Abstract
A 23-year-old woman was admitted for slowly progressive proximal limb muscle weakness from childhood with elevated muscle enzyme levels. Although muscular diseases were suspected, an electromyogram showed remarkable neurogenic changes, and a muscle echogram indicated selective muscle involvement, including dissociation between the soleus and gastrocnemius, which was consistent with previous reports using magnetic resonance imaging (MRI). She was diagnosed with SMA type 3 following genetic testing, and nusinersen was soon initiated. An early diagnosis is mandatory to maximize the benefit of treatment. A muscle echogram may facilitate an early diagnosis in a non-invasive and time-saving manner compared to MRI.
Keywords: SMA, muscle ultrasound, muscle MRI, selective muscle involvement, nusinersen, luminosity ratio
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive neuromuscular disorder caused by a mutation in the survival motor neuron 1 gene (SMN1). After a long period in which no medications were available to treat SMA, nusinersen, an antisense oligonucleotide, was approved for pediatric and adult patients with SMA (1). The efficacy depends on the time when the treatment starts (2). SMA is characterized by progressive muscle atrophy and weakness in the proximal limbs (3), and a disease-specific pattern of muscle involvement is observed (4).
Magnetic resonance imaging (MRI) is useful for detecting such muscle selectivity. The triceps brachii, quadriceps femoris, and iliopsoas are most severely affected, whereas the deltoid, adductor longus, portions of the hamstrings, gracilis, sartorius, and rectus abdominis muscles are preserved in SMA type 3 patients (4). Another report showed that the soleus muscle was more vulnerable than the gastrocnemius (5). Although selective involvement of specific muscles increases the diagnostic value, a physical examination alone is often challenging for the detection of such features; muscle imaging is thus helpful and provides compelling information about selectivity (6). MRI has been used for this purpose, and muscle ultrasound has emerged as a more convenient alternative method that is available at the bedside.
Wu et al. reported the usefulness of muscle ultrasound for assessing the severity of SMA types 2 and 3 by investigating the luminosity in 4 muscles (biceps brachii, wrist extensors, quadriceps, and tibialis anterior) in 25 patients, potentially contributing to an early diagnosis (7). However, no study has described the selectivity of the affected muscles in SMA type 3 using muscle ultrasound.
We herein report an adult patient with SMA type 3 in which muscle ultrasound was quite useful for detecting selective muscle involvement. Ultrasound contributed to the early diagnosis and the initiation of treatment with nusinersen.
Case Report
A 23-year-old woman was admitted to our hospital due to difficulty walking long distances. She had been poor at running in childhood. An orthopedic doctor only noted that she was pigeon-toed. At eight years old, she showed overt difficulty with ambulation on school excursions. About one year before admission, she required a cane for walking due to muscle weakness. A general physician noted proximal weakness in all extremities [manual muscle test (MMT) 3-4] and elevated creatine kinase as high as 900 U/L. Muscular dystrophy was suspected, so the patient was referred to our hospital for a further examination, including a muscle biopsy.
On admission, she was obese with a body mass index as high as 32.1 kg/m2. The MMT revealed moderate proximal limb weakness: deltoid 4/4, biceps 4+/4+, triceps 4/4, forearm and intrinsic hand muscles 5/5, iliopsoas muscles 3/3, quadriceps muscles 3/4, tibialis anterior and gastrocnemius muscles 5/5. Her deep tendon reflex was overall decreased. No atrophy, fasciculation, or myotonia was present. Her sensory and coordination systems were intact. The serum level of creatine kinase was 973 U/L (normal range, 50-230 U/L). Extractable nuclear antigens were negative, and serum thyroid hormone levels were normal.
