ABSTRACT
Filamentous hemagglutinin (FhaB) is a critical virulence factor for both Bordetella pertussis, the causal agent of whooping cough, and the closely related species Bordetella bronchiseptica. FhaB is an adhesin, suppresses inflammatory cytokine production, and protects against phagocytic cell clearance during infection. Regulated degradation of the FhaB C-terminal prodomain is required to establish a persistent infection in mice. Two proteases, CtpA in the periplasm and SphB1 on the bacterial surface, are known to mediate FhaB processing, and we recently determined that CtpA functions before, and controls the FhaB cleavage site of, SphB1. However, the data indicate that another periplasmic protease must initiate degradation of the prodomain by removing a portion of the FhaB C terminus that inhibits CtpA-mediated degradation. Using a candidate approach, we identified DegP as the initiating protease. Deletion of degP or substitution of its predicted catalytic residue resulted in reduced creation of FHA′ (the main product of FhaB processing) and an accumulation of full-length FhaB in whole-cell lysates. Also, FHA′ was no longer released into culture supernatants in degP mutants. Alterations of the FhaB C terminus that relieve inhibition of CtpA abrogate the need for DegP, consistent with DegP functioning prior to CtpA in the processing pathway. DegP is not required for secretion of FhaB through FhaC or for adherence of the bacteria to host cells, indicating that DegP acts primarily as a protease and not a chaperone for FhaB in B. bronchiseptica. Our results highlight a role for HtrA family proteases in activation of virulence factors in pathogenic bacteria.
KEYWORDS: Bordetella, DegP, filamentous hemagglutinin, regulated proteolysis, two-partner secretion
INTRODUCTION
Gram-negative bacteria from the genus Bordetella cause highly contagious respiratory infections in mammals that have adverse impacts on public health and industrial agriculture (1). The human-specific pathogen Bordetella pertussis causes pertussis, also known as whooping cough, and is responsible for infecting an estimated 24 million children annually, causing 160,000 deaths (2). A majority of pertussis cases are from areas of the world with low (<85%) vaccine coverage. However, pertussis incidence has been increasing over the last 30 years even in areas where >90% of the population has received three doses of the dTap/Tdap vaccines, including the United States (3). The resurgence of pertussis correlates with the switch from whole-cell (wP) vaccines to acellular (aP) vaccines, which are composed of up to four purified B. pertussis proteins. While the increased incidence of pertussis is in part due to abiologic factors, such as improved diagnosis and case reporting, studies using baboons indicate that although wP and aP vaccines protect against disease, neither prevents B. pertussis colonization, and the aP vaccine also failed to prevent transmission (4).
The most widely used aP vaccine contains pertussis toxin, pertactin, fimbriae subunits, and a proteolytically processed version of filamentous hemagglutinin (FhaB) called FHA. FhaB is produced by all Bordetella species that infect mammals and is required for bacterial adherence to eukaryotic cells (5–7). Infection of small rodents with Bordetella bronchiseptica, a species closely related to B. pertussis that produces a nearly identical set of virulence factors, indicate that FhaB is functionally interchangeable between B. bronchiseptica and B. pertussis and that FhaB is required for persistent infection in the mammalian lower respiratory tract (LRT) (8–11). The mechanism by which FhaB mediates protection from inflammatory clearance is not understood (11).
FhaB is a member of the broadly distributed two-partner secretion (TPS, also known as type Vb) family of bacterial secretion systems (12–14). It is synthesized as a 379-kDa preproprotein that possesses an extended N-terminal signal sequence that mediates Sec-dependent delivery to the periplasm (15, 16). Following removal of the signal sequence, the conserved N-terminal TPS domain of FhaB interacts with the periplasmic POTRA domains of its cognate transporter FhaC to begin translocation of FhaB across the outer membrane (OM) (17–19). While the FhaB N terminus remains anchored to FhaC, two-thirds of FhaB is secreted through FhaC in the N- to C-terminal direction, emerging on the bacterial surface as a hairpin consisting of a β-helical shaft that is topped by the globular mature C-terminal domain (MCD) (20) (Fig. 1A). The remaining C-terminal third of FhaB (∼1,200 amino acids), called the prodomain (PD), is retained in the periplasm by the prodomain N terminus (PNT), which prevents further secretion through FhaC (20). FhaB undergoes complex regulated processing by multiple proteases. In B. bronchiseptica, the tail-specific family member CtpA mediates degradation of the prodomain, resulting in the generation of the 250-kDa product FHA′, which stays membrane-associated temporarily but is ultimately released from the bacterial surface (Fig. 1A). Under certain conditions (i.e., overnight growth in rich medium), FHA′ can be cleaved by the surface-anchored exoprotease SphB1 to produce the 243-kDa product FHA (Fig. 1A, dashed arrow), which is immediately released. The biological significance of SphB1-dependent cleavage is unclear. Although initially identified as a protease mediating release of FHA (21, 23), it has subsequently been shown that FhaB polypeptides lacking the prodomain, such as FHA′, are efficiently released by both wild-type and SphB1-deficient strains of B. bronchiseptica (12, 22). The fully processed protein (i.e., FHA) was long considered the functional molecule, as the PD is thoroughly degraded and not detectable as a standalone protein (11). However, full-length FhaB plays a vital role during infection, as mutants lacking portions of the PD are rapidly cleared from the murine LRT despite being able to adhere to host cells as effectively as wild-type B. bronchiseptica (11). These findings suggest that full-length FhaB is required specifically for defense against inflammation-mediated clearance from the LRT, and we hypothesize that this activity requires properly regulated degradation of the FhaB PD.
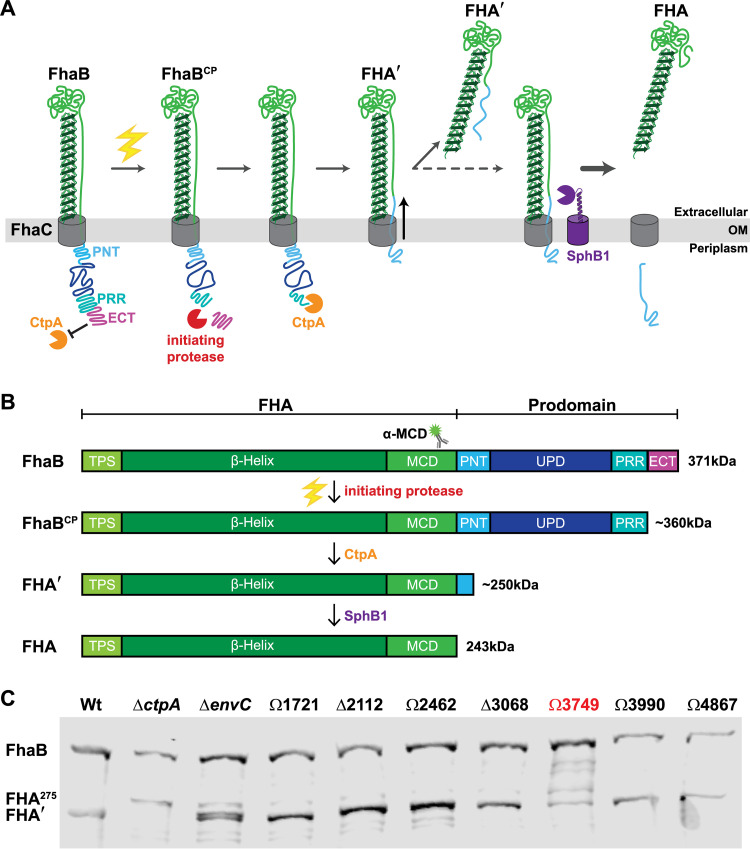
FIG 1.
A screen of FhaB processing in B. bronchiseptica strains that carry mutations of predicted extracytoplasmic proteases identifies BB3749 as encoding a putative FhaB protease. (A) Our current model of regulated FhaB processing by three proteases. An unknown signal (electric bolt) causes the initiating protease to remove the extreme C terminus (ECT) that acts as a guard against CtpA. This clipped product (FhaBCP) is recognized by CtpA, which degrades most of the prodomain to form FHA′. Removal of a portion of the PNT by CtpA causes a shift in the molecule that allows the release of FHA′ into the supernatant. Under certain conditions this shift also exposes the primary SphB1 cleavage site and allows SphB1 to create FHA (dashed arrow), which is immediately released. (B) Linear schematics of FhaB (without the extended signal sequence) and the main polypeptides that result from processing by each protease. Molecular weights (kDa) are listed on the right. (C) Western blot analysis of whole-cell lysates (WCL) from overnight cultures of B. bronchiseptica strains with deletions of (Δ) or plasmid disruptions in (Ω) the indicated ORFs. The antigenic region of the α-MCD polyclonal antibody is indicated by the cartoon of a fluorescent labeled antibody in panel B.
