Abstract
We herein report a case of nonbacterial thrombotic endocarditis (NBTE) in a patient with previously undiagnosed lung cancer. A 62-year-old woman presented to our hospital with multiple cerebral infarctions. There was no evidence of valvular heart disease or vegetations. Whole-leg ultrasonography revealed deep vein thrombosis of the left peroneal vein. We administered direct oral anticoagulants (DOACs) for a presumed diagnosis of paradoxical embolisms caused by patent foramen ovale. Unfortunately, she experienced further embolization and died. At a postmortem examination, she was diagnosed with NBTE and metastatic adenocarcinoma of the lung. Our experience with this patient suggests that DOACs may be an insufficient treatment for NBTE.
Keywords: nonbacterial thrombotic endocarditis, direct oral anticoagulants, echocardiography, lung cancer, postmortem
Introduction
Nonbacterial thrombotic endocarditis (NBTE), also known as marantic endocarditis, is a rare hypercoagulable condition associated with advanced malignancy and other illnesses, including autoimmune disease, sepsis, and burns (1-3). The condition is often not detected until after death; an autopsy series showed an incidence of 0.6% to 1.6% (2,3).
Heparin is reportedly the most effective anticoagulant treatment for NBTE (3), but in recent years, direct oral anticoagulants (DOACs) have been used for patients with cancer-associated venous thromboembolism (CAT). However, whether or not DOACs are an appropriate treatment for NBTE, which can cause arterial embolic events, is unclear.
We herein report an unusual case of NBTE in a patient with previously undiagnosed lung cancer who was undergoing DOAC treatment for presumed paradoxical embolism.
Case Report
One month before her admission, a 62-year-old woman underwent tooth extraction that caused a periapical abscess, resulting in intermittent back pain and dysarthria. She presented to our hospital with a two-day history of a fever and was admitted.
On admission, her temperature was 38℃. Her vital signs were otherwise normal, and a physical examination was unremarkable: there were no cardiac murmurs, Osler's nodes, Janeway lesions, or neurologic findings except for mild dysarthria. There were no significant findings on electrocardiography or chest radiography (no atrial fibrillation or nodular lung lesions). Blood testing revealed mildly elevated neutrophils (8,960/μL), mildly decreased platelets (14.8×103/μL), elevated lactate dehydrogenase (318 IU/L), and elevated C-reactive protein (CRP; 3.3 mg/dL). She had an elevated international normalized ratio (1.15) and D-dimer level (40.0 μg/mL), suggesting a coagulation disorder. Although she did have an elevated rheumatoid factor level, testing for specific antinuclear antibody and antineutrophil cytoplasmic autoantibody was negative. Therefore, systemic lupus erythematosus was excluded as a potential diagnosis.
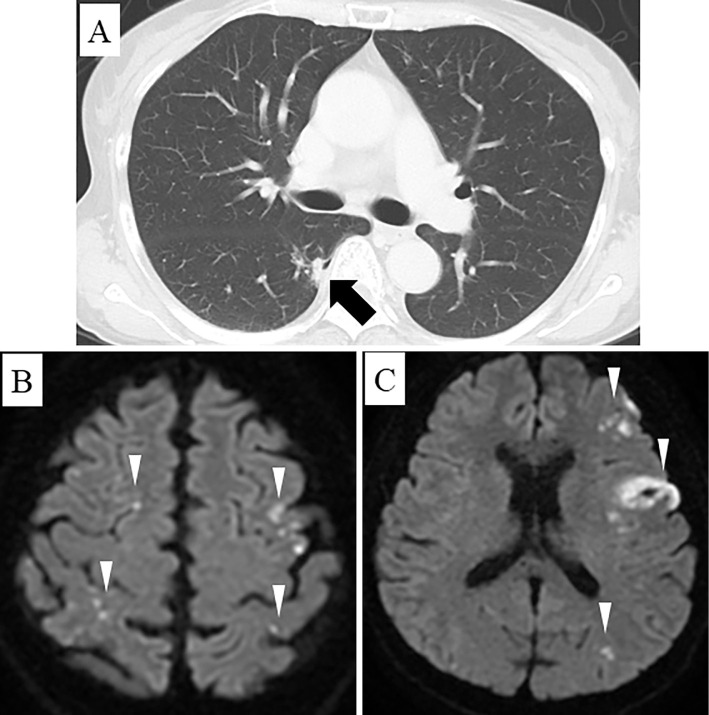
Computed tomography (CT) of the head revealed an area of low density in her left frontal lobe with no evidence of bleeding. Chest CT revealed a 10-mm nodular lesion with pleural indentation in segment 6 of her right lung (Fig. 1A). A pulmonologist was consulted, who advised follow-up because this lesion had not enlarged over four years. Contrast-enhanced CT revealed infarction of her right kidney but no evidence of pulmonary embolism. Diffusion-weighted magnetic resonance imaging demonstrated multiple acute cerebral infarctions involving both hemispheres (Fig. 1B, C).