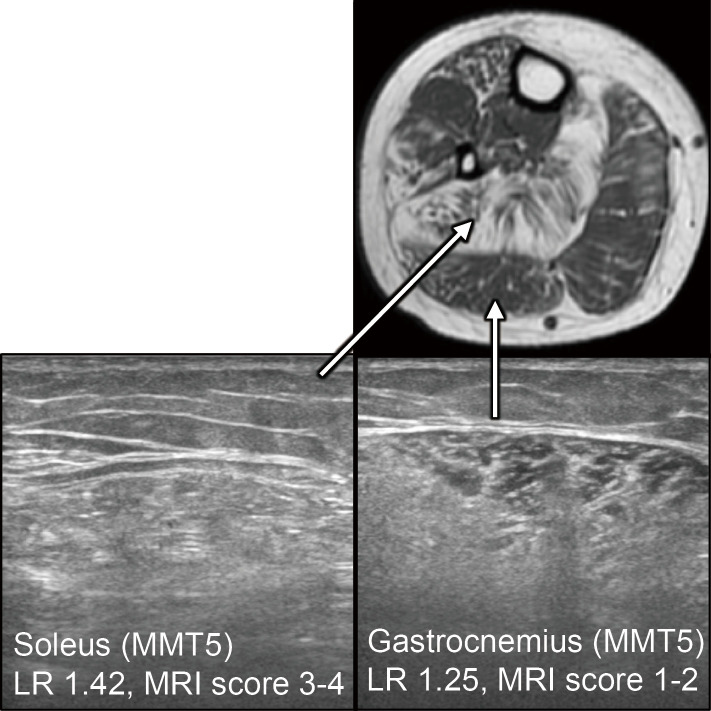
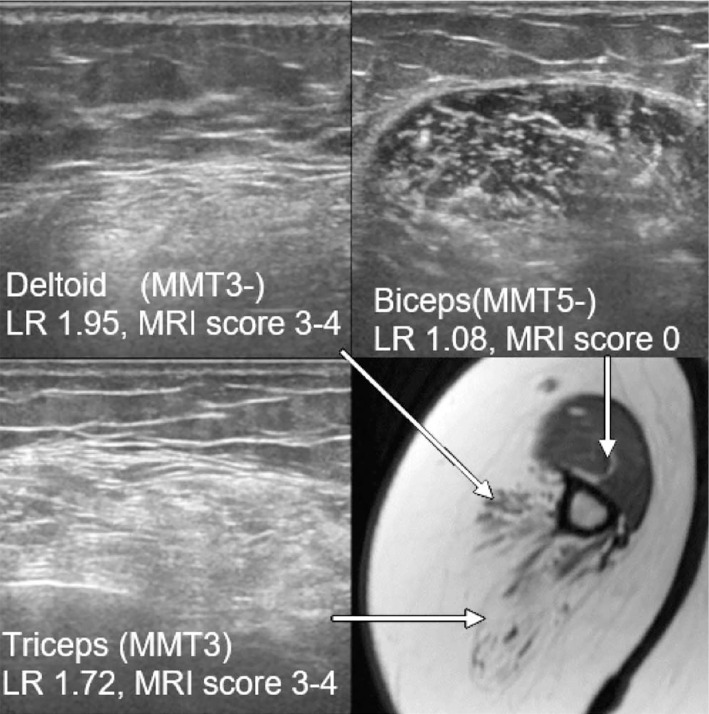
A nerve conduction study was normal, and repetitive nerve stimulation in the right accessory nerve demonstrated a decrementing response (14.6%). An electromyogram showed large motor unit action potentials with very late recruitment. No spontaneous activities, including positive sharp waves, fibrillation, or fasciculation potentials, were detected in the deltoid and quadriceps muscles, indicating chronic neurogenic disease. We conducted muscle ultrasound in the right arm and leg with a standard system using a 5- to 18-MHz probe (Fig. 1-3). The gain, compression, and time gain compensation sonographic settings remained constant for all muscles examined. Images were exported to the ImageJ software program (8) to calculate the luminosity ratio (LR), which was obtained by dividing the luminosity of the target muscle by that of the subcutaneous fat. Regions of interest were placed at the center of muscles or subcutaneous fat, avoiding fascia, as in a previous study (7). We utilized the histogram function, which provided luminosity values for the selected area to record both muscles and the subcutaneous fat. In the upper extremity, LR scores correlated with the physical findings of muscle strength; LRs were remarkably increased in the triceps and the deltoid to a similar extent, whereas those in the biceps were normal (Fig. 2, Table). A normal echo signal was obtained in the forearm muscles, the strength of which was fully preserved. In the thigh, subcutaneous fat prevented us from estimating the luminosity in different muscles (Table). Interestingly, in the lower leg muscles in which full strength was preserved, the ultrasound showed a marked high signal in the soleus but yielded normal signals in the tibialis anterior or gastrocnemius (Fig. 3, Table).