Previous work from our laboratory determined that the last 98 amino acids of FhaB, called the “extreme C terminus” (ECT), negatively regulate CtpA-dependent FhaB processing (11). While characterizing CtpA, we identified a novel polypeptide intermediate called the “FhaB clipped product” (FhaBCP), which lacks ∼90 amino acids (i.e., most of the ECT) from the C terminus (22). FhaBCP is stabilized in whole-cell lysates (WCLs) harvested from B. bronchiseptica ΔctpA cultures, consistent with the discovery that the native FhaB C terminus is a poor substrate for CtpA (22) and the hypothesis that FhaBCP is the preferred substrate for CtpA. The presence of FhaBCP in ΔctpA WCLs indicates that yet another protease, which we are calling the initiating protease, is responsible for converting FhaB into FhaBCP through removal of the ECT. According to our model, CtpA degrades FhaBCP to FHA′, which is either released from the bacterial surface or, if the bacteria are grown for extended periods of time, is cleaved by SphB1 to produce FHA, which is then immediately released (Fig. 1A). We hypothesize that cleavage of the PD to form FhaBCP is the critical control point, as it initiates PD degradation. The goal of this study was to identify the initiating protease and to determine its contribution to FhaB processing and function.
RESULTS
BB3749 (degP) appears to encode the initiating protease.
To identify candidates for the initiating protease, we searched the genome of wild-type B. bronchiseptica strain RB50 for open reading frames (ORFs) predicted to encode proteases containing N-terminal Sec or TAT signal peptides. The 11 ORFs identified included those encoding both of the previously characterized proteases involved in processing FhaB, sphB1 (21) and ctpA (22), as well as nine predicted to encode endopeptidases and carboxy-terminal-acting peptidases (Table 1). We generated B. bronchiseptica strains containing either an in-frame deletion (ΔBB0299, ΔBB3068, ΔBB2112) or an integrated plasmid disruption (ΩBB1398, ΩBB1721, ΩBB2462, ΩBB3749, ΩBB3990, ΩBB4867) in one of the nine ORFs and examined FhaB/FHA′ in whole-cell lysates (WCL) using α-MCD-specific antisera (Fig. 1C). We included as a control the strain carrying an in-frame deletion of ctpA (ΔctpA), which has been previously shown to be required for normal FhaB processing (Fig. 1C). Instead of processing FhaB to the 250 kDa FHA′ product, the ΔctpA strain converts FhaB to the larger FHA275 polypeptide, likely due to aberrant processing by as-yet-unidentified proteases (22). Two of the mutants displayed altered FhaB processing compared to the wild-type strain. BB0299 (envC) is located directly 5′ to, and is cotranscribed with, ctpA (22) and is predicted to encode an M23 family peptidase with a degenerate LytM (dLytM) domain. dLytM homologs in other species, such as EnvC in Escherichia coli, serve as accessory factors that allosterically activate cell wall cleaving hydrolases (24, 25). The phenotype of the ΔenvCBb mutant is similar, but not identical, to that of the ΔctpA strain, suggesting that EnvCBb may function as an accessory factor promoting CtpA activity (Fig. 1C). As envC is in an operon with ctpA, it is also possible that the in-frame deletion mutation in envC could alter expression of ctpA. Characterizing the precise role of EnvCBb in FhaB processing is beyond the scope of the current work and will be addressed with future studies.
TABLE 1.
ORFs encoding initiating protease candidates
| ORF | Superfamily |
|---|---|
| BB0299 (envC) | M23-dLytM |
| BB1398 | M23 |
| BB1721 | Imelysin-like |
| BB2112 | M48_M56 |
| BB2462 | M48_M56 |
| BB3068 | M14c |
| BB3749 (degP) a | HtrA a |
| BB3990 | YfgC |
| BB4867 | HtrA |
Boldface type indicates the candidate ORF of interest.
The strain containing a disruption in BB3749 (ΩBB3749) displayed the expected phenotype for loss of initiating protease activity, i.e., a majority of the FhaB protein remained as the full-length ∼371 kDa form (Fig. 1C). We observed partially degraded FhaB polypeptides in ΩBB3749 WCLs that are not present in lysates of wild-type bacteria, suggesting that in the absence of the BB3749 gene product, unidentified proteases inefficiently degrade FhaB following overnight growth. BB3749 is predicted to encode a high-temperature requirement A (HtrA) family serine protease. Prokaryotic HtrA proteases are extracytoplasmic enzymes initially identified as factors necessary for bacterial survival at elevated temperatures (26–28). They contain trypsin-like serine protease domains at their N termini, and either one or two protein-binding PDZ domains at their C termini. The protein encoded by BB3749 is predicted to contain a serine protease domain with a conserved catalytic triad (a GNSGG motif and nearby histidine and aspartic acid residues), as well as two PDZ domains (Fig. 2A). Although annotated as a homolog of mucD, which encodes a protease responsible for regulating mucoidy (due to alginate overproduction) in Pseudomonas aeruginosa (Fig. S1 in the supplemental material), BB3749 is not associated with mucoidy in B. bronchiseptica. Moreover, the amino acid sequence of the protein encoded by BB3749 is 99.8% identical to that of B. pertussis DegP (29). Hence, we will here refer to BB3749 as degP (or degPBb when distinguishing it from homologs is necessary) and its protein product as DegP (or DegPBb).
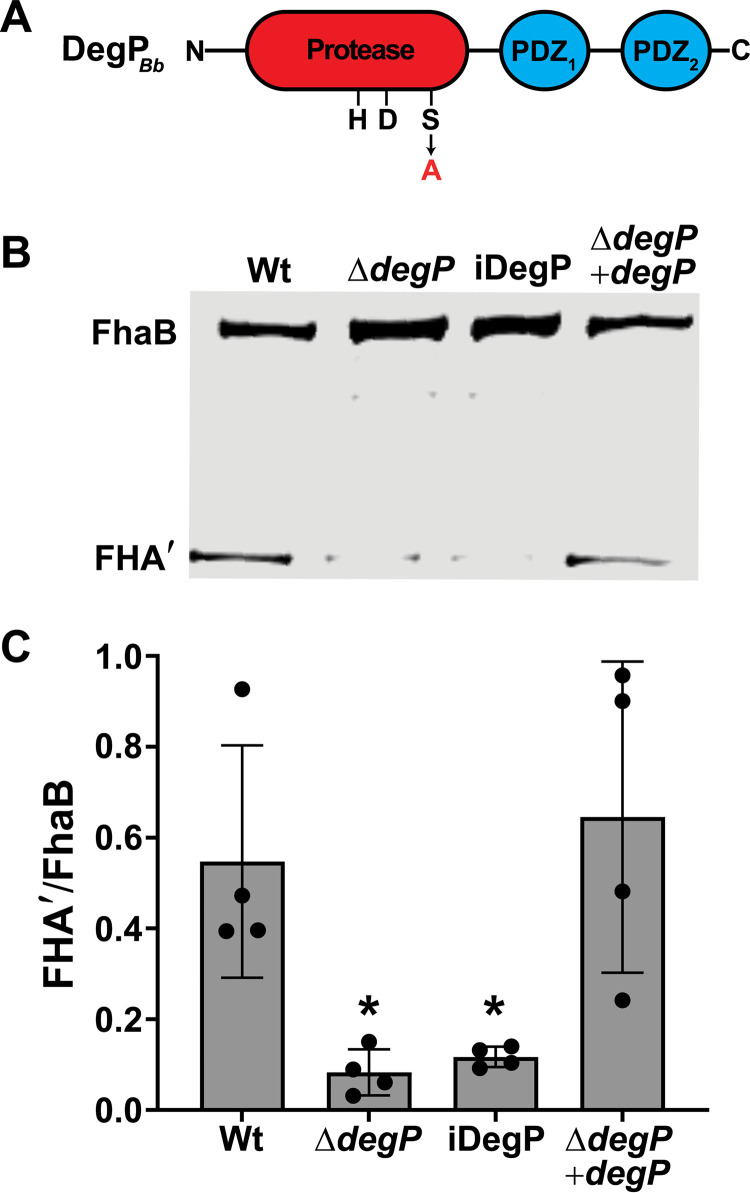
FIG 2.