Figure 1.
A: Computed tomography shows a 10-mm nodular lesion in the right lung (arrow). B, C: Diffusion-weighted magnetic resonance imaging demonstrates multiple cerebral infarctions (arrowheads).
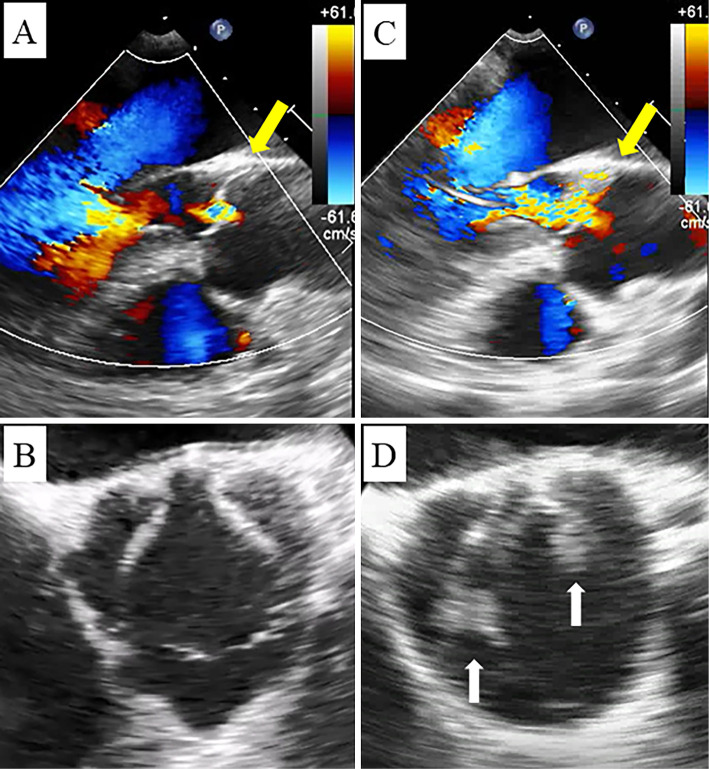
Although infective endocarditis was initially suspected, her blood culture was negative, and transthoracic echocardiography (TTE) demonstrated only trivial aortic regurgitation with no evidence of vegetations. Transesophageal echocardiography showed no evidence of valvular disease or vegetations (Fig. 2A, B) but was suggestive of patent foramen ovale (PFO) when agitated saline bubbles were used for contrast (Grade 1: 1-5 bubbles passing the septum after injection). Whole-leg ultrasonography revealed deep vein thrombosis of her left peroneal vein.
Figure 2.
A, B: Transesophageal echocardiography on admission shows only trivial aortic regurgitation (yellow arrows) without vegetations. C, D: Transesophageal echocardiography on day 24 demonstrates moderate aortic regurgitation (yellow arrow) and vegetations on the noncoronary and left coronary cusps of the aortic valve (white arrows).