Figure 1.
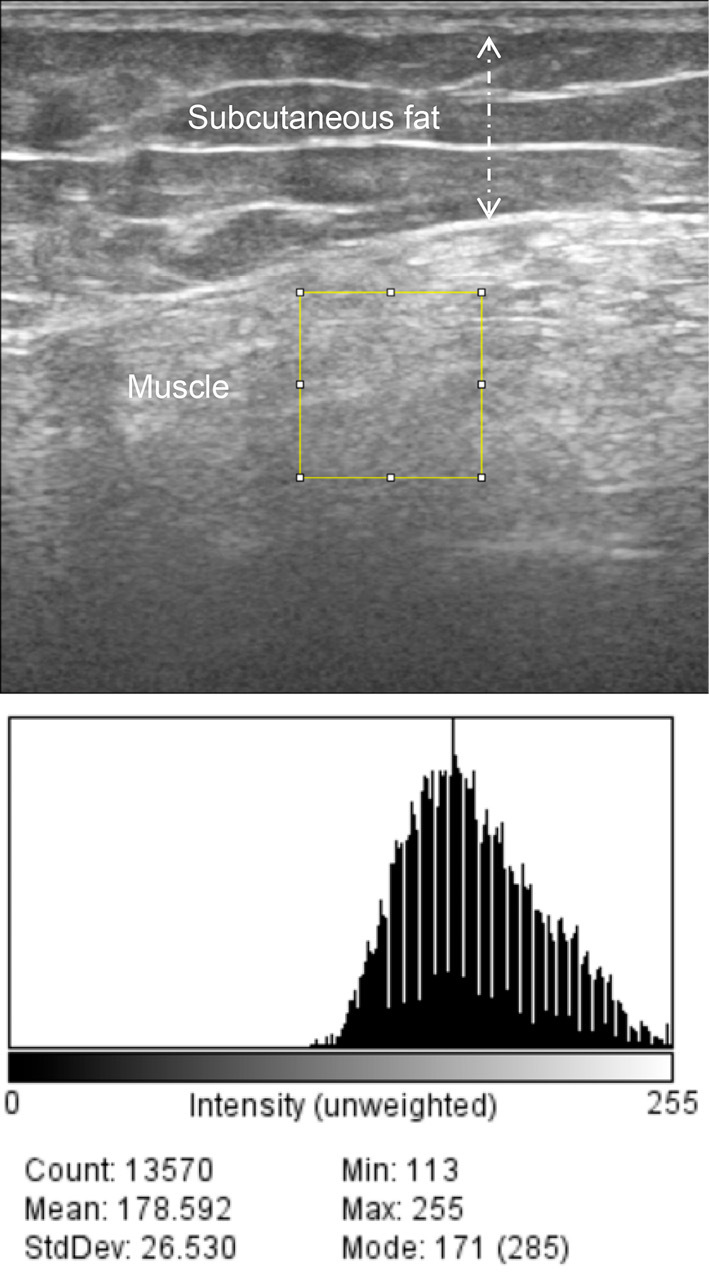
Quantitative ultrasound image analyses using the ImageJ software program: An ultrasound image shows a region of interest in the rectus femoris muscle. The histogram box to the bottom of the image shows the corresponding grayscale luminosity values (mean 178). Subcutaneous fat is also shown (dashed line).
Figure 3.
Muscle MRI (T1WI) and ultrasound in the lower leg: LRs and MRI scores are increased in the soleus compared to the gastrocnemius. Dissociation between the soleus and gastrocnemius is clearly shown.
Figure 2.
MRI (T1WI) and ultrasound images in the humerus: LRs and MRI scores are remarkably increased in the triceps and deltoid to a similar extent, whereas the intensity in the biceps appears normal.
Table.