BB3749 encodes the HtrA family serine protease DegP, and its catalytic activity is required for efficient FhaB processing. (A) Diagram of DegPBb with the catalytic triad (HDS) and two PDZ domains indicated. The catalytic serine at position 237 was changed to an alanine to generate the inactive (iDegP) strain. (B) Western blot analysis of WCL from cultures grown for 4 h in BvgAS-activating medium (SS) after overnight growth in BvgAS-inactivating medium (SS + 20mM MgSO4). (C) Ratio of processed FHA′ to full-length FhaB in the Western blot in panel B and three similar blots. The significant difference of P < 0.05 (as determined by unpaired two-tailed t test) is indicated by an asterisk.
To determine if the FhaB processing defect displayed by the ΩdegP strain was due to the loss of DegP (and not polar effects of the integrated plasmid), we constructed a strain in which codons 6 to 491 of degP were deleted (ΔdegP). We also created a derivative of ΔdegP in which a wild-type copy, driven by the constitutive S12 promoter, was inserted at the attTn7 site (ΔdegP +degP). We grew the bacteria overnight in medium containing 20 mM MgSO4 (which inactivates the BvgAS regulatory system that activates expression of fhaB and other virulence factor-encoding genes) and then outgrew the bacteria in medium without MgSO4 (BvgAS-activating conditions) for 4 h to induce production of FhaB before collecting WCLs, as previously described (22). As with the ΩdegP strain, nearly all of the FhaB protein in the ΔdegP strain was the full-length ∼371 kDa form, and normal processing was restored in the complemented strain (Fig. 2B and C). The intermediate FhaB products that were apparent in WCLs from the ΩBB3749 overnight cultures were less prominent in the WCLs from ΔdegP after 4 h of growth under BvgAS-activating conditions, indicating that these aberrant products accumulate only after extended periods of growth (Fig. 2B). For the remainder of this study we examined FhaB processing following 4 h of growth under BvgAS-activating conditions.
To determine if the catalytic activity of DegP is required for FhaB processing, we constructed a strain (iDegP) in which codon 237 was changed to encode alanine instead of the predicted catalytic serine. The FhaB/FHA′ profile of the iDegP mutant was nearly identical to that of the ΔdegP mutant, indicating that Ser237 and, most likely, the proteolytic activity of DegP are required for efficient FhaB processing (Fig. 2B and C).
DegP is required for B. bronchiseptica growth at elevated temperatures.
DegP homologs from other bacterial species, including B. pertussis (29), are required for bacterial survival and growth at elevated temperatures. To determine if B. bronchiseptica also requires DegP to grow under heat stress, we inoculated BG blood agar plates with wild-type, ΔdegP, iDegP, and ΔdegP +degP strains and incubated them at either 37°C or 42°C for 72 h. Both the ΔdegP and iDegP mutant strains were attenuated for growth at 42°C but not at 37°C (Fig. S2). Failure of the ΔdegP strain to grow at 42°C was restored by expression of a wild-type copy of degP in trans (ΔdegP +degP, Fig. S2). These data indicate that DegP, and its catalytic activity, are required for B. bronchiseptica to grow at 42°C. BvgAS activates (either directly or indirectly) the expression of all genes encoding Bordetella’s known protein virulence factors, including fhaB (30). Many of these factors are surface-localized or secreted into the extracellular environment and most, if not all, are produced at a high level when BvgAS is active. The severe growth defect displayed by degP mutants in response to elevated temperature was greatly alleviated in bacteria grown in the presence of 50 mM MgSO4, which inactivates BvgAS. Magnesium ions (Mg2+) are crucial for envelope stability (31), so we also grew all four strains on plates containing 50 mM MgCl2. Unlike MgSO4, which rescued the growth of the mutant strains, addition of MgCl2 had no effect on bacterial growth at 42°C. Thus, these data suggest the requirement for DegP under heat stress conditions is primarily due to its proteolytic activity on one or more BvgAS-activated virulence factors (29, 32).
DegP acts first in stepwise processing of the prodomain.
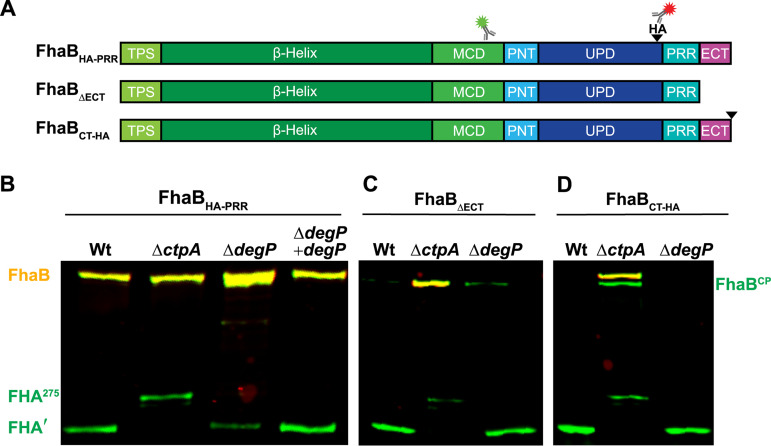
Our current model for FhaB processing involves three proteases: two periplasmic enzymes, CtpA and the initiating protease, and one exoprotease, SphB1 (Fig. 1A). We previously determined that CtpA and SphB1 function in series, with CtpA acting prior to, and determining the cleavage site of, SphB1 (22). In fact, our data suggest that SphB1-dependent processing only occurs after extended time in culture and may have no functional relevance under normal (in vivo) circumstances. We have also shown previously that the ECT inhibits CtpA from degrading the PD, as deletion of the ECT (ΔECT) causes rapid and thorough conversion of FhaB to FHA′ (which we call hyperprocessing) in a CtpA-dependent manner (11, 22). We hypothesized that the initiating protease is responsible for removing the ECT to convert full-length FhaB into the CtpA substrate FhaBCP. To test this hypothesis, we first showed (as we have done previously [22]) that addition of an HA epitope N-terminal to the PRR (Fig. 3A, strain FhaBHA-PRR) has no effect on FhaB processing (Fig. 3B, both full-length FhaB and FHA′ are present in WCL of the FhaBHA-PRR strain). Deletion of degP in this strain resulted in an accumulation of full-length FhaBHA-PRR and a reduced amount of FHA′, just as it did in wild-type bacteria (Fig. 2), and complementation with wild-type degP restored FhaB processing (Fig. 3B). Deletion of ctpA does not alter the abundance of full-length FhaB in WCL, and results in the formation of FHA275 (Fig. 3B and reference 22). FHA275 is an ∼275 kDa polypeptide containing an incompletely degraded PD. We next examined if degP is required for the hyperprocessing of FhaB that occurs in the absence of the ECT. In wild-type bacteria producing FhaB lacking the ECT (FhaBΔECT, Fig. 3A), full-length FhaB is barely detectable (Fig. 3C). The same was true for the ΔdegP strain (Fig. 3C), indicating that DegP does not contribute to the hyperprocessing of FhaB lacking the ECT. In contrast, full-length FhaBΔECT was abundant in the ΔctpA strain (Fig. 3C, yellow band, as this strain also contains an HA epitope immediately N-terminal to the PRR), showing that the hyperprocessing of FhaBΔECT is CtpA-dependent (22). These data indicate that DegP acts prior to CtpA and is required for removal of the ECT to allow degradation of the prodomain by CtpA.
FIG 3.
DegP removes the ECT to permit degradation of the prodomain by CtpA. (A) Linear schematic of FhaBHA-PRR, a version of FhaB that contains an HA tag (black wedge) inserted N-terminal to the proline rich region (PRR). The HA tag at this location is not disruptive to normal FhaB processing. FhaBΔECT lacks the C-terminal 98 amino acids (ECT). FhaBCT-HA has the HA tag located at the C terminus. (B) Western blot analysis of WCLs from strains grown for 4 h in BvgAS-activating medium after overnight growth in BvgAS-inactivating medium. Full-length molecules (FhaBHA-PRR and FhaBCT-HA appear yellow due to recognition by both α-MCD (green) and α-HA (red) antibodies. The intermediate polypeptide FhaBCP and final product FHA appear green due to loss of the HA tag. In the absence of CtpA, a 275 kDa polypeptide, FHA275, is created rather than FHA′.