Based on these findings, we suspected that she had a paradoxical embolism caused by her PFO. We prescribed weight-based edoxaban (30 mg) on day 9 of admission. Testing for thrombophilia (protein C, protein S, antiphospholipid antibody, antithrombin III) was negative. During treatment, her body temperature remained between 36 and 38℃, and her CRP level remained mildly elevated. On day 11, we suspected a drug fever and changed her medication to rivaroxaban (15 mg). Unfortunately, her condition did not improve, and her D-dimer level was still high (30.0 μg/mL). Several sets of blood cultures (days 1, 6, 11, 22, 23) were negative, and TTE was repeatedly negative for vegetations (days 1, 8, 15, 22). On day 24, she complained of sudden-onset back pain and blood in her urine. Enhanced CT showed a new splenic infarction, infarction of the left kidney, and a new cerebral infarction. Another TTE showed worsening aortic regurgitation but no vegetations. However, transesophageal echocardiography on the same day revealed moderate aortic regurgitation and vegetations on the noncoronary and left coronary cusps of the aortic valve (8×5 mm and 6×6 mm, respectively; Fig. 2C, D). At that time, she was given the clinical diagnosis of infective endocarditis despite negative blood cultures.
On day 24, we started antibiotic therapy with ceftriaxone 3 g/day, gentamycin 100 mg/day, and vancomycin 1.5 g/day and stopped rivaroxaban. Although her temperature and CRP levels showed a downward trend, they did not improve to the baseline. Therefore, we ruled out any other organisms as the cause of culture-negative endocarditis during the clinical course of this patient.
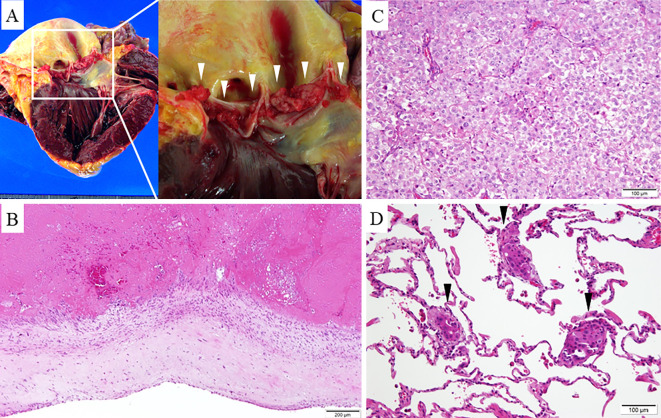
On day 30, she complained of a sudden severe headache, and head CT demonstrated cerebral hemorrhaging in the left occipital lobe with a midline shift. She died on day 31. At her autopsy, vegetations were noted on the leaflets of the aortic valve (Fig. 3A). Although there was inflammatory cell infiltration and thrombus present, there was no evidence of bacterial colonization of these vegetations (Fig. 3B). The nodular lesion in segment 6 of her right lung was determined to be adenocarcinoma, with metastasis to the left lung (Fig. 3C, D) that had not been seen on CT. In addition, her foramen ovale was not patent. Our final diagnosis was NBTE associated with advanced lung adenocarcinoma.
Figure 3.
A: Many vegetations are seen on the aortic leaflets (arrowheads). B: Histopathologic section of the vegetations reveals no visible evidence of microorganisms. C: A histopathologic examination of the right lung mass reveals adenocarcinoma. D: The left lung contains a metastatic adenocarcinoma lesion (arrowheads).
Discussion
Recent trials have indicated the efficacy of DOAC treatment for patients with CAT (4-8); specifically, edoxaban and rivaroxaban are effective for venous thromboembolism in these patients (5,8). However, the efficacy of DOAC treatment for patients with NBTE remains uncertain. Some authors report that patients with NBTE who receive DOACs go on to experience arterial embolisms, including cerebral infarctions (9-11). In patients with NBTE, the most effective anticoagulant therapy is considered to be unfractionated heparin, which has been shown to reduce the incidence of recurrent thromboembolic events (12-14). Our patient experienced recurrent cerebral infarction despite DOAC therapy.