MMT, MRI Scores, and LRs.
| Muscle | MMT | MRI score | LR | |
|---|---|---|---|---|
| Humerus | Deltoid | 3- | 3-4 | 1.95 |
| Biceps brachii | 5- | 0 | 1.08 | |
| Triceps brachii | 3 | 3-4 | 1.72 | |
| Forearm | EDC | 5 | 0 | 1.19 |
| FCU | 5 | 0 | 1.05 | |
| FDP | 5 | 0 | 1.04 | |
| FDI | 5 | * | 1.19 | |
| Thigh | Rectus Femoris | 4 | 3 | 1.67 |
| Vastus Med | 3 | 1.58 | ||
| Vastus Lat | 3 | 1.42 | ||
| Gracilis | 2 | 4 | 2.14 | |
| sartorius | 4 | |||
| adductor longus | 1 | |||
| Biceps, long head | 4+ | 3 | 1.6 | |
| Biceps, shor head | 1 | * | ||
| Lower leg | TA | 5 | 0-1 | 1.24 |
| GC | 5 | 1-2 | 1.25 | |
| Soleus | 3-4 | 1.42 |
Similar results were obtained from muscle MRI; the triceps, deltoid (Fig. 2, Table), quadriceps femoris, gracilis, sartorius, and most of the hamstrings showed marked high intensities, whereas the biceps brachii, adductor longus, and short head of the biceps femoris were relatively spared in T1-weighted images (Table). MRI was superior to ultrasound in the ability to analyze the thigh muscles in our patient. In the lower leg muscles, on the other hand, MRI showed a similar trend to ultrasound, with the marked involvement of the soleus, but not the gastrocnemius or tibialis anterior, demonstrated (Fig. 3, Table).
To perform a quantitative evaluation, MRI findings were also scored from fatty degeneration using a 5-point rating scale based on the amount of fatty degeneration as follows: stage 0, normal; stage 1 (mild), traces of increased signal intensity on T1-weighted MR sequences; stage 2 (moderate), increased signal intensity in <50% of the muscle; stage 3 (severe), increased signal intensity in >50% of an examined muscle; and stage 4 (end-stage), complete fatty degeneration of the entire muscle (4).
The data of LRs and MRI scores are summarized in Figs. 2, 3 and Table. Our patient underwent genetic testing, in which the deletion of SMN1 and four copies of SMN2 were detected, leading to the diagnosis of SMA type 3. The intrathecal administration of nusinersen was started.
Discussion
We herein report the first case of a patient with SMA type 3 in which muscle ultrasound successfully demonstrated the selectivity of muscle involvement. The presence or absence of a high signal in muscles on ultrasound was correlated with muscle strength. Notably, despite the observation that muscle strength of the forearm and lower leg muscles was normal, the soleus muscle exclusively showed high signals on ultrasound, whereas the tibialis anterior and gastrocnemius showed a normal intensity. This “silent contrast” was quite impressive and was also delineated by MRI.
Muscle ultrasound has several advantages over MRI. Ultrasound is a well-tolerated bedside method, requires minimal immobility by the patient, and can survey multiple muscles quickly and non-invasively. Ultrasound is particularly useful for children who have difficulty undergoing time-consuming MRI and painful electromyograms and for patients for whom MRI is contraindicated. However, ultrasound also has a disadvantage in that the results are affected by several potential technical difficulties and recording conditions, such as the depth of the target muscles and thick subcutaneous fat. Indeed, in our case, ultrasound was unable to depict the muscle involvement profiles in the thigh, one of the most specific patterns in SMA, but MRI clearly showed thigh muscle involvement (Fig. 3).
Quantitative muscle ultrasound is suitable for screening of neuromuscular diseases. A prospective study in children showed that quantification of the muscle echo intensity was able to distinguish neuromuscular disorder from non-neuromuscular diseases with a sensitivity of 92% and specificity of 90% when ≥3 muscle groups showed an echo intensity over 0.9 SD above normal (9). Furthermore, patients with myopathies typically have normal or increased muscle thickness and normal subcutaneous fat thickness, whereas patients with neurogenic processes have decreased muscle thickness and increased subcutaneous fat thickness. Another study showed that Duchenne muscular dystrophy results in a severe, homogeneous increase in muscle echo intensity with normal muscle thickness, whereas SMA showed an inhomogeneous increase in echo intensity with severe atrophy (10). In the present case, LRs were useful for the quantitative assessment of the muscle echo intensity, which closely paralleled the muscle weakness (MMT) and MRI intensity (MRI scores) (Table), except for the soleus. This method may be helpful for standardizing and comparing the luminosity of different muscles examined. However, several factors that can affect the ability to obtain LRs, such as the subcutaneous fat thickness and technical errors, should be considered, although the normal range of these factors remains to be established.