Similar to deleting the ECT and, in contrast to inserting an HA epitope N-terminal to the PRR, adding an HA epitope to the C terminus of FhaB (FhaBCT-HA) results in CtpA-dependent hyperprocessing (Fig. 3D and reference 22), i.e., this epitope somehow converts the FhaB C terminus into a substrate for CtpA. We reasoned that addition of the HA epitope to the C terminus could impact FhaB processing in two ways: (i) the HA epitope itself could be a substrate for the tail-specific protease CtpA and/or (ii) addition of the HA epitope could disrupt proper folding of the ECT, causing it to be degraded by DegP. Deletion of degP did not prevent hyperprocessing of FhaBCT-HA, indicating that DegP is not required for CtpA-dependent degradation of the full-length FhaBCT-HA and that CtpA proteolytic activity is not dependent on DegP. Consistent with CtpA-dependent degradation of FhaBCT-HA, we observed an accumulation of the full-length polypeptide (371 kDa yellow band) in WCLs from the ΔctpA strain. We also detected the FhaBCP in ΔctpA WCLs, which we believe is the product formed following removal of the ECT by the initiating protease (∼362 kDa green band). The presence of the FhaBCP in ΔctpA FhaBCT-HA and not in ΔctpA FhaBHA-PRR WCLs indicates that the addition of the C-terminal HA epitope promotes removal of the ECT-HA by the initiating protease (DegP). Taken together, these data indicate that DegP acts first in stepwise processing of the PD.
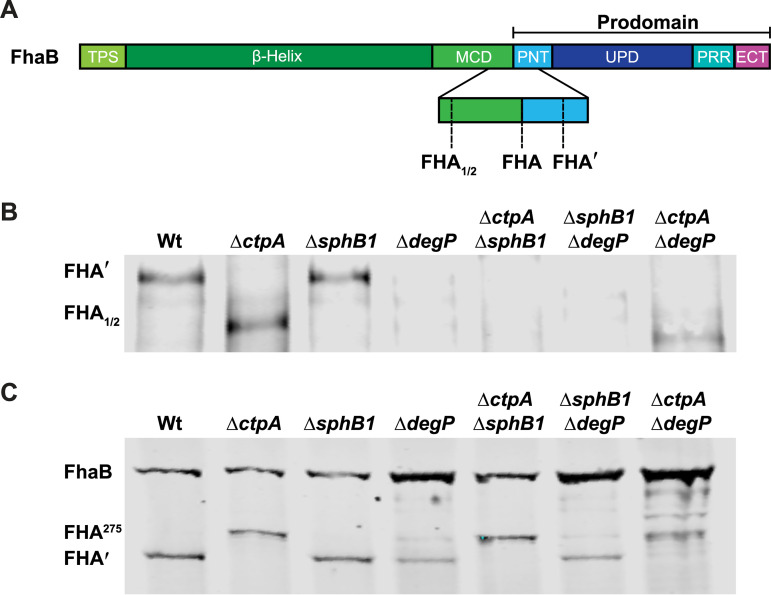
DegP is required for release of FhaB-derived polypeptides from the bacterial surface.
B. bronchiseptica efficiently releases FhaB-derived polypeptides into culture supernatants and the specific polypeptide released depends on the age of the culture and the proteases produced by the bacterial strain (Fig. 4B). Wild-type B. bronchiseptica primarily releases FHA′ during the first 6 h in culture under BvgAS-activating conditions, before SphB1 begins to cleave accumulated FHA′ to cause the formation and immediate release of FHA (22) (Fig. S3). In the absence of CtpA, FHA′ is not produced and, instead, FhaB is converted to the aberrant FHA275 product, which is retained on the cell surface. FHA275 cannot be released due to the presence of the complete PNT. However, FhaB or FHA275 can be cleaved by SphB1 promiscuously at nonpreferred sites to form the smaller polypeptides FHA1/2, which are immediately released (20, 22) (Fig. 4A and B). None of the processed polypeptides (FHA′, FHA, or FHA1/2) were detected in significant amounts in the ΔdegP mutant, demonstrating that DegP is critically important for release of FhaB-derived polypeptides.
FIG 4.
DegPBb is required for release of FhaB-derived polypeptides. (A) Diagram depicting the C termini of the FhaB-derived polypeptides released by B. bronchiseptica. (B and C) Western blot analyses of culture supernatants (B) and WCLs (C) of strains grown for 4 h in BvgAS-activating medium after overnight growth in BvgAS-inactivating medium. Blots were probed with an α-MCD antibody.
As shown in Fig. 4B and in our previous publication (22), either CtpA or SphB1 is required for release of FhaB-derived polypeptides. In the absence of DegP, however, neither CtpA nor SphB1 is sufficient; FHA′ and/or FHA1/2 were barely detectable in supernatants of any strain containing the ΔdegP mutation (Fig. 4B). These results reinforce the conclusion that DegP-mediated cleavage of FhaB occurs first and is required for subsequent degradation by CtpA and then cleavage by SphB1. When considered in conjunction with the WCL data (Fig. 4C), these data are also consistent with our model (12) (Fig. 1 and depicted in greater detail in Fig. S3), which states that the polypeptide formed in the absence of CtpA (FHA275) cannot be released but can serve as a poor substrate for SphB1, cleavage by which generates FHA1/2 (because the PNT prevents movement through the FhaC channel that would allow the preferred SphB1 cleavage site to be exposed on the surface), which is immediately released (Fig. 4A and B). It is important to note that the supernatant samples have been concentrated substantially compared to the WCLs. Therefore, while levels of FHA1/2 in supernatants of the ΔctpA strain appear to be similar to levels of FHA′ in supernatants of wild-type bacteria, no FHA1/2 was detected in WCLs of the ΔctpA mutant and a substantial amount of FHA′ was present in WCLs of wild-type bacteria, indicating that in the absence of CtpA, generation of FHA1/2 by SphB1 is less efficient than generation of FHA′ by wild-type bacteria. In the absence of DegP, FHA275 is not formed and only a small amount of FHA′ is generated, which cannot be detected in culture supernatants (Fig. 4B).
DegP is not required for translocation of FhaB through FhaC.
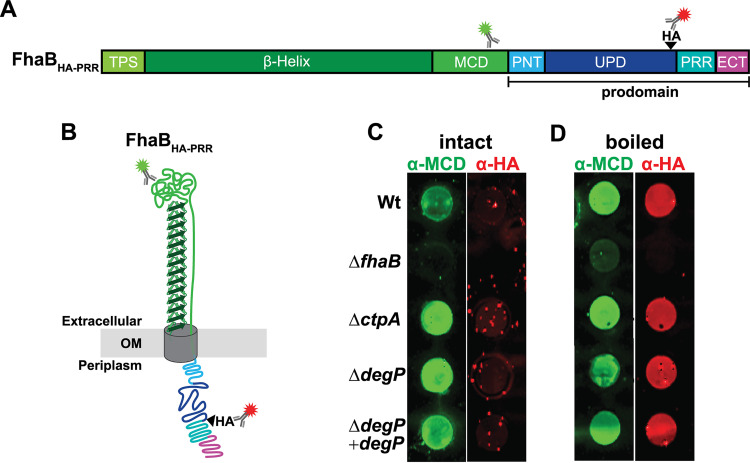
DegP could be indirectly required for FhaB processing by promoting FhaB translocation through FhaC. To determine if loss of DegP prevents efficient translocation of FhaB to the cell surface, we used a dot blot assay to compare relative amounts of FhaB on the surface of intact bacteria following 4 h of growth under BvgAS-activating conditions (Fig. 5). In this assay, only surface-exposed epitopes are detectable by antibodies when cells are intact. When the bacteria are boiled to disrupt the membranes, internal epitopes can additionally be detected by antibodies. The α-MCD antibody (green) detected the MCD in unboiled bacteria, indicating that the MCD was located external to the outer membrane. However, the HA tag was only detected after boiling the bacteria, indicating that the PD remains localized in the periplasm. As expected, neither the MCD nor the HA tag was detected in the ΔfhaB strain. The protease mutants, ΔctpA and ΔdegP, were similar to wild-type bacteria in that the MCD was detected regardless of boiling, while the HA tag was only detected postboiling. These results indicate that neither CtpA nor DegP is required for FhaB to be secreted through the outer membrane or for the PD to remain within the periplasm. Therefore, the reduction in FhaB processing that occurs in degP mutant strains is not due to mislocalization or instability of FhaB.
FIG 5.
DegP is not required for FhaB secretion or retention of the prodomain within the periplasm. (A and B) Linear schematic and folded model of FhaBHA-PRR with α-MCD and α-HA antibody binding regions shown. (C and D) Dot blots of intact and boiled cells. Blots were probed with α-MCD and α-HA antibodies.
DegP is not required for FhaB-mediated adherence to host cells in vitro.
