa PA14 WT approximately 2 orders of magnitude lower than that of free extracellular antibiotic G2637. The dashed line indicates input bacterial concentration at the start of the assay before the 6 h of incubation, which was in the same range for both conditions (approximately 2 × 104 to 5 × 104/ml). Value are averages ± SD for biological triplicates; asterisks indicate significant differences (P < 0.01) compared to free G2637 for each concentration.