Over the last few years, a new disease-modifying drug, nusinersen, which is an antisense oligonucleotide, has become available. To maximize the benefit of nusinersen, an early diagnosis and treatment are mandatory. However, distinguishing SMA from other muscular diseases, such as muscular dystrophy, based solely on clinical symptoms, physical examination findings, and laboratory data without an electromyogram is often difficult. The diagnostic delay for SMA type 3 averages 43.6 months (11). In our case, about 15 years passed from the apparent gait disturbance until a final diagnosis was reached. The patient had been misdiagnosed with muscular dystrophy at other hospitals because proximal muscle weakness gradually progressed starting in childhood, and her creatine kinase level was slightly elevated. Although genetic testing is necessary for a definitive diagnosis of SMA, this presents a delicate ethical issue for patients and their families. Therefore, genetic testing should be prudently conducted with appropriate genetic counselling. Nevertheless, in the era of the emergence of anti-oligonucleotide drugs, their efficacy strongly depend on making an early diagnosis of SMA. Our findings demonstrate the usefulness of muscle ultrasound, a non-invasive and quick bedside method compared to an electromyogram or MRI. Muscle ultrasound has high potential to provide crucial information, especially with regard to the selective vulnerability of affected muscles, which prompted us to recommend genetic testing for the diagnosis of SMA and subsequent nusinersen treatment.
In our case, muscle ultrasound and MRI clearly showed a dissociation between the soleus and gastrocnemius, which is consistent with a recent report using MRI (5). Our report is the first to show that muscle ultrasound can also detect such selectivity in the calf muscles, which is in clear contrast to the findings in patients with inclusion body myositis, who typically show a lower echo intensity in the soleus than in the gastrocnemius (12).
In conclusion, we describe an adult patient in whom muscle ultrasound was useful for detection of selective muscle involvement, leading to early diagnosis and treatment of SMA type 3. LR dissociation with soleus high intensity on ultrasound may be another diagnostic feature of SMA type 3. Further studies are needed to prove the usefulness of ultrasound and LR dissociation with soleus high intensity for the early diagnosis of SMA type 3.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Gidaro T, Servais L. Nusinersen treatment of spinal muscular atrophy: current knowledge and existing gaps. Dev Med Child Neurol 61: 19-24, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 377: 1723-1732, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 371: 2120-2133, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Durmus H, Yilmaz R, Gulsen-Parman Y, et al. Muscle magnetic resonance imaging in spinal muscular atrophy type 3: selective and progressive involvement. Muscle Nerve 55: 651-656, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Brogna C, Cristiano L, Verdolotti T, et al. MRI patterns of muscle involvement in type 2 and 3 spinal muscular atrophy patients. J Neurol 267: 898-912, 2020. [DOI] [PubMed] [Google Scholar]
- 6.Helmy H, Aboumousa A, Abdelmagied A, Alsayyad A, Nasr SA. The role of muscle ultrasound in helping the clinical diagnosis of muscle diseases. Egypt J Neurol Psychiatr Neurosurg 54: 29, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu JS, Darras BT, Rutkove SB. Assessing spinal muscular atrophy with quantitative ultrasound. Neurology 75: 526-531, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671-675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillen S, Scholten RR, Zwarts MJ, Verrips A. Quantitative skeletal muscle ultrasonography in children with suspected neuromuscular disease. Muscle Nerve 27: 699-705, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 37: 679-693, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Lin CW, Kalb SJ, Yeh WS. Delay in diagnosis of spinal muscular atrophy: a systematic literature review. Pediatr Neurol 53: 293-300, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Nodera H, Takamatsu N, Matsui N, et al. Intramuscular dissociation of echogenicity in the triceps surae characterizes sporadic inclusion body myositis. Eur J Neurol 23: 588-596, 2016. [DOI] [PubMed] [Google Scholar]