The incidence of NBTE is particularly associated with mucin-producing adenocarcinomas, including those of the lung and ovary, which account for nearly 50% of patients with NBTE (15). One of the most important factors in NBTE is the hypercoagulable state that involves hypofibrinogenemia, hyperfibrinogenemia, thrombocytopenia, increased tissue factor and factor VIII, circulating fibrin degradation products, increased platelet turnover, and accelerated fibrinolysis (1,3,12). Because many factors are involved in thrombus formation in patients with NBTE, heparin may be more effective than DOACs (oral factor Xa inhibitor or thrombin inhibitor).
Unfortunately, our patient was not diagnosed with NBTE until her autopsy, when advanced lung adenocarcinoma was detected. The diagnosis of NBTE should be suspected in any patient with malignancy and arterial thromboembolic events; the condition has been documented in patients with advanced-stage malignancy (16). We suspected infective endocarditis rather than NBTE in our patient, as her lung cancer remained undiagnosed before her death (CT findings were stable for four years). Because NBTE can occur even in patients who have not been clinically diagnosed with advanced malignancy, NBTE should be suspected in patients with repeated systemic embolisms refractory to anticoagulant therapy and negative blood cultures. Therefore, an aggressive evaluation for malignancy should be considered in patients with embolic strokes of undetermined source or with multiple systemic arterial embolisms.
In our patient, we confused NBTE for paradoxical embolisms caused by PFO, which did not exist. Although the result on the agitated saline bubble study was the reason for our initial diagnosis of paradoxical embolism, only one to five bubbles were seen in the left atrium. Homma et al. (17) reported that the number of microbubbles is significantly greater in patients with cryptogenic stroke than in those with a known cause of stroke. Therefore, we should interpret the results of agitated saline bubble studies with caution; we should confirm the number of microbubbles in the left atrium and the moment when microbubbles pass the atrial septum.
This case indicates that the diagnosis of NBTE requires a high degree of clinical suspicion in patients with cryptogenic stroke, and DOACs may be insufficient as treatment for NBTE. The condition can take on atypical manifestations.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart J 113: 773-784, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Steiner I. Nonbacterial thrombotic endocarditis--a study of 171 case reports. Cesk Patol 29: 58-60, 1993. [PubMed] [Google Scholar]
- 3.El-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 12: 518-523, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res 173: 158-163, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 36: 2017-2023, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Agnelli G, Becattini C, Meyer G, et al. Apixaban for the Treatment of venous thromboembolism associated with cancer. N Engl J Med 382: 1599-1607, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Khorana A, Vadhan-Raj S, Kuderer NM, et al. Ribaroxaban for preventing venous thromboembolism in high-risk ambulatory patients with cancer: rationale and design of the CASSINI trial. Thromb Haemost 117: 2135-2145, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for cancer-associated venous thromboembolism. N Engl J Med 378: 615-624, 2018. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto D, Mochizuki Y, Nakagiri K, Shite J. Unusual rapid progression of non-bacterial thrombotic endocarditis in a patient with bladder cancer despite undergoing intensification treatment with ribaroxaban for acute venous thromboembolism. Eur Heart J 39: 3907, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Mantovani F, Navazio A, Barberi A, Boriani G. A first described case of cancer-associated non-bacterial thrombotic endocarditis in the era of direct oral anticoagulants. Thrombo Res 149: 45-47, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Gundersen H, Molynihan B. An uncommon case of stroke: non-bacterial thrombotic endocarditis. J Stroke Cerebrovasc Dis 25: e163-e164, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Sack GH, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore) 56: 1-37, 1977. [PubMed] [Google Scholar]
- 13.Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 83: 746-756, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Bell WR, Starksen NF, Tong S, Porterfield JK. Trousseau's syndrome. Devastating coagulopathy in the absence of heparin. Am J Med 79: 423-430, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Asopa S, Patel A, Khan O, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur Cardiothorac Surg 32: 696-701, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Edoute Y, Haim N, Rinkevich D, Brenner B, Reisner SA. Cardiac valvular vegetations in cancer: a prospective echocardiographic study of 200 patients. Am J Med 102: 252-258, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Mandri GL, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke. A biplane transesophageal echocardiographic study. Stroke 25: 582-586, 1994. [DOI] [PubMed] [Google Scholar]