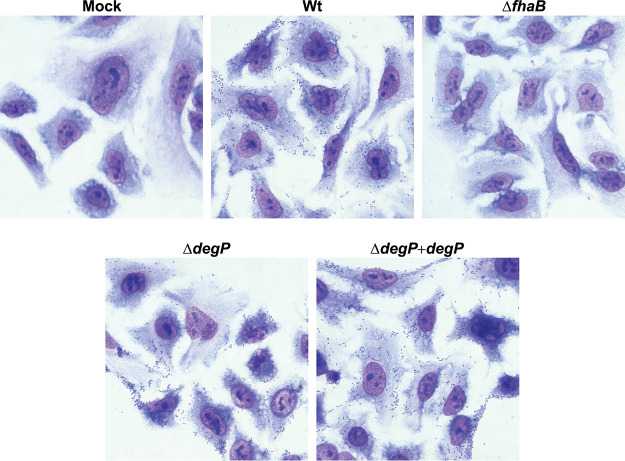
While the dot blot analysis indicates that DegP is not required for translocation of FhaB to the bacterial surface, it cannot distinguish between active or inactive conformations of FhaB. We performed in vitro adherence assays to determine if the FhaB on the surface of ΔdegP bacteria is capable of promoting bacterial adherence to human epithelial cells in vitro. Adherence of the ΔdegP strain was similar to that of wild-type B. bronchiseptica, indicating that DegP is not required for translocation of FhaB to the bacterial surface or for the MCD to fold into an adherence-competent conformation (Fig. 6). These data, along with the finding that strains that produce catalytically inactive DegP are defective for FhaB processing, are consistent with the primary function of DegP being that of a protease, rather than a chaperone, at least with regard to FhaB.
FIG 6.
Deletion of degP does not disrupt FhaB-mediated adherence of B. bronchiseptica to epithelial cells in vitro. Representative images of Giemsa-stained A549 human epithelial cells following a 15-minute inoculation with the indicated strains of B. bronchiseptica at an MOI of 100.
DegP does not signal through the RseA-RpoE pathway to control FhaB processing indirectly.
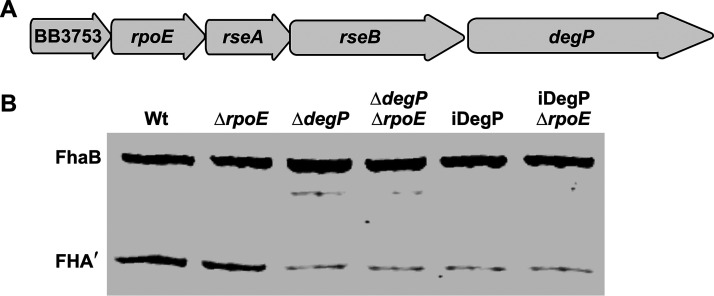
Some HtrA proteins, such as E. coli DegS (DegSEc), are not degradative proteases. Instead, they transduce signals from the periplasm to cytoplasmic effectors, controlling the expression of genes encoding stress response factors indirectly. In the E. coli RseA-RpoE signal transduction system, the anti-sigma factor RseA, which is embedded in the inner membrane, binds and sequesters the RpoE sigma factor (σE) in the cytoplasm, preventing σE from binding to and directing RNA polymerase to activate expression of target genes (33). In E. coli undergoing exponential growth under standard laboratory conditions, DegSEc homotrimers associated with the inner membrane are in an inactive conformation. When E. coli is exposed to conditions that disrupt the folding and localization of proteins in the periplasm, misfolded proteins bind DegSEc’s single PDZ domain, resulting in allosteric activation of DegSEc’s proteolytic activity (34). When active, DegSEc cleaves a specific site on a periplasmic region of RseA, initiating a proteolytic cascade that causes the release of σE and the subsequent activation of stress response genes (33). Collectively, this process is referred to as regulated intramembrane proteolysis (RIP) (35). Among the genes activated in the E. coli σE regulon is degP (36–39). Although the genomes of the Bordetella reference strains Tohama I (B. pertussis) and RB50 (B. bronchiseptica) encode functional Rse-RpoE systems (40–42), they do not encode DegS homologs, as neither of the two HtrA homologs produced by these strains (DegP and the BB4867 gene product) are predicted to include TM domains capable of inserting in the inner membrane. Therefore, the protease responsible for initiating regulated proteolysis of RseA in Bordetella is not known. Unlike E. coli, in which degP is located distally on the chromosome from rpoE, degP is in an operon with rpoE, rseA, and rseB in B. pertussis and B. bronchiseptica (Fig. 7A). These observations led us to ask if DegP could regulate FhaB processing indirectly, by cleaving RseABb and subsequently increasing σE-dependent expression of a gene encoding yet another protease. RpoE is thought to be essential in B. pertussis (41) but is dispensable for growth of B. bronchiseptica under standard laboratory conditions (42). To determine if DegP signals through the RseA-RpoE pathway, we deleted rpoE in wild-type, ΔdegP, and iDegP B. bronchiseptica (Fig. 7B). If DegP signals through RseA-RpoE to activate expression of a gene encoding another protease, then loss of RpoE would cause the ΔrpoE strain to exhibit a similar defect in FhaB/FHA processing seen in WCLs from ΔdegP and iDegP mutants. Instead, we observed that deletion of ΔrpoE had no effect on FhaB processing by any of the strains under the conditions examined, indicating that DegP is not indirectly regulating degradation of the FhaB PD via RpoE (Fig. 7).
FIG 7.
RpoE is not required for FhaB processing in B. bronchiseptica. (A) Diagram of the BB3753-BB3749 (degP) operon in the B. bronchiseptica RB50 genome. (B) Western blot analysis of WCL of strains grown for 4 h in BvgAS-activating medium following overnight growth in BvgAS-inactivating medium. Blots probed with an α-MCD antibody.
DISCUSSION
Our results indicate that the HtrA family protease DegP initiates degradation of the FhaB prodomain in B. bronchiseptica. Three key findings support this conclusion. (i) FhaB processing was dramatically reduced, but not completely abrogated, in strains lacking DegP (ΔdegP) or producing DegP in which the predicted catalytic serine was replaced with alanine (S237A, iDegP). The small amount of FHA′ detected in WCLs of degP mutants was likely due to the prodomain being converted to a CtpA substrate by housekeeping proteases, which is revealed only in the absence of DegP. (ii) DegP was dispensable for degradation of FhaB polypeptides with altered C termini (ΔECT and CT-HA) that are efficiently degraded by CtpA (Fig. 3). These data are consistent with our previous studies, which determined that the native FhaB C terminus is a poor substrate for CtpA (22), and indicate that, in wild-type bacteria, DegP cleaves the prodomain first, generating a C terminus that is then efficiently degraded by CtpA. CtpA-dependent degradation of the ΔECT and CT-HA polypeptides in the absence of DegP also indicate that accumulation of full-length in WCLs from degP mutants is not due to the loss of CtpA activity. (iii) DegP was not required for translocation of FhaB through FhaC (Fig. 5) or for FhaB to mediate adherence to epithelial cells (Fig. 6), consistent with DegP functioning as a protease and not a chaperone for FhaB. Previous studies determined that if FhaB is stalled in the periplasm (i.e., in fhaC mutants), it is degraded (43). The stability of full-length FhaB and the surface-localization of the MCD in degP mutants, therefore, indicate that DegP is not required to preserve FhaB stability or to deliver the TPS domain to FhaC in the periplasm. Although it is formally possible that DegP regulates FhaB processing indirectly by activating another protease, the most parsimonious explanation for our findings is that DegP acts on FhaB directly.
FhaB is required to mediate adherence to mammalian cells, prevent bacterial clearance by innate immune effectors, and control inflammatory signaling during infection. While processed FHA′ is sufficient for adherence, full-length FhaB and regulated degradation of the prodomain is required specifically for B. bronchiseptica to resist phagocytic cell clearance; mutants with altered prodomain processing are cleared rapidly from the lower respiratory tract but are not altered for adherence in vitro or in vivo or for suppression of inflammatory cytokines (11). Consistent with these data, the ΔdegP strain adhered to human A549 epithelial cells as efficiently as do wild-type bacteria (Fig. 6), confirming that regulated prodomain processing is not required for FhaB to function as an adhesin. The fact that FhaB was exported to the cell surface (Fig. 5) and able to mediate adherence in degP mutants (Fig. 6) indicates that DegP does not function as a chaperone required to stabilize FhaB in the periplasm or to bring the N terminus of the TPS domain to FhaC. Furthermore, if DegP was an important chaperone for the FhaB prodomain, then lack of DegP would result in hyperprocessing, similar to the ΔECT strain. However, full-length FhaB was stabilized in degP mutants, indicating that DegP does not promote folding or stability of the prodomain.
Although B. bronchiseptica consistently produces and releases FHA′ (and sometimes FHA) from the bacterial surface during in vitro culture, neither the mechanism by which release occurs, nor its biological relevance, is understood. Our previous data suggested that either CtpA or SphB1 are sufficient for release (22). However, our new data indicate that their sufficiency is dependent on DegP, as no FhaB-derived polypeptides are released by degP mutants (Fig. 4B). Future studies to determine how DegP and CtpA (and potentially EnvC) cooperate to degrade the prodomain should also reveal the mechanism underlying retention versus release of FhaB-derived polypeptides, another important outstanding question for TPS systems, in general.
