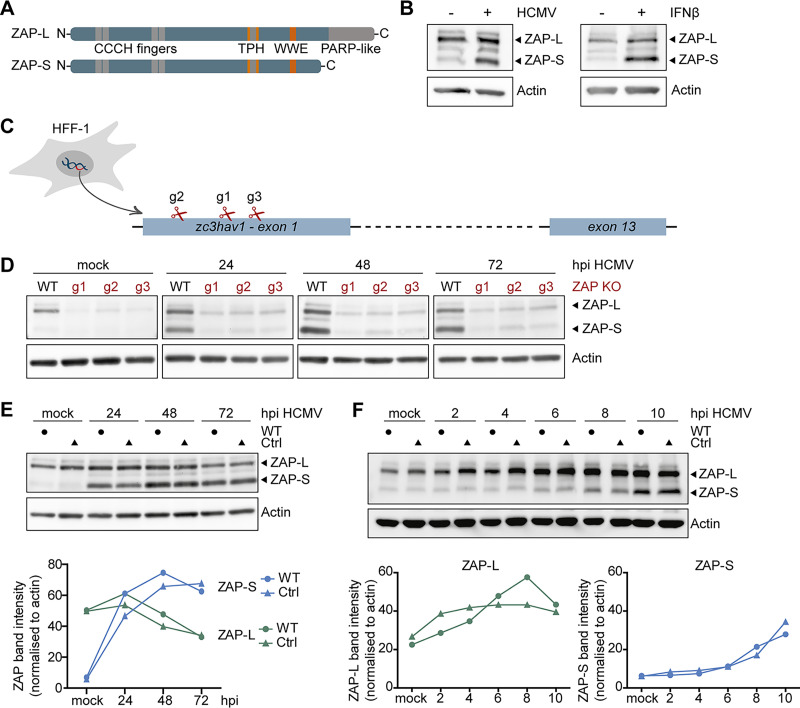
FIG 1.
Expression kinetics of ZAP-S and ZAP-L in HCMV-infected fibroblasts. (A) Schematic representation of the protein domains of the two main isoforms of ZAP, the long isoform, ZAP-L, and the short isoform, ZAP-S. Both isoforms share four CCCH-type zinc finger motifs in the N-terminal domain, as well as a TPH domain containing a fifth zinc finger motif, and a WWE domain, while the C-terminal PARP-like domain is only present in ZAP-L. TPH, TiPARP homology domain; PARP, poly(ADP-ribose)-polymerase. (B) Primary human fibroblasts (HFF-1) were either mock-treated, infected by centrifugal enhancement with HCMV (MOI 0.1), or stimulated by addition of recombinant IFN-β (20 ng/ml), and expression of ZAP and actin was analyzed 24 h later by immunoblotting with a ZAP- or actin-specific antibody. (C) Three independent ZAP KO cell lines were generated by Cas9-mediated gene editing using three different gRNAs (g1, g2, and g3) that target the first exon of the zc3hav1 gene. (D to F) Wild-type HFF-1 and the three ZAP KO (D) or control (E and F) cell lines were mock-treated or infected by centrifugal enhancement with HCMV (MOI 0.1) for the indicated time points, and cell lysates were subjected to immunoblotting with specific antibodies against ZAP and actin. Quantifications of ZAP-L (in green) or ZAP-S (in blue) band intensities normalized to actin are represented in line graphs. hpi, hours postinfection.