DegP’s proteolytic activity is required for initiating FhaB processing in B. bronchiseptica, but it is not known whether the DegP homolog from B. pertussis is involved in degradation of the FhaB prodomain in that organism. A previous study that examined DegP in B. pertussis determined that this protease may have both catalytic and noncatalytic functions which are required for maintaining envelope integrity and are involved with FhaB secretion (29). However, that study did not examine prodomain degradation (29). Comparison between our study and Baud et al. highlights some key differences between B. pertussis and B. bronchiseptica, namely, that B. pertussis degP mutants are more susceptible to elevated temperature than are B. bronchiseptica degP mutants. B. pertussis degP mutants are attenuated for growth at 37°C (29), while B. bronchiseptica strains lacking DegP present a growth defect at 42°C but not at 37°C (Fig. S2).
Baud et al. determined that growth at 37°C of the degP mutants could be partially restored by expression of catalytically inactive DegP (S237A) in trans, which, combined with examination of binding interactions between DegP and nonnative N-terminal FhaB polypeptides in vitro, led them to conclude that DegP functions as a chaperone in B. pertussis (29). In contrast, we found that DegP’s proteolytic activity is required for both FhaB processing and resistance to heat stress. Our iDegP strains, which produce DegP S237A from the native locus, were as attenuated for growth at 42°C and for FhaB processing as was the ΔdegP strain (Fig. S2 and Fig. 2), consistent with DegP in B. bronchiseptica functioning primarily as a protease for both FhaB processing and resistance to envelope stress.
In a follow-up study, Baud et al. determined that DegP can be both soluble and associated with bacterial membranes as trimers, that membrane associated DegP has a higher affinity for nonnative FHA polypeptides (lacking most of the MCD and all of the prodomain), and that membrane-associated DegP has proteolytic activity (44). These findings provide insight into how DegP could be regulating degradation of the FhaB prodomain. We hypothesize that membrane-associated DegP trimers could form a stable complex with the FhaB prodomain until a signal causes a conformational change in the prodomain such that DegP recognizes the prodomain as a substrate and initiates degradation. The signal that initiates degradation of the prodomain is unknown; however, previous work from our lab and others suggests that FhaB may be part of a novel toxin delivery system. The adenylyl cyclase toxin (ACT)—known to inactivate phagocytic cells (45–47)—binds FhaB on the bacterial surface (48, 49), and we hypothesize that regulated degradation of the FhaB prodomain controls delivery of surface-associated ACT to those cells. Our current model states that ACT forms a stable complex with full-length FhaB on the bacterial surface until these FhaB-ACT complexes interact with the ACT receptor (CR3, CD11b/CD18, Mac1) on phagocytic cells (46, 50). We propose that this interaction between FhaB-ACT and CR3 could generate a signal that is propagated through the FhaB MCD, resulting in a conformational change in the prodomain that allows DegP to initiate degradation of the prodomain. Previous data indicate the FhaB ECT—which is 100% identical at the protein level between FhaB homologs in B. pertussis, B. bronchiseptica and Bordetella parapertussis—likely adopts a conformation that stabilizes the prodomain by preventing degradation by CtpA (22). It is interesting to speculate that FhaB-ACT binding the CR3 receptor could trigger the ECT to unfold and therefore become a substrate for DegP, consistent with the preference for DegP homologs, such as those from E. coli and B. pertussis, to degrade unfolded proteins (29, 51). It is also possible that the signal that initiates FhaB processing is envelope stress that B. bronchiseptica faces during infection, which could induce conformational changes in the FhaB prodomain that cause it to become a substrate for DegP. Future studies will focus on identifying the signal that initiates FhaB processing and determining if regulated processing of FhaB is involved in ACT delivery to specific host cells.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains and plasmids are listed in Table S1 in the supplemental material. In-frame deletions were created by allelic exchange using derivatives of the pSS4245 plasmid (52). For complementation, degP was inserted into a derivative of the pUC18 plasmid that contained the S12 promoter and integrated into the chromosome at the att:Tn7 site (53). E. coli strains were used to amplify vectors (DH5α) and to transform B. bronchiseptica (RHO3). Mutations were confirmed by PCR and/or sequencing. As we have published in the past, our “wild-type” strain for FhaB studies is RBX11, a derivative of RB50 in which fhaB is more genetically tractable because the strain lacks fhaS, a gene highly homologous to fhaB but that plays no role in virulence (43).
Culture media and conditions.
Bordetella bronchiseptica strains were streaked from −80°C stocks onto Bordet-Gengou agar (BD Biosciences) supplemented with 6% defibrinated sheep blood (HemoStat Laboratories) and grown at 37°C for 2 to 3 days. Because some combinations of protease deletions, such as ΔctpA ΔdegP, resulted in growth defects that were minimized if BvgAS was not active, protease dual mutants were streaked onto blood plates supplemented with 50 mM MgSO4. Colonies were picked from these plates and cultured overnight in Stainer-Scholte broth (SS) containing MgSO4 (50 mM) to prevent production of Bvg-induced virulence factors, including FhaB. Bacteria were pelleted and washed with cold, sterile Dulbecco’s phosphate-buffered saline (DPBS, Gibco) and then cultured further for 4 h in fresh Stainer-Scholte broth lacking MgSO4 to initiate production and processing of FhaB. This culture method is useful for examining early FhaB production and processing and to compare differences found across bacterial mutants that may be hidden after 16 h of overnight growth (22). For experiments described in Fig. 1, bacteria were simply grown overnight in Bvg-inducing conditions (SS without MgSO4). Escherichia coli strains were grown at 37°C in lysogeny broth or on lysogeny broth agar. Where appropriate, medium was supplemented with streptomycin (20 μg/ml), kanamycin (50 μg/ml), and MgSO4 (50 mM). For heat tolerance experiments (Fig. S2), the indicated strains were grown in Bvg+ phase liquid cultures (SS broth) overnight, normalized to an optical density at 600 nm (OD600) of 1, and then 10-fold serial dilutions of each culture were spotted (5 μl per spot) onto BG blood agar plates alone or onto plates supplemented with 50 mM MgSO4 or 50 mM MgCl2.
A549 human epithelial cells were cultured as described by the supplier (ATCC) in Ham’s F12 medium (Gibco) with 10% fetal bovine serum (FBS) (VWR). Cells were grown until they reached 80% confluence in T75 tissue culture treated flasks (Corning) before passaging, and the cells were passaged no more than 15 times out of a freeze.
Immunoblots.
To examine cell-associated proteins by Western blotting, whole-cell lysates were prepared by boiling pelleted cells in Laemmli buffer and shearing by passage through a 26G needle. For released proteins, culture supernatants were filtered through 0.2-μm filters and proteins were precipitated using 10% trichloroacetic acid, rinsed with cold acetone, resuspended in 1 M Tris-HCl (pH 8.8) and Laemmli buffer mixture, and boiled. Proteins were separated by SDS-PAGE using 4% or 5% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes (GE Healthcare) and the membranes were probed with a rabbit polyclonal antibody generated against the FHA mature C-terminal domain (MCD) (10) and a mouse monoclonal antibody generated against an HA epitope (BioLegend). Corresponding α-rabbit and α-mouse IRDye secondary antibodies (LI-COR Biosciences) were used to detect proteins using a LI-COR Odyssey Classic Blot Imager (LI-COR Biosciences). Protein quantification was performed using LI-COR Image Studio version 5 software. B. bronchiseptica sample volumes were normalized based on optical density of the cultures.
To compare cell surface exoproteins versus internal proteins by dot blot, 100 μl of 0.5 OD600/ml of bacteria were spotted onto nitrocellulose membranes using a 96-well vacuum manifold. The membranes were probed using anti-MCD and anti-HA antibodies. Signal detected on intact bacteria corresponded to surface-exposed epitopes, and boiling disrupted the membrane to allow additional antibody interaction with otherwise inaccessible internal epitopes.
Bacterial adherence assay.
These assays were performed as previously described with minor alterations (5). A549 cells were seeded on sterile coverslips in 12-well plates. Once cells reached a confluence of 50% to 80%, the seeding medium was removed and the cells were inoculated with the indicated B. bronchiseptica strains (wild type [wt], ΔfhaB, ΔdegP, and ΔdegP + degP) in SS broth at a multiplicity of infection (MOI) of 100 CFU per epithelial cell (2.5 × 107 CFU/ml of bacteria). One set of wells was treated with SS broth alone (mock infection control). The bacterial density of each inoculum was confirmed by plating on BG blood agar. The 12-well plates were centrifuged at 500 × g for 5 min at room temperature and then incubated for 15 min at 37°C with 5% CO2. The inoculum was removed from each well and the cells were washed four times with Hanks’ balanced salt solution (Difco). Cells were then fixed with 0.5 ml of ice-cold methanol for 5 min, after which the methanol was removed and the coverslips were air dried for 10 min. The cells were then stained for 15 min with 0.5 ml of 1:20 diluted Giemsa stain (Sigma-Aldrich). The stain was removed and the coverslips were washed twice with water before being air dried and mounted onto slides with permount (Sigma-Aldrich). The slides were imaged at 60× magnification (Keyence).
BB3749 encodes an HtrA family protease. (A) Alignment of the amino acid sequences of DegP of B. bronchiseptica (Uniport ID A0A0H3M188_BORBR), DegP of B. pertussis (Q7VW38_BORPE), MucD of Pseudomonas aeruginosa (G3XD20_PSEAE), DegP (DEGP_ECOLI), DegQ (DEGQ_ECOLI), and DegS (DEGS_ECOLI) of E. coli, and DegQ of B. bronchiseptica (AOAOH3LRV9_BORBR). Alignment was performed with Clustal Omega. (B) Matrix comparing the percent identities between the HtrA amino acid sequences. Download FIG S1, JPG file, 1.4 MB (1.5MB, jpg) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DegP activity is required for B. bronchiseptica to grow at 42°C. Ten-fold serial dilutions of B. bronchiseptica were spotted onto BG blood agar plates without magnesium additives (BvgAS-activating) or containing 50mM MgSO4 (BvgAS-inactivating) or 50mM MgCl2. Bacteria were grown for 72 h at 37°C or 42°C as indicated. Download FIG S2, PDF file, 1.8 MB (1.8MB, pdf) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
FhaB-processing pathways of B. bronchiseptica protease mutants. Linear diagrams of the model for regulated processing of FhaB in wild-type (A), ΔctpA (B), ΔdegP (C), ΔsphB1 (D), ΔctpAΔsphB1 (E), ΔctpAΔdegP (F), and ΔdegPΔsphB1 (G) strains. Polypeptides detected only in culture supernatants are indicated with a silcrow (§), polypeptides present in both WCL and supernatants are indicated with a psi (ψ), and polypeptides that are present in WCL in degP mutant strains, but at greatly reduced levels, are lightly shaded. Download FIG S3, PDF file, 0.7 MB (734.4KB, pdf) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used during this study. Download Table S1, DOCX file, 0.02 MB (18.3KB, docx) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We thank the Cotter lab for helpful discussions, especially Jessica Beauchamp for critically reading the manuscript and providing editorial suggestions. We thank the NIH for financially supporting this work (grants R01-AI129541 and R01-AI153160) and we acknowledge a UNC Infectious Disease and Pathogenesis Training Grant (T32-AI007151). The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We report no conflicts of interest.
R.M.J., Z.M.N., and P.A.C. designed the studies, interpreted data, and wrote the manuscript. R.M.J., Z.M.N., M.R.D., and J.C.S. acquired and analyzed the data.
Footnotes
This article is a direct contribution from Peggy A. Cotter, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Harris Bernstein, NIDDK, NIH, and Andrew Darwin, NYU School of Medicine.
Citation Johnson RM, Nash ZM, Dedloff MR, Shook JC, Cotter PA. 2021. DegP initiates regulated processing of filamentous hemagglutinin in Bordetella bronchiseptica. mBio 12:e01465-21. https://doi.org/10.1128/mBio.01465-21.
Contributor Information
Peggy A. Cotter, Email: peggy_cotter@med.unc.edu.
Jeff F. Miller, UCLA School of Medicine
REFERENCES
- 1.Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. 2017. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 17:974–980. doi: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- 3.Cherry JD. 2012. Epidemic pertussis in 2012—the resurgence of a vaccine-preventable disease. N Engl J Med 367:785–787. doi: 10.1056/NEJMp1209051. [DOI] [PubMed] [Google Scholar]
- 4.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun 66:5921–5929. doi: 10.1128/IAI.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urisu A, Cowell JL, Manclark CR. 1986. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun 52:695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Relman DA, Domenighini M, Tuomanen E, Rappuoli R, Falkow S. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A 86:2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inatsuka CS, Julio SM, Cotter PA. 2005. Bordetella filamentous hemagglutinin plays a critical role in immunomodulation, suggesting a mechanism for host specificity. Proc Natl Acad Sci U S A 102:18578–18583. doi: 10.1073/pnas.0507910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson MW, Inatsuka CS, Sheets AJ, Williams CL, Benaron DJ, Donato GM, Gray MC, Hewlett EL, Cotter PA. 2012. Contribution of Bordetella filamentous hemagglutinin and adenylate cyclase toxin to suppression and evasion of interleukin-17-mediated inflammation. Infect Immun 80:2061–2075. doi: 10.1128/IAI.00148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Julio SM, Inatsuka CS, Mazar J, Dieterich C, Relman DA, Cotter PA. 2009. Natural‐host animal models indicate functional interchangeability between the filamentous haemagglutinins of Bordetella pertussis and Bordetella bronchiseptica and reveal a role for the mature C‐terminal domain, but not the RGD motif, during infection. Mol Microbiol 71:1574–1590. doi: 10.1111/j.1365-2958.2009.06623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melvin JA, Scheller EV, Noël CR, Cotter PA. 2015. New insight into filamentous hemagglutinin secretion reveals a role for full-length FhaB in Bordetella virulence. mBio 6:e01189-15. doi: 10.1128/mBio.01189-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash ZM, Cotter PA. 2019. Bordetella filamentous hemagglutinin, a model for the two-partner secretion pathway. Microbiol Spectr 7. doi: 10.1128/microbiolspec.PSIB-0024-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazar J, Cotter PA. 2007. New insight into the molecular mechanisms of two-partner secretion. Trends Microbiol 15:508–515. doi: 10.1016/j.tim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Jacob-Dubuisson F, Guérin J, Baelen S, Clantin B. 2013. Two-partner secretion: as simple as it sounds? Res Microbiol 164:583–595. doi: 10.1016/j.resmic.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier N, Moser M, Koch H-G, Schimz K-L, Willery E, Locht C, Jacob-Dubuisson F, Müller M. 2004. Membrane targeting of a bacterial virulence factor harbouring an extended signal peptide. J Mol Microbiol Biotechnol 8:7–18. doi: 10.1159/000082076. [DOI] [PubMed] [Google Scholar]
- 16.Lambert‐Buisine C, Willery E, Locht C, Jacob‐Dubuisson F. 1998. N‐terminal characterization of the Bordetella pertussis filamentous haemagglutinin. Mol Microbiol 28:1283–1293. doi: 10.1046/j.1365-2958.1998.00892.x. [DOI] [PubMed] [Google Scholar]
- 17.Clantin B, Hodak H, Willery E, Locht C, Jacob-Dubuisson F, Villeret V. 2004. The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway. Proc Natl Acad Sci U S A 101:6194–6199. doi: 10.1073/pnas.0400291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renauld-Mongénie G, Cornette J, Mielcarek N, Menozzi FD, Locht C. 1996. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J Bacteriol 178:1053–1060. doi: 10.1128/jb.178.4.1053-1060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob-Dubuisson F, Buisine C, Willery E, Renauld-Mongénie G, Locht C. 1997. Lack of functional complementation between Bordetella pertussis filamentous hemagglutinin and Proteus mirabilis HpmA hemolysin secretion machineries. J Bacteriol 179:775–783. doi: 10.1128/jb.179.3.775-783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazar J, Cotter PA. 2006. Topology and maturation of filamentous haemagglutinin suggest a new model for two‐partner secretion. Mol Microbiol 62:641–654. doi: 10.1111/j.1365-2958.2006.05392.x. [DOI] [PubMed] [Google Scholar]
- 21.Coutte L, Antoine R, Drobecq H, Locht C, Jacob-Dubuisson F. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J 20:5040–5048. doi: 10.1093/emboj/20.18.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash ZM, Cotter PA. 2019. Regulated, sequential processing by multiple proteases is required for proper maturation and release of Bordetella filamentous hemagglutinin. Mol Microbiol 112:820–836. doi: 10.1111/mmi.14318. [DOI] [PubMed] [Google Scholar]
- 23.Coutte L, Alonso S, Reveneau N, Willery E, Quatannens B, Locht C, Jacob-Dubuisson F. 2003. Role of adhesin release for mucosal colonization by a bacterial pathogen. J Exp Med 197:735–742. doi: 10.1084/jem.20021153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters NT, Morlot C, Yang DC, Uehara T, Vernet T, Bernhardt TG. 2013. Structure-function analysis of the LytM domain of EnvC, an activator of cell wall remodelling at the Escherichia coli division site. Mol Microbiol 89:690–701. doi: 10.1111/mmi.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang M-J, Yakhnina AA, Bernhardt TG. 2017. NlpD links cell wall remodeling and outer membrane invagination during cytokinesis in Escherichia coli. PLoS Genet 13:e1006888. doi: 10.1371/journal.pgen.1006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauch KL, Johnson K, Beckwith J. 1989. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol 171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipinska B, Fayet O, Baird L, Georgopoulos C. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol 171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipinska B, Zylicz M, Georgopoulos C. 1990. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol 172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baud C, Hodak H, Willery E, Drobecq H, Locht C, Jamin M, Jacob‐Dubuisson F. 2009. Role of DegP for two‐partner secretion in Bordetella. Mol Microbiol 74:315–329. doi: 10.1111/j.1365-2958.2009.06860.x. [DOI] [PubMed] [Google Scholar]
- 30.Carbonetti NH, Khelef N, Guiso N, Gross R. 1993. A phase variant of Bordetella pertussis with a mutation in a new locus involved in the regulation of pertussis toxin and adenylate cyclase toxin expression. J Bacteriol 175:6679–6688. doi: 10.1128/jb.175.20.6679-6688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol Rev 56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings CA, Bootsma HJ, Relman DA, Miller JF. 2006. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol 188:1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ades SE, Connolly LE, Alba BM, Gross CA. 1999. The Escherichia coli sigma(E)-dependent extracytoplasmic stress response is controlled by the regulated proteolysis of an anti-sigma factor. Genes Dev 13:2449–2461. doi: 10.1101/gad.13.18.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilken C, Kitzing K, Kurzbauer R, Ehrmann M, Clausen T. 2004. Crystal structure of the DegS stress sensor: how a PDZ domain recognizes misfolded protein and activates a protease. Cell 117:483–494. doi: 10.1016/S0092-8674(04)00454-4. [DOI] [PubMed] [Google Scholar]
- 35.Brown MS, Ye J, Rawson RB, Goldstein JL. 2000. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 36.Dartigalongue C, Missiakas D, Raina S. 2001. Characterization of the Escherichia coli sigma E regulon. J Biol Chem 276:20866–20875. doi: 10.1074/jbc.M100464200. [DOI] [PubMed] [Google Scholar]
- 37.Erickson JW, Gross CA. 1989. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Gene Dev 3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 38.Bianchi AA, Baneyx F. 1999. Hyperosmotic shock induces the σ32 and σE stress regulons of Escherichia coli. Mol Microbiol 34:1029–1038. doi: 10.1046/j.1365-2958.1999.01664.x. [DOI] [PubMed] [Google Scholar]
- 39.Lipinska B, Sharma S, Georgopoulos C. 1988. Sequence analysis and regulation of the htrA gene of Escherichia coli: a σ 32 -independent mechanism of heat-inducible transcription. Nucleic Acids Res 16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbier M, Boehm DT, Sen-Kilic E, Bonnin C, Pinheiro T, Hoffman C, Gray M, Hewlett E, Damron FH. 2017. Modulation of pertussis and adenylate cyclase toxins by sigma factor RpoE in Bordetella pertussis. Infect Immun 85:e00565-16. doi: 10.1128/IAI.00565-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanawa T, Yonezawa H, Kawakami H, Kamiya S, Armstrong SK. 2013. Role of Bordetella pertussis RseA in the cell envelope stress response and adenylate cyclase toxin release. Pathog Dis 69:7–20. doi: 10.1111/2049-632X.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barchinger SE, Zhang X, Hester SE, Rodriguez ME, Harvill ET, Ades SE. 2012. sigE facilitates the adaptation of Bordetella bronchiseptica to stress conditions and lethal infection in immunocompromised mice. BMC Microbiol 12:179. doi: 10.1186/1471-2180-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Julio SM, Cotter PA. 2005. Characterization of the filamentous hemagglutinin-like protein FhaS in Bordetella bronchiseptica. Infect Immun 73:4960–4971. doi: 10.1128/IAI.73.8.4960-4971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baud C, Gutsche I, Willery E, de Paepe D, Drobecq H, Gilleron M, Locht C, Jamin M, Jacob‐Dubuisson F. 2011. Membrane‐associated DegP in Bordetella chaperones a repeat‐rich secretory protein. Mol Microbiol 80:1625–1636. doi: 10.1111/j.1365-2958.2011.07672.x. [DOI] [PubMed] [Google Scholar]
- 45.Harvill ET, Cotter PA, Yuk MH, Miller JF. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect Immun 67:1493–1500. doi: 10.1128/IAI.67.3.1493-1500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J Exp Med 193:1035–1044. doi: 10.1084/jem.193.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad JN, Cerny O, Linhartova I, Masin J, Osicka R, Sebo P. 2016. cAMP signalling of Bordetella adenylate cyclase toxin through the SHP‐1 phosphatase activates the BimEL‐Bax pro‐apoptotic cascade in phagocytes. Cell Microbiol 18:384–398. doi: 10.1111/cmi.12519. [DOI] [PubMed] [Google Scholar]
- 48.Zaretzky FR, Gray MC, Hewlett EL. 2002. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: a role for toxin-filamentous haemagglutinin interaction. Mol Microbiol 45:1589–1598. doi: 10.1046/j.1365-2958.2002.03107.x. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman C, Eby J, Gray M, Damron FH, Melvin J, Cotter P, Hewlett E. 2017. Bordetella adenylate cyclase toxin interacts with filamentous hemagglutinin to inhibit biofilm formation in vitro. Mol Microbiol 103:214–228. doi: 10.1111/mmi.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osicka R, Osickova A, Hasan S, Bumba L, Cerny J, Sebo P. 2015. Bordetella adenylate cyclase toxin is a unique ligand of the integrin complement receptor 3. Elife 4:e10766. doi: 10.7554/eLife.10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clausen T, Kaiser M, Huber R, Ehrmann M. 2011. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol 12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- 52.Inatsuka CS, Xu Q, Vujkovic-Cvijin I, Wong S, Stibitz S, Miller JF, Cotter PA. 2010. Pertactin is required for Bordetella species to resist neutrophil-mediated clearance. Infect Immun 78:2901–2909. doi: 10.1128/IAI.00188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson MS, Garcia EC, Cotter PA. 2012. The Burkholderia bcpAIOB genes define unique classes of two-partner secretion and contact dependent growth inhibition systems. PLoS Genet 8:e1002877. doi: 10.1371/journal.pgen.1002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BB3749 encodes an HtrA family protease. (A) Alignment of the amino acid sequences of DegP of B. bronchiseptica (Uniport ID A0A0H3M188_BORBR), DegP of B. pertussis (Q7VW38_BORPE), MucD of Pseudomonas aeruginosa (G3XD20_PSEAE), DegP (DEGP_ECOLI), DegQ (DEGQ_ECOLI), and DegS (DEGS_ECOLI) of E. coli, and DegQ of B. bronchiseptica (AOAOH3LRV9_BORBR). Alignment was performed with Clustal Omega. (B) Matrix comparing the percent identities between the HtrA amino acid sequences. Download FIG S1, JPG file, 1.4 MB (1.5MB, jpg) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DegP activity is required for B. bronchiseptica to grow at 42°C. Ten-fold serial dilutions of B. bronchiseptica were spotted onto BG blood agar plates without magnesium additives (BvgAS-activating) or containing 50mM MgSO4 (BvgAS-inactivating) or 50mM MgCl2. Bacteria were grown for 72 h at 37°C or 42°C as indicated. Download FIG S2, PDF file, 1.8 MB (1.8MB, pdf) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
FhaB-processing pathways of B. bronchiseptica protease mutants. Linear diagrams of the model for regulated processing of FhaB in wild-type (A), ΔctpA (B), ΔdegP (C), ΔsphB1 (D), ΔctpAΔsphB1 (E), ΔctpAΔdegP (F), and ΔdegPΔsphB1 (G) strains. Polypeptides detected only in culture supernatants are indicated with a silcrow (§), polypeptides present in both WCL and supernatants are indicated with a psi (ψ), and polypeptides that are present in WCL in degP mutant strains, but at greatly reduced levels, are lightly shaded. Download FIG S3, PDF file, 0.7 MB (734.4KB, pdf) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used during this study. Download Table S1, DOCX file, 0.02 MB (18.3KB, docx) .
Copyright © 2021 Johnson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.